Abstract
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive hematological disease that arises from the oncogenic transformation of developing T cells during T-lymphopoiesis. Although T-ALL prognosis has improved markedly in recent years, relapsing and refractory patients with dismal outcomes still represent a major clinical issue. Consequently, understanding the pathological mechanisms that lead to the appearance of this malignancy and developing novel and more effective targeted therapies is an urgent need. Since the discovery in 2004 that a major proportion of T-ALL patients carry activating mutations that turn NOTCH1 into an oncogene, great efforts have been made to decipher the mechanisms underlying constitutive NOTCH1 activation, with the aim of understanding how NOTCH1 dysregulation converts the physiological NOTCH1-dependent T-cell developmental program into a pathological T-cell transformation process. Several molecular players have so far been shown to cooperate with NOTCH1 in this oncogenic process, and different therapeutic strategies have been developed to specifically target NOTCH1-dependent T-ALLs. Here, we comprehensively analyze the molecular bases of the cross-talk between NOTCH1 and cooperating partners critically involved in the generation and/or maintenance and progression of T-ALL and discuss novel opportunities and therapeutic approaches that current knowledge may open for future treatment of T-ALL patients.
Keywords: NOTCH, T-cell acute lymphoblastic leukemia (T-ALL), thymus, T-cell development, gamma secretase inhibitors (GSI), targeted therapies
1. NOTCH Signaling Pathway
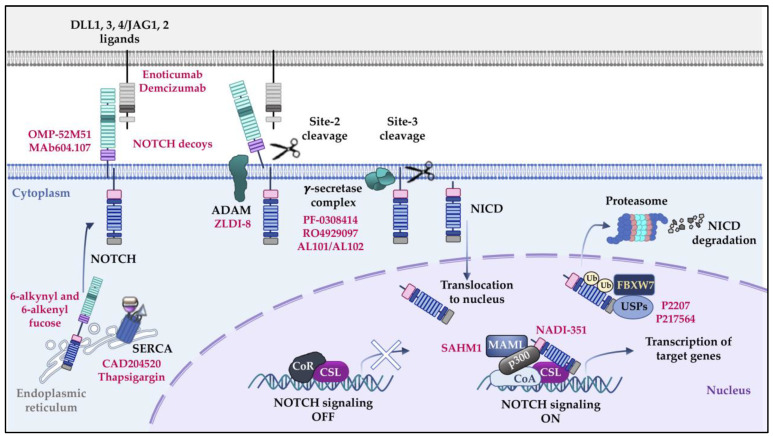
NOTCH comprises a family of highly evolutionary conserved membrane-bound receptors (NOTCH1-4 in mammals), which regulate essential functions in a wide variety of cell types within the organism, such as proliferation, differentiation, or apoptosis, thus controlling fundamental processes in embryonic and adult life, including tissue homeostasis, cell fate decisions or tissue development and regeneration [1]. NOTCH signaling is initiated by cell-to-cell contacts that allow the interaction between NOTCH receptors expressed on the surface of one cell and their ligands, belonging to the DELTA-LIKE (DLL1, DLL3 and DLL4) and JAGGED families (JAG1 and JAG2) in vertebrates [2], expressed on neighboring cells (Figure 1). Ligand recognition by NOTCH receptors triggers a proteolytic cleavage cascade leading to irreversible activation of the pathway. Firstly, ectodomain shedding, mediated by metalloproteases of the ADAM family, occurs within the heterodimerization domain (HD) at the extracellular portion of NOTCH (site-2); and secondly, a protein complex with intrinsic γ-secretase activity enzymatically cleaves the transmembrane domain (TM; site-3), and releases NOTCH intracellular domain (NICD). NICD then translocates to the nucleus and binds to the CSL (CBF1/RBPJ, Su(H), Lag-1) transcription factor, turning it from a repressive into a transcriptionally active complex by displacement of corepressors [SHARP, histone deacetylase-1 (HDAC-1), silencing mediator of retinoic acid and thyroid hormone receptors (SMRT), N-CoR], and recruitment of coactivators [Mastermind-like 1-3 (MAML1-3)] and histone acetyltransferases (p300/CBP) [2]. Several cellular- and context-dependent NOTCH transcriptional targets are then induced, including HES1-5, HEY-1 and -2, PTCRA, MYC, DTX1, IL7R, CD44 and RUNX1 [3]. The shutdown of NICD signaling is finally induced following the recruitment of cyclin-dependent kinase 8 (CDK8) by MAML1 and phosphorylation of residues within the PEST domain [4]. Phosphorylated NICD can then be recognized by FBXW7, an E3 ubiquitin ligase that poly-ubiquitinates NICD and targets it for proteasomal degradation. Several other E3 ubiquitin ligases modulate NOTCH activity in different cellular contexts, either by ubiquitination of NOTCH receptors or ligands, such as suppressor of Deltex [Su(dX)], NEDD4, and MIB1/2 [5]. Recently, deubiquitinases, such as ubiquitin-specific peptidase 7 (USP7) and 10 (USP10), were shown to biochemically interact with and deubiquitinate and stabilize the intracellular NOTCH1 domain, contributing to sustain pathway activation. Thus, inhibition of endogenous USP7 activity results in decreased NOTCH1 protein levels [6,7], and USP10 inactivation also reduces NICD stability and diminishes NOTCH1-induced target gene expression [8], through a mechanism dependent on the hydroxylation status of NOTCH1 [9].
Figure 1.
NOTCH signaling and targeting strategies. Interaction of NOTCH receptors with DLL/JAG ligands exposes site-2 located within the HD domain of extracellular NOTCH, allowing proteolytic cleavage by ADAM metalloproteases. Sequential cleavage by a γ-secretase complex at the transmembrane region (site-3) of the receptor releases the NOTCH intracellular domain (NICD) that translocates to the nucleus and binds to the transcription factor CSL. In the absence of NOTCH signaling (NOTCH signaling OFF), CSL actively represses transcription of NOTCH target genes owing to interaction with corepressors (CoR). Upon NOTCH activation (NOTCH signaling ON), NICD recruits a transcriptional complex (including MAML, p300 and CoA) that displaces CoR from CSL, allowing CSL to become transcriptionally active and to induce NOTCH target gene transcription. Thereafter, FBXW7-dependent ubiquitination and subsequent proteasomal degradation of NICD lead to NOTCH signaling shutdown. NOTCH signaling can be efficiently inhibited by molecular targeting at different levels (red) by: (1) blocking receptor-ligand interactions with monoclonal antibodies (mAbs) [i.e., anti-NOTCH1 (OMP-52M51, Mab604.107) and anti-DLL4 (Enoticumab, Demcizumab) mAbs], or with NOTCH decoys; (2) inhibiting receptor proteolytic cleavage with ADAM inhibitors (ZLDI-8) or γ-secretase inhibitors (i.e., PF-0308414, RO4929097, AL101/AL102); (3) blocking NOTCH transcriptional complex activation with MAML1 inhibitors (SAHM1) or NOTCH ternary complex inhibitors (NADI-351); (4) favoring NOTCH degradation with USP7 inhibitors (P2207, P217564); or (5) interfering with NOTCH receptor maturation with POFUT1 inhibitors (6-alkynyl and 6-alkenyl fucose) and SERCA inhibitors (CAD204520, Thapsigargin).
2. Oncogenic Mutations and Deregulation of NOTCH1 Signaling in T-cell Acute Lymphoblastic Leukemia
T-cell development is one of the best-studied functions of NOTCH signaling in vertebrates. NOTCH ligands expressed by thymic epithelial cells (TECs) provide unique signals required at successive maturation stages for the commitment and development of bone marrow- (BM-) or fetal liver-derived HPCs along the T-cell lineage [10,11,12,13,14,15,16]. Seminal work by Radtke and colleagues demonstrated that the T-cell fate specification of multipotent HPCs is strictly dependent on signaling mediated by the NOTCH1 receptor [17], as conditional Notch1 loss-of-function mice had a defective T-cell production, and accumulated non-T cell lineages including B [17] and dendritic cells [18] in the thymus. Conversely, constitutive expression of active NOTCH1 in HPCs impaired B-cell differentiation and promoted the appearance of ectopic double positive (DP) thymocytes at extrathymic locations, such as BM and peripheral blood (PB) [19,20]. Once T-lineage commitment is induced, maintenance of T-cell specification and further differentiation is dependent on recurrent NOTCH1 signaling [11]. Separate studies showed that DLL4 is the specific NOTCH1 ligand indispensable for triggering both T-cell commitment and differentiation [13,21], although other NOTCH ligands (and receptors) are expressed in the thymus and participate in other processes away from T-cell development [16,22,23,24,25,26].
NOTCH1-DLL4 interactions control the massive expansion that T-cell progenitors undergo at two critical intrathymic checkpoints. At the early CD4− CD8− double negative (DN1-3) stages, thymocyte survival and proliferation are greatly dependent on interleukin 7 (IL-7) produced by TECs. Later, when functional TCRβ rearrangements occur, a TCRβ chain assembles together with the pTα invariant chain and CD3 modules at the cell surface to form the pre-T-cell receptor (pre-TCR), which then signals for survival and proliferation during β-selection [27,28]. NOTCH1 signaling critically contributes to both checkpoints by promoting the transcription and expression first of the IL7R gene encoding the α chain of the IL-7 receptor (IL-7Rα) [29,30,31], and then of the PTCRA gene encoding the pTα chain of pre-TCR [32]. Later in development, NOTCH1 signaling has to be downregulated at the CD4+ CD8+ double positive (DP) stage to allow for αβ T-cell development [15], and NOTCH1 further regulates the CD4 versus CD8 single positive (SP) lineage decision by modulating TCR signaling in DP thymocytes [33,34]. These results highlight the essential role of the NOTCH1 pathway as a master regulator of thymocyte survival and proliferation at crucial checkpoints. It was thus predictable that deregulated NOTCH1 signaling could lead to the aberrant expansion of developing thymocytes and the generation of leukemia. However, the first identified genetic alteration involving the NOTCH1 gene was detected only in less than 1% of T-cell acute lymphoblastic leukemia (T-ALL) patients. It consists of a chromosomal translocation to the TRB locus [t(7;9)(q34;q34.3)], which generates a truncated and constitutively active form of NOTCH1 that lacks site-2 and site-3 cleavage sites [35,36], resulting in γ-secretase-independent activation. Therefore, the seminal discovery in 2004 that more than 60% of T-ALLs carry NOTCH1 gain-of-function (GOF) somatic mutations that also generate a truncated and constitutively active receptor caused an immense revolution in the field, pointing to NOTCH1 as a key regulator of T-ALL pathogenesis [37].
T-ALL is a heterogeneous tumor originating from the malignant transformation of developing T cells that arises from accumulating genetic alterations in key oncogenic, tumor suppressor and developmental pathways, which confer uncontrolled cell proliferation and survival capacity. Notably, NOTCH1 mutations producing aberrant NOTCH1 activation are present in T-ALLs from all major molecular subtypes [38,39]. The classification of clinically relevant biological groups of T-ALL was originally based on the immunophenotypic resemblance of the tumor to particular T-cell maturational stages, indicative of a specific developmental arrest during T-cell transformation. Currently, however, accuracy in the classification and stratification of T-ALL patients preferentially relies on genetic and transcriptomic studies of the specific mutational profile, gene expression signatures and genomic alterations of T-ALL samples [40,41,42,43,44,45,46]. Early T-lineage progenitor (ETP) T-ALL, which is characterized by a very immature CD4− CD8− DN T-cell phenotype and the coexpression of stem cell and myeloid-associated markers [47], commonly harbor mutations in epigenetic regulators (EZH2, IDH1, IDH2, DNMT3A), transcription factors associated to hematopoietic and T-cell development (ETV6, GATA3, RUNX1), and signaling pathways such NRAS and FLT3 [48]. Cortical T-ALLs, characterized by the expression of CD1a and a DP phenotype, are typically associated with genetic aberrations in TLX1, TLX3, NKX.2.1 and NKX.2.2 homeobox genes, and in CDKN2A, TAL1 and LMO1,2 [40,49]. TAL1 and LMO1,2 expression are also typical of mature T-ALL expressing surface CD3 [40,50]. Interestingly, NOTCH1 oncogenic mutations are frequent in cortical T-ALLs and can be found in up to 40% of mature T-ALLs [50], while ETP T-ALLs have a lower prevalence of NOTCH1 mutations [42].
T-ALL activating NOTCH1 mutations are mostly located in the HD or in the LIN12-Notch repeats (LNR) domain within the negative regulatory region (NRR) of the receptor located at the extracellular region. Class I HD mutants disrupt the HD domain, while class II involve short insertions that displace and expose the ADAM-dependent cleavage site-2, allowing for constitutive ligand-independent activation of NOTCH1. Sets of insertions that expand the juxtamembrane region (JME mutants) have also been described [51]. Additionally, a different type of mutation compromises the FBXW7 ubiquitin ligase-binding site located in the C-terminal PEST domain of NICD [37], which impairs the proteasomal degradation of NICD while increasing the half-life. Concordantly, FBXW7 mutants unable to bind NICD have been described to maintain NOTCH1 signaling in T-ALL and to confer resistance to γ-secretase inhibitors (GSI) [52,53]. In addition, high expression of USP7 has been shown to correlate with NOCH1 stabilization and expression in NOTCH1-dependent T-ALLs [6,7]. Although all activating NOTCH1 mutations confer constitutive and/or prolonged NOTCH1 signaling, only some of them, such as those leading to constitutive expression of the NICD form of NOTCH1, promote “strong” NOTCH1 signaling and have the intrinsic capacity to induce T-ALL in murine models [19]. Others, including HD and PEST mutations found in patients, generate weaker NOTCH1 mutant forms that complement other leukemogenic signals or require cooperation with additional oncogenic pathways or with loss-of-function tumor suppressor mutants for driving T-cell transformation [54,55,56,57,58]. These findings support the view that NOTCH1 mutations are secondary oncogenic events in many T-ALL patients [59]. However, NOTCH1 mutations can also act as the initial genetic event driving T-ALL [60]. More importantly, even when NOTCH1 participates secondarily, NOTCH1 activation is an early hallmark of T-cell leukemogenesis and a key regulator of the leukemia-initiating cell (LIC) activity of T-ALL [61,62]. Accordingly, T-ALL tumors show “addiction” to NOTCH1 signaling, reflecting a high selective pressure for NOTCH1 activation [63].
NOTCH1 Targeting Therapeutic Strategies
The realization that NOTCH1 signaling is a major oncogenic driver of T-ALL generated much expectation about the efficacy of pan-NOTCH inhibitors for the treatment of this disease. Several interventional therapies based on compounds that target the activation of NOTCH signaling at different levels have been developed and tested in vitro and in preclinical models (Figure 1, Table 1) [64]. Initial studies demonstrated the therapeutic efficacy of GSIs. However, the observation of severe adverse effects, particularly intestinal toxicities, in the first patients orally receiving GSIs [65,66] hampered the implementation of this therapy. Although these adverse effects could be minimized by coadministration of glucocorticoids [67], and novel GSI compounds have recently entered into clinical trials (i.e., AL101, AL102, PF-03084014, and RO4929097 [64,68,69,70]; Figure 1, Table 1), GSI treatment is still not widely established in the clinic. Indeed, PF-03084014 (Nirogacestat) was evaluated in a Phase I trial, including relapsed/refractory T-ALL patients, and although well tolerated, no conclusive anti-leukemic effects were obtained [70]. AL101, a potent GSI showing very promising anti-tumor efficacy in preclinical models [69]), is currently being evaluated against triple-negative breast cancer (NCT04461600) and adenoid cystic cancer (NCT04973683, NCT03691207), but no T-ALL trials have been opened. On the other hand, RO4929097 was well tolerated in several trials against different types of cancer, but their therapeutic efficacy in T-ALL patients has not been established. Moreover, NOTCH1-mutant but GSI-resistant T-ALLs demand the implementation of alternative strategies that efficiently and safely target NOTCH1-dependent tumor cells without detrimental effects. Promising monoclonal antibodies have been described to specifically bind mutated NOTCH1 receptors [i.e., OMP-52M51 (Brontictuzumab) [71], MAb604.107 [72]], or to block NOTCH1-ligand interactions (i.e., anti-DLL4, Demcizumab [73] and Enoticumab [74]) but their clinical relevance is yet to be established. The safety and tolerability of anti-NOTCH1 OMP-52M51 antibody have been evaluated in clinical trials for refractory solid tumors (NCT01778439) [75] and relapsed/refractory lymphoid malignancies, including T-ALL (NCT01703572) [76]. Both studies have found that OMP-52M51 has moderate anti-tumor activity and is well tolerated, with diarrhea as the main primary toxicity. Regarding anti-DLL4 antibodies, Demcizumab (NCT01189968) [77] and Enoticumab (NCT00871559) [78] have been assayed in Phase Ia trials for advanced solid tumors, showing partial responses and disease stabilization.
Table 1.
NOTCH1 pathway targeting strategies.
| Inhibitor | Target | Mechanism | References |
|---|---|---|---|
| P2207, P217564 | USP7 | Small molecule inhibiting USP7-dependent NOTCH1 deubiquitination |
[6,7] |
| PF-0308414 | γ-secretase complex | Small molecule inhibiting γ-secretase dependent NOTCH1 cleavage |
[64,68,70] |
| RO4929097 | γ-secretase complex | Small molecule inhibiting γ-secretase dependent NOTCH1 cleavage |
[64,68] |
| AL101/AL102 | γ-secretase complex | Small molecule inhibiting γ-secretase dependent NOTCH1 cleavage |
[64,68,69] |
| OMP-52M51 (Brontictuzumab) |
NOTCH1 | Antibody interfering with NOTCH1-ligand interaction |
[71] |
| Mab604.107 | NOTCH1 | Antibody interfering with NOTCH1-ligand interaction |
[72] |
| Demcizumab | DLL4 | Antibody interfering with NOTCH-DLL4 ligand interaction |
[73] |
| Enoticumab | DLL4 | Antibody interfering with NOTCH-DLL4 ligand interaction |
[74] |
| ZLDI-8 | ADAM17 | Small molecule inhibiting ADAM17-dependent NOTCH1 cleavage |
[79] |
| NOTCH decoys | NOTCH1 | Soluble inhibitors interfering with NOTCH1-ligand interaction | [80] |
| SAHM | MAML1 | Stapled α-helical peptides interfering with MAML1 binding to NOTCH transcriptional complex |
[81] |
| NADI-351 | NOTCH Ternary complex (NTC) | Small molecule disrupting NTC | [82] |
| Thapsigargin | SERCA | Small molecule inhibiting SERCA activity | [83] |
| CAD204520 | SERCA | Small molecule inhibiting SERCA activity | [84] |
| 6-alkynyl and 6-alkenyl fucose |
POFUT1 | L-fucose analogs disrupting NOTCH1-DELTA-ligand interactions |
[85] |
Other molecules shown to inhibit NOTCH activation in preclinical settings include ADAM inhibitors [79] and NOTCH decoys [80]. MAML1 and NOTCH ternary complex (NTC) inhibitors, such as SAHM1 [81] and NADI-351 [82], respectively, capable of blocking NOTCH transcriptional complex activity, have demonstrated potential anti-cancer properties. Inducing NOTCH1 intracellular domain degradation by inhibition of endogenous USP7 activity, either by shRNA or with pharmacological inhibitors (i.e., P2207, P217564) [6,7], has been shown to reduce in vitro proliferation, to induce cytotoxicity in T-ALLs, and to inhibit in vivo expansion of NOTCH1-dependent T-ALLs. Novel strategies directed to impair NOTCH1 receptor maturation processes in the endoplasmic reticulum (ER)/Golgi compartments, and subsequent cell surface expression, have proved very efficient in blocking NOTCH1 activation. Among them, SERCA (sarco/endoplasmic reticulum calcium ATPase) inhibitors have been identified as potential regulators of leukemia-associated NOTCH1 mutant signaling and cell cycle progression and expansion of T-ALL xenografts [83]. Improved formulations of SERCA inhibitors, such as CAD204520, have been developed aimed at reducing off-target toxicity or at inducing specific delivery to leukemic cells [84]. Other inhibitors that interfere with the maturation of NOTCH receptors, such as fucose analogs (6-alkynyl and 6-alkenyl fucose), which interfere with the O-fucosyltransferase 1 (POFUT1)-dependent transfer of O-fucose to epidermal growth factor (EGF) repeats of NOTCH receptors, have proved to successfully impair DLL1 and DLL4 interactions with NOTCH receptors [85]. On the other hand, recent phosphoproteomic and expression-based screenings have revealed novel targetable kinases potentially involved in the NOTCH1-launched oncogenesis program and in GSI resistance [86,87,88,89,90,91]. Some examples are Src-family kinases and active cyclin-dependent kinases [90] and protein kinase C (PKC) delta [89].
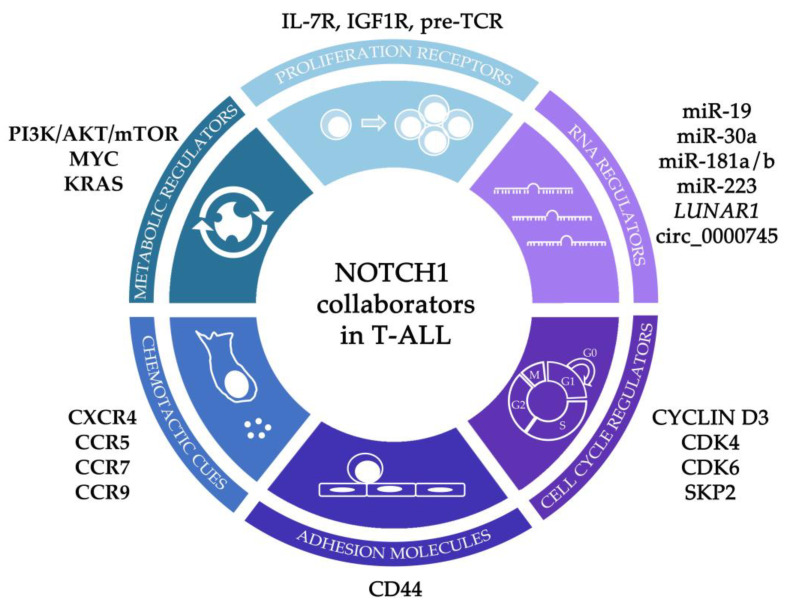
Besides therapeutic strategies directly targeting single components of the NOTCH1 pathway itself (Table 1), the realization that the T-ALL oncogenic process involves multiple NOTCH1 collaborators has revealed new molecular vulnerabilities that offer the opportunity for novel therapeutic developments. NOTCH1 oncogenic collaborators include cell cycle regulators, proliferation and adhesion receptors, chemokine receptors, metabolic regulators or RNA modulators (see below). Therefore, understanding the molecular programs underlying NOTCH1 signaling-dependent T-cell transformation and its multiple steps of regulation is critical for designing new drug combinations that target aberrant NOTCH1 activation in T-ALLs. In addition, deciphering the mechanistic bases of NOTCH1 cross-talk with other signaling pathways and molecular regulators involved in cooperative oncogenesis will help to delineate novel therapeutic approaches to specifically target T-ALL cells while diminishing the risk of undesired collateral effects on healthy cells.
3. Molecular Collaborators of NOTCH1 Signaling in T-ALL Pathogenesis
Multiple signaling pathways and molecular regulators collaborate with NOTCH1 signaling, contributing to T-ALL pathogenesis through several mechanisms, such as favoring cell proliferation and survival through specific surface receptors or by impaired cell cycle control, increasing the fitness of leukemic cells by the modulation of their metabolic demands, or guiding T-ALL cells to specialized niches where they can expand uncontrollably. In this section, we will overview recent findings uncovering some of the molecular collaborators that cooperate in NOTCH1-mediated T-ALL pathogenesis (Figure 2, Table 2). Most experimental strategies approaching this aim have relied on murine models of NOTCH1-mediated T-cell oncogenesis. Widely used is the mouse T-ALL model established by Pear and colleagues, consisting in the transplantation to host mice of murine BM HPCs transduced with the constitutive active form of NOTCH1 (NICD) [19]. The model allows for the generation and follow-up of a murine T-ALL, opening the door to preclinical testing of new therapeutic options to fight disease relapse, which is a main T-ALL clinical issue. However, whether the model recapitulates the earliest stages of human T-ALL pathogenesis has remained elusive, as a retrospective analysis of human T-ALL onset is unfeasible in patients, and no information on such early stages is yet available. Overcoming this limitation, a novel model of human T-ALL generation has recently been developed, consisting of the reconstitution of NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) immunodeficient mice with human CD34+ umbilical cord blood (CB) hematopoietic progenitors expressing constitutively active NOTCH1 [92]. Transplanted mice generate de novo clonal human leukemia that resembles T-ALL in patients, allowing for the delineation of the pathogenic events associated with the onset of the human disease, and revealing the stepwise impact of NOTCH1 mutations on human T-ALL pathogenesis. An additional human T-ALL model has also been described by Weng’s group in NSG mice transplanted with CD34+ CB HPCs cotransduced with NOTCH1-ΔE mutants together with a combination of oncogenes identified in T-ALL patients, i.e., LMO2, TAL1 and BMI1 (LTB) [93], allowing to study the collaboration of these mutations with the NOTCH1 oncogenic program. Therefore, mouse and human T-ALL models provide clinically relevant complementary tools useful for the characterization of NOTCH1 functional interactions with other signaling pathways and molecular regulators in T-ALL, for the identification of human T-ALL LICs and their specific targets, and for the development and validation of improved therapeutic options.
Figure 2.
NOTCH1 pathway collaborators contributing to T-ALL pathogenesis.
3.1. RNA Regulators
In recent years, the non-coding component of the genome has gained great relevance because of its implication in numerous physiological and pathological processes. Non-coding RNAs (ncRNAs) include microRNAs (miRNAs), long non-coding RNAs (lncRNAs), circular RNAs (circRNA), PIWI-interacting RNAs (piRNAs), small nucleolar RNAs (snoRNAs), transcribed ultraconserved regions (T-UCRs), and large intergenic non-coding RNAs (lincRNAs) [94]. While miRNAs have been the most studied ncRNAs in T-ALL to date, lncRNAs and circRNAs have drawn attention lately because of their contribution to T-ALL pathogenesis, and especially to NOTCH1-dependent T-ALL. However, the relationship between NOTCH signaling and ncRNA deregulation in T-ALL is still a matter of intensive study.
Table 2.
RNA regulators and signaling pathways contributing to NOTCH1-induced T-ALL pathogenesis.
| Cellular Process | Molecule | Experimental Model | Effects on T-ALL | References |
|---|---|---|---|---|
| RNA regulators | miR-223 | Cotransduction with NICD in FL-HPCs | Accelerated NICD-dependent leukemia onset | [95] |
| miR-19 | Cotransduction with NICD in FL-HPCs | Accelerated NICD-dependent leukemia onset |
[96] | |
| miR-181a/b | miR-181ab1-/- HPCs + NICD | Increased mice survival & Reduced leukemic burden |
[97] | |
| miR-30a | Retroviral overexpression in T-ALLs | Decreased T-ALL growth rate in vitro | [98] | |
| lncRNAs (LUNAR1) |
LUNAR1 shRNA + T-ALL cell lines | Growth disadvantage | [99] | |
| circRNAs | circ_0000745 overexpression | Promoted proliferation Reduced apoptosis |
[100] | |
|
Survival/
Proliferation |
IGFR1 | IGF1Rneo BM HPCs + NICD | Reduced LIC activity | [101] |
| IL-7R | Il-7r-/- BM HPCs + NICD | In vivo T-ALL impairment | [102] | |
| pre-TCR | Rag2-/-, Slp76-/- BM HPCs + NICD | In vivo T-ALL impairment/delay | [103,104] | |
| Metabolic regulators | KRAS | KrasG12D BM HPCs + NOTCH1 GOF alleles | Accelerated T-ALL development | [54] |
| PI3K/AKT/ mTOR |
Pten-loss-of-function + L1601P Δ-PEST NOTCH1 | Acquisition of in vivo GSI resistance | [105] | |
| GSI + mTOR inhibitor | Decreased in vitro T-ALL proliferation Increased mice survival |
[106] | ||
| [107] | ||||
| MYC | N-Me-/- BM HPCs + ΔE-NOTCH1 | In vivo T-ALL impairment | [108] | |
|
Cell cycle
regulators |
CYCLIN D3 | Ccnd3-/- HPCs + NICD | In vivo T-ALL impairment | [109] |
| CDK6 | Cdk6-/- HPCs + NICD | In vivo T-ALL impairment | [110] | |
| Chemotaxis/Adhesion | CD44 | Anti-CD44 antibody | Impaired BM engraftment and T-ALL progression | [92] |
| CXCL12 | Vascular endothelial cell-specific Cxcl12-/- mice | Reduced Notch-dependent T-ALL expansion in vivo | [111] | |
| CXCR4 | AMD3100 inhibitor Cxcr4-/- FL-HPCs + NICD |
Blocked T-ALL BM colonization In vivo T-ALL impairment |
[112] | |
| CCR7 | Ccr7-/- BM HPCs + NICD | Inhibition of CNS infiltration | [113] | |
| CCR5/9 | GSI/siRNA NOTCH1 | Reduced T-ALL proliferation/migration | [114] |
Abbreviations: T-ALL (T-cell acute lymphoblastic leukemia); NICD (Notch intracellular domain); FL (fetal liver); HPCs hematopoietic progenitor cells); shRNA (short hairpin RNA); BM (bone marrow); GSI (γ-secretase inhibitor); mTOR (mammalian target of rapamycin); GOF (gain of function); siRNA (small interference RNA); CNS (central nervous system).
3.2. miRNAs
The significance of the cross-talk between NOTCH1 and miRNAs in T-ALL was revealed by Junker and colleagues [115], who took advantage of a T-cell-specific Dicer1 conditional loss-of-function mouse model to demonstrate the strict requirement of the miRNA machinery for the generation of NOTCH1-dependent T-ALL. DICER1 is an RNaseIII endoribonuclease that cleaves a precursor miRNA into the 20-22 nucleotide (nt)-long mature regulatory miRNA that will then be incorporated into the RNA-induced silencing complex (RISC), together with the targeted messenger RNA (mRNA), to prevent its translation or to induce its degradation [116]. Loss of miRNAs induced apoptosis in leukemic T cells and impaired leukemia development and progression both at early- and late-stages of T-ALL pathogenesis [115]. Accordingly, several miRNAs (miR-19b, miR-20a, miR-26a, miR-92, and miR-223) accelerate leukemia progression when coexpressed with NICD in a mouse T-ALL model [95] through a mechanism involving specific targeting of T-ALL tumor suppressor genes including Pten, Bim, Nf1, Fbxw7, Ikzf1 and Phf6. Hence, these miRNAs were categorized as T-ALL oncomiRs. Supporting this oncogenic role, miR-19 expression was 5- to 17-fold-increased in T-ALL samples when compared to normal T lymphocytes [96]. In addition, genetic analyses showed that miR-19 is involved in two T-ALL-associated gene rearrangements, comprising T-cell receptor α/γ (TRA/D) locus (t13;14)(q32;q11) and NOTCH1 locus t(9;14)(q34;q11), the latter generating a constitutively active form of NOTCH1. When expressed together with NICD in transplanted fetal liver (FL) HPCs, miR-19 significantly decreased mouse mean survival compared to mice transplanted with HPCs overexpressing NICD alone, suggesting that miR-19 exacerbates the NOTCH1-induced oncogenic program [93]. The identification of several miR-19 targets, including Bim, Pten, Prkaa1, and Ppp2r5e, suggests that miR-19 may sustain lymphocyte survival contributing to leukemogenesis through the regulation of phosphoinositide-3 kinase (PI3K)-related pathways [96].
Several reports have included miR-223 in the list of regulators that cooperate with oncogenic NOTCH1 in T-ALL. Besides its contribution to myeloid development, particularly to granulocyte and macrophage differentiation and to the pathogenesis of acute myeloid leukemia (AML) [117], miR-223 is induced by active NOTCH1 and participates in T-ALL leukemogenesis by repressing the tumor suppressor FBXW7 [118]. Likewise, miR-223-dependent FBXW7 suppression has been described in T-ALLs induced by the oncogenic transcription factor TAL-1 [119]. Accordingly, an inverse correlation of miR-223 and FBXW7 expression has been observed in a panel of T-ALL patient-derived xenografts (PDXs) [118]. In addition to FBXW7, miR-223 targets other regulators of NOTCH1 degradation, such as ARRB1 (Arrestin β1), which serves as an E3 ubiquitin ligase adaptor and facilitates NOTCH1 ubiquitination and degradation via interaction with DTX1 [120]. However, multiple levels of cross-regulation between miR-223 and NOTCH1 may contribute to T-ALL, considering that NOTCH1 signaling can also repress miR-223 leading to upregulation of insulin-like growth factor-1 receptor (IGF1R), which is important for LIC activity in T-ALL [101,121].
Other miRNAs collaborating in NOTCH1-dependent T-ALL pathogenesis include miR-181a-1/b-1 and miR-30a. Deletion of miR-181a-1/b-1 impairs T-cell leukemogenesis induced by either murine NICD or human P12ΔP NOTCH1 alleles, reducing leukemic burden within the thymus and increasing up to 80% mouse survival [97]. At the molecular level, miR-181a-1/b-1 controls the strength and threshold of NOTCH1 receptor activity by dampening multiple negative feedback regulators downstream of both NOTCH1 and pre-TCR [97]. In contrast to the aforementioned miRNAs, miR-30a exhibits tumor-suppressive properties in vitro and inhibits NOTCH1 and NOTCH2 expression in an MYC-dependent manner, and it was also found to participate in a bidirectional regulatory circuitry between NOTCH and MYC in T-ALL [98]. Collectively, available information points to several miRNAs as optimal therapeutic targets for the treatment of NOTCH1-dependent T-ALLs (Figure 3).
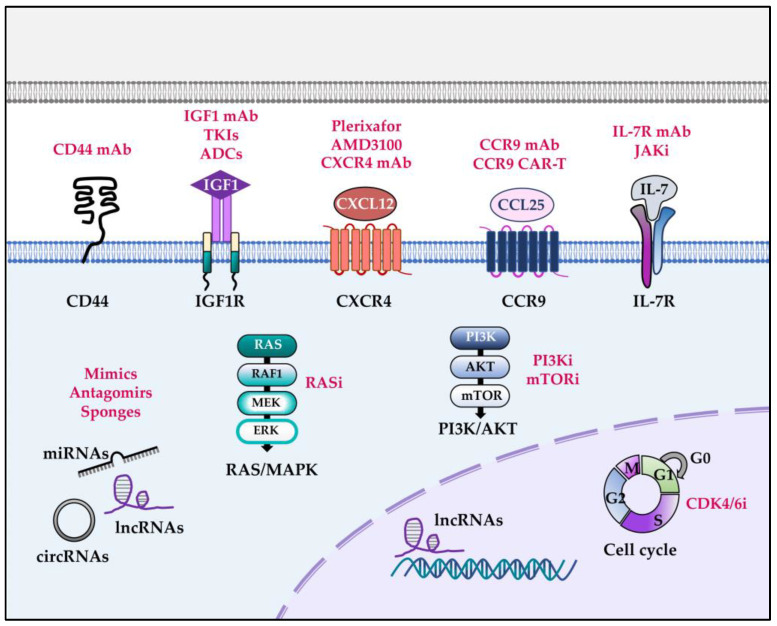
Figure 3.
Preclinical therapies targeting NOTCH1 collaboration partners in T-ALL pathogenesis. Therapeutic approaches (black) specifically targeting signaling pathways and molecular regulators (red) that collaborate with NOTCH1 in T-ALL pathogenesis proved successful in preclinical mouse models. ADCs (antibody-drug conjugates); CAR-T (chimeric antigen receptor T cells); i (inhibitor); mAb (monoclonal antibody); TKIs (tyrosine kinase inhibitors).
3.3. lncRNAs
lncRNAs are a heterogeneous class of ncRNAs that, basically, have a 200 nt minimum length and an apparent lack of protein-coding potential [122]. Although their mechanisms of action are not fully understood, lncRNAs are known to participate in several cellular processes, such as cell cycle progression [123] and epigenetic regulation [124] in different contexts, including hematopoiesis [125,126] and leukemia [127,128,129,130]. Several reports have addressed the NOTCH1-modulated lncRNA repertoire of normal thymocytes and T-ALLs [99,131]. By comprehensive mapping of lncRNAs, Trimarchi and colleagues identified a T-ALL-specific lncRNA signature, including more than 1000 transcripts [99]. Chromatin immunoprecipitation sequencing (ChIP-seq) and GSI treatment assays revealed that a great proportion of these transcripts were regulated by NOTCH1. Of note, expression of the lncRNA, LUNAR1, which displays T-ALL anti-tumor potential, was regulated by NOTCH1 through the IGF1R locus enhancer [99]. In a different study, NALT lncRNA was found to correlate with NOTCH1 expression in pediatric T-ALL and to regulate the NOTCH1 signaling pathway through cis-regulatory mechanisms [132]. Since lncRNAs still remain widely unexplored, it is expected that a large number of new molecules will soon be identified as part of the gene expression network that oncogenic NOTCH1 shapes in T-ALL pathogenesis.
3.4. circRNAs
circRNAs comprise a novel group of ncRNAs generated from back-splicing when an exon is spliced to the preceding instead of to the downstream exon, resulting in a covalently-closed continuous loop structure [133]. Most of them are expressed at low levels in normal cells; however, the higher expression of some circRNAs, and their regulation during cell differentiation processes, suggest a specific yet unknown function. Very few circRNAs have been shown to date to contribute to NOTCH1-dependent oncogenesis. The T-ALL circRNA landscape has been established recently [134], identifying almost 1000 circRNAs with differential expression in T-ALL associated with particular T-ALL genetic signatures. However, their relationship with the NOTCH1 pathway remains to be elucidated. Only circ_0000745 was shown to favor NOTCH1 signaling in T-ALL, acting as a sponge for miR-193-3p and releasing miR-193-3p-mediated NOTCH1 repression through a competing endogenous RNA (ceRNA) mechanism [100].
3.5. Survival/Proliferation Signaling Receptors
3.5.1. Interleukin 7 Receptor (IL-7R)
Interleukin 7 (IL-7) is the essential thymic epithelium-derived cytokine sustaining the first wave of extensive proliferation that immature T cells must undergo during their development in the thymus. Mice deficient in IL-7 (Il7-/-) or in its receptor (Il7r-/-) have an early block in T-cell development, with a dramatic reduction in the generation of mature T cells, and also show an impaired B lymphopoiesis [135,136,137]. In humans, IL-7 receptor (IL-7R) paucity results in a complete lack of T cells, leading to severe combined immunodeficiency (SCID), but does not affect B-cell development [138,139]. IL-7R is commonly expressed in T-ALL, reflecting a developmental block of T-cell lymphoblasts at an IL-7-dependent developmental stage. Accordingly, IL-7-dependency is shared by most T-ALL cells expressing functional IL-7Rs, which respond to IL-7 stimulation by activating Janus kinase (JAK)/signal transducer and activator of transcription (STAT), mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and PI3K/protein kinase B (PKB or AKT)/mammalian target of rapamycin (mTOR) signaling pathways that lead to cell survival and proliferation. Supporting a prominent role for IL-7R in physiological and pathological NOTCH1-dependent T-cell development, NOTCH1 signaling was shown to directly control IL-7R-dependent T-cell expansion by transcriptionally regulating the expression of the IL-7R alpha (IL-7Rα) subunit, via binding to a promoter [29] and enhancer [30,31] regions in the IL7R gene. Also, NOTCH1-mediated T-ALL cell proliferation was shown to rely, to a great extent, on IL-7R signals, as the defective expansion of GSI-treated T-ALL cell lines could be rescued by enforced activation of IL-7/IL-7R signaling [29]. Importantly, it has been recently shown that IL-7R expression is an early functional biomarker of T-ALL cells with LIC potential [102]. Inhibition of endogenous IL-7Rα expression by short-interfering RNA (shRNA) was shown to impair engraftment and progression of IL-7R+ primary human T-ALLs [102], highlighting the relevance that targeting physiological IL-7R signaling may have in future T-ALL therapeutic interventions. More importantly, NOTCH1-dependent T-ALL pathogenesis seems strictly dependent on the IL-7/IL-7R axis, given the failure of NICD to induce T-cell transformation from Il7r-/- BM HPCs [102].
Besides its cooperation with NOTCH1, IL7R can behave itself as an oncogene in T-ALL. Gain-of-function mutations in IL7R exon 6, consisting of in-frame insertions or deletions-insertions, have been described in approximately 10% of T-ALL patients from different cohorts [140,141]. These mutations introduce an unpaired cysteine in the extracellular juxtamembrane-transmembrane region, promoting de novo formation of intermolecular disulfide bonds between mutant IL-7Rα subunits, which drive constitutive IL-7-independent proliferative and survival signaling and contribute to cell transformation and aberrant T-ALL growth. Other studies have shown that wild-type IL-7R overexpression is also able to induce leukemia with features of human T-ALL in mice. Either tetracycline-inducible Il7r transgenic mice or T-cell-specific human IL7R knockin mice (Rosa26-hIL-7R.huCD2-Cre) showed aberrant T-cell differentiation that resulted in the expansion of immature T cells in peripheral organs as mice aged and eventually led to the development of transplantable T-cell malignancies [142]. These data support a prominent pro-oncogenic role of the IL-7R pathway in T-ALL, which concurs with the identification of oncogenic mutations in several IL-7R-signaling downstream molecules such as JAK1, JAK3, and STAT5B in 5–20% of T-ALL cases [43,48,143]. Thus, either by itself or in combination with NOTCH1 signaling, IL-7R represents a major pathogenic pathway in T-ALL and a promising therapeutic target. Accordingly, recently developed anti-IL-7R antibodies capable of binding both wild-type and mutant IL-7Rs have shown therapeutic efficacy in preclinical models, not only by delaying IL-7R+ T-ALL development and reducing tumor burden but also by exerting therapeutic action against chemotherapy-resistant relapsing disease [144,145]. Additionally, JAK inhibitors have shown promising anti-leukemic effects in different assays [86,142] and are currently being tested in clinical trials in combination with other chemotherapeutic drugs (Figure 3).
3.5.2. Insulin Growth Factor 1 Receptor (IGF1R)
Insulin growth factors 1/2 (IGF1/2) bind to the transmembrane receptor IGF1R and activate downstream signaling pathways, including PI3K/AKT/mTOR and RAS/MEK/ERK, regulating proliferation and survival of normal [146] and leukemic stem cells [147]. The contribution of IGF1R to NOTCH1-dependent T-ALL pathogenesis was established using an IGF1R conditional mouse model with reduced expression of IGF1R (IGF1Rneo) [101]. BM cells from IGF1Rneo/neo mice transduced with NOTCH1-ΔE were able to develop leukemia, but their LIC activity was dramatically reduced compared to that of wt transduced BM cells, and T-ALL arising in first recipients was barely transplantable in secondary and tertiary hosts. Molecular analysis identified an NICD/CSL-binding site within intron 20 of IGF1R, suggestive of the presence of a NOTCH1-responsive enhancer, through which NOTCH1 contributes to maintaining high levels of IGF1R expression, rendering T-ALLs more efficient in responding to low levels of IGF1/2 [101]. Additionally, as described above (Section 3.1), both miRNAs (miR-223) [121] and lncRNAs (LUNAR1) [99] can mediate the regulation of NOTCH1 over IGF1R, further supporting that this growth-factor receptor pathway plays an important role in NOTCH1-dependent leukemogenesis.
3.5.3. Pre-TCR
During T-cell development, thymocytes that undergo a productive rearrangement at the T-cell receptor β (TRB) locus express a TCRβ chain that couples to the invariant pTα chain and CD3 molecules (CD3δ, ε, γ and ξ) in the endoplasmic reticulum to form a pre-TCR complex that is exported to the cell surface [148,149]. Early activation events downstream of pre-TCR signaling include the phosphorylation of p56LCK, zeta-chain-associated protein kinase 70 (ZAP-70) and SH2 domain-containing leukocyte protein of 76 KDa (SLP-76), which in turn activates PI3K/AKT, phospholipase C (PLC)γ/nuclear factor of activated T cells (NFAT), PKC and RAS/MAPK pathways [150]. Proper pre-TCR signaling is mandatory to induce the survival and proliferation of committed pre-T cells and their differentiation towards the DP TCRαβ stage, a process known as β-selection [27,28]. Accordingly, T-cell maturation is hindered in mice deficient in any of the components of the pre-TCR (i.e., pTα [151], TCRβ [152], or CD3 chains [153]) or in mice with impaired pre-TCR signaling [154,155]. For many years, pre-TCR has been considered a constitutively active complex, which signals in the absence of ligand by means of oligomerization at the cell membrane [156] and undergoes constitutive internalization and lysosomal degradation for signaling termination [157,158]. In recent years, however, seminal studies by Reinherz’s group have challenged this view and showed that the pre-TCR binds self-peptide-major histocompatibility complex (MHC) complexes at the β-selection checkpoint [159,160,161] and conforms immunological synapses dependent on NOTCH1 and C-X-C chemokine receptor type 4 (CXCR4) signaling [162].
NOTCH1 and pre-TCR act in close collaboration at several levels during T-cell development. Firstly, the Ptcra gene, which encodes the pre-TCR pTα chain, is a direct NOTCH1 transcriptional target [32]. Additionally, mice with a T-cell-specific inhibition of NOTCH activity have a reduction in pre-TCR-associated events and a block in the DN/DP transition, which cannot be rescued by Tcrb or Tcra/b transgene overexpression [163], suggesting that NOTCH1 and pre-TCR act in parallel during β-selection. This cooperation has also been demonstrated in vitro [164]. The mechanism underlying this cross-talk is not completely understood, but NOTCH1 is known to activate a PI3K/AKT-dependent metabolic program to regulate pre-TCR-induced cell growth [165]. NOTCH1 also regulates the expression of FBXL1, and pre-TCR induces FBXL12, two factors of the SCF E3 ligase complex, which promote cyclin-dependent kinase 1B (CDKN1B) degradation resulting in cell cycle progression and proliferation of β-selected thymocytes [166]. While NOTCH1 activation is necessary at the onset of pre-TCR signaling, it needs to be downregulated after β-selection, later at the DN to DP transition, to avoid aberrant DP thymocyte proliferation that could lead to leukemogenesis [103,167,168]. To extinguish NOTCH1 signaling, pre-TCR induces the expression of Bcl6, a transcriptional repressor that diminishes NOTCH1-mediated transcription and NOTCH1 activation [169] and may have a tumor suppressor role in NOTCH1-mediated T-ALL pathogenesis [170]. Pre-TCR signaling also inhibits NOTCH1 transcription via up-regulation of the E2A inhibitor ID3 [171]. These data reveal a direct link between pre-TCR signaling and NOTCH1 expression and suggest that molecular strategies exist during thymocyte development for inhibiting NOTCH1 signaling in pathologic conditions. This is particularly relevant given that NOTCH-induced leukemogenesis in mice has been shown to be dependent on a functional pre-TCR. Indeed, NICD-transduced BM HPCs from mice with impaired pre-TCR function (i.e., Rag2-/-, Slp76-/-) do not develop leukemia [103] or show a delayed leukemic onset [104] depending on the retroviral construct used, and the aggressiveness of NOTCH3-mediated oncogenesis is pre-TCR-dose-dependent as well [172]. In this regard, PTCRA transcription and protein expression have been detected in a high proportion of human T-ALL cases and T-ALL cell lines at different maturational stages [172,173,174]. Also, by comparative immunophenotype, it has been proposed that about half of the αβ-lineage primary human T-ALLs might express the pre-TCR at the cell surface. The availability of a specific mAb against the human pTα could be a valuable T-ALL diagnosis and prognosis tool, although the reduced membrane levels of this complex make its detection challenging [175]. Collectively, the aforementioned studies suggest a key role for NOTCH1-pre-TCR cross-talk in T-ALL pathogenesis, indicating that the signal intensity provided through either the pre-TCR or NOTCH1 could modulate both the latency and the aggressiveness of the disease. Therefore, pre-TCR could be envisaged as a key biomarker in NOTCH1-dependent T-ALL, thus opening new avenues for the design of combinatorial therapies targeting both signaling pathways.
3.6. Metabolic Regulators
The regulation of cellular metabolism by NOTCH has emerged in recent years as an exciting area of research. Although still quite unexplored, strong evidence supports the idea that NOTCH1 signaling promotes T-ALL growth by inducing the activation of anabolic pathways, including glycolysis, glutaminolysis, nucleotide biosynthesis, amino acids metabolism, ribosome biogenesis and protein activation [105,176,177,178]. On the contrary, NOTCH1 signaling inactivation switches metabolism to catabolic pathways, i.e., increased autophagy, ubiquitination and proteasomal degradation, and reduction in glycolytic and glutaminolytic flux [105]. To date, the best-known metabolic routes cooperating with oncogenic NOTCH1 signaling are MYC, PI3K/AKT, and RAS, and they will be discussed below. In addition, very recent studies have uncovered novel players involved in the metabolic reprogramming caused by aberrant NOTCH1 signals, such as the oxidative phosphorylation regulator mitochondrial complex I inhibitor [178] and the nucleotide biosynthesis regulator ubiquitin protein ligase E3 component N-recognin 7 (UBR7) [177]. Given that metabolic reprogramming is an essential mechanism of cancer cell outgrowth and dissemination [179], understanding the multiple levels of interconnections between NOTCH1 and metabolic pathways may help to delineate novel strategies to tackle exacerbated metabolic demand in T-ALL. Several metabolic regulators with a prominent impact on T-ALL oncogenesis will be discussed below.
3.6.1. MYC
MYC is a master regulator involved in many cellular metabolism processes. As a transcriptional regulator, MYC displays the essential ability to directly couple sensing and acquisition of nutrients to molecular pathways that control the growth and proliferation of healthy cells and whose aberrant activation confers growth factor independency to cancer cells [180]. During T-cell development, MYC expression is upregulated in DN3 and DN4 stages [181,182], coincident with NOTCH1 and pre-TCR signaling [183], decreasing later in DP and SP stages. Accordingly, a deficient Myc expression in murine T-cell progenitors was shown to result in the impaired expansion of TCRβ rearranged cells at DN3-DN4 stages and caused a dramatic loss of DP cells, indicating that MYC function is essential for intrathymic T-cell generation [184]. Depletion of Myc later in development, using CD4-Cre conditional mice, demonstrated an essential MYC function in the differentiation of effector and memory T cells [185] associated with TCR- and interleukin-2 (IL-2)-dependent MYC regulation [186].
According to its expression in normal T cells, MYC is also broadly expressed in T-ALL [187], displaying a prominent role in both cell proliferation and survival [108,188,189]. Deregulated MYC expression in T-ALLs is partially attributed to the presence of chromosomal translocations in 6% of T-ALL patients, encompassing the MYC locus and TCR genes or other partners, such as CDK6 [190]. The oncogenicity of MYC deregulation in T cells has been demonstrated in zebrafish [191,192] and mouse models [193], in which transgenic MYC expression was probed sufficiently to induce T-cell lymphomagenesis. However, the inability of sporadic MYC activation to initiate a bonafide murine T-ALL questioned the action of MYC deregulation as a driving oncogene in T-ALL [194]. Despite these findings, several studies have demonstrated the dependency of T-ALL on aberrant MYC signals. Downregulation of MYC activity either by shRNA or by JQ1/CPI203 treatment reduced LIC frequency, impaired T-ALL progression and increased overall survival in murine T-ALL models [188,189,195].
Importantly, both in normal and in neoplastic T cells, MYC expression was directly regulated by NOTCH1 [196]. Although initial experiments failed to determine an active role of NOTCH1 on MYC promoter regulation, deeper chromatin immunoprecipitation (ChIP) analysis in T-ALL led to the identification of a T-cell-specific distal genomic region located >1 Mb 3′ of human and murine MYC locus strongly occupied by NOTCH transcription complexes, which physically interacts with the regulatory sequences in the MYC proximal promoter [108,197]. Recurrent somatic focal duplications of this NOTCH/MYC enhancer (N-Me) region were found in ~5% of T-ALL patients [108]. Supporting the oncogenic role of the N-Me region, leukemia development is impaired in mice transplanted with N-Me-deficient BM progenitors transduced with an oncogenic NOTCH1 allele (ΔE-NOTCH1). N-Me deficiency also leads to a reduction in thymus size and cellularity, mainly caused by a DN3-stage developmental block, alongside the consequent reduction in DP and SP cells [108], a phenotype similar to that described for MYC-deficient T cells [184]. These results further confirm the crucial function of MYC during β-selection and T-cell development. Additional studies by Ferrando’s group uncovered the role of the transcription factor GATA3 in regulating chromatin accessibility at the N-Me, both in thymocytes and in T-ALL [198], and suggested an epistatic function of GATA3 and N-Me over NOTCH1 signaling during T-cell transformation. Moreover, FBXW7- [195] and PTEN-dependent [108,192,199] posttranscriptional regulation of MYC has been described in T-ALL.
Taken together, available data indicate that MYC is a suitable therapeutic target, not only for T-ALL patients with NOTCH1/FBXW7 mutations but also for NOTCH-independent T-ALLs where additional signaling pathways, including PTEN and PI3K/AKT (see below), are deregulated. Nonetheless, since MYC represents a challenging druggable protein because of its multiple levels of regulation, additional studies addressing MYC posttranscriptional modifications and conformational changes would be helpful to obtain more effective and affordable inhibitors to be used in the clinic.
3.6.2. PI3K/AKT/mTOR
PI3K/AKT signaling has been largely associated with cellular metabolism by regulation of different pathways, including expression of nutrient transporters, biosynthesis of macromolecules, maintenance of redox balance and glucose metabolism [200]. Under physiological conditions, the PI3K/AKT pathway is activated by growth factors, insulin or cytokines, resulting in binding to phosphorylated tyrosine residues within activated receptors and adaptor proteins. At the plasma membrane, PI3K activation induces phosphatidylinositol 4,5-bisphosphate (PIP2) phosphorylation and generation of phosphatidylinositol 3,4,5-trisphosphate (PIP3), which accumulates and serves as a docking site for pleckstrin homology (PH) domain-containing proteins, such as AKT. Subsequent phosphorylation events lead to full AKT activation, which in turn activates several metabolic regulators, including mTOR, glycogen synthase kinase 3 (GSK3) and forkhead box protein O (FOXO), and contribute to transcriptional regulation of key effectors of metabolic pathways. Conversely, PI3K signaling is counteracted by the action of phosphatase and tensin homolog (PTEN), which dephosphorylates PIP3 to restore PIP2 levels. Activation of AKT has also been shown to favor glucose uptake by modulation of glucose transporter-1 (GLUT1) trafficking to the cell surface [201,202,203] and by activation of glycolytic enzymes, such as hexokinase 2 (HK2) [204] and 6-phosphofructo-2-kinase/fructose-2,6- biphosphatase (PFKFB2) [205].
A central role has been described for the NOTCH1/PI3K/AKT axis in regulating T-cell metabolism. NOTCH1 withdrawal in DN pre-T cells causes cellular atrophy and programmed cell death, in addition to reduced GLUT1 expression and glycolytic rate, indicating that NOTCH1 signals might promote glucose metabolism in developing T cells [165]. The trophic action of NOTCH1 and pre-TCR at the β-selection checkpoint was shown to be assisted by IL-7R signaling, which induced CD71 (transferrin receptor) and CD98 (neutral amino acid transporter) expression, and collaborated with NOTCH1 in DN4 cell self-renewal [206]. Remarkably, PI3K/AKT activation downstream of IL-7R was shown to induce the expression of GLUT1 in T-ALL cells and stimulated glucose uptake [201], pointing to IL-7R as an essential intermediate in the NOTCH/PI3K/AKT pathway.
Also, NOTCH1 directly regulates PI3K/AKT pathway activity by modulating PTEN expression, as demonstrated by comparative global gene expression analysis showing a specific reduction of PTEN expression in GSI-resistant T-ALLs, as compared with GSI-sensitive NOTCH1-dependent T-ALLs [176]. Molecular studies established that NOTCH1 inhibits PTEN expression via HES1, a transcriptional repressor that mediates many NOTCH1-induced transcriptional programs. Genetic abnormalities, including point mutations, gene deletions, micro-deletions in the PTEN gene, and posttranslational inactivation of the PTEN protein, are frequently found in T-ALL [207,208]. PTEN loss-of-function, leading to constitutive activation of PI3K/AKT-dependent growth signals in T-ALL, might bypass NOTCH1 requirements to maintain cell survival, supporting the generation of T-ALL cells resistant to NOTCH1 inhibition [176]. Seminal studies by Herranz and colleagues using a NOTCH1-dependent TALL mouse model with inducible loss of Pten revealed that metabolic changes were responsible for the resistance to GSI of Pten loss-of-function T-ALLs [105]. They showed that murine T-ALL cells induced by active NOTCH1 were highly dependent on glutaminolysis as a source of carbon. Thus, concomitant administration of GSI and glutaminase (GLS) inhibitors showed synergistic anti-leukemic effects against Pten-positive T-ALLs, while Pten-negative leukemias were resistant not only to GSI but also to the combination of both GSI and GLS inhibitors. Mechanistically, Pten-deleted T-ALLs displayed glycolysis hyperactivation and increased glucose-derived carbon input to the TCA cycle, rendering cells more resistant to glutaminolysis inhibition [105]. Therefore, metabolic unbalance caused by NOTCH1 inhibition in T-ALLs forces cells to rely on catabolic pathways to obtain essential metabolites for cell growth, this finding opening new therapeutic avenues to overcome GSI resistance in T-ALL patients.
Additional studies have uncovered other complex cross-regulation mechanisms between NOTCH1 and PI3K/AKT pathways. It was shown that inhibition of AKT using pharmacological inhibitors decreases NOTCH1 protein levels [209] by inducing NOTCH1 lysosomal degradation through C-CBL (an E3 ubiquitin ligase)-mediated monoubiquitination [210]. Besides regulating PI3K/AKT activity through PTEN inhibition, NOTCH1 can also regulate the PI3K/AKT pathway by modulating PP2A-dependent AKT phosphorylation [211]. Moreover, NOTCH1 regulates the phosphorylation of multiple signaling proteins of the mTOR pathway through a PI3K/AKT-independent but MYC-dependent mechanism [212]. mTOR is an essential regulator of T-ALL cell growth, and its inhibition has been demonstrated to have anti-leukemic effects either alone or in combination with other drugs [106,213,214,215,216,217,218,219]. Notably, dual inhibition of NOTCH1 and mTOR signaling was proved to act synergistically by blocking T-ALL proliferation in vitro [106]. Dual NOTCH1 and mTOR targeting were also beneficial in preclinical models in which human T-ALL xenotransplanted mice treated with a combination of GSI and rapamycin showed increased survival compared to mice treated with GSI alone [107].
In conclusion, PI3K/AKT/mTOR represents a major signaling pathway to be targeted in combination with NOTCH1 inhibitors, especially in T-ALL patients harboring PTEN inactivation where constitutive activation of PI3K/AKT signaling may lead to GSI resistance.
3.6.3. KRAS
KRAS belongs to the RAS protein family of small GTPases that cycle between an active (GTP-bound) and an inactive (GDP-bound) state by means of regulation by guanine-nucleotide-exchange factors (GEFs) and GTPase-activating proteins (GAP)s. Commonly, stimulation of receptor tyrosine kinases leads to recruitment of protein complexes that activate RAS, which subsequently recruits and activates several effectors, including MAPK (RAF/MEK/ERK) and PI3K/AKT, regulating multiple cellular processes such as proliferation, differentiation, survival and cytoskeleton reorganization. KRAS activation has been shown to balance metabolism towards anabolic pathways through metabolic rewiring that involves the up-regulation of enzymes that mediate glycolysis, together with amino acids, fatty acids and nucleotide biosynthesis [220]. Furthermore, KRAS mutations stimulate scavenger pathways, such as macropinocytosis and autophagy, to sustain cancer cell survival under nutrient deprivation [220].
Although KRAS is one of the most frequent mutated oncogenes in cancer, mutations in KRAS appear at low frequency in ALL patients at diagnosis [221]. However, the frequency of KRAS mutations increases in relapses, suggesting either therapy-induced clonal selection or secondarily-acquired mutations [222,223]. Experimentally, conditional hematopoietic expression of an active KrasG12D mutant was shown to induce a fatal monocytic myeloproliferative disorder (MPD) in first-recipient mice that was not transplantable [224]. Instead, a highly penetrant aggressive T-cell disease developed in secondary recipient mice, which showed characteristic extrathymic immature DP CD44+ leukemic cells [55,225]. Mutational screening uncovered the presence of secondarily acquired NOTCH1 mutations, located in the PEST domain and/or the overexpression of NOTCH1, in 100% of mice, which correlated with a high level of transplantability of these leukemias [225]. On the contrary, preleukemic cells at early stages of leukemia development lacked NOTCH1 mutations and were unable to generate leukemia in secondary recipients [225], indicating that the acquisition of NOTCH1 mutations increases KRAS-induced T-ALL aggressiveness. Accordingly, oncogenic KRAS was shown to induce a preleukemic state in HPCs that requires secondarily acquired NOTCH1 mutations to fatally induce the transformation of developing T cells into T-ALL [224]. Secondary acquisition of NOTCH1 mutations was also confirmed in T-ALLs arising in an alternative KrasG12D–induced T-ALL mouse model [226]. Notably, survival and proliferation of T-ALL cell lines derived from those mice showed a great dependency on both NOTCH1 and Ras pathway activation [55,226]. Cooperation between KRAS and NOTCH in T-ALL was directly demonstrated by Pear and colleagues [54] in studies showing that NOTCH1 GOF alleles found in T-ALL patients efficiently generate leukemia in mice when overexpressed in transplanted BM HPCs expressing KrasG12D but not in wild-type BM HPCs.
Collectively, the above results point towards activated KRAS as an important oncogenic pathway cooperating with NOTCH1 in T-ALL, which could potentially be targeted by pharmacological inhibitors. Despite KRAS has been considered a virtually undruggable target for years, several RAS signaling inhibitors have been recently developed [227], and many are currently being tested in clinical trials for different cancers. Therefore, numerous T-ALL patients could still benefit from these novel compounds targeting RAS pathway activation downstream of deregulated signaling from cytokine or growth factor receptors (i.e., IL-7R, IGFR1) frequently found in T-ALL (Figure 3).
3.7. Cell Cycle Regulators
Deregulation of cell cycle progression is widely associated with oncogenic transformation [179] and has a prominent role in T-ALL pathogenesis [43]. D-type cyclins are the ultimate recipients of mitogenic and oncogenic signals. Of them, CYCLIN D3 is required during T-cell development for p56LCK-mediated pre-TCR dependent expansion at the β-selection checkpoint and thus for the proliferation of DN4 and ISP thymocytes and generation of DP thymocytes [228]. Notably, Ccnd3 deficiency in mice impairs the generation of T-ALL induced by NOTCH1, highlighting the relevance of CYCLIN D3-dependent cell cycle progression in NOTCH1-mediated oncogenesis. Supporting a direct role of oncogenic NOTCH1 signaling in G1-S cell cycle progression, CCND3 was confirmed as a transcriptional target of NOTCH1 in T cells [109]. NOTCH function also controls the expression of cyclin-dependent kinases CDK4 and CDK6 that associate with D-type cyclins and regulate retinoblastoma (RB) phosphorylation [109]. Consequently, NOTCH1-induced T-ALL pathogenesis and/or disease maintenance and progression were shown to be dependent on CCND3, CDK4 and CDK6 gene expression [109,110] and on down-regulation of the cyclin-dependent kinase inhibitors CDKN2D (p19INK4d) and CDKN1B (p27Kip1) [229]. In this regard, NOTCH1 was shown to induce transcription of the S-phase kinase-associated protein 2 (SKP2), a negative regulator of CDKN1A and CDKN1B cell cycle inhibitors [230]. Importantly, both CYCLIN D3 and CDK4 are highly overexpressed in NOTCH-dependent T-cell tumors [229]. Moreover, activating mutations in NOTCH1 frequently co-occur with loss of the CDKN2A locus, which encodes the tumor suppressors p16INK4A and p14ARF, and CDKN2A deletions are found in most (>70%) T-ALLs [40,43,231,232], while 15% and 12% of T-ALLs show chromosomal deletions involving the RB1 locus [232,233], and the CDKN1B gene [234], respectively.
Collectively, the reported cross-talk between oncogenic NOTCH1 signaling and cell cycle regulators highlights the need to design accurate combined therapies targeting cell cycle deregulation in NOTCH1-dependent T-ALLs.
3.8. Adhesion/Chemotactic Receptors
3.8.1. Chemokine Receptors
Activation of chemokine receptor CXCR4 by its ligand CXCL12 (stromal cell-derived factor α, SDF1-α) is known to mediate critical interactions of HPCs with the BM niche that regulate BM homing and engraftment [235,236]. Importantly, CXCR4-CXCL12 signaling supports as well the interaction of leukemic cells with the BM microenvironment, regulating the survival and progression of NOTCH1-induced mouse and human T-ALL cells and the expansion of human T-ALL xenografts mediated by LICs [92,111,112,237]. Confirming that LIC activity is dependent on CXCR4 expression, downregulation of CXCR4 in NICD-induced T-ALLs was shown to promote apoptosis, decrease proliferation and reduce BM leukemic burden in vivo [237]. Also, mice with a specific deletion of the Cxcl12 CXCR4 ligand in vascular endothelial cells revealed reduced expansion of NOTCH1-dependent T-ALL cells in BM and peripheral organs [111]. These findings concur with the observation that overexpression of CXCR4 in hematological tumors correlates with disease progression [238,239], pointing to CXCR4 as an important player in the T-cell oncogenic program induced by NOTCH1. Regarding possible crosstalk between NOTCH1 signaling and CXCR4 function, NOTCH1 does not seem to directly control CXCR4 expression [92,113,114]. However, NOTCH3, another member of the NOTCH receptor family with an established T-cell oncogenic capacity [240], has been shown to upregulate CXCR4 expression in mouse T-ALL cells by inhibiting receptor internalization through modulation of ARRESTIN-1β localization and function [241]. Additional regulatory mechanisms have been described using a mouse model of de novo generation of human T-ALL, where NOTCH1 activation was shown to increase the functional competence of CXCR4 in leukemic cells without altering protein expression levels [92]. Therefore, NOTCH1 signaling may promote the interaction of leukemic cells with the supportive BM niche by regulating the activity of the CXCR4/CXCL12 signaling axis.
Of note, CXCR4’s function is not only involved in the BM engraftment of T-ALL cells but also seems to guide them to the colonization of different organs [242], including the central nervous system (CNS). Migration of T-ALL cells to the CNS has also been shown to be dependent on the expression of the C-C- chemokine receptor 7 (CCR7) in oncogenic NOTCH1 mouse models [113]. In this case, constitutively active NOTCH1 was found to upregulate Ccr7 gene expression in murine hematopoietic progenitors, and CCR7 expression in T-ALL cell lines was sensitive to GSI treatment, supporting a direct role of NOTCH1 activation in the regulation of CCR7 expression. In vivo, Ccr7 deletion showed no effect on NICD-induced murine T-ALL generation or T-ALL cell infiltration of most tissues but specifically impaired CNS infiltration [112]. In contrast, deletion of Ccr7 failed to impair CNS infiltration by B-ALLs, thus pointing to a specific function for CCR7 in NOTCH1-dependent T-ALL.
The relevance of the above findings is supported by results derived from primary human T-ALLs, showing that expression of high levels of mRNA of both CCR7 and CXCR4 correlated with a high risk of CNS involvement in non-relapsed patients [243]. Collectively, these data highlight the potential of CXCR4 and CCR7 chemokine receptors as powerful therapeutic targets for the treatment of T-ALL patients with NOTCH1 activating mutations, especially for those with CNS involvement (Figure 3). Other chemokine receptors, including CCR5 and CCR9, which regulate T-ALL proliferation and migration in vitro, are known to be transcriptionally regulated by NOTCH1 and could be likewise considered optimal T-ALL therapeutic targets. While the functional relevance of CCR5 in T-ALL progression has yet to be determined in vivo [114], very recent studies have demonstrated that targeting of CCR9, which is expressed in >70% of cases of T-ALL, has potent anti-leukemic activity in vivo in human T-ALL xenografts and could be a highly effective therapeutic strategy for T-ALL [244,245] (Figure 3).
3.8.2. CD44 Adhesion Receptor
Adhesion molecules that mediate the interaction of T-ALL cells with their specific niches are key contributors to leukemia progression. Of special relevance is CD44, a transmembrane glycoprotein with several isoforms resulting from alternative splicing, whose main ligand, hyaluronan, is present in the extracellular matrix [246]. CD44 plays a key role as a physiological mediator of HPC anchoring within the BM niche during hematopoiesis and also mediates BM engraftment of aberrant hematopoietic cells [247]. Specifically, several studies have highlighted the clinical significance of CD44 as a tumor-initiating marker of chronic (CML) [248] and acute (AML) myeloid leukemias [249], and CD44 expression levels have also been associated with LIC activity in murine models of T-ALL, were a link between NOTCH signaling and CD44 expression was observed [250]. More recently, upregulation of CD44 has been proposed as one of the initial events encompassing human T-ALL generation in mice transplanted with human HPCs expressing NICD, in which CD44 was shown to support BM engraftment and expansion and further progression of human preleukemic cells [92]. This study also demonstrated that CD44 is a direct NOTCH1 target, whose transcription is regulated by MAML1/CSL-binding to its proximal promoter, but also through dynamic interactions with a superenhancer, which is a mechanism shared with other NOTCH1 targets involved in development and cancer, such as IL7R [30,31]. Also, NOTCH1-induced upregulation of CD44 was essential for NOTCH1-dependent human T-ALL pathogenesis since treatment with anti-CD44 antibodies reduced BM engraftment of NICD+ preleukemic cells, and eradicated disease progression of established T-ALL patient-derived xenografts [92].
Supporting a clinical relevance for CD44 targeting in T-ALL, aberrant expression of CD44 has been detected in different T-ALL subtypes, and abnormally high expression levels of CD44 have been associated with organ infiltration in T-ALL patients [251] and with T-ALL chemoresistance [252]. These results suggest that expression of CD44 may provide LICs with a retention capacity to specific niches within the BM, where they could be kept protected from treatment and contribute later to leukemia relapses. In line with this hypothesis, CD44 expression has been identified as a predictor of T-ALL relapse [253]. Therefore, CD44 may be a useful marker in T-ALL patients with NOTCH1 mutations that could help in the prognosis and evaluation of relapse risk and hence can be considered a potential target for future combined therapies (Figure 3).
4. Concluding Remarks and Future Perspectives
Although intensive chemotherapy treatments have greatly improved T-ALL patient survival in recent years, relapses and refractories still have a disheartening prognosis. Since the discovery that constitutive activation of NOTCH1 signaling is a major player in the T-cell oncogenic process, results from ongoing anti-NOTCH1 clinical trials are expected to offer T-ALL patients a more specific, effective and targeted treatment to improve their life expectancy. However, most of the naturally-found NOTCH1 mutants in T-ALL patients are not capable of inducing complete T-cell transformation in animal models, which raised the notion that other players might be acting in concert with NOTCH during the T-ALL oncogenic process. As expected, the majority of these players comprise signaling pathways that have been known for a long time as essential regulators of cell metabolism, proliferation and survival, which are deregulated in many types of cancers. This scenario represents a great opportunity for future therapeutic developments for T-ALL patients since numerous modulators of these pro-oncogenic signaling pathways have been approved for clinical use for other pathologies. Other oncogenic collaborators of NOTCH1, such as RNA regulators, have been revealed as potential targets of therapeutic intervention in recent years, and their potential clinical relevance needs to be confirmed. Therefore, innovative technologies focusing on the identification of the deregulated pathways collaborating with NOTCH1 in T-ALL patients, in combination with human T-ALL in vivo models that provide proof-of-concept of the efficacy and safety of novel combinational therapies targeting both NOTCH1 and associated oncogenic collaborators, will open new avenues for developing in the near future efficacious and safe targeting therapies for T-ALL patients.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Ministerio de Ciencia e Innovación (PID2019-105623RB-I00 and PDC2021-121238-I00; Agencia Estatal de Investigación/European Regional Development Fund, European Union), Fundación Asociación Española Contra el Cáncer (CICPF18030TORI7), Fundación Unoentrecienmil, Fundación Ramón Areces, and Fundación Inocente Inocente. Institutional grants from the Fundación Ramón Areces and Banco de Santander to the Centro de Biología Molecular Severo Ochoa are also acknowledged.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Artavanis-Tsakonas S., Rand M.D., Lake R.J. Notch Signaling: Cell Fate Control and Signal Integration in Development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 2.Kopan R., Ilagan M.X.G. The Canonical Notch Signaling Pathway: Unfolding the Activation Mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.González-García S., García-Peydró M., Alcain J., Toribio M.L. Notch1 and IL-7 Receptor Signalling in Early T-Cell Development and Leukaemia. Curr. Top. Microbiol. Immunol. 2012;360:47–73. doi: 10.1007/82_2012_231. [DOI] [PubMed] [Google Scholar]
- 4.Fryer C.J., White J.B., Jones K.A. Mastermind Recruits CycC:CDK8 to Phosphorylate the Notch ICD and Coordinate Activation with Turnover. Mol. Cell. 2004;16:509–520. doi: 10.1016/j.molcel.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Dutta D., Sharma V., Mutsuddi M., Mukherjee A. Regulation of Notch Signaling by E3 Ubiquitin Ligases. FEBS J. 2022;289:937–954. doi: 10.1111/febs.15792. [DOI] [PubMed] [Google Scholar]
- 6.Shan H., Li X., Xiao X., Dai Y., Huang J., Song J., Liu M., Yang L., Lei H., Tong Y., et al. USP7 Deubiquitinates and Stabilizes NOTCH1 in T-Cell Acute Lymphoblastic Leukemia. Signal Transduct. Target. Ther. 2018;3:29. doi: 10.1038/s41392-018-0028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin Q., Martinez C.A., Arcipowski K.M., Zhu Y., Gutierrez-Diaz B.T., Wang K.K., Johnson M.R., Volk A.G., Wang F., Wu J., et al. USP7 Cooperates with NOTCH1 to Drive the Oncogenic Transcriptional Program in T-Cell Leukemia. Clin. Cancer Res. 2019;25:222–239. doi: 10.1158/1078-0432.CCR-18-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim R., Sugino T., Nolte H., Andrade J., Zimmermann B., Shi C., Doddaballapur A., Ong Y.T., Wilhelm K., Fasse J.W.D., et al. Deubiquitinase USP10 Regulates Notch Signaling in the Endothelium. Science. 2019;364:188–193. doi: 10.1126/science.aat0778. [DOI] [PubMed] [Google Scholar]
- 9.Ferrante F., Giaimo B.D., Friedrich T., Sugino T., Mertens D., Kugler S., Gahr B.M., Just S., Pan L., Bartkuhn M., et al. Hydroxylation of the NOTCH1 Intracellular Domain Regulates Notch Signaling Dynamics. Cell Death Dis. 2022;13:600. doi: 10.1038/s41419-022-05052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felli M.P., Maroder M., Mitsiadis T.A., Campese A.F., Bellavia D., Vacca A., Mann R.S., Frati L., Lendahl U., Gulino A., et al. Expression Pattern of Notch1, 2 and 3 and Jagged1 and 2 in Lymphoid and Stromal Thymus Components: Distinct Ligand-Receptor Interactions in Intrathymic T Cell Development. Int. Immunol. 1999;11:1017–1025. doi: 10.1093/intimm/11.7.1017. [DOI] [PubMed] [Google Scholar]
- 11.Schmitt T.M., Ciofani M., Petrie H.T., Zúñiga-Pflücker J.C. Maintenance of T Cell Specification and Differentiation Requires Recurrent Notch Receptor-Ligand Interactions. J. Exp. Med. 2004;200:469–479. doi: 10.1084/jem.20040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehar S.M., Dooley J., Farr A.G., Bevan M.J. Notch Ligands Delta1 and Jagged1 Transmit Distinct Signals to T-Cell Precursors. Blood. 2005;105:1440–1447. doi: 10.1182/blood-2004-08-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch U., Fiorini E., Benedito R., Besseyrias V., Schuster-Gossler K., Pierres M., Manley N.R., Duarte A., MacDonald H.R., Radtke F. Delta-like 4 Is the Essential, Nonredundant Ligand for Notchl during Thymic T Cell Lineage Commitment. J. Exp. Med. 2008;205:2515–2523. doi: 10.1084/jem.20080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van De Walle I., De Smet G., Gärtner M., De Smedt M., Waegemans E., Vandekerckhove B., Leclercq G., Plum J., Aster J.C., Bernstein I.D., et al. Jagged2 Acts as a Delta-like Notch Ligand during Early Hematopoietic Cell Fate Decisions. Blood. 2011;117:4449–4459. doi: 10.1182/blood-2010-06-290049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van de Walle I., Waegemans E., De Medts J., De Smet G., De Smedt M., Snauwaert S., Vandekerckhove B., Kerre T., Leclercq G., Plum J., et al. Specific Notch Receptor-Ligand Interactions Control Human TCR-αβ/γδ Development by Inducing Differential Notch Signal Strength. J. Exp. Med. 2013;210:683–697. doi: 10.1084/jem.20121798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-León M.J., Fuentes P., de la Pompa J.L., Toribio M.L. Dynamic Regulation of Notch1 Activation and Notch Ligand Expression in Human Thymus Development. Development. 2018;145:dev165597. doi: 10.1242/dev.165597. [DOI] [PubMed] [Google Scholar]
- 17.Radtke F., Wilson A., Stark G., Bauer M., Van Meerwijk J., MacDonald H.R., Aguet M. Deficient T Cell Fate Specification in Mice with an Induced Inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/S1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 18.Feyerabend T.B., Terszowski G., Tietz A., Blum C., Luche H., Gossler A., Gale N.W., Radtke F., Fehling H.J., Rodewald H.R. Deletion of Notch1 Converts Pro-T Cells to Dendritic Cells and Promotes Thymic B Cells by Cell-Extrinsic and Cell-Intrinsic Mechanisms. Immunity. 2009;30:67–79. doi: 10.1016/j.immuni.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Pear W.S., Aster J.C., Scott M.L., Hasserjian R.P., Soffer B., Sklar J., Baltimore D. Exclusive Development of T Cell Neoplasms in Mice Transplanted with Bone Marrow Expressing Activated Notch Alleles. J. Exp. Med. 1996;183:2283–2291. doi: 10.1084/jem.183.5.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pui J.C., Allman D., Xu L., DeRocco S., Karnell F.G., Bakkour S., Lee J.Y., Kadesch T., Hardy R.R., Aster J.C., et al. Notch1 Expression in Early Lymphopoiesis Influences B versus T Lineage Determination. Immunity. 1999;11:299–308. doi: 10.1016/S1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 21.Hozumi K., Mailhos C., Negishi N., Hirano K.I., Yahata T., Ando K., Zuklys S., Holländer G.A., Shima D.T., Habu S. Delta-like 4 Is Indispensable in Thymic Environment Specific for T Cell Development. J. Exp. Med. 2008;205:2507–2513. doi: 10.1084/jem.20080134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martín-Gayo E., González-García S., García-León M.J., Murcia-Ceballos A., Alcain J., García-Peydró M., Allende L., de Andrés B., Gaspar M.L., Toribio M.L. Spatially Restricted JAG1-Notch Signaling in Human Thymus Provides Suitable DC Developmental Niches. J. Exp. Med. 2017;214:3361–3379. doi: 10.1084/jem.20161564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.García-Peydró M., De Yébenes V.G., Toribio M.L. Sustained Notch1 Signaling Instructs the Earliest Human Intrathymic Precursors to Adopt a Gammadelta T-Cell Fate in Fetal Thymus Organ Culture. Blood. 2003;102:2444–2451. doi: 10.1182/blood-2002-10-3261. [DOI] [PubMed] [Google Scholar]
- 24.Li J., Gordon J., Chen E.L.Y., Xiao S., Wu L., Zúniga-Pflücker J.C., Manley N.R. NOTCH1 Signaling Establishes the Medullary Thymic Epithelial Cell Progenitor Pool during Mouse Fetal Development. Development. 2020;147:dev178988. doi: 10.1242/dev.178988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu D., Kousa A.I., O’Neill K.E., Rouse P., Popis M., Farley A.M., Tomlinson S.R., Ulyanchenko S., Guillemot F., Seymour P.A., et al. Canonical Notch Signaling Controls the Early Thymic Epithelial Progenitor Cell State and Emergence of the Medullary Epithelial Lineage in Fetal Thymus Development. Development. 2020;147:dev178582. doi: 10.1242/dev.178582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-León M.J., Mosquera M., Cela C., Alcain J., Zuklys S., Holländer G., Toribio M.L. Abrogation of Notch Signaling in Embryonic TECs Impacts Postnatal MTEC Homeostasis and Thymic Involution. Front. Immunol. 2022;13:867302. doi: 10.3389/fimmu.2022.867302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffman E.S., Passoni L., Crompton T., Leu T.M.J., Schatz D.G., Koff A., Owen M.J., Hayday A.C. Productive T-Cell Receptor Beta-Chain Gene Rearrangement: Coincident Regulation of Cell Cycle and Clonality during Development in Vivo. Genes Dev. 1996;10:948–962. doi: 10.1101/gad.10.8.948. [DOI] [PubMed] [Google Scholar]
- 28.Von Boehmer H., Aifantis I., Azogui O., Feinberg J., Saint-Ruf C., Zober C., Garcia C., Buer J. Crucial Function of the Pre-T-Cell Receptor (TCR) in TCR Beta Selection, TCR Beta Allelic Exclusion and Alpha Beta versus Gamma Delta Lineage Commitment. Immunol. Rev. 1998;165:111–119. doi: 10.1111/j.1600-065X.1998.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 29.González-García S., García-Peydró M., Martín-Gayo E., Ballestar E., Esteller M., Bornstein R., De La Pompa J.L., Ferrando A.A., Toribio M.L. CSL-MAML-Dependent Notch1 Signaling Controls t Lineage-Specifc IL-7Rα Gene Expression in Early Human Thymopoiesis and Leukemia. J. Exp. Med. 2009;206:779–791. doi: 10.1084/jem.20081922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H., Zou J., Zhao B., Johannsen E., Ashworth T., Wong H., Pear W.S., Schug J., Blacklow S.C., Arnett K.L., et al. Genome-Wide Analysis Reveals Conserved and Divergent Features of Notch1/RBPJ Binding in Human and Murine T-Lymphoblastic Leukemia Cells. Proc. Natl. Acad. Sci. USA. 2011;108:14908–14913. doi: 10.1073/pnas.1109023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H., Zang C., Taing L., Arnett K.L., Wong Y.J., Pear W.S., Blacklow S.C., Liu X.S., Aster J.C. NOTCH1-RBPJ Complexes Drive Target Gene Expression through Dynamic Interactions with Superenhancers. Proc. Natl. Acad. Sci. USA. 2014;111:705–710. doi: 10.1073/pnas.1315023111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reizis B., Leder P. Direct Induction of T Lymphocyte-Specific Gene Expression by the Mammalian Notch Signaling Pathway. Genes Dev. 2002;16:295–300. doi: 10.1101/gad.960702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deftos M.L., He Y.W., Ojala E.W., Bevan M.J. Correlating Notch Signaling with Thymocyte Maturation. Immunity. 1998;9:777–786. doi: 10.1016/S1074-7613(00)80643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laky K., Fowlkes B.J. Presenilins Regulate Alphabeta T Cell Development by Modulating TCR Signaling. J. Exp. Med. 2007;204:2115–2129. doi: 10.1084/jem.20070550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellisen L.W., Bird J., West D.C., Soreng A.L., Reynolds T.C., Smith S.D., Sklar J. TAN-1, the Human Homolog of the Drosophila Notch Gene, Is Broken by Chromosomal Translocations in T Lymphoblastic Neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-B. [DOI] [PubMed] [Google Scholar]
- 36.Palomero T., Barnes K.C., Real P.J., Glade Bender J.L., Sulis M.L., Murty V.V., Colovai A.I., Balbin M., Ferrando A.A. CUTLL1, a Novel Human T-Cell Lymphoma Cell Line with t(7;9) Rearrangement, Aberrant NOTCH1 Activation and High Sensitivity to γ-Secretase Inhibitors. Leukemia. 2006;20:1279–1287. doi: 10.1038/sj.leu.2404258. [DOI] [PubMed] [Google Scholar]
- 37.Weng A.P., Ferrando A.A., Lee W., Morris IV J.P., Silverman L.B., Sanchez-Irizarry C., Blacklow S.C., Look A.T., Aster J.C. Activating Mutations of NOTCH1 in Human T Cell Acute Lymphoblastic Leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 38.Pear W.S., Aster J.C. T Cell Acute Lymphoblastic Leukemia/Lymphoma: A Human Cancer Commonly Associated with Aberrant NOTCH1 Signaling. Curr. Opin. Hematol. 2004;11:426–433. doi: 10.1097/01.moh.0000143965.90813.70. [DOI] [PubMed] [Google Scholar]
- 39.Ma X., Liu Y., Liu Y., Alexandrov L.B., Edmonson M.N., Gawad C., Zhou X., Li Y., Rusch M.C., John E., et al. Pan-Cancer Genome and Transcriptome Analyses of 1,699 Paediatric Leukaemias and Solid Tumours. Nature. 2018;555:371–376. doi: 10.1038/nature25795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrando A.A., Neuberg D.S., Staunton J., Loh M.L., Huard C., Raimondi S.C., Behm F.G., Pui C.H., Downing J.R., Gilliland D.G., et al. Gene Expression Signatures Define Novel Oncogenic Pathways in T Cell Acute Lymphoblastic Leukemia. Cancer Cell. 2002;1:75–87. doi: 10.1016/S1535-6108(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 41.Soulier J., Clappier E., Cayuela J.M., Regnault A., García-Peydró M., Dombret H., Baruchel A., Toribio M.L., Sigaux F. HOXA Genes Are Included in Genetic and Biologic Networks Defining Human Acute T-Cell Leukemia (T-ALL) Blood. 2005;106:274–286. doi: 10.1182/blood-2004-10-3900. [DOI] [PubMed] [Google Scholar]
- 42.Belver L., Ferrando A. The Genetics and Mechanisms of T Cell Acute Lymphoblastic Leukaemia. Nat. Rev. Cancer. 2016;16:494–507. doi: 10.1038/nrc.2016.63. [DOI] [PubMed] [Google Scholar]
- 43.Girardi T., Vicente C., Cools J., De Keersmaecker K. The Genetics and Molecular Biology of T-ALL. Blood. 2017;129:1113–1123. doi: 10.1182/blood-2016-10-706465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y., Easton J., Shao Y., Maciaszek J., Wang Z., Wilkinson M.R., McCastlain K., Edmonson M., Pounds S.B., Shi L., et al. The Genomic Landscape of Pediatric and Young Adult T-Lineage Acute Lymphoblastic Leukemia. Nat. Genet. 2017;49:1211–1218. doi: 10.1038/ng.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inaba H., Mullighan C.G. Pediatric Acute Lymphoblastic Leukemia. Haematologica. 2020;105:2524–2539. doi: 10.3324/haematol.2020.247031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fattizzo B., Rosa J., Giannotta J.A., Baldini L., Fracchiolla N.S. The Physiopathology of T- Cell Acute Lymphoblastic Leukemia: Focus on Molecular Aspects. Front. Oncol. 2020;10:273. doi: 10.3389/fonc.2020.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coustan-Smith E., Mullighan C.G., Onciu M., Behm F.G., Raimondi S.C., Pei D., Cheng C., Su X., Rubnitz J.E., Basso G., et al. Early T-Cell Precursor Leukaemia: A Subtype of Very High-Risk Acute Lymphoblastic Leukaemia. Lancet Oncol. 2009;10:147–156. doi: 10.1016/S1470-2045(08)70314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J., Ding L., Holmfeldt L., Wu G., Heatley S.L., Payne-Turner D., Easton J., Chen X., Wang J., Rusch M., et al. The Genetic Basis of Early T-Cell Precursor Acute Lymphoblastic Leukaemia. Nature. 2012;481:157–163. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Homminga I., Vuerhard M.J., Langerak A.W., Buijs-Gladdines J., Pieters R., Meijerink J.P.P. Characterization of a Pediatric T-Cell Acute Lymphoblastic Leukemia Patient with Simultaneous LYL1 and LMO2 Rearrangements. Haematologica. 2012;97:258–261. doi: 10.3324/haematol.2011.051722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bardelli V., Arniani S., Pierini V., Di Giacomo D., Pierini T., Gorello P., Mecucci C., La Starza R. T-Cell Acute Lymphoblastic Leukemia: Biomarkers and Their Clinical Usefulness. Genes. 2021;12:1118. doi: 10.3390/genes12081118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sulis M.L., Williams O., Palomero T., Tosello V., Pallikuppam S., Real P.J., Barnes K., Zuurbier L., Meijerink J.P., Ferrando A.A. NOTCH1 Extracellular Juxtamembrane Expansion Mutations in T-ALL. Blood. 2008;112:733–740. doi: 10.1182/blood-2007-12-130096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Neil J., Grim J., Strack P., Rao S., Tibbitts D., Winter C., Hardwick J., Welcker M., Meijerink J.P., Pieters R., et al. FBW7 Mutations in Leukemic Cells Mediate NOTCH Pathway Activation and Resistance to γ-Secretase Inhibitors. J. Exp. Med. 2007;204:1813–1824. doi: 10.1084/jem.20070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson B.J., Buonamici S., Sulis M.L., Palomero T., Vilimas T., Basso G., Ferrando A., Aifantis I. The SCFFBW7 Ubiquitin Ligase Complex as a Tumor Suppressor in T Cell Leukemia. J. Exp. Med. 2007;204:1825–1835. doi: 10.1084/jem.20070872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiang M.Y., Xu L., Shestova O., Histen G., L’Heureux S., Romany C., Childs M.E., Gimotty P.A., Aster J.C., Pear W.S. Leukemia-Associated NOTCH1 Alleles Are Weak Tumor Initiators but Accelerate K-Ras–Initiated Leukemia. J. Clin. Investig. 2008;118:3181–3194. doi: 10.1172/JCI35090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kindler T., Cornejo M.G., Scholl C., Liu J., Leeman D.S., Haydu J.E., Fröhling S., Lee B.H., Gilliland D.G. K-RasG12D-Induced T-Cell Lymphoblastic Lymphoma/Leukemias Harbor Notch1 Mutations and Are Sensitive to {gamma}-Secretase Inhibitors. Blood. 2008;112:3373–3382. doi: 10.1182/blood-2008-03-147587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hodson D.J., Janas M.L., Galloway A., Bell S.E., Andrews S., Li C.M., Pannell R., Siebel C.W., MacDonald H.R., De Keersmaecker K., et al. Deletion of the RNA-Binding Proteins ZFP36L1 and ZFP36L2 Leads to Perturbed Thymic Development and T Lymphoblastic Leukemia. Nat. Immunol. 2010;11:717–724. doi: 10.1038/ni.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li N., Fassl A., Chick J., Inuzuka H., Li X., Mansour M.R., Liu L., Wang H., King B., Shaik S., et al. Cyclin C Is a Haploinsufficient Tumour Suppressor. Nat. Cell Biol. 2014;16:1080–1091. doi: 10.1038/ncb3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pajcini K.V., Xu L., Shao L., Petrovic J., Palasiewicz K., Ohtani Y., Bailis W., Lee C., Wertheim G.B., Mani R., et al. MAFB Enhances Oncogenic Notch Signaling in T Cell Acute Lymphoblastic Leukemia. Sci. Signal. 2017;10:eaam6846. doi: 10.1126/scisignal.aam6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mansour M.R., Duke V., Foroni L., Patel B., Allen C.G., Ancliff P.J., Gale R.E., Linch D.C. Notch-1 Mutations Are Secondary Events in Some Patients with T-Cell Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2007;13:6964–6969. doi: 10.1158/1078-0432.CCR-07-1474. [DOI] [PubMed] [Google Scholar]
- 60.Eguchi-Ishimae M., Eguchi M., Kempski H., Greaves M. NOTCH1 Mutation Can Be an Early, Prenatal Genetic Event in T-ALL. Blood. 2008;111:376–378. doi: 10.1182/blood-2007-02-074690. [DOI] [PubMed] [Google Scholar]
- 61.Armstrong F., La Grange P.B., Gerby B., Rouyez M.C., Calvo J., Fontenay M., Boissel N., Dombret H., Baruchel A., Landman-Parker J., et al. NOTCH Is a Key Regulator of Human T-Cell Acute Leukemia Initiating Cell Activity. Blood. 2009;113:1730–1740. doi: 10.1182/blood-2008-02-138172. [DOI] [PubMed] [Google Scholar]
- 62.Medyouf H., Gao X., Armstrong F., Gusscott S., Liu Q., Gedman A.L., Matherly L.H., Schultz K.R., Pflumio F., You M.J., et al. Acute T-Cell Leukemias Remain Dependent on Notch Signaling despite PTEN and INK4A/ARF Loss. Blood. 2010;115:1175–1184. doi: 10.1182/blood-2009-04-214718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiang M.Y., Wang Q., Gormley A.C., Stein S.J., Xu L., Shestova O., Aster J.C., Pear W.S. High Selective Pressure for Notch1 Mutations That Induce Myc in T-Cell Acute Lymphoblastic Leukemia. Blood. 2016;128:2229–2240. doi: 10.1182/blood-2016-01-692855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Majumder S., Crabtree J.S., Golde T.E., Minter L.M., Osborne B.A., Miele L. Targeting Notch in Oncology: The Path Forward. Nat. Rev. Drug Discov. 2021;20:125–144. doi: 10.1038/s41573-020-00091-3. [DOI] [PubMed] [Google Scholar]
- 65.Deangelo D.J., Stone R.M., Silverman L.B., Stock W., Attar E.C., Fearen I., Dallob A., Matthews C., Stone J., Freedman S.J., et al. A Phase I Clinical Trial of the Notch Inhibitor MK-0752 in Patients with T-Cell Acute Lymphoblastic Leukemia/Lymphoma (T-ALL) and Other Leukemias. J. Clin. Oncol. 2006;24:6585. doi: 10.1200/jco.2006.24.18_suppl.6585. [DOI] [Google Scholar]
- 66.Paganin M., Ferrando A. Molecular Pathogenesis and Targeted Therapies for NOTCH1-Induced T-Cell Acute Lymphoblastic Leukemia. Blood Rev. 2011;25:83–90. doi: 10.1016/j.blre.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Real P.J., Tosello V., Palomero T., Castillo M., Hernando E., De Stanchina E., Sulis M.L., Barnes K., Sawai C., Homminga I., et al. Gamma-Secretase Inhibitors Reverse Glucocorticoid Resistance in T Cell Acute Lymphoblastic Leukemia. Nat. Med. 2009;15:50–58. doi: 10.1038/nm.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou B., Lin W., Long Y., Yang Y., Zhang H., Wu K., Chu Q. Notch Signaling Pathway: Architecture, Disease, and Therapeutics. Signal Transduct. Target. Ther. 2022;7:95. doi: 10.1038/s41392-022-00934-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferrarotto R., Mishra V., Herz E., Yaacov A., Solomon O., Rauch R., Mondshine A., Motin M., Leibovich-Rivkin T., Davis M., et al. AL101, a Gamma-Secretase Inhibitor, Has Potent Antitumor Activity against Adenoid Cystic Carcinoma with Activated NOTCH Signaling. Cell Death Dis. 2022;13:678. doi: 10.1038/s41419-022-05133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Papayannidis C., DeAngelo D.J., Stock W., Huang B., Shaik M.N., Cesari R., Zheng X., Reynolds J.M., English P.A., Ozeck M., et al. A Phase 1 Study of the Novel Gamma-Secretase Inhibitor PF-03084014 in Patients with T-Cell Acute Lymphoblastic Leukemia and T-Cell Lymphoblastic Lymphoma. Blood Cancer J. 2015;5:e350. doi: 10.1038/bcj.2015.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Agnusdei V., Minuzzo S., Frasson C., Grassi A., Axelrod F., Satyal S., Gurney A., Hoey T., Seganfreddo E., Basso G., et al. Therapeutic Antibody Targeting of Notch1 in T-Acute Lymphoblastic Leukemia Xenografts. Leukemia. 2014;28:278–288. doi: 10.1038/leu.2013.183. [DOI] [PubMed] [Google Scholar]
- 72.Sharma A., Gadkari R.A., Ramakanth S.V., Padmanabhan K., Madhumathi D.S., Devi L., Appaji L., Aster J.C., Rangarajan A., Dighe R.R. A Novel Monoclonal Antibody against Notch1 Targets Leukemia-Associated Mutant Notch1 and Depletes Therapy Resistant Cancer Stem Cells in Solid Tumors. Sci. Rep. 2015;5:11012. doi: 10.1038/srep11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoey T., Yen W.C., Axelrod F., Basi J., Donigian L., Dylla S., Fitch-Bruhns M., Lazetic S., Park I.K., Sato A., et al. DLL4 Blockade Inhibits Tumor Growth and Reduces Tumor-Initiating Cell Frequency. Cell Stem Cell. 2009;5:168–177. doi: 10.1016/j.stem.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 74.Kuhnert F., Chen G., Coetzee S., Thambi N., Hickey C., Shan J., Kovalenko P., Noguera-Troise I., Smith E., Fairhurst J., et al. Dll4 Blockade in Stromal Cells Mediates Antitumor Effects in Preclinical Models of Ovarian Cancer. Cancer Res. 2015;75:4086–4096. doi: 10.1158/0008-5472.CAN-14-3773. [DOI] [PubMed] [Google Scholar]
- 75.Ferrarotto R., Eckhardt G., Patnaik A., LoRusso P., Faoro L., Heymach J.V., Kapoun A.M., Xu L., Munster P. A Phase I Dose-Escalation and Dose-Expansion Study of Brontictuzumab in Subjects with Selected Solid Tumors. Ann. Oncol. 2018;29:1561–1568. doi: 10.1093/annonc/mdy171. [DOI] [PubMed] [Google Scholar]
- 76.Casulo C., Ruan J., Dang N.H., Gore L., Diefenbach C., Beaven A.W., Castro J.E., Porcu P., Faoro L., Dupont J., et al. Safety and Preliminary Efficacy Results of a Phase I First-in-Human Study of the Novel Notch-1 Targeting Antibody Brontictuzumab (OMP-52M51) Administered Intravenously to Patients with Hematologic Malignancies. Blood. 2016;128:5108. doi: 10.1182/blood.V128.22.5108.5108. [DOI] [Google Scholar]
- 77.McKeage M.J., Kotasek D., Markman B., Hidalgo M., Millward M.J., Jameson M.B., Harris D.L., Stagg R.J., Kapoun A.M., Xu L., et al. Phase IB Trial of the Anti-Cancer Stem Cell DLL4-Binding Agent Demcizumab with Pemetrexed and Carboplatin as First-Line Treatment of Metastatic Non-Squamous NSCLC. Target. Oncol. 2018;13:89–98. doi: 10.1007/s11523-017-0543-0. [DOI] [PubMed] [Google Scholar]
- 78.Chiorean E.G., LoRusso P., Strother R.M., Diamond J.R., Younger A., Messersmith W.A., Adriaens L., Liu L., Kao R.J., DiCioccio A.T., et al. A Phase I First-in-Human Study of Enoticumab (REGN421), a Fully Human Delta-like Ligand 4 (Dll4) Monoclonal Antibody in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2015;21:2695–2703. doi: 10.1158/1078-0432.CCR-14-2797. [DOI] [PubMed] [Google Scholar]
- 79.Lu H.Y., Zu Y.X., Jiang X.W., Sun X.T., Liu T.Y., Li R.L., Wu Q., Zhang Y.S., Zhao Q.C. Novel ADAM-17 Inhibitor ZLDI-8 Inhibits the Proliferation and Metastasis of Chemo-Resistant Non-Small-Cell Lung Cancer by Reversing Notch and Epithelial Mesenchymal Transition in Vitro and in Vivo. Pharmacol. Res. 2019;148:104406. doi: 10.1016/j.phrs.2019.104406. [DOI] [PubMed] [Google Scholar]
- 80.Kangsamaksin T., Murtomaki A., Kofler N.M., Cuervo H., Chaudhri R.A., Tattersall I.W., Rosenstiel P.E., Shawber C.J., Kitajewski J. NOTCH Decoys That Selectively Block DLL/NOTCH or JAG/NOTCH Disrupt Angiogenesis by Unique Mechanisms to Inhibit Tumor Growth. Cancer Discov. 2015;5:182–197. doi: 10.1158/2159-8290.CD-14-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moellering R.E., Cornejo M., Davis T.N., Del Bianco C., Aster J.C., Blacklow S.C., Kung A.L., Gilliland D.G., Verdine G.L., Bradner J.E. Direct Inhibition of the NOTCH Transcription Factor Complex. Nature. 2009;462:182–188. doi: 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alvarez-Trotta A., Guerrant W., Astudillo L., Lahiry M., Diluvio G., Shersher E., Kaneku H., Robbins D.J., Orton D., Capobianco A.J. Pharmacological Disruption of the Notch1 Transcriptional Complex Inhibits Tumor Growth by Selectively Targeting Cancer Stem Cells. Cancer Res. 2021;81:3347. doi: 10.1158/0008-5472.CAN-20-3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roti G., Carlton A., Ross K.N., Markstein M., Pajcini K., Su A.H., Perrimon N., Pear W.S., Kung A.L., Blacklow S.C., et al. Complementary Genomic Screens Identify SERCA as a Therapeutic Target in NOTCH1 Mutated Cancer. Cancer Cell. 2013;23:390–405. doi: 10.1016/j.ccr.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pagliaro L., Marchesini M., Roti G. Targeting Oncogenic Notch Signaling with SERCA Inhibitors. J. Hematol. Oncol. 2021;14:8. doi: 10.1186/s13045-020-01015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schneider M., Kumar V., Nordstrøm L.U., Feng L., Takeuchi H., Hao H., Luca V.C., Garcia K.C., Stanley P., Wu P., et al. Inhibition of Delta-Induced Notch Signaling Using Fucose Analogs. Nat. Chem. Biol. 2018;14:65–71. doi: 10.1038/nchembio.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanchez-Martin M., Ambesi-Impiombato A., Qin Y., Herranz D., Bansal M., Girardi T., Paietta E., Tallman M.S., Rowe J.M., De Keersmaecker K., et al. Synergistic Antileukemic Therapies in NOTCH1-Induced T-ALL. Proc. Natl. Acad. Sci. USA. 2017;114:2006–2011. doi: 10.1073/pnas.1611831114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Serafin V., Lissandron V., Buldini B., Bresolin S., Paganin M., Grillo F., Andriano N., Palmi C., Cazzaniga G., Marmiroli S., et al. Phosphoproteomic Analysis Reveals Hyperactivation of MTOR/STAT3 and LCK/Calcineurin Axes in Pediatric Early T-Cell Precursor ALL. Leukemia. 2017;31:1007–1011. doi: 10.1038/leu.2017.13. [DOI] [PubMed] [Google Scholar]
- 88.Degryse S., De Bock C.E., Demeyer S., Govaerts I., Bornschein S., Verbeke D., Jacobs K., Binos S., Skerrett-Byrne D.A., Murray H.C., et al. Mutant JAK3 Phosphoproteomic Profiling Predicts Synergism between JAK3 Inhibitors and MEK/BCL2 Inhibitors for the Treatment of T-Cell Acute Lymphoblastic Leukemia. Leukemia. 2018;32:788–800. doi: 10.1038/leu.2017.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Franciosa G., Smits J.G.A., Minuzzo S., Martinez-Val A., Indraccolo S., Olsen J.V. Proteomics of Resistance to Notch1 Inhibition in Acute Lymphoblastic Leukemia Reveals Targetable Kinase Signatures. Nat. Commun. 2021;12:2507. doi: 10.1038/s41467-021-22787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cordo’ V., Meijer M.T., Hagelaar R., de Goeij-de Haas R.R., Poort V.M., Henneman A.A., Piersma S.R., Pham T.V., Oshima K., Ferrando A.A., et al. Phosphoproteomic Profiling of T Cell Acute Lymphoblastic Leukemia Reveals Targetable Kinases and Combination Treatment Strategies. Nat. Commun. 2022;13:1048. doi: 10.1038/s41467-022-28682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Veltri G., Lovisa F., Cortese G., Pillon M., Carraro E., Cesaro S., Provenzi M., Buffardi S., Francescato S., Biffi A., et al. Phosphoproteomic Analysis Reveals a Different Proteomic Profile in Pediatric Patients With T-Cell Lymphoblastic Lymphoma or T-Cell Acute Lymphoblastic Leukemia. Front. Oncol. 2022;12:913487. doi: 10.3389/fonc.2022.913487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.García-Peydró M., Fuentes P., Mosquera M., García-León M.J., Alcain J., Rodríguez A., De Miguel P.G., Menéndez P., Weijer K., Spits H., et al. The NOTCH1/CD44 Axis Drives Pathogenesis in a T Cell Acute Lymphoblastic Leukemia Model. J. Clin. Investig. 2018;128:2802–2818. doi: 10.1172/JCI92981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kusakabe M., Sun A.C., Tyshchenko K., Wong R., Nanda A., Shanna C., Gusscott S., Chavez E.A., Lorzadeh A., Zhu A., et al. Synthetic Modeling Reveals HOXB Genes Are Critical for the Initiation and Maintenance of Human Leukemia. Nat. Commun. 2019;10:2913. doi: 10.1038/s41467-019-10510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goodall G.J., Wickramasinghe V.O. RNA in Cancer. Nat. Rev. Cancer. 2021;21:22–36. doi: 10.1038/s41568-020-00306-0. [DOI] [PubMed] [Google Scholar]
- 95.Mavrakis K.J., Van Der Meulen J., Wolfe A.L., Liu X., Mets E., Taghon T., Khan A.A., Setti M., Rondou P., Vandenberghe P., et al. A Cooperative MicroRNA-Tumor Suppressor Gene Network in Acute T-Cell Lymphoblastic Leukemia (T-ALL) Nat. Genet. 2011;43:673–678. doi: 10.1038/ng.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mavrakis K.J., Wolfe A.L., Oricchio E., Palomero T., De Keersmaecker K., McJunkin K., Zuber J., James T., Chang K., Khan A.A., et al. Genome-Wide RNA-Mediated Interference Screen Identifies MiR-19 Targets in Notch-Induced T-Cell Acute Lymphoblastic Leukaemia. Nat. Cell Biol. 2010;12:372–379. doi: 10.1038/ncb2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fragoso R., Mao T., Wang S., Schaffert S., Gong X., Yue S., Luong R., Min H., Yashiro-Ohtani Y., Davis M., et al. Modulating the Strength and Threshold of NOTCH Oncogenic Signals by Mir-181a-1/b-1. PLoS Genet. 2012;8:e1002855. doi: 10.1371/journal.pgen.1002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ortega M., Bhatnagar H., Lin A.P., Wang L., Aster J.C., Sill H., Aguiar R.C.T. A MicroRNA-Mediated Regulatory Loop Modulates NOTCH and MYC Oncogenic Signals in B- and T-Cell Malignancies. Leukemia. 2015;29:968–976. doi: 10.1038/leu.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Trimarchi T., Bilal E., Ntziachristos P., Fabbri G., Dalla-Favera R., Tsirigos A., Aifantis I. Genome-Wide Mapping and Characterization of Notch-Regulated Long Noncoding RNAs in Acute Leukemia. Cell. 2014;158:593–606. doi: 10.1016/j.cell.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feng H., Li F., Tang P. Circ_0000745 Regulates NOTCH1-Mediated Cell Proliferation and Apoptosis in Pediatric T-Cell Acute Lymphoblastic Leukemia through Adsorbing MiR-193b-3p. Hematology. 2021;26:885–895. doi: 10.1080/16078454.2021.1997197. [DOI] [PubMed] [Google Scholar]
- 101.Medyouf H., Gusscott S., Wang H., Tseng J.-C., Wai C., Nemirovsky O., Trumpp A., Pflumio F., Carboni J., Gottardis M., et al. High-Level IGF1R Expression Is Required for Leukemia-Initiating Cell Activity in T-ALL and Is Supported by Notch Signaling. J. Exp. Med. 2011;208:1809–1822. doi: 10.1084/jem.20110121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.González-García S., Mosquera M., Fuentes P., Palumbo T., Escudero A., Pérez-Martínez A., Ramírez M., Corcoran A.E., Toribio M.L. IL-7R Is Essential for Leukemia-Initiating Cell Activity of T-Cell Acute Lymphoblastic Leukemia. Blood. 2019;134:2171–2182. doi: 10.1182/blood.2019000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Allman D., Karnell F.G., Punt J.A., Bakkour S., Xu L., Myung P., Koretzky G.A., Pui J.C., Aster J.C., Pear W.S. Separation of Notch1 Promoted Lineage Commitment and Expansion/Transformation in Developing T Cells. J. Exp. Med. 2001;194:99–106. doi: 10.1084/jem.194.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Campese A.F., Garbe A.I., Zhang F., Grassi F., Screpanti I., Von Boehmer H. Notch1-Dependent Lymphomagenesis Is Assisted by but Does Not Essentially Require Pre-TCR Signaling. Blood. 2006;108:305–310. doi: 10.1182/blood-2006-01-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Herranz D., Ambesi-Impiombato A., Sudderth J., Sánchez-Martín M., Belver L., Tosello V., Xu L., Wendorff A.A., Castillo M., Haydu J.E., et al. Metabolic Reprogramming Induces Resistance to Anti-NOTCH1 Therapies in T Cell Acute Lymphoblastic Leukemia. Nat. Med. 2015;21:1182–1189. doi: 10.1038/nm.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Batista A., Barata J.T., Raderschall E., Sallan S.E., Carlesso N., Nadler L.M., Cardoso A.A. Targeting of Active MTOR Inhibits Primary Leukemia T Cells and Synergizes with Cytotoxic Drugs and Signaling Inhibitors. Exp. Hematol. 2011;39:457–472.e3. doi: 10.1016/j.exphem.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 107.Cullion K., Draheim K.M., Hermance N., Tammam J., Sharma V.M., Ware C., Nikov G., Krishnamoorthy V., Majumder P.K., Kelliher M.A. Targeting the Notch1 and MTOR Pathways in a Mouse T-ALL Model. Blood. 2009;113:6172–6181. doi: 10.1182/blood-2008-02-136762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Herranz D., Ambesi-Impiombato A., Palomero T., Schnell S.A., Belver L., Wendorff A.A., Xu L., Castillo-Martin M., Llobet-Navás D., Cordon-Cardo C., et al. A NOTCH1-Driven MYC Enhancer Promotes T Cell Development, Transformation and Acute Lymphoblastic Leukemia. Nat. Med. 2014;20:1130–1137. doi: 10.1038/nm.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Joshi I., Minter L.M., Telfer J., Demarest R.M., Capobianco A.J., Aster J.C., Sicinski P., Fauq A., Golde T.E., Osborne B.A. Notch Signaling Mediates G1/S Cell-Cycle Progression in T Cells via Cyclin D3 and Its Dependent Kinases. Blood. 2009;113:1689–1698. doi: 10.1182/blood-2008-03-147967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jena N., Sheng J., Hu J.K., Li W., Zhou W., Lee G., Tsichlis N., Pathak A., Brown N., Deshpande A., et al. CDK6-Mediated Repression of CD25 Is Required for Induction and Maintenance of Notch1-Induced T-Cell Acute Lymphoblastic Leukemia. Leukemia. 2016;30:1033–1043. doi: 10.1038/leu.2015.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pitt L.A., Tikhonova A.N., Hu H., Trimarchi T., King B., Gong Y., Sanchez-Martin M., Tsirigos A., Littman D.R., Ferrando A.A., et al. CXCL12-Producing Vascular Endothelial Niches Control Acute T Cell Leukemia Maintenance. Cancer Cell. 2015;27:755–768. doi: 10.1016/j.ccell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jost T.R., Borga C., Radaelli E., Romagnani A., Perruzza L., Omodho L., Cazzaniga G., Biondi A., Indraccolo S., Thelen M., et al. Role of CXCR4-Mediated Bone Marrow Colonization in CNS Infiltration by T Cell Acute Lymphoblastic Leukemia. J. Leukoc. Biol. 2016;99:1077–1087. doi: 10.1189/jlb.5MA0915-394R. [DOI] [PubMed] [Google Scholar]
- 113.Buonamici S., Trimarchi T., Ruocco M.G., Reavie L., Cathelin S., Mar B.G., Klinakis A., Lukyanov Y., Tseng J.C., Sen F., et al. CCR7 Signalling as an Essential Regulator of CNS Infiltration in T-Cell Leukaemia. Nature. 2009;459:1000–1004. doi: 10.1038/nature08020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mirandola L., Chiriva-Internati M., Montagna D., Locatelli F., Zecca M., Ranzani M., Basile A., Locati M., Cobos E., Kast W.M., et al. Notch1 Regulates Chemotaxis and Proliferation by Controlling the CC-Chemokine Receptors 5 and 9 in T Cell Acute Lymphoblastic Leukaemia. J. Pathol. 2012;226:713–722. doi: 10.1002/path.3015. [DOI] [PubMed] [Google Scholar]
- 115.Junker F., Chabloz A., Koch U., Radtke F. Dicer1 Imparts Essential Survival Cues in Notch-Driven T-ALL via MiR-21-Mediated Tumor Suppressor Pdcd4 Repression. Blood. 2015;126:993–1004. doi: 10.1182/blood-2014-12-618892. [DOI] [PubMed] [Google Scholar]
- 116.Treiber T., Treiber N., Meister G. Regulation of MicroRNA Biogenesis and Its Crosstalk with Other Cellular Pathways. Nat. Rev. Mol. Cell Biol. 2019;20:5–20. doi: 10.1038/s41580-018-0059-1. [DOI] [PubMed] [Google Scholar]
- 117.Haneklaus M., Gerlic M., O’Neill L.A.J., Masters S.L. MiR-223: Infection, Inflammation and Cancer. J. Intern. Med. 2013;274:215–226. doi: 10.1111/joim.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kumar V., Palermo R., Talora C., Campese A.F., Checquolo S., Bellavia D., Tottone L., Testa G., Miele E., Indraccolo S., et al. Notch and NF-KB Signaling Pathways Regulate MiR-223/FBXW7 Axis in T-Cell Acute Lymphoblastic Leukemia. Leukemia. 2014;28:2324–2335. doi: 10.1038/leu.2014.133. [DOI] [PubMed] [Google Scholar]
- 119.Mansour M.R., Sanda T., Lawton L.N., Li X., Kreslavsky T., Novina C.D., Brand M., Gutierrez A., Kelliher M.A., Jamieson C.H.M., et al. The TAL1 Complex Targets the FBXW7 Tumor Suppressor by Activating MiR-223 in Human T Cell Acute Lymphoblastic Leukemia. J. Exp. Med. 2013;210:1545–1557. doi: 10.1084/jem.20122516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shu Y., Wang Y., Lv W.Q., Peng D.Y., Li J., Zhang H., Jiang G.J., Yang B.J., Liu S., Zhang J., et al. ARRB1-Promoted NOTCH1 Degradation Is Suppressed by OncomiR MiR-223 in T-Cell Acute Lymphoblastic Leukemia. Cancer Res. 2020;80:988–998. doi: 10.1158/0008-5472.CAN-19-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gusscott S., Kuchenbauer F., Humphries R.K., Weng A.P. Notch-Mediated Repression of MiR-223 Contributes to IGF1R Regulation in T-ALL. Leuk. Res. 2012;36:905–911. doi: 10.1016/j.leukres.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 122.Guttman M., Russell P., Ingolia N.T., Weissman J.S., Lander E.S. Ribosome Profiling Provides Evidence That Large Noncoding RNAs Do Not Encode Proteins. Cell. 2013;154:240–251. doi: 10.1016/j.cell.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hung T., Wang Y., Lin M.F., Koegel A.K., Kotake Y., Grant G.D., Horlings H.M., Shah N., Umbricht C., Wang P., et al. Extensive and Coordinated Transcription of Noncoding RNAs within Cell-Cycle Promoters. Nat. Genet. 2011;43:621–629. doi: 10.1038/ng.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee J.T. Epigenetic Regulation by Long Noncoding RNAs. Science. 2012;338:1435–1439. doi: 10.1126/science.1231776. [DOI] [PubMed] [Google Scholar]
- 125.Luo M., Jeong M., Sun D., Park H.J., Rodriguez B.A.T., Xia Z., Yang L., Zhang X., Sheng K., Darlington G.J., et al. Long Non-Coding RNAs Control Hematopoietic Stem Cell Function. Cell Stem Cell. 2015;16:426–438. doi: 10.1016/j.stem.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu Z., Gao S., Zhao X., Chen J., Keyvanfar K., Feng X., Kajigaya S., Young N.S. Long Noncoding RNAs of Single Hematopoietic Stem and Progenitor Cells in Healthy and Dysplastic Human Bone Marrow. Haematologica. 2019;104:894–906. doi: 10.3324/haematol.2018.208926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Luo H., Zhu G., Xu J., Lai Q., Yan B., Guo Y., Fung T.K., Zeisig B.B., Cui Y., Zha J., et al. HOTTIP LncRNA Promotes Hematopoietic Stem Cell Self-Renewal Leading to AML-like Disease in Mice. Cancer Cell. 2019;36:645–659.e8. doi: 10.1016/j.ccell.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li G., Gao L., Zhao J., Liu D., Li H., Hu M. LncRNA ANRIL/MiR-7-5p/TCF4 Axis Contributes to the Progression of T Cell Acute Lymphoblastic Leukemia. Cancer Cell Int. 2020;20:335. doi: 10.1186/s12935-020-01376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu G., Luo H., Feng Y., Guryanova O.A., Xu J., Chen S., Lai Q., Sharma A., Xu B., Zhao Z., et al. HOXBLINC Long Non-Coding RNA Activation Promotes Leukemogenesis in NPM1-Mutant Acute Myeloid Leukemia. Nat. Commun. 2021;12:1956. doi: 10.1038/s41467-021-22095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li G., Lei X., Zhang Y., Liu Z., Zhu K. LncRNA PPM1A-AS Regulate Tumor Development Through Multiple Signal Pathways in T-Cell Acute Lymphoblastic Leukemia. Front. Oncol. 2021;11:761205. doi: 10.3389/fonc.2021.761205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Durinck K., Wallaert A., Van de Walle I., Van Loocke W., Volders P.J., Vanhauwaert S., Geerdens E., Benoit Y., Van Roy N., Poppe B., et al. The Notch Driven Long Non-Coding RNA Repertoire in T-Cell Acute Lymphoblastic Leukemia. Haematologica. 2014;99:1808–1816. doi: 10.3324/haematol.2014.115683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Y., Wu P., Lin R., Rong L., Xue Y., Fang Y. LncRNA NALT Interaction with NOTCH1 Promoted Cell Proliferation in Pediatric T Cell Acute Lymphoblastic Leukemia. Sci. Rep. 2015;5:13749. doi: 10.1038/srep13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu C.X., Chen L.L. Circular RNAs: Characterization, Cellular Roles, and Applications. Cell. 2022;185:2016–2034. doi: 10.1016/j.cell.2022.04.021. [DOI] [PubMed] [Google Scholar]
- 134.Buratin A., Paganin M., Gaffo E., Dal Molin A., Roels J., Germano G., Siddi M.T., Serafin V., de Decker M., Gachet S., et al. Large-Scale Circular RNA Deregulation in T-ALL: Unlocking Unique Ectopic Expression of Molecular Subtypes. Blood Adv. 2020;4:5902–5914. doi: 10.1182/bloodadvances.2020002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sudo T., Nishikawa S., Ohno N., Akiyama N., Tamakoshi M., Yoshida H., Nishikawa S.I. Expression and Function of the Interleukin 7 Receptor in Murine Lymphocytes. Proc. Natl. Acad. Sci. USA. 1993;90:9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peschon J.J., Morrissey P.J., Grabstein K.H., Ramsdell F.J., Maraskovsky E., Gliniak B.C., Park L.S., Ziegler S.F., Williams D.E., Ware C.B., et al. Early Lymphocyte Expansion Is Severely Impaired in Interleukin 7 Receptor-Deficient Mice. J. Exp. Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Von Freeden-Jeffry U., Vieira P., Lucian L.A., McNeil T., Burdach S.E.G., Murray R. Lymphopenia in Interleukin (IL)-7 Gene-Deleted Mice Identifies IL-7 as a Nonredundant Cytokine. J. Exp. Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Puel A., Ziegler S.F., Buckley R.H., Leonard W.J. Defective IL7R Expression in T(-)B(+)NK(+) Severe Combined Immunodeficiency. Nat. Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 139.Leonard W.J. Cytokines and Immunodeficiency Diseases. Nat. Rev. Immunol. 2001;1:200–208. doi: 10.1038/35105066. [DOI] [PubMed] [Google Scholar]
- 140.Shochat C., Tal N., Bandapalli O.R., Palmi C., Ganmore I., te Kronnie G., Cario G., Cazzaniga G., Kulozik A.E., Stanulla M., et al. Gain-of-Function Mutations in Interleukin-7 Receptor-α (IL7R) in Childhood Acute Lymphoblastic Leukemias. J. Exp. Med. 2011;208:901–908. doi: 10.1084/jem.20110580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zenatti P.P., Ribeiro D., Li W., Zuurbier L., Silva M.C., Paganin M., Tritapoe J., Hixon J.A., Silveira A.B., Cardoso B.A., et al. Oncogenic IL7R Gain-of-Function Mutations in Childhood T-Cell Acute Lymphoblastic Leukemia. Nat. Genet. 2011;43:932–941. doi: 10.1038/ng.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Silva A., Almeida A.R.M., Cachucho A., Neto J.L., Demeyer S., de Matos M., Hogan T., Li Y., Meijerink J., Cools J., et al. Overexpression of Wild-Type IL-7Rα Promotes T-Cell Acute Lymphoblastic Leukemia/Lymphoma. Blood. 2021;138:1040–1052. doi: 10.1182/blood.2019000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vicente C., Schwab C., Broux M., Geerdens E., Degryse S., Demeyer S., Lahortiga I., Elliott A., Chilton L., La Starza R., et al. Targeted Sequencing Identifies Associations between IL7R-JAK Mutations and Epigenetic Modulators in T-Cell Acute Lymphoblastic Leukemia. Haematologica. 2015;100:1301–1310. doi: 10.3324/haematol.2015.130179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Akkapeddi P., Fragoso R., Hixon J.A., Ramalho A.S., Oliveira M.L., Carvalho T., Gloger A., Matasci M., Corzana F., Durum S.K., et al. A Fully Human Anti-IL-7Rα Antibody Promotes Antitumor Activity against T-Cell Acute Lymphoblastic Leukemia. Leukemia. 2019;33:2155–2168. doi: 10.1038/s41375-019-0434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hixon J.A., Andrews C., Kashi L., Kohnhorst C.L., Senkevitch E., Czarra K., Barata J.T., Li W., Schneider J.P., Walsh S.T.R., et al. New Anti-IL-7Rα Monoclonal Antibodies Show Efficacy against T Cell Acute Lymphoblastic Leukemia in Pre-Clinical Models. Leukemia. 2020;34:35–49. doi: 10.1038/s41375-019-0531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bertrand F.E., Steelman L.S., Chappell W.H., Abrams S.L., Shelton J.G., White E.R., Ludwig D.L., McCubrey J.A. Synergy between an IGF-1R Antibody and Raf/MEK/ERK and PI3K/Akt/MTOR Pathway Inhibitors in Suppressing IGF-1R-Mediated Growth in Hematopoietic Cells. Leukemia. 2006;20:1254–1260. doi: 10.1038/sj.leu.2404217. [DOI] [PubMed] [Google Scholar]
- 147.Xu D.D., Wang Y., Zhou P.J., Qin S.R., Zhang R., Zhang Y., Xue X., Wang J., Wang X., Chen H.C., et al. The IGF2/IGF1R/Nanog Signaling Pathway Regulates the Proliferation of Acute Myeloid Leukemia Stem Cells. Front. Pharmacol. 2018;9:687. doi: 10.3389/fphar.2018.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Von Boehmer H., Fehling H.J. Structure and Function of the Pre-T Cell Receptor. Annu. Rev. Immunol. 1997;15:433–452. doi: 10.1146/annurev.immunol.15.1.433. [DOI] [PubMed] [Google Scholar]
- 149.Carrasco Y.R., Navarro M.N., De Yébenes V.G., Ramiro A.R., Toribio M.L. Regulation of Surface Expression of the Human Pre-T Cell Receptor Complex. Semin. Immunol. 2002;14:325–334. doi: 10.1016/S1044-5323(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 150.Von Boehmer H. Unique Features of the Pre-T-Cell Receptor Alpha-Chain: Not Just a Surrogate. Nat. Rev. Immunol. 2005;5:571–577. doi: 10.1038/nri1636. [DOI] [PubMed] [Google Scholar]
- 151.Fehling H.J., Krotkova A., Saint-Ruf C., von Boehmer H. Crucial Role of the Pre-T-Cell Receptor α Gene in Development of αβ but Not γδ T Cells. Nature. 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 152.Mombaerts P., Clarke A.R., Rudnicki M.A., Iacomini J., Itohara S., Lafaille J.J., Wang L., Ichikawa Y., Jaenisch R., Hooper M.L., et al. Mutations in T-Cell Antigen Receptor Genes α and β Block Thymocyte Development at Different Stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 153.Wang B., Wang N., Whitehurst C.E., She J., Chen J., Terhorst C. T Lymphocyte Development in the Absence of CD3 Epsilon or CD3 Gamma Delta Epsilon Zeta. J. Immunol. 1999;162:88–94. doi: 10.4049/jimmunol.162.1.88. [DOI] [PubMed] [Google Scholar]
- 154.Molina T.J., Kishihara K., Siderovskid D.P., Van Ewijk W., Narendran A., Timms E., Wakeham A., Paige C.J., Hartmann K.U., Veillette A., et al. Profound Block in Thymocyte Development in Mice Lacking P56lck. Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 155.Clements J.L., Yang B., Ross-Barta S.E., Eliason S.L., Hrstka R.F., Williamson R.A., Koretzky G.A. Requirement for the Leukocyte-Specific Adapter Protein SLP-76 for Normal T Cell Development. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 156.Yamasaki S., Ishikawa E., Sakuma M., Ogata K., Sakata-Sogawa K., Hiroshima M., Wiest D.L., Tokunaga M., Saito T. Mechanistic Basis of Pre-T Cell Receptor-Mediated Autonomous Signaling Critical for Thymocyte Development. Nat. Immunol. 2006;7:67–75. doi: 10.1038/ni1290. [DOI] [PubMed] [Google Scholar]
- 157.Panigada M., Porcellini S., Barbier E., Hoeflinger S., Cazenave P.A., Gu H., Band H., Von Boehmer H., Grassi F. Constitutive Endocytosis and Degradation of the Pre-T Cell Receptor. J. Exp. Med. 2002;195:1585–1597. doi: 10.1084/jem.20020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Carrasco Y.R., Navarro M.N., Toribio M.L. A Role for the Cytoplasmic Tail of the Pre-T Cell Receptor (TCR) Alpha Chain in Promoting Constitutive Internalization and Degradation of the Pre-TCR. J. Biol. Chem. 2003;278:14507–14513. doi: 10.1074/jbc.M204944200. [DOI] [PubMed] [Google Scholar]
- 159.Mallis R.J., Bai K., Arthanari H., Hussey R.E., Handley M., Li Z., Chingozha L., Duke-Cohan J.S., Lu H., Wang J.H., et al. Pre-TCR Ligand Binding Impacts Thymocyte Development before AβTCR Expression. Proc. Natl. Acad. Sci. USA. 2015;112:8373–8378. doi: 10.1073/pnas.1504971112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Das D.K., Mallis R.J., Duke-Cohan J.S., Hussey R.E., Tetteh P.W., Hilton M., Wagner G., Lang M.J., Reinherz E.L. Pre-T Cell Receptors (Pre-TCRs) Leverage Vβ Complementarity Determining Regions (CDRs) and Hydrophobic Patch in Mechanosensing Thymic Self-Ligands. J. Biol. Chem. 2016;291:25292–25305. doi: 10.1074/jbc.M116.752865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li X., Mizsei R., Tan K., Mallis R.J., Duke-Cohan J.S., Akitsu A., Tetteh P.W., Dubey A., Hwang W., Wagner G., et al. Pre–T Cell Receptors Topologically Sample Self-Ligands during Thymocyte b-Selection. Science. 2021;371:181–185. doi: 10.1126/science.abe0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Allam A.H., Charnley M., Pham K., Russell S.M. Developing T Cells Form an Immunological Synapse for Passage through the β-Selection Checkpoint. J. Cell Biol. 2021;220:e201908108. doi: 10.1083/jcb.201908108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Maillard I., Tu L., Sambandam A., Yashiro-Otani Y., Millholland J., Keeshan K., Shestova O., Xu L., Bhandoola A., Pear W.S. The Requirement for Notch Signaling at the Beta-Selection Checkpoint in Vivo Is Absolute and Independent of the Pre-T Cell Receptor. J. Exp. Med. 2006;203:2239–2245. doi: 10.1084/jem.20061020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ciofani M., Schmitt T.M., Ciofani A., Michie A.M., Çuburu N., Aublin A., Maryanski J.L., Zúñiga-Pflücker J.C. Obligatory Role for Cooperative Signaling by Pre-TCR and Notch during Thymocyte Differentiation. J. Immunol. 2004;172:5230–5239. doi: 10.4049/jimmunol.172.9.5230. [DOI] [PubMed] [Google Scholar]
- 165.Ciofani M., Zúñiga-Pflücker J.C. Notch Promotes Survival of Pre-T Cells at the β-Selection Checkpoint by Regulating Cellular Metabolism. Nat. Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- 166.Zhao B., Yoganathan K., Li L.Q., Lee J.Y., Zúñiga-Pflücker J.C., Love P.E. Notch and the Pre-TCR Coordinate Thymocyte Proliferation by Induction of the SCF Subunits Fbxl1 and Fbxl12. Nat. Immunol. 2019;20:1381–1392. doi: 10.1038/s41590-019-0469-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Huang E.Y., Gallegos A.M., Richards S.M., Lehar S.M., Bevan M.J. Surface Expression of Notch1 on Thymocytes: Correlation with the Double-Negative to Double-Positive Transition. J. Immunol. 2003;171:2296–2304. doi: 10.4049/jimmunol.171.5.2296. [DOI] [PubMed] [Google Scholar]
- 168.Michie A.M., Chan A.C., Ciofani M., Carleton M., Lefebvre J.M., He Y., Allman D.M., Wiest D.L., Zúñiga-Pflücker J.C., Izon D.J. Constitutive Notch Signalling Promotes CD4- CD8—Thymocyte Differentiation in the Absence of the Pre-TCR Complex, by Mimicking Pre-TCR Signals. Int. Immunol. 2007;19:1421–1430. doi: 10.1093/intimm/dxm113. [DOI] [PubMed] [Google Scholar]
- 169.Solanki A., Yánez D.C., Lau C.I., Rowell J., Barbarulo A., Ross S., Sahni H., Crompton T. The Transcriptional Repressor Bcl6 Promotes Pre-TCR-Induced Thymocyte Differentiation and Attenuates Notch1 Activation. Development. 2020;147:dev192203. doi: 10.1242/dev.192203. [DOI] [PubMed] [Google Scholar]
- 170.Tanaka T., Nakajima-Takagi Y., Aoyama K., Tara S., Oshima M., Saraya A., Koide S., Si S., Manabe I., Sanada M., et al. Internal Deletion of BCOR Reveals a Tumor Suppressor Function for BCOR in T Lymphocyte Malignancies. J. Exp. Med. 2017;214:2901–2913. doi: 10.1084/jem.20170167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yashiro-Ohtani Y., He Y., Ohtani T., Jones M.E., Shestova O., Xu L., Fang T.C., Chiang M.Y., Intlekofer A.M., Blacklow S.C., et al. Pre-TCR Signaling Inactivates Notch1 Transcription by Antagonizing E2A. Genes Dev. 2009;23:1665–1676. doi: 10.1101/gad.1793709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bellavia D., Campese A.F., Checquolo S., Balestri A., Biondi A., Cazzaniga G., Lendahl U., Fehling H.J., Hayday A.C., Frati L., et al. Combined Expression of PTα and Notch3 in T Cell Leukemia Identifies the Requirement of PreTCR for Leukemogenesis. Proc. Natl. Acad. Sci. USA. 2002;99:3788–3793. doi: 10.1073/pnas.062050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Asnafi V., Beldjord K., Boulanger E., Comba B., Le Tutour P., Estienne M.H., Davi F., Landman-Parker J., Quartier P., Buzyn A., et al. Analysis of TCR, PTα, and RAG-1 in T-Acute Lymphoblastic Leukemias Improves Understanding of Early Human T-Lymphoid Lineage Commitment. Blood. 2003;101:2693–2703. doi: 10.1182/blood-2002-08-2438. [DOI] [PubMed] [Google Scholar]
- 174.Ivanyi P., Morgan M., Piao W., Ukena S.N., Steube K., Ganser A., Franzke A. Pre T-Cell Receptor Alpha (PTalpha) Expression Patterns and Functional Analysis in Human T-Cell Lymphoblastic Leukemia. Cell Oncol. 2010;32:101–108. doi: 10.3233/CLO-2009-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ramiro A.R., Navarro M.N., Carreira A., Carrasco Y.R., de Yébenes V.G., Carrillo G., San Millán J.L., Rubin B., Toribio M.L. Differential Developmental Regulation and Functional Effects on Pre-TCR Surface Expression of Human PTalpha(a) and PTalpha(b) Spliced Isoforms. J. Immunol. 2001;167:5106–5114. doi: 10.4049/jimmunol.167.9.5106. [DOI] [PubMed] [Google Scholar]
- 176.Palomero T., Sulis M.L., Cortina M., Real P.J., Barnes K., Ciofani M., Caparros E., Buteau J., Brown K., Perkins S.L., et al. Mutational Loss of PTEN Induces Resistance to NOTCH1 Inhibition in T-Cell Leukemia. Nat. Med. 2007;13:1203–1210. doi: 10.1038/nm1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Srivastava S., Sahu U., Zhou Y., Hogan A.K., Sathyan K.M., Bodner J., Huang J., Wong K.A., Khalatyan N., Savas J.N., et al. NOTCH1-Driven UBR7 Stimulates Nucleotide Biosynthesis to Promote T Cell Acute Lymphoblastic Leukemia. Sci. Adv. 2021;7:eabc9781. doi: 10.1126/sciadv.abc9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Baran N., Lodi A., Dhungana Y., Herbrich S., Collins M., Sweeney S., Pandey R., Skwarska A., Patel S., Tremblay M., et al. Inhibition of Mitochondrial Complex I Reverses NOTCH1-Driven Metabolic Reprogramming in T-Cell Acute Lymphoblastic Leukemia. Nat. Commun. 2022;13:2801. doi: 10.1038/s41467-022-30396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hanahan D., Weinberg R.A. Hallmarks of Cancer: The next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 180.Stine Z.E., Walton Z.E., Altman B.J., Hsieh A.L., Dang C.V. MYC, Metabolism, and Cancer. Cancer Discov. 2015;5:1024. doi: 10.1158/2159-8290.CD-15-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Huang C.Y., Bredemeyer A.L., Walker L.M., Bassing C.H., Sleckman B.P. Dynamic Regulation of C-Myc Proto-Oncogene Expression during Lymphocyte Development Revealed by a GFP-c-Myc Knock-in Mouse. Eur. J. Immunol. 2008;38:342–349. doi: 10.1002/eji.200737972. [DOI] [PubMed] [Google Scholar]
- 182.Mingueneau M., Kreslavsky T., Gray D., Heng T., Cruse R., Ericson J., Bendall S., Spitzer M.H., Nolan G.P., Kobayashi K., et al. The Transcriptional Landscape of Aβ T Cell Differentiation. Nat. Immunol. 2013;14:619–632. doi: 10.1038/ni.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dose M., Khan I., Guo Z., Kovalovsky D., Krueger A., Von Boehmer H., Khazaie K., Gounari F. C-Myc Mediates Pre-TCR-Induced Proliferation but Not Developmental Progression. Blood. 2006;108:2669–2677. doi: 10.1182/blood-2006-02-005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Douglas N.C., Jacobs H., Bothwell A.L.M., Hayday A.C. Defining the Specific Physiological Requirements for C-Myc in T Cell Development. Nat. Immunol. 2001;2:307–315. doi: 10.1038/86308. [DOI] [PubMed] [Google Scholar]
- 185.Nozais M., Loosveld M., Pankaew S., Grosjean C., Gentil N., Quessada J., Nadel B., Mionnet C., Potier D., Payet-Bornet D. MYC Deficiency Impairs the Development of Effector/Memory T Lymphocytes. iScience. 2021;24:102761. doi: 10.1016/j.isci.2021.102761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Preston G.C., Sinclair L.V., Kaskar A., Hukelmann J.L., Navarro M.N., Ferrero I., MacDonald H.R., Cowling V.H., Cantrell D.A. Single Cell Tuning of Myc Expression by Antigen Receptor Signal Strength and Interleukin-2 in T Lymphocytes. EMBO J. 2015;34:2008–2024. doi: 10.15252/embj.201490252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bonnet M., Loosveld M., Montpellier B., Navarro J.-M., Quilichini B., Picard C., Di Cristofaro J., Bagnis C., Fossat C., Hernandez L., et al. Posttranscriptional Deregulation of MYC via PTEN Constitutes a Major Alternative Pathway of MYC Activation in T-Cell Acute Lymphoblastic Leukemia. Blood. 2011;117:6650–6659. doi: 10.1182/blood-2011-02-336842. [DOI] [PubMed] [Google Scholar]
- 188.Loosveld M., Castellano R., Gon S., Goubard A., Crouzet T., Pouyet L., Prebet T., Vey N., Nadel B., Collette Y., et al. Therapeutic Targeting of C-Myc in T-Cell Acute Lymphoblastic Leukemia (T-ALL) Oncotarget. 2014;5:3168–3172. doi: 10.18632/oncotarget.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Roderick J.E., Tesell J., Shultz L.D., Brehm M.A., Greiner D.L., Harris M.H., Silverman L.B., Sallan S.E., Gutierrez A., Look A.T., et al. C-Myc Inhibition Prevents Leukemia Initiation in Mice and Impairs the Growth of Relapsed and Induction Failure Pediatric T-ALL Cells. Blood. 2014;123:1040–1050. doi: 10.1182/blood-2013-08-522698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.La Starza R., Borga C., Barba G., Pierini V., Schwab C., Matteucci C., Lema Fernandez A.G., Leszl A., Cazzaniga G., Chiaretti S., et al. Genetic Profile of T-Cell Acute Lymphoblastic Leukemias with MYC Translocations. Blood. 2014;124:3577–3582. doi: 10.1182/blood-2014-06-578856. [DOI] [PubMed] [Google Scholar]
- 191.Langenau D.M., Traver D., Ferrando A.A., Kutok J.L., Aster J.C., Kanki J.P., Lin S., Prochownik E., Trede N.S., Zon L.I., et al. Myc-Induced T Cell Leukemia in Transgenic Zebrafish. Science. 2003;299:887–890. doi: 10.1126/science.1080280. [DOI] [PubMed] [Google Scholar]
- 192.Gutierrez A., Grebliunaite R., Feng H., Kozakewich E., Zhu S., Guo F., Payne E., Mansour M., Dahlberg S.E., Neuberg D.S., et al. Pten Mediates Myc Oncogene Dependence in a Conditional Zebrafish Model of T Cell Acute Lymphoblastic Leukemia. J. Exp. Med. 2011;208:1595–1603. doi: 10.1084/jem.20101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Smith D.P., Bath M.L., Metcalf D., Harris A.W., Cory S. MYC Levels Govern Hematopoietic Tumor Type and Latency in Transgenic Mice. Blood. 2006;108:653–661. doi: 10.1182/blood-2006-01-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Loosveld M., Bonnet M., Gon S., Montpellier B., Quilichini B., Navarro J.M., Crouzet T., Goujart M.A., Chasson L., Morgado E., et al. MYC Fails to Efficiently Shape Malignant Transformation in T-Cell Acute Lymphoblastic Leukemia. Genes. Chromosomes Cancer. 2014;53:52–66. doi: 10.1002/gcc.22117. [DOI] [PubMed] [Google Scholar]
- 195.King B., Trimarchi T., Reavie L., Xu L., Mullenders J., Ntziachristos P., Aranda-Orgilles B., Perez-Garcia A., Shi J., Vakoc C., et al. The Ubiquitin Ligase FBXW7 Modulates Leukemia-Initiating Cell Activity by Regulating MYC Stability. Cell. 2013;153:1552–1566. doi: 10.1016/j.cell.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Weng A.P., Millholland J.M., Yashiro-Ohtani Y., Arcangeli M.L., Lau A., Wai C., del Bianco C., Rodriguez C.G., Sai H., Tobias J., et al. C-Myc Is an Important Direct Target of Notch1 in T-Cell Acute Lymphoblastic Leukemia/Lymphoma. Genes Dev. 2006;20:2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yashiro-Ohtani Y., Wang H., Zang C., Arnett K.L., Bailis W., Ho Y., Knoechel B., Lanauze C., Louis L., Forsyth K.S., et al. Long-Range Enhancer Activity Determines Myc Sensitivity to Notch Inhibitors in T Cell Leukemia. Proc. Natl. Acad. Sci. USA. 2014;111:E4946–E4953. doi: 10.1073/pnas.1407079111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Belver L., Yang A.Y., Albero R., Herranz D., Brundu F.G., Quinn S.A., Pérez-Durán P., Álvarez S., Gianni F., Rashkovan M., et al. GATA3-Controlled Nucleosome Eviction Drives MYC Enhancer Activity in T-Cell Development and Leukemia. Cancer Discov. 2019;9:1774–1791. doi: 10.1158/2159-8290.CD-19-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Guo W., Lasky J.L., Chang C.J., Mosessian S., Lewis X., Xiao Y., Yeh J.E., Chen J.Y., Iruela-Arispe M.L., Varella-Garcia M., et al. Multi-Genetic Events Collaboratively Contribute to Pten-Null Leukaemia Stem-Cell Formation. Nature. 2008;453:529–533. doi: 10.1038/nature06933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hoxhaj G., Manning B.D. The PI3K-AKT Network at the Interface of Oncogenic Signalling and Cancer Metabolism. Nat. Rev. Cancer. 2020;20:74–88. doi: 10.1038/s41568-019-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Barata J.T., Silva A., Brandao J.G., Nadler L.M., Cardoso A.A., Boussiotis V.A. Activation of PI3K Is Indispensable for Interleukin 7-Mediated Viability, Proliferation, Glucose Use, and Growth of T Cell Acute Lymphoblastic Leukemia Cells. J. Exp. Med. 2004;200:659–669. doi: 10.1084/jem.20040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wieman H.L., Wofford J.A., Rathmell J.C. Cytokine Stimulation Promotes Glucose Uptake via Phosphatidylinositol-3 Kinase/Akt Regulation of Glut1 Activity and Trafficking. Mol. Biol. Cell. 2007;18:1437–1446. doi: 10.1091/mbc.e06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wofford J.A., Wieman H.L., Jacobs S.R., Zhao Y., Rathmell J.C. IL-7 Promotes Glut1 Trafficking and Glucose Uptake via STAT5-Mediated Activation of Akt to Support T-Cell Survival. Blood. 2008;111:2101–2111. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Roberts D.J., Tan-Sah V.P., Smith J.M., Miyamoto S. Akt Phosphorylates HK-II at Thr-473 and Increases Mitochondrial HK-II Association to Protect Cardiomyocytes. J. Biol. Chem. 2013;288:23798–23806. doi: 10.1074/jbc.M113.482026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Deprez J., Vertommen D., Alessi D.R., Hue L., Rider M.H. Phosphorylation and Activation of Heart 6-Phosphofructo-2-Kinase by Protein Kinase B and Other Protein Kinases of the Insulin Signaling Cascades. J. Biol. Chem. 1997;272:17269–17275. doi: 10.1074/jbc.272.28.17269. [DOI] [PubMed] [Google Scholar]
- 206.Boudil A., Matei I.R., Shih H.Y., Bogdanoski G., Yuan J.S., Chang S.G., Montpellier B., Kowalski P.E., Voisin V., Bashir S., et al. IL-7 Coordinates Proliferation, Differentiation and Tcra Recombination during Thymocyte β-Selection. Nat. Immunol. 2015;16:397–405. doi: 10.1038/ni.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Silva A., Yunes J.A., Cardoso B.A., Martins L.R., Jotta P.Y., Abecasis M., Nowill A.E., Leslie N.R., Cardoso A.A., Barata J.T. PTEN Posttranslational Inactivation and Hyperactivation of the PI3K/Akt Pathway Sustain Primary T Cell Leukemia Viability. J. Clin. Investig. 2008;118:3762. doi: 10.1172/JCI34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mendes R.D., Canté-Barrett K., Pieters R., Meijerink J.P.P. The Relevance of PTEN-AKT in Relation to NOTCH1-Directed Treatment Strategies in T-Cell Acute Lymphoblastic Leukemia. Haematologica. 2016;101:1010–1017. doi: 10.3324/haematol.2016.146381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Calzavara E., Chiaramonte R., Cesana D., Basile A., Sherbet G.V., Comi P. Reciprocal Regulation of Notch and PI3K/Akt Signalling in T-ALL Cells In Vitro. J. Cell Biochem. 2008;103:1405–1412. doi: 10.1002/jcb.21527. [DOI] [PubMed] [Google Scholar]
- 210.Platonova N., Manzo T., Mirandola L., Colombo M., Calzavara E., Vigolo E., Cermisoni G.C., De Simone D., Garavelli S., Cecchinato V., et al. PI3K/AKT Signaling Inhibits NOTCH1 Lysosome-Mediated Degradation. Genes. Chromosomes Cancer. 2015;54:516–526. doi: 10.1002/gcc.22264. [DOI] [PubMed] [Google Scholar]
- 211.Hales E.C., Orr S.M., Gedman A.L., Taub J.W., Matherly L.H. Notch1 Receptor Regulates AKT Protein Activation Loop (Thr308) Dephosphorylation through Modulation of the PP2A Phosphatase in Phosphatase and Tensin Homolog (PTEN)-Null T-Cell Acute Lymphoblastic Leukemia Cells. J. Biol. Chem. 2013;288:22836–22848. doi: 10.1074/jbc.M113.451625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chan S.M., Weng A.P., Tibshirani R., Aster J.C., Utz P.J. Notch Signals Positively Regulate Activity of the MTOR Pathway in T-Cell Acute Lymphoblastic Leukemia. Blood. 2007;110:278–286. doi: 10.1182/blood-2006-08-039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Evangelisti C., Ricci F., Tazzari P., Tabellini G., Battistelli M., Falcieri E., Chiarini F., Bortul R., Melchionda F., Pagliaro P., et al. Targeted Inhibition of MTORC1 and MTORC2 by Active-Site MTOR Inhibitors Has Cytotoxic Effects in T-Cell Acute Lymphoblastic Leukemia. Leukemia. 2011;25:781–791. doi: 10.1038/leu.2011.20. [DOI] [PubMed] [Google Scholar]
- 214.Lonetti A., Cappellini A., Bertaina A., Locatelli F., Pession A., Buontempo F., Evangelisti C., Evangelisti C., Orsini E., Zambonin L., et al. Improving Nelarabine Efficacy in T Cell Acute Lymphoblastic Leukemia by Targeting Aberrant PI3K/AKT/MTOR Signaling Pathway. J. Hematol. Oncol. 2016;9:114. doi: 10.1186/s13045-016-0344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gazi M., Moharram S.A., Marhäll A., Kazi J.U. The Dual Specificity PI3K/MTOR Inhibitor PKI-587 Displays Efficacy against T-Cell Acute Lymphoblastic Leukemia (T-ALL) Cancer Lett. 2017;392:9–16. doi: 10.1016/j.canlet.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 216.Evangelisti C., Chiarini F., Cappellini A., Paganelli F., Fini M., Santi S., Martelli A.M., Neri L.M., Evangelisti C. Targeting Wnt/β-Catenin and PI3K/Akt/MTOR Pathways in T-Cell Acute Lymphoblastic Leukemia. J. Cell Physiol. 2020;235:5413–5428. doi: 10.1002/jcp.29429. [DOI] [PubMed] [Google Scholar]
- 217.Kong D., Fan S., Sun L., Chen X., Zhao Y., Zhao L., Guo Z., Li Y. Growth Inhibition and Suppression of the MTOR and Wnt/β-Catenin Pathways in T-Acute Lymphoblastic Leukemia by Rapamycin and MYCN Depletion. Hematol. Oncol. 2021;39:222–230. doi: 10.1002/hon.2831. [DOI] [PubMed] [Google Scholar]
- 218.Laukkanen S., Bacquelaine Veloso A., Yan C., Oksa L., Alpert E.J., Do D., Hyvärinen N., McCarthy K., Adhikari A., Yang Q., et al. Therapeutic Targeting of LCK Tyrosine Kinase and MTOR Signaling in T-Cell Acute Lymphoblastic Leukemia. Blood. 2022;140:1891–1906. doi: 10.1182/blood.2021015106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pham L.T., Peng H., Ueno M., Kohno S., Kasada A., Hosomichi K., Sato T., Kurayoshi K., Kobayashi M., Tadokoro Y., et al. RHEB Is a Potential Therapeutic Target in T Cell Acute Lymphoblastic Leukemia. Biochem. Biophys. Res. Commun. 2022;621:74–79. doi: 10.1016/j.bbrc.2022.06.089. [DOI] [PubMed] [Google Scholar]
- 220.Pupo E., Avanzato D., Middonti E., Bussolino F., Lanzetti L. KRAS-Driven Metabolic Rewiring Reveals Novel Actionable Targets in Cancer. Front. Oncol. 2019;9:848. doi: 10.3389/fonc.2019.00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Perentesis J.P., Bhatia S., Boyle E., Shao Y., Shu X.O., Steinbuch M., Sather H.N., Gaynon P., Kiffmeyer W., Envall-Fox J., et al. RAS Oncogene Mutations and Outcome of Therapy for Childhood Acute Lymphoblastic Leukemia. Leukemia. 2004;18:685–692. doi: 10.1038/sj.leu.2403272. [DOI] [PubMed] [Google Scholar]
- 222.Irving J., Matheson E., Minto L., Blair H., Case M., Halsey C., Swidenbank I., Ponthan F., Kirschner-Schwabe R., Groeneveld-Krentz S., et al. Ras Pathway Mutations Are Prevalent in Relapsed Childhood Acute Lymphoblastic Leukemia and Confer Sensitivity to MEK Inhibition. Blood. 2014;124:3420–3430. doi: 10.1182/blood-2014-04-531871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Oshima K., Khiabanian H., Da Silva-Almeida A.C., Tzoneva G., Abate F., Ambesi-Impiombato A., Sanchez-Martin M., Carpenter Z., Penson A., Perez-Garcia A., et al. Mutational Landscape, Clonal Evolution Patterns, and Role of RAS Mutations in Relapsed Acute Lymphoblastic Leukemia. Proc. Natl. Acad. Sci. USA. 2016;113:11306–11311. doi: 10.1073/pnas.1608420113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Van Meter M.E.M., Díaz-Flores E., Archard J.A., Passegué E., Irish J.M., Kotecha N., Nolan G.P., Shannon K., Braun B.S. K-RasG12D Expression Induces Hyperproliferation and Aberrant Signaling in Primary Hematopoietic Stem/Progenitor Cells. Blood. 2007;109:3945–3952. doi: 10.1182/blood-2006-09-047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kong G., Du J., Liu Y., Meline B., Chang Y.I., Ranheim E.A., Wang J., Zhang J. Notch1 Gene Mutations Target KRAS G12D-Expressing CD8+ Cells and Contribute to Their Leukemogenic Transformation. J. Biol. Chem. 2013;288:18219–18227. doi: 10.1074/jbc.M113.475376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dail M., Li Q., McDaniel A., Wong J., Akagi K., Huang B., Kang H.C., Kogan S.C., Shokat K., Wolff L., et al. Mutant Ikzf1, KrasG12D, and Notch1 Cooperate in T Lineage Leukemogenesis and Modulate Responses to Targeted Agents. Proc. Natl. Acad. Sci. USA. 2010;107:5106–5111. doi: 10.1073/pnas.1001064107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Punekar S.R., Velcheti V., Neel B.G., Wong K.K. The Current State of the Art and Future Trends in RAS-Targeted Cancer Therapies. Nat. Rev. Clin. Oncol. 2022;19:637–655. doi: 10.1038/s41571-022-00671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sicinska E., Aifantis I., Le Cam L., Swat W., Borowski C., Yu Q., Ferrando A.A., Levin S.D., Geng Y., Von Boehmer H., et al. Requirement for Cyclin D3 in Lymphocyte Development and T Cell Leukemias. Cancer Cell. 2003;4:451–461. doi: 10.1016/S1535-6108(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 229.Rao S.S., O’Neil J., Liberator C.D., Hardwick J.S., Xudong D., Zhang T., Tyminski E., Yuan J., Kohl N.E., Richon V.M., et al. Inhibition of NOTCH Signaling by Gamma Secretase Inhibitor Engages the RB Pathway and Elicits Cell Cycle Exit in T-Cell Acute Lymphoblastic Leukemia Cells. Cancer Res. 2009;69:3060–3068. doi: 10.1158/0008-5472.CAN-08-4295. [DOI] [PubMed] [Google Scholar]
- 230.Dohda T., Maljukova A., Liu L., Heyman M., Grandér D., Brodin D., Sangfelt O., Lendahl U. Notch Signaling Induces SKP2 Expression and Promotes Reduction of P27Kip1 in T-Cell Acute Lymphoblastic Leukemia Cell Lines. Exp. Cell Res. 2007;313:3141–3152. doi: 10.1016/j.yexcr.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 231.Hebert J., Cayuela J.M., Berkeley J., Sigaux F. Candidate Tumor-Suppressor Genes MTS1 (P16INK4A) and MTS2 (P15INK4B) Display Frequent Homozygous Deletions in Primary Cells From T-But Not From B-Cell Lineage Acute Lymphoblastic Leukemias. Blood. 1994;84:4038–4044. doi: 10.1182/blood.V84.12.4038.bloodjournal84124038. [DOI] [PubMed] [Google Scholar]
- 232.Mullighan C.G., Goorha S., Radtke I., Miller C.B., Coustan-Smith E., Dalton J.D., Girtman K., Mathew S., Ma J., Pounds S.B., et al. Genome-Wide Analysis of Genetic Alterations in Acute Lymphoblastic Leukaemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 233.Van Vlierberghe P., Ambesi-Impiombato A., De Keersmaecker K., Hadler M., Paietta E., Tallman M.S., Rowe J.M., Forne C., Rue M., Ferrando A.A. Prognostic Relevance of Integrated Genetic Profiling in Adult T-Cell Acute Lymphoblastic Leukemia. Blood. 2013;122:74–82. doi: 10.1182/blood-2013-03-491092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Remke M., Pfister S., Kox C., Toedt G., Becker N., Benner A., Werft W., Breit S., Liu S., Engel F., et al. High-Resolution Genomic Profiling of Childhood T-ALL Reveals Frequent Copy-Number Alterations Affecting the TGF-Beta and PI3K-AKT Pathways and Deletions at 6q15-16.1 as a Genomic Marker for Unfavorable Early Treatment Response. Blood. 2009;114:1053–1062. doi: 10.1182/blood-2008-10-186536. [DOI] [PubMed] [Google Scholar]
- 235.Peled A., Petit I., Kollet O., Magid M., Ponomaryov T., Byk T., Nagler A., Ben-Hur H., Many A., Shultz L., et al. Dependence of Human Stem Cell Engraftment and Repopulation of NOD/SCID Mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 236.Lapidot T., Kollet O. The Essential Roles of the Chemokine SDF-1 and Its Receptor CXCR4 in Human Stem Cell Homing and Repopulation of Transplanted Immune-Deficient NOD/SCID and NOD/SCID/B2m(Null) Mice. Leukemia. 2002;16:1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- 237.Passaro D., Irigoyen M., Catherinet C., Gachet S., Da Costa De Jesus C., Lasgi C., Tran Quang C., Ghysdael J. CXCR4 Is Required for Leukemia-Initiating Cell Activity in T Cell Acute Lymphoblastic Leukemia. Cancer Cell. 2015;27:769–779. doi: 10.1016/j.ccell.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 238.Du W., Lu C., Zhu X., Hu D., Chen X., Li J., Liu W., Zhu J., He Y., Yao J. Prognostic Significance of CXCR4 Expression in Acute Myeloid Leukemia. Cancer Med. 2019;8:6595–6603. doi: 10.1002/cam4.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Su L., Hu Z., Yang Y.G. Role of CXCR4 in the Progression and Therapy of Acute Leukaemia. Cell Prolif. 2021;54:e13076. doi: 10.1111/cpr.13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bellavia D., Campese A.F., Alesse E., Vacca A., Felli M.P., Balestri A., Stoppacciaro A., Tiveron C., Tatangelo L., Giovarelli M., et al. Constitutive Activation of NF-KappaB and T-Cell Leukemia/Lymphoma in Notch3 Transgenic Mice. EMBO J. 2000;19:3337–3348. doi: 10.1093/emboj/19.13.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ferrandino F., Bernardini G., Tsaouli G., Grazioli P., Campese A.F., Noce C., Ciuffetta A., Vacca A., Besharat Z.M., Bellavia D., et al. Intrathymic Notch3 and CXCR4 Combinatorial Interplay Facilitates T-Cell Leukemia Propagation. Oncogene. 2018;37:6285–6298. doi: 10.1038/s41388-018-0401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gao X., Qin S., Wu Y., Chu C., Jiang B., Johnson R.H., Kuang D., Zhang J., Wang X., Mehta A., et al. Nuclear PFKP Promotes CXCR4-Dependent Infiltration by T Cell Acute Lymphoblastic Leukemia. J. Clin. Investig. 2021;131:e143119. doi: 10.1172/JCI143119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Alsadeq A., Fedders H., Vokuhl C., Belau N.M., Zimmermann M., Wirbelauer T., Spielberg S., Vossen-Gajcy M., Cario G., Schrappe M., et al. The Role of ZAP70 Kinase in Acute Lymphoblastic Leukemia Infiltration into the Central Nervous System. Haematologica. 2017;102:346–355. doi: 10.3324/haematol.2016.147744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Maciocia P.M., Wawrzyniecka P.A., Maciocia N.C., Burley A., Karpanasamy T., Devereaux S., Hoekx M., O’Connor D., Leon T., Rapoz-D’Silva T., et al. Anti-CCR9 Chimeric Antigen Receptor T Cells for T-Cell Acute Lymphoblastic Leukemia. Blood. 2022;140:25–37. doi: 10.1182/blood.2021013648. [DOI] [PubMed] [Google Scholar]
- 245.Santamaria S., Delgado M., Botas M., Castellano E., Corraliza-Gorjon I., Lafuente P., Muñoz-Calleja C., Toribio M.L., Kremer L., Garcia-Sanz J.A. Therapeutic Potential of an Anti-CCR9 MAb Evidenced in Xenografts of Human CCR9+ Tumors. Front. Immunol. 2022;13:4139. doi: 10.3389/fimmu.2022.825635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zöller M. CD44: Can a Cancer-Initiating Cell Profit from an Abundantly Expressed Molecule? Nat. Rev. Cancer. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 247.Ghaffari S., Smadja-Joffe F., Oostendorp R., Lévesque J.P., Dougherty G., Eaves A., Eaves C. CD44 Isoforms in Normal and Leukemic Hematopoiesis. Exp. Hematol. 1999;27:978–993. doi: 10.1016/S0301-472X(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 248.Krause D.S., Lazarides K., Von Andrian U.H., Van Etten R.A. Requirement for CD44 in Homing and Engraftment of BCR-ABL–Expressing Leukemic Stem Cells. Nat. Med. 2006;12:1175–1180. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- 249.Jin L., Hope K.J., Zhai Q., Smadja-Joffe F., Dick J.E. Targeting of CD44 Eradicates Human Acute Myeloid Leukemic Stem Cells. Nat. Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 250.Giambra V., Jenkins C.R., Wang H., Lam S.H., Shevchuk O.O., Nemirovsky O., Wai C., Gusscott S., Chiang M.Y., Aster J.C., et al. NOTCH1 Promotes T Cell Leukemia-Initiating Activity by RUNX-Mediated Regulation of PKC-θ and Reactive Oxygen Species. Nat. Med. 2012;18:1693–1698. doi: 10.1038/nm.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Marques L.V.C., Noronha E.P., Andrade F.G., dos Santos-Bueno F.V., Mansur M.B., Terra-Granado E., Pombo-de-Oliveira M.S. CD44 Expression Profile Varies According to Maturational Subtypes and Molecular Profiles of Pediatric T-Cell Lymphoblastic Leukemia. Front. Oncol. 2018;8:488. doi: 10.3389/fonc.2018.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hoofd C., Wang X., Lam S., Jenkins C., Wood B., Giambra V., Weng A.P. CD44 Promotes Chemoresistance in T-ALL by Increased Drug Efflux. Exp. Hematol. 2016;44:166–171.e17. doi: 10.1016/j.exphem.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 253.Yeoh E.J., Ross M.E., Shurtleff S.A., Williams W.K., Patel D., Mahfouz R., Behm F.G., Raimondi S.C., Relling M.V., Patel A., et al. Classification, Subtype Discovery, and Prediction of Outcome in Pediatric Acute Lymphoblastic Leukemia by Gene Expression Profiling. Cancer Cell. 2002;1:133–143. doi: 10.1016/S1535-6108(02)00032-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.