Abstract
To ensure optimal prescribing practices in the dolutegravir-era in Cameroon, we compared first-line virological response (VR) under tenofovir + lamivudine + dolutegravir (TLD) according to prior exposure to tenofovir + lamivudine + efavirenz (TLE). A facility-based survey was conducted among patients initiating antiretroviral therapy (ART) with TLD (I-TLD) versus those transitioning from TLE to TLD (T-TLD). HIV viral load was performed and unsuppressed participants (VL > 1000 copies/mL) had genotyping performed by Sanger sequencing. Of the 12,093 patients followed, 310 (mean-age: 41 ± 11 years; 52.26% female) complied with study criteria (171 I-TLD vs. 139 T-TLD). The median ART-duration was 14 (12–17) months among I-TLDs versus 28 (24.5–31) months among T-TLDs (15 (11–19) on TLE and 14 (9–15) on TLD), and 83.15% (148/178) were at WHO clinical stages I/II. The viral suppression rate (<1000 copies/mL) was 96.45%, with 97.08% among I-TLDs versus 95.68% among T-TLDs (p = 0.55). VR was similar in I-TLD versus T-TLD at <400 copies/mL (94.15% versus 94.42%) and age, gender, residence, ART-duration, and WHO stages were not associated with VR (p > 0.05). Genotyping was successful for 72.7% (8/11), with no major mutations to integrase inhibitors found. VR is optimal under first-line TLD after 14 months, even among TLE-exposed, thus confirming the effectiveness of transitioning from TLE to TLD in similar settings, supported by strong pharmacological potency and genetic barrier of dolutegravir.
Keywords: HIV, antiretrovirals, first-line, TLD, virological response, Cameroon
1. Introduction
In the frame of HIV treatment, the five main goals of antiretroviral therapy (ART) are (i) maximal and durable control of the viral replication, (ii) prevention of HIV transmission, (iii) restoration of immunological function, (iv) reduction of HIV-related mortality, and (v) increased duration and quality of life for people living with HIV [1]. Regarding treatment regimens, first-generation non-nucleoside reverse-transcriptase inhibitors (NNRTIs), namely efavirenz (EFV) and nevirapine (NVP), were the recommended first-line-based regimens, in combination with nucleoside reverse-transcriptase inhibitors (NRTIs) such as lamivudine (3TC) and tenofovir (TDF) [2,3,4,5,6,7]. However, given the low genetic barrier of NNRTIs to HIV drug resistance and the emergence of EFV/NVP pretreatment drug resistance above the threshold of 10% in several resource-limited settings (RLS), the use of NNRTI-sparing regimens has become a high priority [8,9,10]. In effect, people with NNRTI resistance are less likely to achieve viral suppression, more likely to experience virological failure or death, more likely to discontinue treatment, and more likely to acquire new HIV drug-resistant mutations [11]. In this context, transitioning from EFV/NVP-based therapy to dolutegravir (DTG)-based regimens was of paramount importance [10,12,13,14,15,16,17]. Of note, DTG is an integrase strand-transfer inhibitor (INSTI) with a high potency, a high genetic barrier to HIV drug resistance, and is widely available in a fixed-dose combination consisting of tenofovir (TDF) + lamivudine (3TC) + DTG (TLD) [10,18,19,20]. Therefore, TLD is the current preferred regimen for ART initiation in RLS in replacement of tenofovir (TDF) + lamivudine (3TC) + EFV (TLE) [12,21]. ART guidelines in Cameroon follow the WHO public health approach, and consist of two NRTIs and one NNRTI as a preferred first-line regimen before 2020 [3]. The introduction of TLD as a first-line regimen for treatment initiation was officially launched for newly infected patients as of 1 January 2020 [22], and the transition of the formal first-line regimen (like TLE) to TLD was done in health facilities at the discretion of clinicians; current guidelines recommend a switch to second-line regimens consisting of two NRTIs and one ritonavir-boosted protease inhibitor (PI/r) in the event of first-line ART failures. Importantly, many authors have already reported early findings on the effectiveness of DTG-based first-line ART, including modelling studies and clinical trials in sub-Saharan Africa and beyond [13,21,23,24]. However, despite the predicted high effectiveness of TLD, the effect of previous exposure to the same backbone (TDF + 3TC) gives room for possible existing drug-resistant mutants in the current transition from TLE to TLD (specifically with the K65R and M184V mutations known as major DRMs to TDF and 3TC/FTC, respectively). In effect, Inzaule et al. recently reported that high levels of resistance to the NRTI backbone were common in patients failing NNRTI-based first-line ART in RLS [25]. Moreover, accumulation of NRTI-resistance mutations in patients transitioning to TLD would lead to a “functional” monotherapy of DTG [26], which would further enhance rapid emergence of DTG resistance among such patients. Thus, the transition to TLD in patients with prior exposure to TLE may be accompanied by a suboptimal response in RLS and sub-Saharan Africa in particular. Understanding the treatment outcomes among patients on the recently introduced first-line (TLD) regimen would shed light on potential disparities between those with previous exposure to ART and those initiating ART with DTG within the Cameroonian context. To address this gap, and in order to ensure the long-term efficacy of DTG, we evaluated the virological response under TLD according to previous exposure to TLE in first-line patients in Cameroon.
2. Materials and Methods
2.1. Study Setting and Site
A facility-based survey was conducted from June through November 2021 among first-line patients living with HIV (PLWH) initiating ART directly with TLD versus those transitioning from TLE to TLD, all followed at the daycare Hospital of the Yaoundé Central Hospital (YCH) and the Douala General Hospital (DGH), the two main hospitals of the capital cities of Cameroon. Laboratory analyses within the frame of the study were performed at the Chantal Biya International Reference Centre for research on HIV/AIDS prevention and management (CIRCB) in Yaoundé, Cameroon.
The CIRCB is a government institution of the Ministry of Public Health dedicated to HIV research and patient monitoring in several aspects. Among these aspects are (a) HIV early infant diagnosis in the frame of the national PMTCT program, (b) diagnosis of coinfections with HIV, (c) viral load measurement, (d) CD4 and CD8 T lymphocytes counts, (e) biochemical and hematological tests for drug safety, and (f) genotypic HIVDR testing (GRT) at subsidized costs, with quality-control programs conducted in partnership with the Quality Assessment and Standardization of Indicators (QASI) and other international agencies [27].
2.2. Study Population
Eligible participants were HIV-1 infected patients currently under TLD first-line ART, having been on treatment for at least six months and who consented to participate in the study. Through an exhaustive sampling strategy, two groups of patients were recruited: (i) those initiating ART directly with TLD (I-TLD), and (ii) those transitioning from TLE or TLN to TLD (T-TLD). WHO clinical stages were those reported in patients’ medical reports at treatment initiation. Participants excluded were PLWH with a nondetailed treatment history and those who had received prior ART other than TLE or TDF + 3TC + NVP (TLN). Children and adolescents (≤18 years) were not considered for inclusion in this study as per national guidelines (i.e., current recommendations for treatment initiation are not TLD in this target population).
2.3. Viral Load Measurement
Plasma viral load (PVL) at the CIRCB was performed by using the Abbott® m2000rt HIV platform (Abbott Molecular Inc. 1300 E. Touhy Ave. Des Plaines, IL 60018 200680-105, USA) according to manufacturer’s instructions [28]. Briefly, a protocol using 0.6 mL of plasma was used for RNA extraction, followed by a simultaneous amplification and detection on a real-time polymerase chain reaction (RT-PCR). The lower and upper detection thresholds of the assay were <40 and >10,000,000 HIV-1 RNA copies/mL, respectively. Viral suppression was considered as a viral load <1000 copies/mL, as previously defined by WHO guidelines for resource-limited settings [18].
2.4. HIV-1 Genotypic Drug-Resistance Testing
HIV-1 genotypic resistance testing (GRT) was performed on both HIV-1 plasma RNA and proviral DNA following an in-house integrase genotyping assay as described and published elsewhere [29]. Briefly, viral nucleic acids were extracted by using the Qiagen protocol with a viral RNA extraction kit for plasma and DNA extraction for buffy-coat samples. For RNA extracts, reverse-transcriptase and PCR were performed by using the following conditions (1 cycle at 50° for 30 min, 1 cycle at 94 °C for 2 min, 40 cycles (95 °C, 30 s; 51 °C, 30 s, 72 °C, 2 min and 30 s); 1 cycle at 72 °C for 10 min, 1 cycle at 4 °C for 30 min, and 1 cycle at 10 °C forever). For DNA extracts, a direct PCR was performed by using the following conditions (1 cycle at 94 °C for 12 min, 40 cycles (95 °C, 30 s, 51 °C, 30 s, 72 °C, 2 min and 30 s), 1 cycle at 72 °C for 10 min, and 1 cycle at 4 °C forever). Gel electrophoresis, purification, and sequencing reaction were performed in a similar manner.
2.5. Interpretation of Drug-Resistance Mutations and HIV Subtyping
Regarding the analysis of drug-resistance mutations (DRMs), sequences obtained after capillary electrophoresis on the 3500 Genetic Analyzer (Applied Biosystems™, California, USA) were assembled and manually edited by using RECall (CDC, Atlanta, GA, USA). They were then analyzed for interpreting DRMs by using Stanford HIVdb v9. All variants at amino acid positions associated with a decreased INSTI susceptibility (i.e., at least a Stanford penalty score ≥ 10) were considered as resistant variants. Samples with resistant mutant or mixture of wild type and mutant at an amino acid position were considered resistant.
For subtyping, sequences were aligned in BioEdit version 7.2.6 (Tom Hall, Raleigh, NC, USA) by using CLUSTAL W, and compared with reference sequences for the major HIV-1 subtypes and circulating recombinant forms (CRFs), available in the Los Alamos database [30]; gaps were then removed from the final alignment. The phylogenetic tree was inferred by using maximum likelihood method on the MEGA software v7.0.26 primarily for subtyping and to ensure that there was no cross-contamination of samples.
2.6. Statistical Analysis
Data were analyzed by using Excel 2016, Epi-info v7. Chi square or Fisher’s exact test was used to describe the associations between variables wherever appropriate, with p-values < 0.05 considered statistically significant; logistic regression was used for multivariate multivariable analyses for variables showing a p-values ≤ 0.2 from bivariable analyses.
3. Results
3.1. General Characteristics of Study Population
3.1.1. Sociodemographic and Basic Clinical Characteristics
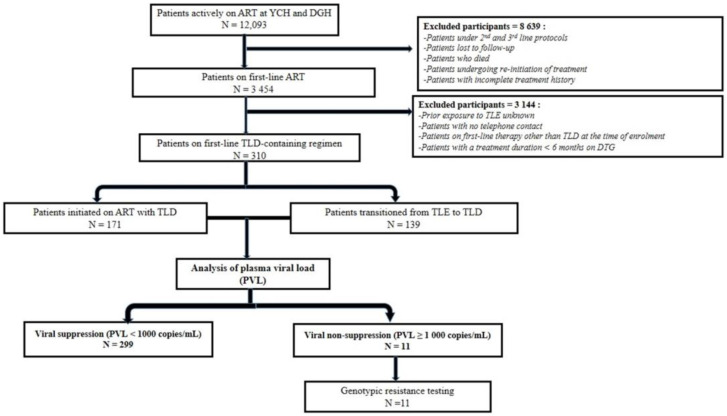
Out of 12,093 patients receiving ART at the reference treatment centres (YCH and DGH), 310 had been on a DTG-based first-line ART for at least six months. The participants had a median (IQR) age of 41 (34–49) years. The most represented age group included those of 35–44 years of age (34.19%), followed by those 25–34 years of age (21.29%), those 45–54 years of age (20.32%), those 55–64 years of age (10.65%), those 15–24 years of age (4.52%), those 65–74 years of age (2.26%), and finally those 75–85 years of age (0.32%). There was a nearly equal distribution of male and female participants (52.26% female; 162/310) in the study population and the majority of participants (about 2/3) lived in Yaoundé. Only 178/310 (57.4%) of participants had data on WHO clinical stage at treatment initiation, and the majority of them were in clinical stages I and II (67.41%; 120/178 and 15.73%; 28/178, respectively), as shown in Table 1.
Table 1.
Baseline characteristics of study participants.
| Characteristics | Overall N = 310 |
I-TLD N = 171 |
T-TLD N = 139 |
|---|---|---|---|
| Female | 162 | 87 | 75 |
| Male | 148 | 84 | 64 |
| Median age in years | 41 (34–49) | 39 (32–48) | 42 (36–49) |
| Region of residence: | |||
| Yaoundé | 209 | 128 | 81 |
| Douala | 101 | 43 | 58 |
| WHO clinical stage (n = 178) *: | |||
| I | 120 | 84 | 36 |
| II | 28 | 21 | 07 |
| III | 18 | 11 | 07 |
| IV | 12 | 07 | 05 |
| Median ART-duration on first-line [IQR] (months) | 19 (13–27) |
14 (12–17) |
28 (24.5–31) |
| Median ART-duration on TLD [IQR] (months) | 14 (11–15) |
14 (12–17) |
14 (9–15) |
| Median viremia before transition [IQR] (copies/mL) | 3258 (831–59,276) |
/ | 3258 (831–59,276) |
Legend: I-TLD refers to participants initiated on ART with TLD; T-TLD refers to participants transitioned from TLE to TLD; IQR refers to interquartile range. * Data regarding the WHO clinical stages at treatment initiation was available only for 178 participants.
3.1.2. Description of ART History
The overall median ART duration on a first-line regimen was 19 (13–27) months (Table 1). Regarding ART exposure, 171/310 (55.16%) participants initiated ART directly with TLD (I-TLD) and the remaining 139/310 (44.84%), initiated ART with TLE before subsequent transition to TLD (T-TLD). We did not find any participant transitioning from TLN to TLD. The median viremia before transition to TLD-based first-line therapy among T-TLD participants was 3258 (831–59,276) copies/mL (Table 1) and 89.93% (125/139) had a detectable viral load before the transition. We did not have any record of CD4 count in both arms. ART duration among I-TLD was 14 (12–17) months compared to 28 (24.5–31) months (divided as 15 (11–19) months on TLE and 14 (9–15) months on TLD) among T-TLD. Figure 1 details the algorithm used to enroll and track patients.
Figure 1.
Flow chart of patients’ enrolment and follow-up in the study population. Legend. Bold represents subtitles.
3.2. Virological Response
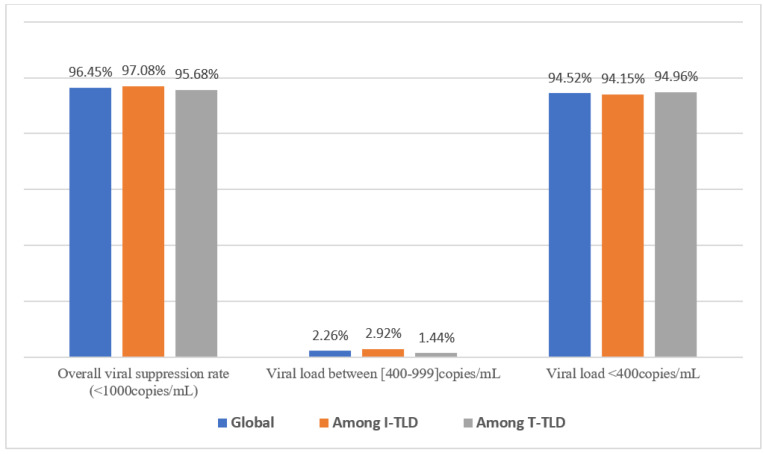
The overall rate of viral suppression (PVL < 1000 copies/mL) after approximately 14 months was 96.45% (299/310), without any significant disparity (p = 0.55) between I-TLD vs. T-TLD, as shown in Figure 2. Importantly, median viral load for TLD’s non-suppression was 2970 (1334–8866) copies/mL, 1262 (1096–2970) copies/mL, and 8605 (3339–16,417) copies/mL, respectively, among I-TLD and T-TLD.
Figure 2.
Virological response under TLD in first-line treatment among participants.
3.2.1. Factors Associated to Virological Response
Following bi- and multivariate analyses, neither age, nor gender, city of residence, WHO clinical stage, or duration on TLD were found to be associated with viral suppression in the present study (Table 2).
Table 2.
Evaluation of sociodemographic factors potentially associated with virological suppression in Cameroonian individuals treated with tenofovir + lamivudine + dolutegravir (TLD).
| Variables | Virological Suppression on TLD | Odd Ratio [IC = 95%] (p-Value) |
* Adjusted Odd Ratio [IC = 95%] (p-Value) |
|
|---|---|---|---|---|
| Age | Yes | No | ||
| ≤41 yrs | 150 | 9 | 0.26 [0.05–1.22] (0.06) | 1.64 (0.45–6.00) (0.45) |
| >41 yrs | 129 | 2 | ||
| Sexe | ||||
| Female | 159 | 3 | 3.03 [0.79–11.63] (0.08) | 0.44 (0.12–1.63) (0.22) |
| Male | 140 | 8 | ||
| Region of residence | ||||
| Douala | 100 | 1 | 5.02 [0.63–39.81] (0.07) | 1.27 (0.30–5.34) (0.74) |
| Yaoundé | 199 | 10 | ||
| WHO stage | ||||
| I and II III and IV |
139 | 29 | 0.53 [0.06–4.37] (0.47) | / |
| 29 | 1 | |||
| Duration on TLD | ||||
| ≤14 months | 179 | 6 | 1.24 [0.37–4.17] (0.76) |
/ |
| >14 months | 120 | 5 | ||
Legend: * Adjustment was made through multivariable analysis.
3.2.2. HIV-1 Genotyping and Genetic Diversity in the Integrase Coding Region
Among the 11 patients with unsuppressed viremia (PVL ≥ 1000 copies/mL), HIV-1 integrase genotyping was successful for 72.7% (8/11) of the study participants. Two viral strains were identified, 87.5% CRF02_AG (7/8) and 12.5% F2 (1/8). No major mutations to integrase inhibitors were found.
4. Discussion
In view of achieving UNAIDS’ target by 2030, 85% of all people living with HIV worldwide knew their status; 88% had access to ART and 92% achieved viral suppression in 2021 [31,32]. In Cameroon specifically, 94.1% of all people living with HIV know their status, 82.7% of them have access to ART, and 94.1% of those on ART with access to viral load testing achieved viral suppression by 2021 [33]. This progress was a result of rapid ART scale-up which has dramatically reduced HIV-related morbidity and mortality even in RLS [18,34,35,36]. However, despite the tremendous successes of ART scale-up, it is very unfortunate that there has been an increase in levels of pretreatment HIVDR among patients initiating ART [9,25]. The burden of HIVDR is significantly associated with poor virological outcomes and with increased mortality and morbidity [9]. Regimens with greater potency and higher genetic barrier to resistance might be essential in this area. Thus, understanding the treatment outcomes among patients on the recently introduced first-line regimen (TLD) would shed light on potential disparities between those with previous exposure to ART and those initiating ART with DTG within the context of countries similar to Cameroon.
Participants in this study were aged 41 years on average, with nearly equal distribution of male and female participants, which is in line with the national HIV epidemiology [37], even though other studies highlight female vulnerability to HIV infection [38,39,40]. This highlights the representativeness of our findings to the target population at the country level. Even though the information was absent for some participants, the high number of early WHO clinical stages at treatment initiation translates into overall good clinical condition among first-line patients in our clinical practice, and this is supported by the test and treatment strategy implemented nationwide [18,41].
Regarding virological response to first-line TLD, very high rates of viral suppression (>95%) were observed in real-life clinical settings after 14 months of TLD uptake, even with prior exposure to TLE. It is important to note that for homogeneity in the trend of data and for an optimal clinical relevance, data on viral load have been harmonized to showcase the rate of detectable VL before transition, which dropped down to 5.04% (at the threshold of >400 copies/mL) after 14 months of transitioning to TLD; this is in line with recent findings on TLD effectiveness worldwide [10,12,13,14,15,16,17,24,42,43,44,45,46,47]. TLE and TLD are once-daily fixed-dose combinations; hence, among similar adherence features, the major difference relies essentially on the regimen potency/genetic barrier likely driven by the DTG. Furthermore, with a median duration of 14 months and a viral nonsuppression rate of approximately 3%, it would be commendable to ensure a long-term monitoring of viremic patients in order to determine cases of infrequent nonadherence from cases of failure due to emerging DRMs to TLD, while emphasizing pharmacovigilance for an efficient patient outcome in the long run [48,49]. Importantly, neither age, gender, patient clinical status, nor ART duration were found to be associated with the viral suppression in these patients, indicating that the good virological response obtained is the primary outcome of an effective ART regimen, due essentially to the absence of preexisting DTG-resistance and possibly the scarcity of NRTI-DRMs in the current ART combination (indeed, exposure to TLE was relatively short to enable emergence or archiving of NRTI drug resistance). This finding is reassuring, encouraging a timely switch from TLE to TLD and warranting the extension of DTG-containing regimens even for pediatric populations in RLS just as recently reported [24,50]. Additionally, the few cases of nonsuppression were seemingly related to recent poor treatment adherence. Notably, recent cohorts in RLS reported the high acceptability and/or adherence to DTG-based regimens to be associated with the high rates of viral suppression observed with TLD-based first-line ART [43,44]. These findings suggest that enhanced therapeutic education and psychosocial support are key for a rapid decay of the viremia among patients initiating TLD-based first-line ART. Thus, this study brings additional emphasis on the high potency (i.e., high viral inhibitory capacity) of DTG observed within real-life clinical settings.
Moreover, no integrase resistance-associated mutations among virologically unsuppressed patients were reported when using a highly sensitive integrase-genotyping protocol that was validated locally [29]. The absence of INSTI resistance therefore confirms the high genetic barrier of DTG while encouraging the use of the current genotyping approach. The predominance of CRF02_AG subtype found in this study is in agreement with several other local studies [29,51,52,53,54,55,56], thus confirming the reliability from genotyping.
Although these findings are overall very encouraging, there is a need to continue monitoring the emergence of DRMs in this population, taking into consideration the suboptimal viral load coverage and risk of drug stock outs in RLS [9,11,49,53,57]. Moreover, pharmacovigilance of TLD remains a key component with major gaps in the current programmatic era, to ensure long-term safety alongside the reported efficacy of TLD in RLS [58,59,60].
The limited amount of data on the WHO clinical stages at treatment initiation might have led to some information bias. Nonetheless, the very high rates of clinical stages groups 1 and 2 suggest that this variable might not change even with full data coverage. Furthermore, as per national guidelines, viral load and NRTI resistance were not performed at treatment initiation, which could have helped to mitigate potential effects of baseline viremia and/or preexisting drug resistance mutations on the current TLD efficacy in both groups. This study was conducted during the COVID-19 pandemic, leading to some cases of exclusion due to limited clinic attendance for ART monitoring; however, the sample population was statistically sufficient for assessing the study primary outcomes.
5. Conclusions
In a nutshell, viral suppression is very high under first-line treatment after approximately 14 months following TLD initiation, even among patients with prior exposure to TLE in Cameroon. Our finding suggests that previous exposure to TLE is seemingly not a counterindication for transitioning from TLE to TLD as a public health approach in RLS. Interestingly, no independent factor was found to be associated with these high rates of virological control, suggesting that this observation is exclusively related to the strong pharmacological potency of dolutegravir (DTG). The current transition plan from TLE to TLD in similar African settings should therefore be encouraged, pending long-term ART monitoring for efficacy and drug-resistance surveillance, as well as safety monitoring through pharmacovigilance.
Acknowledgments
We thank the Chantal BIYA International Reference Centre for research on HIV/AIDS prevention and management (CIRCB), for hosting the present study and for all the facilitations. We are also very appreciative to the daycare Hospital of the Yaoundé Central Hospital (HDJ/HCY) and the Douala General Hospital (HGD), for their technical assistance in reaching out to the patients during the early phase of this study.
Author Contributions
Conception and design of the study, E.N.J.S., N.-K.E., E.M., D.T., M.M.S., F.C.-S. and J.F; collection and analysis of the data, E.N.J.S., N.-K.E., E.M., B.D., G.T., A.D.N., A.C.K., G.A.B., S.C.D.N., A.A., A.M.N.K., M.C.T.T. and S.M.S.; drafting of the manuscript, E.N.J.S., N.-K.E., E.M., E.N.J.S., N.-K.E., E.M., A.D.N., L.M., A.P.M., C.A.C., B.Y. and J.F.; involvement in critically revising the protocol for important intellectual content, E.N.J.S., N.-K.E., E.M., A.D.N., L.M., A.P.M., C.A.C., B.Y., M.M.S., J.F., C.-F.P., V.C., M.M.S., F.C.-S. and A.N.; approving the final version of the submitted manuscript, all authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Administrative authorizations were issued by the CIRCB directorate (reference N°0191/019L/CIRCB/DG/SAA/BRH); ethical clearance for the study was obtained from the institutional research ethics committee for human health of the School of Health Sciences of the Catholic University of Central Africa (Authorization Number: N°2021/020373/CEIRSH/ESS/MBV).
Informed Consent Statement
Written informed consent for each participant was obtained from parents or legal guardians; data were protected by the use of specific identifiers for purpose of confidentiality and stored in a password encrypted computer; all laboratory results were freely returned to participants for benefit in their personal clinical management.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The study was co-sponsored by the Chantal BIYA international Reference Centre for research on HIV/AIDS prevention and management (CIRCB; under the annual budget plan 2021) and the Royal Society of Tropical Medicine and Hygiene (RSTMH under the small grant programme 2021).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.NIH-National Institute of Health Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. [(accessed on 28 May 2022)]; Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/treatment-goals.
- 2.MINSANTE . Guide National De Prise En Charge Des Personnes Vivant Avec Le VIH/SIDA-Cameroun. Ministère de la Santé Publique; Yaoundé, Cameroun: 2012. [Google Scholar]
- 3.MINSANTE . Plan National Multisectoriel De Lutte Contre Le Vih, Le Sida Et Les Ist (Pnm) Annee 2014–2017. Ministère de la Santé Publique; Yaoundé, Cameroun: 2013. pp. 1–163. [Google Scholar]
- 4.MINSANTE . Directives Nationales De Prevention. Ministère de la Santé Publique; Yaoundé, Cameroun: 2014. p. 193. [Google Scholar]
- 5.WHO . Lignes Directrices Unifiées Sur l’utilisation Des Antirétroviraux Pour Le Traitement et La Prévention De l’infection à VIH: Recommandations Pour Une Approche De Santé Publique. World Health Organization; Geneve, Switzerland: 2013. [(accessed on 2 March 2022)]. Available online: https://apps.who.int/iris/handle/10665/101196. [Google Scholar]
- 6.WHO . Notes d’orientation: Lignes Directrices Unifiées Relatives à L’utilisation De Médicaments Antirétroviraux Pour LE Traitement et La Prévention De L’infection à VIH: Dernières Informations. World Health Organization; Geneve, Switzerland: 2015. [(accessed on 2 March 2022)]. Available online: https://apps.who.int/iris/handle/10665/206448. [Google Scholar]
- 7.Dinesha T.R., Gomathi S., Boobalan J., Sivamalar S., Solomon S.S., Pradeep A., Poongulali S., Solomon S., Balakrishnan P., Saravanan S. Genotypic HIV-1 Drug Resistance among Patients Failing Tenofovir-Based First-Line HAART in South India. AIDS Res. Hum. Retrovir. 2016;32:1234–1236. doi: 10.1089/aid.2016.0110. [DOI] [PubMed] [Google Scholar]
- 8.Rusine J., Asiimwe-Kateera B., van de Wijgert J., Boer K.R., Mukantwali E., Karita E., Gasengayire A., Jurriaans S., de Jong M., Ondoa P. Low Primary and Secondary HIV Drug-Resistance after 12 Months of Antiretroviral Therapy in Human Immune-Deficiency Virus Type 1 (HIV-1)-Infected Individuals from Kigali, Rwanda. PLoS ONE. 2013;8:e64345. doi: 10.1371/journal.pone.0064345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO . Combating HIV Drug Resistance, a Little Known but Growing Threat. World Health Organization; Geneve, Switzerland: 2017. [Google Scholar]
- 10.Scidev L’Afrique s’offre Un Plan d’action Contre La Résistance Aux ARV-Afrique Sub-Saharienne. [(accessed on 28 May 2022)]. Available online: https://www.scidev.net/afrique-sub-saharienne/news/afrique-plan-d-action-la-resistance-arv-20122019/
- 11.WHO . HIV Drug Resistance Report 2017. World Health Organization; Geneve, Switzerland: Elsevier Ltd./Inc./BV; Cambridge, MA, USA: 2017. [Google Scholar]
- 12.Llibre J.M. Time to Get Serious with HIV-1 Resistance in Sub-Saharan Africa. Lancet Infect. Dis. 2017;17:241–243. doi: 10.1016/S1473-3099(16)30447-9. [DOI] [PubMed] [Google Scholar]
- 13.Charles K., Mireille M.-E., Pierrette O.B., Sabrina E.-D.D., Sandrine L., Sylvie B., Martine P., Alexandra C., Eric D., Group T.N.A. 12313 S. Dolutegravir-Based or Low-Dose Efavirenz–Based Regimen for the Treatment of HIV-1. N. Engl. J. Med. 2019;381:816–826. doi: 10.1056/nejmoa1904340. [DOI] [PubMed] [Google Scholar]
- 14.WHO . Updated Recommendations on First-Line and Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV-Interim Guidance. World Health Organization; Geneve, Switzerland: 2018. [Google Scholar]
- 15.Wainberg M.A., Han Y.-S., Mesplède T. Might Dolutegravir Be Part of a Functional Cure for HIV? Can. J. Microbiol. 2016;62:375–382. doi: 10.1139/cjm-2015-0725. [DOI] [PubMed] [Google Scholar]
- 16.Inzaule S.C., Hamers R.L., Doherty M., Shafer R.W., Bertagnolio S., Wit T.F.R. De Personal View Curbing the Rise of HIV Drug Resistance in Low-Income and Middle-Income Countries: The Role of Dolutegravir-Containing Regimens. Lancet Infect. Dis. 2019;19:e246–e252. doi: 10.1016/S1473-3099(18)30710-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitoria M., Hill A., Ford N., Doherty M., Clayden P., Venter F., Ripin D., Flexner C., Domanico P.L. The Transition to Dolutegravir and Other New Antiretrovirals in Low-Income and Middle-Income Countries: What Are the Issues? Volume 32. Lippincott Williams and Wilkins; Philadelphia, PA, USA: 2018. [DOI] [PubMed] [Google Scholar]
- 18.WHO . Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating And Preventing HIV Infection. World Health Organization; Geneve, Switzerland: 2016. [PubMed] [Google Scholar]
- 19.Dow D.E., Bartlett J.A. Dolutegravir, the Second-Generation of Integrase Strand Transfer Inhibitors (INSTIs) for the Treatment of HIV. Infect. Dis. Ther. 2014;3:83–102. doi: 10.1007/s40121-014-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fantauzzi A., Mezzaroma I. Dolutegravir: Clinical Efficacy and Role in HIV Therapy. Ther. Adv. Chronic Dis. 2014;5:164–177. doi: 10.1177/2040622314530461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips A.N., Bansi-Matharu L., Venter F., Havlir D., Pozniak A., Kuritzkes D.R., Wensing A., Lundgren J.D., Pillay D., Mellors J., et al. Updated Assessment of Risks and Benefits of Dolutegravir versus Efavirenz in New Antiretroviral Treatment Initiators in Sub-Saharan Africa: Modelling to Inform Treatment Guidelines. Lancet HIV. 2020;7:e193–e200. doi: 10.1016/S2352-3018(19)30400-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Republic of Cameroon. Ministry of Public Health. CIRCB New HIV Guidelines and Strategies against Drug Resistance. (November 22, 2019) [(accessed on 15 February 2021)]. Available online: http://www.circb.cm/
- 23.Venter W.D.F., Clayden P., Serenata C. The ADVANCE Study: A Groundbreaking Trial to Evaluate a Candidate Universal Antiretroviral Regimen. Curr. Opin. HIV AIDS. 2017;12:351–354. doi: 10.1097/COH.0000000000000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore C.L., Turkova A., Mujuru H., Kekitiinwa A., Lugemwa A., Kityo C.M., Barlow-Mosha L.N., Cressey T.R., Violari A., Variava E., et al. ODYSSEY Clinical Trial Design: A Randomised Global Study to Evaluate the Efficacy and Safety of Dolutegravir-Based Antiretroviral Therapy in HIV-Positive Children, with Nested Pharmacokinetic Sub-Studies to Evaluate Pragmatic WHO-Weight-Band Based Doluteg. BMC Infect. Dis. 2021;21:5. doi: 10.1186/s12879-020-05672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inzaule S.C., Hamers R.L., Bertagnolio S., Siedner M.J., Rinke T.F., Wit D., Gupta R.K. Pretreatment HIV Drug Resistance in Low- and Middle-Income Countries. Future Virol. 2019;14:427–440. doi: 10.2217/fvl-2018-0208. [DOI] [Google Scholar]
- 26.Wijting I., Rokx C., Boucher C., van Kampen J., Pas S., de Vries-Sluijs T., Schurink C., Bax H., Derksen M., Andrinopoulou E.R., et al. Dolutegravir as Maintenance Monotherapy for HIV (DOMONO): A Phase 2, Randomised Non-Inferiority Trial. Lancet HIV. 2017;4:e547–e554. doi: 10.1016/S2352-3018(17)30152-2. [DOI] [PubMed] [Google Scholar]
- 27.Centre International de Référence Chantal Biya (CIRCB) [(accessed on 15 July 2021)]. Available online: http://www.circb.cm/btc_circb/web/
- 28.Abbott Infectious Disease Products. [(accessed on 20 May 2022)]. Available online: www.abbottmolecular.com/products/infectious-diseases/realtime-pcr/hiv-1-assay.
- 29.Fokam J., Ngoufack Jagni Semengue E., Armenia D., Takou D., Dambaya B., Teto G., Chenwi C.A., Nka A.D., Beloumou G.A., Ndjeyep S.C.D., et al. High Performance of Integrase Genotyping on Diverse HIV-1 Clades Circulating in Cameroon: Toward a Successful Transition to Dolutegravir-Based Regimens in Low and Middle-Income Countries. Diagn. Microbiol. Infect. Dis. 2022;102:115574. doi: 10.1016/j.diagmicrobio.2021.115574. [DOI] [PubMed] [Google Scholar]
- 30.Los Alamos National Repository. [(accessed on 13 October 2022)]; Available online: http://www.hiv.lanl.gov.
- 31.ONUSIDA Fiche d’information—Dernières Statistiques Sur l’état de l’épidémie de Sida. [(accessed on 13 April 2022)]. Available online: https://www.unaids.org/fr/resources/fact-sheet.
- 32.UNAIDS Global HIV & AIDS Statistics—Fact Sheet. [(accessed on 16 July 2022)]. Available online: https://www.unaids.org/en/resources/fact-sheet.
- 33.NACC . N.A.C.C. Rapport Annuel Des Activités De Lutte Contre Le VIH & Le SIDA 2021. National AIDS Control Committe; Yaoundé II, Cameroon: 2021. pp. 1–163. [Google Scholar]
- 34.Bland R.M. Management of HIV-Infected Children in Africa: Progress and Challenges. Arch. Dis. Child. 2011;96:911–915. doi: 10.1136/adc.2010.193789. [DOI] [PubMed] [Google Scholar]
- 35.Billong S.C., Fokam J., Aghokeng A.F., Milenge P., Kembou E., Abessouguie I., Meva F.B., Bissek A.C.Z.-K., Meva’a-Onglene F.B., Bissek A.C.Z.-K., et al. Population-Based Monitoring of Emerging HIV-1 Drug B Resistance on Antiretroviral Therapy and Associated Factors in a Sentinel Site in Cameroon: Low Levels of Resistance but Poor Programmatic Performance Serge. PLoS ONE. 2013;8:e72680. doi: 10.1371/journal.pone.0072680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fokam J., Nangmo A., Wandum C., Takou D., Santoro M.M., Nlend A.E.N., Ateba F.N., Ndombo P.K., Kamgaing N., Kamta C., et al. Programme Quality Indicators of HIV Drug Resistance among Adolescents in Urban versus Rural Settings of the Centre Region of Cameroon. AIDS Res. Ther. 2020;17:14. doi: 10.1186/s12981-020-00270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MINSANTE . Cameroon-Enquête Démographique et De Santé 2018. Institut National De La Statistique; Montrouge, France: 2018. [(accessed on 28 May 2022)]. Available online: https://microdata.worldbank.org/index.php/catalog/3717. [Google Scholar]
- 38.Duarte M.T.C., de Lima Parada C.M.G., do Rosário de Souza L. Vulnerabilidade de Mulheres Vivendo Com HIV/Aids. Rev. Lat. Am. Enferm. 2014;22:68–75. doi: 10.1590/0104-1169.2837.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.UNAIDS Women and Girls Still Vulnerable to HIV Due to Gender Inequality: UNAIDS. UN News. [(accessed on 1 July 2022)]. Available online: https://news.un.org/en/story/2020/03/1058751.
- 40.Aidsmap Study Finds That Women Are Much More Vulnerable to HIV Infection during Pregnancy and in the Months after Giving Birth. [(accessed on 1 July 2022)]. Available online: https://www.aidsmap.com/news/mar-2018/study-finds-women-are-much-more-vulnerable-hiv-infection-during-pregnancy-and-months.
- 41.Phanuphak N., Seekaew P., Phanuphak P. Optimising Treatment in the Test-and-Treat Strategy: What Are We Waiting For? Lancet HIV. 2019;6:e715–e722. doi: 10.1016/S2352-3018(19)30236-X. [DOI] [PubMed] [Google Scholar]
- 42.Deng L., Li C., Chen P., Luo X., Zheng X., Zhou L., Zhou Y., Xia J., Hong Z. Dolutegravir plus Lamivudine versus Efavirenz plus Tenofovir Disoproxil Fumarate and Lamivudine in Antiretroviral-Naive Adults with HIV-1 Infection. BMC Infect. Dis. 2022;22:17. doi: 10.1186/s12879-021-06991-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nabitaka V.M., Nawaggi P., Campbell J., Conroy J., Harwell J., Magambo K., Middlecote C., Caldwell B., Katureebe C., Namuwenge N., et al. High Acceptability and Viral Suppression of Patients on Dolutegravir-Based First-Line Regimens in Pilot Sites in Uganda: A Mixed-Methods Prospective Cohort Study. PLoS ONE. 2020;15:e0232419. doi: 10.1371/journal.pone.0232419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehari E.A., Muche E.A., Gonete K.A. Virological Suppression and Its Associated Factors of Dolutegravir Based Regimen in a Resource-Limited Setting: An Observational Retrospective Study in Ethiopia. HIV/AIDS-Res. Palliat. Care. 2021;13:709–717. doi: 10.2147/HIV.S316776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagami E.H., Thakarar K., Sax P.E. Sustained HIV Viral Suppression with Dolutegravir, Tenofovir, and Emtricitabine as Initial Therapy Despite High-Level Transmitted Multiclass Resistance. Open Forum Infect. Dis. 2022;9:ofab648. doi: 10.1093/ofid/ofab648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schramm B., Temfack E., Descamps D., Nicholas S., Peytavin G., Bitilinyu-bangoh J.E., Storto A., Lê M.P. Viral Suppression and HIV-1 Drug Resistance 1 Year after Pragmatic Transitioning to Dolutegravir First-Line Therapy in Malawi: A Prospective Cohort Study. Lancet HIV. 2022;9:544–553. doi: 10.1016/S2352-3018(22)00136-9. [DOI] [PubMed] [Google Scholar]
- 47.Ndashimye E., Arts E.J. Dolutegravir Response in Antiretroviral Therapy Naïve and Experienced Patients with M184V/I: Impact in Low-and Middle-Income Settings. Int. J. Infect. Dis. 2021;105:298–303. doi: 10.1016/j.ijid.2021.03.018. [DOI] [PubMed] [Google Scholar]
- 48.Daar E.S. HIV-1 Virulence, Fitness and Replication Capacity. Therapy. 2005;2:131–140. doi: 10.2217/14750708.2.1.131. [DOI] [Google Scholar]
- 49.Fokam J., Elat J.-B.N., Billong S.C., Kembou E., Nkwescheu A.S., Obam N.M., Essiane A., Torimiro J.N., Ekanmian G.K., Ndjolo A., et al. Monitoring HIV Drug Resistance Early Warning Indicators in Cameroon: A Study Following the Revised World Health Organization Recommendations. PLoS ONE. 2015;10:e0129210. doi: 10.1371/journal.pone.0129210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Golin R., Samuel J.M., Phelps B.R., Persaud U., Malati C.Y., Siberry G.K. The Promise of Paediatric Dolutegravir. J. Int. AIDS Soc. 2021;24:10–11. doi: 10.1002/jia2.25660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Semengue E.N.J., Armenia D., Inzaule S., Santoro M.M., Dambaya B., Takou D., Teto G., Nka A.D., Yagai B., Fabeni L., et al. Baseline Integrase Drug Resistance Mutations and Conserved Regions across HIV-1 Clades in Cameroon: Implications for Transition to Dolutegravir in Resource-Limited Settings. J. Antimicrob. Chemother. 2021;76:1277–1285. doi: 10.1093/jac/dkab004. [DOI] [PubMed] [Google Scholar]
- 52.Fokam J., Salpini R., Santoro M.M., Cento V., D’Arrigo R., Gori C., Perno C.F., Colizzi V., Nanfack A., Gwom L.C., et al. Performance Evaluation of an In-House Human Immunodeficiency Virus Type-1 Protease-Reverse Transcriptase Genotyping Assay in Cameroon. Arch. Virol. 2011;156:1235–1243. doi: 10.1007/s00705-011-0982-3. [DOI] [PubMed] [Google Scholar]
- 53.Fokam J., Santoro M.M., Takou D., Njom-Nlend A.E., Ndombo P.K., Kamgaing N., Kamta C., Essiane A., Sosso S.M., Ndjolo A., et al. Evaluation of Treatment Response, Drug Resistance and HIV-1 Variability among Adolescents on First- And Second-Line Antiretroviral Therapy: A Study Protocol for a Prospective Observational Study in the Centre Region of Cameroon (EDCTP READY-Study) BMC Pediatr. 2019;19:226. doi: 10.1186/s12887-019-1599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santoro M.M., Perno C.F. HIV-1 Genetic Variability and Clinical Implications. Int. Sch. Res. Not. 2013;2013:481314. doi: 10.1155/2013/481314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mphahlele M.K. Ph.D. Thesis. Faculty of Health Sciences, University of the Witwater; Johannesburg, South Afica: 2015. In Vitro Selection and Characterisation of Human Immunodeficiency Virus Type-1 Subtype C Integrase Strand Transfer Inhibitor Resistant Mutants. [Google Scholar]
- 56.Wenk B.M., Mbunkah H.A., Nsanwe N.N., Mbu E.T., Besong L.M., Sama B.A., Orock E., Leemann C., Metzner K.J. Prevalence of Integrase Strand Transfer Inhibitor Resistance Mutations in Antiretroviral-Naive HIV-1-Infected Individuals in Cameroon. J. Antimicrob. Chemother. 2021;76:124–129. doi: 10.1093/jac/dkaa383. [DOI] [PubMed] [Google Scholar]
- 57.Fokam J., Takou D., Semengue E.N.J., Teto G., Beloumou G., Dambaya B., Santoro M.M., Mossiang L., Billong S.C., Cham F., et al. First Case of Dolutegravir and Darunavir/r Multi Drug-Resistant HIV-1 in Cameroon Following Exposure to Raltegravir: Lessons and Implications in the Era of Transition to Dolutegravir-Based Regimens. Antimicrob. Resist. Infect. Control. 2020;9:143. doi: 10.1186/s13756-020-00799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adeiza M.A., Kekulah I., Wachekwa I., Ogbuagu O. Dolutegravir Hypersensitivity Reaction: The Need for Strengthening Pharmacovigilance Systems with Optimization of Antiretroviral Therapy in HIV Programs in Resource-Limited Settings: Case Report. PAMJ Clin. Med. 2022;8:47. doi: 10.11604/pamj-cm.2022.8.47.33930. [DOI] [Google Scholar]
- 59.PEPFAR . Considerations for the Introduction of TLD in National Programs: PEPFAR Guidance on Developing Clinical and Programmatic Recommendations December 2018. PEPFAR; Washington, DC, USA: 2018. [Google Scholar]
- 60.Mulenga L. Introduction, Roll out and Pharmacovigilance of Dolutegravir in the Zambia National HIV Program; Proceedings of the 3rd IeDEAAll Africa Meeting 2019; Johannesburg, South Africa. 30–31 October 2019; pp. 4–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.