Abstract
To elucidate the mechanisms of immunostimulation by bacterial DNA and synthetic oligonucleotides, the effects of heat shock protein 90 (Hsp90) inhibitors on the activation of murine spleen cells and macrophages by these molecules were investigated. Murine spleen cells and J774 and RAW264.7 macrophages responded to a CpG-containing oligodeoxynucleotide (CpG ODN) and Escherichia coli DNA by increased production of interleukin 6 (IL-6), IL-12, tumor necrosis factor alpha, and nitric oxide (NO). Pretreatment with any of the three Hsp90 inhibitors geldanamycin, radicicol, and herbimycin A resulted in a dose-dependent suppression of cytokine production from the spleen cells and macrophages and of NO from macrophages stimulated with CpG ODN or E. coli DNA. These Hsp90 inhibitors, however, had no effect on Staphylococcus aureus Cowan strain 1-induced IL-12 production from either the murine spleen cells or macrophages. CpG ODN and E. coli DNA induced increased intracellular levels of phosphorylated extracellular signal-regulated kinases (ERK1 and -2), which are members of the mitogen-activated protein (MAP) kinase family, while geldanamycin and radicicol blocked the phosphorylation of ERK1 and -2 in J774 and RAW264.7 cells. These data indicate that DNA-induced activation of murine spleen cells and macrophages is mediated by Hsp90 and that Hsp90 inhibitor suppression of DNA-induced macrophage activation is associated with disruption of the MAP kinase signaling pathway. Our findings suggest that Hsp90 inhibitors may provide a useful means of elucidating the mechanisms of immunostimulation by bacterial DNA and CpG ODN as well as a strategy for preventing adverse effects of bacterial DNA as well as lipopolysaccharide.
DNA is a complex macromolecule whose biological activities encompass immune activation. Depending on base sequence and backbone structure, DNA can cause potent immune response stimulation, with DNAs from bacteria displaying activities similar to those of lipopolysaccharide (LPS) (13, 15, 16, 20, 33, 37; T. Sparwasser, T. Miethke, G. Lipford, K. Borschert, H. Hacker, K. Heeg, and H. Wagner, Letter, Nature 386:336–337, 1997). These activities result from short sequence motifs called CpG motifs or immunostimulatory sequences that have the general structure of two 5′ purines, an unmethylated CpG motif, and two 3′ pyrimidines (15, 39). These sequences occur much more commonly in bacterial DNA than in mammalian DNA and provide a signal that, in code-like fashion, can activate the innate immune system (2, 14, 24). In addition to natural DNA, synthetic oligodeoxynucleotides (ODN) with CpG motifs (collectively known as CpG DNA) display immune activities, providing the basis for new classes of adjuvants and immunomodulators (7, 15, 23, 28).
While CpG DNA exerts widespread effects on immune cells, the mechanisms for its action are unclear. Current data indicate that DNA must be internalized for stimulation of murine cells (15, 18) and that CpG DNA-induced intracellular signaling includes the activation of mitogen-activated protein (MAP) kinases and the transcription factor NF-κB (10, 33, 42). The nature of the internal receptor that binds DNA matter is unknown, although both DNA-dependent protein kinase and Toll-like receptor 9 may have a role in this process (6, 12). Whatever the internal receptor for DNA, cellular uptake by CpG DNA is required for its activation; this process appears to be independent of sequence and occurs by endocytosis (15, 18).
To assess further the mechanisms of stimulation by CpG DNA, we have explored the role of heat shock protein 90 (Hsp90) in immune cell activation. Hsp90, a member of the heat shock protein family, is a ubiquitous molecular chaperone present in the cytoplasms of all eukaryotic cells (3). Through its role in protein folding, Hsp90 constitutes an essential component in several signaling transduction systems, including nuclear receptors for steroid hormones, such as glucocorticoids, progesterone, and estrogen, and a variety of protein kinases, such as Raf, extracellular signal-regulated kinases (ERK), and MAP-ERK kinase (MEK) in the MAP kinase family (26). Studies have shown that Hsp90 plays a crucial role in LPS-mediated macrophage activation (4) and anti-CD3- and -CD28-mediated T-lymphocyte activation (17, 29).
Because of the similarity of immune activation by CpG DNA and LPS, we questioned whether Hsp90 is involved in immune stimulation by CpG DNA. To determine the role of Hsp90 in immune stimulation by DNA, we tested the in vitro effects of three inhibitors of Hsp90, geldanamycin, radicicol, and herbimycin A, previously known as protein tyrosine kinase inhibitors (27). These inhibitors, while differing structurally, all bind Hsp90 and inhibit its chaperone function for signaling proteins in the MAP kinase pathway (30, 32, 41). With data presented herein with murine cell preparations, we show that Hsp90 inhibitors can block the production of interleukin 6 (IL-6), IL-12, tumor necrosis factor alpha (TNF-α), and nitric oxide (NO) induced by CpG-containing ODN (CpG ODN) and bacterial DNA. We further show that the Hsp90 inhibitors block phosphorylation of the MAP kinases ERK1 and -2 under these conditions. These findings emphasize the important role of Hsp90 in immune stimulation by CpG DNA and provide further evidence for common activation pathways by LPS and CpG DNA.
MATERIALS AND METHODS
Synthetic oligonucleotides and bacterial and mammalian DNA.
Phosphorothioate oligonucleotides were purchased from Midland Certified Reagent Company (Midland, Tex.). The sequences for a CpG ODN (5′-TCCATGACGTTCCTGACGTT-3′) and a non-CpG-containing ODN (non-CpG ODN) (5′-TCCATGAGCTTCCTGAGTCT-3′) were taken from reference 5. Escherichia coli DNA and calf thymus DNA (CT DNA) were purchased from Sigma Chemical Co. (St. Louis, Mo.).
To prepare fluorescently labeled oligonucleotides, oligonucleotides containing an amino terminus were mixed with fluorescein isothiocyanate (FITC; Sigma Chemical Co.) in a ratio of 0.1 mg of FITC to 10 mg of oligonucleotide in carbonate-bicarbonate buffer, pH 9.5. After a 2-h reaction at room temperature, unreacted FITC was removed by gel filtration through a Sephadex G-25 column (Amersham Pharmacia Biotech, Piscataway, N.J.) followed by ethanol precipitation.
Mice.
BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, Maine) and were housed under conventional conditions in the animal facility of the Durham Veterans Administration Hospital.
Cell culture.
The murine macrophage cell lines J774 and RAW264.7 (American Type Culture Collection, Manassas, Va.) were maintained in a culture medium consisting of RPMI 1640 medium (Life Technologies, Grand Island, N.Y.) supplemented with 10% fetal bovine serum (HyClone, Logan, Utah) and 50 μg of gentamicin per ml. To prepare single-cell suspensions of spleen cells, the spleens were removed aseptically from BALB/c mice and nonstromal cells were expressed with flame-sterilized microscope slides into RPMI 1640 medium. The cell suspension was centrifuged at 400 × g for 5 min. The cell pellet was resuspended in lysis medium (1 volume of 0.17 M Tris [pH 7.6], 9 volumes of 0.16 M NH4Cl) to eliminate red blood cells and repelleted. Cells were then washed twice with RPMI 1640 medium and were suspended in the culture medium.
Activation of murine spleen cells and macrophages.
Spleen cells (1 × 106/well) or J774 and RAW264.7 cells (5 × 104/well) in 96-well culture plates were pretreated with medium alone or a range of doses (0.01 to 1 μg/ml) of geldanamycin (A.G. Scientific, Inc., San Diego, Calif.), radicicol (Sigma Chemical Co.), and herbimycin A (Sigma Chemical Co.) at 37°C for 1 h, followed by further incubation with synthetic or natural DNA (10 μg/ml) for up to 48 h, with the Hsp90 inhibitors being continuously present. In some experiments, spleen cells and macrophages were stimulated with 0.1% (wt/vol) killed Staphylococcus aureus Cowan strain 1 organisms purchased from Sigma Chemical Co. The Hsp90 inhibitors were continuously present during the whole incubation period in all our experiments in this study.
To assess the time course of cellular activation, culture supernatants were collected at different time points and kept at −20°C for later assays for cytokines and nitrite (NO2−), the stable metabolite of NO. The cell viability of the spleen cells and macrophages subjected to the above-described treatments was assessed by the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay (21). All three Hsp90 inhibitors showed minimal effects on the viability of these cells at the dose range (0.01 to 1 μg/ml) used in the present study, although considerable reduction of growth was observed in cells treated with 5- to 10-fold-higher doses of the compounds in our preliminary experiments (data not shown).
Cytokine ELISA.
IL-6, IL-12, and TNF-α levels in the supernatant samples were measured by enzyme-linked immunosorbent assay (ELISA) as previously described (11). Briefly, ELISA plates (Immulon II HB; Dynex Technologies, Chantilly, Va.) were prepared by coating each well with 100 μl of capture antibody diluted to 1 to 5 μg/ml in phosphate-buffered saline (PBS; pH 8.5). After overnight incubation at 4°C, the plates were washed with PBS (pH 7.4) using an automated plate washer (Skatron Instruments Inc., Sterling, Va.). One hundred microliters of the culture supernatants diluted in PBS (pH 7.4) containing 0.5% bovine serum albumin and 0.5% Tween 20 (Sigma Chemical Co.) was added to each well. Recombinant cytokines were diluted in duplicate and run on each plate as standards.
Diluted samples and standards were allowed to incubate for 2 h at room temperature. After washing of the plates, 100 μl of diluted biotinylated detection antibody (0.1 to 1 μg/ml) was added to each well. Following another 2-h incubation at room temperature, the plates were washed and 100 μl of diluted peroxidase avidin conjugate (Zymed, San Francisco, Calif.) was added to each well. After a 30-min incubation, plates were washed and 100 μl of 0.1 M sodium citrate buffer (pH 4.0) containing 0.015% 3,3′,5,5′-tetramethylbenzidine hydrochloride and 0.01% hydrogen peroxide (Sigma Chemical Co.) was added. Plates were read at 380 nm with a microtiter plate reader (Molecular Devices, Menlo Park, Calif.), and cytokine concentrations were derived from standard curves. All the capture and detection antibodies for the cytokine ELISA described here were purchased from PharMingen (San Diego, Calif.).
Nitrite assay.
NO production in the supernatant samples was quantified by using the Griess method (35) to measure nitrite, a stable breakdown product of NO. Briefly, 50-μl samples were transferred to a 96-well microtiter plate, followed by the addition of 100 μl of Griess reagent I (1% sulfanilamide in 2.5% phosphoric acid) and 100 μl of Griess reagent II (0.1% naphthylenediamine in 2.5% phosphoric acid). The absorbency was read within 5 min at 550 nm on a microtiter plate reader. Nitrite concentrations were calculated by comparison with a standard curve for sodium nitrite.
Immunoblotting analysis.
J774 or RAW264.7 cells (5 × 106/well) were cultured in a six-well culture plate and pretreated with 1 μg of geldanamycin, radicicol, or herbimycin A per ml for 1 h, followed by stimulation with 10 μg of CpG ODN or E. coli DNA per ml or 1 μg of LPS per ml in the presence of the Hsp90 inhibitors for 30 min. For controls, cells were treated with medium alone or 10 μg of non-CpG ODN or CT DNA per ml. The stimulation was stopped by adding a 3-ml/well solution of cold PBS (pH 7.4) containing 1 mM Na3VO4. The cells were lysed on ice for 10 min with 300 μl of lysis buffer (25 mM HEPES, 300 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, 5 mM Na3VO4, 10 mM NaF, 50 μg of leupeptin per ml, 50 μg of pepstatin A per ml, 1 mM phenylmethylsulfonyl fluoride, and 0.5 mM dithiothreitol) per well. The lysates were cleared by centrifugation at 16,000 × g for 15 min in a cold room, and protein concentrations were determined by the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, Calif.). The lysates (10 μg of protein/lane) were subjected to 12% polyacrylamide gel electrophoresis and transferred to Hybond enhanced-chemiluminescence (ECL) nitrocellulose membranes (Amersham Pharmacia Biotech). The membranes were blocked in 5% nonfat dry milk–0.1% Triton X-100–Tris-buffered saline (pH 6.8), followed by staining with monoclonal anti-mouse phosphorylated ERK1 and -2 (dilution, 1:200) purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). After being washed with 0.1% Tween 20–Tris-buffered saline three times, the membranes were incubated with horseradish peroxidase-conjugated anti-mouse whole-molecule immunoglobulin G (dilution, 1:5,000; Sigma Chemical Co.). The specific bands were detected with an ECL Western blotting detection kit and Hyperfilm-ECL according to the instructions of the manufacturer (Amersham Pharmacia Biotech).
Confocal microscopy.
J774 cells were cultured on glass slide coverslips in a six-well plate overnight. The cells were pretreated with medium alone or 1 μg of geldanamycin per ml at 37°C for 1 h, followed by incubation with 10 μg of FITC-labeled CpG ODN or non-CpG ODN per ml or 1 μg of FITC-labeled LPS (Sigma Chemical Co.) per ml at 37°C for 30 min. After three washes with cold 0.5% bovine serum albumin with 0.1% NaN3 in PBS, the slides were viewed for intracellular distribution of labeled oligonucleotides or LPS under a laser confocal microscope (LSM 510; Carl Zeiss, Oberkochen, Germany).
Statistical analysis.
Statistical analysis was performed with a paired Student t test to compare the cytokine and nitrite levels of stimulated cells treated with Hsp90 inhibitors with those of the cells not treated with the inhibitors.
RESULTS
Hsp90 inhibitors block cytokine production by murine spleen cells.
To determine the role of Hsp90 in mediating cell activation by CpG DNA, we first tested the effects of the Hsp90 inhibitor geldanamycin on activation of murine spleen cells in culture. Spleen cells were treated with various doses of geldanamycin for 1 h, followed by stimulation with 10 μg of CpG ODN or E. coli DNA per ml for 12 h. For negative controls, cells were treated in parallel with medium or geldanamycin alone or with 10 μg of non-CpG ODN or CT DNA per ml. For positive controls, cells were treated with 10 μg of LPS per ml.
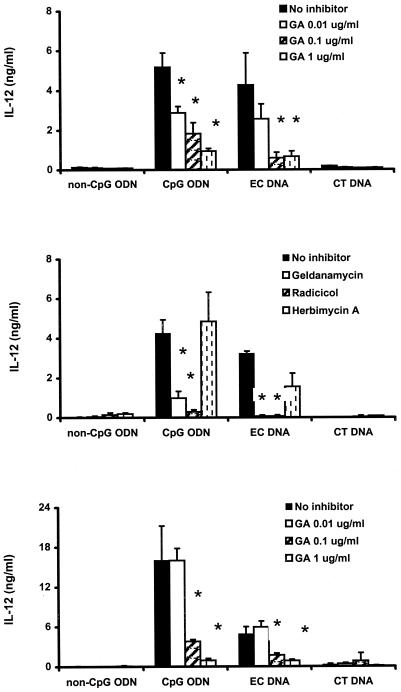
As shown in Fig. 1 (upper panel), murine spleen cells treated with CpG ODN and E. coli DNA showed increased production of IL-12 while non-CpG ODN and CT DNA at the same dose were inactive. Treatment of the spleen cells with 0.01 to 1 μg of geldanamycin per ml resulted in a dose-dependent inhibition of IL-12, while geldanamycin alone had no effect on cytokine production. To confirm the results with geldanamycin, spleen cells were treated with two different Hsp90 inhibitors, radicicol and herbimycin A, as well as geldanamycin. Radicicol significantly blocked IL-12 production, while herbimycin A did not affect IL-12 production under these conditions (Fig. 1, middle panel). These results suggest possible differences among these inhibitors in their actions.
FIG. 1.
Effects of Hsp90 inhibitors on IL-12 production in DNA-stimulated murine spleen cells (upper and middle panels) and J774 macrophages (lower panel). Cells were pretreated with the indicated concentrations of geldanamycin (GA) or 0.1 μg of geldanamycin, radicicol, or herbimycin A per ml for 1 h and then stimulated with CpG ODN or E. coli DNA (10 μg/ml) in the presence or absence of the inhibitor for 12 h. The controls were incubated with medium alone or non-CpG ODN and CT DNA (10 μg/ml). IL-12 levels in the culture supernatants were determined by ELISA. Each value is the mean of triplicate results. Error bars indicate the standard deviations. ∗, P < 0.05 in a comparison of stimulated cells with and without the inhibitors.
Under the same conditions, geldanamycin, radicicol, and herbimycin A inhibited production of IL-6 and TNF-α from spleen cells stimulated with CpG ODN or E. coli DNA (data not shown). Similarly, these reagents blocked cytokine production by spleen cells stimulated with LPS, as 0.1 μg of geldanamycin, radicicol, and herbimycin A per ml blocked IL-12 production by 80, 87, and 56%, respectively, in murine spleen cells stimulated with 10 μg of LPS per ml for 12 h. These results indicate that inhibition of Hsp90 has significant effects on cytokine production by murine spleen cells.
Hsp90 inhibitors block cytokine and NO production by murine macrophage cell lines.
To assess the role of Hsp90 in CpG DNA-induced macrophage activation, we treated J774 cells with 0.01 to 1 μg of geldanamycin per ml for 1 h, followed by stimulation with CpG ODN or E. coli DNA for 12 h. CpG ODN or E. coli DNA at 10 μg/ml induced IL-12 production, while neither non-CpG ODN nor CT DNA was active (Fig. 1, lower panel). Geldanamycin significantly blocked the production of IL-12 in a dose-dependent fashion (Fig. 1, lower panel). This Hsp90 inhibitor also affected production of IL-6 and TNF-α stimulated by CpG DNA as well as IL-6 and IL-12 and TNF-α production from J774 cells stimulated with 1 μg of LPS per ml (data not shown).
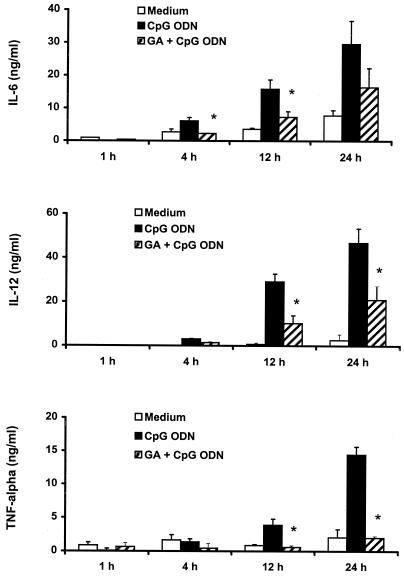
We further examined the time course for geldanamycin-mediated inhibition of cytokine production from J774 cells stimulated with CpG ODN. As shown in Fig. 2, 10 μg of CpG ODN per ml stimulated J774 cells to produce increased levels of IL-6, IL-12, and TNF-α in a time-dependent fashion, while geldanamycin at a dose of 0.1 μg/ml significantly blocked the production of the cytokines from 12 to 24 h.
FIG. 2.
Time course for IL-6, IL-12, and TNF-α production induced by CpG ODN and inhibited by geldanamycin. J774 cells were pretreated with 0.1 μg of geldanamycin (GA) per ml for 1 h and then stimulated with 10 μg of CpG ODN per ml in the presence or absence of geldanamycin. Culture supernatants were collected at the indicated time intervals and examined for their levels of IL-6, IL-12, and TNF-α. Each value is the mean of triplicate results. Error bars indicate the standard deviations. ∗, P < 0.05 in a comparison of stimulated cells with and without the inhibitors.
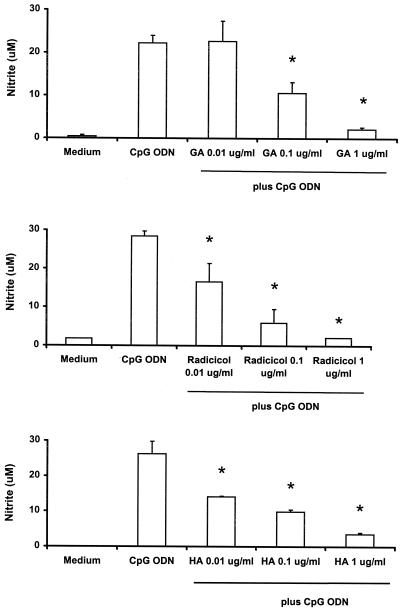
NO is an important inflammatory mediator that is produced by monocytes and macrophages in response to LPS and other stimuli (40). A previous study showed that macrophages respond to the stimulation by plasmid DNA by increased expression of nitric oxide synthase (33). In the present study, J774 cells were stimulated with 10 μg of CpG ODN per ml in the presence or absence of geldanamycin, radicicol, or herbimycin A for 48 h. CpG ODN induced an increased production of nitrite, an indicator of NO, while all three Hsp90 inhibitors significantly blocked nitrite production in a dose-dependent fashion (Fig. 3). The three Hsp90 inhibitors had similar effects on IL-6, IL-12, TNF-α, and NO production from RAW264.7 cells stimulated with CpG ODN or E. coli DNA (data not shown).
FIG. 3.
Inhibition of nitrite production from J774 cells by Hsp90 inhibitors. J774 cells were treated as described in the legend to Fig. 1. Culture supernatants were collected at 48 h and examined for their levels of nitrite by the Griess method. Geldanamycin (GA), radicicol, and herbimycin A (HA) inhibited nitrite production in J774 cells in a dose-dependent fashion. Each value is a mean of triplicate results. Error bars indicate the standard deviations. ∗, P < 0.05 in a comparison of stimulated cells with and without the inhibitors.
Effects of Hsp90 inhibitors on S. aureus Cowan strain 1-induced activation of murine spleen cells and macrophages.
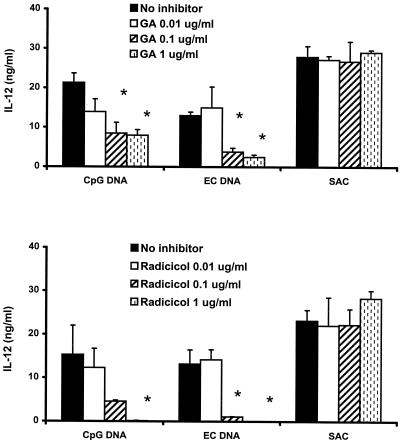
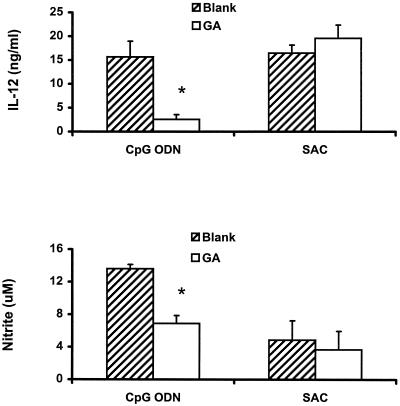
To assess the specificity of inhibition of the Hsp90 inhibitors, we stimulated murine spleen cells and J774 cells with a 0.1% concentration of killed S. aureus bacteria (S. aureus Cowan strain 1) as well as 10 μg of CpG ODN or E. coli DNA per ml in the presence or absence of geldanamycin or radicicol. These two Hsp90 inhibitors significantly blocked CpG DNA-induced IL-12 production but did not significantly affect S. aureus Cowan strain 1-induced cytokine production by spleen cells (Fig. 4). In a similar fashion, geldanamycin failed to block S. aureus Cowan strain 1-induced IL-12 and nitrite production by J774 cells, while it significantly inhibited CpG ODN-induced IL-12 and nitrite production (Fig. 5). Radicicol had effects on S. aureus Cowan strain 1-induced cytokine and nitrite production by J774 cells similar to those produced by geldanamycin, although both of the Hsp90 inhibitors showed some inhibition of IL-6 and TNF-α production by murine spleen cells and macrophages (data not shown). These observations support the notion that Hsp90 inhibitors do not uniformly inhibit cellular activation.
FIG. 4.
Effects of geldanamycin and radicicol on IL-12 production in murine spleen cells treated with stimulatory DNA and S. aureus Cowan strain 1 (SAC). The spleen cells were pretreated with 0.1 μg of geldanamycin (GA) and radicicol per ml for 1 h, followed by stimulation with 10 μg of CpG DNA or E. coli DNA per ml or a 0.1% concentration of S. aureus Cowan strain 1 cells for 12 h. Each value is the mean of triplicate results. Error bars indicate the standard deviations. ∗, P < 0.05 in a comparison of stimulated cells with and without the inhibitors.
FIG. 5.
Effects of geldanamycin on IL-12 and NO production by J774 cells. J774 cells were pretreated with 0.1 μg of geldanamycin (GA) per ml for 1 h and then stimulated with 10 μg of CpG ODN per ml in the presence or absence of the inhibitor for 12 h. Each value is the mean of triplicate results. ∗, P < 0.05 in a comparison of stimulated cells with and without the inhibitors. SAC, S. aureus Cowan strain 1.
Effects of Hsp90 inhibitors on CpG ODN-induced phosphorylation of ERK1 and -2.
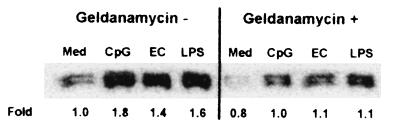
ERK1 and -2 constitute one family of MAP kinases that are downstream effector kinases in a signaling pathway activated by a wide range of extracellular stimulants, including LPS and CpG-containing oligonucleotides (10, 19). In the present study, increased levels of phosphorylated ERK1 and -2 were detected in J774 cells stimulated by 10 μg of CpG ODN or E. coli DNA per ml or 1 μg of LPS per ml for 30 min (Fig. 6) while non-CpG ODN and CT DNA at the same dose were inactive (data not shown). Pretreatment with 1 μg of geldanamycin per ml resulted in the suppression of phosphorylated ERK1 and -2 levels in J774 cells stimulated with CpG ODN, E. coli DNA, or LPS (Fig. 6). Similarly inhibitory effects on CpG DNA-induced activation of ERK1 and -2 were also observed in RAW264.7 cells pretreated with geldanamycin (data not shown). Another Hsp90 inhibitor, radicicol (1 μg/ml), displayed similarly inhibitory effects on the phosphorylation of ERK1 and -2 in both J774 and RAW264.7 cells stimulated with CpG ODN, E. coli DNA, and LPS (data not shown).
FIG. 6.
DNA-mediated activation of the MAP kinases ERK1 and -2 and their inhibition by geldanamycin in murine macrophages. J774 cells were pretreated with or without 1 μg of geldanamycin per ml for 1 h and then stimulated with 10 μg of CpG ODN (CpG) or E. coli DNA (EC) per ml or 1 μg of LPS per ml in the presence or absence of geldanamycin for 30 min. Cell lysates were analyzed for the presence of phosphorylated ERK1 and -2 by immunoblotting. Membranes were exposed using ECL, the images were scanned, and optical densities were determined. Values are presented as the fold increases in optical density against that of a medium control without geldanamycin treatment (Med). The data are representative of four separate experiments.
Effect of geldanamycin on CpG ODN internalization.
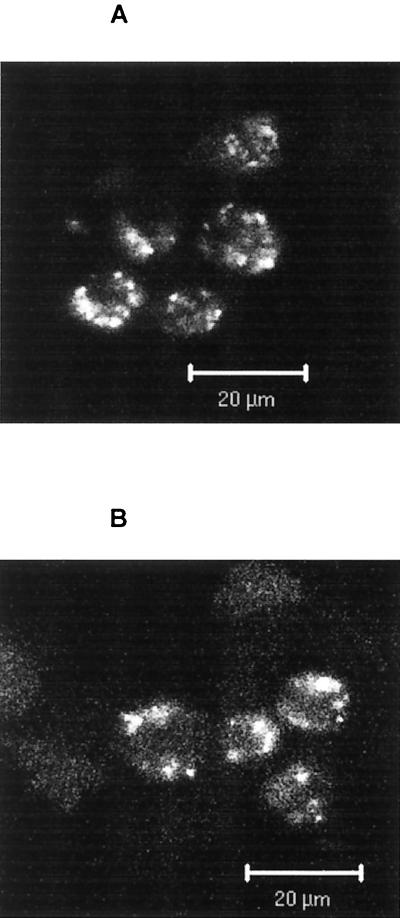
Since the internalization of CpG DNA is necessary for cell activation (15, 18), we asked whether Hsp90 inhibitors exerted their inhibitory effects by blocking the cellular uptake of CpG DNA. As shown in Fig. 7, geldanamycin (1 μg/ml) had no major effect on the uptake or intracellular distribution of fluorescently labeled CpG ODN (10 μg/ml) in J774 cells. Geldanamycin also had a minimal effect on the internalization of fluorescently labeled LPS (1 μg/ml), which has CD14-dependent and -independent pathways for its uptake (data not shown) (38). These results indicate that the inhibitory effects of geldanamycin are related to its inhibition of the signaling process rather than of the internalization of CpG DNA or LPS.
FIG. 7.
Effects of geldanamycin on CpG ODN internalization. J774 cells were pretreated with or without 1 μg of geldanamycin per ml for 1 h, followed by incubation with 10 μg of FITC-labeled CpG ODN per ml at 37°C for 30 min. Cells were washed and examined under a confocal microscope. Note the similarity of uptake and peripheral distribution patterns of labeled CpG ODN in the cells with (B) or without (A) geldanamycin pretreatment. The data shown are from a representative experiment that was repeated with identical results.
DISCUSSION
The results presented herein clarify mechanisms of immune activation by CpG ODN and bacterial DNA as well as the role of Hsp90 in signal transduction induced by foreign molecules. As shown in in vitro experiments, three Hsp90 inhibitors, geldanamycin, radicicol, and herbimycin A, all inhibited CpG DNA-induced responses in murine spleen cells and J774 and RAW264.7 cells, although there were some differences in the effects of these compounds depending on the cell system. Furthermore, these compounds, while not affecting DNA uptake, blocked activation of the MAP kinase family. Taken together, these results suggest that Hsp90 plays a key role in mediating the stimulatory effects of CpG ODN and E. coli DNA on murine spleen cells and macrophages.
Heat shock proteins are a set of ubiquitous proteins present in the cytoplasms of all eukaryotic cells. Based on their molecular weights and function, these proteins are characterized as Hsp100, Hsp90, Hsp70, Hsp60, Hsp40, and small Hsp (1). Hsp90 plays an essential role in cell signal transduction by forming multimolecular complexes with several signal proteins, including the protein kinases Raf, ERK, and MEK in the MAP kinase signaling pathway (26, 34). Receptors for a variety of extracellular stimulants including LPS transduce signals via the MAP kinase pathway, with activation of this pathway involving the cascade Raf→MEK→ERK and nuclear translocation of transcription factors such as NF-κB.
As probes of this pathway, we have used a number of Hsp90 inhibitors that differ in their chemical compositions. Both geldanamycin and herbimycin A belong to the benzoquinone ansamycin family, while radicicol is a macrocyclic antibiotic (8, 9, 22). These compounds inhibit the function of Hsp90 by binding to its NH2-terminal domain, leading to disruption of the Raf-Hsp90 complexes and depletion of Raf (30). As a result, the activation and phosphorylation of the MAP kinases ERK1 and -2 is inhibited, leading to the blockade of the MAP kinase signaling pathway (32).
In the present study, CpG ODN and E. coli DNA induced IL-6, IL-12, TNF-α, and NO in murine spleen cells and macrophage cell lines. As shown by immunoblotting analysis, stimulation by these agents was associated with the activation of the MAP kinase pathway, resulting in elevated levels of phosphorylated ERK1 and -2 in J774 and RAW264.7 cells. These observations are consistent with previous findings on the induction of cytokines and ERK1 and -2 phosphorylation in murine macrophages stimulated with either bacterial DNA or CpG-containing oligonucleotides (10, 31). With treatment of spleen cells or macrophages with Hsp90 inhibitors, however, there was a dose-dependent inhibition of the cytokine and NO production induced by CpG ODN and E. coli DNA depending on the cell system. We further observed that pretreatment with geldanamycin or radicicol resulted in reduced levels of phosphorylated ERK1 and -2 in stimulated J774 or RAW264.7 cells. Since the reduction in activated ERK1 and -2 was correlated with reduced production of cytokines and NO, these data suggest a critical role for Hsp90 in the MAP kinase-mediated signal transduction pathway in CpG DNA-stimulated murine macrophages.
In concert with a previous study (4), our findings provide evidence for a similarity in the mechanisms of immune stimulation by LPS and CpG DNA. Both LPS and CpG DNA utilize a Toll-like receptor and MyD88 to induce a range of cell responses, including activation of the MAP kinase cascade and the nuclear translocation of NF-κB (12, 25). Since Hsp90 inhibitors can affect activation by both LPS and CpG DNA, our findings suggest the utilization of a common signaling pathway by these bacterial products; they do not exclude, however, the roles of signaling pathways that are not closely associated with Hsp90 but may be activated by CpG DNA.
Compared with the inhibition of CpG DNA-induced responses by geldanamycin and radicicol, the Hsp90 inhibitors had much less effect on the IL-12 and NO production induced by S. aureus Cowan strain 1. Although activation by S. aureus Cowan strain 1 may involve a variety of immunostimulatory components (e.g., protein A, peptidoglycan, and lipoteichoic acid), the effects of Hsp90 inhibitors observed in this study point to specific effects on the cell activation triggered by immunostimulatory DNA and LPS rather than a global inhibition of spleen cell and macrophage function. Observations from our confocal microscopic experiment indicate further that the inhibitory effects of the Hsp90 inhibitors are not likely mediated through blocking the internalization of CpG ODN, since geldanamycin did not influence the uptake or intracellular distribution of fluorescently labeled CpG ODN.
While indicating the utility of Hsp90 inhibitors in elucidating the signaling pathway in cell activation (26), our findings are also relevant to disease treatment. The ability of Hsp90 inhibitors to suppress immune cell activation and cytokine production suggests strategies to block immune-mediated diseases, including autoimmunity, by interdicting these signaling pathways. Indeed, one recent report showed that geldanamycin suppressed the progression of adjuvant-induced arthritis in rats (36). Studies are therefore in progress to investigate further the role of bacterial DNA in the pathogenesis of autoimmune and inflammatory diseases and to develop approaches to overcome its deleterious actions.
ACKNOWLEDGMENTS
We thank Charles F. Reich III, Mary A. Misukonis, J. Brice Weinberg, Farshid Guilak, and Douglas J. Perkins for their assistance and advice in this study.
This work was supported by grants from the VA Merit Review, the Arthritis Foundation, and the NIH (grant no. 5405940000740578).
REFERENCES
- 1.Becker J, Craig E A. Heat-shock proteins as molecular chaperones. Eur J Biochem. 1994;219:11–23. doi: 10.1007/978-3-642-79502-2_2. [DOI] [PubMed] [Google Scholar]
- 2.Bird A P. Functions for DNA methylation in vertebrates. Cold Spring Harbor Symp Quant Biol. 1993;58:281–285. doi: 10.1101/sqb.1993.058.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Buchner J. Hsp90 & Co.—a holding for folding. Trends Biochem Sci. 1999;24:136–141. doi: 10.1016/s0968-0004(99)01373-0. [DOI] [PubMed] [Google Scholar]
- 4.Byrd C A, Bornmann W, Erdjument-Bromage H, Tempst P, Pavletich N, Rosen N, Nathan C F, Ding A. Heat shock protein 90 mediates macrophage activation by Taxol and bacterial lipopolysaccharide. Proc Natl Acad Sci USA. 1999;96:5645–5650. doi: 10.1073/pnas.96.10.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chace J H, Hooker N A, Mildenstein K L, Krieg A M, Cowdery J S. Bacterial DNA-induced NK cell IFN-gamma production is dependent on macrophage secretion of IL-12. Clin Immunol Immunopathol. 1997;84:185–193. doi: 10.1006/clin.1997.4380. [DOI] [PubMed] [Google Scholar]
- 6.Chu W M, Gong X, Li Z W, Takabayashi K, Ouyang H H, Lois A, Chen D J, Li G C, Karin M, Raz E. DNA-PKcs is required for activation of innate immunity by immunostimulatory DNA. Cell. 2000;103:909–918. doi: 10.1016/s0092-8674(00)00194-x. [DOI] [PubMed] [Google Scholar]
- 7.Davis H L, Weeratna R, Waldschmidt T J, Tygrett L, Schorr J, Krieg A M, Weeranta R. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol. 1998;160:870–876. [PubMed] [Google Scholar]
- 8.DeBoer C, Meulman P A, Wnuk R J, Peterson D H. Geldanamycin, a new antibiotic. J Antibiot (Tokyo) 1970;23:442–447. doi: 10.7164/antibiotics.23.442. [DOI] [PubMed] [Google Scholar]
- 9.Demotte P, Demotte-Plaquee J. A new antifungal substance of fungal origin. Nature. 1953;171:344. doi: 10.1038/171344a0. [DOI] [PubMed] [Google Scholar]
- 10.Hacker H, Mischak H, Hacker G, Eser S, Prenzel N, Ullrich A, Wagner H. Cell type-specific activation of mitogen-activated protein kinases by CpG-DNA controls interleukin-12 release from antigen-presenting cells. EMBO J. 1999;18:6973–6982. doi: 10.1093/emboj/18.24.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halpern M D, Pisetsky D S. In vitro inhibition of murine IFN gamma production by phosphorothioate deoxyguanosine oligomers. Immunopharmacology. 1995;29:47–52. doi: 10.1016/0162-3109(95)00043-s. [DOI] [PubMed] [Google Scholar]
- 12.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 13.Klinman D M, Yi A K, Beaucage S L, Conover J, Krieg A M. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krieg A M. An innate immune defense mechanism based on the recognition of CpG motifs in microbial DNA. J Lab Clin Med. 1996;128:128–133. doi: 10.1016/s0022-2143(96)90004-9. [DOI] [PubMed] [Google Scholar]
- 15.Krieg A M, Yi A K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 16.Liang H, Nishioka Y, Reich C F, Pisetsky D S, Lipsky P E. Activation of human B cells by phosphorothioate oligodeoxynucleotides. J Clin Investig. 1996;98:1119–1129. doi: 10.1172/JCI118894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Losiewicz M D, Kaur G, Sausville E A. Different early effects of tyrphostin AG957 and geldanamycins on mitogen-activated protein kinase and p120cbl phosphorylation in anti CD-3-stimulated T-lymphoblasts 1. Biochem Pharmacol. 1999;57:281–289. doi: 10.1016/s0006-2952(98)00293-7. [DOI] [PubMed] [Google Scholar]
- 18.Manzel L, Macfarlane D E. Lack of immune stimulation by immobilized CpG-oligodeoxynucleotide. Antisense Nucleic Acid Drug Dev. 1999;9:459–464. doi: 10.1089/oli.1.1999.9.459. [DOI] [PubMed] [Google Scholar]
- 19.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 20.Messina J P, Gilkeson G S, Pisetsky D S. Stimulation of in vitro murine lymphocyte proliferation by bacterial DNA. J Immunol. 1991;147:1759–1764. [PubMed] [Google Scholar]
- 21.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Omura S, Iwai Y, Takahashi Y, Sadakane N, Nakagawa A, Oiwa H, Hasegawa Y, Ikai T. Herbimycin, a new antibiotic produced by a strain of Streptomyces. J Antibiot (Tokyo) 1979;32:255–261. doi: 10.7164/antibiotics.32.255. [DOI] [PubMed] [Google Scholar]
- 23.Pisetsky D S, Reich C. Stimulation of in vitro proliferation of murine lymphocytes by synthetic oligodeoxynucleotides. Mol Biol Rep. 1993;18:217–221. doi: 10.1007/BF01674433. [DOI] [PubMed] [Google Scholar]
- 24.Pisetsky D S, Reich C, Crowley S D, Halpern M D. Immunological properties of bacterial DNA. Ann N Y Acad Sci. 1995;772:152–163. doi: 10.1111/j.1749-6632.1995.tb44740.x. [DOI] [PubMed] [Google Scholar]
- 25.Poltorak A, Smirnova I, He X, Liu M Y, Van Huffel C, McNally O, Birdwell D, Alejos E, Silva M, Du X, Thompson P, Chan E K, Ledesma J, Roe B, Clifton S, Vogel S N, Beutler B. Genetic and physical mapping of the Lps locus: identification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol Dis. 1998;24:340–355. doi: 10.1006/bcmd.1998.0201. [DOI] [PubMed] [Google Scholar]
- 26.Pratt W B. The role of the hsp90-based chaperone system in signal transduction by nuclear receptors and receptors signaling via MAP kinase. Annu Rev Pharmacol Toxicol. 1997;37:297–326. doi: 10.1146/annurev.pharmtox.37.1.297. [DOI] [PubMed] [Google Scholar]
- 27.Roe S M, Prodromou C, O'Brien R, Ladbury J E, Piper P W, Pearl L H. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42:260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 28.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen M D, Silverman G J, Lotz M, Carson D A, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 29.Schnaider T, Somogyi J, Csermely P, Szamel M. The Hsp90-specific inhibitor geldanamycin selectively disrupts kinase-mediated signaling events of T-lymphocyte activation. Cell Stress Chaperones. 2000;5:52–61. doi: 10.1043/1355-8145(2000)005<0052:THSIGS>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulte T W, Blagosklonny M V, Ingui C, Neckers L. Disruption of the Raf-1–Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1–Ras association. J Biol Chem. 1995;270:24585–24588. doi: 10.1074/jbc.270.41.24585. [DOI] [PubMed] [Google Scholar]
- 31.Sester D P, Beasley S J, Sweet M J, Fowles L F, Cronau S L, Stacey K J, Hume D A. Bacterial/CpG DNA down-modulates colony stimulating factor-1 receptor surface expression on murine bone marrow-derived macrophages with concomitant growth arrest and factor-independent survival. J Immunol. 1999;163:6541–6550. [PubMed] [Google Scholar]
- 32.Soga S, Kozawa T, Narumi H, Akinaga S, Irie K, Matsumoto K, Sharma S V, Nakano H, Mizukami T, Hara M. Radicicol leads to selective depletion of Raf kinase and disrupts K-Ras-activated aberrant signaling pathway. J Biol Chem. 1998;273:822–828. doi: 10.1074/jbc.273.2.822. [DOI] [PubMed] [Google Scholar]
- 33.Stacey K J, Sweet M J, Hume D A. Macrophages ingest and are activated by bacterial DNA. J Immunol. 1996;157:2116–2122. [PubMed] [Google Scholar]
- 34.Stancato L F, Silverstein A M, Owens-Grillo J K, Chow Y H, Jove R, Pratt W B. The hsp90-binding antibiotic geldanamycin decreases Raf levels and epidermal growth factor signaling without disrupting formation of signaling complexes or reducing the specific enzymatic activity of Raf kinase. J Biol Chem. 1997;272:4013–4020. doi: 10.1074/jbc.272.7.4013. [DOI] [PubMed] [Google Scholar]
- 35.Stuehr D J, Nathan C F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989;169:1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugita T, Tanaka S, Murakami T, Miyoshi H, Ohnuki T. Immunosuppressive effects of the heat shock protein 90-binding antibiotic geldanamycin. Biochem Mol Biol Int. 1999;47:587–595. doi: 10.1080/15216549900201633. [DOI] [PubMed] [Google Scholar]
- 37.Sun S, Beard C, Jaenisch R, Jones P, Sprent J. Mitogenicity of DNA from different organisms for murine B cells. J Immunol. 1997;159:3119–3125. [PubMed] [Google Scholar]
- 38.Tapping R I, Gegner J A, Kravchenko V V, Tobias P S. Roles for LBP and soluble CD14 in cellular uptake of LPS. Prog Clin Biol Res. 1998;397:73–78. [PubMed] [Google Scholar]
- 39.Tokunaga T, Yano O, Kuramoto E, Kimura Y, Yamamoto T, Kataoka T, Yamamoto S. Synthetic oligonucleotides with particular base sequences from the cDNA encoding proteins of Mycobacterium bovis BCG induce interferons and activate natural killer cells. Microbiol Immunol. 1992;36:55–66. doi: 10.1111/j.1348-0421.1992.tb01642.x. [DOI] [PubMed] [Google Scholar]
- 40.Weinberg J B. Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes: a review. Mol Med. 1998;4:557–591. doi: 10.1007/BF03401758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitesell L, Mimnaugh E G, De Costa B, Myers C E, Neckers L M. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yi A K, Krieg A M. CpG DNA rescue from anti-IgM-induced WEHI-231 B lymphoma apoptosis via modulation of IκBα and IκBβ and sustained activation of nuclear factor-κB/c-Rel. J Immunol. 1998;160:1240–1245. [PubMed] [Google Scholar]