Abstract
Background and Objectives: The heart is the organ with the highest metabolic demand in the body, and it relies on high ATP turnover and efficient energy substrate utilisation in order to function normally. The derangement of myocardial energetics may lead to abnormalities in cardiac metabolism, which herald the symptoms of heart failure (HF). In addition, phosphorus magnetic resonance spectroscopy (31P MRS) is the only available non-invasive method that allows clinicians and researchers to evaluate the myocardial metabolic state in vivo. This review summarises the importance of myocardial energetics and provides a systematic review of all the available research studies utilising 31P MRS to evaluate patients with a range of cardiac pathologies. Materials and Methods: We have performed a systematic review of all available studies that used 31P MRS for the investigation of myocardial energetics in cardiovascular disease. Results: A systematic search of the Medline database, the Cochrane library, and Web of Science yielded 1092 results, out of which 62 studies were included in the systematic review. The 31P MRS has been used in numerous studies and has demonstrated that impaired myocardial energetics is often the beginning of pathological processes in several cardiac pathologies. Conclusions: The 31P MRS has become a valuable tool in the understanding of myocardial metabolic changes and their impact on the diagnosis, risk stratification, and prognosis of patients with cardiovascular diseases.
Keywords: phosphorus magnetic resonance spectroscopy (31P MRS), myocardial energetics, cardiovascular disease, cardiovascular imaging, cardiac magnetic resonance imaging
1. Introduction
Myocardial energetics represent one of the most important biochemical processes in the human body. A heart that functions well also has a normal cardiac metabolic profile. Derangement of this metabolic profile heralds the pathophysiological processes that subsequently lead to heart failure symptoms. This “engine out of fuel,” as the failing heart has previously been described [1], needs to be diagnosed appropriately and in a timely fashion, but even more importantly, the mechanism behind this failure needs to be understood.
The correlation of biochemical pathways with metabolic function and energetic state in active tissues is a challenging project that researchers have acknowledged since the late 1970s [2]. At the time, the very first studies using 31-phosphorus magnetic resonance spectroscopy (31P MRS) began. These were ex vivo studies that, given the limitations of spatial localization techniques, focused on the energetics of animal hearts [2,3,4,5]. However, with the advancement of surface coils and improvements in spatial localization techniques, the first in vivo studies of human hearts emerged [6,7]. Since then, 31P MRS has significantly contributed to clinical research, and by providing invaluable insights on cardiac energetics, it has become a powerful non-invasive tool that enables the study of the most metabolically demanding organ in vivo, the heart.
This comprehensive review examines the link between cardiac energetics and cardiovascular disease and the important role that 31P MRS plays in the thorough assessment of this link. It also provides a systematic review of the available studies in the literature that have used 31P MRS for the assessment of cardiovascular diseases in humans and the prognostic implications of their findings.
2. Materials and Methods
2.1. Search Strategy
We have performed a systematic review of all available studies that used 31P MRS for the investigation of myocardial energetics in cardiovascular disease. The two independent investigators (V.T. and G.B.) performed a systematic search in the Medline database, the Cochrane library, and Web of Science from inception to 30 September 2022. No limitations were used in the search strategy. The following algorithm was used to retrieve all relevant studies for “cardiovascular disease AND (31P spectroscopy OR phosphorus spectroscopy)”.
2.2. Eligibility Criteria
We considered eligible all studies that used 31P MRS for the investigation of myocardial energetics in cardiovascular disease. In addition, we excluded studies that were conducted in animals or ex vivo.
2.3. Data Collection Process
The following data were extracted for each included study: publication data (first author, year of publication), patient characteristics (number of patients, mean age, gender, type of cardiomyopathy), and the reported outcomes.
3. Results
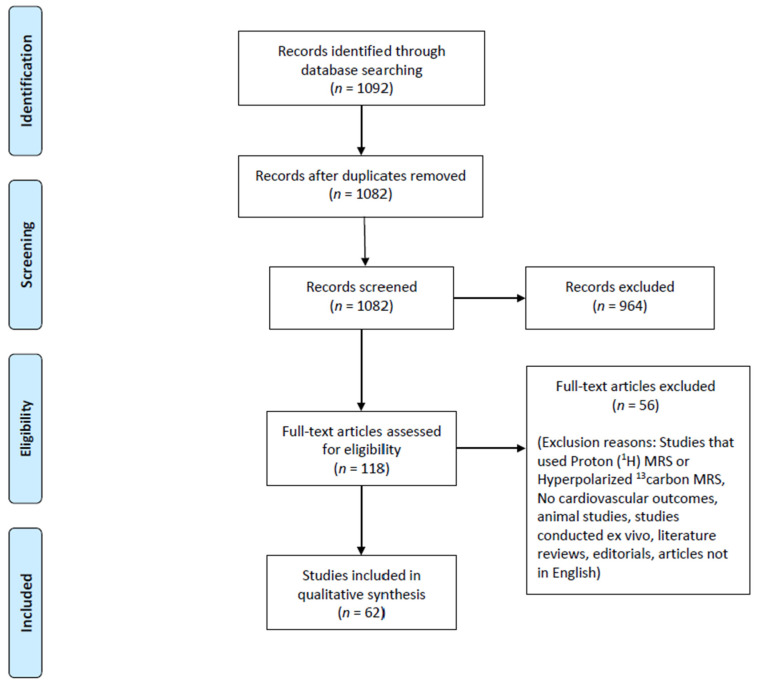
Our search strategy returned 1092 possible relevant studies. Of them, 10 studies were excluded as duplicate records, 964 studies were excluded at the title/abstract level, while 56 studies were excluded at the full-text level. As a result, 62 studies were finally included in the review. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram of the study population process is shown in Figure 1. Table 1 provides a detailed description of all the included studies, their study population cohorts, the cardiovascular diseases examined, and their major outcomes.
Figure 1.
PRISMA flow diagram of the study selection process.
Table 1.
Table of studies included in the systematic review and their characteristics.
| Study | Year | Participants | Major Findings |
|---|---|---|---|
| Myocardial Infarction (MI) and Ischaemic Cardiomyopathy (ICM) | |||
| Weiss et al. [8] | 1990 | 11 healthy controls 16 patients with CAD, of which 5 patients were re-examined after successful revascularisation 9 patients with non-ischaemic heart disease |
31P NMR spectra recorded from the anterior myocardium before, during and after isometric hand-grip exercise
|
| Hardy et al. [9] | 1991 | 12 healthy controls 9 patients with ICM 11 patients with NICM |
|
| Neubauer et al. [10] | 1992 | 19 healthy controls 14 patients with CAD 19 patients with DCM |
|
| Mitsunami et al. [11] | 1992 | 9 healthy controls 12 patients with old MI |
|
| Yabe et al. [12] | 1994 | 27 patients with CAD (severe LAD) 11 healthy controls |
|
| Yabe et al. [13] | 1995 | 41 patients with CAD (severe LAD) 11 healthy controls |
|
| Kalil-Filho et al. [14] | 1997 | 8 healthy controls 29 patients with MI and successful reperfusion within 6 h of onset of chest pain |
|
| Beer et al. [15] | 2000 | 15 patients post MI (3 weeks post MI) 10 healthy controls |
|
| Buchthal et al. [16] | 2000 | 35 women with normal coronary angiograms hospitalised for chest pain |
|
| Beer et al. [17] | 2004 | 8 patients with subacute inferior wall MI |
|
| Bottomley et al. [18] | 2009 | 15 patients with MI 15 healthy controls |
|
| Dilated Cardiomyopathy (DCM) | |||
| Schaefer et al. [6] | 1990 | 14 healthy controls 11 patients with LVH, 6 patients with DCM |
|
| Schaefer et al. [19] | 1992 | 7 normal controls 8 patients with DCM (EF < 30%) |
Subjects underwent dobutamine stress test
|
| de Roos et al. [20] | 1992 | 9 healthy controls 9 patients with DCM 8 patients with HCM |
|
| Neubauer et al. [21] | 1995 | 14 healthy controls 23 patients with DCM |
|
| Neubauer et al. [22] | 1997 | 39 patients with DCM (EF < 50%) |
|
| Beer et al. [23] | 2002 | 10 healthy controls 10 patients with DCM |
|
| Weiss et al. [24] | 2005 | 16 healthy controls 16 patients with heart failure |
|
| Hansch et al. [25] | 2005 | 20 healthy volunteers 25 patients with DCM (15 with LVEF < 30% and severe symptoms, 10 with LVEF > 30% and moderate symptoms) |
|
| Chida et al. [26] | 2005 | 20 patients with global myocardial dysfunction (13 DCM, 3 HCM, 3 HHD, 1 AR) |
|
| Bottomley et al. [27] | 2013 | 17 healthy subjects 58 patients with NICM |
|
| Dass et al. [28] | 2012 | 12 healthy 28 patients with HCM 18 patients with DCM |
|
| Dass et al. [29] | 2015 | 12 healthy volunteers 14 patients with DCM |
|
| Schär et al. [30] | 2015 | 12 healthy controls 17 patients with HF (LVEF < 40%) |
|
| Stoll et al. [31] | 2016 | 10 healthy controls 25 patients with DCM |
|
| Gabr et al. [32] | 2018 | 14 healthy volunteers 27 patients with mild-to-moderate HF (EF ≤ 45%) |
|
| Rayner et al. [33] | 2022 | 26 healthy controls 16 patients with DCM 27 patients with DCM and obesity |
|
| Hypertrophic Cardiomyopathy (HCM) | |||
| Sakuma et al. [34] | 1993 | 6 healthy controls 19 patients with HCM |
|
| Sieverding et al. [35] | 1997 | 16 healthy controls 13 patients with HCM |
|
| Jung et al. [36] | 1998 | 11 healthy controls 14 patients with HCM |
|
| Okada et al. [37] | 1998 | 30 healthy control subjects 10 patients with HCM |
|
| Grilley et al. [38] | 2003 | 24 healthy controls 31 patients with HCM mutation positive (beta-myosin heavy chain, cardiac troponin T, or myosin-binding protein C) |
|
| Shivu et al. [39] | 2008 | 37 healthy volunteers 26 patients with hypertrophic cardiomyopathy (HCM) |
|
| Esposito et al. [40] | 2009 | 19 healthy controls 19 patients with HCM |
|
| Dass et al. [28] | 2012 | 12 healthy controls 28 patients with HCM 18 patients with DCM |
|
| Abraham et al. [41] | 2013 | 17 healthy controls 9 patients with HCM with point mutation (Arg403Gln) in the cardiac β-myosin heavy-chain (MHC) gene |
|
| Dass et al. [42] | 2015 | 20 healthy volunteers 35 patients with HCM |
|
| Valkovic et al. [43] | 2019 | 10 healthy controls 3 patients with HCM |
|
| Heart Failure with Preserved Ejection Fraction (HFpEF), Left ventricular hypertrophy, Hypertensive Heart Disease (HHD), Diabetic Cardiomyopathy, Obesity | |||
| Okada et al. [37] | 1998 | 30 healthy control subjects 8 patients with HHD |
|
| Lamb et al. [44] | 1999 | 11 patients with HTN 13 healthy controls |
|
| Beer et al. [23] | 2002 | 10 healthy controls 10 patients with HHD |
|
| Heyne et al. [45] | 2006 | 20 healthy controls 36 patients with HHD (11 systolic dysfunction) |
|
| Smith et al. [46] | 2006 | 14 healthy controls 20 patients with LVH (10 of which LVH + CHF) |
|
| Phan et al. [47] | 2009 | 20 controls 37 patients with HFpEF |
|
| Levelt et al. [48] | 2016 | 20 healthy controls 46 patients with DMII |
|
| Rayner et al. [49] | 2020 | 80 volunteers (35 controls, 45 obese) |
|
| Burrage et al. [50] | 2021 | 11 healthy controls 32 patients with diastolic dysfunction and HFpEF (9 with DMII, 14 with HFpEF, 9 with severe diastolic dysfunction attributable to cardiac amyloidosis) |
|
| Thirunavukarasu et al. [51] | 2021 | Empagliflozin on DMII 18 DMII 12 weeks |
|
| Rayner et al. [33] | 2022 | 26 healthy controls 16 patients with DCM 27 patients with DCM and obesity |
|
| Valvular Heart Disease | |||
| Conway et al. [52] | 1991 | 13 healthy controls 8 patients with AS, 8 patients with AR (out of which 6 had HF symptoms, for which they were on medical treatment) |
|
| Neubauer et al. [53] | 1997 | 13 patients with AS 9 patients with AR |
|
| Conway et al. [54] | 1998 | 13 healthy controls 22 patients with chronic MR |
|
| Beer et al. [23] | 2002 | 10 healthy controls 10 patients with aortic stenosis (AS) |
|
| Mannacio et al. [55] | 2012 | 30 patients post AVR (Medtronic Mosaic bioprosthesis) 15 with PPM, 15 without PPM |
|
| Mahmod et al. [56] | 2014 | 15 healthy controls 28 patients with severe AS |
|
| Peterzan et al. [57] | 2020 | 30 healthy 13 patients with moderate AS 37 patients with severe AS and EF ≥ 55% 15 patients with severe AS and EF < 55% 7 pressure-loaded heart biopsies |
|
| Heart transplant | |||
| Evanochko et al. [58] | 2002 | 11 healthy controls 25 heart transplant patients |
|
| Caus et al. [59] | 2006 | 9 healthy controls 8 patients with CAV (cardiac allograft vasculopathy) 18 patients without CAV |
|
| Pharmaceutical Interventions and Research Trials | |||
| Lee et al. [60] | 2005 | Double blind RCT in patients with CHF 28 patients received placebo 28 patients received perhexiline (antianginal drug that augments glucose metabolism by blocking muscle mitochondrial free fatty acid uptake and thereby increasing metabolic efficiency) |
|
| Fragasso et al. [61] | 2006 | 12 patients with HF randomized in a double-blind, cross-over study to Placebo or trimetazidine (20 mg t.i.d.) for two periods of 90 days |
|
| Hirsch et al. [62] | 2012 | Double blind randomised placebo-control study 16 patients with NICM randomised to allopurinol (with a ratio 4:1) |
|
| Spoladore et al. [63] | 2013 | 10 HF patients had beta blocker (bisoprolol or carvedilol) |
|
| Beadle et al. [64] | 2015 | Double blind randomised placebo-control study 50 patients with NICM (symptomatic and LVEF < 40%) randomised to placebo (25) and perhexiline (25) |
|
| Thirunavukarasu et al. [51] | 2021 | Empagliflozin for 18 patients with DMII for 12 weeks |
|
| Additional Studies | |||
| Leme et al. [65] | 2013 | 28 patients with Chagas disease 8 healthy controls |
|
| Solaiyappan et al. [66] | 2019 | 109 patients with NICM (38 DCM, 32 HCM) 15 patients with CAD 45 healthy controls |
|
AS aortic stenosis; AR aortic regurgitation; AVR aortic valve replacement; CAD Coronary Artery Disease; CAV Cardiac allograft vasculopathy; CHF Congestive Heart Failure; DMII Diabetes Mellitus Type II; EF ejection fraction; HCM Hypertrophic cardiomyopathy; HHD Hypertensive Heart Disease; ICM Ischaemic Cardiomyopathy; LAD (Left Anterior Descending Artery); LVH (Left Ventricular Hypertrophy); MI (Myocardial Infarction); MR (Mitral Regurgitation); NICM (Non-ischaemic Cardiomyopathy); PPM (Patient-Prosthesis Mismatch)
4. Discussion
4.1. Myocardial Energetics
The heart has been described as the organ with the highest metabolic demand, consuming 8% of the total body adenosine triphosphate (ATP) [67,68]. Considering that the heart only accounts for ~0.5% of the total body weight, it is estimated that it uses 20 to 30 times its weight in ATP to maintain its metabolic homeostasis [68,69]. The cardiac metabolic phenotype is not only highly demanding but also quite dynamic, as its function depends on the continuous production of ATP. If this copious and uninterrupted production of ATP is not maintained, then the heart would be deprived of energy within less than 10 s [67,70].
The healthy adult heart primarily uses free fatty acids and glucose as its main energy substrates [71,72,73], while the rest of the energy requirements are fulfilled by alternative energy substrates such as lactate, ketones, and amino acids [67,70]. The alterations in cardiac energy demands and availability of the substrates lead to shifts in energy substrate utilisation, a feature described as “metabolic flexibility” [70,71]. This reflects the ability of the heart to switch to different energy substrates in order to maintain its requirements [70,71].
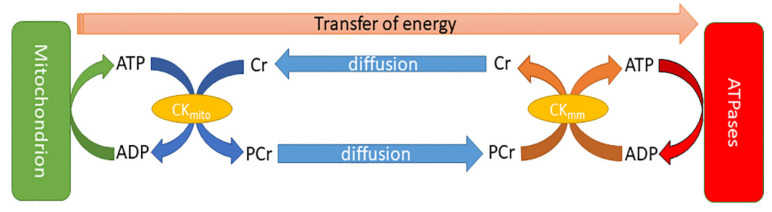
In order to meet these high metabolic demands, the adult heart uses the above energy substrates to generate ample amounts of ATP, mainly through mitochondrial oxidative phosphorylation, which makes up to 95% of myocardial ATP, while a smaller amount is generated through glycolysis [70,71]. The mitochondrial oxidative phosphorylation has a key role in the generation, transfer, and utilisation of ATP by the myofibrils of the cardiomyocytes. In addition, through the creatine kinase (CK) “shuttle” system, high-energy phosphate is transferred from the ATP produced in the mitochondria to creatine (Cr), generating in this way phosphocreatine (PCr) and adenosine diphosphate (ADP). The PCr, a much smaller molecule than ATP, is then rapidly diffused from the mitochondria to the sarcomeres that comprise the myofibrils. In the myofibrils, CK catalyses the formation of ATP and Cr. The ATP is then used by the ATPases and contributes to cell contraction, while Cr is diffused back into the mitochondria [1,74] (Figure 2).
Figure 2.
Schematic of energy transfer through the cell. Reproduced with permission from Watson et al. [75] under a Creative Commons Attribution 4.0 International License.
The PCr is the primary energy reserve metabolite in the heart, with its concentration being twice that of ATP phosphorylation [76]. However, through the CK-mediated reaction, ATP production with the use of PCr is ~10 times faster than the rate of ATP production by oxidative phosphorylation [77]. In situations of high metabolic demand, when the rate of ATP use exceeds that of its production, the PCr/CK system therefore works as a buffer to maintain homeostasis, as the use of PCr via the CK reaction/catalysis helps in maintaining ATP at a stable normal level of phosphorylation [1,76]. Indeed, in heart failure—regardless of the aetiology—the PCr level falls in order to help maintain the ATP at a normal level [1]. While this may seem like an appropriate compensatory mechanism, it is certainly not sufficient to maintain the high metabolic demand of the cardiomyocytes. In a disease state, the failing heart is much less energy efficient, as mitochondrial dysfunction and shifts in energy substrate utilisation lead to a significant reduction in ATP production [73,78]. Additionally, PCr has been shown to decrease in pathological cardiac hypertrophy and failure as a result of the significant ATP supply-demand mismatch and partly due to a loss of creatine levels [76]. This is followed by a progressive ATP decrease [74,77]. It is therefore clear that a change in the PCr or ATP levels, or the ratio PCr/ATP, signifies a metabolic derangement that proclaims the failure of the heart. In the failing myocardium, the decrease in creatine levels occurs earlier and is faster than the reduction in ATP levels [77,79,80]. The ATP level is ~30% lower than in the normal myocardium, with the respective reduction of creatine levels being up to 50–70% in severely failing myocardium [77,80]. As creatine, PCr, and ATP levels all reduce significantly, it is likely that the PCr/ATP ratio underestimates the severity of the PCr decrease [77,79]. In addition, although previous studies have shown that the PCr/ATP ratio in the healthy myocardium is maintained, there is evidence to suggest that stress may lead to its reduction even in the absence of any underlying cardiac pathology [33,81].
4.2. Assessment of Myocardial Energetics with 31P MRS
The 31P MRS is a non-invasive technique that provides unique insights into the abovementioned intracellular parameters through the detection of phosphorus-containing metabolites. It therefore allows an accurate evaluation of the myocardial energetic state through measurement of the myocardial PCr/ATP ratio as well as absolute levels of high-energy phosphates [82,83]. On most magnetic resonance imaging (MRI) scanners, specialised scanner software and additional hardware, including a broadband amplifier, receive system, and radiofrequency (RF) coils, are needed to permit the acquisition of 31P MRS data [84]. In addition, for optimised data collection and correct metabolite quantification, the RF excitation pulse bandwidth must be large enough to excite all the relevant metabolites homogeneously [84].
The excitation of 31P nuclei with RF signals is required for 31P MRS. However, in following the excitation of the nuclei, the induced signal is recorded in the receiver coils of the scanner after the RF energy is switched off [85]. The amplitude of the received signal reflects the number of nuclei present in the interrogated tissue [85]. The resonance frequency of the nuclei being interrogated depends on their chemical environment; a phenomenon described as ‘chemical shift’ [75,85]. This is a result of different nuclei being surrounded by a different number and unique spatial location of the surrounding electrons (i.e., different shielding environments) [75,85]. Further, although the resonance frequency depends on the strength of the magnetic field, the chemical shift, as quantified in parts per million (ppm), does not alter.
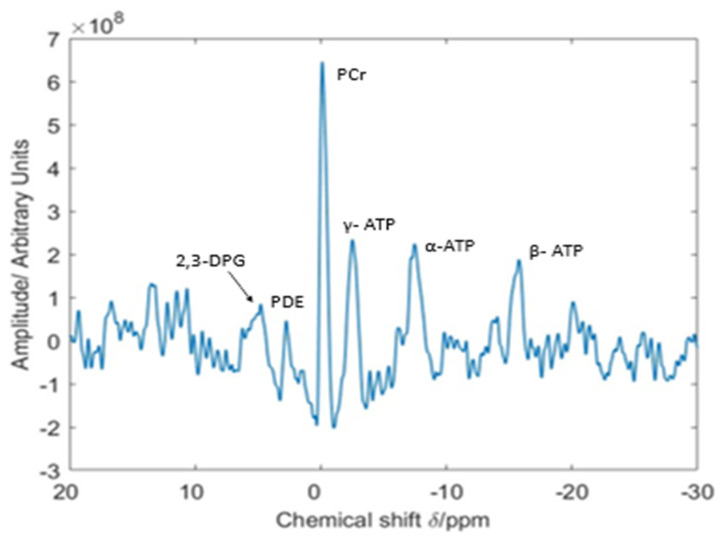
The typical 31P cardiac spectrum demonstrates peaks for phosphocreatine and the three phosphorus nuclei of ATP (γ-ATP, α-ATP, and β-ATP). The area under the peak is proportional to the relative concentrations of these metabolites [1] (Figure 3).
Figure 3.
Example of a typical 31P cardiac spectrum demonstrating peaks for PCr, 2,3-DPG, PDE, and the three phosphorus nuclei of ATP (γ-ATP, α-ATP, and β-ATP). Image courtesy of the University of East Anglia. 2,3-DPG, 2,3-Diphosphoglycerate; PDE, phosphodiesters; PCr, phosphocreatine; ATP, adenosine triphosphate.
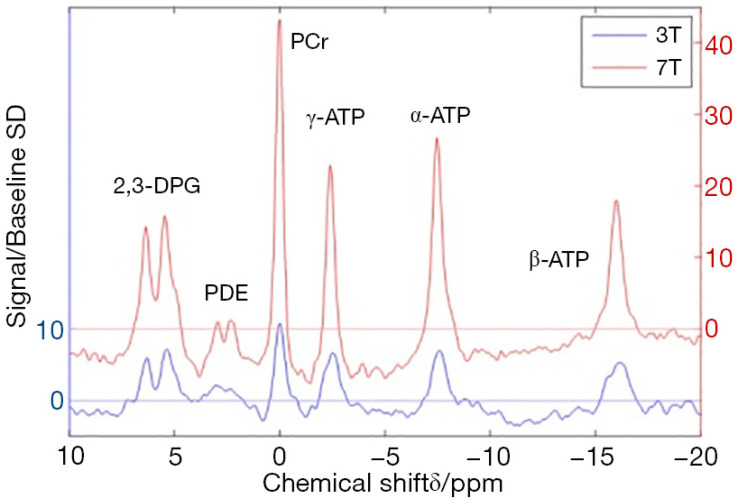
The cardiac 31P MRS is typically performed on MR scanners with a field strength of 1.5 or 3 Tesla(T), although studies using a higher field strength of 7T have been reported [86,87]. As the field strength increases, the frequencies, as reflected by the spectral peaks, are more separated and therefore better quantified [75,87] (Figure 4).
Figure 4.
Comparison of cardiac 31Phosphorus spectra acquired from the same individual at 3 and 7 Tesla field strengths real part of the spectrum from a voxel in the middle of the interventricular septum. The spectra were apodized with an exponential filter (matched to the fitted PCr linewidth), first order phase corrected, and normalized to the resulting baseline noise standard deviation. Higher magnetic field yields higher signal-to-noise ratio (SNR). The SNR is markedly higher for the 7T data. Note that the y-axes are offset for clarity. Reproduced with permission from Rodgers et al. [87] under a Creative Commons Attribution 4.0 International License. SD: standard deviation; 2,3-DPG, 2,3-Diphosphoglycerate; PDE, phosphodiesters; PCr, phosphocreatine; ATP, adenosine triphosphate; ppm, parts per million.
A cardiac 31P spectrum can be obtained using a variety of techniques that help in the identification of signals from a region of interest. In brief, these include the following: (a) Depth-resolved surface coil spectroscopy (DRESS): a single slice parallel to the coil is selectively excited by an MRI gradient. (b) Image selected in-vivo spectroscopy (ISIS): data are acquired using data from inversion pulses from consecutive cycles, and voxel localisation is achieved using a slice-based intersection strategy. (c) One-, two- and three- dimensional (1D, 2D, and 3D) chemical shift imaging (CSI): this technique uses phase encoding for spatial localisation, and the spectra can be acquired in a column of voxels (1D), a plane (2D), or a block (3D) of voxels [75,88,89]. The CSI has seen more widespread use in recent years, and is often applied with saturation bands to minimise 31P contributions from skeletal muscle and the liver [75,89].
Using one or a combination of the aforementioned techniques, a localised cardiac 31P spectrum can be acquired. However, through analysis of this spectrum, the PCr/ATP ratio can be determined, allowing in this way an evaluation of the cardiac energetic state. In addition, the cellular pH (from the chemical shift of inorganic phosphate (Pi) relative to PCr) can be indirectly deduced from the acquired spectra [20,85,90]. Apart from absolute measurements, cardiac 31P MRS can also provide a unique insight into dynamic changes in the cellular metabolism through assessment of the CK flux and the rate of ATP generation [18,24,30]. Furthermore, by the measurement of the CK flux, the pseudo–first-order unidirectional rate constant (kf) of CK in the ATP-generating (forward) direction is first measured. This is then multiplied by the PCr/ATP ratio to give the forward CK flux, i.e., the ATP delivery rate [24,57].
The major limitation of 31P MRS is its intrinsically low SNR (approximately 105-fold lower than 1H-MRS), which is typically compensated via coarser spatial and temporal resolutions [85,87,89]. This is primarily because of the low concentrations of the metabolites being studied compared to water [85,87,89]. This drawback is reflected in longer acquisition times that may be up to approximately 30 min [87,89]. Nevertheless, the disadvantage of the low SNR can be mitigated with the use of higher stronger magnetic fields, such as 7T, if available [31,87].
4.3. The Role of 31P MRS in the Evaluation of Cardiovascular Diseases
Numerous studies have shown that, in a cardiovascular disease state, there is significant impairment of energy production mediated by mitochondrial dysfunction, alterations in energy substrate utilisation, and impaired ATP transfer and utilisation [1,68]. The 31P MRS is the only technique available with the ability to study this pathological alteration in cellular metabolism in vivo, which is reflected in changes in cellular ATP and PCr concentrations, the PCr/ATP ratio, as well as the rate of ATP production as reflected by the CK flux. The research community has provided promising evidence that the role of 31P MRS is crucial in the early detection of pathological changes of the myocardial energetics through an exponential growth of clinical research studies over the last four decades.
4.3.1. Myocardial Infarction (MI) and Ischaemic Cardiomyopathy (ICM)
The cardiac energy metabolism appears to be significantly impaired both in the infarcted and in the ischaemic myocardium. Following myocardial infarction, the concentrations of both PCr and ATP metabolites fall significantly [13,18]. In addition, the CK ATP supply, reflected by the CK flux, is also significantly reduced, which is likely attributable to myocyte loss [18]. The PCr/ATP ratio is very much dependent on the presence or absence of myocardial ischaemia and heart failure secondary to ischaemic cardiomyopathy. In a study that included 27 patients with severe left anterior descending artery (LAD) disease, Yabe et al. found that the PCr/ATP ratio was significantly reduced in those with reversible ischaemia compared to those with fixed defects or healthy volunteers [12]. Weiss et al. also demonstrated that the PCr/ATP ratio significantly decreased during isometric hand-grip exercise in patients with significant coronary artery disease [8]. Interestingly, the PCr/ATP ratio was not reduced in the patients that underwent successful revascularisation, suggesting the normalisation of the metabolic parameters after a timely successful clinical intervention and normalisation of the blood supply [8]. This is in keeping with another study in which 15 patients underwent 31P MRS 3 weeks post MI and the PCr/ATP ratio was found to be normal in viable myocardium [15].
There is only one study that investigated the potential changes in myocardial energetics in patients with normal epicardial coronary arteries. Buchthal et al. recruited 35 female patients who had been admitted to the hospital with cardiac chest pain and had normal invasive coronary angiograms. A total of Seven of the 35 women (20%) had significantly reduced cardiac PCr/ATP ratios during handgrip exercise, suggesting potentially significant abnormal myocardial metabolism in this population [16].
4.3.2. Dilated Cardiomyopathy (DCM)
The DCM exhibits impaired myocardial energetics, with reduced PCr and ATP concentrations, and PCr/ATP ratios [10,21,22,23,25,26,33]. The PCr/ATP ratio has attracted a lot of research interest in this population. However, with both the PCr and ATP metabolites being significantly reduced simultaneously, it has been noted that a seemingly mild reduction in the PCr/ATP ratio may underestimate the true impairment of myocardial metabolism [23]. Despite this weakness, it has been shown to have potentially strong implications for risk stratification and prognosis. More specifically, there is evidence suggesting that the PCr/ATP ratio has a strong correlation with the clinical severity of heart failure, as estimated by the New York Heart Association (NYHA) class, as well as the left ventricular ejection fraction (LVEF) [21,23,25]. In a study that included 39 patients with DCM, Neubauer et al. demonstrated that the PCr/ATP ratio is a significant predictor of cardiovascular mortality [53]. The CK flux is also not only markedly reduced in DCM, but it also carries important prognostic implications. In a study that included 58 patients, Bottomley et al. found that reduced myocardial CK flux was a significant predictor of all-cause mortality and HF outcomes, even after correction for NYHA class, LVEF, and race [27].
4.3.3. Hypertrophic Cardiomyopathy (HCM)
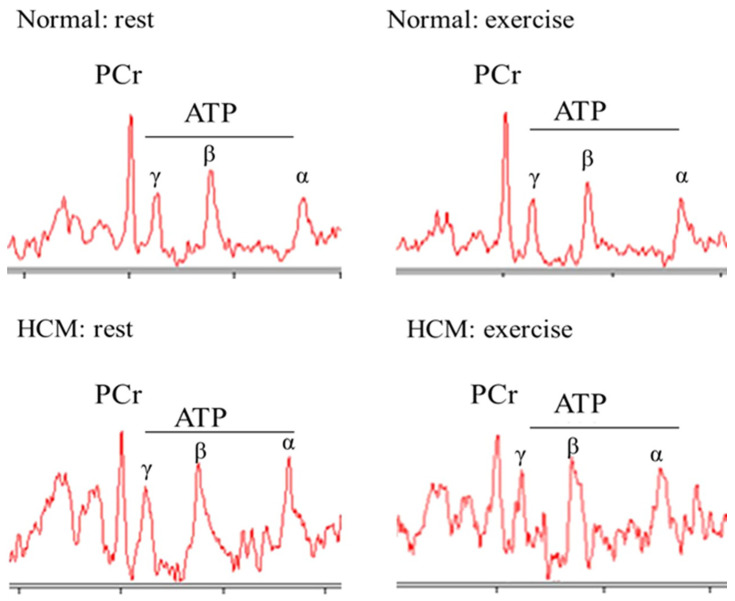
The PCr/ATP ratio is significantly reduced in patients with HCM, highlighting the abnormal myocardial metabolic changes in this population too. Early studies from almost three decades ago demonstrated unanimously that HCM is associated with impaired myocardial energetics [20,34,37,90]. Consequently, following those early studies, research focused on specific mutations associated with the disease. In a study that included 31 patients with HCM positive for 3 mutations (beta-myosin heavy chain, cardiac troponin T, and myosin-binding protein C), Grilley et al. found that the PCr/ATP ratio was significantly reduced, and the reduction was of similar magnitude in all three disease-gene groups [38]. Another study that included 9 patients with a point mutation (Arg403Gln) in the beta-myosin heavy chain gene found significantly reduced PCr concentrations and CK flux while the PCr/ATP ratio showed a trend towards significance [41]. Furthermore, during exercise there is a pathological reduction of the PCr/ATP ratio in patients with HCM, a finding that may explain the exercise-related diastolic dysfunction in these patients [42] (Figure 5). In addition to the above, the PCr/ATP ratio has been shown to have a significant correlation with other imaging parameters derived from cardiac magnetic resonance (CMR) imaging, including T1 values and late gadolinium enhancement [28,40].
Figure 5.
Examples of spectra in normal and hypertrophic cardiomyopathy. Reproduced with permission from Dass et al. [42] under a Creative Commons Attribution 4.0 International License.
4.3.4. Heart Failure with Preserved Ejection Fraction (HFpEF), Hypertensive Heart Disease (HHD), Diabetic Cardiomyopathy, and Obesity
Despite the complex and heterogeneous cohort of patients that comprise the clinical entity of HFpEF, the myocardial energetics display a uniform impairment, reflected by a significant reduction in the PCr/ATP ratio [47,50]. In addition, there is evidence suggesting that this ratio is significantly correlated with the log N-terminal pro b-type natriuretic peptide (NT-proBNP), the echocardiographic e/E’, the NYHA class, and the exercise induced pulmonary congestion that occurs in these patients [50]. Significantly reduced PCr/ATP ratios are also present in diabetic cardiomyopathy [48,51] and in hypertensive heart disease associated with systolic dysfunction [26,45]. Similarly, obesity is associated with impairment of myocardial energetics with a reduced PCr/ATP ratio at rest and failure of CK flux to increase during increased workload [49]. Weight loss appears to ameliorate the dysfunction of the cellular metabolism as it leads to an increase in the PCr/ATP ratio as an well as increase in CK flux during increased workload [49].
4.3.5. Valvular Cardiomyopathy
Both aortic and mitral valve disease have been shown to have a significant impact on the myocardium [91]. This is depicted in the cardiac metabolic profile of these patients as well. More specifically, PCr/ATP is significantly decreased in symptomatic patients with aortic valve disease, and it improves post aortic valve replacement [23,52,53,55,56]. Notably, in a study that included 65 patients with aortic stenosis, Peterzan et al. found that the PCr/ATP ratio and CK flux were significantly reduced both in patients with moderate aortic stenosis, as well as those with severe aortic stenosis and normal left ventricular ejection fraction, suggesting in this way that pressure loading conditions are associated with deranged myocardial energetics even before the disease progresses to the severe stage or the presentation of symptoms [57]. In patients with mitral regurgitation, myocardial energetics are significantly impaired in symptomatic patients and in those with severe disease, while the PCr/ATP ratio is correlated with left ventricular dilatation [54].
4.3.6. Heart Transplantation
The 31P MRS has also revealed valuable insights in the pathophysiology of cardiac allografts and is a promising non-invasive tool that can efficiently assess cardiac allograft vasculopathy (CAV). In a study that included 25 heart transplant recipients, Evanochko et al. used a 31P MRS stress test and found that certain patients have abnormal cardiac energetics after transplantation, as reflected by a significant change in the PCr/ATP ratio [58]. This appeared to be unrelated to the timing of the transplantation and was present in patients with normal coronary arteries as well. The authors highlight that the 31P MRS stress test may have an important role in the diagnosis of CAV as it is a much more sensitive means of assessing the microvasculature [58]. Similarly, Caus et al. suggest that 31P MRS is a promising tool able to detect impairment of myocardial energetics related to CAV, with a PCr/ATP value of 1.59 being their proposed optimal cut-off value to predict CAV with specificity and sensitivity of 100% and 72%, respectively [59]. It has to be noted, however, that 31P MRS is not standard as yet; hence, such cut-offs only apply to the particular methodology and the specific MRI scanner used in this study.
4.3.7. Cardiovascular Research and Future Directions
As noted above, the metabolism has a central role in the pathogenesis of a range of cardiovascular diseases. As such, medical therapies targeted at the metabolic pathways that have a key role in these diseases are extremely beneficial for the patients. This has been shown for several medications that have been proven to be not only of symptomatic but also of prognostic benefit. For example, beta blockers have been shown to increase the PCr/ATP ratio by 33% in patients with heart failure after only 3 months of treatment [63], and, similarly, perhexiline has also been shown to improve the PCr/ATP ratio by 30% and the left ventricular systolic function [64]. More recently, sodium-glucose co-transporter-2 inhibitors (SGLT2i) have shown an impact on mortality and morbidity in patients with heart failure [92]. As research focuses on their mechanism of action, there is evidence suggesting that they alter myocardial energetics and improve cellular metabolism [93], as recent evidence reveals their positive impact on the PCr/ATP ratio [51].
The cardiovascular research has now shifted to treatments that focus on the improvement of myocardial energetics and metabolism, which play a pivotal role in the pathogenesis of several cardiovascular diseases. The 31P MRS will continue to have an instrumental part in this journey as it provides a non-invasive and accurate evaluation of the cardiac metabolic profile that helps researchers gain important insights into the complex pathophysiology of cardiac disorders. The current limiting factors in the widespread use of 31P MRS include the requirement of both expensive hardware and clinical expertise. Nevertheless, as research emphasises the importance of myocardial energy reserve in the pathophysiology of disease and pharmacotherapy, these issues will gradually recede.
5. Conclusions
The myocardial energetic compromise has proved to be an important feature in the pathophysiological process of several conditions. The 31P MRS is the only available non-invasive technique with the capacity to quantify metabolism in vivo, and it is, therefore, an excellent tool in the evaluation of the myocardial metabolic profile of patients with cardiac pathologies. Future studies assessing myocardial energetic phenotypes and how these are associated with clinical outcomes and prognosis will help further in understanding the impact of altered metabolism in clinical practice.
Author Contributions
V.T. executed the systematic review and wrote the first draft. D.C. and R.S. significantly amended the manuscript. G.B. executed a systematic review and significantly amended the manuscript. V.S.V. planned and designed the study, supervised the systematic review, and significantly amended the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study as this is a meta-analysis of already published data.
Informed Consent Statement
Patient consent was waived as this is a meta-analysis of already published data.
Data Availability Statement
All data are available from the corresponding author on request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Neubauer S. The Failing Heart—An Engine Out of Fuel. N. Engl. J. Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 2.Jacobus W.E., Taylor G.J., IV, Hollis D.P., Nunnally R.L. Phosphorus nuclear magnetic resonance of perfused working rat hearts. Nature. 1977;265:756–758. doi: 10.1038/265756a0. [DOI] [PubMed] [Google Scholar]
- 3.Hollis D.P., Nunnally R.L., Taylor G.J., Weisfeldt M.L., Jacobus W.E. Phosphorus nuclear magnetic resonance studies of heart physiology. J. Magn. Reson. (1969) 1978;29:319–330. doi: 10.1016/0022-2364(78)90156-7. [DOI] [Google Scholar]
- 4.Pieper G.M., Todd G.L., Wu S.T., Salhany J.M., Clayton F.C., Eliot R.S. Attenuation of myocardial acidosis by propranolol during ischaemic arrest and reperfusion: Evidence with 31P nuclear magnetic resonance. Cardiovasc. Res. 1980;14:646–653. doi: 10.1093/cvr/14.11.646. [DOI] [PubMed] [Google Scholar]
- 5.Ackerman J.J.H., Grove T.H., Wong G.G., Gadian D.G., Radda G.K. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980;283:167–170. doi: 10.1038/283167a0. [DOI] [PubMed] [Google Scholar]
- 6.Schaefer S., Gober J.R., Schwartz G.G., Twieg D.B., Weiner M.W., Massie B. In vivo phosphorus-31 spectroscopic imaging in patients with global myocardial disease. Am. J. Cardiol. 1990;65:1154–1161. doi: 10.1016/0002-9149(90)90331-T. [DOI] [PubMed] [Google Scholar]
- 7.Bottomley P.A. Noninvasive Study of High-Energy Phosphate Metabolism in Human Heart by Depth-Resolved 31 P NMR Spectroscopy. Science. 1985;229:769–772. doi: 10.1126/science.4023711. [DOI] [PubMed] [Google Scholar]
- 8.Weiss R.G., Bottomley P.A., Hardy C.J., Gerstenblith G. Regional Myocardial Metabolism of High-Energy Phosphates during Isometric Exercise in Patients with Coronary Artery Disease. N. Engl. J. Med. 2010;323:1593–1600. doi: 10.1056/NEJM199012063232304. [DOI] [PubMed] [Google Scholar]
- 9.Hardy C.J., Weiss R.G., Bottomley P.A., Gerstenblith G. Altered myocardial high-energy phosphate metabolites in patients with dilated cardiomyopathy. Am. Hear. J. 1991;122:795–801. doi: 10.1016/0002-8703(91)90527-O. [DOI] [PubMed] [Google Scholar]
- 10.Neubauer S., Krahe T., Schindler R., Horn M., Hillenbrand H., Entzeroth C., Mader H., Kromer E.P., A Riegger G., Lackner K. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation. 1992;86:1810–1818. doi: 10.1161/01.CIR.86.6.1810. [DOI] [PubMed] [Google Scholar]
- 11.Mitsunami K., Okada M., Inoue T., Hachisuka M., Kinoshita M., Inubushi T. In Vivo 31P Nuclear Magnetic Resonance Spectroscopy in Patients with Old Myocardial Infarction. Jpn. Circ. J. 1992;56:614–619. doi: 10.1253/jcj.56.614. [DOI] [PubMed] [Google Scholar]
- 12.Yabe T., Mitsunami K., Okada M., Morikawa S., Inubushi T., Kinoshita M. Detection of myocardial ischemia by 31P magnetic resonance spectroscopy during handgrip exercise. Circulation. 1994;89:1709–1716. doi: 10.1161/01.CIR.89.4.1709. [DOI] [PubMed] [Google Scholar]
- 13.Yabe T., Mitsunami K., Inubushi T., Kinoshita M. Quantitative Measurements of Cardiac Phosphorus Metabolites in Coronary Artery Disease by 31 P Magnetic Resonance Spectroscopy. Circulation. 1995;92:15–23. doi: 10.1161/01.CIR.92.1.15. [DOI] [PubMed] [Google Scholar]
- 14.Kalil-Filho R., de Albuquerque C.P., Weiss R.G., Mocelim A., Bellotti G., Cerri G., Pileggi F. Normal High Energy Phosphate Ratios in “Stunned” Human Myocardium. J. Am. Coll. Cardiol. 1997;30:1228–1232. doi: 10.1016/S0735-1097(97)00306-9. [DOI] [PubMed] [Google Scholar]
- 15.Beer M., Sandstede J., Landschütz W., Viehrig M., Harre K., Horn M., Meininger M., Pabst T., Kenn W., Haase A., et al. Altered energy metabolism after myocardial infarction assessed by 31 P-MR-spectroscopy in humans. Eur. Radiol. 2000;10:1323–1328. doi: 10.1007/s003300000316. [DOI] [PubMed] [Google Scholar]
- 16.Buchthal S.D., Hollander J.A.D., Merz C.N.B., Rogers W.J., Pepine C.J., Reichek N., Sharaf B.L., Reis S., Kelsey S.F., Pohost G.M. Abnormal Myocardial Phosphorus-31 Nuclear Magnetic Resonance Spectroscopy in Women with Chest Pain but Normal Coronary Angiograms. N. Engl. J. Med. 2000;342:829–835. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 17.Beer M., Spindler M., Sandstede J.J., Remmert H., Beer S., Köstler H., Hahn D. Detection of myocardial infarctions by acquisition-weighted31P-MR spectroscopy in humans. J. Magn. Reson. Imaging. 2004;20:798–802. doi: 10.1002/jmri.20185. [DOI] [PubMed] [Google Scholar]
- 18.Bottomley P.A., Wu K.C., Gerstenblith G., Schulman S.P., Steinberg A., Weiss R.G. Reduced myocardial creatine kinase flux in human myocardial infarction an in vivo phosphorus magnetic resonance spectroscopy study. Circulation. 2009;119:1918–1924. doi: 10.1161/CIRCULATIONAHA.108.823187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaefer S., Schwartz G.G., Steinman S.K., Meyerhoff D., Massie B.M., Weiner M.W. Metabolic response of the human heart to inotropic stimulation:In vivo phosphorus-31 studies of normal and cardiomyopathic myocardium. Magn. Reson. Med. 1992;25:260–272. doi: 10.1002/mrm.1910250205. [DOI] [PubMed] [Google Scholar]
- 20.De Roos A., Doornbos J., Luyten P.R., Bsc L.J.M.P.O., Hollander J.A.D., van der Wall E.E. Cardiac metabolism in patients with dilated and hypertrophic cardio-myopathy: Assessment with proton-decoupled P-31 MR spectroscopy. J. Magn. Reson. Imaging. 1992;2:711–719. doi: 10.1002/jmri.1880020616. [DOI] [PubMed] [Google Scholar]
- 21.Neubauer S., Horn M., Pabst T., Godde M., Lubke D., Jilling B., Hahn D., Ertl G. Contributions of 31P-magnetic resonance spectroscopy to the understanding of dilated heart muscle disease. Eur. Hear. J. 1995;16:115–118. doi: 10.1093/eurheartj/16.suppl_O.115. [DOI] [PubMed] [Google Scholar]
- 22.Neubauer S., Horn M., Cramer M., Harre K., Newell J.B., Peters W., Pabst T., Ertl G., Hahn D., Ingwall J.S., et al. Myocardial Phosphocreatine-to-ATP Ratio Is a Predictor of Mortality in Patients With Dilated Cardiomyopathy. Circulation. 1997;96:2190–2196. doi: 10.1161/01.CIR.96.7.2190. [DOI] [PubMed] [Google Scholar]
- 23.Beer M., Seyfarth T., Sandstede J., Landschütz W., Lipke C., Köstler H., von Kienlin M., Harre K., Hahn D., Neubauer S. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with 31P-SLOOP magnetic resonance spectroscopy. J. Am. Coll. Cardiol. 2002;40:1267–1274. doi: 10.1016/S0735-1097(02)02160-5. [DOI] [PubMed] [Google Scholar]
- 24.Weiss R.G., Gerstenblith G., Bottomley P.A. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc. Natl. Acad. Sci. USA. 2005;102:808–813. doi: 10.1073/pnas.0408962102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansch A., Rzanny R., Heyne J.-P., Leder U., Reichenbach J.R., Kaiser W.A. Noninvasive measurements of cardiac high-energy phosphate metabolites in dilated cardiomyopathy by using 31P spectroscopic chemical shift imaging. Eur. Radiol. 2005;15:319–323. doi: 10.1007/s00330-004-2504-0. [DOI] [PubMed] [Google Scholar]
- 26.Chida K., Otani H., Saito H., Nagasaka T., Kagaya Y., Kohzuki M., Zuguchi M., Shirato K. Feasibility of rapid-sequence 31p magnetic resonance spectroscopy in cardiac patients. Acta Radiol. 2005;46:386–390. doi: 10.1080/02841850510021283. [DOI] [PubMed] [Google Scholar]
- 27.Bottomley P.A., Panjrath G.S., Lai S., Hirsch G.A., Wu K., Najjar S.S., Steinberg A., Gerstenblith G., Weiss R.G. Metabolic Rates of ATP Transfer Through Creatine Kinase (CK Flux) Predict Clinical Heart Failure Events and Death. Sci. Transl. Med. 2013;5:215re3. doi: 10.1126/scitranslmed.3007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dass S., Suttie J.J., Piechnik S.K., Ferreira V.M., Holloway C.J., Banerjee R., Mahmod M., Cochlin L., Karamitsos T.D., Robson M.D., et al. Myocardial Tissue Characterization Using Magnetic Resonance Noncontrast T1 Mapping in Hypertrophic and Dilated Cardiomyopathy. Circ. Cardiovasc. Imaging. 2012;5:726–733. doi: 10.1161/CIRCIMAGING.112.976738. [DOI] [PubMed] [Google Scholar]
- 29.Dass S., Holloway C.J., Cochlin L.E., Rider O.J., Mahmod M., Robson M., Sever E., Clarke K., Watkins H., Ashrafian H., et al. No Evidence of Myocardial Oxygen Deprivation in Nonischemic Heart Failure. Circ. Hear. Fail. 2015;8:1088–1093. doi: 10.1161/CIRCHEARTFAILURE.114.002169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schär M., Gabr R.E., El-Sharkawy A.-M.M., Steinberg A., Bottomley P.A., Weiss R.G. Two repetition time saturation transfer (TwiST) with spill-over correction to measure creatine kinase reaction rates in human hearts. J. Cardiovasc. Magn. Reson. 2015;17:1–11. doi: 10.1186/s12968-015-0175-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoll V.M., Clarke W.T., Levelt E., Liu A., Myerson S.G., Robson M.D., Neubauer S., Rodgers C.T. Dilated Cardiomyopathy: Phosphorus 31 MR Spectroscopy at 7 T. Radiology. 2016;281:409–417. doi: 10.1148/radiol.2016152629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabr R.E., El-Sharkawy A.-M.M., Schär M., Panjrath G.S., Gerstenblith G., Weiss R.G., Bottomley P.A. Cardiac work is related to creatine kinase energy supply in human heart failure: A cardiovascular magnetic resonance spectroscopy study. J. Cardiovasc. Magn. Reson. 2018;20:1–11. doi: 10.1186/s12968-018-0491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rayner J.J., A Peterzan M., Clarke W.T., Rodgers C.T., Neubauer S., Rider O.J. Obesity modifies the energetic phenotype of dilated cardiomyopathy. Eur. Hear. J. 2021;43:868–877. doi: 10.1093/eurheartj/ehab663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakuma H., Takeda K., Tagami T., Nakagawa T., Okamoto S., Konishi T., Nakano T. 31P MR spectroscopy in hypertrophic cardiomyopathy: Comparison with Tl-201 myocardial perfusion imaging. Am. Hear. J. 1993;125:1323–1328. doi: 10.1016/0002-8703(93)91002-V. [DOI] [PubMed] [Google Scholar]
- 35.Sieverding L., Jung W.-I., Breuer J., Widmaier S., Staubert A., van Erckelens F., Schmidt O., Bunse M., Hoess T., Lutz O., et al. Proton-Decoupled Myocardial 31P NMR Spectroscopy Reveals Decreased PCr/Pi in Patients with Severe Hypertrophic Cardiomyopathy. Am. J. Cardiol. 1997;80:34A–40A. doi: 10.1016/S0002-9149(97)00456-6. [DOI] [PubMed] [Google Scholar]
- 36.Jungab W.I., Sieverdingc L., Breuerc J., Schmidtab O., Widmaierab S., Bunseab M., van Erckelens F., Apitz J., Dietze G.J., Lutza O. Detection of Phosphomonoester Signals in Proton-Decoupled31P NMR Spectra of the Myocardium of Patients with Myocardial Hypertrophy. J. Magn. Reson. 1998;133:232–235. doi: 10.1006/jmre.1998.1454. [DOI] [PubMed] [Google Scholar]
- 37.Okada M., Mitsunami K., Inubushi T., Kinoshita M. Influence of aging or left ventricular hypertrophy on the human heart: Contents of phosphorus metabolites measured by31P MRS. Magn. Reson. Med. 1998;39:772–782. doi: 10.1002/mrm.1910390515. [DOI] [PubMed] [Google Scholar]
- 38.Crilley J.G., A Boehm E., Blair E., Rajagopalan B., Blamire A.M., Styles P., McKenna W.J., Östman-Smith I., Clarke K., Watkins H. Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J. Am. Coll. Cardiol. 2003;41:1776–1782. doi: 10.1016/S0735-1097(02)03009-7. [DOI] [PubMed] [Google Scholar]
- 39.Shivu G.N., Abozguia K., Phan T.T., Ahmed I., Henning A., Frenneaux M. 31P magnetic resonance spectroscopy to measure in vivo cardiac energetics in normal myocardium and hypertrophic cardiomyopathy: Experiences at 3T. Eur. J. Radiol. 2010;73:255–259. doi: 10.1016/j.ejrad.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 40.Esposito A., De Cobelli F., Perseghin G., Pieroni M., Belloni E., Mellone R., Canu T., Gentinetta F., Scifo P., Chimenti C., et al. Impaired left ventricular energy metabolism in patients with hypertrophic cardiomyopathy is related to the extension of fibrosis at delayed gadolinium-enhanced magnetic resonance imaging. Heart. 2008;95:228–233. doi: 10.1136/hrt.2008.142562. [DOI] [PubMed] [Google Scholar]
- 41.Abraham M.R., Bottomley P.A., Dimaano V.L., Pinheiro A., Steinberg A., Traill T.A., Abraham T.P., Weiss R.G. Creatine Kinase Adenosine Triphosphate and Phosphocreatine Energy Supply in a Single Kindred of Patients With Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2013;112:861–866. doi: 10.1016/j.amjcard.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dass S., Cochlin L.E., Suttie J.J., Holloway C.J., Rider O.J., Carden L., Tyler D.J., Karamitsos T.D., Clarke K., Neubauer S., et al. Exacerbation of cardiac energetic impairment during exercise in hypertrophic cardiomyopathy: A potential mechanism for diastolic dysfunction. Eur. Hear. J. 2015;36:1547–1554. doi: 10.1093/eurheartj/ehv120. [DOI] [PubMed] [Google Scholar]
- 43.Valkovič L., Clarke W.T., Schmid A.I., Raman B., Ellis J., Watkins H., Robson M.D., Neubauer S., Rodgers C.T. Measuring inorganic phosphate and intracellular pH in the healthy and hypertrophic cardiomyopathy hearts by in vivo 7T 31P-cardiovascular magnetic resonance spectroscopy. J. Cardiovasc. Magn. Reson. 2019;21:1–11. doi: 10.1186/s12968-019-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamb H.J., Beyerbacht H.P., van der Laarse A., Stoel B.C., Doornbos J., van der Wall E.E., de Roos A. Diastolic Dysfunction in Hypertensive Heart Disease Is Associated With Altered Myocardial Metabolism. Circulation. 1999;99:2261–2267. doi: 10.1161/01.CIR.99.17.2261. [DOI] [PubMed] [Google Scholar]
- 45.Heyne J.-P., Rzanny R., Hansch A., Leder U., Reichenbach J.R., Kaiser W.A. 31P-MR spectroscopic imaging in hypertensive heart disease. Eur. Radiol. 2006;16:1796–1802. doi: 10.1007/s00330-006-0170-0. [DOI] [PubMed] [Google Scholar]
- 46.Smith C.S., Bottomley P.A., Schulman S.P., Gerstenblith G., Weiss R.G. Altered Creatine Kinase Adenosine Triphosphate Kinetics in Failing Hypertrophied Human Myocardium. Circulation. 2006;114:1151–1158. doi: 10.1161/CIRCULATIONAHA.106.613646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phan T.T., Abozguia K., Shivu G.N., Mahadevan G., Ahmed I., Williams L., Dwivedi G., Patel K., Steendijk P., Ashrafian H., et al. Heart Failure With Preserved Ejection Fraction Is Characterized by Dynamic Impairment of Active Relaxation and Contraction of the Left Ventricle on Exercise and Associated With Myocardial Energy Deficiency. J. Am. Coll. Cardiol. 2009;54:402–409. doi: 10.1016/j.jacc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Levelt E., Mahmod M., Piechnik S.K., Ariga R., Francis J.M., Rodgers C.T., Clarke W.T., Sabharwal N., Schneider J.E., Karamitsos T.D., et al. Relationship Between Left Ventricular Structural and Metabolic Remodeling in Type 2 Diabetes. Diabetes. 2015;65:44–52. doi: 10.2337/db15-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rayner J.J., Peterzan M.A., Watson W.D., Clarke W., Neubauer S., Rodgers C.T., Rider O.J. Myocardial Energetics in Obesity. Circulation. 2020;141:1152–1163. doi: 10.1161/CIRCULATIONAHA.119.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burrage M.K., Hundertmark M., Valkovič L., Watson W.D., Rayner J., Sabharwal N., Ferreira V.M., Neubauer S., Miller J.J., Rider O.J., et al. Energetic Basis for Exercise-Induced Pulmonary Congestion in Heart Failure With Preserved Ejection Fraction. Circulation. 2021;144:1664–1678. doi: 10.1161/CIRCULATIONAHA.121.054858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thirunavukarasu S., Jex N., Chowdhary A., Hassan I.U., Straw S., Craven T.C., Gorecka M., Broadbent D., Swoboda P., Witte K.K., et al. Empagliflozin Treatment Is Associated With Improvements in Cardiac Energetics and Function and Reductions in Myocardial Cellular Volume in Patients With Type 2 Diabetes. Diabetes. 2021;70:2810–2822. doi: 10.2337/db21-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conway M., Allis J., Ouwerkerk R., Niioka T., Rajagopalan B., Radda G. Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet. 1991;338:973–976. doi: 10.1016/0140-6736(91)91838-L. [DOI] [PubMed] [Google Scholar]
- 53.Neubauer S., Horn M., Pabst T., Harre K., Strömer H., Bertsch G., Sandstede J., Ertl G., Hahn D., Kochsiek K. Cardiac high-energy phosphate metabolism in patients with aortic valve disease assessed by 31P-magnetic resonance spectroscopy. J. Investig. Med. 1997;45:453–462. [PubMed] [Google Scholar]
- 54.Conway M.A., Bottomley P.A., Ouwerkerk R., Radda G.K., Rajagopalan B. Impaired Systolic Function, Eccentric Hypertrophy, and Increased Severity Are Linked to Lower Phosphocreatine/ATP Ratios in Humans. Circulation. 1998;97:1716–1723. doi: 10.1161/01.CIR.97.17.1716. [DOI] [PubMed] [Google Scholar]
- 55.Mannacio V., Di Tommaso L., Stassano P., De Amicis V., Vosa C. Myocardial metabolism and diastolic function after aortic valve replacement for aortic stenosis: Influence of patient-prosthesis mismatch. Eur. J. Cardio-Thoracic Surg. 2011;41:316–321. doi: 10.1016/j.ejcts.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 56.Mahmod M., Francis J.M., Pal N., Lewis A., Dass S., De Silva R., Petrou M., Sayeed R., Westaby S., Robson M.D., et al. Myocardial perfusion and oxygenation are impaired during stress in severe aortic stenosis and correlate with impaired energetics and subclinical left ventricular dysfunction. J. Cardiovasc. Magn. Reson. 2014;16:29. doi: 10.1186/1532-429X-16-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peterzan M.A., Clarke W.T., Lygate C.A., Lake H.A., Lau J.Y., Miller J.J., Johnson E., Rayner J.J., Hundertmark M.J., Sayeed R., et al. Cardiac Energetics in Patients With Aortic Stenosis and Preserved Versus Reduced Ejection Fraction. Circulation. 2020;141:1971–1985. doi: 10.1161/CIRCULATIONAHA.119.043450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Evanochko W., Buchthal S.D., den Hollander J.A., Katholi C.R., Bourge R.C., Benza R.L., Kirklin J.K., Pohost G.M. Cardiac transplant patients response to the 31P MRS stress test. J. Hear. Lung Transplant. 2002;21:522–529. doi: 10.1016/S1053-2498(01)00412-0. [DOI] [PubMed] [Google Scholar]
- 59.Caus T., Kober F., Marin P., Mouly-Bandini A., Quilici J., Métras D., Cozzone P.J., Bernard M. Non-invasive diagnostic of cardiac allograft vasculopathy by 31P magnetic resonance chemical shift imaging. Eur. J. Cardio-Thoracic Surg. 2006;29:45–49. doi: 10.1016/j.ejcts.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 60.Lee L., Campbell R., Scheuermann-Freestone M., Taylor R., Gunaruwan P., Williams L., Ashrafian H., Horowitz J., Fraser A.G., Clarke K., et al. Metabolic modulation with perhexiline in chronic heart failure: A randomized, controlled trial of short-term use of a novel treatment. Circulation. 2005;112:3280–3288. doi: 10.1161/CIRCULATIONAHA.105.551457. [DOI] [PubMed] [Google Scholar]
- 61.Fragasso G., Perseghin G., De Cobelli F., Esposito A., Palloshi A., Lattuada G., Scifo P., Calori G., Del Maschio A., Margonato A. Effects of metabolic modulation by trimetazidine on left ventricular function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur. Hear. J. 2006;27:942–948. doi: 10.1093/eurheartj/ehi816. [DOI] [PubMed] [Google Scholar]
- 62.Hirsch G.A., Bottomley P.A., Gerstenblith G., Weiss R.G. Allopurinol Acutely Increases Adenosine Triphospate Energy Delivery in Failing Human Hearts. J. Am. Coll. Cardiol. 2012;59:802–808. doi: 10.1016/j.jacc.2011.10.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spoladore R., Fragasso G., Perseghin G., De Cobelli F., Esposito A., Maranta F., Calori G., Locatelli M., Lattuada G., Scifo P., et al. Beneficial effects of beta-blockers on left ventricular function and cellular energy reserve in patients with heart failure. Fundam. Clin. Pharmacol. 2012;27:455–464. doi: 10.1111/j.1472-8206.2012.01029.x. [DOI] [PubMed] [Google Scholar]
- 64.Beadle R.M., Williams L.K., Kuehl M., Bowater S., Abozguia K., Leyva F., Yousef Z., Wagenmakers A.J., Thies F., Horowitz J., et al. Improvement in Cardiac Energetics by Perhexiline in Heart Failure Due to Dilated Cardiomyopathy. JACC: Hear. Fail. 2015;3:202–211. doi: 10.1016/j.jchf.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 65.Leme A.M.B.P., Salemi V.M.C., Weiss R.G., Parga J.R., Ianni B.M., Mady C., Kalil-Filho R. Exercise-Induced Decrease in Myocardial High-Energy Phosphate Metabolites in Patients With Chagas Heart Disease. J. Card. Fail. 2013;19:454–460. doi: 10.1016/j.cardfail.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 66.Solaiyappan M., Weiss R.G., Bottomley P.A. Neural-network classification of cardiac disease from 31P cardiovascular magnetic resonance spectroscopy measures of creatine kinase energy metabolism. J. Cardiovasc. Magn. Reson. 2019;21:1–11. doi: 10.1186/s12968-019-0560-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lopaschuk G.D., Karwi Q.G., Tian R., Wende A.R., Abel E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021;128:1487–1513. doi: 10.1161/CIRCRESAHA.121.318241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown D.A., Perry J.B., Allen M.E., Sabbah H.N., Stauffer B.L., Shaikh S.R., Cleland J.G.F., Colucci W.S., Butler J., Voors A.A., et al. Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2016;14:238–250. doi: 10.1038/nrcardio.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhatt K.N., Butler J. Myocardial Energetics and Heart Failure: A Review of Recent Therapeutic Trials. Curr. Hear. Fail. Rep. 2018;15:191–197. doi: 10.1007/s11897-018-0386-8. [DOI] [PubMed] [Google Scholar]
- 70.Karwi Q.G., Uddin G.M., Ho K.L., Lopaschuk G.D. Loss of Metabolic Flexibility in the Failing Heart. Front. Cardiovasc. Med. 2018;5:68. doi: 10.3389/fcvm.2018.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lopaschuk G.D., Ussher J.R., Folmes C.D.L., Jaswal J.S., Stanley W.C. Myocardial Fatty Acid Metabolism in Health and Disease. Physiol. Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 72.Johnson B.B., Reinhold J., Holmes T.L., Moore J.A., Cowell V., Bernardo A.S., Rushworth S.A., Vassiliou V., Smith J.G.W. Modelling Metabolic Shifts during Cardiomyocyte Differentiation, Iron Deficiency and Transferrin Rescue Using Human Pluripotent Stem Cells. Metabolites. 2021;12:9. doi: 10.3390/metabo12010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fillmore N., Mori J., Lopaschuk G.D. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br. J. Pharmacol. 2014;171:2080–2090. doi: 10.1111/bph.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guimarães-Ferreira L. Role of the phosphocreatine system on energetic homeostasis in skeletal and cardiac muscles. Einstein. 2014;12:126–131. doi: 10.1590/S1679-45082014RB2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Watson W.D., Miller J.J.J., Lewis A., Neubauer S., Tyler D., Rider O.J., Valkovič L. Use of cardiac magnetic resonance to detect changes in metabolism in heart failure. Cardiovasc. Diagn. Ther. 2020;10:583–597. doi: 10.21037/cdt.2019.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ingwall J.S., Weiss R.G. Is the Failing Heart Energy Starved? On using chemical energy to support cardiac function. Circ. Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 77.Ingwall J.S. Energy metabolism in heart failure and remodelling. Cardiovasc. Res. 2008;81:412–419. doi: 10.1093/cvr/cvn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nickel A., Löffler J., Maack C. Myocardial energetics in heart failure. Basic Res. Cardiol. 2013;108:1–20. doi: 10.1007/s00395-013-0358-9. [DOI] [PubMed] [Google Scholar]
- 79.Ingwall J.S. ATP and the Heart: An Overview. ATP Heart. 2002;11:3–6. doi: 10.1007/978-1-4615-1093-2_1. [DOI] [Google Scholar]
- 80.Shen W., Asai K., Uechi M., Mathier M.A., Shannon R.P., Vatner S.F., Ingwall J.S. Progressive Loss of Myocardial ATP Due to a Loss of Total Purines During the Development of Heart Failure in Dogs A Compensatory Role for the Parallel Loss of Creatine. Circulation. 1999;100:2113–2118. doi: 10.1161/01.CIR.100.20.2113. [DOI] [PubMed] [Google Scholar]
- 81.Watson W.D., Green P.G., Valkovič L., Herring N., Neubauer S., Rider O.J. Myocardial Energy Response to Glyceryl Trinitrate: Physiology Revisited. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.790525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsampasian V., Swift A.J., Assadi H., Chowdhary A., Swoboda P., Sammut E., Dastidar A., Cabrero J.B., Del Val J.R., Nair S., et al. Myocardial inflammation and energetics by cardiac MRI: A review of emerging techniques. BMC Med Imaging. 2021;21:1–9. doi: 10.1186/s12880-021-00695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bottomley P.A., Hardy C.J., Roemer P.B. Phosphate metabolite imaging and concentration measurements in human heart by nuclear magnetic resonance. Magn. Reson. Med. 1990;14:425–434. doi: 10.1002/mrm.1910140302. [DOI] [PubMed] [Google Scholar]
- 84.Meyerspeer M., Boesch C., Cameron D., Dezortová M., Forbes S.C., Heerschap A., Jeneson J.A., Kan H.E., Kent J., Layec G., et al. 31P magnetic resonance spectroscopy in skeletal muscle: Experts’ consensus recommendations. NMR Biomed. 2020;34:e4246. doi: 10.1002/nbm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peterzan M.A., Lewis A.J.M., Neubauer S., Rider O.J. Non-invasive investigation of myocardial energetics in cardiac disease using 31P magnetic resonance spectroscopy. Cardiovasc. Diagn. Ther. 2020;10:625–635. doi: 10.21037/cdt-20-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wampl S., Körner T., Valkovič L., Trattnig S., Wolzt M., Meyerspeer M., Schmid A.I. Investigating the effect of trigger delay on cardiac 31P MRS signals. Sci. Rep. 2021;11:1–8. doi: 10.1038/s41598-021-87063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rodgers C.T., Clarke W.T., Snyder C., Vaughan J.T., Neubauer S., Robson M.D. Human cardiac31P magnetic resonance spectroscopy at 7 tesla. Magn. Reson. Med. 2013;72:304–315. doi: 10.1002/mrm.24922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keevil S.F. Spatial localization in nuclear magnetic resonance spectroscopy. Phys. Med. Biol. 2006;51:R579–R636. doi: 10.1088/0031-9155/51/16/R01. [DOI] [PubMed] [Google Scholar]
- 89.Bottomley P.A. MRS Studies of Creatine Kinase Metabolism in Human Heart. eMagRes. 2016;5:1183–1202. doi: 10.1002/9780470034590.emrstm1488. [DOI] [Google Scholar]
- 90.Jung W.-I., Sieverding L., Breuer J., Hoess T., Widmaier S., Schmidt O., Bunse M., van Erckelens F., Apitz J., Lutz O., et al. 31 P NMR Spectroscopy Detects Metabolic Abnormalities in Asymptomatic Patients With Hypertrophic Cardiomyopathy. Circulation. 1998;97:2536–2542. doi: 10.1161/01.CIR.97.25.2536. [DOI] [PubMed] [Google Scholar]
- 91.Tsampasian V., Hothi S.S., Ravindrarajah T., Swift A.J., Garg P., Vassiliou V.S. Valvular Cardiomyopathy: The Value of Cardiovascular Magnetic Resonance Imaging. Cardiol. Res. Pr. 2022;2022:1–9. doi: 10.1155/2022/3144386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Braunwald E. SGLT2 inhibitors: The statins of the 21st century. Eur. Hear. J. 2021;43:1029–1030. doi: 10.1093/eurheartj/ehab765. [DOI] [PubMed] [Google Scholar]
- 93.Santos-Gallego C.G., Mayr M., Badimon J. SGLT2 Inhibitors in Heart Failure: Targeted Metabolomics and Energetic Metabolism. Circulation. 2022;146:819–821. doi: 10.1161/CIRCULATIONAHA.122.060805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available from the corresponding author on request.