Abstract
Blood is the most widely used matrix for biomonitoring of persistent organic pollutants (POPs). It is assumed that POPs are homogenously distributed within body lipids at steady state; however, the variability underlying the partitioning of POPs between fat compartments is poorly understood. Hence, the objective of this study was to review the state of the science about the relationships of POPs between adipose tissue and serum in humans. We conducted a narrative literature review of human observational studies reporting concentrations of POPs in paired samples of adipose tissue with other lipid-based compartments (e.g., serum lipids). The searches were conducted in SCOPUS and PUBMED. A meta-regression was performed to identify factors responsible for variability. All included studies reported high variability in the partition coefficients of POPs, mainly between adipose tissue and serum. The number of halogen atoms was the physicochemical variable most strongly and positively associated with the partition ratios, whereas body mass index was the main biological factor positively and significantly associated. To conclude, although this study provides a better understanding of partitioning of POPs to refine physiologically based pharmacokinetic and epidemiological models, further research is still needed to determine other key factors involved in the partitioning of POPs.
Keywords: adipose tissue, biomonitoring, meta-regression, partition coefficients, persistent organic pollutants
1. Introduction
Persistent organic pollutants (POPs) are chemicals from a large class of substances heavily produced and released in the environment during the last century, peaking in the 1970s and switching to declining trends when regulations and bans began to be enforced [1]. These chemicals attracted international attention because of their high persistence in the environment and capacity to be transported over long distances and absorbed in the fat tissues of living organisms, prompting their magnification along trophic chains [2,3]. Additionally, many POPs may elicit adverse health effects on animals and humans, motivating global regulatory frameworks such as the Stockholm Convention, adopted in 2004, intended to reduce and monitor their worldwide production. Despite regulatory efforts, substantial concentrations of POPs can still be found in ecosystems, or in fatty tissues and specimens from animals and humans across the globe, decades after the banning period [1,4,5]. The vast family of POPs includes legacy chemicals intentionally produced for agricultural applications, like pesticides (e.g., organochlorinated pesticides), or for industrial uses, including polychlorinated biphenyls (PCBs), which can also be unintentionally released through thermal processes [6]. In addition, other classes, like polybrominated diphenyl ethers (PBDE) used as additive flame retardants, are among the substances of emerging concern [2]. Some POPs have shown the capacity for interacting with and disrupting a variety of molecular pathways, triggering pathogenic processes involved in human health, including impaired reproduction [7,8], carcinogenesis [9,10] or metabolic dysregulation [11,12], among others. Experimental and epidemiological studies on POPs have mainly relied on the effects of a few individual congeners, whereas the effect of low-dose mixtures of POPs resembling realistic background exposures remains scarcely explored [13]. Computational systems biology modeling also offers a new way of investigating potential toxicological effects of POPs [14].
Globally, POPs are characterized by their hydrophobic properties, dissolving in lipid-based foodstuffs like fatty fish or butter, favoring diet becoming a major entry pathway in humans [15]. The mechanism of absorption for many hydrophobic pollutants is the same as for dietary lipids, including the lymphatic pathway or the portal vein to the liver, and is determined by the octanol–water partition coefficients (log Kow) of chemicals [16]. For instance, the absorption of the highly lipophilic pesticide hexachlorobenzene (HCB, log Kow =5.7) mostly happens in the intestine; it is transported through the lymph to the tissues within chylomicrons, being mainly stored in adipose tissue [17]. POPs stored in adipocytes are mobilized with neutral lipids to systemic circulation upon energy demands and packed within lipoproteins with lipids and other hydrophobic chemicals. Thus, blood is often used as a convenient matrix for biomonitoring purposes, yet adipose tissue is considered the gold-standard matrix for long-term POP exposure assessment [18]. Elimination of POPs is conducted through bile acids into the intestine where they can ultimately be evacuated within feces, whereas they can be reabsorbed within the enterohepatic recycling process of conjugated bile acids [19]. The dynamics of lipids, and thus of POPs, may be impaired through metabolic disorders (e.g., obesity, diabetes), and POPs may disrupt lipid metabolism and energy balance [20]. In addition, the anthropometry of individuals and variations of fat pad volumes during the lifespan may modify the kinetics of POPs and the chemical exposure biomarkers in environmental health studies [21].
In the last decade, pharmacokinetic models, and especially physiologically based pharmacokinetic (PBPK) models, have been developed to refine risk assessment of chemicals [22]. PBPK models have also been used in epidemiological studies to refine the estimation of individual exposures at different critical life stages and/or in different target tissues [23]. They allow the simulation of toxicokinetic profiles for different sub-populations exposed to lipophilic pollutants from single-time-point measurements of POP concentrations in different matrices (e.g., serum, milk), potentially highlighting critical periods of vulnerability to the toxicity of POPs [24,25,26]. These models incorporate physiological parameters such as tissue-specific blood flows, metabolic processes, and tissue partitioning. An assumption usually made for those models is a homogeneous distribution of POPs within body lipids at steady state, thus a partitioning between adipose tissue and blood lipids close to the unit [27,28]. The same assumption has also been generalized to the partitioning of POPs between blood and breast milk if the concentrations are reported in lipid units, yet some authors have reported that this approach may be too simplistic [29,30]. Compound-specific physicochemical properties (e.g., log Kow, molar volume) and individual physiological parameters (e.g., age, body mass index) may influence such assumptions in the case of breast milk [30]. Nonetheless, little attention has been paid to the partitioning of POPs between adipose tissue and blood lipids; hence, further characterization of POPs’ dynamics through lipid compartments is therefore needed to refine the parametrization of PBPK models and right interpretation of blood biomarkers in epidemiological settings.
Recent publications have previously addressed the interest in adipose tissue for biomonitoring of environmental chemicals, with a special focus on women [31] and also in epidemiological studies [12], highlighting that adipocytes are also the target of POP. To the best of our knowledge, there are no published studies reporting biomonitoring data on POPs in matched lipid-based matrices (i.e., adipose tissues and blood). Another research gap concerns the biological and physicochemical factors influencing the variability in partitioning of POPs among lipid-based tissues reported in individual studies. Thus, the main objective of this study was to review the state of the science of interrelationships in POP concentrations in adipose tissue with other internal lipid-based compartments including blood lipids, excluding breast milk. Hence, we conducted a narrative literature review of primary articles reporting concentrations of POPs in paired samples of adipose tissue with blood (e.g., serum) or other measured internal compartments. For those articles with reported partition coefficients (e.g., adipose tissue: serum) in comparable metrics, a meta-regression was conducted to identify factors responsible for the variability, otherwise a qualitative synthesis is provided.
2. Methods
A literature search strategy was developed to identify recent human studies that measured POPs in serum and adipose tissue from the same individuals. Although the review is essentially narrative, we used the principles of systematic review methodology to ensure the transparency and reproducibility of the process and PRISMA guidelines for reporting [32]. Hence, the bibliographic search was carried out using harmonized standards [33] to develop the search protocol. In this regard, the search strategy was developed to reflect the main key elements of the PECO statement (Table 1), including search strings developed to cover the relevant articles in Scopus and PubMed. The search strings detailed in Table S1 were used in Scopus and PUBMED on 05/08/2022. A ‘publication date’ filter was applied from 2011 to July 2021 in order to focus on the most recent studies that use highly sensitive analytical methods. Manual searches were conducted to retrieve studies published before 2011, based on references cited in the selected studies and the expert knowledge of the panel.
Table 1.
PECO statement.
| PECO | Description |
|---|---|
| Population | Human populations (including men, women, pregnant women, foetuses exposed in utero, and children exposed). |
| Exposure | All type of persistent organic pollutants (e.g., PBDEs, PCBs, etc) |
| Comparator | Not applicable. |
| Outcome | Simultaneous determination in adipose tissues and serum (or different adipose tissue locations). |
To be included, studies had to be conducted in human populations, measuring internal levels of any type of POP in at least one adipose tissue location and serum or another different adipose tissue location. Hence, articles were excluded when concentrations of POPs in human adipose tissue, either subcutaneous adipose tissue or visceral adipose tissue and serum or in different adipose tissue compartments, were not reported. The search was restricted to articles in English and primary studies (review and conference publications were excluded).
Synthesis and Meta-Analysis
Studies with comparative metrics (e.g., ratio of POP concentrations in adipose tissue: serum-determined lipid-based weight, p.e. ng/g lipid weight) were pooled in a meta-analysis. The effect sizes were the adipose tissue ratio means and the respective standard deviations. The means and standard deviations were estimated from the median and interquartile range using the Wan method [34] for two studies [35,36]. Meta-estimates were appraised using a random-effect meta-analysis approach implemented with the metamean function in the “meta” R package [37,38]. Higgins & Thompson’s I2 statistic was used to quantify the between-study heterogeneity. The influence of variables on meta-estimates was assessed by linear meta-regression using the restricted maximum likelihood method and implemented with the metareg function of the same package. In this regard, individual variables (e.g., age, body mass index), year of collection and physicochemical properties (summarized in Table 2) were assessed in single and multivariate models. A narrative synthesis displaying the study design and main outputs was conducted for the rest of studies excluded from the meta-analysis, i.e., those for which no ratio of POP concentrations in adipose tissue: serum was provided.
Table 2.
Physicochemical properties of included chemicals retrieved from PUBCHEM database [39] and adapted from previous publications [40,41,42].
| Name | CAS N° | Molecular Mass |
Log Kow | Half-Life (Year) |
N° Halogen |
Molar Volume |
|---|---|---|---|---|---|---|
| Dioxin-like Polychlorinated Biphenyls | ||||||
| PCB77 | 32598-13-3 | 292 | 6.34 | 0.1 | 4 | 268.2 |
| PCB81 | 70362-50-4 | 292 | 6.34 | 0.7 | 4 | 268.2 |
| PCB126 | 57465-28-8 | 326.4 | 6.98 | 1.6 | 5 | 289.1 |
| PCB169 | 32774-16-6 | 360.9 | 7.62 | 7.3 | 6 | 310 |
| PCB105 | 32598-14-4 | 326.4 | 6.98 | 2.4 | 5 | 289.1 |
| PCB114 | 74472-37-0 | 326.4 | 6.98 | 10 | 5 | 289.1 |
| PCB118 | 31508-00-6 | 326.4 | 6.98 | 3.8 | 5 | 289.1 |
| PCB123 | 65510-44-3 | 326.4 | 6.98 | 7.4 | 5 | 289.1 |
| PCB156 | 38380-08-4 | 360.9 | 7.62 | 16 | 6 | 310 |
| PCB157 | 69782-90-7 | 360.9 | 7.62 | 18 | 6 | 310 |
| PCB167 | 52663-72-6 | 360.9 | 7.62 | 12 | 6 | 310 |
| PCB189 | 39635-31-9 | 395.3 | 7.27 | 22 | 7 | 330.9 |
| Non-dioxin-like Polychlorinated Biphenyls | ||||||
| PCB28 | 7012-37-5 | 257.5 | 5.62 | 16 | 3 | 247.3 |
| PCB52 | 35693-99-3 | 292 | 6.34 | 10 | 4 | 268.2 |
| PCB101 | 37680-73-2 | 326.4 | 6.98 | 11 | 5 | 289.1 |
| PCB138 | 35065-28-2 | 360.9 | 7.44 | 27 | 6 | 310 |
| PCB153 | 35065-27-1 | 360.9 | 7.62 | 14.4 | 6 | 310 |
| PCB180 | 35065-29-3 | 395.3 | 8.27 | 11.5 | 7 | 330.9 |
| Organochlorinated pesticides | ||||||
| HCB | 118-74-1 | 284.8 | 5.73 | 6 | 6 | 221.4 |
| p,p’-DDE | 72-55-9 | 318 | 6.51 | 10 | 4 | 305.2 |
| p,p’-DDT | 50-29-3 | 354.5 | 6.91 | 7 | 5 | 333.5 |
| Brominated flame retardants | ||||||
| BDE28 | 41318-75-6 | 406.89 | 5.88 | 0.94 | 3 | 365.5 |
| BDE47 | 5436-43-1 | 485.79 | 6.77 | 0.37 | 4 | 288.8 |
| BDE99 | 60348-60-9 | 564.7 | 7.66 | 8.2 | 5 | 312.1 |
| BDE100 | 189084-64-8 | 564.7 | 7.66 | 2 | 5 | 312.1 |
| BDE153 | 68631-49-2 | 643.6 | 8.55 | 3.5 | 6 | 335.4 |
| BDE154 | 207122-15-4 | 643.6 | 7.82 | 3.3 | 6 | 335.4 |
| BDE183 | 207122-16-5 | 722.5 | 9.44 | 0.25 | 7 | 358.7 |
| BDE209 | 1163-19-5 | 959.2 | 12.11 | 0.04 | 10 | 428.6 |
| PBB153 | 59080-40-9 | 627.6 | 9.1 | 6.2 | 6 | 335.4 |
3. Results
3.1. Study Characteristics
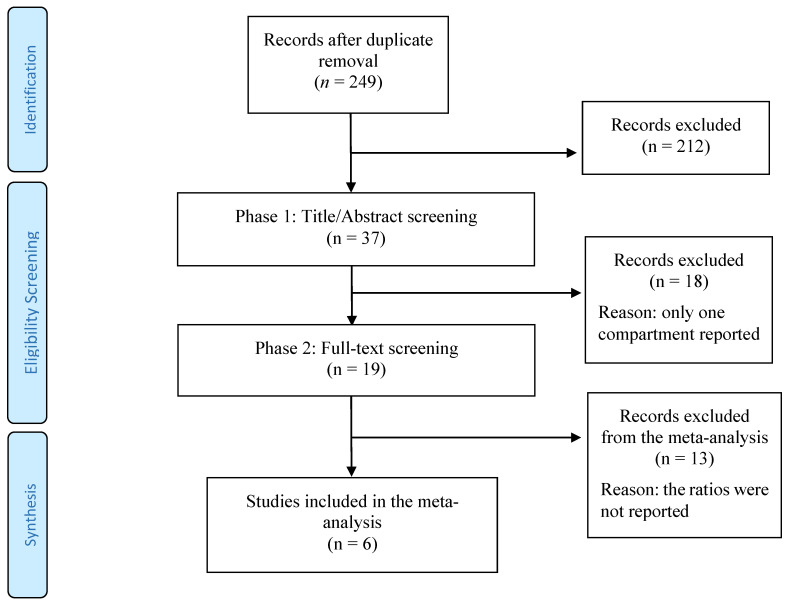
Finally, 249 publications were initially gathered from PubMed and SCOPUS after duplicate removal, among which 212 did not match the selection criteria and thus were excluded based on title and abstract (Figure 1). The remaining 37 references were evaluated based on the full text, of which 18 studies were considered eligible articles. A final list of 19 relevant articles reporting concentrations of POPs in adipose tissue and serum was included, of which 6 provided comparable metrics to conduct a meta-analysis (Table 2).
Figure 1.
PRISMA flowchart of search strategy and study selection with exclusion criteria.
The main characteristics of 19 included studies are displayed in Table 3; they were published between 1999 [43] and 2021 [44]. Among these studies, 11 were performed in Europe and 8 outside Europe. It should be noticed that, for most of these studies, the sampling was conducted several years before publication, extending the exposure timeframe by several years. Different families of compounds belonging to POPs were measured depending on the study: the most frequently measured compounds were PCBs, either as individual congeners such as PCB 153, 138, or 180, or as a sum of PCBs, followed by 1,1,1-Trichloro-2,2-bis(4-chlorophenyl)ethane (p,p’-DDT), 1,1-dichloro-2,2-bis(p-chlorophenyl) ethylene (p,p’-DDE), HCB, beta hexachlorocyclohexane (β-HCH) and PBDE. Most studies represented a clinical population undergoing surgery exposed to background levels of POPs, but several involved occupational exposures [36] or highly contaminated areas [45]. The study participants provided adipose tissue biopsies for the surgical intervention during caesareans [36], breast cancer [10,43] or hepatocarcinoma interventions [45], for endometriosis [46,47], or for bariatric surgery [48,49]. Hence, the majority of the population was represented by females with a minor presence of males. Mean ages ranged between 29 and 59 years, and BMI was between 21 and 48 kg/m2. Subcutaneous adipose tissue (SAT) was the most frequent location for biopsies, yet some other locations were also reported, including visceral adipose tissue (VAT), breast (BAT) and the gluteal region (GAT). A few studies measured concentrations of POPs in two different adipose tissue types/locations simultaneously [49,50,51]. Nine studies reported or provided the adipose tissue: serum ratios, of which one only reported ratios expressing serum concentrations in wet weight [47], three were either in lipid weight or wet weight, and six were in lipid weight.
Table 3.
Summary of study characteristics.
| Reference | Country | n | Condition | Gender | Age a | BMI a | Year Collection | Adipose Tissue | Serum | Ratio c | Meta d | Dioxin | PCB | OCP | BFR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Years) | (kg/m2) | Fasting | Units | |||||||||||||
| [52] | France | 86 | Caesarean | F | 33 (20–46) | NA | 2004–2006 | SAT | Yes | LW | 25 | |||||
| [35] | Spain | 103 | NA | F | 54 (SD:12) | 27 | 2012–2014 | BAT | Yes | WW | X | 3 | 2 | |||
| [35] | Spain | 103 | NA | F | 54 (SD:12) | 27 | 2012–2014 | BAT | Yes | LW | X | X | 3 | 2 | ||
| [51] | Bolivia | 112 | NA | M/F+ | 31 (18–70) | 27 | 2010 | VAT | NA | WW | X | 3 | 3 | |||
| [51] | Bolivia | 112 | NA | M/F+ | 31 (18–70) | 27 | 2010 | VAT | NA | LW | X | X | 3 | 3 | ||
| [53] | Spain | 387 | NA | M/F | 52 b (37–63) | 27 | 2003–2004 | SAT | No | LW | 1 | |||||
| [54] | Italy | 70 | Caesarean | F | 33 b (SD:4) | 24 | 2006 | SAT | NA | LW | 30 | 3 | ||||
| [55] | Sweden | 20 | Stillbirths | F | 25–40 | 21–35 | 2015–2016 | FAT | NA | WW | X | 10 | 9 | 3 | ||
| [50] | Qatar | 34 | IR | M/F | 32 b (SD: 10) | 45 | NA | SAT, VAT | No blood | 28 | ||||||
| [48] | France | 42 | Obesity/H | M/F | 44/40 | 48/22 | 2006–2008 | SAT(VAT) | NA | W/LW | 17 | 18 | ||||
| [56] | Korea | 50 | T2D/N-T2D | M/F | 66/62 (8/11) | 23 (3) | NA | SAT,VAT | No blood | 19 | 13 | |||||
| [10] | France | 48 | BC | F | 64 | 24 | 2015 | BAT | No | LW | X | X | 18 | 8 | ||
| [36] | China | 37–55 | Caesarean | F | 29 | 21 | 2011 | SAT | Yes | LW | X | X | 6 | 8 | ||
| [36] | China | 37–55 | Caesarean | F | 29 | 21 | 2011 | SAT | Yes | WW | X | 6 | 8 | |||
| [43] | Mexico | 198 | BC/H | F | 40 (19–78) | NA | 1994–1996 | BAT | No | LW | 1 | |||||
| [49] | Belgium | 52 | Obesity | M/F | 40 (18–58) | 42 | 2010–2012 | SAT, VAT | No blood | 28 | 2 | |||||
| [57] | Belgium | 101 | Infertile | F | 32 (24–42) | 1996–1998 | SAT | LW | 7 | 7 | ||||||
| [58] | Portugal | 189 | Obesity | M/F+ | 43 (19–65) | 45 (5) | 2010–2011 | SAT, VAT | No blood | 1 | 11 | |||||
| [46] | France | 67 | ENDO/H | M+/F | 34 (48–80) | 24 | 2013–2015 | VAT, SAT | NA | LW | X | X | 18 | 8 | ||
| [47] | US | 473 | ENDO/H | F | 33 (IQR:11) | 26(IQR:10) | 2007–2009 | VAT | No | WW | X | 13 | 5 | 5 | ||
| [59] | India | 29 | BC | F | 24–65 | NA | 1996–1997 | BAT | Yes | WW | X | 2 | 3 | |||
| [59] | India | 29 | BC | F | 24–65 | NA | 1996–1997 | GAT | Yes | WW | X | 2 | 3 | |||
| [59] | India | 29 | BC | F | 24–65 | NA | 1996–1997 | BAT | Yes | LW | X | 2 | 3 | |||
| [59] | India | 29 | BC | F | 24–65 | NA | 1996–1997 | GAT | Yes | LW | X | 2 | 3 | |||
| [44] | Australia | 32 | NA | M/F | 55 (22–89) | 25 | 2016 | SAT | Yes | LW | X | 3 | ||||
| [45] | Italy | 59 | HCC | M+/F | 69 (48–80) | NA | NA | VAT | Yes | LW | X | X | 7 | |||
a Mean age or body mass index and range, standard deviation (SD) or interquartile range (IQR), b geometric mean or median, c identifier of studies that reported ratios, d identifier of studies included in the meta-regression. Abbreviations: BAT, breast adipose tissue; BC, breast cancer; BFR, brominated flame retardants; BMI, body mass index; FAT, fetal adipose tissue; GAT, gluteal adipose tissue; F, female; F+, overrepresentation of female; H, healthy subjects; HCC, hepatocellular carcinoma; IQR, interquartile range; IR, insulin resistance; LW, lipid weight; M, male; NA, not available; OCP, organochlorinated pesticides; PCBs, polychlorinated biphenyls; R, identifier for studies reporting calculated partition ratios; SAT, subcutaneous adipose tissue; SD, standard deviation; VAT, visceral adipose tissue; WW, wet weight.
3.2. Partition Coefficients of Persistent Organic Pollutants between Adipose Tissue and Serum
The studies tended to report the ratios considering both metrics on a lipid weight basis, but in some cases (Table 4) reported both lipid weight and wet weight biomarkers [35,36,51,59]. One study chose to present the values of ratios in wet weight [47]. Among PCBs, the congeners 138, 153 and 180 were the most reported ones in the literature because of their abundance (eight studies). In most cases, the congeners showed adipose tissue: serum ratios between 1.5 and 2; however, some exceptions were found. For instance, a few studies showed mean ratios of PCB 153 close to 5 [35,44], whereas others were close to 0.7–0.9 for the same congener [35,59]. In turn, the metabolite of the insecticide DDT, p,p’-DDE, was the organochlorinated pesticide (OCP) most widely reported in the six studies. As previously described for PCBs, the mean levels of ratios of OCP were mostly in the range 1.5–2, with several exceptions. The adipose tissue: serum ratios of brominated flame retardants (BFRs) were reported in three studies. Two French studies tended to show consistent values for most PBDEs [10,46], showing mean values in the range between two and three for most congeners with the exception of BDE209, which showed the highest values (4.6–8.0). Conversely, in one study conducted in an e-waste facility area with expectant women, the ratios tended to be below the unit for most congeners (0.4–1.1) [36]. In summary, for most studies, the reported adipose tissue: serum ratios displayed a similar pattern, with a 1.5-3-fold increased concentration of POPs in the adipose tissue, considering the measures based on lipid weight.
Table 4.
Congener-specific summary of mean coefficient partitions of persistent organic pollutants between adipose tissue and serum, both normalized on the lipid weight basis.
| Chemical | [52] | [51] | [35] | [10] | [43] | [36] | [46] | [44] | [59] BAT |
[59] GAT | [45] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCB101 | 1.8 | 1.5 | |||||||||
| PCB105 | 2.1 | 2.1 | |||||||||
| PCB114 | 2.1 | 2.3 | |||||||||
| PCB118 | 1.8 | 1.4 | 1.8 | 1.2 | |||||||
| PCB123 | 1.6 | 1.9 | |||||||||
| PCB126 | 1.7 | 1.7 | |||||||||
| PCB138 | 3.6 | 4.9 | 2.1 | 1.6 | 2.3 | 5.4 | 2.0 | ||||
| PCB153 | 0.9 | 4.7 | 2.1 | 1.7 | 2.3 | 5.4 | 0.7 | 0.7 | 2.0 | ||
| PCB156 | 2.1 | 2.6 | 1.7 | ||||||||
| PCB157 | 1.6 | 1.8 | |||||||||
| PCB167 | 1.5 | 1.7 | |||||||||
| PCB169 | 1.8 | 2.0 | |||||||||
| PCB169 | 2.2 | ||||||||||
| PCB180 | 1.7 | 2.2 | 2.0 | 1.5 | 2.4 | 4.4 | 0.8 | 0.8 | 2 | ||
| PCB189 | 2.6 | 2.6 | |||||||||
| PCB194 | 2.1 | ||||||||||
| PCB28 | 1.7 | 1.2 | 1.3 | ||||||||
| PCB52 | 1.5 | 1.2 | |||||||||
| PCB77 | 1.6 | 0.4 | |||||||||
| PCB81 | 1.2 | 0.7 | |||||||||
| PCB99 | 1.4 | ||||||||||
| p,p’-DDE | 1.9 | 1.7 | 4.2 | 3.4 | 0.7 | 0.9 | |||||
| p,p’-DDT | 2 | 1.1 | 1.5 | ||||||||
| HCB | 1.9 | 2.2 | |||||||||
| β-HCH | 1.1 | 1.6 | |||||||||
| BDE100 | 0.91 | 2.3 | 0.7 | 3.2 | |||||||
| BDE153 | 2.12 | 2.4 | 1.0 | 3.0 | |||||||
| BDE154 | 1.24 | 1.3 | 0.9 | 2.3 | |||||||
| BDE183 | 3.0 | 1.1 | 3.2 | ||||||||
| BDE209 | 0.16 | 4.6 | 0.4 | 7.9 | |||||||
| BDE28 | 1.00 | 2.7 | 0.6 | 2.5 | |||||||
| BDE47 | 0.97 | 2.5 | 0.5 | 3.7 | |||||||
| BDE99 | 0.36 | 3.0 | 0.5 | 4.1 | |||||||
| PBB153 | 2.5 |
Abbreviations: BAT, brown adipose tissue; BDE, bromodiphenyl ether; β-HCH, β-hexachlorocyclohexane; GAT, gluteal adipose tissue; HCB, hexachlorobenzene; PCB, polychlorinated biphenyl; PBB153, 2,2′,4,4′,5,5′-hexabromobiphenyl; p,p’-DDT, 1,1,1-Trichloro-2,2-bis(4-chlorophenyl)ethane; p,p’-DDE, 1,1-dichloro-2,2-bis(p-chlorophenyl) ethylene.
3.3. Physicochemical Determinants of the Variability in Adipose Tissue: Serum Partition Coefficients
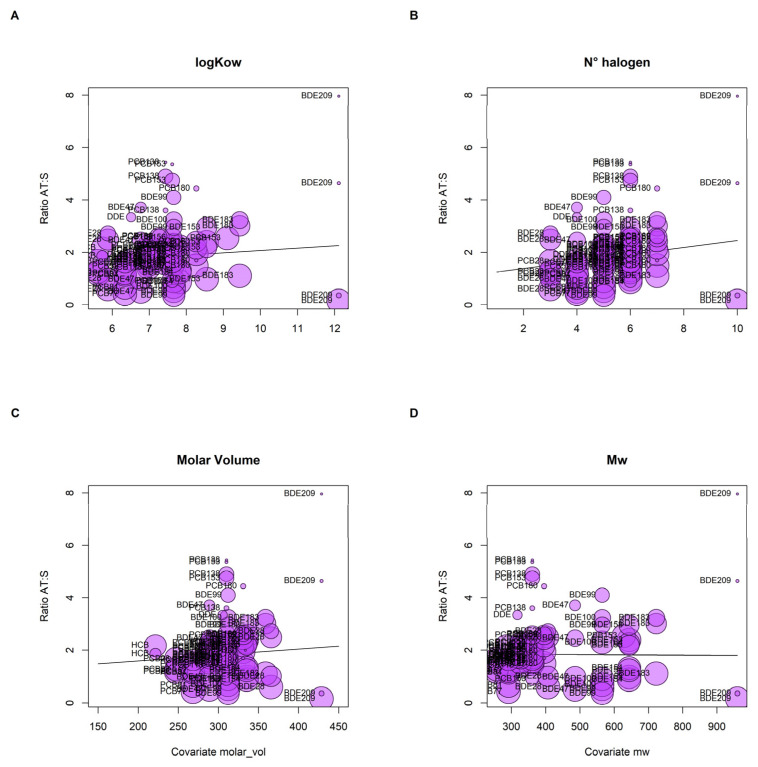
In order to gain insight into the physicochemical factors potentially affecting the variability in adipose tissue: serum ratios, we conducted a meta-regression based on available summary partitioning data reported in the selected studies. The meta-regression slopes, graphically depicted by bubble plots, are displayed in Figure 2A–D. The number of halogens was the variable most strongly associated with adipose tissue: serum ratios (β 0.25 95% CI (0.17–0.34), p = 0.05 (Figure 2B)). Log Kow, molar weight (Mw) and molar volume showed null or mild associations with the ratios (Figure 2A,C,D).
Figure 2.
Bubble plots representing the associations between the mean partitioning ratios of persistent organic pollutants between adipose tissue and serum reported in the literature and physicochemical properties, including the partition octanol–water (Log Kow, (A)), number of halogen atoms (B), molar volume (C) and molar weight (Mw, (D)).
3.4. Biological/Individual/Instrumental Determinants of the Variability in Adipose Tissue: Serum Partition Coefficients
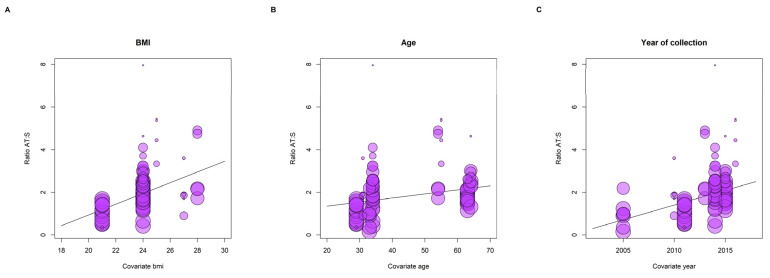
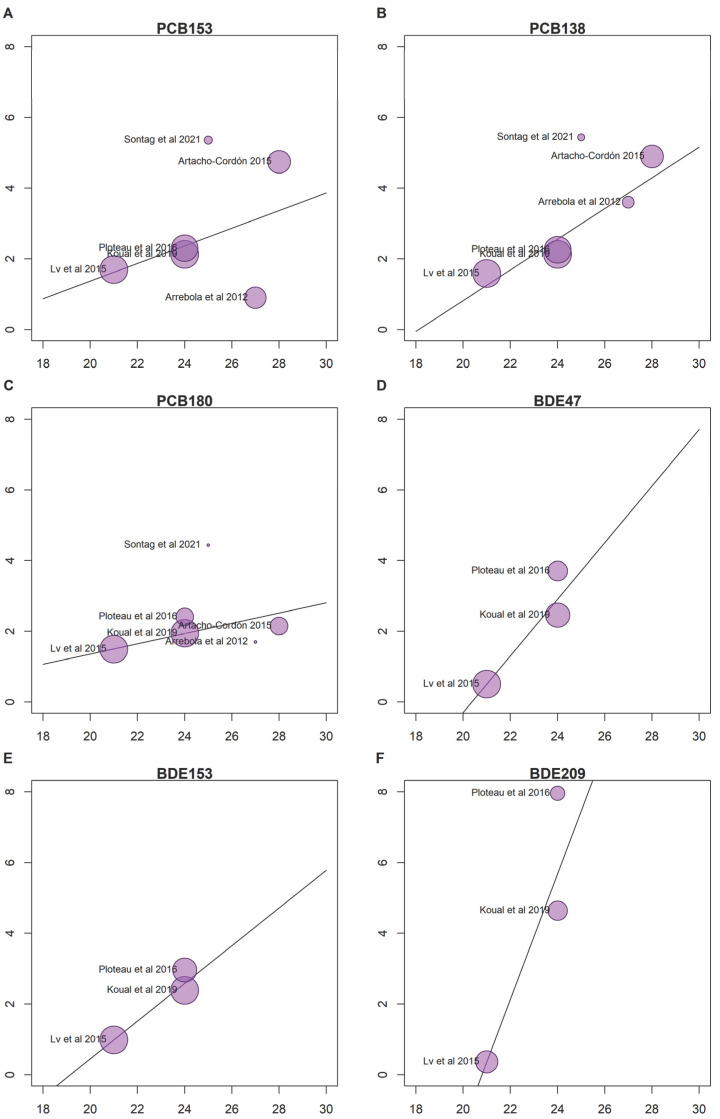
Among individual variables, BMI was the most strongly associated with the adipose tissue: serum ratios for all POPs combined (Figure 3A), β 0.26 95% CI (0.17–0.34), p < 0.001, whereas age showed a more flattened slope than BMI, yet was statistically significant, β 0.02 95% CI (0.00–0.03), p = 0.03 (Figure 3B). Due to the close relationship between age and BMI, we also considered both variables in the same meta-regression model, suggesting a potential confounding effect of age, resulting in null associations for age with a minor impact on BMI coefficient. The year of sample collection (Figure 3C) also showed a positive association with the ratios β 0.26 95% CI (0.17–0.34), p < 0.001. For the congeners with at least three studies reporting the adipose tissue: serum ratios and BMI, we conducted a detailed meta-regression for individual congeners of PCBs and BDEs (Figure 4A–F). These stratified results supported the existence of congener-specific relationships between the ratios and BMI. For instance, BDE 209 was associated with BMI with the steepest slope, whereas PCB180 showed the most flattened one (Figure 4).
Figure 3.
Bubble plots representing the associations between adipose tissue: serum ratios with body mass index (BMI, (A)), age (B), and year of collection (C) from the random effects meta-regression model. The study [52] was excluded from this analysis because the BMI was not reported.
Figure 4.
Bubble plots representing the associations between adipose tissue: serum ratios and body mass index (BMI) from the random effects meta-regression model for specific congeners including PCB 153 (A), PCB 138 (B), PCB 180 (C), BDE 47 (D), BDE 153 (E), and BDE 209 (F). The study [52] was excluded from this analysis because the BMI was not reported.
3.5. Variability in Levels of POPs between Adipose Tissue Types/Location
Seven studies analysed POPs in different adipose tissue locations (mainly visceral and subcutaneous) matched from the same individuals, including obese populations and women with endometriosis [46,48,49,50,56,58,59]. Globally, concentrations of organochlorinated and brominated chemicals analysed in paired VAT and SAT matrices showed high correlations, for instance with R Pearson correlation >0.9 (p < 0.001) for all POPs from France [46] or R2 0.7–0.6 (p < 0.0001) for PCBs from Belgium [49]. Congener-specific and strong sex-specific differences were noticed for the correlation slopes, showing lower correlations of POPs between VAT and SAT among females [49]. Noteworthy, two studies reported high correlations for individual POPs or ∑POPs between VAT and SAT locations but statistically different concentrations, with higher concentrations of individual POPs or ∑POPs in VAT than SAT [56,58]. Concentrations of organochlorinated pesticides (p,p’-DDE, p,p’-DDT, β-HCH) and PCBs (153 and 180) were also highly correlated between breast and gluteal fat pads (rho > 0.7, p < 0.001), showing comparable concentration levels [59]. The relationships between POPs in human foetal adipose tissue and maternal serum or placenta have been addressed by one study, showing in general high correlations for all PCBs and pesticides analysed (HCB, β-HCH, p,p’-DDE and transnonachlor) [55]. The authors reported sex-specific patterns of tissue accumulation, reporting higher tissue ratios among male foetuses than among females and lower among those with placental insufficiency.
4. Discussion
To the best of our knowledge, this is the first study attempting to summarize the available scientific evidence on partitioning of POPs between adipose tissue and serum from human studies. We have also aimed at characterizing and gaining insight into the determining factors of variability for partitions, as was already done for partitioning between serum and breast milk [30]. Data were retrieved mostly from studies conducted with non-occupationally exposed populations, reporting matched samples on lipid-based compartments, thus illustrating chronic low-dose exposure to POPs. Adipose tissue is acknowledged to be the gold-standard matrix for biomonitoring internal POP levels, yet blood (lipids) has been used as a surrogate for many years, assuming that blood POPs reflect their adipose tissue levels in equilibrated states [60]. Consequently, blood concentrations of POPs are extensively used for biomonitoring and epidemiological research. The widespread presence of low doses of POPs in internal biological matrices urges a comprehensive understanding of their kinetics for accurate use of exposure biomarkers in biomonitoring and epidemiological studies.
4.1. Overall Variability in Adipose Tissue: Serum Partition Coefficients among POPs
All individual studies included in this review reported high variability in the partition coefficients of POPs between adipose tissue and serum, at different levels of magnitude depending on the specific congeners. Whereas it has often been assumed that highly lipophilic chemicals would distribute homogeneously within lipid compartments of the human body, similarly to 2,3,7,8-tetrachlorodibenzo-p-dioxin [60], the studies included in this review illustrate that the mean ranges between 0.4 and 8 in a congener-specific base. Most ratios were above 1, suggesting a higher concentration of POPs in adipose tissue than in blood; nonetheless, in a few cases the ratios were inversed, reflecting the highest concentrations in serum. Among the different physicochemical properties investigated in the meta-regression, the number of halogen atoms was the most strongly and positively associated variable with the partition ratios, at the limit of statistical significance (p = 0.05), with the rest being statistically null (Log Kow, Mw, and molar volume). The bubble plots reveal the strong dispersion of data for BDE209 and the underlying correlation of physicochemical variables (e.g., BDE209 showed the largest physicochemical properties: Log Kow = 12.11, number of halogens = 10, molar volume = 428.6 m3/mol). In addition, the bubble plots reflect that the linear meta-regression may be strongly influenced by two dominant studies reporting low ratios for BDE209, suggesting a non-monotonic relationship of the ratios with these physicochemical properties. These trends are consistent with the previous literature. For instance, positive associations between log Kow and adipose tissue: serum ratios were reported for most POPs with the exception of PCB180 and BDE209, with higher log Kow values (8.27 and 12.11, respectively) and a higher number of halogens [36]. Similar trends were reported for the blood/milk partitioning, which decreased among substances with larger log Kow (>8), higher molecular weight (>400 for PCB/PCDD/Fs, >700 for PBDEs), and more halogen compounds (>7) [30]. A bioaccumulation factor increase correlated with increased Kow was reported for lipophilic compounds with Kow < 7 in wild aquatic species [61]: the bioaccumulation factor would increase for Kow < 7, then decline for Kow > 7. A parabolic relationship was also observed between log biomagnification factor and log Kow, with a peak for Kow of approximately 8 [62]. Lv et al. (2015) also noted that compounds with similar log Kow but from different families (PCB vs. PBDE) exhibited different partition ratios, lower for BDEs than PCBs [36]. It was assumed that deposition of PBDE in human adipose tissues was lower than deposition of PCB with similar Kow; however, these trends were not consistent in other studies [10,46].
Globally, these data support that physicochemical parameters, such as number of halogens, may have an impact on POP partitioning between adipose tissue and serum, but likely in a non-monotonic manner and influenced by other parameters. For instance, the tridimensional structure and associated degree of rigidity of the different compounds could also have an impact on the diffusion-limited partition of POPs [30]
4.2. Congener-Specific Variability in Adipose Tissue: Serum Partition Coefficients between Studies
Table 4 summarizes the high variability in partition ratios determined for the same congener among different studies. For instance, PCB138’s reported mean ratios ranged from 1.59 to 5.44, a three-fold difference for the same compound. Systematic differences were appreciated between studies, in some cases even switching the direction in the case of BDEs [36]. These differences may be influenced by specific study characteristics, such as timing of sampling, differences in analytical procedures introducing analytical uncertainties for POPs, or the different determinations of lipid content derived from different equations [15].
Among the individual biological factors analyzed, BMI was the main determinant, positively associated with the partition ratios (β 0.26 95% CI (0.17–0.34), p < 0.001), while age was associated to a weaker degree and non-associated when both BMI and age were considered in the multivariate meta-regression model. Globally, these findings suggest that the larger the fat mass, the higher the concentration of POPs in adipose tissue relative to blood lipids, independently of age. These findings would empirically support the hypothesis that populations with high adipose fractions may be sequestering more POPs in their adipocytes, preventing their release into circulation and thus protecting other organs [63]. Most studies that reported different types of adipose tissue deposits did not support differences between locations (e.g., VAT vs. SAT), as high or similar concentrations were reported. In any case, the number of studies was very limited to include the adipose tissue location as a co-variable in the meta-regression or to conduct a stratification analysis. One study showed different trends, with high correlations between ∑POP and AT, with the highest concentrations in VAT rather than SAT [56,58]. These differences may be due to the dissimilarities in lipid content [58], differences in blood flow, or in the density of blood vessels and/or differences in metabolism, with VAT being more metabolically active [64,65]. A PBPK model was proposed for γ-HBCD in mice [66], including an additional compartment corresponding to the deep fat fraction of the body. For POPs with log now >4, it was suggested that ratio of the neutral lipid-equivalent content (corresponding to neutral lipids and phospholipids) of adipose tissue and blood was the main determinant of POP partitioning between those tissues [67]: different types of lipids and compositions of non-lipid molecules might play a role in POP dynamics within AT, and interactions between POPs and lipid compounds at the molecular scale should be further investigated [46], especially for biomonitoring with blood. It was also suggested that extracellular and lymphatic circulation might contribute to POP storage [66]. Metabolic status and disease condition may also play a role in POP partitioning [15], but those parameters were not investigated in the meta-regression due to the limited number of studies. The level and the route of exposure could also have an impact on the metabolic disruption of adipocytes, and thus the kinetics of POPs. In this regard, the exposure scenario may also contribute to explain the variabilities of coefficients. Nonetheless, formal statistics were not applicable to compare background exposures with occupational or highly contaminated sites. For instance, one occupational e-waste study [36] showed serum levels of some BFRs 20 times higher than in populations exposed at background levels [10,46], which would explain ratios systematically lower, below the unit.
4.3. Limitations of the Review
First, the current review was essentially narrative, even if a robust methodology based on systematic review principles was applied. If our review is not exhaustive, we believe that most studies with AT and blood POP measurements published during the last decade have been included. Because of this criterion, only a limited number of studies were eligible in the meta-analysis. In addition, each individual study involved a modest sample size. In order to strengthen the statistical power, studies with larger sample size will be required. Furthermore, the selected studies explored different sets of congeners, limiting the ability to compare ratios among studies for the same compounds and to conclude about the influence of population biological characteristics. Actually, the studies provided few individual variables that could be integrated in the meta-regression, but other factors may likely impact on the kinetics of POPs and consequently on the ratios (e.g., parity, breastfeeding, physical activity). In addition, we pooled some summary metrics through approximations, because not all studies reported the means and standard deviations. Optimally, statistical analyses combining raw data from individual studies would improve the accuracy of estimates. Considering the invasive character of adipose tissue biopsies collected from the populations with cancer or obesity the data may not be representative of the general population. This review also stressed the need for further studies on dioxins scarcely reported in the literature. Per/polyfluorinated substances (PFAS) are a wide class of chemicals with some congeners listed as POPs in the Stockholm Convention commonly characterized in blood due to their amphiphilic properties; hence, they are not represented in the present review because they are more present in livers than in AT. Nonetheless, some studies have shown that even non-persistent chemicals like bisphenol A or parabens can be found in adipose tissue [68,69]. New approaches based on artificial intelligence, which used text mining to automatically screen large literature sources and databases, could also be considered in order to get as much information as possible. As an example, the AOP-helpFinder tool [70,71], which helps the development of adverse outcome pathways, could be adapted in future meta-analysis studies.
4.4. Impacts of Findings
We believe that the findings of this study are of high relevance due to the widespread use of exposure biomarkers of POPs for biomonitoring and epidemiological research. Biomonitoring of chemicals has been progressively integrated as a novel tool within the chemical risk assessment process, notably by using PBPK modelling and the development of biomonitoring equivalents [72,73,74]. PBPK models of POPs are often based on the assumption of a homogeneous distribution of POP in lipid compartments within the body (i.e., that adipose tissue: blood ratios are comparable). Thus, a better understanding of partitioning of POPs between fat compartments will help the refinement of those models and reduce uncertainty within chemical risk assessment and regulatory decision-making. Likewise, in epidemiological research, the extensive use of blood biomarkers of POPs has not been supported by a deep understanding of the complex relationship of POPs in blood and adipose tissue [15]. Nowadays, different statistical methods have been proposed to use the blood biomarkers, addressing the different causal structures depicted when the perturbation of lipid metabolism is in the causal pathway between the exposure and the health outcomes [75]. Although some methods may attenuate the statistical bias, none of the methods using a single-spot blood biomarker may fully recapitulate the complex dynamics of POPs. The problem becomes more complex from the perspective of POP mixtures, where individual congeners may impact the kinetics of the others. Hence, a better understanding of the dynamics of partitions between fat compartments is of key relevance for accurate use and interpretation of surrogates’ measures in environmental health studies.
5. Conclusions
This review supports the fact that partitioning of POPs between fat compartments is highly variable. These findings also show that the partition ratios of POPs between fat compartments systematically depart from the unit, even at equilibrated states, as extensively assumed. BMI and number of halogens were the main determinants of the variability in the adipose tissue: serum ratio of POP among the selected studies. Finally, this study highlights that further research is still needed to better understand the behavior of POPs in the main matrices used for biomonitoring, especially for those scarcely studied congeners.
Acknowledgments
The authors would like to acknowledge the CREATIvE project, as this project has received funding from the French National Research Agency (ANR-18-CE34-0001-01). This work was supported by the Université de Paris and INSERM.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxics11010041/s1, Table S1. Search strings.
Author Contributions
Conceptualization, G.C.-S., M.K., X.C., J.-P.A. (Juan-Pedro Arrebola), C.E. and C.R.; methodology, G.C.-S., M.K., X.C., J.-P.A. (Jean-Philippe Antignac) and C.R.; software, G.C.-S.; validation, M.-A.M., G.C.-S. and C.R.; formal analysis, M.-A.M., G.C.-S. and C.R.; resources, K.A., C.R.; data curation, M.-A.M. and G.C.-S.; writing—original draft preparation, M.-A.M., G.C.-S. and C.R.; writing—review and editing, all authors.; visualization, G.C.-S.; supervision, C.R.; project administration, C.R., K.A.; funding acquisition, C.R., K.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare they have no actual or potential competing financial interests.
Funding Statement
The project has been funded by the French National Research Agency (ANR-18-CE34-0001-01, Creative Project). Juan Pedro Arrebola is under contract within the Ramón y Cajal Program (RYC-2016-20155, Ministerio de Economía, Industria y Competitividad, Spain).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.van den Berg M., Kypke K., Kotz A., Tritscher A., Lee S.Y., Magulova K., Fiedler H., Malisch R. WHO/UNEP global surveys of PCDDs, PCDFs, PCBs and DDTs in human milk and benefit-risk evaluation of breastfeeding. Arch. Toxicol. 2017;91:83–96. doi: 10.1007/s00204-016-1802-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., He C., Han W., Song J., Li H., Zhang Y., Jing X., Wu W. Exposure pathways, levels and toxicity of polybrominated diphenyl ethers in humans: A review. Environ. Res. 2020;187:109531. doi: 10.1016/j.envres.2020.109531. [DOI] [PubMed] [Google Scholar]
- 3.Long M., Wielsøe M., Bonefeld-Jørgensen E.C. Time Trend of Persistent Organic Pollutants and Metals in Greenlandic Inuit during 1994–2015. Int. J. Environ. Res. Public Health. 2021;18:2774. doi: 10.3390/ijerph18052774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan H.-Y., Li J.-F., Li X.-H., Yang Y.-L., Qin Z.-F., Li J.-B., Li Y.-Y. Transfer of dechlorane plus between human breast milk and adipose tissue and comparison with legacy lipophilic compounds. Environ. Pollut. 2020;265:115096. doi: 10.1016/j.envpol.2020.115096. [DOI] [PubMed] [Google Scholar]
- 5.Nøst T.H., Berg V., Hanssen L., Rylander C., Gaudreau E., Dumas P., Breivik K., Sandanger T.M. Time trends of persistent organic pollutants in 30 year olds sampled in 1986, 1994, 2001 and 2007 in Northern Norway: Measurements, mechanistic modeling and a comparison of study designs. Environ. Res. 2019;172:684–692. doi: 10.1016/j.envres.2019.02.047. [DOI] [PubMed] [Google Scholar]
- 6.Quinete N., Schettgen T., Bertram J., Kraus T. Occurrence and distribution of PCB metabolites in blood and their potential health effects in humans: A review. Environ. Sci. Pollut. Res. Int. 2014;21:11951–11972. doi: 10.1007/s11356-014-3136-9. [DOI] [PubMed] [Google Scholar]
- 7.Kahn L.G., Harley K.G., Siegel E.L., Zhu Y., Factor-Litvak P., Porucznik C.A., Klein-Fedyshin M., Hipwell A.E. Persistent organic pollutants and couple fecundability: A systematic review. Hum. Reprod. Update. 2021;27:339–366. doi: 10.1093/humupd/dmaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefebvre T., Fréour T., Ploteau S., Le Bizec B., Antignac J.P., Cano-Sancho G. Associations between human internal chemical exposure to Persistent Organic Pollutants (POPs) and In Vitro Fertilization (IVF) outcomes: Systematic review and evidence map of human epidemiological evidence. Reprod. Toxicol. 2021;105:184–197. doi: 10.1016/j.reprotox.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Ennour-Idrissi K., Ayotte P., Diorio C. Persistent Organic Pollutants and Breast Cancer: A Systematic Review and Critical Appraisal of the Literature. Cancers. 2019;11:1063. doi: 10.3390/cancers11081063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koual M., Cano-Sancho G., Bats A.-S., Tomkiewicz C., Kaddouch-Amar Y., Douay-Hauser N., Ngo C., Bonsang H., Deloménie M., Lecuru F., et al. Associations between persistent organic pollutants and risk of breast cancer metastasis. Environ. Int. 2019;132:105028. doi: 10.1016/j.envint.2019.105028. [DOI] [PubMed] [Google Scholar]
- 11.Mendes V., Ribeiro C., Delgado I., Peleteiro B., Aggerbeck M., Distel E., Annesi-Maesano I., Sarigiannis D., Ramos E. The association between environmental exposures to chlordanes, adiposity and diabetes-related features: A systematic review and meta-analysis. Sci. Rep. 2021;11:14546. doi: 10.1038/s41598-021-93868-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mustieles V., Pérez-Carrascosa F.M., León J., Lange T., Bonde J.-P., Gómez-Peña C., Artacho-Cordón F., Barrios-Rodríguez R., Olmedo-Requena R., Expósito J., et al. Adipose Tissue Redox Microenvironment as a Potential Link between Persistent Organic Pollutants and the 16-Year Incidence of Non-hormone-Dependent Cancer. Environ. Sci. Technol. 2021;55:9926–9937. doi: 10.1021/acs.est.0c08180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee D.H., Jacobs D.R., Jr., Park H.Y., Carpenter D.O. A role of low dose chemical mixtures in adipose tissue in carcinogenesis. Environ. Int. 2017;108:170–175. doi: 10.1016/j.envint.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Wu Q., Achebouche R., Audouze K. Computational systems biology as an animal-free approach to characterize toxicological effects of persistent organic pollutants. Altex. 2020;37:287–299. doi: 10.14573/altex.1910161. [DOI] [PubMed] [Google Scholar]
- 15.Cano-Sancho G., Labrune L., Ploteau S., Marchand P., Le Bizec B., Antignac J.-P. The challenging use and interpretation of circulating biomarkers of exposure to persistent organic pollutants in environmental health: Comparison of lipid adjustment approaches in a case study related to endometriosis. Chemosphere. 2018;200:388–396. doi: 10.1016/j.chemosphere.2018.02.120. [DOI] [PubMed] [Google Scholar]
- 16.Jandacek R.J., Rider T., Yang Q., Woollett L.A., Tso P. Lymphatic and portal vein absorption of organochlorine compounds in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G226–G234. doi: 10.1152/ajpgi.90517.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jandacek R.J., Zheng S., Yang Q., Tso P. Rapid clearance of hexachlorobenzene from chylomicrons. Lipids. 2004;39:993–995. doi: 10.1007/s11745-004-1321-4. [DOI] [PubMed] [Google Scholar]
- 18.Vorkamp K., Castaño A., Antignac J.P., Boada L.D., Cequier E., Covaci A., Esteban López M., Haug L.S., Kasper-Sonnenberg M., Koch H.M., et al. Biomarkers, matrices and analytical methods targeting human exposure to chemicals selected for a European human biomonitoring initiative. Environ. Int. 2021;146:106082. doi: 10.1016/j.envint.2020.106082. [DOI] [PubMed] [Google Scholar]
- 19.Genuis S.J., Lane K., Birkholz D. Human Elimination of Organochlorine Pesticides: Blood, Urine, and Sweat Study. BioMed Res. Int. 2016;2016:1624643. doi: 10.1155/2016/1624643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee D.-H., Porta M., Jacobs D.R., Vandenberg L.N. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr. Rev. 2014;35:557–601. doi: 10.1210/er.2013-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L., Wania F. Mechanistic Pharmacokinetic Modeling of the Bioamplification of Persistent Lipophilic Organic Pollutants in Humans during Weight Loss. Environ. Sci. Technol. 2017;51:5563–5571. doi: 10.1021/acs.est.7b00055. [DOI] [PubMed] [Google Scholar]
- 22.Desalegn A., Bopp S., Asturiol D., Lamon L., Worth A., Paini A. Role of Physiologically Based Kinetic modelling in addressing environmental chemical mixtures—A review. Comput. Toxicol. 2019;10:158–168. doi: 10.1016/j.comtox.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen M.E., Mallick P., Clewell H.J., 3rd, Yoon M., Olsen G.W., Longnecker M.P. Using quantitative modeling tools to assess pharmacokinetic bias in epidemiological studies showing associations between biomarkers and health outcomes at low exposures. Environ. Res. 2021;197:111183. doi: 10.1016/j.envres.2021.111183. [DOI] [PubMed] [Google Scholar]
- 24.Verner M.A., Ayotte P., Muckle G., Charbonneau M., Haddad S. A physiologically based pharmacokinetic model for the assessment of infant exposure to persistent organic pollutants in epidemiologic studies. Environ. Health Perspect. 2009;117:481–487. doi: 10.1289/ehp.0800047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verner M.A., Hart J.E., Sagiv S.K., Bellinger D.C., Altshul L.M., Korrick S.A. Measured Prenatal and Estimated Postnatal Levels of Polychlorinated Biphenyls (PCBs) and ADHD-Related Behaviors in 8-Year-Old Children. Environ. Health Perspect. 2015;123:888–894. doi: 10.1289/ehp.1408084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pruvost-Couvreur M., Béchaux C., Rivière G., Le Bizec B. Impact of sociodemographic profile, generation and bioaccumulation on lifetime dietary and internal exposures to PCBs. Sci. Total Environ. 2021;800:149511. doi: 10.1016/j.scitotenv.2021.149511. [DOI] [PubMed] [Google Scholar]
- 27.Emond C., Charbonneau M., Krishnan K. Physiologically based modeling of the accumulation in plasma and tissue lipids of a mixture of PCB congeners in female Sprague-Dawley rats. J. Toxicol. Environ. Health Part A. 2005;68:1393–1412. doi: 10.1080/15287390590956551. [DOI] [PubMed] [Google Scholar]
- 28.Emond C., Krishnan K. A physiological pharmacokinetic model based on tissue lipid content for simulating inhalation pharmacokinetics of highly lipophilic volatile organic chemicals. Toxicol. Mech. Methods. 2006;16:395–403. doi: 10.1080/15376510600860474. [DOI] [PubMed] [Google Scholar]
- 29.Needham L.L., Grandjean P., Heinzow B., Jørgensen P.J., Nielsen F., Patterson D.G., Jr., Sjödin A., Turner W.E., Weihe P. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ. Sci. Technol. 2011;45:1121–1126. doi: 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannetje A., Coakley J., Mueller J.F., Harden F., Toms L.M., Douwes J. Partitioning of persistent organic pollutants (POPs) between human serum and breast milk: A literature review. Chemosphere. 2012;89:911–918. doi: 10.1016/j.chemosphere.2012.06.049. [DOI] [PubMed] [Google Scholar]
- 31.Sousa S., Maia M.L., Delerue-Matos C., Calhau C., Domingues V.F. The role of adipose tissue analysis on Environmental Pollutants Biomonitoring in women: The European scenario. Sci. Total Environ. 2022;806:150922. doi: 10.1016/j.scitotenv.2021.150922. [DOI] [PubMed] [Google Scholar]
- 32.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ Clin. Res. Ed. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.EFSA Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA J. 2010;8:1637. doi: 10.2903/j.efsa.2010.1637. [DOI] [Google Scholar]
- 34.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Artacho-Cordón F., Fernández-Rodríguez M., Garde C., Salamanca E., Iribarne-Durán L., Torné P., Expósito J., Papay-Ramírez L., Fernández M., Olea N., et al. Serum and adipose tissue as matrices for assessment of exposure to persistent organic pollutants in breast cancer patients. Environ. Res. 2015;142:633–643. doi: 10.1016/j.envres.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Lv Q.-X., Wang W., Li X.-H., Yu L., Zhang Y., Tian Y. Polychlorinated biphenyls and polybrominated biphenyl ethers in adipose tissue and matched serum from an E-waste recycling area (Wenling, China) Environ. Pollut. 2015;199:219–226. doi: 10.1016/j.envpol.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Luo D., Wan X., Liu J., Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 38.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: A practical tutorial. Evid.-Based Ment. Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S., Chen J., Cheng T., Gindulyte A., He J., He S., Li Q., Shoemaker B.A., Thiessen P.A., Yu B., et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021;49:D1388–D1395. doi: 10.1093/nar/gkaa971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sjödin A., Mueller J.F., Jones R., Schütze A., Wong L.-Y., Caudill S.P., Harden F.A., Webster T.F., Toms L.-M. Serum elimination half-lives adjusted for ongoing exposure of tri-to hexabrominated diphenyl ethers: Determined in persons moving from North America to Australia. Chemosphere. 2020;248:125905. doi: 10.1016/j.chemosphere.2020.125905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bu Q., MacLeod M., Wong F., Toms L.-M.L., Mueller J.F., Yu G. Historical intake and elimination of polychlorinated biphenyls and organochlorine pesticides by the Australian population reconstructed from biomonitoring data. Environ. Int. 2015;74:82–88. doi: 10.1016/j.envint.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Milbrath M.O., Wenger Y., Chang C.W., Emond C., Garabrant D., Gillespie B.W., Jolliet O. Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast-feeding. Environ. Health Perspect. 2009;117:417–425. doi: 10.1289/ehp.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.López-Carrillo L., Torres-Sánchez L., López-Cervantes M., Blair A., Cebrián M.E., Uribe M. The adipose tissue to serum dichlorodiphenyldichloroethane (DDE) ratio: Some methodological considerations. Environ. Res. 1999;81:142–145. doi: 10.1006/enrs.1999.3961. [DOI] [PubMed] [Google Scholar]
- 44.Sontag N.-J., Banks A.P.W., Aylward L.L., O’Rourke N.A., Cavallucci D.J., Mueller J.F., Drage D.S. Comparison of lipid-normalised concentrations of persistent organic pollutants (POPs) between serum and adipose tissue. Int. J. Hyg. Environ. Health. 2021;236:113801. doi: 10.1016/j.ijheh.2021.113801. [DOI] [PubMed] [Google Scholar]
- 45.Zani C., Gelatti U., Donato F., Capelli M., Portolani N., Bergonzi R., Apostoli P. Polychlorinated biphenyls in serum, liver and adipose tissue of subjects with hepatocellular carcinoma living in a highly polluted area. Chemosphere. 2013;91:194–199. doi: 10.1016/j.chemosphere.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 46.Ploteau S., Antignac J.-P., Volteau C., Marchand P., Vénisseau A., Vacher V., Le Bizec B. Distribution of persistent organic pollutants in serum, omental, and parietal adipose tissue of French women with deep infiltrating endometriosis and circulating versus stored ratio as new marker of exposure. Environ. Int. 2016;97:125–136. doi: 10.1016/j.envint.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 47.Pollack A.Z., Krall J.R., Kannan K., Louis G.M.B. Adipose to serum ratio and mixtures of persistent organic pollutants in relation to endometriosis: Findings from the ENDO Study. Environ. Res. 2021;195:110732. doi: 10.1016/j.envres.2021.110732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim M.J., Marchand P., Henegar C., Antignac J.-P., Alili R., Poitou C., Bouillot J.-L., Basdevant A., Le Bizec B., Barouki R., et al. Fate and complex pathogenic effects of dioxins and polychlorinated biphenyls in obese subjects before and after drastic weight loss. Environ. Health Perspect. 2011;119:377–383. doi: 10.1289/ehp.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malarvannan G., Dirinck E., Dirtu A.C., Pereira-Fernandes A., Neels H., Jorens P.G., Van Gaal L., Blust R., Covaci A. Distribution of persistent organic pollutants in two different fat compartments from obese individuals. Environ. Int. 2013;55:33–42. doi: 10.1016/j.envint.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 50.Helaleh M., Diboun I., Al-Tamimi N., Al-Sulaiti H., Al-Emadi M., Madani A., Mazloum N.A., Latiff A., Elrayess M.A. Association of polybrominated diphenyl ethers in two fat compartments with increased risk of insulin resistance in obese individuals. Chemosphere. 2018;209:268–276. doi: 10.1016/j.chemosphere.2018.06.108. [DOI] [PubMed] [Google Scholar]
- 51.Arrebola J., Cuellar M., Claure E., Quevedo M., Antelo S., Mutch E., Ramirez E., Fernandez M., Olea N., Mercado L. Concentrations of organochlorine pesticides and polychlorinated biphenyls in human serum and adipose tissue from Bolivia. Environ. Res. 2012;112:40–47. doi: 10.1016/j.envres.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Antignac J.-P., Cariou R., Zalko D., Berrebi A., Cravedi J.-P., Maume D., Marchand P., Monteau F., Riu A., Andre F., et al. Exposure assessment of French women and their newborn to brominated flame retardants: Determination of tri- to deca- polybromodiphenylethers (PBDE) in maternal adipose tissue, serum, breast milk and cord serum. Environ. Pollut. 2009;157:164–173. doi: 10.1016/j.envpol.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Arrebola J.P., Fernández M.F., Olea N., Ramos R., Martin-Olmedo P. Human exposure to p,p’-dichlorodiphenyldichloroethylene (p,p’-DDE) in urban and semi-rural areas in southeast Spain: A gender perspective. Sci. Total Environ. 2013;458–460:209–216. doi: 10.1016/j.scitotenv.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Bergonzi R., Specchia C., Dinolfo M., Tomasi C., De Palma G., Frusca T., Apostoli P. Distribution of persistent organochlorine pollutants in maternal and foetal tissues: Data from an Italian polluted urban area. Chemosphere. 2009;76:747–754. doi: 10.1016/j.chemosphere.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 55.Björvang R.D., Vinnars M.-T., Papadogiannakis N., Gidlöf S., Mamsen L.S., Mucs D., Kiviranta H., Rantakokko P., Ruokojärvi P., Lindh C.H., et al. Mixtures of persistent organic pollutants are found in vital organs of late gestation human fetuses. Chemosphere. 2021;283:131125. doi: 10.1016/j.chemosphere.2021.131125. [DOI] [PubMed] [Google Scholar]
- 56.Kim K.-S., Lee Y.-M., Kim S.G., Lee I.-K., Lee H.-J., Kim J.-H., Kim J., Moon H.-B., Jacobs D.R., Lee D.-H. Associations of organochlorine pesticides and polychlorinated biphenyls in visceral vs. subcutaneous adipose tissue with type 2 diabetes and insulin resistance. Chemosphere. 2014;94:151–157. doi: 10.1016/j.chemosphere.2013.09.066. [DOI] [PubMed] [Google Scholar]
- 57.Pauwels A., Schepens P.J., D’Hooghe T., Delbeke L., Dhont M., Brouwer A., Weyler J. The risk of endometriosis and exposure to dioxins and polychlorinated biphenyls: A case-control study of infertile women. Hum. Reprod. 2001;16:2050–2055. doi: 10.1093/humrep/16.10.2050. [DOI] [PubMed] [Google Scholar]
- 58.Pestana D., Faria G., Sá C., Fernandes V.C., Teixeira D., Norberto S., Faria A., Meireles M., Marques C., Correia-Sá L., et al. Persistent organic pollutant levels in human visceral and subcutaneous adipose tissue in obese individuals--depot differences and dysmetabolism implications. Environ. Res. 2014;133:170–177. doi: 10.1016/j.envres.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 59.Rusiecki J.A., Matthews A., Sturgeon S., Sinha R., Pellizzari E., Zheng T., Baris D. A correlation study of organochlorine levels in serum, breast adipose tissue, and gluteal adipose tissue among breast cancer cases in India. Cancer Epidemiol. Biomark. Prev. 2005;14:1113–1124. doi: 10.1158/1055-9965.EPI-04-0356. [DOI] [PubMed] [Google Scholar]
- 60.Patterson D.G., Jr., Needham L.L., Pirkle J.L., Roberts D.W., Bagby J., Garrett W.A., Andrews J.S., Jr., Falk H., Bernert J.T., Sampson E.J., et al. Correlation between serum and adipose tissue levels of 2,3,7,8-tetrachlorodibenzo-p-dioxin in 50 persons from Missouri. Arch. Environ. Contam. Toxicol. 1988;17:139–143. doi: 10.1007/BF01056017. [DOI] [PubMed] [Google Scholar]
- 61.Wu J.P., Luo X.J., Zhang Y., Luo Y., Chen S.J., Mai B.X., Yang Z.Y. Bioaccumulation of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in wild aquatic species from an electronic waste (e-waste) recycling site in South China. Environ. Int. 2008;34:1109–1113. doi: 10.1016/j.envint.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Wu J.P., Luo X.J., Zhang Y., Chen S.J., Mai B.X., Guan Y.T., Yang Z.Y. Residues of polybrominated diphenyl ethers in frogs (Rana limnocharis) from a contaminated site, South China: Tissue distribution, biomagnification, and maternal transfer. Environ. Sci. Technol. 2009;43:5212–5217. doi: 10.1021/es901103y. [DOI] [PubMed] [Google Scholar]
- 63.La Merrill M., Emond C., Kim M.J., Antignac J.-P., Le Bizec B., Clément K., Birnbaum L.S., Barouki R. Toxicological function of adipose tissue: Focus on persistent organic pollutants. Environ. Health Perspect. 2013;121:162–169. doi: 10.1289/ehp.1205485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Freedland E.S. Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: Implications for controlling dietary carbohydrates: A review. Nutr. Metab. 2004;1:12. doi: 10.1186/1743-7075-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Preis S.R., Massaro J.M., Robins S.J., Hoffmann U., Vasan R.S., Irlbeck T., Meigs J.B., Sutherland P., D’Agostino R.B., Sr., O’Donnell C.J., et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity. 2010;18:2191–2198. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Emond C., DeVito M.J., Birnbaum L.S. A PBPK model describing the pharmacokinetics of γ-HBCD exposure in mice. Toxicol. Appl. Pharmacol. 2021;428:115678. doi: 10.1016/j.taap.2021.115678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haddad S., Poulin P., Krishnan K. Relative lipid content as the sole mechanistic determinant of the adipose tissue:blood partition coefficients of highly lipophilic organic chemicals. Chemosphere. 2000;40:839–843. doi: 10.1016/S0045-6535(99)00279-9. [DOI] [PubMed] [Google Scholar]
- 68.Artacho-Cordón F., Arrebola J.P., Nielsen O., Hernández P., Skakkebaek N.E., Fernández M.F., Andersson A.M., Olea N., Frederiksen H. Assumed non-persistent environmental chemicals in human adipose tissue; matrix stability and correlation with levels measured in urine and serum. Environ. Res. 2017;156:120–127. doi: 10.1016/j.envres.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 69.Venisse N., Cambien G., Robin J., Rouillon S., Nadeau C., Charles T., Rabouan S., Migeot V., Dupuis A. Development and validation of an LC-MS/MS method for the simultaneous determination of bisphenol A and its chlorinated derivatives in adipose tissue. Talanta. 2019;204:145–152. doi: 10.1016/j.talanta.2019.05.103. [DOI] [PubMed] [Google Scholar]
- 70.Jornod F., Jaylet T., Blaha L., Sarigiannis D., Tamisier L., Audouze K. AOP-helpFinder webserver: A tool for comprehensive analysis of the literature to support adverse outcome pathways development. Bioinformatics. 2021;38:1173–1175. doi: 10.1093/bioinformatics/btab750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carvaillo J.C., Barouki R., Coumoul X., Audouze K. Linking Bisphenol S to Adverse Outcome Pathways Using a Combined Text Mining and Systems Biology Approach. Environ. Health Perspect. 2019;127:47005. doi: 10.1289/EHP4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakayama S.F., St-Amand A., Pollock T., Apel P., Bamai Y.A., Barr D.B., Bessems J., Calafat A.M., Castaño A., Covaci A., et al. Interpreting biomonitoring data: Introducing the international human biomonitoring (i-HBM) working group’s health-based guidance value (HB2GV) dashboard. Int. J. Hyg. Environ. Health. 2022;247:114046. doi: 10.1016/j.ijheh.2022.114046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hays S.M., Aylward L.L. Interpreting human biomonitoring data in a public health risk context using Biomonitoring Equivalents. Int. J. Hyg. Environ. Health. 2012;215:145–148. doi: 10.1016/j.ijheh.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 74.Audouze K., Sarigiannis D., Alonso-Magdalena P., Brochot C., Casas M., Vrijheid M., Babin P.J., Karakitsios S., Coumoul X., Barouki R. Integrative Strategy of Testing Systems for Identification of Endocrine Disruptors Inducing Metabolic Disorders-An Introduction to the OBERON Project. Int. J. Mol. Sci. 2020;21:2988. doi: 10.3390/ijms21082988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Brien K.M., Upson K., Cook N.R., Weinberg C.R. Environmental Chemicals in Urine and Blood: Improving Methods for Creatinine and Lipid Adjustment. Environ. Health Perspect. 2016;124:220–227. doi: 10.1289/ehp.1509693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.