Abstract
Genome integrity is critical for proper cell functioning, and chromosome instability can lead to age-related diseases, including cancer and neurodegenerative disorders. Chromosome instability is caused by multiple factors, including replication stress, chromosome missegregation, exposure to pollutants, and viral infections. Although many studies have investigated the effects of environmental or lifestyle genotoxins on chromosomal integrity, information on the effects of viral infections on micronucleus formation and other chromosomal aberrations is still limited. Currently, HIV infection is considered a chronic disease treatable by antiretroviral therapy (ART). However, HIV-infected individuals still face important health problems, such as chronic inflammation and age-related diseases. In this context, this article reviews studies that have evaluated genomic instability using micronucleus assays in the context of HIV infection. In brief, HIV can induce chromosome instability directly through the interaction of HIV proteins with host DNA and indirectly through chronic inflammation or as a result of ART use. Connections between HIV infection, immunosenescence and age-related disease are discussed in this article. The monitoring of HIV-infected individuals should consider the increased risk of chromosome instability, and lifestyle interventions, such as reduced exposure to genotoxins and an antioxidant-rich diet, should be considered. Therapies to reduce chronic inflammation in HIV infection are needed.
Keywords: antiretroviral therapy, chromosomal aberration, DNA damage, immunosenescence, inflammation, chromosome instability, HIV, micronucleus
1. Introduction
The maintenance of genome integrity is a key factor in proper cell functioning and disease prevention. An accumulation of DNA damage accelerates aging by impairing cell metabolism, causing senescence and immunosenescence, apoptosis, stem-cell exhaustion, and inflammation, among other deleterious effects to cells, thus increasing the risk of age-related diseases [1,2]. Genomic instability, which is the tendency of the genome to undergo mutation or chemical modification [1], is caused by replication stress, chromosome missegregation via defective mitosis, impaired homologous recombination, environmental insults (e.g., exposure to pollutants, pesticides, or radiation), and lifestyle factors (e.g., diet, physical activity, smoking, and alcohol consumption). Genomic instability can result in a loss or amplification of genes, rearrangements, extrachromosomal DNA, and micronuclei formation, among other molecular breakdowns, with multiple pathological consequences, including various types of cancer [3,4,5].
The micronucleus (MN) is a small and rounded DNA-containing nuclear structure observed to be isolated in the cytoplasm, located adjacent to the main nucleus. Mitotic errors or DNA damage can produce lagging chromosomes or chromosome fragments, which are deposited in the cytoplasm in the form of a MN [6]. Increased MN frequency is associated with age, occupational exposure to genotoxins, a heavy smoking habit, and cancer [7,8]. Micronuclei are well-accepted biomarkers of DNA damage and chromosomal instability in multiple organisms, including humans [9].
The rupture of the nuclear envelope of a MN and the consequent exposure of DNA to the cytoplasm has deleterious consequences for cells, such as inflammation and chromothripsis [6]. Of note, chromothripsis is a mutational process that promotes cancer development due to tumor suppressor loss, oncogenic translocations, or oncogene amplification [10]. Inflammation derived from MN formation and rupture is an increasingly recognized issue, associated with various diseases. Therefore, micronuclei are biomarkers of DNA damage and chromosomal instability, as well as inducers of hypermutation and inflammation [9].
Viruses can also damage the genetic material and impair DNA repair processes in host cells directly by interaction with DNA and the proteins of DNA repair machinery, and indirectly by the production of reactive oxygen species (ROS) and oncoproteins, replication stress, exacerbation of inflammatory responses, and impairment of cellular integrity or function. Viruses are therefore recognized inducers of genome instability in host cells, with some viral species (Epstein–Barr virus, hepatitis B virus, Kaposi’s sarcoma-associated herpesvirus, and human papillomaviruses, among others) inducing carcinogenesis in humans [11,12].
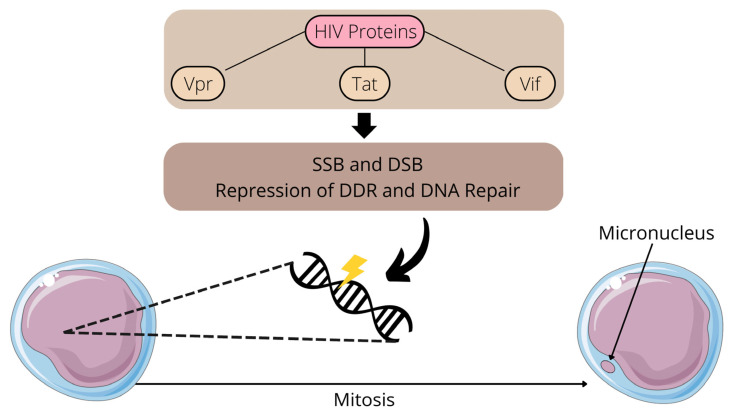
Retroviruses damage DNA through multiple mechanisms, including genome integration, replication, inflammation, and the direct interaction of viral proteins with DNA. For example, HIV-1 lentiviral protein Vpr induces single-strand DNA breaks (SSBs) and double-strand DNA breaks (DBSs), potentially by inducing replication fork collapse after the inhibition of DNA replication [13]. In addition to promoting SSBs and DBSs, Vpr and other HIV-1 proteins (Tat and Vif) may damage DNA by other mechanisms, including by repressing DNA damage response (DDR) and DNA repair [13]. As a consequence, HIV Vpr can induce MN formation and other chromosomal aberrations [14,15]. Of note, HIV proteins Tat, Vif, and Vpr promote cellular arrest, potentially benefiting viral replication, and latent HIV-infected cells are more susceptible to DNA damage [13]. Figure 1 summarizes the connections between HIV proteins and MN formation.
Figure 1.
Effects of HIV proteins on DNA integrity and micronucleus formation. SSB: single-strand DNA break; DSB: double-strand DNA break; DDR: DNA damage response.
HIV drugs are also associated with chromosome instability [16]. People living with HIV, even on antiretroviral therapy (ART), suffer from chronic diseases and conditions associated with aging (e.g., cancer and cardiovascular and neurodegenerative diseases) at an increased rate compared to uninfected people. This indicates that HIV facilitates the occurrence of age-related diseases, independently or in association with other risk factors, potentially through the exacerbation of chronic inflammation, cellular senescence, mitochondrial dysfunction, telomere attrition, stem-cell exhaustion, DNA damage, and genomic instability [17]. Chronic inflammation is a major issue for HIV-infected individuals, and is caused by dysbiosis, microbial translocation, co-infections, and residual HIV replication, among other factors. As mentioned above, these conditions are commonly observed even in HIV-infected individuals undergoing ART [18,19,20,21].
Micronuclei formation and the release of DNA into the cytosol can trigger inflammatory cascades [22]. Infection-related inflammation produces reactive oxygen species (ROS) and reactive nitrogen species which, on one hand, are important in protecting the host from pathogens but, on the other hand, cause DNA damage. Consequently, DNA damage and DDR trigger further inflammation, creating a vicious circle of inflammation and genetic damage [22]. Other cellular processes also influence MN formation during HIV infection. Autophagy promotes cellular recycling [23,24] and can limit the amount of cytoplasmic DNA derived from DNA insults (e.g., MN can be degraded by the autophagy–lysosomal pathway), thus determining cell fate following genomic instability events. In brief, autophagy is a protective mechanism against MN formation and related consequences [25,26] and this can explain, at least partially, the “genome-stabilizing effects of autophagy” [27]. Autophagy also contributes to the maintenance of the innate immune homeostasis, protecting humans from inflammatory conditions [24]. HIV can inhibit autophagy [23,28,29,30] and, consequently, such inhibition contributes to chromosome instability and chronic inflammation. Deciphering the connections between viral infection, genome instability, and DDR can help understandings of viral pathogenesis and the development of better antiviral therapies [13].
The buccal micronucleus cytome (BMCyt) assay is a method used to study chromosomal instability, DNA damage, and cell death using cells exfoliated from oral mucosal tissue, based on the microscopic analysis of cytoplasmic and nuclear morphology [31]. The BMCyt assay is considered a minimally invasive method [31]; therefore, it is widely used by laboratories from different parts of the world to evaluate genetic damage in human populations [8]. The BMCyt assay yields the quantification of the frequency of chromosome breakage or loss due to incorrect mitosis (observed microscopically in the cytoplasm as a MN), gene amplification (viewed as nuclear buds), cytokinesis failure or arrest (observed as binucleated cells), and cell death (observed as condensed chromatin, karyorrhectic, pyknotic, or karyolitic cells) [8,31]. Of note, MN frequency in exfoliated buccal cells highly correlates with MN frequency in peripheral blood lymphocytes [8]. The estimated spontaneous MN frequency in (healthy) human buccal exfoliated cells is 0.74‰ (95% CI 0.52–1.05) [8]. The BMCyt assay is commonly called the “MN test” or “MN assay”, and variations of this technique also exist, including the evaluation of micronuclei and other nuclear anomalies in non-buccal epithelial cells (e.g., nasal and cervical cells) [9].
Considering the information mentioned above and the poorly explored connections between HIV infection, inflammation, and chromosomal instability, we reviewed studies from multiple countries that evaluate genomic instability, using MN as a biomarker, in the context of HIV infection. This narrative review also discusses the connections between HIV infection, immunosenescence, and age-related diseases.
2. Impacts of HIV Infection and Treatment on Chromosomal Integrity: A Focus on Human Studies
In Brazil, Lima et al. [32] investigated micronuclei in exfoliated oral cells of HIV-infected individuals undergoing ART and non-infected controls (n = 30 each group). In addition to measuring MN frequency, the authors separated micronuclei into two categories: (I) single MN and (II) multiple micronuclei. The total number of micronucleated cells and the MN number were not statistically different between groups, and no statistical correlation between CD4+ T cell counts, and MN frequency was observed. Considering the two MN categories mentioned, a statistically significant increased mean of single MN in the cells of the controls compared to those of HIV-infected individuals and a non-significant increase in the occurrence of multiple micronuclei in the cells of the HIV group compared to the controls were reported. However, the small sample size of this study and the absence of difference in overall MN frequency between the groups may limit interpretations of these findings [32].
In South Africa, Baeyens et al. [33] evaluated the chromosomal radiosensitivity of HIV-infected individuals (n = 49) and controls (n = 29) using the MN assay. Blood samples from both groups were exposed to doses of 6 MV X-rays in vitro, ranging from 1 to 4 Gy. MN frequencies were significantly higher in irradiated lymphocytes of HIV-infected individuals compared to the controls at all exposure doses (1, 2, 3, and 4 Gy), which can be at least partially attributed to differences in CD4+/CD8+ T cell ratios between HIV-infected individuals and controls, in addition to the direct effects of HIV on chromosomal instability (increased DNA damage and impaired DNA repair and apoptosis, among others). In brief, this result suggests that cells of HIV-infected individuals have increased radiosensitivity [33]. Subsequently, other studies confirmed these findings, also describing increased MN frequencies in the blood cells of South African HIV-infected individuals exposed to radiation in vitro [34,35]. Increased radiosensitivity can be problematic, especially in HIV-infected individuals with cancer who need to undergo radiotherapy [35].
Zizza et al. [36] evaluated MN frequency in peripheral blood mononuclear cells of HIV-infected individuals undergoing ART (n = 52) and non-infected controls (n = 55), and both groups were from Italy. They found an increased MN frequency in the HIV-infected group, with HCV co-infection and HIV-RNA load being risk factors for increased MN frequency. Individuals with undetectable viremia showed a reduced MN frequency compared to those with uncontrolled viremia. These results indicate that MN in HIV-infected individuals undergoing ART is not a feature exclusively derived from ART or HIV infection per se, but that it is also due to problems associated with chronic infection (i.e., co-infections). However, the absence of a group composed of HIV-infected but ART-naive individuals precludes the inferring of the degree of contribution of HIV infection and ART use on MN frequency, separately [36].
Several in vitro studies, as well as studies performed in animals, have evidenced genotoxic effects (e.g., formation of MN and nucleoplasmic bridges) of various drugs used to treat HIV infection, including zidovudine, tenofovir disoproxil fumarate, lamivudine, and efavirenz. Taken together, these reports indicate that HIV drugs can indeed induce clastogenic and aneugenic effects on chromosomes [16,37,38,39,40,41].
Zidovudine-based ART is commonly used to prevent mother-to-child HIV transmissions. In this context, Witt et al. [42] evaluated chromosomal damage in children exposed to zidovudine (transplacentary and post-partum) in a study performed in the USA. Micronucleated reticulocyte frequencies were evaluated by flow cytometry in children (n = 16, all subjects received prophylactic post-partum zidovudine for 6 weeks) and mothers on zidovudine-based ART (n = 13) or ART without zidovudine (n = 3, a small control group) pre-natal. In women, samples were obtained from venous blood. In children, samples were obtained from both cord blood at birth and subsequently from venous blood. Controls were obtained from HIV-negative cord-blood samples (n = 10). A 10-fold increase in micronucleated reticulocyte frequencies was observed in mothers and children pre-natal with zidovudine-based ART compared to the controls [42]. These results confirm, in humans, the genotoxic effects of zidovudine observed in vitro [37] and in animals [38].
In India, Shah et al. [43] evaluated MN frequency in oral cells of HIV-infected women undergoing ART and healthy controls (n = 25 each group) using Papanicolaou staining smears. The HIV-infected group showed a significant increase in MN rate (~two-fold) compared to the controls. Papanicolaou stain is considered effective in detecting and evaluating micronuclei [43]. However, it is important to stress that an abnormal increase in MN frequency can be observed in non-DNA-specific stains. For example, Giemsa and aceto-orcein stains can produce higher MN frequencies. Feulgen-Fast Green is considered the most specific and versatile DNA-specific staining method; therefore, it is the standard staining method used in the BMCyt assay [8].
In Mexico, Gutiérrez-Sevilla et al. [44] evaluated genomic instability markers in HIV-infected individuals undergoing ART (n = 46, with two sub-groups, each receiving a specific ART combination), HIV-infected but ART-naive individuals (n = 13), and HIV-negative controls (n = 8). Genomic instability markers were accessed by nuclear abnormality analyses (MN, binucleated cells, nuclear buds, karyorrhexis, karyolysis, and pyknosis) of buccal mucosal samples using the BMCyt assay [31]. No difference in MN frequencies was observed between the groups. However, higher frequencies of binucleated cells and nuclear buds were observed in both HIV-infected ART-naive individuals and HIV-infected individuals undergoing ART compared to the HIV-negative control group. This result suggests that the effect of HIV on genomic instability can occur independently of ART. However, differences in genomic instability caused by variations of ART regimens can occur. Karyorrhexis, binucleated cells, and nuclear buds were found to be increased in the subgroup of HIV-infected individuals receiving reverse transcriptase inhibitors (RTIs) as ART compared to the controls. Such a difference was not observed between the subgroup of HIV-infected individuals receiving protease inhibitors (PIs) and controls. This suggest that ART based on PIs can produce less cytotoxic damage than RTIs. Finally, the authors also found a positive correlation between the nuclear buds and CD4+/CD8+ ratio among the HIV-infected individuals, suggesting a role of CD4+ T cells in genomic instability occurrence [44].
As mentioned previously [16,37,38,39,40,41] and reinforced by the results of Gutiérrez-Sevilla et al. [44], different antiretroviral combinations have varying toxic effects on DNA. Since the United States Food and Drug Administration (FDA) approved Zidovudine in 1987 for the treatment of HIV infection [45], many other antiretrovirals have been developed and HIV therapy has greatly advanced, with important improvements in terms of decreased toxicity and side effects. However, even modern antiretrovirals have some cellular toxicity, damaging DNA and mitochondria to some extent. Maraviroc is an antiretroviral approved for clinical use in 2007. This drug inhibits HIV infection by interfering with virus interaction with the human C-C chemokine receptor type 5 (CCR5), the main HIV-1 co-receptor [46]. Other new CCR5 antagonists for the treatment of HIV infection and other conditions (i.e., cancers) are under investigation, including Vicriviroc and Leronlimab [47,48]. The main function of CCR5 is to regulate the activity of inflammatory cells [46], but it has recently been demonstrated that CCR5 also participates in the control of DNA repair [47]. Consequently, CCR5 antagonists (i.e., Maraviroc, Vicriviroc, and Leronlimab) can lead to genomic instability by impairing DNA repair and through other CCR5-related mechanisms [47,48,49]. However, it is important to emphasize that evidence of the participation of CCR5 in DNA damage/repair comes from cancer studies, in which the participation of other drugs is present [47,48,49]. The clinical significance of this effect of CCR5 antagonists on DNA damage in HIV-infected individuals is still speculative. Beyond CCR5 antagonists, other HIV drugs (e.g., Dolutegravir) can cause mitochondrial ROS production, mtDNA damage, mitochondrial dysfunction, and cell death [50,51,52,53].
Lazarde-Ramos et al. [54] observed, also in Mexico, an increased frequency of MN and other nuclear abnormalities (binucleated and karyorrhectic cells) in HIV-infected individuals (n = 22) undergoing ART (ATRIPLA: efavirenz, 600 mg; emtricitabine, 200 mg; and tenofovir disoproxil fumarate, 300 mg) compared to a control group (n = 22) using the BMCyt assay. In this same study [54], the administration of ART in combination with aqueous (n = 22) or methanolic (n = 23) extracts of rosemary (Rosmarinus officinalis, a plant species with anti-inflammatory and antioxidant properties [55]), significantly reduced the frequency of MN (in the methanolic extract group) and abnormally condensed chromatin, karyorrhexis, and binucleated cells (in both the methanolic and aqueous extract groups), compared to the use of ART alone. These findings suggest that the prescription of antioxidants or plant extracts with antioxidant properties could be beneficial for HIV-infected individuals undergoing ART [54]. However, more studies on these aspects need to be performed before any clinical recommendation, especially considering the potential adverse effects of herbal medicines and herb–drug interactions. Finally, Table 1 summarizes the impacts of HIV infection and treatment on chromosomal integrity based on human studies cited in this section.
Table 1.
Summary of the impacts of HIV infection and treatment on chromosomal integrity based on studies with humans.
| Country | Cell Type Investigated | Main Findings | References |
|---|---|---|---|
| Brazil | Exfoliated oral cells | Increased mean of single MN in cells of controls compared to those of HIV-infected individuals; non-significant increase in the occurrence of multiple micronuclei in cells of HIV group compared to controls | Lima et al. [32] |
| South Africa | Blood cells | MN frequencies were significantly higher in irradiated lymphocytes from HIV-infected individuals compared to controls | Baeyens et al. [33]; Herd et al. [34]; Herd et al. [35] |
| Italy | Blood cells | Increased MN frequency in the HIV-infected group (HCV co-infection and HIV-RNA load being risk factors for increased MN frequency); HIV-infected individuals with undetectable viremia showed reduced MN frequency compared to those with uncontrolled viremia | Zizza et al. [36] |
| USA | Reticulocytes | A 10-fold increase in micronucleated reticulocyte frequencies was observed in mothers and children pre-natal with zidovudine-based ART compared to controls | Witt et al. [42] |
| India | Exfoliated oral cells | HIV-infected individuals showed significantly increased in MN rate (~two-fold) compared to controls | Shah et al. [43] |
| Mexico | Exfoliated oral cells | Higher frequencies of binucleated cells and nuclear buds in both HIV-infected ART-naive individuals and HIV-infected individuals undergoing ART compared to HIV-negative controls; karyorrhexis, binucleated cells, and nuclear buds were found to be increased in a subgroup of HIV-infected individuals receiving RTIs as ART compared to controls | Gutiérrez-Sevilla et al. [44] |
| Mexico | Exfoliated oral cells | Increased frequency of MN and other nuclear abnormalities in HIV-infected individuals undergoing ART compared to controls | Lazarde-Ramos et al. [54] |
MN: micronucleus. RTIs: reverse transcriptase inhibitors.
Potential Lifestyle and Nutritional Interventions to Be Used in Association with ART
In addition to the potential benefits of plant-based extracts highlighted by Lazarde-Ramos et al. [54], some lifestyles and nutritional habits can help to control genome instability, thus benefiting HIV-infected individuals undergoing ART. For example, reduced MN frequency is associated with daily fruit consumption [8]. In this sense, proper intake of micronutrients (e.g., antioxidant vitamins, selenium), regular physical activity, and other behavior interventions (e.g., UV protection, cessation of tobacco smoking, and adequate sleep/rest) can help reduce oxidative stress and HIV-related aging manifestations, including genome instability [1,2,17,56,57]. Specifically concerning micronutrients, it is essential to consider the potential deleterious effects of some nutrients, especially when used in inappropriate doses. For example, selenium in excess can be detrimental (even toxic) to humans [58,59]. High vitamin D levels can trigger inflammation in HIV-infected individuals [60], and vitamin D supplementation in individuals with proper plasma levels of this micronutrient does not provide health benefits [61]. Recommendations for the therapeutic use of micronutrients during HIV infection should be made by a qualified health professional (e.g., physician, nutritionist), and it is essential to pay special attention to the prescribed doses for each micronutrient.
Some studies suggest that a diet rich in anti-inflammatory compounds (food-derived fibers, ω-3, magnesium, flavonoids, and carotenoids, among others), commonly observed in Mediterranean diet patterns and ‘plant-based foods’, may help to control chronic inflammation [62,63], being potentially beneficial for HIV-infected individuals undergoing ART concerning inflammatory, metabolic, and immune markers [64,65,66,67]. However, therapeutic strategies (from nutritional therapy to regular drugs) to control inflammation in chronic HIV infection are still limited, indicating the need for more studies to focus on this issue.
3. Immunosenescence, HIV Infection and Chromosome Instability
The concept of immunosenescence includes a set of processes that culminate in a weakening of the immune system following the course of aging, associated with increased morbidity and mortality risks [68,69,70]. Immunosenescence-related processes include changes in the innate and adaptive immune systems, with atrophy or involution of the thymus being the first observed and best characterized process related to immunosenescence [69,70]. Thymus involution leads to a decrease in the production of naive T cells, which results in an activation of further replication of pre-existing memory T cells in an attempt to maintain a meaningful and still diverse repertoire [71,72]. Such increased replication results in ‘replicative senescence’, in which cells lose the ability to replicate over time until they reach exhaustion [69,70]. T cell exhaustion also occurs when cells are chronically exposed to high levels of antigens, leading to severe T cell dysfunction. This exhaustion state triggers a deficient immune control of HIV infection. Consequently, chronic HIV infection creates a vicious circle of infection-associated antigen production and a loss of control of HIV infection [73]. Of note, inhibitory signals of T cell activation (e.g., PD-1, TIGIT, LAG-3) [73] are linked to T cell exhaustion, persistent HIV infection, and disease progression, even in individuals undergoing ART [74,75,76]. Following thymus involution, the secretion of pro-inflammatory cytokines occurs, which correlates with a state of chronic inflammation and, at the same time, a greater susceptibility to infections, autoimmunity, and cardiovascular diseases, as well as other outcomes present in elderly individuals. Furthermore, HIV-infected individuals with immunosenescence features show an acceleration of progression to AIDS [69,70].
The profile of immune cells can be used as indicators of aging and to determine the immunosenescence state [77]. The epigenetic clock (i.e., DNA methylation data, DNA methylation-based estimate of telomere length) is a useful biomarker in detecting HIV-related aging, a marker that usually indicates accelerated aging in HIV-infected individuals [78,79]. Furthermore, telomere length is a pivotal marker of replicative history and an indicator of biological aging, and telomere shortening is associated with chromosome instability and immunosenescence [80,81,82]. In this context, multiple studies have shown that inflammation, immune activation, and other factors related to HIV infection are associated with telomere shortening, which can contribute to immunosenescence, aging, and age-related diseases in HIV-infected individuals [80,83,84,85,86,87,88]. The ‘oxi-inflamm-aging theory’ is a concept of aging that describes this biological process as an association of chronic, low-grade inflammation in association with oxidative stress, which prejudices the homeostasis of the nervous, endocrine, and immune systems. This culminates in higher morbidity and mortality [89].
Many causes of ‘inflamm-aging’ are related to chromosome instability, such as defective autophagy/mitophagy, the activation of inflammasome by cell debris and misplaced self-molecules, and DDR activation [90]. Cell senescence is, at least partially, a result of chromosomal damage accumulation and defective cell-cycle functioning. Genome instability, expressed by nuclear anomalies and chromosomal aberrations, can lead to defective cell division, apoptosis, cell-cycle arrest, and carcinogenesis [91,92,93]. Furthermore, MN frequency increases with aging and is associated with less proliferative lymphocytes [94]. Additionally, increased genomic instability and MN formation have an impact on lymphocyte function and immunosenescence [93]. Chromosome instability and immunosenescence are therefore connected processes that can form a vicious circle, which can be aggravated by HIV infection.
Immunoscenence is also associated with insertions of mitochondrial sequences into the nuclear DNA of T lymphocytes, which increased progressively with aging [93]. ROS generation in the mitochondria (mitROS) could damage/fragment mtDNA, with such fragments accumulating into the nuclear genome. mtDNA insertions may compromise chromosome segregation and cause increasing genomic instability and MN formation in lymphocytes, forming cells with a reduced proliferation capacity and an increase in apoptosis, which are typical immunosenescence markers. These processes may affect the balance of cell division, differentiation, senescence, and death, which is essential for the maintenance of tissue homeostasis. These processes could be a major contributing factor to aging and even cancer formation [93,95,96]. A robust body of evidence supports the occurrence of increased mtDNA damage and mitochondrial dysfunction in different cell types of HIV-infected individuals, including neuronal cells [97,98,99,100,101,102,103]. It is worth noting that mtDNA damage may be associated with HIV-related neurocognitive disorders [100,101].
The immune system in the context of HIV infection is severely weakened in individuals who progress to AIDS, usually in the absence of ART. However, even in individuals who receive adequate treatment and maintain an undetectable viral load, there are important and broad health consequences that cannot be overlooked [69,104]. In the context of immunosenescence, there is an acceleration of the processes that lead to the premature aging of the immune system, mainly because there is an increased activation of the response mechanisms due to the infection and associated features (e.g., co-infections, inflammation, and comorbidities). This increased activation results in greater cell replication and death associated with antiviral responses, which lead to the exhaustion of the immune system [105,106]. Furthermore, even the main pathway that leads to immunosenescence, which is thymus involution, is present at an early stage in HIV-infected individuals, as well as other damages in tissues that are important for the proper functioning of the immune system, such as bone marrow and liver tissue. Taken together, there is a prominent and premature aging of the immune system in HIV-infected individuals, with young people sometimes presenting clinical signs or immune features of individuals in their 40s [69]. This reverberates on multiple health aspects, including increased chromosome instability and associated risks.
4. Conclusions
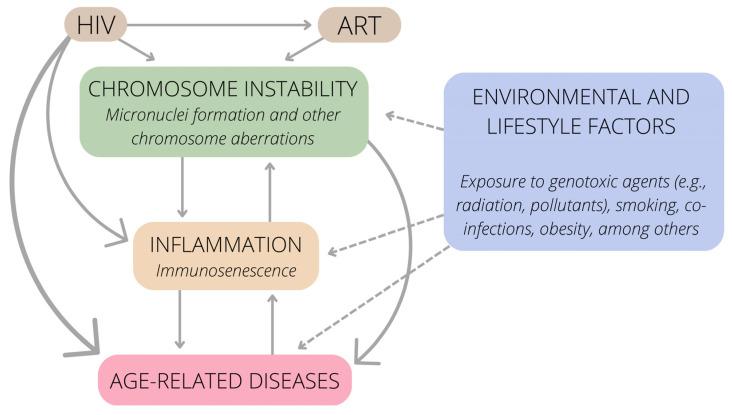
The impact of HIV infection on chronic inflammation and chromosome instability is an emerging topic, especially because HIV infection is currently considered a chronic disease in many countries. In conclusion, both HIV and ART can cause chromosome instability, leading to MN formation and other chromosome aberrations (Table 1). Inflammation and immunosenescence are both the cause and consequence of chromosome instability. HIV infection also triggers inflammation by other mechanisms. Age-related diseases result, directly or indirectly, from (I) chromosome instability, (II) inflammation, and (III) HIV infection. These connections are summarized in Figure 2. Of note, these connections must be interpreted while taking into account classic environmental and lifestyle factors that can also trigger chromosomal instability, inflammation, and associated diseases (smoking habits, heavy metals, pollutants, and obesity, among many others) [8,107,108,109]. Dietary and lifestyle interventions, including reduced exposure to genotoxins and an antioxidant-rich diet, could be considered to mitigate the deleterious effects of HIV infection on DNA integrity.
Figure 2.
Connections between HIV infection, chromosome instability, inflammation, and age-related diseases.
Finally, the number of human studies that evaluate chromosome instability in the context of HIV infection is limited, which is noteworthy. Additionally, in general, the sample size in such studies is small, which represents another limitation. New studies concerning these topics can contribute to the clinical management of HIV-infected individuals undergoing ART, reducing chromosome instability and related health consequences.
Acknowledgments
Figures were created with Canva [110] and Servier Medical Art [111] templates.
Author Contributions
Conceptualization, J.H.E.; Writing—original draft, J.H.E., B.K.-L. and M.Z; Writing—review and editing, J.H.E., B.K.-L., M.Z. and J.A.B.C.; Visualization, J.H.E. and B.K.-L.; Project administration, J.H.E.; Supervision, J.A.B.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
Joel Henrique Ellwanger receives a postdoctoral fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Programa Nacional de Pós-Doutorado–PNPD/CAPES, Brazil). Bruna Kulmann-Leal receives a doctoral fellowship from CAPES (Brazil). Marina Ziliotto receives a master fellowship from CAPES (Brazil). José Artur Bogo Chies receives a research fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (Bolsa de Produtividade em Pesquisa-Nível 1A, CNPq, Brazil) and has research funded by CAPES (CAPES AUXPE 686/2020; Brazil).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Schumacher B., Pothof J., Vijg J., Hoeijmakers J.H.J. The central role of DNA damage in the ageing process. Nature. 2021;592:695–703. doi: 10.1038/s41586-021-03307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yousefzadeh M., Henpita C., Vyas R., Soto-Palma C., Robbins P., Niedernhofer L. DNA damage-how and why we age? Elife. 2021;10:e62852. doi: 10.7554/eLife.62852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valverde M., Rojas E. Environmental and occupational biomonitoring using the Comet assay. Mutat. Res. 2009;681:93–109. doi: 10.1016/j.mrrev.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Fenech M., Bonassi S. The effect of age, gender, diet and lifestyle on DNA damage measured using micronucleus frequency in human peripheral blood lymphocytes. Mutagenesis. 2011;26:43–49. doi: 10.1093/mutage/geq050. [DOI] [PubMed] [Google Scholar]
- 5.Drews R.M., Hernando B., Tarabichi M., Haase K., Lesluyes T., Smith P.S., Morrill Gavarró L., Couturier D.L., Liu L., Schneider M., et al. A pan-cancer compendium of chromosomal instability. Nature. 2022;606:976–983. doi: 10.1038/s41586-022-04789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon M., Leibowitz M.L., Lee J.H. Small but mighty: The causes and consequences of micronucleus rupture. Exp. Mol. Med. 2020;52:1777–1786. doi: 10.1038/s12276-020-00529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonassi S., Znaor A., Ceppi M., Lando C., Chang W.P., Holland N., Kirsch-Volders M., Zeiger E., Ban S., Barale R., et al. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis. 2007;28:625–631. doi: 10.1093/carcin/bgl177. [DOI] [PubMed] [Google Scholar]
- 8.Bonassi S., Coskun E., Ceppi M., Lando C., Bolognesi C., Burgaz S., Holland N., Kirsh-Volders M., Knasmueller S., Zeiger E., et al. The HUman MicroNucleus project on eXfoLiated buccal cells (HUMNXL): The role of life-style, host factors, occupational exposures, health status, and assay protocol. Mutat. Res. 2011;728:88–97. doi: 10.1016/j.mrrev.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Fenech M., Knasmueller S., Bolognesi C., Holland N., Bonassi S., Kirsch-Volders M. Micronuclei as biomarkers of DNA damage, aneuploidy, inducers of chromosomal hypermutation and as sources of pro-inflammatory DNA in humans. Mutat. Res. Rev. Mutat. Res. 2020;786:108342. doi: 10.1016/j.mrrev.2020.108342. [DOI] [PubMed] [Google Scholar]
- 10.Tang S., Stokasimov E., Cui Y., Pellman D. Breakage of cytoplasmic chromosomes by pathological DNA base excision repair. Nature. 2022;606:930–936. doi: 10.1038/s41586-022-04767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weitzman M.D., Weitzman J.B. What’s the damage? The impact of pathogens on pathways that maintain host genome integrity. Cell Host Microbe. 2014;15:283–294. doi: 10.1016/j.chom.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katerji M., Duerksen-Hughes P.J. DNA damage in cancer development: Special implications in viral oncogenesis. Am. J. Cancer Res. 2021;11:3956–3979. [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez A., Nichols Doyle R., Sandoval C., Nisson K., Yang V., Fregoso O.I. Viral modulation of the DNA damage response and innate immunity: Two sides of the same coin. J. Mol. Biol. 2022;434:167327. doi: 10.1016/j.jmb.2021.167327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimura M., Tanaka Y., Nakamura S., Minemoto Y., Yamashita K., Hatake K., Takaku F., Ishizaka Y. Micronuclei formation and aneuploidy induced by Vpr, an accessory gene of human immunodeficiency virus type 1. FASEB J. 1999;13:621–637. doi: 10.1096/fasebj.13.6.621. [DOI] [PubMed] [Google Scholar]
- 15.Chang F., Re F., Sebastian S., Sazer S., Luban J. HIV-1 Vpr induces defects in mitosis, cytokinesis, nuclear structure, and centrosomes. Mol. Biol. Cell. 2004;15:1793–1801. doi: 10.1091/mbc.e03-09-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moraes Filho A.V., Carvalho C.J.S., Carneiro C.C., Vale C.R., Lima D.C.S., Carvalho W.F., Vieira T.B., Silva D.M., Cunha K.S., Chen-Chen L. Genotoxic and cytotoxic effects of antiretroviral combinations in mice bone marrow. PLoS ONE. 2016;11:e0165706. doi: 10.1371/journal.pone.0165706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montano M., Oursler K.K., Xu K., Sun Y.V., Marconi C.V. Biological ageing with HIV infection: Evaluating the geroscience hypothesis. Lancet Healthy Longev. 2022;3:e194–e205. doi: 10.1016/S2666-7568(21)00278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deeks S.G., Lewin S.R., Havlir D.V. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valverde-Villegas J.M., de Medeiros R.M., Ellwanger J.H., Santos B.R., Melo M.G., Almeida S.E.M., Chies J.A.B. High CXCL10/IP-10 levels are a hallmark in the clinical evolution of the HIV infection. Infect. Genet. Evol. 2018;57:51–58. doi: 10.1016/j.meegid.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Zicari S., Sessa L., Cotugno N., Ruggiero A., Morrocchi E., Concato C., Rocca S., Zangari P., Manno E.C., Palma P. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses. 2019;11:200. doi: 10.3390/v11030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellwanger J.H., Valverde-Villegas J.M., Kaminski V.L., de Medeiros R.M., Almeida S.E.M., Santos B.R., de Melo M.G., Hackenhaar F.S., Chies J.A.B. Increased IL-8 levels in HIV-infected individuals who initiated ART with CD4+ T cell counts <350 cells/mm3-A potential hallmark of chronic inflammation. Microbes Infect. 2020;22:474–480. doi: 10.1016/j.micinf.2020.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Kay J., Thadhani E., Samson L., Engelward B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair. 2019;83:102673. doi: 10.1016/j.dnarep.2019.102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nardacci R., Ciccosanti F., Marsella C., Ippolito G., Piacentini M., Fimia G.M. Role of autophagy in HIV infection and pathogenesis. J. Intern. Med. 2017;281:422–432. doi: 10.1111/joim.12596. [DOI] [PubMed] [Google Scholar]
- 24.Chauhan S., Jena K.K., Mehto S., Chauhan N.R., Sahu R., Dhar K., Yadav R., Krishna S., Jaiswal P., Chauhan S. Innate immunity and inflammophagy: Balancing the defence and immune homeostasis. FEBS J. 2022;289:4112–4131. doi: 10.1111/febs.16298. [DOI] [PubMed] [Google Scholar]
- 25.Talens F., Van Vugt M.A.T.M. Inflammatory signaling in genomically instable cancers. Cell Cycle. 2019;18:1830–1848. doi: 10.1080/15384101.2019.1638192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krupina K., Goginashvili A., Cleveland D.W. Causes and consequences of micronuclei. Curr. Opin. Cell Biol. 2021;70:91–99. doi: 10.1016/j.ceb.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rello-Varona S., Lissa D., Shen S., Niso-Santano M., Senovilla L., Mariño G., Vitale I., Jemaá M., Harper F., Pierron G., et al. Autophagic removal of micronuclei. Cell Cycle. 2012;11:170–176. doi: 10.4161/cc.11.1.18564. [DOI] [PubMed] [Google Scholar]
- 28.Van Grol J., Subauste C., Andrade R.M., Fujinaga K., Nelson J., Subauste C.S. HIV-1 inhibits autophagy in bystander macrophage/monocytic cells through Src-Akt and STAT3. PLoS ONE. 2010;5:e11733. doi: 10.1371/journal.pone.0011733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinkins C., Pilli M., Kehrl J.H. Roles of autophagy in HIV infection. Immunol. Cell Biol. 2015;93:11–17. doi: 10.1038/icb.2014.88. [DOI] [PubMed] [Google Scholar]
- 30.Sardo L., Iordanskiy S., Klase Z., Kashanchi F. HIV-1 Nef blocks autophagy in human astrocytes. Cell Cycle. 2015;14:3781–3782. doi: 10.1080/15384101.2015.1105700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas P., Holland N., Bolognesi C., Kirsch-Volders M., Bonassi S., Zeiger E., Knasmueller S., Fenech M. Buccal micronucleus cytome assay. Nat. Protoc. 2009;4:825–837. doi: 10.1038/nprot.2009.53. [DOI] [PubMed] [Google Scholar]
- 32.Lima C.F., Alves M.G.O., Furtado J.J.D., Marcucci M., Balducci I., Almeida J.D. Effect of HIV infection in the micronuclei frequency on the oral mucosa. J. Oral Pathol. Med. 2017;46:644–648. doi: 10.1111/jop.12527. [DOI] [PubMed] [Google Scholar]
- 33.Baeyens A., Slabbert J.P., Willem P., Jozela S., Van Der Merwe D., Vral A. Chromosomal radiosensitivity of HIV positive individuals. Int. J. Radiat. Biol. 2010;86:584–592. doi: 10.3109/09553001003734576. [DOI] [PubMed] [Google Scholar]
- 34.Herd O., Francies F., Slabbert J., Baeyens A. The effect of HIV and antiretroviral therapy on chromosomal radiosensitivity. J. AIDS Clin. Res. 2014;5:12. doi: 10.4172/2155-6113.1000397. [DOI] [Google Scholar]
- 35.Herd O., Francies F., Kotzen J., Smith T., Nxumalo Z., Muller X., Slabbert J., Vral A., Baeyens A. Chromosomal radiosensitivity of human immunodeficiency virus positive/negative cervical cancer patients in South Africa. Mol. Med. Rep. 2016;13:130–136. doi: 10.3892/mmr.2015.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zizza A., Grima P., Andreassi M.G., Tumolo M.R., Borghini A., De Donno A., Negro P., Guido M. HIV infection and frequency of micronucleus in human peripheral blood cells. J. Prev. Med. Hyg. 2019;60:E191–E196. doi: 10.15167/2421-4248/jpmh2019.60.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stern M., Cid M.G., Larripa I., Slavutsky I. AZT-induction of micronuclei in human lymphocyte subpopulations. Toxicol. Lett. 1994;70:235–242. doi: 10.1016/0378-4274(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 38.Ayers K.M., Clive D., Tucker W.E., Jr., Hajian G., de Miranda P. Nonclinical toxicology studies with zidovudine: Genetic toxicity tests and carcinogenicity bioassays in mice and rats. Fundam. Appl. Toxicol. 1996;32:148–158. doi: 10.1006/faat.1996.0118. [DOI] [PubMed] [Google Scholar]
- 39.Bayram S., Topaktaş M. Confirmation of the chromosome damaging effects of lamivudine in in vitro human peripheral blood lymphocytes. Environ. Mol. Mutagen. 2008;49:328–333. doi: 10.1002/em.20393. [DOI] [PubMed] [Google Scholar]
- 40.Olivero O.A., Vazquez I.L., Cooch C.C., Ming J., Keller E., Yu M., Borojerdi J.P., Braun H.M., McKee E., Poirier M.C. Long-term AZT exposure alters the metabolic capacity of cultured human lymphoblastoid cells. Toxicol. Sci. 2010;115:109–117. doi: 10.1093/toxsci/kfq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grando A.C., Guimarães N.N., de Souza A.P., Lehmann M., Cunha K.S., Dihl R.R. Assessment of complex genomic alterations induced by AZT, 3TC, and the combination AZT +3TC. Drug Chem. Toxicol. 2020;43:429–434. doi: 10.1080/01480545.2018.1504959. [DOI] [PubMed] [Google Scholar]
- 42.Witt K.L., Cunningham C.K., Patterson K.B., Kissling G.E., Dertinger S.D., Livingston E., Bishop J.B. Elevated frequencies of micronucleated erythrocytes in infants exposed to zidovudine in utero and postpartum to prevent mother-to-child transmission of HIV. Environ. Mol. Mutagen. 2007;48:322–329. doi: 10.1002/em.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah S., Singaraju S., Bertin E.T., Singaraju M., Sharma A. Quantification of micronuclei in exfoliated cells of human immunodeficiency virus/AIDS-infected female patients. J. Oral Maxillofac. Pathol. 2019;23:301. doi: 10.4103/jomfp.JOMFP_251_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gutiérrez-Sevilla J.E., Cárdenas-Bedoya J., Escoto-Delgadillo M., Zúñiga-González G.M., Pérez-Ríos A.M., Gómez-Meda B.C., González-Enríquez G.V., Figarola-Centurión I., Chavarría-Avila E., Torres-Mendoza B.M. Genomic instability in people living with HIV. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2021;865:503336. doi: 10.1016/j.mrgentox.2021.503336. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X. Anti-retroviral drugs: Current state and development in the next decade. Acta Pharm. Sin. B. 2018;8:131–136. doi: 10.1016/j.apsb.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ellwanger J.H., Kulmann-Leal B., Kaminski V.L., Rodrigues A.G., Bragatte M.A.S., Chies J.A.B. Beyond HIV infection: Neglected and varied impacts of CCR5 and CCR5Δ32 on viral diseases. Virus Res. 2020;286:198040. doi: 10.1016/j.virusres.2020.198040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiao X., Velasco-Velázquez M.A., Wang M., Li Z., Rui H., Peck A.R., Korkola J.E., Chen X., Xu S., DuHadaway J.B., et al. CCR5 governs DNA damage repair and breast cancer stem cell expansion. Cancer Res. 2018;78:1657–1671. doi: 10.1158/0008-5472.CAN-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiao X., Wang M., Zhang Z., Li Z., Ni D., Ashton A.W., Tang H.Y., Speicher D.W., Pestell R.G. Leronlimab, a humanized monoclonal antibody to CCR5, blocks breast cancer cellular metastasis and enhances cell death induced by DNA damaging chemotherapy. Breast Cancer Res. 2021;23:11. doi: 10.1186/s13058-021-01391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casagrande N., Borghese C., Aldinucci D. In classical Hodgkin lymphoma the combination of the CCR5 antagonist maraviroc with trabectedin synergizes, enhances DNA damage and decreases three-dimensional tumor-stroma heterospheroid viability. Haematologica. 2022;107:287–291. doi: 10.3324/haematol.2021.279389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W.J., Mao L.F., Lai H.L., Wang Y.W., Jiang Z.B., Li W., Huang J.M., Xie Y.J., Xu C., Liu P., et al. Dolutegravir derivative inhibits proliferation and induces apoptosis of non-small cell lung cancer cells via calcium signaling pathway. Pharmacol. Res. 2020;161:105129. doi: 10.1016/j.phrs.2020.105129. [DOI] [PubMed] [Google Scholar]
- 51.Schank M., Zhao J., Moorman J.P., Yao Z.Q. The impact of HIV- and ART-induced mitochondrial dysfunction in cellular senescence and aging. Cells. 2021;10:174. doi: 10.3390/cells10010174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jung I., Tu-Sekine B., Jin S., Anokye-Danso F., Ahima R.S., Brown T.T., Kim S.F. Dolutegravir suppresses thermogenesis via disrupting uncoupling protein 1 expression and mitochondrial function in brown/beige adipocytes in preclinical models. J. Infect. Dis. 2022;226:1626–1636. doi: 10.1093/infdis/jiac175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ajaykumar A., Caloren L.C., Povshedna T., Hsieh A.Y.Y., Zakaria A., Cai R., Smith M.R., Thompson C.A.H., Becquart P., Uday P., et al. Dolutegravir-containing HIV therapy reversibly alters mitochondrial health and morphology in cultured human fibroblasts and peripheral blood mononuclear cells. AIDS. 2023;37:19–32. doi: 10.1097/QAD.0000000000003369. [DOI] [PubMed] [Google Scholar]
- 54.Lazalde-Ramos B.P., Zamora-Perez A.L., Ortega-Guerrero A.I., Quintero-Fraire S.Z., Palacios-Lara O., Quirarte-Báez S.M., Galaviz-Hernández C., Sosa-Macías M., Ortiz-García Y.M., Morales-Velazquez G. Genomic instability decreases in HIV patient by complementary therapy with Rosmarinus officinalis extracts. J. Med. Food. 2020;23:1070–1076. doi: 10.1089/jmf.2019.0199. [DOI] [PubMed] [Google Scholar]
- 55.Borges R.S., Ortiz B.L.S., Pereira A.C.M., Keita H., Carvalho J.C.T. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2019;229:29–45. doi: 10.1016/j.jep.2018.09.038. [DOI] [PubMed] [Google Scholar]
- 56.Jaruga P., Jaruga B., Gackowski D., Olczak A., Halota W., Pawlowska M., Olinski R. Supplementation with antioxidant vitamins prevents oxidative modification of DNA in lymphocytes of HIV-infected patients. Free Radic. Biol. Med. 2002;32:414–420. doi: 10.1016/S0891-5849(01)00821-8. [DOI] [PubMed] [Google Scholar]
- 57.Ellwanger J.H., Prá D., Rieger A., Franke S.I.R. Influência do estado nutricional de selênio sobre a progressão da infecção pelo HIV. Nutrire. 2011;36:109–122. [Google Scholar]
- 58.Ellwanger J.H., Franke S.I.R., Bordin D.L., Prá D., Henriques J.A.P. Biological functions of selenium and its potential influence on Parkinson’s disease. An. Acad. Bras. Cienc. 2016;88:1655–1674. doi: 10.1590/0001-3765201620150595. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L., Zeng H., Cheng W.H. Beneficial and paradoxical roles of selenium at nutritional levels of intake in healthspan and longevity. Free Radic. Biol. Med. 2018;127:3–13. doi: 10.1016/j.freeradbiomed.2018.05.067. [DOI] [PubMed] [Google Scholar]
- 60.Gangcuangco L.M.A., Kohorn L.B., Chow D.C., Keating S.M., Norris P.J., Nagamine L.S., Ndhlovu L.C., Souza S.A., Kallianpur K.J., Shikuma C.M. High 25-hydroxyvitamin D is associated with unexpectedly high plasma inflammatory markers in HIV patients on antiretroviral therapy. Medicine. 2016;95:e5270. doi: 10.1097/MD.0000000000005270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bouillon R., Manousaki D., Rosen C., Trajanoska K., Rivadeneira F., Richards J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022;18:96–110. doi: 10.1038/s41574-021-00593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Galland L. Diet and inflammation. Nutr. Clin. Pract. 2010;25:634–640. doi: 10.1177/0884533610385703. [DOI] [PubMed] [Google Scholar]
- 63.Ricker M.A., Haas W.C. Anti-inflammatory diet in clinical practice: A review. Nutr. Clin. Pract. 2017;32:318–325. doi: 10.1177/0884533617700353. [DOI] [PubMed] [Google Scholar]
- 64.Tsiodras S., Poulia K.A., Yannakoulia M., Chimienti S.N., Wadhwa S., Karchmer A.W., Mantzoros C.S. Adherence to Mediterranean diet is favorably associated with metabolic parameters in HIV-positive patients with the highly active antiretroviral therapy-induced metabolic syndrome and lipodystrophy. Metabolism. 2009;58:854–859. doi: 10.1016/j.metabol.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dokmanović S.K., Kolovrat K., Laškaj R., Jukić V., Vrkić N., Begovac J. Effect of extra virgin olive oil on biomarkers of inflammation in HIV-infected patients: A randomized, crossover, controlled clinical trial. Med. Sci. Monit. 2015;21:2406–2413. doi: 10.12659/MSM.893881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manzano M., Talavera-Rodríguez A., Moreno E., Madrid N., Gosalbes M.J., Ron R., Dronda F., Pérez-Molina J.A., Lanza V.F., Díaz J., et al. Relationship of diet to gut microbiota and inflammatory biomarkers in people with HIV. Nutrients. 2022;14:1221. doi: 10.3390/nu14061221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pastor-Ibáñez R., Blanco-Heredia J., Etcheverry F., Sánchez-Palomino S., Díez-Fuertes F., Casas R., Navarrete-Muñoz M.Á., Castro-Barquero S., Lucero C., Fernández I., et al. Adherence to a supplemented mediterranean diet drives changes in the gut microbiota of HIV-1-infected individuals. Nutrients. 2021;13:1141. doi: 10.3390/nu13041141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sansoni P., Vescovini R., Fagnoni F., Biasini C., Zanni F., Zanlari L., Telera A., Lucchini G., Passeri G., Monti D., et al. The immune system in extreme longevity. Exp. Gerontol. 2008;43:61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 69.Deeks S.G., Verdin E., McCune J.M. Immunosenescence and HIV. Curr. Opin. Immunol. 2012;24:501–506. doi: 10.1016/j.coi.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 70.Pawelec G., Akbar A., Caruso C., Solana R., Grubeck-Loebenstein B., Wikby A. Human immunosenescence: Is it infectious? Immunol. Rev. 2005;205:257–268. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 71.Agarwal S., Busse P.J. Innate and adaptive immunosenescence. Ann. Allergy Asthma Immunol. 2010;104:183–190. doi: 10.1016/j.anai.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Thomas R., Wang W., Su D.M. Contributions of age-related thymic involution to immunosenescence and inflammaging. Immun. Ageing. 2020;17:2. doi: 10.1186/s12979-020-0173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fenwick C., Joo V., Jacquier P., Noto A., Banga R., Perreau M., Pantaleo G. T-cell exhaustion in HIV infection. Immunol. Rev. 2019;292:149–163. doi: 10.1111/imr.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Day C.L., Kaufmann D.E., Kiepiela P., Brown J.A., Moodley E.S., Reddy S., Mackey E.W., Miller J.D., Leslie A.J., DePierres C., et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 75.Chew G.M., Fujita T., Webb G.M., Burwitz B.J., Wu H.L., Reed J.S., Hammond K.B., Clayton K.L., Ishii N., Abdel-Mohsen M., et al. TIGIT marks exhausted t cells, correlates with disease progression, and serves as a target for immune restoration in HIV and SIV infection. PLoS Pathog. 2016;12:e1005349. doi: 10.1371/journal.ppat.1005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fromentin R., Bakeman W., Lawani M.B., Khoury G., Hartogensis W., DaFonseca S., Killian M., Epling L., Hoh R., Sinclair E., et al. CD4+ T cells expressing PD-1, TIGIT and LAG-3 contribute to HIV persistence during ART. PLoS Pathog. 2016;12:e1005761. doi: 10.1371/journal.ppat.1005761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martínez de Toda I., Maté I., Vida C., Cruces J., De la Fuente M. Immune function parameters as markers of biological age and predictors of longevity. Aging. 2016;8:3110–3119. doi: 10.18632/aging.101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horvath S., Levine A.J. HIV-1 infection accelerates age according to the epigenetic clock. J. Infect. Dis. 2015;212:1563–1573. doi: 10.1093/infdis/jiv277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sehl M.E., Breen E.C., Shih R., Chen L., Wang R., Horvath S., Bream J.H., Duggal P., Martinson J., Wolinsky S.M., et al. Increased rate of epigenetic aging in men living with HIV prior to treatment. Front. Genet. 2022;12:796547. doi: 10.3389/fgene.2021.796547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Appay V., Sauce D. Immune activation and inflammation in HIV-1 infection: Causes and consequences. J. Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 81.Dock J.N., Effros R.B. Role of CD8 T cell replicative senescence in human aging and in HIV-mediated immunosenescence. Aging Dis. 2011;2:382–397. [PMC free article] [PubMed] [Google Scholar]
- 82.Paul L. Diet, nutrition and telomere length. J. Nutr. Biochem. 2011;22:895–901. doi: 10.1016/j.jnutbio.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 83.Malan-Müller S., Hemmings S.M., Spies G., Kidd M., Fennema-Notestine C., Seedat S. Shorter telomere length-A potential susceptibility factor for HIV-associated neurocognitive impairments in South African women. PLoS ONE. 2013;8:e58351. doi: 10.1371/journal.pone.0058351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Auld E., Lin J., Chang E., Byanyima P., Ayakaka I., Musisi E., Worodria W., Davis J.L., Segal M., Blackburn E., et al. HIV infection is associated with shortened telomere length in Ugandans with suspected tuberculosis. PLoS ONE. 2016;11:e0163153. doi: 10.1371/journal.pone.0163153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Minami R., Takahama S., Yamamoto M. Correlates of telomere length shortening in peripheral leukocytes of HIV-infected individuals and association with leukoaraiosis. PLoS ONE. 2019;14:e0218996. doi: 10.1371/journal.pone.0218996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iyengar S., Cȏté H.C.F., Fitch K.V., Torriani M., Feldpausch M., Srinivasa S. Relationship of telomere length to fat redistribution in HIV. Open Forum Infect. Dis. 2020;7:ofaa523. doi: 10.1093/ofid/ofaa523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mehta S.R., Iudicello J.E., Lin J., Ellis R.J., Morgan E., Okwuegbuna O., Cookson D., Karris M., Saloner R., Heaton R., et al. Telomere length is associated with HIV infection, methamphetamine use, inflammation, and comorbid disease risk. Drug Alcohol Depend. 2021;221:108639. doi: 10.1016/j.drugalcdep.2021.108639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chauvin M., Sauce D. Mechanisms of immune aging in HIV. Clin. Sci. 2022;136:61–80. doi: 10.1042/CS20210344. [DOI] [PubMed] [Google Scholar]
- 89.Martínez de Toda I., Ceprián N., Díaz-Del Cerro E., De la Fuente M. The role of immune cells in oxi-inflamm-aging. Cells. 2021;10:2974. doi: 10.3390/cells10112974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fulop T., Larbi A., Dupuis G., Le Page A., Frost E.H., Cohen A.A., Witkowski J.M., Franceschi C. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or foes? Front. Immunol. 2018;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ginaldi L., De Martinis M., D’Ostilio A., Marini L., Loreto M.F., Corsi M.P., Quaglino D. Cell proliferation and apoptosis in the immune system in the elderly. Immunol. Res. 2000;21:31–38. doi: 10.1385/IR:21:1:31. [DOI] [PubMed] [Google Scholar]
- 92.George A., Dey R., Bhuria V., Banerjee S., Ethirajan S., Siluvaimuthu A., Saraswathy R. Nuclear anomalies, chromosomal aberrations and proliferation rates in cultured lymphocytes of head and neck cancer patients. Asian Pac. J. Cancer Prev. 2014;15:1119–1123. doi: 10.7314/APJCP.2014.15.3.1119. [DOI] [PubMed] [Google Scholar]
- 93.González-Sánchez M., García-Martínez V., Bravo S., Kobayashi H., Martínez de Toda I., González-Bermúdez B., Plaza G.R., De la Fuente M. Mitochondrial DNA insertions into nuclear DNA affecting chromosome segregation: Insights for a novel mechanism of immunosenescence in mice. Mech. Ageing Dev. 2022;207:111722. doi: 10.1016/j.mad.2022.111722. [DOI] [PubMed] [Google Scholar]
- 94.Bolognesi C., Lando C., Forni A., Landini E., Scarpato R., Migliore L., Bonassi S. Chromosomal damage and ageing: Effect on micronuclei frequency in peripheral blood lymphocytes. Age Ageing. 1999;28:393–397. doi: 10.1093/ageing/28.4.393. [DOI] [PubMed] [Google Scholar]
- 95.Richter C. Do mitochondrial DNA fragments promote cancer and aging? FEBS Lett. 1988;241:1–5. doi: 10.1016/0014-5793(88)81018-4. [DOI] [PubMed] [Google Scholar]
- 96.Sikora E. Activation-induced and damage-induced cell death in aging human T cells. Mech. Ageing Dev. 2015;151:85–92. doi: 10.1016/j.mad.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 97.Fiala M., Murphy T., MacDougall J., Yang W., Luque A., Iruela-Arispe L., Cashman J., Buga G., Byrns R.E., Barbaro G., et al. HAART drugs induce mitochondrial damage and intercellular gaps and gp120 causes apoptosis. Cardiovasc. Toxicol. 2004;4:327–337. doi: 10.1385/CT:4:4:327. [DOI] [PubMed] [Google Scholar]
- 98.Foster C., Lyall H. HIV and mitochondrial toxicity in children. J. Antimicrob. Chemother. 2008;61:8–12. doi: 10.1093/jac/dkm411. [DOI] [PubMed] [Google Scholar]
- 99.Garrabou G., López S., Morén C., Martínez E., Fontdevila J., Cardellach F., Gatell J.M., Miró O. Mitochondrial damage in adipose tissue of untreated HIV-infected patients. AIDS. 2011;25:165–170. doi: 10.1097/QAD.0b013e3283423219. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y., Wang M., Li H., Zhang H., Shi Y., Wei F., Liu D., Liu K., Chen D. Accumulation of nuclear and mitochondrial DNA damage in the frontal cortex cells of patients with HIV-associated neurocognitive disorders. Brain. Res. 2012;1458:1–11. doi: 10.1016/j.brainres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 101.Kallianpur K.J., Gerschenson M., Mitchell B.I., LiButti D.E., Umaki T.M., Ndhlovu L.C., Nakamoto B.K., Chow D.C., Shikuma C.M. Oxidative mitochondrial DNA damage in peripheral blood mononuclear cells is associated with reduced volumes of hippocampus and subcortical gray matter in chronically HIV-infected patients. Mitochondrion. 2016;28:8–15. doi: 10.1016/j.mito.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Darbinian N., Darbinyan A., Merabova N., Selzer M.E., Amini S. HIV-1 and HIV-1-Tat induce mitochondrial DNA damage in human neurons. J. HIV AIDS. 2020;6:176. doi: 10.16966/2380-5536.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roca-Bayerri C., Robertson F., Pyle A., Hudson G., Payne B.A.I. Mitochondrial DNA damage and brain aging in human immunodeficiency virus. Clin. Infect. Dis. 2021;73:e466–e473. doi: 10.1093/cid/ciaa984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Siegel K., Lekas H.M. AIDS as a chronic illness: Psychosocial implications. AIDS. 2002;4:S69–S76. doi: 10.1097/00002030-200216004-00010. [DOI] [PubMed] [Google Scholar]
- 105.Deeks S.G. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nasi M., Pinti M., De Biasi S., Gibellini L., Ferraro D., Mussini C., Cossarizza A. Aging with HIV infection: A journey to the center of inflammAIDS, immunosenescence and neuroHIV. Immunol. Lett. 2014;162:329–333. doi: 10.1016/j.imlet.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 107.Langie S.A.S., Koppen G., Desaulniers D., Al-Mulla F., Al-Temaimi R., Amedei A., Azqueta A., Bisson W.H., Brown D.G., Brunborg G., et al. Causes of genome instability: The effect of low dose chemical exposures in modern society. Carcinogenesis. 2015;36:S61–S88. doi: 10.1093/carcin/bgv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kirsch-Volders M., Bolognesi C., Ceppi M., Bruzzone M., Fenech M. Micronuclei, inflammation and auto-immune disease. Mutat. Res. Rev. Mutat. Res. 2020;786:108335. doi: 10.1016/j.mrrev.2020.108335. [DOI] [PubMed] [Google Scholar]
- 109.Ye C.J., Sharpe Z., Heng H.H. Origins and consequences of chromosomal instability: From cellular adaptation to genome chaos-mediated system survival. Genes. 2020;11:1162. doi: 10.3390/genes11101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Canva. [(accessed on 26 December 2022)]. Available online: https://www.canva.com/
- 111.Servier Medical Art. [(accessed on 25 November 2022)]. Available online: https://smart.servier.com/)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.