Abstract
Chronic pain is reportedly associated with the transient receptor potential canonical 3 (TRPC3) gene. The present study examined the genetic associations between the single-nucleotide polymorphisms (SNPs) of the TRPC3 gene and chronic pain. The genomic samples from 194 patients underwent linkage disequilibrium (LD) analyses of 29 SNPs within and around the vicinity of the TRPC3 gene. We examined the associations between the SNPs and the susceptibility to chronic pain by comparing the genotype distribution of 194 patients with 282 control subjects. All SNP genotype data were extracted from our previous whole-genome genotyping results. Twenty-nine SNPs were extracted, and a total of four LD blocks with 15 tag SNPs were observed within and around the TRPC3 gene. We further analyzed the associations between these tag SNPs and chronic pain. The rs11726196 SNP genotype distribution of patients was significantly different from the control subjects even after multiple-testing correction with the number of SNPs. The TT + TG genotype of rs11726196 is often carried by chronic pain patients, suggesting a causal role for the T allele. These results contribute to our understanding of the genetic risk factors for chronic pain.
Keywords: TRPC3 gene, SNP, chronic pain
1. Introduction
Pain is the fifth vital sign, comprising an alert system that is necessary for human survival. However, prolonged pain causes a significant reduction in the quality of life. Chronic pain has deleterious effects on socioeconomic activity. In the United States, common pain conditions resulted in USD 60 billion (GBP 32.34 billion) in lost productivity each year, of which 77% was caused by lower performance rather than work absence [1]. In Australia, absent workdays are estimated to be 9.9 million work days annually, and the lower effectiveness of workdays is estimated to be 36.5 million work days, which raises productivity costs from AUD 1.4 billion (GBP 0.65 billion) to AUD 5.1 billion (GBP 2.35 billion) when absenteeism and presenteeism are considered [1]. In Japan, back pain (72.10%) and stiff shoulders (54.90%) were the most common types of pain. The respondents with these types of chronic pain reported more prolonged absenteeism (4.74% and 2.74%, respectively), incapacity for current employment (30.19% and 15.19%, respectively), overall work disability (31.70% and 16.82%, respectively), and indirect costs (JPY 1,488,385 and JPY 804,634, respectively) in 2016 [2]. The International Association for the Study of Pain revised the definition of pain in 2020 as “an unpleasant sensory and emotional experience that is associated with or similar to actual or potential tissue damage”, defining chronic pain as “pain that persists or recurs for more than 3 months” [3]. Chronic pain is associated with various diseases, and its incidence ranges from 8% to 60%. The International Statistical Classification of Diseases and Related Health Problems, 11th revision, was the first to include a classification code for chronic pain, classifying it according to condition and body site, but some chronic pain conditions, such as fibromyalgia and complex regional pain syndrome, have no known cause, and effective treatments and therapies have not been developed [3].
Various factors, including biological, physiological, and psychological factors, are involved in pain sensitivity and chronic pain, producing individual differences in chronic pain. The contribution of genetic factors to chronic pain has been reported by twin studies for each classification code. The contribution of the genetic factors to the incidence of chronic pain and severe chronic pain was reported to be 0.16 and 0.30, respectively [4]. Chronic pain has a strong genetic component. Inbred mice and rats exhibit varying pain sensitivities, and chronic pain runs in families in humans [5].
Several genetic polymorphisms have been reported to be involved in various chronic pain conditions. Fibromyalgia has been reported to be affected by single-nucleotide polymorphisms (SNPs) of such genes as COMT, DRD4, MAO-A, GTPCH, and genes that encode γ-aminobutyric acid-A (GABAA) receptors [6]. Chronic low back pain has been reported to be affected by SNPs of such genes as OPRM1 and COMT [7]. A meta-analysis of all potential genetic variants that are associated with neuropathic pain identified 28 genes that were significantly associated with neuropathic pain, involving neurotransmission, the immune response, and metabolism. The genetic variants of the HLA, COMT, OPRM1, TNFA, IL6, and GCH1 genes have been associated with neuropathic pain in multiple studies [7]. The genetic variants that were reported to be associated with chronic postoperative pain (postsurgical pain) include COMT, GCH1, ABCB1, 5HTR2A, IFNG1, IL1R1, IL1R2, IL4, IL10, IL13, NFKB1, HLA-DRB1*4, DQB1, PRKCA, CDH18, ATXN1, DRD2, NFKB1A, CHRNA6, KCND2, KCNJ3, KCNJ6, KCNK3, KCNK9, CACNG2, P2X7R, KCNS1, and TNFA (Supplementary Table S1) [8]. Chronic widespread musculoskeletal pain has been reported to be related to the RNF123 and ATP2C1 loci [9]. The genetic polymorphisms of the exonuclease 3′–5′ domain containing 3 (EXD3) and solute carrier family 39 member 8 (SLC39A3) are associated with multiple chronic pain conditions [10]. A genome-wide association study meta-analysis of chronic low back pain reported associations between chronic low back pain and SNPs of the SOX5, CCDC26/GSDMC, and DCC genes [11]. A meta-analysis found the strongest association for the rs887797 SNP of the gene that encodes the protein kinase Cα (PRKCA) [12]. Genes that were significantly associated with multisite chronic pain in males, included CENPW, MTCH2, NICN1, AMIGO3, DNAJA4, CTBP2, and NOP14, and genes that were significantly associated with multisite chronic pain in females, included NCAN, SPATS2L, TBC1D9, CAMK1D, SOX11, GON4L, and DAGLB [13]. We also identified several SNPs that are possibly associated with chronic pain [14,15], in which several candidate SNPs, including SNPs within and around the PRKCQ and HS3ST4 genes, were identified, and the most potent SNP was rs4773840 that was associated with postherpetic neuralgia (PHN). Various SNPs and genes have been reported to be associated with various types of chronic pain, but no clear picture has emerged for all genetic factors that are associated with chronic pain. This suggests that no single SNP or gene is responsible for chronic pain; instead, multiple SNPs and genes are involved.
The transient receptor potential (TRP) protein superfamily is composed of cation-permeable channels that are expressed in mammalian cells. As part of the oxidative stress response, there are three subfamilies of TRPs: the TRP cation channel subfamily C (TRPC; which contains calcium entry channels), the TRP cation channel subfamily M (TRPM; which is involved in biological proliferation and death), and the TRP cation channel subfamily V (TRPV; which is activated by chemical, mechanical, and physical stimuli) [16]. Among these, at least six channels (TRPV1-4, TRPM8, and TRPA1) are expressed in the nociceptors, where they act as transducers of signals from thermal, chemical, and mechanical stimuli and play crucial roles in the generation and development of pathological pain perception [17,18]. With regard to chronic pain, TRPV1, TRPV3, TRPM3, and TRPA1 are particularly interesting [19,20,21,22]. The antagonists of TRPV1, TRPV3, and TRPA1 have been advanced into clinical trials for the treatment of inflammatory, neuropathic, and visceral pain [20]. Additionally, TRPV4 and TRPM8 were reported to be involved in the mechanisms of chronic pain [23,24] and are suggested to be putative treatment targets for chronic low back pain [23]. For the TRPC channels, TRPC1, TRPC4, and TRPC5 are activated directly by inositol-1,4,5-trisphosphate, and TRPC3, TRPC6, and TRPC7 are activated by diacylglycerol (DAG), independent of the storage reduction of intracellular calcium [16]. The TRPC channels have been reported to play important roles in both the nociceptor signaling and the sensitization of the nociceptors by the inflammatory mediators [25]. This previous study [25] revealed a major contribution of TRPC to the neuronal calcium homeostasis in the somatosensory pathways. These channels are non selectively permeable to cations, with variable selectivity for calcium over sodium among the different members. Here, TRPC engages in both the store-operated calcium entry (SOCE) and the receptor-operated calcium entry (ROCE) in the control of the calcium influx that triggers the calcium-dependent pathways and the peripheral sensitization. Therefore, TRPC is functionally coupled to several inflammatory transduction mechanisms, including the UTP/P2Y2 and protease/PAR2 signaling complexes. This unique dual contribution to SOCE and ROCE defines the calcium-permeable TRPC, including TRPC3, as a key regulator of the calcium homeostasis in the dorsal root ganglia (DRGs) neurons under normal and pathological pain conditions. Xia et al. (2015) suggested that TRPC3 may be a novel therapeutic target for chronic pain [26]. We previously reported that a SNP near the TRPC3 gene was associated with the postoperative fentanyl requirements and pain [27]. Several studies of the TRPC3 gene polymorphisms and chronic itch have been reported [25,28], but no studies have evaluated the associations between the TRPC3 gene polymorphisms and chronic pain. In the present study, we investigated the genetic polymorphisms within and around the TRPC3 gene and analyzed the TRPC3 gene polymorphisms that influence chronic pain.
2. Results
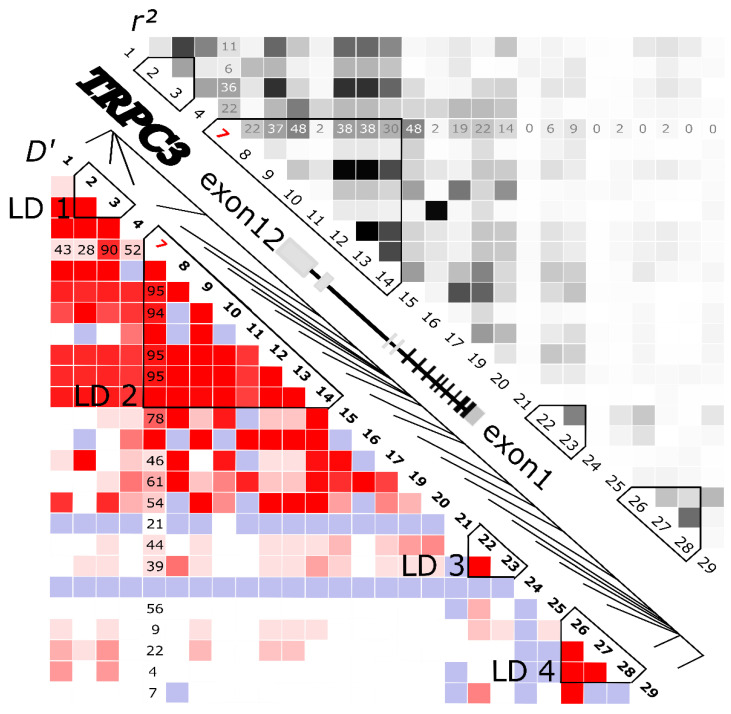
The sample groups in the present study were the neuropathic pain patient group (NPPG) and the nociceptive control group (NOCG), without any diseases or chronic pain, as detailed in a previous report [14]. The genotype data for the 50 kilobase pair (kbp) regions upstream and downstream of the TRPC3 gene were extracted from the whole-genome genotyping data (Supplementary Table S2). The linkage disequilibrium (LD) blocks were made with HaploView for 29 SNPs in this region using the data from the NPPG. Among the 29 SNPs that were calculated by HaploView, three SNPs were excluded because they had a minor allele frequency of 0 (Table 1). The r2 value, a measure of the LD strength, was calculated. As a result, 15 SNPs were selected as tag SNPs because they had other SNPs with a greater LD between them (Table 1). The TRPC3 gene has been shown to be located on the complementary strand side, according to the National Center for Biotechnology Information dbSNP database. The alleles in each SNP were displayed, based on the dbSNP annotation. A total of four LD blocks with 15 tag SNPs (rs1507994, rs1314213, rs11726196, rs2135976, rs11732666, rs17517624, rs3762839, rs884701, rs13127488, rs906496, rs1358229, rs6838639, rs6817255, rs11098653, and rs13130390) were observed in the region within and around the TRPC3 gene (Figure 1, Table 1). Among the 15 tag SNPs, rs11732666 and rs6838639 showed a significant difference, with Hardy–Weinberg equilibrium p values of 0.0406 and 0.0449, respectively, but the other SNPs were under the Hardy–Weinberg equilibrium (Table 1).
Table 1.
Results of the Hardy–Weinberg equilibrium test for each SNP.
| LD Number | # | Tag | SNP | Position (hg19) | ObsHET | PredHET | HWpval | %Geno | FamTrio | MendErr | MAF | Rating | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | rs1048433 | 122748996 | 0.481 | 0.465 | 0.771 | 99 | 0 | 0 | 0.368 | Used | |||
| LD1 | 2 | Tag | rs1507994 | 122749541 | 0.382 | 0.346 | 0.2236 | 100 | 0 | 0 | 0.223 | Used | |
| LD1 | 3 | Tag | rs13145213 | 122750079 | 0.527 | 0.493 | 0.4445 | 98.4 | 0 | 0 | 0.439 | Used | |
| 4 | rs12499222 | 122774917 | 0.361 | 0.34 | 0.5514 | 100 | 0 | 0 | 0.217 | Used | |||
| 5 | rs6838198 | 122781263 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | BAD | - | ||
| 6 | rs10518290 | 122805872 | 0 | 0 | 1 | 100 | 0 | 0 | 0 | BAD | - | ||
| LD2 | 7 | Tag | rs11726196 | 122806228 | 0.361 | 0.379 | 0.616 | 100 | 0 | 0 | 0.254 | Used | |
| LD2 | 8 | Tag | rs2135976 | 122816181 | 0.131 | 0.131 | 1 | 100 | 0 | 0 | 0.071 | Used | |
| LD2 | 9 | Tag | rs11732666 | 122824052 | 0.577 | 0.496 | 0.0406 | 99 | 0 | 0 | 0.458 | Used | |
| LD2 | 10 | Tag | rs17517624 | 122824591 | 0.257 | 0.261 | 0.9592 | 100 | 0 | 0 | 0.154 | Used | |
| LD2 | 11 | Tag | rs3762839 | 122825364 | 0.157 | 0.145 | 0.5814 | 100 | 0 | 0 | 0.079 | Used | |
| LD2 | 12 | Tag | rs884701 | 122830406 | 0.56 | 0.495 | 0.0973 | 100 | 0 | 0 | 0.448 | Used | |
| LD2 | 13 | Tag | rs13127488 | 122830800 | 0.56 | 0.495 | 0.0973 | 100 | 0 | 0 | 0.448 | Used | |
| LD2 | 14 | Tag | rs906496 | 122833314 | 0.539 | 0.498 | 0.335 | 100 | 0 | 0 | 0.469 | Used | |
| 15 | rs4292355 | 122842267 | 0.45 | 0.423 | 0.4946 | 100 | 0 | 0 | 0.304 | Used | |||
| 16 | rs12644087 | 122844819 | 0.152 | 0.14 | 0.6334 | 100 | 0 | 0 | 0.076 | Used | |||
| 17 | rs6841843 | 122857568 | 0.366 | 0.36 | 1 | 100 | 0 | 0 | 0.236 | Used | |||
| 18 | rs4833779 | 122861426 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | BAD | - | ||
| 19 | rs950574 | 122864241 | 0.487 | 0.463 | 0.6025 | 100 | 0 | 0 | 0.364 | Used | |||
| 20 | rs970349 | 122871632 | 0.225 | 0.246 | 0.3395 | 100 | 0 | 0 | 0.144 | Used | |||
| 21 | rs4001038 | 122886240 | 0.026 | 0.026 | 1 | 100 | 0 | 0 | 0.013 | Used | |||
| LD3 | 22 | Tag | rs1358229 | 122896734 | 0.555 | 0.499 | 0.1734 | 100 | 0 | 0 | 0.482 | Used | |
| LD3 | 23 | Tag | rs6838639 | 122899165 | 0.524 | 0.452 | 0.0449 | 100 | 0 | 0 | 0.346 | Used | |
| 24 | rs13109219 | 122900529 | 0.005 | 0.005 | 1 | 100 | 0 | 0 | 0.003 | Used | |||
| 25 | rs9999992 | 122902084 | 0.314 | 0.325 | 0.7639 | 100 | 0 | 0 | 0.204 | Used | |||
| LD4 | 26 | Tag | rs6817255 | 122902923 | 0.455 | 0.489 | 0.399 | 100 | 0 | 0 | 0.427 | Used | |
| LD4 | 27 | Tag | rs11098653 | 122903090 | 0.257 | 0.254 | 1 | 100 | 0 | 0 | 0.149 | Used | |
| LD4 | 28 | Tag | rs13130390 | 122903206 | 0.173 | 0.166 | 1 | 100 | 0 | 0 | 0.092 | Used | |
| 29 | rs11098654 | 122909415 | 0.33 | 0.282 | 0.0251 | 100 | 0 | 0 | 0.17 | Used |
# Matches the number in Figure 1. Tag SNPs are indicated with a circle. MAF, minor allele frequency (those with a value of 0 were excluded [=BAD], and finally 26 [=Used] in the results column were used); ObsHET, observed heterozygosity p value; PredHET, predicted heterozygosity p value; HWpval, Hardy–Weinberg equilibrium p value; %Geno, percentage of the non-missing genotypes for this marker; FamTrio, number of fully genotyped family trios for this marker (0 for datasets with unrelated individuals); MendErr, number of the observed Mendelian inheritance errors (0 for datasets with unrelated individuals).
Figure 1.
Schematic representation of the TRPC3 gene structure and the LD. The gene structure of the TRPC3 gene is shown with exons 1–12. The four linkage disequilibrium (LD 1–4) blocks were generated from 29 SNPs using the LD statistics and HaploView. Linkage disequilibrium was measured using the D’ and r2 correlation coefficients and is displayed according to the standard color schemes for D’ (white: LOD < 2; D’ < 1; blue: LOD < 2; D’ = 1; shades of pink/red: LOD ≥ 2; D’ < 1; bright red: LOD ≥ 2; D’ = 1) and r2 (white: r2 = 0; shades of gray: 0 < r2 <1; black: r2 = 1). The D’ × 100 and r2 × 100 values of only the SNP pairs between rs11726196 (#7, red) and other SNPs are shown in each cell. Empty cells between these pairs of SNPs in the standard color scheme indicate D’ = 1. Red #7 indicates the tag SNP rs11726196.
We further analyzed the associations between these tag SNPs and the chronic pain status. Even after correcting for multiple testing, such as the Bonferroni adjustments with the number of SNPs (the significance level after the Bonferroni correction was p < 0.0033 [0.05/15]), the genotype distribution for the rs11726196 SNP in the patient subjects was significantly different from the control subjects in the genotypic model (Pearson χ2 test, p = 0.00089; Table 2). This result suggests that rs11726196 is significantly associated with chronic pain. No other SNPs were significantly associated with the chronic pain status (Table 2).
Table 2.
Associations between the tag SNPs in and around the TRPC3 gene and chronic pain, comparing the genotype distributions between patients and control subjects.
| # Corresponding to Figure 1 | SNP | Genotype | Number of Participants | χ 2 | |
|---|---|---|---|---|---|
| NPPG | NOCG | p | |||
| #2 | rs1507994 | GG | 112 | 177 | 0.816 |
| GA | 73 | 97 | 0.665 | ||
| AA | 6 | 8 | |||
| #3 | rs13145213 | GG | 33 | 46 | 0.888 |
| GA | 99 | 135 | 0.642 | ||
| AA | 56 | 93 | |||
| #7 | rs11726196 | TT | 14 | 5 | 14.0559 |
| TG | 69 | 80 | 0.00089 * | ||
| GG | 108 | 197 | |||
| #8 | rs2135976 | GG | 165 | 259 | 4.584 |
| GA | 25 | 23 | 0.101 | ||
| AA | 1 | 0 | |||
| #9 | rs11732666 | AA | 48 | 102 | 7.395 |
| AG | 109 | 129 | 0.025 | ||
| GG | 32 | 51 | |||
| #10 | rs17517624 | AA | 5 | 3 | 4.471 |
| AG | 49 | 55 | 0.107 | ||
| GG | 137 | 224 | |||
| #11 | rs3762839 | TT | 161 | 229 | 2.439 |
| TG | 30 | 50 | 0.295 | ||
| GG | 0 | 3 | |||
| #12 | rs884701 | TT | 52 | 102 | 5.324 |
| TC | 107 | 129 | 0.070 | ||
| CC | 32 | 51 | |||
| #13 | rs13127488 | TT | 32 | 50 | 5.098 |
| TG | 107 | 130 | 0.078 | ||
| GG | 52 | 102 | |||
| #14 | rs906496 | AA | 38 | 74 | 3.123 |
| AG | 103 | 132 | 0.210 | ||
| GG | 50 | 76 | |||
| #22 | rs1358229 | AA | 39 | 72 | 5.687 |
| AG | 106 | 125 | 0.058 | ||
| GG | 46 | 85 | |||
| #23 | rs6838639 | TT | 16 | 33 | 4.109 |
| TC | 100 | 122 | 0.128 | ||
| CC | 75 | 127 | |||
| #26 | rs6817255 | AA | 66 | 82 | 1.194 |
| AC | 87 | 136 | 0.551 | ||
| CC | 38 | 57 | |||
| #27 | rs11098653 | AA | 4 | 4 | 0.705 |
| AG | 49 | 80 | 0.703 | ||
| GG | 138 | 197 | |||
| #28 | rs13130390 | CC | 1 | 3 | 0.692 |
| CT | 33 | 54 | 0.708 | ||
| TT | 157 | 225 | |||
# Matches the number in Figure 1. NPPG and NOCG indicate the neuropathic pain patient group and the nociceptive control subject group, respectively. Three × 2 χ2 tests were conducted to compare the neuropathic pain patients and control subjects among the genotypes in each SNP. * Statistically significant differences between the neuropathic pain patients and control subjects. The p values in the table are the crude values without the Bonferroni correction.
The ratios of the TT/TG/GG genotypes of rs11726196 were 0.018/0.284/0.699 in the NOCG and 0.073/0.361/0.565 in the NPPG (Table 2, Supplementary Table S2). The T allele or TT + TG genotype ratio was higher in the NPPG than in the NOCG, compared with the C allele or GG genotype. This result suggests that the T allele of rs11726196 is considered to be the causative allele in the risk for chronic pain.
To examine in more detail the association between the TRPC3 SNPs and chronic pain, a haplotype-based test was performed for the selected neighboring tag SNPs. As shown in Supplementary Table S3, strong associations were found for the haplotypes including the rs11726196 SNP with chronic pain. Although the strongest associations were observed between the haplotypes that consisted of one or two SNPs, including the rs11726196 SNP and chronic pain (χ2 = 12.7800, p = 0.0003), the associations were compromised when three SNPs, including the rs11726196 SNP, were incorporated in the analysis (Supplementary Table S3), and the trend was similar when four or more SNPs, including the rs11726196 SNP, were incorporated in the analysis (data not shown).
3. Discussion
The data distribution showed that postherpetic neuralgia (PHN) was the most common type of pain (94 patients [48.4%]), followed by spinal stenosis (20 patients [9.8%]), chronic postoperative pain (12 patients [6.2%]), cervical spondylosis (11 patients [5.7%]), disk herniation (seven patients [3.6%]), and traumatic nerve injury (seven patients [3.6%]; Table 3). Kosson et al. (2019) reported that the types of pain were osteoarticular pain (146 patients [44.92%]), neuropathic pain (139 patients [42.77%]), headache (43 patients [13.23%]), and other types of pain (21 patients [6.46%]) [29]. Their findings were similar to our data, in which 48.4% of patients had neuropathic pain. Bone and joint pain, for which our data were categorized separately, were less common (10.4% for scoliosis, 5.7% for cervical disc herniation, 4.7% for lower back pain, 3.7% for disc herniation, and 1% each for buttock pain, groin pain, olecranon joint pain, knee pain, and thoracic pain; total of 32.2%). No noteworthy differences were found between the groups of patients who attended the pain clinics in any of the study groups.
Table 3.
Distribution of the main diagnostic names and number/ratio of patients.
| ICD-11 | Diagnosis | Number/Ratio of Patients | Age (Avg)/Range | Sex (Male/Female/Unknown) | |
|---|---|---|---|---|---|
| MG30.5 | PHN (post-herpetic neuralgia) | 94 | 0.485 | 71.8/32–89 | 45/45/4 |
| FA82 | Spinal canal stenosis | 20 | 0.103 | 65.2/22–75 | 8/12/0 |
| MG30.21 | Chronic postoperative pain | 12 | 0.062 | 59.9/28–88 | 3/9/0 |
| FA8Z | Cervical spondylosis | 11 | 0.057 | 59.8/30–74 | 5/6/0 |
| MG30.02 | Low back pain | 9 | 0.046 | 60.0/41–72 | 4/5/0 |
| FB1Y | Herniated disc | 7 | 0.036 | 56.1/37–75 | 2/5/0 |
| MG30.20 | Post-traumatic pain | 7 | 0.036 | 56.1/33–70 | 5/2/0 |
| VV12 | Hip pain | 2 | 0.010 | 40.0/30–56 | 0/2/0 |
| MD81.12 | Inguinal pain | 2 | 0.010 | 61.5/61–62 | 0/2/0 |
| ME82 | Knee joint pain | 2 | 0.010 | 41.0/39–43 | 1/1/0 |
| 4B20 | Sarcoidosis | 2 | 0.010 | 51.0/33–69 | 0/2/0 |
| ME84.3 | Sciatica | 2 | 0.010 | 64.5/54–75 | 1/1/0 |
| Thalamic pain | 2 | 0.010 | 65.0/63–67 | 1/1/0 | |
| 8B82.0 | Trigeminal neuralgia | 2 | 0.010 | 73.0/64–82 | 2/0/0 |
| MD81 | Abdominal pain | 1 | 0.005 | 63/63 | 0/1/0 |
| 9A05 | Blepharospasm | 1 | 0.005 | 57/57 | 0/1/0 |
| MD30 | Chest pain | 1 | 0.005 | 29/29 | 1/0/0 |
| MG30.03 | Facial pain | 1 | 0.005 | 56/56 | 0/1/0 |
| MG30.01 | Fibromyalgia | 1 | 0.005 | 68/68 | 0/1/0 |
| Fibula neuralgia | 1 | 0.005 | 60/60 | 0/1/0 | |
| VV12 | Generalized pain | 1 | 0.005 | 35/35 | 0/1/0 |
| MG30.02 | Shoulder pain | 1 | 0.005 | 65/65 | 0/1/0 |
| MG30.03 | Temporomandibular joint pain | 1 | 0.005 | 63/63 | 0/1/0 |
| others | 11 | 0.057 | 54.5/30–71 | 5/5/1 | |
| Total | 194 | 1.000 | 66.1/22–89 | 83/106/5 | |
ICD-11, International Statistical Classification of Diseases and Related Health Problems, 11th revision; avg, average.
The present results suggest that the T allele of rs11726196 is a causative allele in the risk for chronic pain. Although this SNP is located in the intron, the SNP may exist in a functional region, such as a region that is involved in the transcription regulation. We searched for the functional region (i.e., promoter and enhancer) in the 5 kbp regions upstream and downstream of the rs11726196 SNP in a public database. The enhancers existed in the 2.2 kbp region upstream of rs11726196 and the 1.3 kbp region downstream of rs11726196 (Supplementary Table S4) [30]. The expression quantitative trait loci (eQTLs), identified by GTEx, showed that the TRPC3 mRNA expression depends on the rs11726196 genotypes in the peripheral nerve, such as the tibial nerve, with TT > GG, which is the same trend as most peripheral tissues (Supplementary Figure S1) [31]. The tibial nerve projects to the DRGs that are located in the peripheral nervous system. The dorsal root ganglion neurons may express TRPC3 in a similar trend as the tibial nerve. In the DRG neurons, the T allele carriers may exhibit an upregulation of the TRPC3 expression, possibly in the peripheral trigeminal ganglion (TGG) as well, which increases the intracellular calcium levels, possibly exacerbating the nociception and chronic pain, which is consistent with the present results. This suggests that the high TRPC3 expression in the peripheral nerves or an increase in the T allele number was associated with the higher risk of chronic pain in the present study. In the thoracolumbar DRGs in rats, the prolonged elevations of intracellular calcium can cause neuronal hyperexcitability and hypersensitivity, leading to nociception and chronic pain [26].
According to the 1000 genomes study in the dbSNP database, the allele frequencies for the T allele (i.e., complementary A allele) of the rs11726196 SNP are 0.5265, 0.1845, 0.3350, 0.492, and 0.281 in African, East Asian, European, South Asian, and American populations, respectively (https://www.ncbi.nlm.nih.gov/snp/rs11726196; accessed on 25 November 2022). Although the T allele frequency of this SNP appears to be relatively low in the East Asian population, including Japan, compared with other populations, the SNP would not be too uncommon in the East Asian population to detect some associations with susceptibility to chronic pain. Meanwhile, considering the relatively low T allele frequency, there might be a possibility that the East Asian population is less susceptible to chronic pain than other populations, in terms of the rs11726196 SNP, if this SNP actually contributes to the susceptibility to chronic pain.
The TRPC3 channels, as well as other TRP channels (e.g., TRPV1, TRPV3, TRPV4, TRPA1, TRPM3, and TRPM8), possibly play an important role in nociception [17,18,19,20,21,22,23,24,26]. In humans, TRPC3 mRNA is highly expressed in the brain, including the cervical spinal cord and heart [32]. In the cervical spinal cord, TRPC3 mRNA is most highly expressed in the dorsal horn, which contains ascending sensory neuron pathways. A single cell type analysis revealed that excitatory neurons express TRPC3 mRNA [33]. The highest TRPC3 mRNA expression was found in TGGs and DRGs from C1 to S1 in mice (seven cervical segments, 13 thoracic segments, six lumbar segments, and one sacral segment) [34]. The mechanotransduction complex of TRPC3 and TRPC6 is thought to be present in the sensory nerves [35]. The TRPC3 overexpression activates the P2Y2 purinergic receptors and protease-activated receptor-2 (PAR2/F2RL1), increasing the intracellular calcium levels [26]. In rat thoracic/lumbar DRGs, prolonged elevations of intracellular calcium are commonly associated with neuronal hyperexcitability or hypersensitivity, contributing to nociception and chronic pain [26]. However, in humans, the T allele carriers may exhibit an upregulation of the TRPC3 expression in the DRG neurons, possibly in TGGs as well, which increases the intracellular calcium levels, possibly leading to the exacerbation of nociception and chronic pain, as mentioned above. The activation of the P2Y2 receptors and PAR2 strongly elevates the intracellular calcium levels via the overexpression of TRPC3 [36]. Furthermore, a strong association was found between DAG and the activation of TRPC3 through the overexpression of the inflammatory receptors [36]. Diacylglycerol mediates the calcium influx by activating both SOCE and ROCE. This suggests that TRPC3 binds SOCE/ROCE and DAG as an inflammatory metabotropic receptor and is involved in the peripheral nerve sensitization [36]. Altogether, these reports suggest important roles for TRPC3 in the mechanisms of inflammation and pain.
We previously reported associations between the rs1465040 SNP near the TRPC3 gene and postoperative analgesic requirements after the sagittal split ramous osteotomy and open abdominal surgery [27]. The TT + TC genotypes of the rs1465040 SNP of the TRPC3 gene contributes to an increase in the postoperative fentanyl requirements and exacerbates pain, compared with the CC genotype. However, further investigations are required to understand the functional contribution of this TRPC3 SNP to the postoperative analgesic requirements. Similar to rs1465040, a strong LD was observed for rs6534331 (r2 = 0.96) and rs4833787 (r2 = 1.00) in the sagittal split ramous osteotomy patient samples, based on the genotype data that were obtained in our previous study [27]. However, when eQTLs of these three SNPs were examined in GTEx, no significant association with the TRPC3 gene expression was found. Therefore, based on the GTEx database, it is unlikely that these SNPs affect individual differences in opioid analgesia and pain through alterations of the gene expression. TRPC3 is considered to be important in analgesia/pain, but future studies are required to elucidate the mechanisms by which the SNPs influence the channel function or expression to lead to individual differences in the susceptibility to chronic pain.
In the additional haplotype-based test that was performed to examine in more detail the association between the TRPC3 SNPs and chronic pain for selected neighboring tag SNPs, the strongest associations were observed for the haplotypes that consisted of one or two SNPs, including the rs11726196 SNP and chronic pain (χ2 = 12.7800, p = 0.0003; Supplementary Table S3). However, the associations were compromised when three SNPs, including the rs11726196 SNP, were incorporated in the analysis (Supplementary Table S3), and the trend was similar when four or more SNPs, including the rs11726196 SNP, were incorporated in the analysis (data not shown). The results suggest that the rs11726196 SNP, and no other SNPs, solely contributes to the susceptibility to chronic pain among the 15 tag SNPs that were examined, and this SNP would be the most plausible causal SNP.
4. Materials and Methods
4.1. Participants
The present study used patient group recruitment and sample collection methods that were approved by the ethics committees of the respective universities and hospitals (see the institutional review board statement section below). Patients who were enrolled in the study were 194 adult patients who suffered from chronic pain or pain-related disorders, including neuropathic pain, such as PHN (NPPG), and who visited the JR Tokyo General Hospital (Tokyo, Japan), Juntendo University Hospital (Tokyo, Japan), or Nihon University Itabashi Hospital (Tokyo, Japan) for chronic pain treatment. The detailed demographic data for the subjects and their statistics of attributes (age, sex) are provided in Table 3 and in a previous report [14].
Enrolled in the study as controls were 282 adult (20–70 years old, 158 males and 124 females), apparently healthy volunteers (NOCG) without any diseases or chronic pain who lived in or near the Kanto area in Japan. The detailed demographic data for the subjects and their statistics are provided in a previous report [14].
4.2. Genotyping
Genotyping was performed using HumanOmni1-Quad v. 1.0 (total markers: 11, 34, 514) and HumanOmniExpress-12 v. 1.1 (total makers: 7, 19, 665) for the 194 participants in the NPPG and HumanOmniExpressExome-8 v. 1.2 (total makers: 9, 64, 193) for the 282 subjects in the NOCG, respectively [14]. The genotype data for the SNPs in the 50 kbp region upstream and downstream of the TRPC3 gene were extracted from microarray data for the NPPG and NOCG.
4.3. Linkage Disequilibrium Block Analysis
Single-nucleotide polymorphisms within and around the TRPC3 gene were subjected to the LD statistics using HaploView 4.2 [37]. Single-nucleotide polymorphisms with minor allele frequencies < 0.01 were excluded from the LD analysis. The linkage disequilibrium was examined between all pairs of biallelic loci to calculate the correlation coefficients (D’ and r2) and the logarithm of odds (LOD). D’ and r2 were used to measure the LD strength and absolute LD between the pairs of SNPs, respectively.
4.4. Statistical Analysis
The statistical tests for the individual SNPs were performed using SPSS v. 21 software (IBM Japan, Tokyo, Japan). The associations between the genotypic variances of SNPs and chronic pain were statistically evaluated using the χ2 test. To ensure that the observed genotype frequencies were in accordance with the expected frequencies for the entire population, we tested for deviations from the Hardy–Weinberg equilibrium. Two SNPs, rs11732666 and rs6838639, were outliers (Table 1). Multiple-testing corrections were performed using Bonferroni’s method to adjust the p values that resulted from the association analyses. In all of the association analyses, the criterion for significance was set at the corrected p values (i.e., crude p < 0.0033 [0.05/15]), and the criterion for significance was set at a crude p < 0.05 in the Hardy–Weinberg equilibrium test.
gPLINK v. 2.050 and PLINK v. 1.07 (https://zzz.bwh.harvard.edu/plink/index.shtml; accessed on 28 July 2022) [38] were used for the haplotype-specific tests that involved several SNPs among the 15 selected tag SNPs, with the “--hap-window” option to specify all haplotypes in sliding windows of a fixed number of SNPs (shifting one SNP at a time).
5. Conclusions
The present findings improve our understanding of the genetic risk factors for chronic pain. More studies are necessary to elucidate the genetic mechanisms of the individual differences in chronic pain sensitivity. The rs11726196 SNP of the TRPC3 gene may be useful for predicting chronic pain susceptibility.
Acknowledgments
The authors thank Michael Arends for his assistance with editing the manuscript. We are grateful to the volunteers for their participation in the study and the anesthesiologists and pain clinicians for collecting the clinical data and blood and oral mucosa samples at Juntendo University Hospital, Nihon University Itabashi Hospital, and JR Tokyo General Hospital. All individuals included in this section agree to the acknowledgments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24021028/s1.
Author Contributions
Conceptualization, H.A., M.I. and K.I.; methodology, Y.A., D.N., J.H., K.N. and Y.E.; software, Y.A. and D.N.; validation, Y.A., D.N., J.H., K.N. and Y.E.; formal analysis, Y.A.; investigation, Y.A., D.N., S.O. (Seii Ohka) and S.K.; resources, H.A., K.H., C.Y., M.I., J.K., S.O. (Setsuro Ogawa) and A.H.; data curation, Y.A., D.N., J.H., K.N. and Y.E.; writing—original draft preparation, Y.A., D.N., S.O. (Seii Ohka), S.K. and K.I.; writing—review and editing, Y.A., D.N., S.O. (Seii Ohka), S.K. and K.I.; visualization, Y.A., D.N., S.O. (Seii Ohka) and S.K.; supervision, T.I., M.H., K.-i.F. and K.I.; project administration, K.I.; funding acquisition, D.N., M.H. and K.I. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of JR Tokyo General Hospital (protocol code none and date of approval: 14 January 2012), the Institutional Review Board of Juntendo University Hospital (protocol code 429 and date of approval: 27 February 2012), the Institutional Review Board of Nihon University Itabashi Hospital (protocol code none and date of approval: 30 September 2008), and the Institutional Review Board of Tokyo Metropolitan Institute of Medical Science (protocol code: 22-12, date of approval: 31 March 2022; protocol code: 21-24, date of approval: 31 March 2021; protocol code: 21-22, date of approval: 31 March 2021; protocol code: 22-9, date of approval: 31 March 2022).
Informed Consent Statement
Informed consent was obtained from all subjects who were involved in the study. Written informed consent was obtained from the patients to publish this paper.
Data Availability Statement
The data in this study are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by grants from the Japan Society for the Promotion of Science (JSPS) KAKENHI (no. 21H03028, JP22H04922 [AdAMS], 20K07774, 17K09052, 20K09259, 17K08970, 18K08829, and 17H04324) and the Japan Agency for Medical Research and Development (AMED; no. JP19ek0610011).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Phillips C.J. The cost and burden of chronic pain. Rev. Pain. 2009;3:2–5. doi: 10.1177/204946370900300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takura T., Ushida T., Kanchiku T., Ebata N., Fujii K., DiBonaventura M., Taguchi T. The societal burden of chronic pain in Japan: An internet survey. J. Orthop. Sci. 2015;20:750–760. doi: 10.1007/s00776-015-0730-8. [DOI] [PubMed] [Google Scholar]
- 3.Treede R.D., Rief W., Barke A., Aziz Q., Bennet M.I., Benoliel R., Cohen M., Evers S., Finnerup N.B., First M.B., et al. A classification of chronic pain for ICD-11. Pain. 2015;156:1003–1007. doi: 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hocking L.J., Scotland G., Morris A.D., Dominiczak A.F., Porteous D.J., Smith B.H. Heritability of chronic pain in 2195 extended families. Eur. J. Pain. 2012;16:1053–1063. doi: 10.1002/j.1532-2149.2011.00095.x. [DOI] [PubMed] [Google Scholar]
- 5.Diatchenko L., Fillingim R.B., Smith S.B., Maixner W. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nat. Rev. Rheumatol. 2013;9:340–350. doi: 10.1038/nrrheum.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knezevic N.N., Tverdohleb T., Knezevic I., Candido K.D. The role of genetic polymorphisms in chronic pain patients. Int. J. Mol. Sci. 2018;19:1707. doi: 10.3390/ijms19061707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veluchamy A., Hébert H.L., Meng W., Palmer C.N.A., Smith B.H. Systematic review and meta-analysis of genetic risk factors for neuropathic pain. Pain. 2018;159:825–848. doi: 10.1097/j.pain.0000000000001164. [DOI] [PubMed] [Google Scholar]
- 8.Chidambaran V., Gang Y., Pilipenko V., Ashton M., Ding L. Systematic review and meta-analysis of genetic risk of developing chronic postsurgical pain. J. Pain. 2020;21:2–24. doi: 10.1016/j.jpain.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman M.S., Winsvold B.S., Chavez S.O.C., Børte S., Tsepilov Y.A., Sharapov S.Z., HUNT All-In Pain. Aulchenko Y.S., Hagen K., Fors E.A., et al. Genome-wide association study identifies RNF123 locus as associated with chronic widespread musculoskeletal pain. Ann. Rheum. Dis. 2021;80:1227–1235. doi: 10.1136/annrheumdis-2020-219624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston K.J.A., Adams M.J., Nicholl B.I., Ward J., Strawbridge R.J., Ferguson A., McIntosh A.M., Bailey M.E.S., Smith D.J. Genome-wide association study of multisite chronic pain in UK Biobank. PLoS Genet. 2019;15:e1008164. doi: 10.1371/journal.pgen.1008164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suri P., Palmer M.R., Tsepilov Y.A., Freidin M.B., Boer C.G., Yau M.S., Evans D.S., Gelemanovic A., Bartz T.M., Nethander M., et al. Genome-wide meta-analysis of 158,000 individuals of European ancestry identifies three loci associated with chronic back pain. PLoS Genet. 2018;14:e1007601. doi: 10.1371/journal.pgen.1007601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warner S.C., van Meurs J.B., Schiphof D., Bierma-Zeinstra S.M., Hofman A., Uitterlinden A.G., Richardson H., Jenkins W., Doherty M., Valdes A.M. Genome-wide association scan of neuropathic pain symptoms post total joint replacement highlights a variant in the protein-kinase C gene. Eur. J. Hum. Genet. 2017;25:446–451. doi: 10.1038/ejhg.2016.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston K.J.A., Ward J., Ray P.R., Adams M.J., Mclntosh A.M., Smith B.H., Strawbridge R.J., Price T.J., Smith D.J., Nicholl B.I., et al. Sex-stratified genome-wide association study of multisite chronic pain in UK Biobank. PLoS Genet. 2021;17:e1009428. doi: 10.1371/journal.pgen.1009428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishizawa D., Iseki M., Arita H., Hanaoka K., Yajima C., Kato J., Ogawa S., Hiranuma A., Kasai S., Hasegawa J., et al. Genome-wide association study identifies candidate loci associated with chronic pain and postherpetic neuralgia. Mol. Pain. 2021;17:1744806921999924. doi: 10.1177/1744806921999924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohka S., Yamada S., Nishizawa D., Fukui Y., Arita H., Hanaoka K., Iseki M., Kato J., Ogawa S., Hiranuma A., et al. Heparan sulfate 3-O-sulfotransferase 4 is genetically associated with herpes zoster and enhances varicella-zoster virus-mediated fusogenic activity. Mol. Pain. 2021;17:17448069211052171. doi: 10.1177/17448069211052171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishida M., Kuwahara K., Kozai D., Sakaguchi R., Mori Y. TRP channels: Their function and potentiality as drug Targets. In: Nakao K., Minato N., Uemoto S., editors. Innovative Medicine: Basic Research and Development. Springer; Tokyo, Japan: 2015. pp. 195–218. [PubMed] [Google Scholar]
- 17.Levine J.D., Alessandri-Haber N. TRP channels: Targets for the relief of pain. Biochim. Biophys. Acta. 2007;1772:989–1003. doi: 10.1016/j.bbadis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Dai Y. TRPs and pain. Semin. Immunopathol. 2016;38:277–291. doi: 10.1007/s00281-015-0526-0. [DOI] [PubMed] [Google Scholar]
- 19.Bamps D., Vriens J., de Hoon J., Voets T. TRP channel cooperation for nociception: Therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 2021;6:655–677. doi: 10.1146/annurev-pharmtox-010919-023238. [DOI] [PubMed] [Google Scholar]
- 20.Brederson J.D., Kym P.R., Szallasi A. Targeting TRP channels for pain relief. Eur. J. Pharmacol. 2013;716:61–76. doi: 10.1016/j.ejphar.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 21.de Almeida A.S., Bernardes L.B., Trevisan G. TRP channels in cancer pain. Eur. J. Pharmacol. 2021;904:174185. doi: 10.1016/j.ejphar.2021.174185. [DOI] [PubMed] [Google Scholar]
- 22.Souza Monteiro de Araujo D., Nassini R., Geppetti P., De Logu F. TRPA1 as a therapeutic target for nociceptive pain. Expert Opin. Ther. Targets. 2020;24:997–1008. doi: 10.1080/14728222.2020.1815191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fozzato S., Baranzini N., Bossi E., Cinquetti R., Grimaldi A., Campomenosi P., Surace M.F. TRPV4 and TRPM8 as putative targets for chronic low back pain alleviation. Pflügers Arch. 2021;473:151–165. doi: 10.1007/s00424-020-02460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu Y.J., Zhang X., Fan Z.Z., Huai J., Teng Y.B., Zhang Y., Yue S.W. Effect of TRPV4-p38 MAPK pathway on neuropathic pain in rats with chronic compression of the dorsal root ganglion. BioMed Res. Int. 2016;2016:6978923. doi: 10.1155/2016/6978923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S.A., Jang J.H., Kim W., Lee P.R., Kim Y.H., Vang H., Lee K., Oh S.B. Mitochondrial reactive oxygen species elicit acute and chronic itch via transient receptor potential canonical 3 activation in mice. Neurosci. Bull. 2022;38:373–385. doi: 10.1007/s12264-022-00837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia M., Liu D., Yao C. TRPC3: A new target for therapeutic strategies in chronic pain-DAG-mediated activation of non-selective cation currents and chronic pain. J. Neurogastroenterol. Motil. 2015;21:445–447. doi: 10.5056/jnm15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoki Y., Nishizawa D., Hasegawa J., Kasai S., Yoshida K., Koukita Y., Ichinohe T., Nagashima M., Katoh R., Satoh Y., et al. Association between the rs1465040 single-nucleotide polymorphism close to the transient receptor potential subfamily C member 3 (TRPC3) gene and postoperative analgesic requirements. J. Pharmacol. Sci. 2015;127:391–393. doi: 10.1016/j.jphs.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y., Liu Y., Limjunyawong N., Narang C., Jamaldeen H., Yu S., Patiram S., Nie H., Caterina M.J., Dong X., et al. Sensory neuron expressed TRPC3 mediates acute and chronic itch. Pain. 2023;164:98–110. doi: 10.1097/j.pain.0000000000002668. [DOI] [PubMed] [Google Scholar]
- 29.Kosson D., Kołacz M., Gałązkowski R., Rzońca P., Lisowska B. The effect of the treatment at a pain clinic on the patients’ assessment of their pain intensity and the incidence of mental disorders in the form of anxiety, depression, and aggression. Int. J. Environ. Res. Public Health. 2019;16:586. doi: 10.3390/ijerph16040586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DNaseI-Accessible Regulatory Regions Defined by Roadmap Consortiums. [(accessed on 28 July 2022)]. Available online: https://fantom.gsc.riken.jphttps://fantom.gsc.riken.jp/zenbu/gLyphs/index.html#config=9O1caUYdJRqglKrQ1IUVu;loc=hg19::chr5:76126999.76127192+
- 31.GTEx Portal eQTL. [(accessed on 28 July 2022)]. Available online: https://www.gtexportal.org/home/snp/rs11726196.
- 32.Martin-Trujillo A., Iglesias-Platas I., Coto E., Corral-Juan M., Nicolás H.S., Corral J., Volpini V., Matilla-Dueñas A., Monk D. Genotype of an individual single nucleotide polymorphism regulates DNA methylation at the TRPC3 alternative promoter. Epigenetics. 2011;6:1236–1241. doi: 10.4161/epi.6.10.17654. [DOI] [PubMed] [Google Scholar]
- 33.The Human Protein Atlas. [(accessed on 28 July 2022)]. Available online: https://www.proteinatlas.org/ENSG00000138741-TRPC3.
- 34.Vandewauw I., Owsianik G., Voets T. Systematic and quantitative mRNA expression analysis of TRP channel genes at the single trigeminal and dorsal root ganglion level in mouse. BMC Neurosci. 2013;14:21. doi: 10.1186/1471-2202-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quick K., Zhao J., Eijkelkamp N., Linley J.E., Rugiero F., Cox J.J., Raouf R., Gringhuis M., Sexton J.E., Abramowitz J., et al. TRPC3 and TRPC6 are essential for normal mechanotransduction in subsets of sensory neurons and cochlear hair cells. Open Biol. 2012;2:120068. doi: 10.1098/rsob.120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alkhani H., Ase A.R., Grant R., O’Donnell D., Groschner K., Séguéla P. Contribution of TRPC3 to store-operated calcium entry and inflammatory transductions in primary nociceptors. Mol. Pain. 2014;10:43. doi: 10.1186/1744-8069-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrett J.C., Fry B., Maller J., Daly M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 38.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A., Bender D., Maller J., Sklar P., de Bakker P.I., Daly M.J., et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in this study are available in the Supplementary Materials.