Abstract
Toxoplasma gondii is an important pathogen in the central nervous system, causing a severe and often fatal encephalitis in patients with AIDS. Gamma interferon (IFN-γ) is the main cytokine preventing reactivation of Toxoplasma encephalitis in the brain. Microglia are important IFN-γ-activated effector cells controlling the growth of T. gondii in the brain via a nitric oxide (NO)-mediated mechanism. IFN-γ can also activate astrocytes to inhibit the growth of T. gondii. Previous studies found that the mechanism in murine astrocytes is independent of NO and all other known anti-Toxoplasma mechanisms. In this study we investigated the role of IGTP, a recently identified IFN-γ-regulated gene, in IFN-γ inhibition of T. gondii in murine astrocytes. Primary astrocytes were cultivated from IGTP-deficient mice, treated with IFN-γ, and then tested for anti-Toxoplasma activity. In wild-type astrocytes T. gondii growth was significantly inhibited by IFN-γ, whereas in astrocytes from IGTP-deficient mice IFN-γ did not cause a significant inhibition of growth. Immunoblot analysis confirmed that IFN-γ induced significant levels of IGTP in wild-type murine astrocytes within 24 h. These results indicate that IGTP plays a central role in the IFN-γ-induced inhibition of T. gondii in murine astrocytes.
Toxoplasma gondii is an important pathogen in the central nervous system, where it causes a severe and often fatal encephalitis in patients with AIDS. Cytokines play an important role in the regulation of T. gondii replication in the central nervous system (5, 10, 11). Gamma interferon (IFN-γ) has been shown to be the main cytokine preventing reactivation of Toxoplasma encephalitis in the brain (17, 18). Several studies have demonstrated that IFN-γ can control the growth of T. gondii in the brain via the activation of microglia (3, 4). The anti-Toxoplasma activity in microglia is via a nitric oxide (NO)-mediated mechanism (7). IFN-γ can also activate astrocytes to inhibit the growth of T. gondii (8). The mechanism of IFN-γ-mediated anti-Toxoplasma activity in murine astrocytes has been found to be independent of the mechanisms previously demonstrated in other cells, e.g., mechanisms involving NO, tryptophan starvation, reactive oxygen intermediates, and iron deprivation (9).
IFN-γ is thought to exert its effects largely by activation of IFN-γ-responsive genes, of which over 200 have been identified (2). For most of these genes, their contributions in mediating the effects of IFN-γ are unknown. One recently identified IFN-γ-regulated gene is IGTP (19). It is representative of a family of at least six genes encoding 47- to 48-kDa proteins that contain a GTP-binding sequence and which are expressed at high levels in immune and nonimmune cells after exposure to IFN-γ. Several of these proteins, including IGTP, localize to the endoplasmic reticulum (ER) of cells, suggesting that they may be involved in the processing or trafficking of immunologically relevant proteins, such as antigens or cytokines (20). Recently it has been found that IGTP-deficient (ΔIGTP) mice display a loss of host resistance to acute infection with T. gondii (21). In this study, we investigated the potential involvement of IGTP in IFN-γ inhibition of T. gondii in murine astrocytes using primary astrocytes cultivated from IGTP-deficient mice.
MATERIALS AND METHODS
Primary astrocyte culture.
Murine astrocytes from C57BL/6 × SV129 mice or syngeneic mice, deficient in IGTP (21), were cultivated from the brains of neonatal (less-than-24-h-old) mice. Murine pups were sacrificed, the brains were removed from the cranium, the forebrains dissected, and the meninges were removed. The tissue was minced and incubated in 0.25% trypsin for 5 min at 37°C. After 5 min, the trypsin was inactivated with a solution containing DNase and soybean trypsinase inhibitors, and the tissue was further disrupted by trituration in a 20-ml pipette. The dissociated cells were filtered through a 74-μm-pore-size Nitex mesh, centrifuged at 200 × g, and suspended in growth medium (GIBCO-BRL, Gaithersburg, Md.) supplemented with 20% fetal bovine serum (FBS) (GIBCO-BRL), 5% glucose, and 1% penicillin and streptomycin (GIBCO-BRL) per ml. The growth medium was changed every 3 days. After 7 days in vitro, a confluent layer of 1 × 104 to 2 × 104 cells/cm was reached. By this method, cells were found to be >95% astrocytes, as judged by positive staining for glial fibrillary acidic protein (GFAP). Cultures contained <5% microglia, as identified by staining with the lectin BS1-B4 (L-2895; Sigma, St. Louis, Mo.). Astrocytes were dissociated in trypsin-EDTA, replated onto poly-l-lysine-coated coverslips or 24-well plates at 104 cells/cm, and cultured for 7 to 10 days after replating. These astrocytes were then infected with T. gondii ME49 as described below.
Cryopreservation of primary murine astrocytes.
Murine astrocyte cultures prepared from mouse brain were cultivated in growth medium as described above. At 7 to 10 days after plating, cultures were trypsinized and then resuspended in growth medium with 10% dimethyl sulfoxide. Cells were then frozen to −70°C at −1°C/min using a Nalgene Cryo Freezing Container (catalog no. 5100-001; Nalge Nunc International, Rochester, N.Y.) and were stored in liquid nitrogen. Astrocytes frozen by this method were stable for months and routinely could be replated to attain a monolayer within 7 days that was GFAP positive.
Culture of T. gondii.
Parasites were maintained by serial passage in confluent monolayers of human foreskin fibroblasts (ATCC CCD-27SK) grown in Dulbecco's modified Eagle's medium (pH 7.1) (GIBCO-BRL) supplemented with 10% FBS and a 1% penicillin-streptomycin solution (GIBCO-BRL). Parasites were harvested at 5 to 6 days postinfection, resuspended in minimal essential medium supplemented with 10% FBS, and used for infection of murine astrocyte cultures.
Cytokine treatments.
Murine astrocytes were stimulated with IFN-γ (Calbiochem) at 64 to 500 U/ml for 72 h prior to infection. Cultures were washed and then infected with 5 × 104 T. gondii tachyzoites per well (a 5:1 tachyzoite/host cell ratio) for 2 h. Cultures were then washed to remove extracellular parasites and incubated for 48 h without IFN-γ. Growth of T. gondii was assessed microscopically as detailed below. All cultures were incubated in endotoxin-free media, and no endotoxin contamination was detected in any experimental cultures.
Microscopic analysis of T. gondii intracellular replication.
The percentage of infected astrocytes was determined by counting the number of infected cells per 500 cells under both phase and immunofluorescent microscopies. Each condition was determined in triplicate. Immunofluorescence was performed using a 1:50 dilution of a commercial polyclonal rabbit anti-Toxoplasma antibody (DAKO, Carpinteria, Calif.) followed by detection with anti-rabbit fluorescein immunoglobulin G (IgG; Boehringer-Mannheim, Indianapolis, Ind.) as previously described (8, 9).
Western blotting.
Total cellular protein lysates were prepared by washing the cells three times with ice-cold phosphate-buffered saline and then scraping them into ice-cold lysis buffer (1% [vol/vol] Nonidet P-40, 50 mM Tris [pH 7.5], 0.15 M NaCl, 5 mM EDTA) with plastic cell scrapers. Cellular protein lysates were separated on sodium dodecyl sulfate–10% polyacrylamide gels; the gels were transferred to an Immobilon-P membrane (Millipore, Bedford, Mass.) with a TEX50 wet transfer apparatus (Hoefer, San Francisco, Calif.) using 25 mM Tris, 480 mM glycine, and 20% (vol/vol) methanol buffer. Western blotting and detection were carried out using the ECL Detection System (Amersham International, Buckinghamshire, United Kingdom) according to the manufacturer's protocols. The rabbit polyclonal anti-IGTP antiserum (19–21) was used at a 1:1,000 dilution; the peroxidase-conjugated anti-rabbit IgG secondary antibody (Boehringer Mannheim) was used at a 1:5,000 dilution.
Statistics.
Within each experiment all conditions were repeated in triplicate wells, and each experiment was replicated two to three times (as indicated in the tables). Data were analyzed by the Student t test method using Sigma Stat Version 1.0 (Jandel Scientific, San Rafael, Calif.).
RESULTS
Growth of Toxoplasma in IFN-γ-stimulated wild-type astrocytes versus growth in ΔIGTP astrocytes.
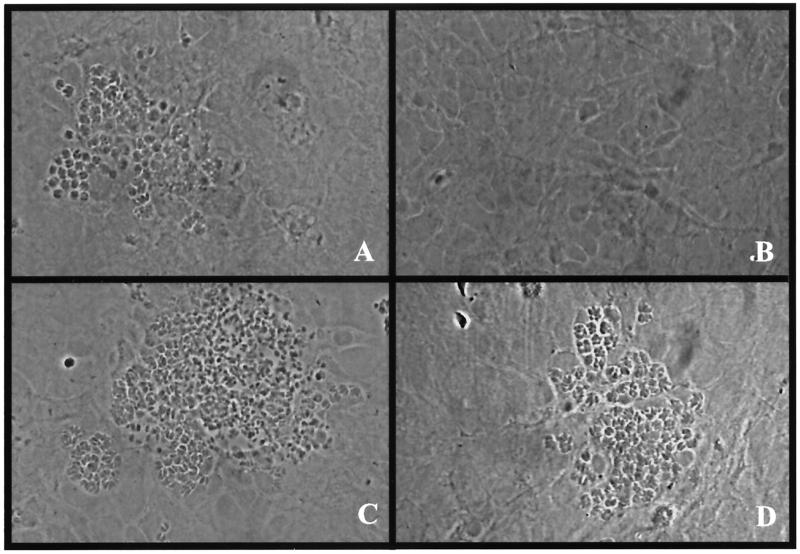
Wild-type and ΔIGTP murine astrocytes were stimulated with IFN-γ for 72 h and infected with T. gondii, and growth of the parasite was assessed 24 h after infection. There was no difference in the rate of infection or growth of T. gondii in the untreated ΔIGTP astrocytes compared to that of the wild-type astrocytes. At 48 h after infection, both wild-type and ΔIGTP untreated astrocytes contained vacuoles with numerous tachyzoites (Fig. 1A versus C). In the IFN-γ-treated astrocytes, however, a significant difference was observed between wild-type astrocytes and ΔIGTP astrocytes. At 48 h after infection, the IFN-γ-treated wild-type astrocytes had few visible parasites, while the IFN-γ-treated ΔIGTP astrocytes had numerous parasites and cells contained vacuoles with numerous tachyzoites (Fig. 1B versus D). The growth of T. gondii in IFN-γ-treated ΔIGTP astrocytes was comparable to that of untreated wild-type astrocytes, indicating that the IFN-γ-mediated inhibition was reversed in ΔIGTP astrocytes.
FIG. 1.
Phase contrast of untreated and IFN-γ-treated wild-type astrocytes versus untreated and IFN-γ-treated ΔIGTP murine astrocytes. (A) Untreated wild-type astrocytes; (B) IFN-γ-treated wild-type astrocytes; (C) untreated ΔIGTP astrocytes; (D) IFN-γ-treated ΔIGTP astrocytes, 48 h after infection. Almost no organisms were present in IFN-γ-treated control astrocytes, but extensive replication was present in IFN-γ-treated ΔIGTP astrocytes.
Immunoblotting of IFN-γ induction of IGTP in wild-type astrocytes.
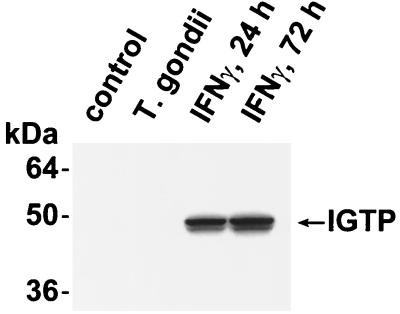
Wild-type astrocytes were stimulated with IFN-γ (100 U/ml) for 24 and 72 h and tested for induction of IGTP via Western blotting. IGTP was not detected either under control conditions or following infection with T. gondii (Fig. 2). However, wild-type astrocytes treated with 100 U of IFN-γ for 24 or 72 h demonstrated strong IGTP induction (Fig. 2, lanes 3 and 4).
FIG. 2.
Accumulation of IGTP in astrocytes exposed to IFN-γ. Primary astrocytes were infected with T. gondii for 24 h at a multiplicity of infection of 5:1, were exposed to 100 U of IFN-γ/ml for 24 or 72 h, or were exposed to control conditions. Protein lysates were then prepared and used for sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis and blotting with IGTP antisera.
Dose response of treated wild-type versus IFN-γ-treated ΔIGTP astrocytes.
A dose response of IFN-γ stimulation in wild-type versus ΔIGTP astrocytes was done to further investigate if IFN-γ-mediated inhibition occurred in ΔIGTP astrocytes at higher doses of IFN-γ. Wild-type and ΔIGTP astrocytes were pretreated with IFN-γ at 64, 125, 250, and 500 U/ml for 72 h prior to infection. In wild-type astrocytes, T. gondii growth was significantly inhibited (10.6% of control) at 64 U/ml, whereas in ΔIGTP astrocytes, 64 to 500 U of IFN-γ/ml caused a minimal inhibition of growth (75 to 88% of control) (Table 1).
TABLE 1.
Dose response of IFN-γ-mediated inhibition in wild-type and ΔIGTP astrocytesa
| IFN-γ treatment (U/ml) |
T. gondii growth (% of control) in:
|
|
|---|---|---|
| Wild-type astrocytes | ΔIGTP astrocytes | |
| 64 | 10.6 ± 2.2* | 88.8 ± 14.5 |
| 125 | 3.6 ± 0.2* | 88.2 ± 8.1 |
| 250 | 0.9 ± 0.05* | 77.7 ± 12.9 |
| 500 | 0.9 ± 0.05* | 75.6 ± 6.2 |
Means ± standard errors of the means of three separate experiments for wild-type and ΔIGTP astrocyte cultures treated with IFN-γ are shown. Control cultures were incubated in medium alone. Cells were incubated with cytokines for 72 h prior to infection. *, P < 0.05 for wild-type astrocytes versus IFN-γ-treated astrocytes. There was no statistical difference between IFN-γ-treated ΔIGTP cells and untreated wild-type cells.
DISCUSSION
IFN-γ has been shown to be the main cytokine preventing reactivation of Toxoplasma encephalitis in the brain (17, 18). Macrophages and microglia are phagocytic cells of hemopoietic origin and are important IFN-γ-activated effector cells against T. gondii that exert potent anti-Toxoplasma activity via the induction of inducible NO synthase and the production of NO (1, 3, 4). NO is believed to be directly toxoplasmacidal, resulting in intracellular killing and/or stasis of parasites. IFN-γ has been shown to induce anti-Toxoplasma activity in a variety of nonhemopoietic cells via NO-independent mechanisms. For example, in human fibroblasts and retinal epithelial cells and rat retinal epithelial cells, IFN-γ inhibition is due to the induction of indoleamine 2,3-dioxygenase, an enzyme that degrades intracellular tryptophan (14, 15, 16). In rat enterocytes, IFN-γ inhibition was found to be due to iron starvation (6), while in human endothelial cells, IFN-γ inhibition was found to be independent of reactive nitrogen intermediates, reactive oxygen species, or tryptophan starvation (22). In murine astrocytes we have found IFN-γ inhibition was independent of all of the above mechanisms (8, 9). The anti-Toxoplasma mechanism operating in murine astrocytes, and possibly in other nonhemopoietic effector cells, remains to be elucidated.
In this paper we investigated the role of IGTP, a recently identified IFN-γ-regulated gene, in the IFN-γ-induced inhibition of T. gondii in murine astrocytes. We found that the inhibitory effect of IFN-γ is ablated in astrocytes lacking the protein IGTP. In wild-type astrocytes, T. gondii growth was significantly inhibited at 64 U/ml, whereas in ΔIGTP astrocytes, concentrations of up to 500 U of IFN-γ/ml caused minimal growth inhibition. IGTP was highly expressed in wild-type astrocytes within 24 h of exposure to IFN-γ, and expression remained high to at least 72 h. These data suggest that IGTP plays a central role in the IFN-γ-induced inhibition of T. gondii in murine astrocytes. It is possible that small amounts of IFN-γ in the central nervous system are important in maintaining astrocytes in an activated condition.
The function of IGTP is not known. IGTP is expressed at high levels in many IFN-γ-stimulated cells, including immune cells such as macrophages, T cells, and B cells as well as nonimmune effector cells such as fibroblasts, hepatocytes (18), and as we have shown in this paper, astrocytes. IGTP is a GTP-binding protein that is membrane bound, and it localizes predominantly to the ER (20). There are many families of GTPases that associate with the ER and that are involved in protein processing or trafficking; therefore, it has been suggested that IGTP may regulate vesicular trafficking within the cell (20, 21).
T. gondii has a unique relationship with its host cell. Unlike other intracellular pathogens that reside in the cytoplasm, endosome, or lysosomal compartments, T. gondii resides within an intracellular compartment called the parasitophorous vacuole. The parasitophorous vacuole is a nonfusogenic compartment that does not intersect with the host cell endocytic or exocytic pathways (13). The nonfusogenic nature of the parasitophorous vacuole is crucial to survival of the intracellular stage, as fusion with lysosomes results in degradation of the parasite. Alterations in the host cell that induce trafficking of the host cell endocytic or exocytic pathways to the parasitophorous vacuole could conceivably be detrimental to intracellular survival. IGTP, for example, may control parasite clearance through regulation of vesicular movement of antigen, cytokines, or other molecules to the parasitophorous vacuole.
The ability of nonhematopoietic cells to control T. gondii in vivo has, until recently, been unclear. A study employing chimeric mice demonstrated that resistance to the acute and chronic phases of T. gondii requires nonhematopoietic cells as well as those of hematopoietic origin (23). Host control of T. gondii and other pathogens is dependent on the induction of diverse effector molecules by IFN-γ in a variety of nonhematopoietic cells. Consequently, murine knockouts of different IFN-γ-induced effectors display various pathogen-specific susceptibilities, depending on the cells targeted by the pathogen and the cells in which the effectors are expressed. For example, IGTP-deficient mice are susceptible to T. gondii but not to Listeria. Our data demonstrate that IGTP is particularly important for astrocyte-mediated control of T. gondii, and they further underscore the critical importance of nonhematopoietic cells in resistance to toxoplasmosis. T. gondii infects several types of nonhematopoietic cells, including epithelial, endothelial, mesodermal, and neuronal cells, and the ability of the host to elicit effector mechanisms in these cells may relate to the pathogenesis of toxoplasmosis. For example, epithelial cells may be important effector cells in the acute phase of infection, endothelial cells in congenital toxoplasmosis, and astrocytes in cerebral toxoplasmosis. A better understanding of IFN-γ-induced mechanisms in these cell types may be important in devising better treatment strategies for toxoplasmosis.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant AI39454 (L.M.W.).
We thank Yan Fen Ma for assistance with tissue culture.
REFERENCES
- 1.Adams L B, Hibbs J B, Taintor R R, Kranhenbuhl J L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990;144:2725–2729. [PubMed] [Google Scholar]
- 2.Boehne U, Klaamp T, Groot M, Howard J C. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 3.Chao C C S H, Gekker G, Novick W J, Jr, Remington J S, Peterson P K. Effects of cytokines on multiplication of Toxoplasma gondii in microglia cells. J Immunol. 1993;150:3404–3410. [PubMed] [Google Scholar]
- 4.Chao C C, Gekker G, Hu S, Peterson P K. Human microglia cell defense against Toxoplasma gondii. The role of cytokines. J Immunol. 1994;152:1246–1252. [PubMed] [Google Scholar]
- 5.Deckert-Schluter S, Albrect H, Hor H, Wiestler O D, Schluter D. Dynamics of the intracerebral and splenic cytokine mRNA production in Toxoplasma gondii-resistant and -susceptible congenic strains of mice. Immunology. 1995;85:408–418. [PMC free article] [PubMed] [Google Scholar]
- 6.Dimier I H, Bout D T. Interferon-γ-activated primary enterocytes inhibit Toxoplasma gondii replication: a role for intracellular iron. Immunology. 1998;94:488–495. doi: 10.1046/j.1365-2567.1998.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazzinelli R T, Eltoum I, Wynn T A, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNFα and correlates with the down regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:3672–3681. [PubMed] [Google Scholar]
- 8.Halonen S K, Chiu F C, Weiss L M. Effect of cytokines on growth of Toxoplasma gondii in murine astrocytes. Infect Immun. 1998;66:4989–4993. doi: 10.1128/iai.66.10.4989-4993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halonen S K, Weiss L M. Investigation into the mechanism of gamma interferon-mediated inhibition of Toxoplasma gondii in murine astrocytes. Infect Immun. 2000;68:3426–3430. doi: 10.1128/iai.68.6.3426-3430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter C A, Roberts C W, Murray M, Alexander J. Detection of cytokine mRNA in the brains of mice with toxoplasmic encephalitis. Parasite Immunol. 1992;14:405–413. doi: 10.1111/j.1365-3024.1992.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 11.Hunter C A, Litton M J, Remington J S, Abrams J S. Immunocytochemical detection of cytokines in the lymph nodes and brains of mice resistant or susceptible to toxoplasmic encephalitis. J Infect Dis. 1994;170:939–945. doi: 10.1093/infdis/170.4.939. [DOI] [PubMed] [Google Scholar]
- 12.Hunter C A, Remington J S. Immunopathogenesis of toxoplasmic encephalitis. J Infect Dis. 1994;170:1057–1067. doi: 10.1093/infdis/170.5.1057. [DOI] [PubMed] [Google Scholar]
- 13.Mordue D G, Häkansson S, Niesman I, Sibley L D. Toxoplasma gondii reside in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Exp Parasitol. 1999;92:87–99. doi: 10.1006/expr.1999.4412. [DOI] [PubMed] [Google Scholar]
- 14.Nagineni C N, Pardhasaradhi K, Martins M C, Detrick B, Hooks J J. Mechanisms of interferon-induced inhibition of Toxoplasma gondii replication in human retinal pigment epithelial cells. Infect Immun. 1996;64:4188–4196. doi: 10.1128/iai.64.10.4188-4196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfefferkorn E R. Interferon-γ blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfefferkorn E R, Eckel M, Rebhun S. Interferon-γ suppresses the growth of Toxoplasma gondii in human fibroblasts through starvation for tryptophan. Mol Biochem Parasitol. 1986;20:215–224. doi: 10.1016/0166-6851(86)90101-5. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki Y, Orellana M A, Schreiber R D, Remington J S. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki Y, Conley F K, Remington J S. Importance of endogenous IFN-γ for prevention of toxoplasmic encephalitis in mice. J Immunol. 1989;143:2045–2050. [PubMed] [Google Scholar]
- 19.Taylor G A, Jeffers M, Largasespada D A, Jenkins N A, Copeland N G, Vande Woude G F. Identification of a novel GTPase, the inducibly expressed GTPase, that accumulates in response to interferon γ. J Biol Chem. 1996;271:20399–20405. doi: 10.1074/jbc.271.34.20399. [DOI] [PubMed] [Google Scholar]
- 20.Taylor G A, Stauber R, Rulong S, Hudson E, Pei V, Pavlakis G N, Resau J H, Vande Woude G F. The inducibly expressed GTPase localizes to the endoplasmic reticulum, independently of GTP binding. J Biol Chem. 1997;272:10639–10645. doi: 10.1074/jbc.272.16.10639. [DOI] [PubMed] [Google Scholar]
- 21.Taylor G A, Collazo C M, Yap G S, Nguyen K, Gregorio T A, Taylor L S, Eagleson B, Secrest L, Southon E A, Reid S W, Tessarollo L, Bray M, McVicar D W, Komschlies K L, Young H A, Biron C A, Sher A, Vande Woude G F. Pathogen-specific loss of host resistance in mice lacking the IFN-γ-inducible gene IGTP. Proc Natl Acad Sci USA. 2000;97:751–755. doi: 10.1073/pnas.97.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodman J P, Dimier I H, Bout D T. Human endothelial cells are activated by IFN-γ to inhibit Toxoplasma gondii replication. J Immunol. 1991;147:2019–2023. [PubMed] [Google Scholar]
- 23.Yap G S, Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-γ and tumor necrosis factor (TNF)-α dependent host resistance to the intracellular pathogen Toxoplasma gondii. J Exp Med. 1999;189:1083–1091. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]