Abstract
The urogenital microbiota is dominated by Lactobacillus that, together with Bifidobacterium, creates a physiological barrier counteracting pathogen infections. The aim of this study was to evaluate the efficacy of a multi-strain probiotic formulation (Lactiplantibacillus plantarum PBS067, Lacticaseibacillus rhamnosus LRH020, and Bifidobacterium animalis subsp. lactis BL050) to inhibit adhesion and growth of urogenital pathogens. The antimicrobial and antiadhesive properties of the probiotic strains and their mixture were evaluated on human vaginal epithelium infected with Candida glabrata, Neisseria gonorrheae, Trichomonas vaginalis, and Escherichia coli-infected human bladder epithelium. The epithelial tissue permeability and integrity were assessed by transepithelial/transendothelial electrical resistance (TEER). Co-aggregation between probiotics and vaginal pathogens was also investigated to elucidate a possible mechanism of action. The multi-strain formulation showed a full inhibition of T. vaginalis, and a reduction in C. glabrata and N. gonorrheae growth. A relevant antimicrobial activity was observed for each single strain against E. coli. TEER results demonstrated that none of the strains have negatively impaired the integrity of the 3D tissues. All the probiotics and their mixture were able to form aggregates with the tested pathogens. The study demonstrated that the three strains and their mixture are effective to prevent urogenital infections.
Keywords: urogenital microbiota, probiotics, pathogens, infection, antimicrobial activity, co-aggregation
1. Introduction
Vaginal microbiota (VMB) is generally defined as a complex association of heterogeneous microorganisms which can influence the vaginal microenvironment. Indeed, the close interaction among these microorganisms can positively or negatively affect the host through mechanisms of commensalism, mutualism, and pathogenicity [1]. VMB composition and structure has been largely studied and it is widely acknowledged that it is subjected to important physiological modifications throughout the lifespan of women, from birth to menopause. Such changes can be correlated to different glycogen concentrations in the vagina, hormones levels, and the consequent pH alteration [2].
In the vagina, interactions between its microbiota and the human host represent the first line of defence against opportunistic pathogens. In normal conditions, a balanced VMB composition is considered in eubiosis. When this equilibrium is disrupted, opportunistic pathogens can prevail, leading to dysbiosis, a state of alteration of normal functionality that involves innate and immune-mediated responses, often leading to chronic inflammation [3], such as urinary tract infections (UTIs), common clinical conditions in which VMB plays an important role [4].
Generally, an eubiotic VMB is characterized by the dominant presence of different Lactobacillus species [3]. They produce lactic acid and thus are defined as lactic acid bacteria (LAB). Lactobacillus are responsible for the acidic environment of the vagina which, along with other mechanisms such as antimicrobial substances secretion and competitive exclusion, prevents pathogen growth in the urogenital tract. Lactic acid is a potent bactericidal compound since it causes the pH lowering, thus inhibiting most of the pathogens [5]. It is normally present in the vaginal environment in two racemic forms, D (−) and L (+) lactic acid. Such isomers, produced in different ratios depending on resident Lactobacillus spp., have shown specific inhibition effect against opportunistic microorganisms [3,5]. Previous studies stated that VMB with a predominance of Lactobacillus crispatus is characterized by higher levels of D (−) lactic acid, while L (+) lactic acid has specific immunological properties. Therefore, both isomers play an important role in the maintenance of a healthy vaginal environment [6]. Additionally, Lactobacillus are known to produce bacteriocins with inhibitory capacity against pathogens. These substances are small peptides, classified according to their molecular size, mode of action, presence of modified amino acids, and morphological traits [7,8]. Different studies on several Lactobacillus strains demonstrated their ability to produce a broad spectrum of antimicrobial bacteriocins, such as plantaricin, reuterin, and nisin [7]. Finally, Lactobacillus could block urogenital pathogen adhesion to mucosa epithelial cells throughout different mechanisms, such as exclusion, competition, or displacement [9]. During the exclusion process, LAB are able to colonize the epithelium and to occupy the binding sites, leaving no free surface for urological pathogen adhesion. Competition occurs instead when LAB and pathogens compete for nutrients in the environment, while displacement indicates microorganisms’ ability to remove pathogens from epithelial cells [10]. For all these reasons, a microbiota composition particularly enriched in Lactobacillus plays a pivotal role in the maintenance of eubiosis [11]. When an unbalanced situation occurs, their presence is reduced, and the consequent increase in pH causes overgrowth of undesirable or pathogenic microorganisms [12].
UTIs are frequent disorders characterized by pathogenic colonization of vagina, urethra, and bladder, which sometimes reaches kidneys, causing infection [4]. The proximity of these districts allows a gradual cross-contamination which can lead to more severe infections [13]. Escherichia coli, Atopobium vaginae, Gardnerella vaginalis, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecalis, and Staphylococcus saprophyticus species have been recognized as the most common responsible for urinary tract diseases [14,15]. In particular, the Gram-negative bacterium E. coli is able to produce several soluble metabolic products with potential tissue-damaging effects, such as pore forming toxins and proteases. Its adherence ability is mediated by slime production, which confer to E. coli higher virulence, higher resistance to the phagocytosis, prevention of antimicrobial substances access, and improved adherence to host tissues. Moreover, E. coli along with Candida spp. are considered as main etiological agents of common diseases in the vagina [16].
Antimicrobials compounds such as fluconazole, metronidazole and clindamycin are used as common therapy for urogenital infections [17,18]. Nevertheless, long-term antimicrobial drug administration is reported to be related to antibiotic resistance and consequent recurrences [19,20]. In the last years, probiotic use for the treatment and prevention of vaginal infections has considerably increased [21]. Indeed, probiotics are reported not only to restore normal vaginal homeostasis by promoting the proliferation of beneficial microorganisms but also to reduce the associated symptoms (pain, inflammation, discomfort, etc.) [22,23]. Lactobacillus-based probiotics are also known to be particularly effective in counteracting urogenital pathologies [24]. Furthermore, some studies suggest the co-aggregation of Lactobacillus with pathogens as one of the mechanisms of action in the intestinal district [25,26].
The aim of this research was to determine the antimicrobial and co-adhesion activity of a multi-strain probiotic composition containing Lactiplantibacillus plantarum PBS067, Lacticaseibacillus rhamnosus LRH020, and Bifidobacterium animalis subsp. lactis BL050 against some common urogenital pathogens. This formulation (SynBalance® Femme) was previously characterized for its probiotic properties both as single strains [27] and as their mixture [28,29]. In this work, further evaluations were performed on 3D reconstructed human vaginal epithelium (HVE) and human bladder epithelium (HBE). Moreover, in order to more deeply investigate the mechanism of action of SynBalance® Femme on pathogens, its interaction as mixture and single strains, and possible co-aggregation with Candida albicans, G. vaginalis, and E. coli were also studied at the ultrastructural level by Scanning Electron Microscope (SEM) analysis, demonstrating the efficacy of the probiotic treatment.
2. Results
2.1. Antimicrobial and Antiadhesive Efficacy on HVE
HVE tissues were infected in vitro with three pathogenic strains: Candida glabrata, Neisseria gonhorreae, and Trichomonas vaginalis.
In the antimicrobial efficacy protocol, the probiotic formulation was applied on HVE after being colonized by the different pathogens. In all cases, viability and integrity of the tissues and probiotic concentration were not altered at the end of the experiment (Table 1).
Table 1.
In vitro model of antimicrobial efficacy of SynBalance® Femme vs. C. glabrata ATCC 15126, N. gonhorreae ATCC 43069 or T. vaginalis ATCC 30238 on HVE. The amount of SynBalance® Femme and each pathogen was expressed as log10 CFU/mL. The epithelium viability was expressed in percentage, while the TEER in Ohm × cm2. All the experiments were carried out in triplicate.
| Protocol A | SynBalance® Femme |
C. glabrata | SynBalance® Femme | N. gonhorreae | SynBalance® Femme | T. vaginalis |
|---|---|---|---|---|---|---|
| Initial count | 7.74 | 7.75 | 7.47 | 7.81 | 7.96 | 7.49 |
| Non-adhered microrganism (washing) | 6.68 | 7.75 | 0 | 0 | 4.60 | 0 |
| Adhered microrganism (homogenates) | 7.30 | 5.43 | 6.10 | 4.69 | 6.68 | 0 |
| Microrganism penetrated into the epithelium (medium) | 0 | 0 | 0 | 0 | 0 | 0 |
| Epithelium viability (%) | 99.0 | - | 98.7 | - | 98.7 | - |
| Epithelium integrity (TEER, Ω) | 173.3 | - | 175.4 | - | 173.8 | - |
- not performed.
C. glabrata colonized the tissues with an initial mean count of 7.75 log10 CFU/mL. After exposure to probiotic, an amount of 7.75 log10 and 5.43 log10 CFU/mL was found in tissue washing wastes and tissue homogenates, respectively. In the case of N. gonhorreae, the initial mean count was 7.81 log10 CFU/mL. No viable microorganisms were found in tissue washing wastes, while an amount of 4.69 log10 CFU/mL was found in tissue homogenates. T. vaginalis colonized the tissue with an initial mean count of 7.49 log10 CFU/mL. In this case, no viable microorganisms were found neither in the tissue washing wastes nor in the tissue homogenates (Table 1).
To evaluate the preventive-antiadhesive activity (Protocol B), SynBalance® Femme (7.74 log10 CFU/mL initial count) was applied directly on HVE; after incubation, an inoculum of the three pathogenic microorganisms was carried out (Table 2).
Table 2.
In vitro model of preventive and antiadhesive efficacy of SynBalance® Femme vs. C. glabrata ATCC 15126, N. gonhorreae ATCC 43069 or T. vaginalis ATCC 30238 on HVE. The amount of SynBalance® Femme or each pathogen was expressed as log10 CFU/mL. The epithelium viability was expressed in percentage, while the TEER in Ohm × cm2. All the experiments were carried out in triplicate.
| Protocol B | SynBalance® Femme |
C. glabrata | SynBalance® Femme | N. gonhorreae | SynBalance® Femme | T. vaginalis |
|---|---|---|---|---|---|---|
| Initial count | 7.74 | 7.75 | 7.47 | 7.81 | 7.96 | 7.49 |
| Non-adhered microrganism (washing) | 6.20 | 6.17 | 0 | 0 | 4.84 | 2.17 |
| Adhered microrganism (homogenated) | 7.83 | 0 | 5.95 | 5.14 | 3.95 | 3.48 |
| Microrganism penetrated into epithelium (medium) | 0 | 0 | 0 | 0 | 0 | 0 |
| Epithelium viability (%) | 99.1 | - | 96.8 | - | 95.1 | - |
| Epithelium integrity (TEER, Ω) | 170.7 | - | 171.0 | - | 170.9 | - |
- not performed.
As for Protocol A, the amount of probiotics was not affected by infection of pathogens. C. glabrata was applied with an initial mean concentration of 7.75 log10 CFU/mL. At the end of the experiment, the fungal concentration found in the tissue washing waste was 6.17 log10 CFU/mL, while no viable microorganisms were found in the tissue homogenates. Likewise, starting from 7.81 log10 CFU/mL of N. gonhorreae, the anti-adhesion activity was evaluated. No viable microorganisms were found in tissue washing waste and in the tissue media, while in tissue homogenates a count of 5.14 log10 CFU/mL was found. HVE, pretreated with the probiotic formulation, was also tested for T. vaginalis adhesion. Starting from an initial amount of 7.49 log10 CFU/mL, only 2.17 log10 CFU/mL and 3.48 log10 CFU/mL in tissue washing waste and in tissue homogenates were detected, respectively. In summary, on HVE the amount of viable pathogens was significantly reduced. Moreover, no significant changes in the epithelium viability and integrity was observed among treatments (Table 2).
2.2. Antibacterial and Antiadhesive Efficacy on HBE
In the antibacterial efficacy test (Protocol A), each strain of the probiotic mixture was applied on HBE, already colonized by E. coli. After incubation, the concentration of viable probiotics and pathogen was counted both in apical compartment and homogenate tissue. B. animalis subsp. lactis BL050 totally inhibited E. coli non-adherent cells (apical compartment) and significantly reduced the viable bacteria in the homogenate tissue by 1 log10, indicating antibacterial activity on E. coli cells. Both L. plantarum PBS067 and L. rhamnosus LRH020 showed an optimal performance, with a significant reduction in non-adherent cells and total depletion of E. coli cells in the homogenate tissues (Table 3, left part).
Table 3.
Antimicrobial and preventive-antiadhesive efficacy of B. animalis subsp. lactis BL050, L. plantarum PBS067 and L. rhamnosus LRH020 vs. E. coli ATCC 8739 on HBE. The amount of each strain and the pathogen was expressed as log10/CFU/mL.
| Protocol A | Protocol B | |||
|---|---|---|---|---|
| APICAL log10 CFU/mL |
HOMOGENATE log10 CFU/mL |
APICAL log10 CFU/mL |
HOMOGENATE log10 CFU/mL |
|
| E. coli | 7.18 | 5.90 | 6.56 | 5.96 |
| B. animalis subsp. lactis BL050 | 0.00 | 4.38 | 6.38 | 4.08 |
| L. plantarum PBS067 | 4.63 | 0.00 | 6.63 | 4.93 |
| L. rhamnosus LRH020 | 5.11 | 0.00 | 5.45 | 0.00 |
In order to evaluate the preventive-antiadhesive activity (Protocol B), the three probiotic strains were applied on HBE separately; after incubation, the tissue was inoculated with E. coli. For B. animalis subsp. lactis BL050 and L. plantarum PBS067 the reduction in viable E. coli cells in the tissue homogenates was less evident than in L. rhamnosus, while no decrease in non-adherent E. coli cells was found. For L. rhamnosus LRH020, 1 log10 reduction in non-adherent E. coli was observed in the apical compartment, while a total inhibition of E. coli cell growth was obtained in the HBE homogenates (Table 3, right part).
In parallel, the overall resistance of the tissue linked both to its thickness and to the integrity of tight junctions was assessed by TEER measurements. In Protocol A, TEER values, registered after 4 h of colonization with E. coli and 16 h treatment with the probiotic strains, did not shown any modification of the tissue barrier function compared to the negative control. As observed in Protocol A, no differences in the TEER values were registered also in Protocol B (Table 4).
Table 4.
Trans-Epithelial-Electrical-Resistance (TEER) expressed in Ohm × cm2 at baseline (only in the controls), at the end of the colonization with E. coli, and at the end of treatment with probiotics (20 h).
| Protocol A | Protocol B | |||
|---|---|---|---|---|
| Baseline | T = 20 h | Baseline | T = 20 h | |
| Negative control | 71.00 ± 0.94 | 76.25 ± 0.35 | 71.08 ± 1.53 | 75.58 ± 0.59 |
| E. coli | 71.08 ± 1.77 | 73.83 ± 1.41 | 71.42 ± 1.06 | 69.92 ± 3.18 |
| B. animalis subsp. lactis BL050 | - | 71.58 ± 4.12 | - | 68.33 ± 1.65 |
| L. plantarum PBS067 | - | 73.08 ± 2.24 | - | 70.75 ± 5.30 |
| L. rhamnosus LRH020 | - | 69.25 ± 1.06 | - | 72.17 ± 1.18 |
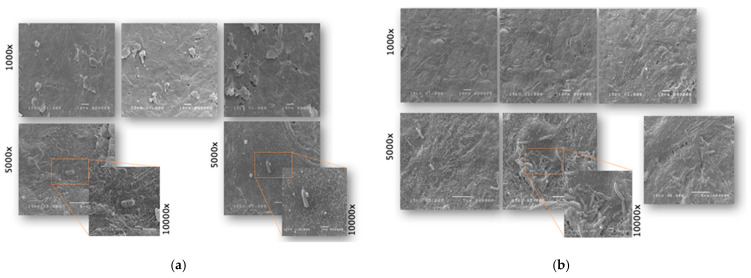
The ultrastructural analysis by SEM showed the bacterial phenotype’s, defined as density, ability to adhere to the epithelium and to form biofilms. The HBE tissue colonized by E. coli showed a mild level of dryness. E. coli was strongly attached to the tissue and kept adherent to the surface by mucus (Figure 1a).
Figure 1.
Representative SEM images of HBE tissue (a) colonized by E. coli (control) and (b) pretreated with L. rhamnosus LRH020. SEM image magnifications are reported near the figures.
Pretreatment with L. rhamnosus LRH020 on HBE counteracted the observed E. coli-induced damages related to hydration, as demonstrated by a greater and more regular distribution of microvilli on the surface (Figure 1b) than in the control (E. coli only).
LAB were clearly visible on the surface, adhering closely to the HBE surface due to the production of extracellular matrix that allowed their aggregation and colonization. E. coli was not detected on the surface (Table 5).
Table 5.
Results of viable count obtained by the decrease in E. coli viability on HBE, expressed as log10 variation with respect to the colonized control, for both protocols.
| Protocol A | Protocol B | |||
|---|---|---|---|---|
| Strain Tested | APICAL | HOMOGENATE | APICAL | HOMOGENATE |
| B. animalis supsp. lactis BL050 | >7 log10 (total depletion) | >1 log10 | No variation | >1 log10 |
| L. plantarum PBS067 | >2 log10 | >5 log10 (total depletion) | No variation | 1 log10 |
| L. rhamnosus LRH020 | >1 log10 | >5 log10 (total depletion) | >1 log10 | >5 log10 (total depletion) |
2.3. Probiotic Microorganisms and Vaginal Pathogens Co-Aggregation
After mixing SynBalance® Femme with the three different pathogens, G. vaginalis, E. coli, and C. albicans, flocculation times were recorded and clustered in four different groups of response: less than 15 min, 15–30 min, more than 30 min, and no evidence of precipitate (Table 6).
Table 6.
The visual check of precipitate was expressed as cluster quantity (−, +, ++, +++) and the flocculation time was recorded in minutes (min).
| Probiotic Strain/Formulation | Pathogen | Precipitate Formation | Flocculation Time |
|---|---|---|---|
| B. animalis subsp. lactis BL050 | Gardnerella vaginalis | + | >30 min |
| Escherichia coli | + | >30 min | |
| Candida albicans | ++ | 15 < min <30 | |
| L. plantarum PBS067 | Gardnerella vaginalis | − | >30 min |
| Escherichia coli | − | >30 min | |
| Candida albicans | − | >30 min | |
| L. rhamnosus LRH020 | Gardnerella vaginalis | +++ | <15 min |
| Escherichia coli | +++ | <15 min | |
| Candida albicans | +++ | <15 min | |
| SynBalance® Femme | Gardnerella vaginalis | +++ | <15 min |
| Escherichia coli | +++ | <15 min | |
| Candida albicans | +++ | <15 min |
All precipitates were collected and fixed for SEM analysis. Magnifications were selected based on pathogen morphology and dimensions to highlight the formation of aggregates within the precipitate.
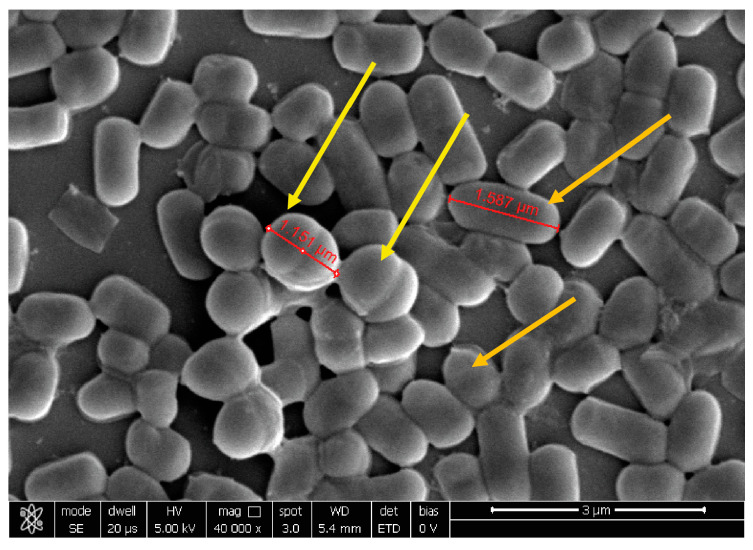
At 20,000×, G. vaginalis morphology showed a round shape of about 1 µm of diameter, while, at 10,000× C. albicans seemed organized in characteristic clusters formed by ovoidal cells with 3–5 µm of diameter. At 40,000×, E. coli exhibited its typical rod shape of 1.8 µm long and wide 583 nm.
Co-aggregation with G. vaginalis was well visible for B. animalis subsp. lactis BL050 and L. plantarum PBS067 (Figure 2), highlighting a dense carpet where pathogen and probiotic bacteria interplayed, while for L. rhamnosus LRH020 the two different population are organized individually.
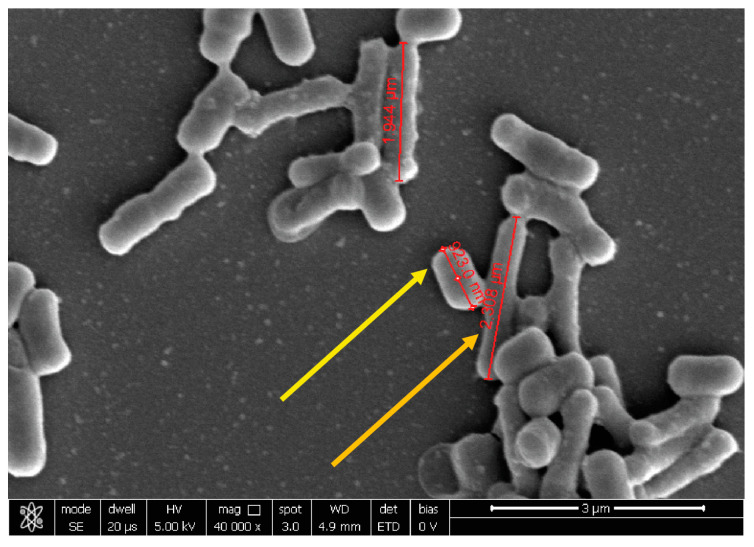
Figure 2.
Representative image of L. plantarum PBS067 and G. vaginalis observed at SEM. The yellow arrows indicate G. vaginalis, the orange arrows L. plantarum PBS067. Magnification 40,000×.
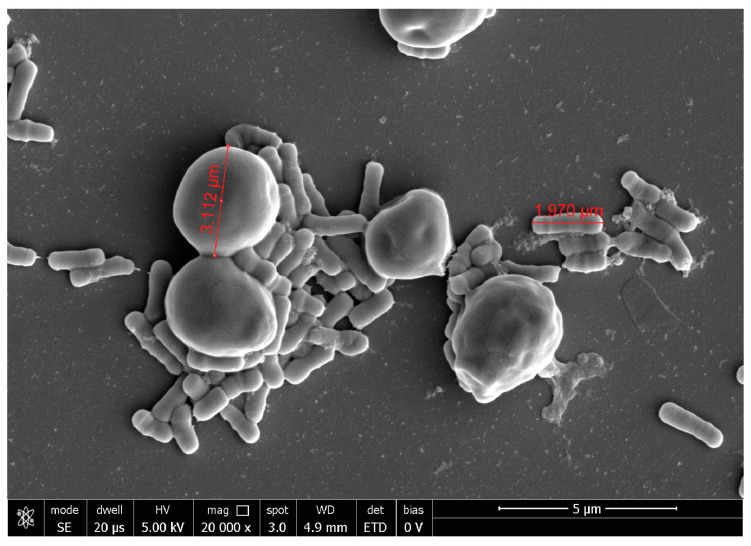
Due to the different morphology and size, the co-aggregation with C. albicans was very clear both for the single strains and their mixture. L. rhamnosus LRH020 was able to surround isolated C. albicans cells forming partial co-aggregates (Figure 3).
Figure 3.
Representative image of L. rhamnosus LRH020 and C. albicans observed at SEM. Magnification 20,000×.
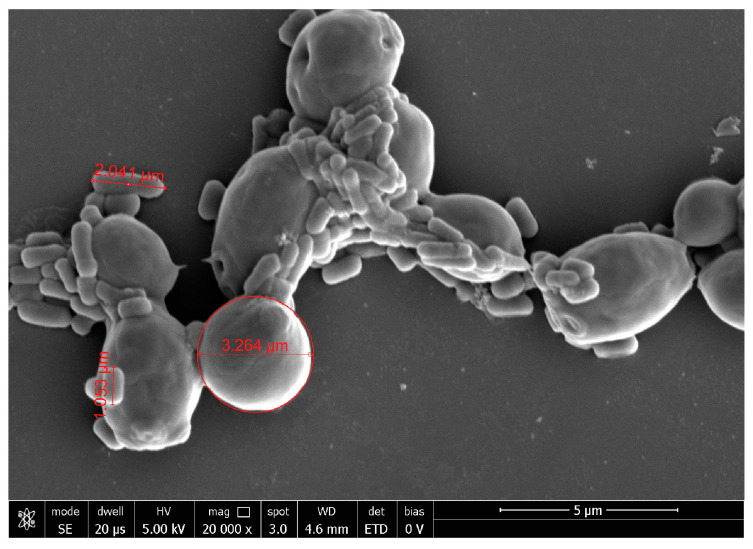
B. animalis subsp. lactis BL050 and L. plantarum PBS067 formed well visible clusters; similarly, a complete co-aggregation was observed for SynBalance® Femme: fungal cells resulted embedded into the probiotic biofilm (Figure 4).
Figure 4.
Representative image of SynBalance® Femme mixture and C. albicans observed at SEM. Magnification 20,000×.
The assessment of co-aggregation against E. coli was very difficult. For all the tested probiotic strains, the dense organization of the precipitate was evident but, due to the similar shape, the two populations are not clearly distinguishable. In the suspension of probiotic formulation and E. coli, the precipitates interact closely but it was difficult to appraise the distinct bacteria (Figure 5).
Figure 5.
Representative image obtained with SEM of SynBalance® Femme mixture and E. coli. The yellow arrow indicates the pathogen cell, while the orange arrow a probiotic bacterium. Magnification 40,000×.
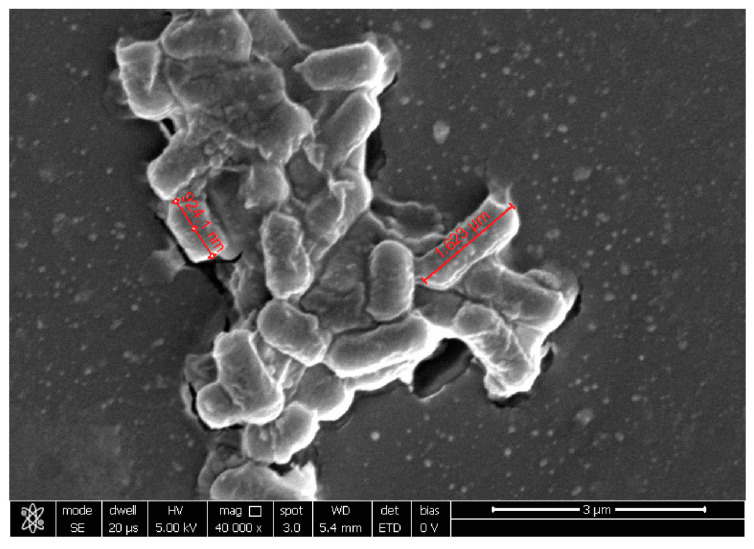
In the case of L. plantarum PBS067, however, the presence of a solid aggregate (Figure 6) was evident.
Figure 6.
Representative image obtained with SEM of L. plantarum PBS067 and E. coli aggregates. Magnification 40,000×.
3. Discussion
The maintenance of the VMB equilibrium has a pivotal role in keeping a eubiotic and wholesome status [30]. The prevalence of good bacteria, in particular belonging to Lactobacillus genus, exerts an antimicrobial activity which inhibits the growth of different pathogenic microorganisms. When this balance is lost, the use of probiotics to restore a healthy microbiota is reported to be effective as an adjutant or alternative to antibiotic treatment [31]. Many studies on antimicrobial ability of probiotic bacteria have been carried out demonstrating that the efficacy against different pathogenic microorganisms is strain-specific [32]. Many authors have reported that urogenital infections can originate from lurked reservoirs present in the gut [33,34], confirming that an unhealthy gut microbiota can be responsible for infections such as vaginitis, cystitis, and pyelonephritis, due to the crosstalk between vaginal and intestinal district [35]. This connection is fundamental to understand the pathogenesis of UTIs and, consequently, to design a correct protocol to prevent them.
In this study, three probiotic strains and their mixture were investigated for their antimicrobial effects against different pathogens that cause urogenital infections.
Previously, the same probiotic formulation had already been extensively studied both in vitro for its antimicrobial activity and in clinical trials for several applications. In a randomized placebo-controlled pilot study, Mezzasalma et al. showed that the formulation possessed antimicrobial activity against both E. coli and C. albicans, and determined an enhancement in the amount of the specific strains due to its persistence in the vaginal microbiota after a wash out period of seven days [29]. Furthermore, the oral administration of SynBalance® Femme formulation in women with recurrent bacterial vaginosis drove to a Lactobacillus-dominated vaginal microbiota, reducing dysbiosis condition and recurrence rate of bacterial vaginosis (BV) in the active group (16%) compared to the control group (40%) [36].
In order to understand the mechanisms of action of the probiotics tested and their mixture, two different protocols were applied to explore their antimicrobial efficacy, and preventive and anti-adhesive activity on vaginal and bladder reconstructed epithelia. These two different approaches were designed to mimic acute or chronic infections and to verify the effectiveness of tested probiotics in such conditions.
The results obtained in the experiment on HVE showed that only a part of C. glabrata remained attached to the epithelium after the addition of probiotic formulation to the system; a similar mechanism was observed for N. gonorrhoeae, demonstrating for these two pathogens the antimicrobial activity of the probiotic formulation tested. Different behavior was observed for T. vaginalis, where the pathogen has not been found in its viable form neither in the homogenate nor in the tissue washing phase; a full antimicrobial activity was registered, demonstrating the efficacy of SynBalance® Femme for both adherent and non-adherent cells. Therefore, only a fraction of the initial pathogenic microorganism concentration remained attached to the epithelium treated with probiotics, showing a remarkable antimicrobial and anti-adhesion activity of SynBalance® Femme.
A similar performance was observed on the bladder epithelia for each strain of the probiotic formulation, suggesting an antibacterial activity and the ability to counteract E. coli adhesion, both on apical compartment and in the tissue homogenates.
B. animalis supsp. lactis BL050 showed a strong antibacterial ability when applied on the established E. coli-colonized model, suggesting that this strain exerts its activity in the first hours of treatment. In the preventive model, the lack of activity could be explained as possible sensitivity to the tissue cultivation conditions, losing its efficacy after 16 h of incubation.
L. plantarum PBS067 showed a high efficacy in counteracting E. coli adhesion by detaching it from the HBE when it has already colonized the mucosa. The mechanism of action may involve the displacement of E. coli from the epithelial surface by competition with the same adhesion sites on the mucosa or by interference with the slime [37]. This ability was partially lost when this strain was applied as pretreatment. This outcome could be caused by a higher sensitivity of this probiotic to the tissue cultivation conditions, as observed for B. lactis BL050, thus limiting its efficacy against E. coli. However, a competition mechanism in the adhesion process of E. coli by displacement of the pathogenic bacterium is evident. On the contrary, L. rhamnosus LRH020 determined a total inhibition of E. coli adhesion when applied as preventive and antimicrobial agent. The absence of E. coli on the surface of HBE confirmed the efficacy of L. rhamnosus LRH020 in displacing E. coli after colonization. This event was also confirmed by SEM analysis, where a high colonization capacity by L. rhamnosus LRH020 was observed, with relative eradication of E. coli. A preservation of the microvilli structure was also observed, which are useful for the physiological role they play in preventing E. coli-induced tissue drying [38].
Another possible mechanism of action for the antagonistic activity against pathogen could be co-aggregation, i.e., the microorganisms’ ability to cluster together, forming stable multi-cellular associations. This phenomenon has been observed for the first time in human oral bacteria and it can occur among different genera and species [39]. It has been suggested that cellular aggregation could promote the colonization of beneficial microorganisms on human tissues, such as the intestinal and the vaginal tract, and it has been reported both for Lactobacillus and Bifidobacterium genera [40,41,42]. Co-aggregation ability represents a barrier to prevent surface colonization by pathogenic microorganisms [43,44]. In our study, the probiotic strains and their mixture showed a high capability to co-aggregate with different vaginal pathogens, suggesting that this property could allow them to survive at sufficiently high number and colonize the urogenital tract. The ability to co-aggregate with pathogens and to adhere to the epithelial cell surface is probably due to the presence of specific molecules involved in the mechanisms of binding microorganisms or cells [45]. This characteristic is not related only to the genera or species but it is a strain-dependent mechanism [25].
4. Materials and Methods
4.1. Bacterial Strains
Lactiplantibacillus plantarum PBS067 (DSM 24937), Lacticaseibacillus rhamnosus LRH020 (DSM 25568) and Bifidobacterium animalis subsp. lactis BL050 (DSM 25566) freeze-dried probiotic powders and their mixture called SynBalance® Femme were provided by Roelmi HPC Srl (Origgio, VA, Italy). Lactobacillus were grown in homofermentative-heterofermentative differential (HHD) medium, while HHD supplemented with 0.3 g/L L-cysteine hydrochloride monohydrate (cys-HHD) medium was used for Bifidobacterium and SynBalance® Femme formulation. The cultures were incubated at 37 °C under anaerobic conditions.
As antagonistic microorganisms, Escherichia coli (ATCC 8739), Gardnerella vaginalis (ATCC 14018), Neisseria gonhorreae (ATCC 43069), Trichomonas vaginalis (ATCC 30238), Candida glabrata (ATCC 15126), and Candida albicans (ATCC 10231) were investigated. E. coli was cultivated in Tryptic Soy Broth (TSB) or Eosin Methylene Blue (EMB) agar at 37 °C for 24 h (h); G. vaginalis in NYC III broth at 37 °C in of 5% CO2 atmosphere for 24 h; N. gonhorreae was grown on Chocolate Enriched agar and T. vaginalis on 2154 ATCC Medium at 35 °C for 3–5 days; C. glabrata and C. albicans were cultivated in Sabouraud Dextrose (SD) broth at 30 °C in aerobiosis.
Each fresh culture of pathogen strain was collected and resuspended in saline solution to obtain a concentration range between 107–108 CFU/mL. The optical densities of the bacterial cultures at 600 nm were measured.
4.2. 3D Reconstructed Human Epithelia
Reconstructed human vaginal epithelium (HVE) of 0.5 cm2 size (STERLAB, batch n° 2008 VAG01, Vallauris, France) was cultivated for 5 days starting from A431 cell line, reconstituted by airlifted culture on insert polycarbonate filter 0,4 µm in a specific maintenance medium.
3D human reconstructed bladder epithelium (HBE) of 0.5 cm2 size (EPISKIN SAS, batch n° 18SMM022, Lyon, France) was formed after 5 days of air-lift culture of immortalized cell line (RT-112) in a chemically defined medium.
On the day of arrival, the HVE and HBE tissues were immediately transferred to a 6-well plate with 1 mL of maintenance medium and placed in incubator at 37 ± 1 °C and 5% CO2 for 24 h before the experiments. HVE and HBE batches were tested for the absence of Human Immunodeficiency virus (HIV), Hepatitis B and C, and mycoplasma.
4.3. Antimicrobial and Antiadhesive Efficacy on Reconstructed HVE
Two different protocols were applied to assess SynBalance® Femme efficacy: the first for antimicrobial efficacy, and the second for preventive and antiadhesive performance. Each pathogen was tested with both protocols in parallel and each condition was investigated in triplicate.
Antimicrobial efficacy protocol (Protocol A): SynBalance® Femme, at 107 CFU/tissue was applied for 16 h on HVE previously colonized by 30 µL of C. glabrata, N. gonhorreae or T. vaginalis (107 CFU/tissue, contact time of 4 h to induce an infection).
Preventive and antiadhesive performance protocol (Protocol B): SynBalance® Femme, at 107 CFU/tissue, was applied directly on HVE tissues for 16 h and then the infection was induced by 30 µL of each three pathogens (107 CFU/tissue for 4 h of contact).
The total viable bacteria count was performed at the end of the treatment on non-adherent microorganisms (washing), attached microorganisms (tissue homogenates), and microorganism penetrated the epithelium and found in the underlying maintenance medium (medium), according to an internal procedure. After incubation, microorganisms of the washing fraction were obtained by adding 2 mL of Dulbecco’s Phosphate Buffered Saline (DPBS, Biowest, Nuaillé, France) to each tissue. To recollect adherent microorganisms, 2 mL of Tryton X-100 Solution 0.1% (MatTek, Ashland, OH, USA) was added to the tissue that was then homogenized. The tissue maintenance medium was also collected. To perform a cell count of each strain, the suspensions obtained were diluted and plated on selective media. For T. vaginalis the count was performed after staining with Trypan Blue solution (Sigma Aldrich, St. Louis, MO, USA), by microscope observation in a Bürker chamber to distinguish viable cells from non-viable ones.
At the end of the procedure, the integrity and viability of the tissues were assessed. The integrity of the epithelium barrier was determined by measuring the transepithelial/transendothelial electrical resistance (TEER) using the MilliCell®-ERS-2 Volt-Ohm meter and electrode system (Merck Millipore, Burlington, NJ, USA). Tissue viability was assessed based on the ability of the yellow dye MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, thiazolyl blue) to be reduced to purple formazan crystals by metabolically active cells. Results are expressed as a percentage of initial value (baseline).
4.4. Antimicrobial and Antiadhesive Efficacy on HBE
The same protocols previously described were applied to HBE tissues, analyzing the strains contained in the SynBalance® Femme against E. coli. In particular, each probiotic strain was tested for its putative antimicrobial efficacy (Protocol A) and antimicrobial adhesion efficacy (Protocol B) in parallel and each condition was analyzed in triplicate.
Protocol A: the HBE tissues were exposed to 30 μL of E. coli (about 7.60 log10 CFU/tissue, OD600 nm = 0.8) for 4 h; then, the infected HBE tissues were treated with 30 μL of probiotic strain suspension (OD600 nm = 0.8) for 16 h to mimic a realistic daily exposure time.
Protocol B: the HBE tissues were pre-treated with 30 μL of probiotic strain suspension (OD600 nm = 0.8) for 16 h; then, the HBE tissues were infected with 30 μL of E. coli (OD600 nm = 0.8) for 4 h.
The results obtained on the tissues colonized by E. coli and treated with the probiotic suspensions were compared with a positive control, colonized by E. coli only, and a negative sterile control.
The total viable bacteria count was performed on apical (non-adherent bacteria) and tissue (adherent bacteria) homogenates fractions, according to an internal procedure. The viable counts on apical and homogenate samples were performed using appropriate 10-fold dilutions in saline solution. A volume of 10 µL of each dilution (as well as the starting suspension) were plated by drop plate method on EMB agar and incubated at 37 °C for 24–48 h, in duplicate. The CFU/mL data were then converted in CFU/cm2.
Tissue integrity was assessed at the end of each experiment. TEER was measured before infection, after 4 h of colonization and 16 h of probiotic treatment. To perform the measurement, 500 μL of saline solution was directly applied on the tissues that were then placed into a plate containing 5 mL of saline solution. Three measurements for each tissue were carried out. Experiments were performed on duplicate tissues and results were expressed as mean ± SD.
Treated HBE tissues were collected for ultrastructural analysis by Field Emission Scanning Electron Microscope (FE-SEM) (in simplicate). HBE samples were fixed with 2.5% glutaraldehyde solution in PBS 0.1 mol/L for 24 h. Before the analysis, the samples were washed in 0.1 mol/L sodium cacodylate buffer at pH 7.4 and then carried out in 1% osmium tetraoxide (OsO4) in the same buffer (2 h at room temperature, RT). They were dehydrated in increasing concentration of ethanol and hesamethyldisilazane overnight at RT. Finally, samples were placed on pins, with carbon tablets coated with a layer of gold, using Polaron Equipment limited SEM coating unit E5100 and then transferred to the FE-SEM for viewing and image acquisition.
4.5. Co-Aggregation Evaluation
To evaluate a possible co-aggregation of the probiotic strains with vaginal pathogens G. vaginalis, E. coli, and C. albicans, 100 mg of each probiotic strain or SynBalance® Femme were resuspended in a buffer solution at pH 5.5. A concentration of 109 CFU/mL was checked by spectrophotometer determination at OD600 nm in comparison with buffer solution (blank). Similarly, each of the three pathogen strains, grown on agar, was resuspended in saline solution and 108 CFU/mL were spectrophotometrically checked in comparison with saline solution (blank). Each test was prepared by mixing 1:1 the two suspensions (probiotics and pathogens) on a slide and leaving them until the formation of aggregates. A visual check of precipitate formation was performed and the flocculation time (precipitate forming) was recorded in order to define the time range of co-aggregation. Then, each sample was prepared for SEM analysis.
Precipitates were collected after eliminating the excess of diluent and an aliquot was transferred onto a glass support designated for SEM analysis; each precipitate was covered with 10 µL of fixative buffer solution and stored at 2–8 °C. Samples were placed on pins with carbon tabs coated with a layer of gold using Polaron Equipment limited SEM coating unit E5100 and then transferred to the Electronic Microscope: FEI Nova Nano SEM 600 (FEI, Beaverton, USA; Field Emission Gun (FEG)-SEM acquisitions by SE dwell 20 ms HV5 Kv). The magnifications were selected based on the morphology and dimensions of the pathogen and the probiotic strains to highlight the formation of aggregates within the precipitate. SEM acquisition was performed by Service Biotech, Naples, Italy.
5. Conclusions
In conclusion, the presented in vitro data confirm that L. plantarum PBS067, L. rhamnosus LRH020, B. animalis subsp. lactis BL050, and their combination SynBalance® Femme are able to compete and prevent the development of the most common urogenital pathogens. In particular, the production of antimicrobial substances, the competition in adhesion to the epithelial cells, and the co-aggregation ability could explain their mechanism of action in counteracting pathogens infection. However, further investigations are necessary to confirm these activities against other pathogenic microorganisms involved in urinary tract and vaginal infections.
Acknowledgments
All the authors are grateful to Salvatore Del Prete, Service Biotech, Naples, Italy, for his expertise and helpful support in performing SEM analysis.
Author Contributions
Conceptualization, P.M.; methodology, S.G. and M.M. (Marisa Meloni); validation, L.B. and M.M. (Martina Masciarelli); formal analysis, S.G., L.B. and M.M. (Marisa Meloni); investigation, L.B. and M.M. (Martina Masciarelli); writing—original draft preparation, P.M. and M.M. (Marisa Meloni); writing—review and editing, D.F.S.; supervision, P.M., F.C. and M.M. (Marisa Meloni); project administration, P.M.; funding acquisition, F.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are within the manuscript.
Conflicts of Interest
P.M. and D.F.S. are employee of SynBalance srl and F.C. of Roelmi HPC Srl. S.G., M.M. (Martina Masciarelli), L.B. and M.M. (Marisa Meloni) declare no conflict of interest.
Funding Statement
This research was partially funded by Lombardy Region (2014IT16RFOP012-POR FESR 2014-2020-Project ID226149).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Martin D.H. The microbiota of the vagina and its influence on women’s health and disease. Am. J. Med. Sci. 2012;343:2–9. doi: 10.1097/MAJ.0b013e31823ea228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farage M., Maibach H. Lifetime changes in the vulva and vagina. Arch. Gynecol. Obstet. 2006;273:195–202. doi: 10.1007/s00404-005-0079-x. [DOI] [PubMed] [Google Scholar]
- 3.Kalia N., Singh J., Kaur M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: A critical review. Ann. Clin. Microbiol. Antimicrob. 2020;19:5. doi: 10.1186/s12941-020-0347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stapleton A.E. The Vaginal Microbiota and Urinary Tract Infection. Microbiol. Spectr. 2016;4 doi: 10.1128/microbiolspec.UTI-0025-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muzny C.A., Łaniewski P., Schwebke J.R., Herbst-Kralovetz M.M. Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis. 2020;33:59–65. doi: 10.1097/QCO.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witkin S.S., Mendes-Soares H., Linhares I.M., Jayaram A., Ledger W.J., Forney L.J. Influence of vaginal bacteria and D- and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: Implications for protection against upper genital tract infections. mBio. 2013;4:e00460-13. doi: 10.1128/mBio.00460-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mokoena M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules. 2017;22:255. doi: 10.3390/molecules22081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutiérrez-Cortés C., Suarez H., Buitrago G., Nero L.A., Todorov S.D. Enhanced Bacteriocin Production by Pediococcus pentosaceus 147 in Co-culture With Lactobacillus plantarum LE27 on Cheese Whey Broth. Front. Microbiol. 2018;9:2952. doi: 10.3389/fmicb.2018.02952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coman M.M., Verdenelli M.C., Cecchini C., Silvi S., Orpianesi C., Caspani M., Mondello F., Cresci A. In vitro evaluation on HeLa cells of protective mechanisms of probiotic lactobacilli against Candida clinical isolates. J. Appl. Microbiol. 2015;119:1383–1390. doi: 10.1111/jam.12947. [DOI] [PubMed] [Google Scholar]
- 10.Vagios S., Hesham H., Mitchell C. Understanding the potential of lactobacilli in recurrent UTI prevention. Microb. Pathog. 2020;148:104544. doi: 10.1016/j.micpath.2020.104544. [DOI] [PubMed] [Google Scholar]
- 11.Amabebe E., Anumba D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018;5:181. doi: 10.3389/fmed.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hattiholi A., Tendulkar S., Dodamani S. Probiotic Research in Therapeutics. Springer; Berlin/Heidelberg, Germany: 2021. An Update on the Probiotic Usage in Bacterial Vaginosis; pp. 191–213. [Google Scholar]
- 13.Minardi D., d’Anzeo G., Cantoro D., Conti A., Muzzonigro G. Urinary tract infections in women: Etiology and treatment options. Int. J. Gen. Med. 2011;4:333–343. doi: 10.2147/IJGM.S11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aldunate M., Srbinovski D., Hearps A.C., Latham C.F., Ramsland P.A., Gugasyan R., Cone R.A., Tachedjian G. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 2015;6:164. doi: 10.3389/fphys.2015.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flores-Mireles A.L., Walker J.N., Caparon M., Hultgren S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015;13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tariq N., Jaffery T., Ayub R., Alam A.Y., Javid M.H., Shafique S. Frequency and antimicrobial susceptibility of aerobic bacterial vaginal isolates. J. Coll. Physicians Surg Pak. 2006;16:196–199. [PubMed] [Google Scholar]
- 17.Córdoba S., Taverna C., Vivot W., Szusz W., Vivot M., Isla G., Davel G. Emergence of Resistance to Fluconazole in Candida albicans Isolated From Vaginal Discharge. Curr. Fungal. Infect. Rep. 2018;12:155–160. doi: 10.1007/s12281-018-0329-6. [DOI] [Google Scholar]
- 18.Vicariotto F., Mogna L., Del Piano M. Effectiveness of the two microorganisms Lactobacillus fermentum LF15 and Lactobacillus plantarum LP01, formulated in slow-release vaginal tablets, in women affected by bacterial vaginosis: A pilot study. J. Clin. Gastroenterol. 2014;48((Suppl. S1)):S106–S112. doi: 10.1097/MCG.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 19.Sobel J.D., Sobel R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin. Pharmacother. 2018;19:971–977. doi: 10.1080/14656566.2018.1476490. [DOI] [PubMed] [Google Scholar]
- 20.Bradshaw C.S., Sobel J.D. Current Treatment of Bacterial Vaginosis-Limitations and Need for Innovation. J. Infect. Dis. 2016;214((Suppl. S1)):S14–S20. doi: 10.1093/infdis/jiw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J.M., Park Y.J. Probiotics in the Prevention and Treatment of Postmenopausal Vaginal Infections: Review Article. J. Menopausal. Med. 2017;23:139–145. doi: 10.6118/jmm.2017.23.3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmeira-de-Oliveira R., Palmeira-de-Oliveira A., Martinez-de-Oliveira J. New strategies for local treatment of vaginal infections. Adv. Drug Deliv. Rev. 2015;92:105–122. doi: 10.1016/j.addr.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 23.De Seta F., Parazzini F., De Leo R., Banco R., Maso G.P., De Santo D., Sartore A., Stabile G., Inglese S., Tonon M., et al. Lactobacillus plantarum P17630 for preventing Candida vaginitis recurrence: A retrospective comparative study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;182:136–139. doi: 10.1016/j.ejogrb.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Borges S., Silva J., Teixeira P. The role of Lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 2014;289:479–489. doi: 10.1007/s00404-013-3064-9. [DOI] [PubMed] [Google Scholar]
- 25.Campana R., van Hemert S., Baffone W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog. 2017;9:12. doi: 10.1186/s13099-017-0162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collado M.C., Meriluoto J., Salminen S. Measurement of aggregation properties between probiotics and pathogens: In vitro evaluation of different methods. J. Microbiol. Methods. 2007;71:71–74. doi: 10.1016/j.mimet.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Presti I., D’Orazio G., Labra M., La Ferla B., Mezzasalma V., Bizzaro G., Giardina S., Michelotti A., Tursi F., Vassallo M., et al. Evaluation of the probiotic properties of new Lactobacillus and Bifidobacterium strains and their in vitro effect. Appl. Microbiol. Biotechnol. 2015;99:5613–5626. doi: 10.1007/s00253-015-6482-8. [DOI] [PubMed] [Google Scholar]
- 28.Mezzasalma V., Manfrini E., Ferri E., Sandionigi A., La Ferla B., Schiano I., Michelotti A., Nobile V., Labra M., Di Gennaro P. A Randomized, Double-Blind, Placebo-Controlled Trial: The Efficacy of Multispecies Probiotic Supplementation in Alleviating Symptoms of Irritable Bowel Syndrome Associated with Constipation. Biomed. Res. Int. 2016;2016:4740907. doi: 10.1155/2016/4740907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mezzasalma V., Manfrini E., Ferri E., Boccarusso M., Di Gennaro P., Schiano I., Michelotti A., Labra M. Orally administered multispecies probiotic formulations to prevent uro-genital infections: A randomized placebo-controlled pilot study. Arch. Gynecol. Obstet. 2017;295:163–172. doi: 10.1007/s00404-016-4235-2. [DOI] [PubMed] [Google Scholar]
- 30.Barrientos-Durán A., Fuentes-López A., de Salazar A., Plaza-Díaz J., García F. Reviewing the Composition of Vaginal Microbiota: Inclusion of Nutrition and Probiotic Factors in the Maintenance of Eubiosis. Nutrients. 2020;12:419. doi: 10.3390/nu12020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scillato M., Spitale A., Mongelli G., Privitera G.F., Mangano K., Cianci A., Stefani S., Santagati M. Antimicrobial properties of Lactobacillus cell-free supernatants against multidrug-resistant urogenital pathogens. Microbiologyopen. 2021;10:e1173. doi: 10.1002/mbo3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fijan S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health. 2014;11:4745–4767. doi: 10.3390/ijerph110504745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thänert R., Reske K.A., Hink T., Wallace M.A., Wang B., Schwartz D.J., Seiler S., Cass C., Burnham C.A., Dubberke E.R., et al. Comparative Genomics of Antibiotic-Resistant Uropathogens Implicates Three Routes for Recurrence of Urinary Tract Infections. mBio. 2019;10:e01977-19. doi: 10.1128/mBio.01977-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meštrović T., Matijašić M., Perić M., Čipčić Paljetak H., Barešić A., Verbanac D. The Role of Gut, Vaginal, and Urinary Microbiome in Urinary Tract Infections: From Bench to Bedside. Diagnostics. 2020;11:7. doi: 10.3390/diagnostics11010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amabebe E., Anumba D.O.C. Female Gut and Genital Tract Microbiota-Induced Crosstalk and Differential Effects of Short-Chain Fatty Acids on Immune Sequelae. Front. Immunol. 2020;11:2184. doi: 10.3389/fimmu.2020.02184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murina F., Vicariotto F. Evaluation of an Orally Administered Multistrain Probiotic Supplement in Reducing Recurrences Rate of Bacterial Vaginosis: A Clinical and Microbiological Study. Adv. Inf. Dis. 2019;9:151–161. doi: 10.4236/aid.2019.93011. [DOI] [Google Scholar]
- 37.Javanshir N., Hosseini G.N.G., Sadeghi M., Esmaeili R., Satarikia F., Ahmadian G., Allahyari N. Evaluation of the Function of Probiotics, Emphasizing the Role of their Binding to the Intestinal Epithelium in the Stability and their Effects on the Immune System. Biol. Proced. Online. 2021;23:23. doi: 10.1186/s12575-021-00160-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liévin-Le Moal V., Servin A.L. Anti-infective activities of lactobacillus strains in the human intestinal microbiota: From probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin. Microbiol. Rev. 2014;27:167–199. doi: 10.1128/CMR.00080-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibbons R.J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch. Oral Biol. 1970;15:1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- 40.Vlková E., Rada V., Smehilová M., Killer J. Auto-aggregation and co-aggregation ability in Bifidobacteria and Clostridia. Folia. Microbiol. 2008;53:263–269. doi: 10.1007/s12223-008-0040-z. [DOI] [PubMed] [Google Scholar]
- 41.Atassi F., Servin A.L. Individual and co-operative roles of lactic acid and hydrogen peroxide in the killing activity of enteric strain Lactobacillus johnsonii NCC933 and vaginal strain Lactobacillus gasseri KS120.1 against enteric, uropathogenic and vaginosis-associated pathogens. FEMS Microbiol. Lett. 2010;304:29–38. doi: 10.1111/j.1574-6968.2009.01887.x. [DOI] [PubMed] [Google Scholar]
- 42.Bujnáková D., Vlková E., Rada V., Kmet V. Aggregation of Lactobacilli and Bifidobacteria with Escherichia coli O157. Folia. Microbiol. 2004;49:143–146. doi: 10.1007/BF02931389. [DOI] [PubMed] [Google Scholar]
- 43.Kos B., Susković J., Vuković S., Simpraga M., Frece J., Matosić S. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 2003;94:981–987. doi: 10.1046/j.1365-2672.2003.01915.x. [DOI] [PubMed] [Google Scholar]
- 44.Collado M.C., Meriluoto J., Salminen S. Adhesion and aggregation properties of probioticand pathogen strains. Eur. Food Res. Technol. 2008;226:1065–1073. doi: 10.1007/s00217-007-0632-x. [DOI] [Google Scholar]
- 45.Sengupta R., Altermann E., Anderson R.C., McNabb W.C., Moughan P.J., Roy N.C. The role of cell surface architecture of lactobacilli in host-microbe interactions in the gastrointestinal tract. Mediat. Inflamm. 2013;2013:237921. doi: 10.1155/2013/237921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.