Abstract
Nematophagous fungi (NF) are a group of diverse fungal genera that benefit plants. The aim of this review is to increase comprehension about the importance of nematophagous fungi and their role in phosphorus solubilization to favor its uptake in agricultural ecosystems. They use different mechanisms, such as acidification in the medium, organic acids production, and the secretion of enzymes and metabolites that promote the bioavailability of phosphorus for plants. This study summarizes the processes of solubilization, in addition to the mechanisms of action and use of NF on crops, evidencing the need to include innovative alternatives for the implementation of microbial resources in management plans. In addition, it provides information to help understand the effect of NF to make phosphorus available for plants, showing how these biological means promote phosphorus uptake, thus improving productivity and yield.
Keywords: biocontrol, nematophagous fungi, phosphorus, solubilization
1. Introduction
Pathogenic threats and nutritional deficiency are the main problems that affect yield and productivity in plant crops [1], increasing the need to apply new microbial agents to solve both obstacles [2]. Thus, NF have been investigated because of their dual ability as nematode controllers and as plant growth promoters that increase the bioavailabilty of nutrients [3].
Phosphorus (P) is the second most important element for plants after nitrogen. It regulates metabolism and plant development and growth, and an adequate supply is needed to keep these metabolic functions [4]. In addition, it is found in abundance in soil, although its uptake is low, which results in a limitation for the productivity of agricultural crops [5].
P can be solubilized by several soil microorganisms (saprotrophic bacteria and fungi) [6]. Phosphate-solubilizing microorganisms (PSM) play an important role in soil by mineralizing organic P, solubilizing inorganic P minerals, and storing P in biomass [7], thus improving plant growth and yield [8]. PSM can solubilize phosphorus by secreting protons and producing organic anions, such as citrate, oxalate, and gluconate [9]. However, the amount of P solubilization depends on the microbial strain and the relationship between carbon sources, P, and organic acid production [10].
Bacteria can release organic acids that solubilize P or produce acid and alkaline phosphatases that mineralize organic P [11]. There are bacterial genera that are known to be very efficient in these mechanisms, such as Arthrobacter, Bacillus, Burkholderia, Natrinema, Pseudomonas, Rhizobium, and Serratia [12]. Bacteria are superior to fungi in colonizing plant roots, but are less tolerant to acids [13], so fungi have great potential to solubilize P in acidic conditions [14]. Fungi, in fact, have a higher capacity to release P compared with bacteria [15]. They use different mechanisms such as the secretion of fatty acids, the production of enzymes, and the discharge of metabolic substances (siderophores) that can rapidly metabolize P [16].
Despite the numerous studies on NF as biocontrol agents, the comprehensive and complete understanding of the mechanisms of action is still incipient [17]. In general, the interactions of NF have been evaluated to reduce the densities of plant pathogenic nematodes [18]. Yet, they present other benefits such as the synthesis of phytohormones, as well as favoring the absorption of P [19]. The inoculation of P solubilizing microbial agents in seeds, crops, and soil aims at improving agricultural production without affecting soil health [20]. Bioformulations based on fungal strains that increase plant yield and are also effective at solubilizing nutritional elements are needed [21]. Therefore, the objective of this review is to broaden the understanding of NF and their potential use in P solubilization, focusing on their mechanisms of solubilization and the benefits provided in the nutrition of agricultural crops.
2. Mechanisms of P solubilization by NF
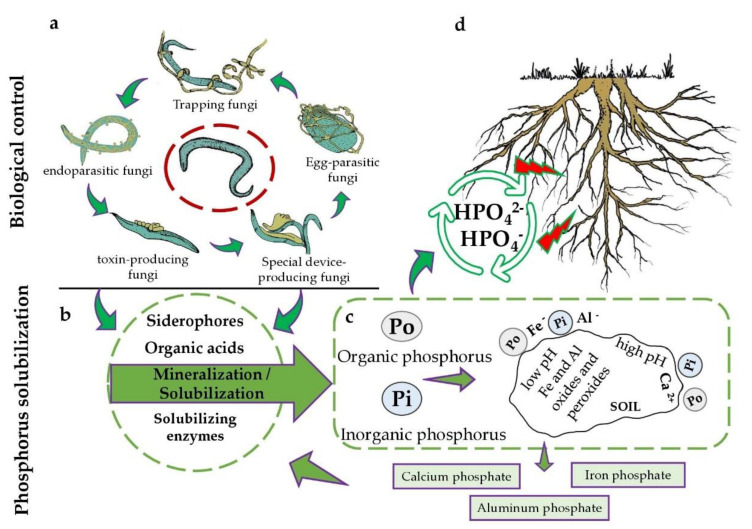
Plants can harness various forms of P; nevertheless, roots take up negatively charged forms of orthophosphate ions (HPO42−- and HPO4−) depending on the soil pH [22]. Although agriculture practices introduce considerable amounts of phosphate in soil, only 15 to 30% of P is taken up by plants [23]. It generally binds to iron and aluminum oxides and hydroxides, or to calcium in calcareous soils, becoming unavailable for roots due to chemical precipitation or physical adsorption [24]. Therefore, the application of P itself does not contribute to the improvement of agricultural production systems (Figure 1).
Figure 1.
Systematic diagram of P solubilization by fungi used in the biological control of nematodes. (a) Nematodes can be parasitized and predated by (in clockwise order) trapping fungi, egg-parasite fungi, special device-producing, or toxin-producing and endoparasitic species. (b) Compounds produced by fungi. (c) Chemical reactions of P in soil, binding with elements such as calcium, iron, and aluminum that can then be mineralized or solubilized by the NF compounds. (d) Roots absorbing negatively charged orthophosphate ions.
Several microorganisms have been studied in relation to the solubilization of P. Fungi and bacteria use different mechanisms on soluble phosphates, especially acidification of the medium, chelation, exchange reactions, and the production of solubilizing enzymes and of various organic acids [4,8,20,25]. Therefore, some species are able to solubilize and mobilize P for plants, including, several nematode controlling fungi. An example is Trichoderma harzianum, which can acidify the medium, produce chelating metabolites, and produce a redox activity (capable of reducing Fe and Cu) [26].
2.1. Organic Acids
Organic acids or anions range from fatty acids to secondary metabolites [22]. They are by-products derived from bacteria and fungi that are able to mobilize P in their microenvironment. Organic acids improve P release so that it can be absorbed by plants [27]. Filamentous fungi use several mechanisms to solubilize minerals and they produce high amounts of organic acids [28]. Species of the genera Aspergillus, Penicillium, Trichoderma, and Fusarium solubilize P by producing different types of organic acids [14,29,30,31,32]. Furthermore, it is possible that some filamentous fungi such as Aspergillus niger solubilize P when exposed to high salt contents (4% NaCl) [33]. Even on ferric phosphate substrates, A. niger can secrete large amounts of citric and oxalic acids to release P [34]. Therefore, the potential of filamentous fungi in the agrobiotechnology industry is increased, especially because of their properties, for the production of organic acids [35].
2.2. Solubilizing Enzymes
Organic P from animal and plant residues can be mineralized by the action of phosphatases [36], such as phytases that hydrolyze the phospho-monoester bonds present in phytates [37]. Nematode controlling fungi can produce phytases during their development or the production of trapping structures [38]. The nematode-capturing fungus Arthrobotrys oligospora forms phytases while building adhesive networks, significantly increasing the release of P [39]. Among the genera of NF that mainly produce phytases and phosphatases under laboratory conditions are the filamentous fungi Aspergillus, Penicillium, and Trichoderma [40].
2.3. Siderophore Production
These are low molecular weight organic iron chelating compounds. They can be produced by bacteria, fungi, and plants [40]. Among the most studied fungi for siderophore production are Aspergillus fumigatus and Aspergillus nidulans, which have 55 similar types of siderophores [41,42]. In addition, Penicillium produces siderophores, capable of solubilizing tricalcium phosphate, even in contaminated soils, opening new opportunities in the field of phytoremediation and agrobiotechnology [43]. Therefore, the use of siderophores is increasing in use as a new alternative for agriculture, to replace pesticides and synthetic fertilizers [44].
3. Potential of NF in P Solubilization
NF can be used in agriculture through the massive production of infective spores or through formulations that improve growth, viability, and efficacy [45]. They use several mechanisms of recognition, signaling, differentiation, and penetration of the cuticle or egg shell of nematodes through mechanical and enzymatic actions [46]. For the purposes of this study, NF are classified according to their mechanism of attack, including trapping, endoparasitic, egg parasitic, toxin-producing, and special device-producing species [47]. NF interact with the environment, performing essential functions to maintain the stability of food webs and the cycling of nutrients in soil [48].
NF widely used in management plans as biological control agents include Purpureocillium lilacinum, Pochonia chlamydosporia, Trichoderma harzianum, Arthrobotrys spp., Hirsutella spp., etc. [49]. Some of these fungi are widely used in agriculture due to their ability to infect and kill arthropods [50]. They are recognized as biopesticides in the management of destructive pests. Some notable examples include Beauveria bassiana [49], Aspergillus fijiensis [51], Cladosporium tenuissimum, and Penicillium citrinum [52]. Among NF, some have been reported as P solubilizers. Trichoderma is one of the most studied genera and is considered as a growth promoter, pathogen controller, and nutritional promoter of plants [53,54]. Furthermore, some NF can solubilize P, even under low temperature conditions [55].
4. Trapping Nematodes—Predatory Fungi
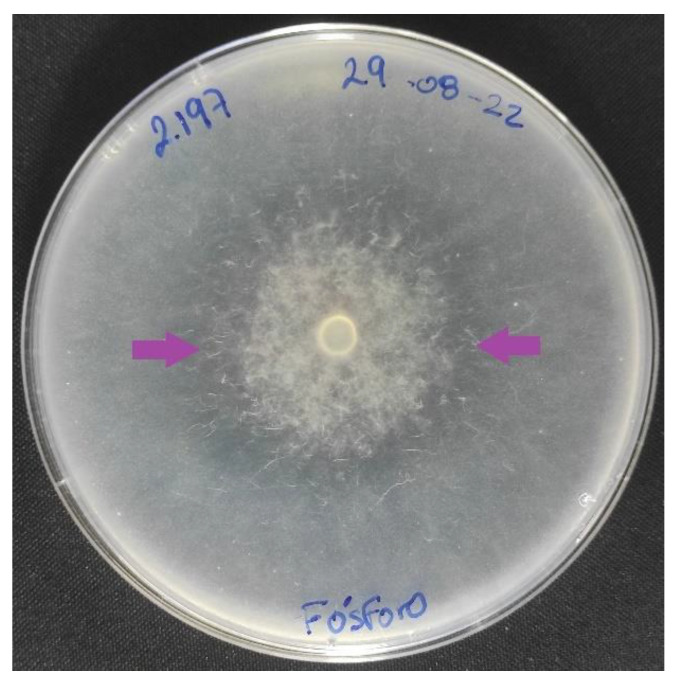
Fungi that capture nematodes have a double function: controlling the populations of infective nematodes and also acting as biofertilizers when applied in soil, being able to solubilizing and mobilize some important nutrients [56,57]. The adhesive networks building fungus Duddingtonia flagrans have been shown to solubilize P both in laboratory [56] and in greenhouse conditions [2]. Likewise, A. oligospora has been used successfully in the solubilization of phosphate rock in in vitro and in vivo conditions (Figure 2), showing promising results in the uptake of P by plants [58].
Figure 2.
Nematode-capturing fungus Arthrobotrys oligospora. Phosphate solubilization zone on Pikovskaya agar plates, as shown by the halo (arrows).
5. Endoparasitic and Egg-parasitic Fungi
Endoparasitic fungi are obligate pathogens that infect nematodes. Conidia of the obligate endoparasitic fungus Drechmeria coniospora are ingested or adhere to the cuticle of its hosts [59]. The spores produce a germination tube that enters the nematode, producing a mycelium that, by degrading internal tissues, exploits the host nutrients for growth [60]. One of the most studied fungal parasites of nematode eggs is the fungus Pochonia chlamydosporia, able to reduce populations of several plant parasitic nematodes, including species of Globodera, Heterodera, Meloidogyne, Nacobbus, and Rotylenchulus [61]. Pochonia chlamydosporia benefits the plant, as it is able to control nematode infections, shortening the flowering and fruiting times (up to 5 and 12 days), as well as significantly increasing root growth [62]. This parasitic fungus produces phosphatases, organic acids, and propionic acid, which promote the depolymerization of phosphate compounds [63,64]. Furthermore, in association with the predatory fungus D. flagrans, they can significantly increase nutrient uptake, especially P, by up to 70% in tomato plants [3]. Therefore, research studies suggest the use of bioformulated P. chlamydosporia for the management of phytoparasitic nematodes, with the potential to support plant nutrition [65].
Purpureocillium is a further fungal genus used for the reduction of phytoparasitic nematode eggs and females. Some species attack the gall-forming nematodes Meloidogyne spp. [66], and show a P solubilization capability in laboratory conditions [67], as well as promoting tomato seedling growth in greenhouse conditions [68]. These fungi may secrete hydrolytic enzymes and siderophores that solubilize P and promote plant growth [69].
6. Toxin Production
Toxin-producing fungi are characterized by the release of compounds that paralyze nematodes and produce a rapid and systemic cell necrosis in multiple tissues throughout their bodies. In the order of the Agaricales, one representative is Pleurotus ostreatus, which is a toxin-producing fungus capable of producing necrosis after the contact of hyphae with the cilia of the sensory system of nematodes [70]. The fungus has P solubilizing properties [71] and is applied as a biofertilizer based on biomasses obtained as mycelial by-products [72].
Piriformospora indica is an endosymbiotic fungus belonging to the order Sebacinales (Basidiomycota), capable of producing metabolites that inhibit nematode populations [73]. This species has shown synergy with phosphate-solubilizing bacteria, sustaining the growth of chickpea plants [74]. Studies have indicated that the transfer of P by P. indica to the host plant has a significant effect on its development [75].
7. Filamentous Fungi with Nematophagous Activity
In addition to the examples mentioned above, there are a variety of fungi that do not use mechanisms based on capture, parasitism, toxins, or special devices, so it is presumed that hydrolytic enzymes are a key in the nematode infection process [76]. Among the known genera that work as both biocontrol and P solubilizers are Trichoderma, Fusarium, Penicillium, and Aspergillus. Filamentous fungi can secrete hydrolytic enzymes, organic acids, and low molecular weight natural products, which confer several functions, including P solubilization [77], and are able to solubilize calcium and iron phosphates [78]. Thus, they hold great potential for the development of biofertilizers, contributing to soil fertility and promoting plant growth [79], which are essential in sustainable agriculture (Table 1) [80].
Filamentous fungi such as Trichoderma spp. are often dominant in soil microbial communities and have the ability to colonize roots [81]. Trichoderma spp. are marketed all over the world for their potential as biocontrol and biostimulant agents for numerous agricultural crops. The application of Trichoderma spp. and related metabolites allegedly improves crop productivity, nutrient supply, and defense against plant pathogens [54,82], as it combines P solubilization and nematode predation.
Table 1.
Fungi known as nematophagous and P solubilizers.
| Type of Fungi | Fungi | Structure Involved | Substance That Is Solubilized | Solubilization Mechanism | Reference |
|---|---|---|---|---|---|
| Predatory | Arthrobotrys oligospora | Adhesive networks | Phosphate rock | pH of the culture medium. | [58] |
| Arthrobotrys conoides and Duddingtonia flagrans | Adhesive networks Adhesive networks | Tricalcium, zinc, and aluminum phosphate phosphate rock | pH of the culture medium. Production of organic acids |
[56] | |
| Duddingtonia flagrans | Adhesive networks | Phosphorus | - | [2,3] | |
| Nematode egg-parasitic | Pochonia chlamydosporia | Appressoria and hyphae | Phosphorus | Phosphatases and organic acids | [3,62,63] |
| Purpureocillium variotii | Toxic metabolites | Phosphorus | Siderophores and pH of the culture medium | [68] | |
| Purpureocillium hepiali | Toxic metabolites | Phosphate | pH of the culture medium | [67] | |
| Purpureocillium lilacinum | Toxic metabolites | Calcium and iron phosphate | pH of the culture medium and organic acids | [83] | |
| Toxin producing | Piriformospora indica | Metabolites | Organic and inorganic phosphorus | pH of the culture medium | [73,75,84] |
| Pleurotus ostreatus | Toxins | Phosphate rock | Organic acids: tartaric, malic, citric, lactic, succinic and four unknown acids | [71,85] | |
| Filamentous fungi with indetermined nematophagous mechanism | Trichoderma harzianum | Hydrolytic enzymes | Calcium phosphate |
pH of the culture medium, production of chelating metabolites, and redox activity | [26] |
| Trichoderma asperellum | Hydrolytic enzymes | Monopotassium phosphate Phytate Phosphate rock |
pH of the culture medium and organic anions | [86] | |
| Trichoderma spp. | Hydrolytic enzymes | Phosphate | Organic acids | [87] | |
|
Aspergillus niger, Penicillium canescens, Eupenicillium ludwigii, and Penicillium islandicum |
Hydrolytic enzymes | Phosphate rock | pH of the culture medium, acids: oxalic, citric, and gluconic | [31] | |
|
Aspergillus, Penicillium, Trichoderma, Fusarium, Mucor, Ovularopsis, Tritirachium, and Geotrichum |
Hydrolytic enzymes | Phosphate rock Tricalcium phosphate |
Acids: fumaric, acetic, gluconic, lactic, and succinic | [29] | |
|
Penicillium expansum, Mucor ramosissimus, and Candida krissii |
Hydrolytic enzymes | Phosphate rock | Acids: citric, oxalic, and gluconic | [79] | |
| Penicillium guanacastense | Hydrolytic enzymes | Organic and inorganic phosphorus | Acids: gluconic, oxalic, lactic, and malonic | [32] | |
| Penicillium bilaji and Penicillium cf.fuscum | Hydrolytic enzymes | Phosphate rock | Organic acids | [30] | |
| Aspergillus niger | Hydrolytic enzymes | Various forms of soluble P calcium iron aluminum phosphate | Acids: gluconic, oxalic, tartaric | [14,33] | |
| Aspergillus aculeatus | Hydrolytic enzymes | Phosphate rock | pH of the culture medium | [88] | |
| Rhizopus stolonifer, Aspergillus niger, and Alternaria alternata | Hydrolytic enzymes | Calcium phosphate | Organic acids | [89] | |
| Aspergillus tubingensis | Hydrolytic enzymes | Phosphate rock | pH of the culture medium | [90] | |
| Penicillium oxalicum and Aspergillus niger | Hydrolytic enzymes | Tricalcium phosphate | Organic acids | [91] | |
| Penicillium bilaii | Hydrolytic enzymes | Calcium phosphate | Acids: citric and oxalic | [92] | |
| Aspergillus niger and Penicillium italicum | Hydrolytic enzymes | Tricalcium phosphate | pH of the culture medium | [93] | |
| Penicillium oxalicum, Trichoderma virens, and Aspergillus | Hydrolytic enzymes | Insoluble tricalcium phosphate, soluble dipotassium hydrogen phosphate | pH of the culture medium and organic acids | [94] | |
| Penicillium | Hydrolytic enzymes | Phosphate | pH of the culture medium | [95] | |
| Penicillium oxalicum | Hydrolytic enzymes | Phosphate | Organic acids | [96] | |
| Paecilomyces, Trichoderma, Aspergillus, Fusarium, and Gongronella | Hydrolytic enzymes | Calcium phosphate Iron phosphate | [78] |
8. Agricultural Solutions: Application of NF in P Solubilization
Although P is added to the soil of agricultural crops, chemical fertilizer synthesis is an energy-consuming process with long-term impacts on the environment in terms of eutrophication, fertility decrease, and carbon footprint [97]. Nematophagous species have been used for plant growth promotion and to sustain yields, especially members of the genera Trichoderma, Purpureocillium, Pochonia, Fusarium, Arthrobotrys, and Verticillium [98].
Several NF are beneficial because of the production of phytohormones, antibiotics, or siderophores, which benefit long-cycle and short-cycle crops [99] (Table 2), as shown by the P solubilization activity in alkaline soils, which increase the yields of corn and wheat [100,101]. These NF potentially shorten the maturity period of crops, improve fruit quality, increase the availability of soluble P, and improve soil biodiversity [102]. Thus, there is a positive link between the application of these fungal strains and the P content in soil and plants [103].
Currently, several investigations suggest the use of fungi as bioinoculants to increase the yield in agricultural crops [104], as well as ornamentals. P solubilization has been in fact also reported in shrubs within ornamental greenhouses, as demonstrated by the application of Mortierella sp. on seedlings of Leucaena leucocephala [105].
Table 2.
List of P-solubilizing NF and related benefits in agricultural crops.
| P-Solubilizing Species | Crop | Plant Benefits | Reference |
|---|---|---|---|
|
Geomyces pannorum, Paecilomyces carneus |
Avena sativa | Increase availability of phosphorus in the soil and mitigate phytoparasitic nematodes | [106] |
|
Duddingtonia flagrans
Pochonia chlamydosporia |
Soy bean and tomato | Reductions in the number of eggs and galls per gram of root and increased nutrient content in roots | [2,3] |
| Pochonia chlamydosporia | Tomato | Increase in secondary roots and increase in the total weight root of seedlings Reduction in flowering and fruiting times and greater weight of mature fruits |
[62] |
| P. chlamydosporia | Maize Cowpea |
Promoting root growth | [107] |
|
Purpureocillium lilacinum
Purpureocillium lavendulum Metarhizium marquandii |
Maize Beans Soy bean |
Plant growth promotion and availability of P and N | [104] |
| Purpureocillium lilacinum | Tomato | Plant growth promotion and phosphorus solubilization | [69] |
| Pleurotus ostreatus | Maize | Increase in root and shoot lengths, fresh and dry root weights, fresh and dry shoot weights, chlorophyll content, and nutrient uptake | [71] |
|
Trichoderma, Purpureocillium |
Banana | Active production of indole-3-acetic acid IAA and solubilize insoluble phosphate | [99] |
| Trichoderma | Soy bean | Plant growth promotion increase of P uptake efficiency | [82,87] |
|
Aspergillus niger, A. fumigatus, Penicillium pinophilum |
Wheat and faba bean | Yield of wheat grains and faba bean seed production | [108] |
| Penicillium oxalicum | Wheat and maize | Replace chemical fertilizer in alkaline soils. Improved crop production |
[101] |
| Penicillium sp. Penicillium oxalicum | Maize | Increased uptake of P by plants and increased availability of P in soil | [100] |
|
Penicillium sp. Aspergillus foetidus |
Sorghum bicolor L. | P uptake and increased growth | [109] |
|
Penicillium expansum, Mucor ramosissimus, Candida krissii |
Wheat | Plant growth promotion, P available in soil, and P absorption | [79] |
| Penicillium oxalicum | Rapeseed | Solubilize inorganic P and mineralize organic P | [110] |
| Mortierella capitata | Maize | Increased biomass, chlorophyll, and gibberellic acid | [111] |
| Mortierella sp. | Avocado | Plant growth promotion and P uptake | [112] |
9. Future Research Perspectives
The main P source in the world, phosphate rock, is fundamental for food production. It is strongly threatened as it is a finite resource. P, for the most part, is limited to a few countries in the world, particularly Morocco, which holds 75% of the world reserve [113]. Therefore, in recent years, the challenge of ensuring sustainable global P management to achieve world food security has been evidenced [114]. Given the current agricultural practices dependent on the continuous supply of commercial P-based fertilizers [115], the need to search for new sustainable strategies to manage the P availability in agricultural fields has increased [116]. Therefore, biological activators must be used to accelerate the bioavailability of P for plants, making NF important not only for the biological control of nematodes, but also for P solubilization. Their applications are also in accordance with sustainable agricultural practices for developing countries [117].
Currently, microbial-based fertilizers are not only considered for productivity and economic benefits, but also for their use as environmental-friendly products [18]. The aim is to guarantee less damage to water quality, increase nutrients recycling, reduce the consumption of resources, improve soil health, and increase the biodiversity of beneficial microorganisms [118]. Therefore, there is a demand to join efforts towards the search and discovery of microfungi, especially from little-explored natural regions [119], with the potential to sustain crop nutrition [120]. In fact, the excessive use of fertilizers affects soil health, adding to the presence of pathogens and pests [121].
Filamentous fungi with a dual activity (nematicide and P solubilizers) have versatile capacities to synthesize biocompounds such as enzymes, organic acids, and metabolites [122]. Especially, for P bioavailability, they can biologically produce high concentrations of organic acids, offering new knowledge through the detection of genes related to the mechanisms of phosphate solubilization [24,120,123]. Biotechnology applications may offer sustainable solutions based on microorganisms that are tolerant to new environmental conditions, including the microbiota that favors plant nutrition [124].
10. Conclusions
Several NF have the ability to suppress nematode parasites and improve the uptake of nutritional elements in order to promote plant growth and development. They have been successfully applied as biofertilizers and biocontrol agents to establish beneficial ecological relationships within their environment. For P solubilization, they rely on different mechanisms, such as a decrease in pH and the production of siderophors, organic acids, and enzymes. The wide variety of mechanisms for P solubilization in NF can be harnessed to reduce the dependence on P-based fertilizers in agriculture. The availability of new enzymes such as phytases promotes the search for beneficial microorganisms to develop environmentally-friendly and sustainable management plans.
Acknowledgments
The authors want to acknowledge Rafael F. Castañeda Ruiz and Daynet Sosa, who pioneered the studies of nematophagous fungi in Ecuador. In addition, a special acknowledgement is given to Enoy Leiva-Pantoja for his collaboration in drawings.
Author Contributions
M.V.-M.: reviewed the database, structured the sections of the article, wrote the article, and created and elaborated the figures. S.E.L.M.: wrote a section of the article, and revised and corrected text. J.N.-M.: wrote a section of the article, and revised and corrected the text. A.Q.: wrote a section of the article, and revised and corrected the text. M.F.R.: wrote a section of the article, and revised and corrected the text. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hallama M., Pekrun C., Lambers H., Kandeler E. Hidden Miners—The Roles of Cover Crops and Soil Microorganisms in Phosphorus Cycling through Agroecosystems. Plant Soil. 2019;434:7–45. doi: 10.1007/s11104-018-3810-7. [DOI] [Google Scholar]
- 2.Balbino H.M., Monteiro T.S.A., Coutinho R.R., Pacheco P.V.M., de Freitas L.G. Association of Duddingtonia flagrans with Microorganisms for Management of Meloidogyne javanica and Acquisition of Nutrients in Soybean. Biol. Control. 2021;159:104626. doi: 10.1016/j.biocontrol.2021.104626. [DOI] [Google Scholar]
- 3.Monteiro T.S.A., Valadares S.V., de Mello I.N.K., Moreira B.C., Kasuya M.C.M., de Araújo J.V., de Freitas L.G. Nematophagus Fungi Increasing Phosphorus Uptake and Promoting Plant Growth. Biol. Control. 2018;123:71–75. doi: 10.1016/j.biocontrol.2018.05.003. [DOI] [Google Scholar]
- 4.Kumar A., Kumar A., Patel H. Role of Microbes in Phosphorus Availability and Acquisition by Plants. Int. J. Curr. Microbiol. Appl. Sci. 2018;7:1344–1347. doi: 10.20546/ijcmas.2018.705.161. [DOI] [Google Scholar]
- 5.Maharana R., Singh B.S.M., Mandal K., Dhal N.K. Microbial-Mediated Mechanism to Improve Rock Phosphate Solubilization and Its Agronomic Implications. In: Mulla S.I., Bharagava R.N., editors. Enzymes for Pollutant Degradation. Springer Nature; Singapore: 2022. pp. 327–339. Microorganisms for Sustainability. [Google Scholar]
- 6.El Maaloum S., Elabed A., Alaoui-Talibi Z.E., Meddich A., Filali-Maltouf A., Douira A., Ibnsouda-Koraichi S., Amir S., El Modafar C. Effect of Arbuscular Mycorrhizal Fungi and Phosphate-Solubilizing Bacteria Consortia Associated with Phospho-Compost on Phosphorus Solubilization and Growth of Tomato Seedlings (Solanum lycopersicum L.) Commun. Soil Sci. Plant Anal. 2020;51:622–634. doi: 10.1080/00103624.2020.1729376. [DOI] [Google Scholar]
- 7.Tian J., Ge F., Zhang D., Deng S., Liu X. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology. 2021;10:158. doi: 10.3390/biology10020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalayu G. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int. J. Agron. 2019;2019:e4917256. doi: 10.1155/2019/4917256. [DOI] [Google Scholar]
- 9.Raymond N.S., Gómez-Muñoz B., van der Bom F.J.T., Nybroe O., Jensen L.S., Müller-Stöver D.S., Oberson A., Richardson A.E. Phosphate-Solubilising Microorganisms for Improved Crop Productivity: A Critical Assessment. New Phytol. 2021;229:1268–1277. doi: 10.1111/nph.16924. [DOI] [PubMed] [Google Scholar]
- 10.Marra L.M., de Oliveira-Longatti S.M., Soares C.R.F.S., Olivares F.L., Moreira F.M.d.S. The Amount of Phosphate Solubilization Depends on the Strain, C-Source, Organic Acids and Type of Phosphate. Geomicrobiol. J. 2019;36:232–242. doi: 10.1080/01490451.2018.1542469. [DOI] [Google Scholar]
- 11.Billah M., Khan M., Bano A., Hassan T.U., Munir A., Gurmani A.R. Phosphorus and Phosphate Solubilizing Bacteria: Keys for Sustainable Agriculture. Geomicrobiol. J. 2019;36:904–916. doi: 10.1080/01490451.2019.1654043. [DOI] [Google Scholar]
- 12.Kour D., Rana K.L., Kaur T., Yadav N., Yadav A.N., Kumar M., Kumar V., Dhaliwal H.S., Saxena A.K. Biodiversity, Current Developments and Potential Biotechnological Applications of Phosphorus-Solubilizing and -Mobilizing Microbes: A Review. Pedosphere. 2021;31:43–75. doi: 10.1016/S1002-0160(20)60057-1. [DOI] [Google Scholar]
- 13.Puente M.E., Bashan Y., Li C.Y., Lebsky V.K. Microbial Populations and Activities in the Rhizoplane of Rock-Weathering Desert Plants. I. Root Colonization and Weathering of Igneous Rocks. Plant Biol. 2004;6:629–642. doi: 10.1055/s-2004-821100. [DOI] [PubMed] [Google Scholar]
- 14.Chuang C.-C., Kuo Y.-L., Chao C.-C., Chao W.-L. Solubilization of Inorganic Phosphates and Plant Growth Promotion by Aspergillus niger. Biol. Fertil. Soils. 2007;43:575–584. doi: 10.1007/s00374-006-0140-3. [DOI] [Google Scholar]
- 15.Zhang Y., Chen F.-S., Wu X.-Q., Luan F.-G., Zhang L.-P., Fang X.-M., Wan S.-Z., Hu X.-F., Ye J.-R. Isolation and characterization of two phosphate-solubilizing fungi from rhizosphere soil of moso bamboo and their functional capacities when exposed to different phosphorus sources and pH environments. PLoS ONE. 2018;13:e0199625. doi: 10.1371/journal.pone.0199625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rawat P., Das S., Shankhdhar D., Shankhdhar S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2021;21:49–68. doi: 10.1007/s42729-020-00342-7. [DOI] [Google Scholar]
- 17.Sun H., Wu Y., Zhou J., Yu D., Chen Y. Microorganisms Drive Stabilization and Accumulation of Organic Phosphorus: An Incubation Experiment. Soil Biol. Biochem. 2022;172:108750. doi: 10.1016/j.soilbio.2022.108750. [DOI] [Google Scholar]
- 18.Quevedo A., Magdama F., Castro J., Vera-Morales M. Interacciones ecológicas de los hongos nematófagos y su potencial uso en cultivos tropicales. Sci. Agropecu. 2022;13:97–108. doi: 10.17268/sci.agropecu.2022.009. [DOI] [Google Scholar]
- 19.Soares F.E.d.F., Gôlo P.S., Fernandes É.K.K. Chapter 23—Nematophagous and Entomopathogenic Fungi: New Insights into the Beneficial Fungus-Plant Interaction. In: Sharma V., Salwan R., Al-Ani L.K.T., editors. Molecular Aspects of Plant Beneficial Microbes in Agriculture. Academic Press; Cambridge, MA, USA: 2020. pp. 295–304. [Google Scholar]
- 20.Alori E.T., Glick B.R., Babalola O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017;8:971. doi: 10.3389/fmicb.2017.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bargaz A., Lyamlouli K., Chtouki M., Zeroual Y., Dhiba D. Soil Microbial Resources for Improving Fertilizers Efficiency in an Integrated Plant Nutrient Management System. Front. Microbiol. 2018;9:1606. doi: 10.3389/fmicb.2018.01606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Lambers H. Root-Released Organic Anions in Response to Low Phosphorus Availability: Recent Progress, Challenges and Future Perspectives. Plant Soil. 2020;447:135–156. doi: 10.1007/s11104-019-03972-8. [DOI] [Google Scholar]
- 23.Syers J.K., Johnston A.E., Curtin D. Efficiency of Soil and Fertilizer Phosphorus Use. Reconciling Changing Concepts of Soil Phosphorus Behaviour with Agronomic Information. FAO Fertilizer and Plant Nutrition Bulletin; Rome, Italy: 2008. [Google Scholar]
- 24.Alaylar B., Egamberdieva D., Gulluce M., Karadayi M., Arora N.K. Integration of Molecular Tools in Microbial Phosphate Solubilization Research in Agriculture Perspective. World J. Microbiol. Biotechnol. 2020;36:93. doi: 10.1007/s11274-020-02870-x. [DOI] [PubMed] [Google Scholar]
- 25.Thakur D., Kaushal R., Shyam V. Phosphate Solubilising Microorganisms: Role in Phosphorus Nutrition of Crop Plants- a Review. Agric. Rev. 2014;35:159–171. doi: 10.5958/0976-0741.2014.00903.9. [DOI] [Google Scholar]
- 26.Altomare C., Norvell W., Björkman T., Harman G. Solubilization of Phosphates and Micronutrients by the Plant-Growth-Promoting and Biocontrol Fungus Trichoderma harzianum Rifai 1295-22. Appl. Environ. Microbiol. 1999;65:2926–2933. doi: 10.1128/AEM.65.7.2926-2933.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X., Kong Y., Guo E., Chen X., Li L. ·Organic Acid Regulation of Inorganic Phosphorus Release from Mollisols with Different Organic Matter Contents. Soil Use Manag. 2022;38:576–583. doi: 10.1111/sum.12710. [DOI] [Google Scholar]
- 28.Magnuson J.K., Lasure L.L. Organic Acid Production by Filamentous Fungi. In: Tkacz J.S., Lange L., editors. Advances in Fungal Biotechnology for Industry, Agriculture, and Medicine. Springer; Boston, MA, USA: 2004. pp. 307–340. [Google Scholar]
- 29.Akintokun A.K., Akande G.A., Akintokun P.O., Popoola T.O.S., Babalola A.O. Solubilization of Insoluble Phosphate by Organic Acid-Producing Fungi Isolated from Nigerian Soil. Int. J. Soil Sci. 2007;2:301–307. doi: 10.3923/ijss.2007.301.307. [DOI] [Google Scholar]
- 30.Asea P.E.A., Kucey R.M.N., Stewart J.W.B. Inorganic Phosphate Solubilization by Two Penicillium Species in Solution Culture and Soil. Soil Biol. Biochem. 1988;20:459–464. doi: 10.1016/0038-0717(88)90058-2. [DOI] [Google Scholar]
- 31.De Oliveira G., Moreira de Freitas A.L., Liparini Pereira O., Ribeiro da Silva I., Bojkov Vassilev N., Dutra Costa M. Mechanisms of Phosphate Solubilization by Fungal Isolates When Exposed to Different P Sources. Ann. Microbiol. 2014;64:239–249. doi: 10.1007/s13213-013-0656-3. [DOI] [Google Scholar]
- 32.Qiao H., Sun X.-R., Wu X.-Q., Li G.-E., Wang Z., Li D.-W. The Phosphate-Solubilizing Ability of Penicillium guanacastense and Its Effects on the Growth of Pinus massoniana in Phosphate-Limiting Conditions. Biol. Open. 2019;8:bio046797. doi: 10.1242/bio.046797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X., Luo L., Yang J., Li B., Yuan H. Mechanisms for Solubilization of Various Insoluble Phosphates and Activation of Immobilized Phosphates in Different Soils by an Efficient and Salinity-Tolerant Aspergillus niger Strain An2. Appl. Biochem. Biotechnol. 2015;175:2755–2768. doi: 10.1007/s12010-014-1465-2. [DOI] [PubMed] [Google Scholar]
- 34.Tian D., Wang L., Hu J., Zhang L., Zhou N., Xia J., Xu M., Yusef K.K., Wang S., Li Z., et al. A Study of P Release from Fe-P and Ca-P via the Organic Acids Secreted by Aspergillus niger. J. Microbiol. 2021;59:819–826. doi: 10.1007/s12275-021-1178-5. [DOI] [PubMed] [Google Scholar]
- 35.Zhang K., Zhang B., Yang S.-T. Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals, and Polymers. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2013. Production of Citric, Itaconic, Fumaric, and Malic Acids in Filamentous Fungal Fermentations; pp. 375–398. [Google Scholar]
- 36.Sadaf N., Haider M.Z., Iqbal N., Abualreesh M.H., Alatawi A. Harnessing the Phytase Production Potential of Soil-Borne Fungi from Wastewater Irrigated Fields Based on Eco-Cultural Optimization under Shake Flask Method. Agriculture. 2022;12:103. doi: 10.3390/agriculture12010103. [DOI] [Google Scholar]
- 37.Azeem M., Riaz A., Chaudhary A.N., Hayat R., Hussain Q., Tahir M.I., Imran M. Microbial Phytase Activity and Their Role in Organic P Mineralization. Arch. Agron. Soil Sci. 2015;61:751–766. doi: 10.1080/03650340.2014.963796. [DOI] [Google Scholar]
- 38.Kaur P., Vohra A., Satyanarayana T. Multifarious Applications of Fungal Phytases. In: Zaragoza Ó., Casadevall A., editors. Encyclopedia of Mycology. Elsevier; Oxford, UK: 2021. pp. 358–369. [Google Scholar]
- 39.Hou X., Shen Z., Li N., Kong X., Sheng K., Wang J., Wang Y. A Novel Fungal Beta-Propeller Phytase from Nematophagous Arthrobotrys oligospora: Characterization and Potential Application in Phosphorus and Mineral Release for Feed Processing. Microb. Cell Factories. 2020;19:84. doi: 10.1186/s12934-020-01346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hider R.C., Kong X. Chemistry and Biology of Siderophores. Nat. Prod. Rep. 2010;27:637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- 41.Oberegger H., Schoeser M., Zadra I., Abt B., Haas H. SREA Is Involved in Regulation of Siderophore Biosynthesis, Utilization and Uptake in Aspergillus nidulans. Mol. Microbiol. 2001;41:1077–1089. doi: 10.1046/j.1365-2958.2001.02586.x. [DOI] [PubMed] [Google Scholar]
- 42.Schrettl M., Ibrahim-Granet O., Droin S., Huerre M., Latgé J.-P., Haas H. The Crucial Role of the Aspergillus fumigatus Siderophore System in Interaction with Alveolar Macrophages. Microbes Infect. 2010;12:1035–1041. doi: 10.1016/j.micinf.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamariz-Angeles C., Huamán G.D., Palacios-Robles E., Olivera-Gonzales P., Castañeda-Barreto A. Characterization of Siderophore-Producing Microorganisms Associated to Plants from High-Andean Heavy Metal Polluted Soil from Callejón de Huaylas (Ancash, Perú) Microbiol. Res. 2021;250:126811. doi: 10.1016/j.micres.2021.126811. [DOI] [PubMed] [Google Scholar]
- 44.Ghosh S.K., Bera T., Chakrabarty A.M. Microbial Siderophore—A Boon to Agricultural Sciences. Biol. Control. 2020;144:104214. doi: 10.1016/j.biocontrol.2020.104214. [DOI] [Google Scholar]
- 45.Al-Ani L.K.T., Soares F.E.d.F., Sharma A., de los Santos-Villalobos S., Valdivia-Padilla A.V., Aguilar-Marcelino L. Strategy of Nematophagous Fungi in Determining the Activity of Plant Parasitic Nematodes and Their Prospective Role in Sustainable Agriculture. Front. Fungal Biol. 2022;3:863198. doi: 10.3389/ffunb.2022.863198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.López-Llorca L.V., Maciá-Vicente J.G., Jansson H.-B. Mode of Action and Interactions of Nematophagous Fungi. In: Ciancio A., Mukerji K.G., editors. Integrated Management and Biocontrol of Vegetable and Grain Crops Nematodes. Springer; Dordrecht, The Netherlands: 2008. pp. 51–76. [Google Scholar]
- 47.Vera-Morales M., Castañeda-Ruiz R.F., Sosa D., Quevedo A., Naranjo-Morán J., Serrano L., Ratti M.F. Mecanismos de captura, colonización y alimentación empleados por parásitos y predadores de nematodos. Ecosistemas. 2022;31:2390. doi: 10.7818/ECOS.2390. [DOI] [Google Scholar]
- 48.Zhang Y., Li S., Li H., Wang R., Zhang K.-Q., Xu J. Fungi–Nematode Interactions: Diversity, Ecology, and Biocontrol Prospects in Agriculture. J. Fungi. 2020;6:206. doi: 10.3390/jof6040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Islam W., Adnan M., Shabbir A., Naveed H., Abubakar Y.S., Qasim M., Tayyab M., Noman A., Nisar M.S., Khan K.A., et al. Insect-Fungal-Interactions: A Detailed Review on Entomopathogenic Fungi Pathogenicity to Combat Insect Pests. Microb. Pathog. 2021;159:105122. doi: 10.1016/j.micpath.2021.105122. [DOI] [PubMed] [Google Scholar]
- 50.Idrees A., Afzal A., Qadir Z.A., Li J. Bioassays of Beauveria bassiana Isolates against the Fall Armyworm, Spodoptera frugiperda. J. Fungi. 2022;8:717. doi: 10.3390/jof8070717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan J., Liu H., Idrees A., Chen F., Lu H., Ouyang G., Meng X. First Record of Aspergillus fijiensis as an Entomopathogenic Fungus against Asian Citrus Psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae) J. Fungi. 2022;8:1222. doi: 10.3390/jof8111222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Idrees A., Qadir Z.A., Akutse K.S., Afzal A., Hussain M., Islam W., Waqas M.S., Bamisile B.S., Li J. Effectiveness of Entomopathogenic Fungi on Immature Stages and Feeding Performance of Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) Larvae. Insects. 2021;12:1044. doi: 10.3390/insects12111044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.França D.V.C., Kupper K.C., Magri M.M.R., Gomes T.M., Rossi F. Trichoderma spp. Isolates with Potential of Phosphate Solubilization and Growth Promotion in Cherry Tomato1. Pesqui. Agropecuária Trop. 2017;47:360–368. doi: 10.1590/1983-40632017v4746447. [DOI] [Google Scholar]
- 54.Joo J.H., Hussein K.A. Biological Control and Plant Growth Promotion Properties of Volatile Organic Compound-Producing Antagonistic Trichoderma spp. Front. Plant Sci. 2022;13:897668. doi: 10.3389/fpls.2022.897668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rinu K., Pandey A., Palni L.M.S. Utilization of Psychrotolerant Phosphate Solubilizing Fungi under Low Temperature Conditions of the Mountain Ecosystem. In: Satyanarayana T., Johri B.N., editors. Microorganisms in Sustainable Agriculture and Biotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. 77–90. [Google Scholar]
- 56.Pandit R., Kunjadia P., Mukhopadhyaya P., Kunjadia A. Inorganic Phosphate Solubilizing Potential of Arthrobotrys conoides and Duddingtonia flagrans, a Nematode Trapping Fungi a Potential Biocontrol Agent. Int. J. Agric. Technol. 2014;10:559–570. [Google Scholar]
- 57.Quevedo A., Vera-Morales M., Espinoza-Lozano F., Castañeda-Ruiz R., Sosa D., Magdama F. Assessing the Predatory Activity of Arthrobotrys oligosporus Strain C-2197 as Biocontrol of the Root-Knot Nematode Meloidogyne spp. Bionatura. 2021;6:1586–1592. doi: 10.21931/RB/2021.06.01.22. [DOI] [Google Scholar]
- 58.Duponnois R., Kisa M., Plenchette C. Phosphate-Solubilizing Potential of the Nematophagous Fungus Arthrobotrys oligospora. J. Plant Nutr. Soil Sci. 2006;169:280–282. doi: 10.1002/jpln.200520551. [DOI] [Google Scholar]
- 59.Wan J., Dai Z., Zhang K., Li G., Zhao P. Pathogenicity and Metabolites of Endoparasitic Nematophagous Fungus Drechmeria coniospora YMF1.01759 against Nematodes. Microorganisms. 2021;9:1735. doi: 10.3390/microorganisms9081735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lebrigand K., He L.D., Thakur N., Arguel M.-J., Polanowska J., Henrissat B., Record E., Magdelenat G., Barbe V., Raffaele S., et al. Comparative Genomic Analysis of Drechmeria coniospora Reveals Core and Specific Genetic Requirements for Fungal Endoparasitism of Nematodes. PLoS Genet. 2016;12:e1006017. doi: 10.1371/journal.pgen.1006017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manzanilla-López R.H., Esteves I., Finetti-Sialer M.M., Hirsch P.R., Ward E., Devonshire J., Hidalgo-Díaz L. Pochonia chlamydosporia: Advances and Challenges to Improve Its Performance as a Biological Control Agent of Sedentary Endo-Parasitic Nematodes. J. Nematol. 2013;45:1–7. [PMC free article] [PubMed] [Google Scholar]
- 62.Zavala-Gonzalez E.A., Escudero N., Lopez-Moya F., Aranda-Martinez A., Exposito A., Ricaño-Rodríguez J., Naranjo-Ortiz M.A., Ramírez-Lepe M., Lopez-Llorca L.V. Some Isolates of the Nematophagous Fungus Pochonia chlamydosporia Promote Root Growth and Reduce Flowering Time of Tomato. Ann. Appl. Biol. 2015;166:472–483. doi: 10.1111/aab.12199. [DOI] [Google Scholar]
- 63.Gouveia A.D.S., Monteiro T.S.A., Valadares S.V., Sufiate B.L., de Freitas L.G., Ramos H.J.D.O., de Queiroz J.H. Understanding How Pochonia chlamydosporia Increases Phosphorus Availability. Geomicrobiol. J. 2019;36:747–751. doi: 10.1080/01490451.2019.1616857. [DOI] [Google Scholar]
- 64.Rosso L.c., Colagiero M., Salatino N., Ciancio A. Observations on the Effect of Trophic Conditions on Pochonia chlamydosporia Gene Expression. Ann. Appl. Biol. 2014;164:232–243. doi: 10.1111/aab.12099. [DOI] [Google Scholar]
- 65.Sellitto V.M., Curto G., Dallavalle E., Ciancio A., Colagiero M., Pietrantonio L., Bireescu G., Stoleru V., Storari M. Effect of Pochonia chlamydosporia-Based Formulates on the Regulation of Root-Knot Nematodes and Plant Growth Response. Front. Life Sci. 2016;9:177–181. doi: 10.1080/21553769.2016.1193827. [DOI] [Google Scholar]
- 66.Perveen Z., Shahzad S. A Comparative Study of the Efficacy of Paecilomyces species against Root-Knot Nematode Meloidogyne incognita. Pak. J. Nematol. 2013;31:125–135. [Google Scholar]
- 67.Rinu K., Pandey A. Slow and Steady Phosphate Solubilization by a Psychrotolerant Strain of Paecilomyces hepiali (MTCC 9621) World J. Microbiol. Biotechnol. 2011;27:1055–1062. doi: 10.1007/s11274-010-0550-0. [DOI] [Google Scholar]
- 68.Moreno-Gavíra A., Diánez F., Sánchez-Montesinos B., Santos M. Paecilomyces variotii as A Plant-Growth Promoter in Horticulture. Agronomy. 2020;10:597. doi: 10.3390/agronomy10040597. [DOI] [Google Scholar]
- 69.Constantin M., Raut I., Gurban A.-M., Doni M., Radu N., Alexandrescu E., Jecu L. Exploring the Potential Applications of Paecilomyces lilacinus 112. Appl. Sci. 2022;12:7572. doi: 10.3390/app12157572. [DOI] [Google Scholar]
- 70.Lee C.-H., Chang H.-W., Yang C.-T., Wali N., Shie J.-J., Hsueh Y.-P. Sensory Cilia as the Achilles Heel of Nematodes When Attacked by Carnivorous Mushrooms. Proc. Natl. Acad. Sci. USA. 2020;117:6014–6022. doi: 10.1073/pnas.1918473117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maharana R., Basu A., Dhal N.K., Adak T. Biosolubilization of Rock Phosphate by Pleurotus ostreatus with Brewery Sludge and Its Effect on the Growth of Maize (Zea mays L.) J. Plant Nutr. 2021;44:395–410. doi: 10.1080/01904167.2020.1822397. [DOI] [Google Scholar]
- 72.Owaid M.N., Abed I.A., Al-Saeedi S.S.S. Applicable Properties of the Bio-Fertilizer Spent Mushroom Substrate in Organic Systems as a Byproduct from the Cultivation of Pleurotus spp. Inf. Process. Agric. 2017;4:78–82. doi: 10.1016/j.inpa.2017.01.001. [DOI] [Google Scholar]
- 73.Gill S.S., Gill R., Trivedi D.K., Anjum N.A., Sharma K.K., Ansari M.W., Ansari A.A., Johri A.K., Prasad R., Pereira E., et al. Piriformospora indica: Potential and Significance in Plant Stress Tolerance. Front. Microbiol. 2016;7:332. doi: 10.3389/fmicb.2016.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meena K.K., Mesapogu S., Kumar M., Yandigeri M.S., Singh G., Saxena A.K. Co-Inoculation of the Endophytic Fungus Piriformospora indica with the Phosphate-Solubilising Bacterium Pseudomonas striata Affects Population Dynamics and Plant Growth in Chickpea. Biol. Fertil. Soils. 2010;46:169–174. doi: 10.1007/s00374-009-0421-8. [DOI] [Google Scholar]
- 75.Kumar M., Yadav V., Kumar H., Sharma R., Singh A., Tuteja N., Johri A.K. Piriformospora indica Enhances Plant Growth by Transferring Phosphate. Plant Signal. Behav. 2011;6:723–725. doi: 10.4161/psb.6.5.15106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soares F.E.d.F., Sufiate B.L., de Queiroz J.H. Nematophagous Fungi: Far beyond the Endoparasite, Predator and Ovicidal Groups. Agric. Nat. Resour. 2018;52:1–8. doi: 10.1016/j.anres.2018.05.010. [DOI] [Google Scholar]
- 77.Cairns T.C., Zheng X., Zheng P., Sun J., Meyer V. Turning inside out: Filamentous Fungal Secretion and Its Applications in Biotechnology, Agriculture, and the Clinic. J. Fungi. 2021;7:535. doi: 10.3390/jof7070535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vera D.F., Pérez H., Valencia H. Aislamiento de hongos solubilizadores de fosfatos de la rizósfera de Arazá (Eugenia stipitata, Myrtaceae) Acta Biológica Colomb. 2002;7:33–40. [Google Scholar]
- 79.Xiao C., Chi R., He H., Qiu G., Wang D., Zhang W. Isolation of Phosphate-Solubilizing Fungi from Phosphate Mines and Their Effect on Wheat Seedling Growth. Appl. Biochem. Biotechnol. 2009;159:330–342. doi: 10.1007/s12010-009-8590-3. [DOI] [PubMed] [Google Scholar]
- 80.Alves G.S., Bertini S.C.B., Barbosa B.B., Pimentel J.P., Ribeiro Junior V.A., Mendes G.d.O., Azevedo L.C.B. Fungal Endophytes Inoculation Improves Soil Nutrient Availability, Arbuscular Mycorrhizal Colonization and Common Bean Growth. Rhizosphere. 2021;18:100330. doi: 10.1016/j.rhisph.2021.100330. [DOI] [Google Scholar]
- 81.Tyśkiewicz R., Nowak A., Ozimek E., Jaroszuk-Ściseł J. Trichoderma: The Current Status of its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022;23:2329. doi: 10.3390/ijms23042329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marra R., Lombardi N., d’Errico G., Troisi J., Scala G., Vinale F., Woo S.L., Bonanomi G., Lorito M. Application of Trichoderma Strains and Metabolites Enhances Soybean Productivity and Nutrient Content. J. Agric. Food Chem. 2019;67:1814–1822. doi: 10.1021/acs.jafc.8b06503. [DOI] [PubMed] [Google Scholar]
- 83.Hernández-Leal T.I., Carrión G., Heredia G. Solubilización in vitro de fosfatos por una cepa de Paecilomyces lilacinus (Thom) Samson. Agrociencia. 2011;45:881–892. [Google Scholar]
- 84.Ngwene B., Boukail S., Söllner L., Franken P., Andrade-Linares D.R. Phosphate Utilization by the Fungal Root Endophyte Piriformospora indica. Plant Soil. 2016;405:231–241. doi: 10.1007/s11104-015-2779-8. [DOI] [Google Scholar]
- 85.Jayasinghearachchi H.S., Seneviratne G. Fungal Solubilization of Rock Phosphate Is Enhanced by Forming Fungal–Rhizobial Biofilms. Soil Biol. Biochem. 2006;38:405–408. doi: 10.1016/j.soilbio.2005.06.004. [DOI] [Google Scholar]
- 86.García-López A.M., Avilés M., Delgado A. Plant Uptake of Phosphorus from Sparingly Available P- Sources as Affected by Trichoderma asperellum T34. Agric. Food Sci. 2015;24:249–260. doi: 10.23986/afsci.49532. [DOI] [Google Scholar]
- 87.Bononi L., Chiaramonte J.B., Pansa C.C., Moitinho M.A., Melo I.S. Phosphorus-Solubilizing Trichoderma spp. from Amazon Soils Improve Soybean Plant Growth. Sci. Rep. 2020;10:2858. doi: 10.1038/s41598-020-59793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Narsian V., Patel H.H. Aspergillus aculeatus as a Rock Phosphate Solubilizer. Soil Biol. Biochem. 2000;32:559–565. doi: 10.1016/S0038-0717(99)00184-4. [DOI] [Google Scholar]
- 89.Ceci A., Pinzari F., Russo F., Maggi O., Persiani A.M. Saprotrophic Soil Fungi to Improve Phosphorus Solubilisation and Release: In Vitro Abilities of Several Species. Ambio. 2018;47:30–40. doi: 10.1007/s13280-017-0972-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jamshidi R., Jalili B., Bahmanyar M.A., Salek-Gilani S. Isolation and Identification of a Phosphate Solubilising Fungus from Soil of a Phosphate Mine in Chaluse, Iran. Mycology. 2016;7:134–142. doi: 10.1080/21501203.2016.1221863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Z., Bai T., Dai L., Wang F., Tao J., Meng S., Hu Y., Wang S., Hu S. A Study of Organic Acid Production in Contrasts between Two Phosphate Solubilizing Fungi: Penicillium oxalicum and Aspergillus niger. Sci. Rep. 2016;6:25313. doi: 10.1038/srep25313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cunningham J., Kuiack C. Production of Citric and Oxalic Acids and Solubilization of Calcium Phosphate by Penicillium bilaii. Appl. Environ. Microbiol. 1992;58:1451–1458. doi: 10.1128/aem.58.5.1451-1458.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.El-Azouni I. Effect of Phosphate Solubilizing Fungi on Growth and Nutrient Uptake of Soybean (Glycine max L.) Plants. J. Appl. Sci. Res. 2008;4:592–598. [Google Scholar]
- 94.Kumari P.D.S.U., Nanayakkara C.M. Phosphate-Solubilizing Fungi for Efficient Soil Phosphorus Management. Sri Lanka J. Food Agric. 2017;3:1–9. doi: 10.4038/sljfa.v3i2.46. [DOI] [Google Scholar]
- 95.Pandey A., Das N., Kumar B., Rinu K., Trivedi P. Phosphate Solubilization by Penicillium spp. Isolated from Soil Samples of Indian Himalayan Region. World J. Microbiol. Biotechnol. 2008;24:97–102. doi: 10.1007/s11274-007-9444-1. [DOI] [Google Scholar]
- 96.Yang T., Li L., Wang B., Tian J., Shi F., Zhang S., Wu Z. Isolation, Mutagenesis, and Organic Acid Secretion of a Highly Efficient Phosphate-Solubilizing Fungus. Front. Microbiol. 2022;13:793122. doi: 10.3389/fmicb.2022.793122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sharma S.B., Sayyed R.Z., Trivedi M.H., Gobi T.A. Phosphate Solubilizing Microbes: Sustainable Approach for Managing Phosphorus Deficiency in Agricultural Soils. SpringerPlus. 2013;2:587. doi: 10.1186/2193-1801-2-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peiris P.U.S., Li Y., Brown P., Xu C. Fungal Biocontrol against Meloidogyne spp. in Agricultural Crops: A Systematic Review and Meta-Analysis. Biol. Control. 2020;144:104235. doi: 10.1016/j.biocontrol.2020.104235. [DOI] [Google Scholar]
- 99.Napitupulu T.P., Ramadhani I., Kanti A., Sudiana I.M. Diversity, Phosphate Solubilizing, and IAA Production of Culturable Fungi Associated with Healthy and Wilt Banana. Arch. Phytopathol. Plant Prot. 2021;54:2306–2332. doi: 10.1080/03235408.2021.1983362. [DOI] [Google Scholar]
- 100.Qarni A., Billah M., Hussain K., Shah S.H., Ahmed W., Alam S., Sheikh A.A., Jafri L., Munir A., Malik K.M., et al. Isolation and Characterization of Phosphate Solubilizing Microbes from Rock Phosphate Mines and Their Potential Effect for Sustainable Agriculture. Sustainability. 2021;13:2151. doi: 10.3390/su13042151. [DOI] [Google Scholar]
- 101.Singh H., Reddy M.S. Effect of Inoculation with Phosphate Solubilizing Fungus on Growth and Nutrient Uptake of Wheat and Maize Plants Fertilized with Rock Phosphate in Alkaline Soils. Eur. J. Soil Biol. 2011;47:30–34. doi: 10.1016/j.ejsobi.2010.10.005. [DOI] [Google Scholar]
- 102.Chang C.-H., Yang S.-S. Thermo-Tolerant Phosphate-Solubilizing Microbes for Multi-Functional Biofertilizer Preparation. Bioresour. Technol. 2009;100:1648–1658. doi: 10.1016/j.biortech.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 103.Djuuna I.A.F., Prabawardani S., Massora M. Population Distribution of Phosphate-Solubilizing Microorganisms in Agricultural Soil. Microbes Environ. 2022;37:ME21041. doi: 10.1264/jsme2.ME21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baron N.C., Pollo A.d.S., Rigobelo E.C. Purpureocillium lilacinum and Metarhizium marquandii as Plant Growth-Promoting Fungi. PeerJ. 2020;8:e9005. doi: 10.7717/peerj.9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Osorio N.W., Habte M. Effect of a Phosphate-Solubilizing Fungus and an Arbuscular Mycorrhizal Fungus on Leucaena Seedlings in Tropical Soils with Contrasting Phosphate Sorption Capacity. Plant Soil. 2015;389:375–385. doi: 10.1007/s11104-014-2357-5. [DOI] [Google Scholar]
- 106.Lima-Rivera D.L., Lopez-Lima D., Desgarennes D., Velázquez-Rodríguez A.S., Carrion G. Phosphate Solubilization by Fungi with Nematicidal Potential. J. Soil Sci. Plant Nutr. 2016;16:507–524. doi: 10.4067/S0718-95162016005000042. [DOI] [Google Scholar]
- 107.Gomezjurado M.E., de Abreu L.M., Marra L.M., Pfenning L.H., de S. Moreira F.M. Phosphate Solubilization by Several Genera of Saprophytic Fungi and Its Influence on Corn and Cowpea Growth. J. Plant Nutr. 2015;38:675–686. doi: 10.1080/01904167.2014.934480. [DOI] [Google Scholar]
- 108.Abdul O.A., Mehana T.A. Impact of Phosphate-Solubilizing Fungi on the Yield and Phosphorus-Uptake by Wheat and Faba Bean Plants. Microbiol. Res. 2000;155:221–227. doi: 10.1016/S0944-5013(00)80036-1. [DOI] [PubMed] [Google Scholar]
- 109.Salih H.M., Yahya A.I., Abdul-Rahem A.M., Munam B.H. Availability of Phosphorus in a Calcareous Soil Treated with Rock Phosphate or Superphosphate as Affected by Phosphate-Dissolving Fungi. Plant Soil. 1989;120:181–185. doi: 10.1007/BF02377067. [DOI] [Google Scholar]
- 110.Wang J., Zhao Y.-G., Maqbool F. Capability of Penicillium oxalicum Y2 to Release Phosphate from Different Insoluble Phosphorus Sources and Soil. Folia Microbiol. (Praha) 2021;66:69–77. doi: 10.1007/s12223-020-00822-4. [DOI] [PubMed] [Google Scholar]
- 111.Li F., Zhang S., Wang Y., Li Y., Li P., Chen L., Jie X., Hu D., Feng B., Yue K., et al. Rare Fungus, Mortierella capitata, Promotes Crop Growth by Stimulating Primary Metabolisms Related Genes and Reshaping Rhizosphere Bacterial Community. Soil Biol. Biochem. 2020;151:108017. doi: 10.1016/j.soilbio.2020.108017. [DOI] [Google Scholar]
- 112.Tamayo-Velez A., Osorio N.W. Co-Inoculation with an Arbuscular Mycorrhizal Fungus and a Phosphate-Solubilizing Fungus Promotes the Plant Growth and Phosphate Uptake of Avocado Plantlets in a Nursery. Botany. 2017;95:539–545. doi: 10.1139/cjb-2016-0224. [DOI] [Google Scholar]
- 113.Cordell D., White S. Tracking Phosphorus Security: Indicators of Phosphorus Vulnerability in the Global Food System. Food Secur. 2015;7:337–350. doi: 10.1007/s12571-015-0442-0. [DOI] [Google Scholar]
- 114.Chowdhury R.B., Moore G.A., Weatherley A.J., Arora M. Key Sustainability Challenges for the Global Phosphorus Resource, Their Implications for Global Food Security, and Options for Mitigation. J. Clean. Prod. 2017;140:945–963. doi: 10.1016/j.jclepro.2016.07.012. [DOI] [Google Scholar]
- 115.Condron L.M., Spears B.M., Haygarth P.M., Turner B.L., Richardson A.E. Role of Legacy Phosphorus in Improving Global Phosphorus-Use Efficiency. Environ. Dev. 2013;8:147–148. doi: 10.1016/j.envdev.2013.09.003. [DOI] [Google Scholar]
- 116.Zhu J., Li M., Whelan M. Phosphorus Activators Contribute to Legacy Phosphorus Availability in Agricultural Soils: A Review. Sci. Total Environ. 2018;612:522–537. doi: 10.1016/j.scitotenv.2017.08.095. [DOI] [PubMed] [Google Scholar]
- 117.Chittora P., Sharma D., Aseri G.K., Sohal J.S., Singh D., Khare N., Jain N. Chapter 9—Fungi as Phosphate-Solubilizing Microorganisms in Arid Soil: Current Perspective. In: Singh J., Gehlot P., editors. New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; Amsterdam, The Netherlands: 2020. pp. 99–108. [Google Scholar]
- 118.Bruulsema T.W., Peterson H.M., Prochnow L.I. The Science of 4R Nutrient Stewardship for Phosphorus ManAgement across Latitudes. J. Environ. Qual. 2019;48:1295–1299. doi: 10.2134/jeq2019.02.0065. [DOI] [PubMed] [Google Scholar]
- 119.Landínez-Torres A.Y., Becerra Abril J.L., Tosi S., Nicola L. Soil Microfungi of the Colombian Natural Regions. Int. J. Environ. Res. Public. Health. 2020;17:E8311. doi: 10.3390/ijerph17228311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hyde K.D., Xu J., Rapior S., Jeewon R., Lumyong S., Niego A.G.T., Abeywickrama P.D., Aluthmuhandiram J.V.S., Brahamanage R.S., Brooks S., et al. The Amazing Potential of Fungi: 50 Ways We Can Exploit Fungi Industrially. Fungal Div. 2019;97:1–136. doi: 10.1007/s13225-019-00430-9. [DOI] [Google Scholar]
- 121.Vassileva M., Mocali S., Canfora L., Malusá E., García Del Moral L.F., Martos V., Flor-Peregrin E., Vassilev N. Safety Level of Microorganism-Bearing Products Applied in Soil-Plant Systems. Front. Plant Sci. 2022;13:862875. doi: 10.3389/fpls.2022.862875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Madhavan A., Arun K., Sindhu R., Alphonsa Jose A., Pugazhendhi A., Binod P., Sirohi R., Reshmy R., Kumar Awasthi M. Engineering Interventions in Industrial Filamentous Fungal Cell Factories for Biomass Valorization. Bioresour. Technol. 2022;344:126209. doi: 10.1016/j.biortech.2021.126209. [DOI] [PubMed] [Google Scholar]
- 123.Dörsam S., Fesseler J., Gorte O., Hahn T., Zibek S., Syldatk C., Ochsenreither K. Sustainable Carbon Sources for Microbial Organic Acid Production with Filamentous Fungi. Biotechnol. Biofuels. 2017;10:242. doi: 10.1186/s13068-017-0930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vassilev N., Eichler-Löbermann B., Vassileva M. Stress-Tolerant P-Solubilizing Microorganisms. Appl. Microbiol. Biotechnol. 2012;95:851–859. doi: 10.1007/s00253-012-4224-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.