Abstract
Background: Celiac disease (CD) is an autoimmune enteropathy affecting approximately 1% of the population and is associated with an increased risk of enteropathy-associated T-cell lymphoma and small bowel adenocarcinoma, whereas the association between CD and other malignancies is unclear. Since pancreatic cancer (PC) remains one of the most lethal neoplasms and its incidence is increasing despite numerous ongoing research on diagnostic biomarkers and novel therapies, we aimed to investigate whether CD has an impact on the risk of PC. Material and Methods: We performed a systematic review of the literature published from January 2000 to March 2022 in two databases: Web of Science and Scopus and a meta-analysis of eligible studies. Results: Our search identified eight publications included in the systematic review. A total of five studies involving 47,941 patients, including 6399 CD patients with malignancies and 1231 PC cases were included in the meta-analysis and 221 cases of PC in CD patients with other cancers were recognized. The pooled OR for PC was 1.46 (95% CI 1.26–1.7) with significant heterogeneity (89.1%; p < 0.05), suggesting that CD patients with malignancies were at higher risk for PC. Conclusions: The association between CD and PC is uncertain. However, the results of the current meta-analysis may indicate an increased risk of PC in the group of patients with CD and other cancers. Further multicenter studies are warranted.
Keywords: pancreatic cancer, celiac disease, autoimmune diseases
1. Introduction
Celiac disease (CD) is an immune-mediated disorder that primarily affects the small intestine and occurs in genetically predisposed individuals with the presence of leukocyte antigen HLA-DQ2/HLA-DQ8 haplotypes [1]. Consumption of gluten in susceptible individuals contributes to inflammation of the small intestinal mucosa and villous atrophy [2]. The worldwide seroprevalence and prevalence of CD were recently estimated to be 1.4% and 0.7%, respectively [3]. However, approximately 70% of cases remain undiagnosed and untreated [4]. CD can occur at any age, but the highest prevalence rates have been observed in children [5]. The incidence of CD has increased by 7.5% per year in recent decades and CD has become a significant health problem worldwide [3]. The increase in new diagnoses is due to growing awareness of the disease and better availability of testing, but also because the increase in actual incidence is a result of environmental changes that may promote loss of tolerance to gluten in the diet [4,6,7]. Diagnostic criteria for CD have evolved, yet there is a high degree of agreement among guidelines published over the past few decades [8]. Despite the intensive development of new treatment strategies [9], a gluten-free diet (GFD) is the only available, but not universally effective, therapeutic option for CD patients. CD shows a wide spectrum of intestinal and extraintestinal manifestations and may be associated with other autoimmune diseases such as type 1 diabetes, inflammatory bowel diseases and genetic disorders. The relationship between CD and malignancies is still under investigation. Enteropathy-associated T-cell lymphoma (relative risk, (RR) = 3.1) and small bowel adenocarcinoma (RR = 3.05) have been for decades the best-known malignancies connected to CD [10]. However, the relationship between CD and other types of neoplasms is still unclear [11,12].
At the same time, cancer is one of the leading causes of death worldwide and will be responsible for nearly 10 million deaths in 2020 [13], a position shared with malnutrition and hunger which kill almost 10 million people each year, amongst whom three million are children [14]. Pancreatic cancer (PC) is the seventh leading cause of cancer-related deaths worldwide due to its extremely poor prognosis [15]. Based on GLOBOCAN data 458,918 new cases of PC were reported worldwide in 2018, accounting for 2.5% of all cancers. Moreover, PC is becoming an increasingly common cause of cancer mortality and a 2.3-fold increase in the global number of cases and deaths from PC has been demonstrated [16]. The incidence of PC varies by region and population group. The highest age-standardized rate incidence was observed in Europe (7.7 per 100,000 population) and North America (7.6 per 100,000), while the lowest was observed in Africa (2.2 per 100,000 population). Varying exposure to risk factors may explain these differences between regions [17,18]. However, the lowest prevalence in Africa may be due to the effects of malnutrition and starvation on that continent, where people die of hunger before exposure resulting in PC [19,20]. PC is more common in men than in women. Noticeably, the incidence rate increases with age in both sexes. The highest incidence occurs in women aged 75–79 years and in men aged 65–69 years [17]. Known risk factors for PC include tobacco smoking, diabetes mellitus, obesity, dietary factors, alcohol abuse, age, ethnicity, family history and genetic factors, Helicobacter pylori infection, non-O blood group and chronic pancreatitis, while inherited risk factors contribute to 5–10% of PC cases [17]. Dietary factors are estimated to have an impact on up to 30–50% of PC cases [21,22]. Early diagnosis and treatment of PC remain a formidable challenge. Approximately one-third of patients have locally advanced disease and 50% of patients are found to have metastatic disease at the time of diagnosis [23].
Our systematic review and meta-analysis investigated the possible relationship between CD and PC. There were three phenomena motivating us to conduct this analysis: 1. The explosion of new cases of CD and PC. 2. The known association of CD with intestinal tumors and suggested connection of CD to other tumors. 3. CD–related factors, mainly immunity-related [24] and metabolic [25,26,27], that may potentially impact PC development. Therefore, we attempted to conduct a systematic review of the current knowledge on the association between CD and PC and a meta-analysis of the available studies. Clarification and better understanding of such an association could have clinical implications and guide the future direction of research on CD and PC.
2. Materials and Methods
2.1. Protocol and Search Strategy
A systematic review was conducted in March 2022 and included manuscripts published from January 2000 to March 2022 in English. Two respective databases, Web of Science and Scopus were queried. To prepare queries, the following expressions ‘‘celiac disease’’ OR ‘‘coeliac disease’’ AND ‘‘cancer*’’ OR ‘‘tumor*’’ OR ‘‘carcinoma*’’ OR ‘‘neoplasm*’’ AND “pancreas” OR “pancreatic” were used. The asterisks allowed us to retrieve records where query words appeared with suffixes (e.g., cancer|s). The reporting of this systematic review was guided by the standards of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) Statement [28]. The review was not registered.
2.2. Eligibility Criteria
Cohort studies that reported PC in patients with CD were reviewed and included if eligible. The following studies were excluded: experimental studies, animal studies, editorials, conference papers and book chapters.
2.3. Study Selection and Data Extraction
Study selections were performed by the authors based on the eligibility criteria. Any disagreement on study selection between two authors was discussed for consensus. Firstly, the potentially relevant studies were identified from two databases; then, duplicates were removed. Secondly, the remaining studies’ titles and abstracts were screened; unrelated studies were excluded. Thirdly, the remaining studies were examined for full texts; unrelated studies were excluded. Finally, eligible studies were included for further review. Data from the included studies were independently extracted into the pilot standardized datasheet by two authors. The following data were extracted from each study: author, year of publication, study site, study design, number of patients with CD, number of patients with PC, main findings, characteristics of the participants, age and gender.
2.4. Quality of the Included Studies
Quality assessment of the selected studies was performed using the Newcastle–Ottawa Quality Assessment Scale (NOS) [29]. NOS is scored by awarding points for fulfilling criteria related to selection (representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, a demonstration that outcome of interest was not present at the start of study, comparability (comparability of cohorts based on the design or analysis) and outcomes of studies. Possible totals are 4 points for selection, 2 points for comparability and 3 points for outcomes.) Both authors (I.G. and K.N.) independently performed data abstraction and quality assessment. Discrepancies between authors’ assessments were discussed until a consensus was reached.
2.5. Statistical Analysis
EPIINFO version 7.2.4.0 (The Centers for Disease Control and Prevention, Atlanta, GA, USA) and Meta Win version 3.0.6 (Center for Biological Data Science, Richmond, VA, USA) beta were used for the statistical analysis. The overall odds ratio (OR) and relative risk (RR) were pooled using a random-effects model. Ors and RRs of each study, at 95% confidence intervals (CIs), were calculated and Mantel–Haenszel test was conducted. I2 statistics with CIs were used to assess statistical heterogeneity. Statistical significance was set at p < 0.05.
3. Results
3.1. Search Results
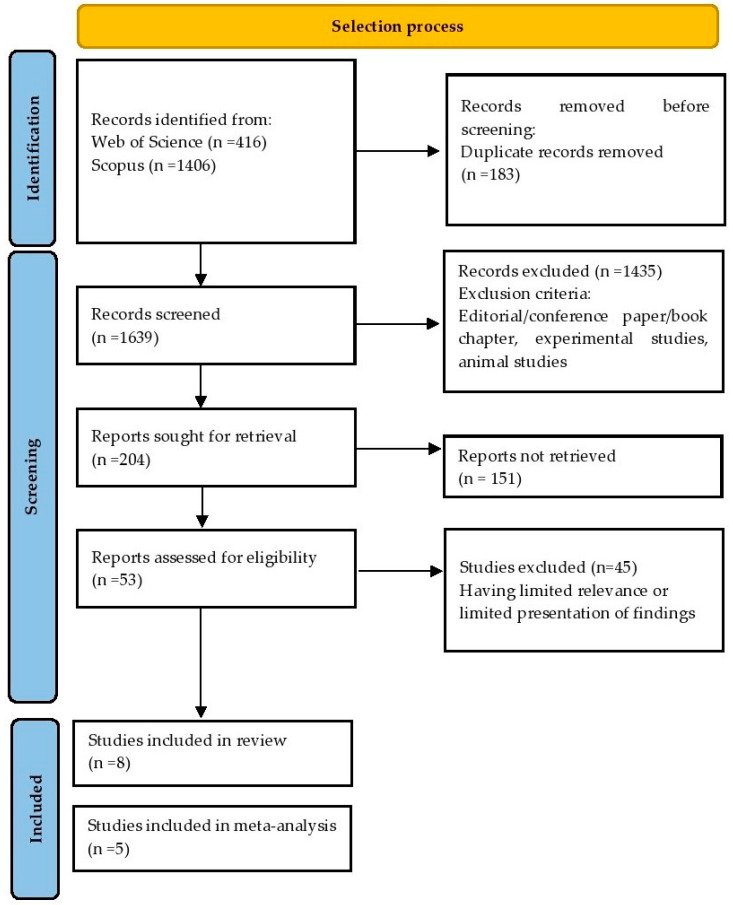
Our primary search using the selected key phrases resulted in 1822 publications, of which 1639 entered the title screening phase. Exclusion criteria removed 1435 entries leaving 204 records in our database for full-text reading. Cross-search and a manual search revealed an additional two eligible articles. Finally, this systematic review was prepared based on eight publications. Five studies were included in the meta-analysis. The selection process is presented in Figure 1.
Figure 1.
Flowchart presenting the selection process.
3.2. Quality Assessment
All the studies proved appropriate representativeness of the exposed cohort and appropriate selection of the non-exposed cohort. No study could prove ascertainment of exposure. Two studies [30,31] showed no outcome of interest at the start of the study. All studies were controlled for confounders. All studies demonstrated assessment for the outcome and follow-up that was long enough for outcomes. One study [30] showed adequacy of follow-up cohort (Table 1).
Table 1.
Quality assessment of the selected studies using the Newcastle–Ottawa Quality Assessment Scale for cohort studies.
| Study | Askling et al. 2002 [32] | Card et al. 2004 [33] | Goldacre et al. 2008 [34] | Landgren et al. 2011 [35] | Elfstrom et al. 2012 [31] | Ilus et al. 2014 [36] | Koskinen et al. 2020 [37] | Lebwohl et al. 2022 [30] | |
|---|---|---|---|---|---|---|---|---|---|
| SELECTION (maximum four) | Representativeness of The Exposed Cohort | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Selection of The Non-Exposed Cohort | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Ascertainment of Exposure | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| No Outcome of Interest at Start of The Study | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| COMPARABILITY (maximum two) | Controlled for confounders | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| OUTCOME (maximum three) | Assessment of outcome | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Follow-Up Long Enough for Outcomes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Adequacy of Follow Up of Cohorts | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Score | 6 | 6 | 6 | 6 | 7 | 6 | 6 | 8 | |
3.3. Celiac Disease and Pancreatic Cancer
In recent decades, several studies have investigated cancer risk in CD patients. Only a few studies have examined this risk in relation to PC. Our search revealed eight papers that included data on PC in CD. The characteristics of the studies included in the systematic review are presented in Table 2.
Table 2.
Characteristics of the studies included in the systematic review and/or meta-analysis.
| Author Year Country |
Study Design | CD/PC (n) | Age and Gender of Participants | Inclusion Criteria | Exclusion Criteria | Relevant Comorbidities | Main Findings |
|---|---|---|---|---|---|---|---|
| Askling et al. 2002 [32] Sweden |
A population-based prospective cohort study | 11,019/9 | All age categories (0–60+) 6521 F 4498 M |
Individuals discharged at least once with a diagnosis of CD | Nonmatching national registration numbers, other data irregularities. Authors excluded cancers occurring during the first year after entry into the cohort |
diabetes mellitus, gastroenteritis, unspecified abdominal pain, anemia, ulcerative colitis, asthma, vertigo, constipation, pneumonia, congestive heart failure, failure to thrive, Down’s syndrome, rheumatoid arthritis, atrial fibrillation, angina pectoris |
An elevated risk of PC (SIR, 1.9; 95% CI, 0.9–3.6) was observed. One of the PC patients had diabetes. |
| Card et al. 2004 [33] England |
A population-based prospective cohort study | 865/2 | All age categories (0–60+) 444 F 193 M |
Patients with the diagnosis of CD based on small bowel biopsy (severe or total VA as a result). The diagnosis date of patients diagnosed clinically in childhood but who did not have an intestinal biopsy until adult life was considered as the date of the intestinal biopsy. |
Patients referred from other secondary care centers. | not reported | No overall increase in the rate of incident malignancy in patients with CD compared with the general population. No increase in the risk of GI carcinomas in general. |
| Goldacre et al. 2008 [34] England |
A hospital-based retrospective cohort study | 1997/2 | All age categories (0–75+) Gender not Reported |
Records of all hospital admissions with ICD codes relevant to CD | In the main findings, the data are shown excluding cancers in the first year after admission for CD |
not reported | The overall risk of cancer was increased when the first-year cases were included and the increased risk was reduced when first year cases were excluded |
| Landgren et al. 2011 [35] USA |
A hospital-based retrospective cohort study | 63/13 | 18–100 Only M |
Discharge diagnoses as defined by the 8th and 9th revisions of the International Classification of Diseases (ICDA, ICD9-CM) | No prior malignancy | Smoking-related diagnoses: emphysema, bronchitis, COPD excluding asthma, COPD including asthma hypertension, alcoholism, diabetes mellitus, obesity, HIV, hepatitis viral, hepatitis (acute, chronic), GERD infectious mononucleosis |
The risk of PC was significantly increased (RR = 2.27 (95% CI, 1.22–4.23) |
| Elfstrom et al. 2012 [31] Sweden |
Population-based cohort study | 28,882/64 | All age categories (0–60+) 17,893 F 10,989 M |
Patients with the diagnosis of CD based on small bowel biopsy: 29,148 patients with CD diagnosis based on a small intestinal biopsy with VA, (equivalent to Marsh 3) and 13,446 individuals with inflammation but without VA (Marsh 1–2). |
Individuals with the biopsy originated from the ileum, individuals without matched reference individuals, Diagnosis of GI cancer before biopsy and study entry. |
not reported | Only in the first year of follow-up patients with CD (HR, 1.50; 95% CI, 1.33–1.68), inflammation (HR, 2.04; 95% CI, 1.80–2.32) and latent CD (HR, 2.06; 95% CI, 1.44–2.96) were at increased risk of GI cancer. The highest relative risks for GI cancer in patients with CD, inflammation and latent CD were seen for small intestinal cancer and PC (HR, 10.7; 95% CI, 5.77–19.7). |
| Ilus et al. 2014 [36] Finland |
A population-based prospective cohort study | 11,991/45 | 15–60+ 21,158 F 11,281 M |
Duodenal biopsy showing typical VA with crypt hyperplasia |
Cancers that occurred before entry to the registerwere excluded | DH | The SIR for malignancies was increased after 5 years from the diagnosis of CD. The SIRs for PC were decreased (0.73; 0.53–0.97). The risk was decreased especially in females (SIR = 0.59, 95% CI 0.36–0.89). |
| Koskinen et al. 2020 [37] Finland |
Population-based cohort study | 12,803/26 | All age categories (0–60+) 7943 F 4860 M |
Patients with the diagnosis of CD based on biopsy. | Inadequate baseline data | not reported | Mortality from all malignancies (HR 1.11, 95% CI 0.96–1.27) and GI malignancies (HR 1.21, 95% CI 0.56–1.71) was not increased among patients with CD. |
| Lebwohl et al. 2022 [30] Sweden |
Population-based cohort study | 47,241/152 | 0.0–95.4 29,381 F 17,860 M |
Diagnosis of CD was defined as having relevant SnoMed codes corresponding to VA in the small intestine (other than the ileum) through 31 December 2016. |
CD patients and controls with a record of cancer preceding the date of CD diagnosis (defined as the time of the first small intestinal biopsy showing VA) or the corresponding date for controls |
type 1 diabetes, autoimmune thyroid disease, rheumatoid arthritis, and inflammatory bowel disease were more common in patients with CD than in controls |
The overall risk of cancer was increased and was significantly elevated in the first year after CD diagnosis. The association between CD and cancer was noted among the others in PC (HR 2.30; 95% CI, 1.87–2.82). The elevated risk of PC persisted in long-term observation. |
CD, celiac disease; PC, pancreatic cancer; NHL; non-Hodgkin lymphoma; GI, gastrointestinal; DH, dermatitis herpetiformis; GERD, gastroesophageal reflux disease; VA, villous atrophy; ICD, International Classification of Diseases; SnoMed, Systematized Nomenclature of Medicine; F, females; M, males; SIR, standardized incident ratio; HR, hazard ratio; RR, relative risk; CI, confidence interval.
Several papers on malignancies in CD did not report data on PC [38,39,40,41,42,43]. In the study in the Lothian region of Scotland, the overall risk of malignancy in CD decreased with time after diagnosis and was not significantly increased after 15 years. The study included data on esophagal and colorectal tumors as well as general GI cancers, yet PC was not specifically addressed [38]. Further, in the study of Green et al. on 381 CD patients (43 cases of cancer), PC was not recognized [39]. In another study on the risk of malignancy and mortality in CD among 4732 people with CD compared with 23,620 matched controls there were 134 patients with at least one malignancy; however, the GI tumors were combined in one group. The authors concluded that people with CD have a modest increase in overall risks of malignancy and mortality. Similar to other studies, most of this excess risk occurred in the year of follow-up after diagnosis of CD [41]. In the study of Viljamaa, the prevalence of malignancies was similar to that in the general population [42]. An Italian study suggested an increased risk of cancer related to age [44].
3.4. Meta-Analysis
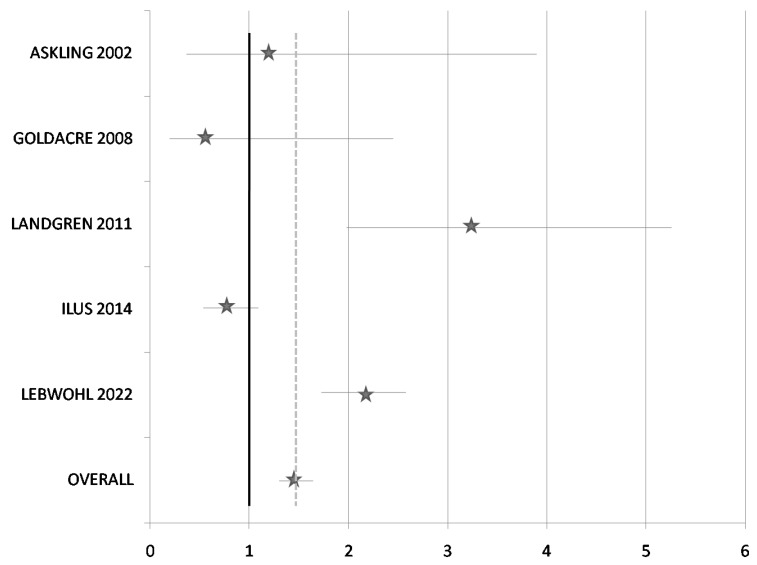
A total of five studies were included in the meta-analysis (Table 3). All five studies with 47,941 patients, including 6399 patients with CD and malignancies and 1213 PC cases, including 221 cases in CD patients and other cancers, were recognized. The pooled OR for PC was 1.46 (95% CI 1.26–1.7) and the pooled RR was 1.4 (95% CI 1.25–1.67), suggesting that patients with CD had a slightly higher risk of PC. Figure 2 shows the forest plot of the association between CD and PC. However, a high degree of heterogeneity was found (I2 = 89.1%; p < 0.05). Subgroup analysis was not possible due to the small number of studies included in our meta-analysis. In consequence, the results should be prudently considered and more detailed studies with adequate sample size are needed to determine the association between CD and the risk of PC.
Table 3.
Analysis of the association between celiac disease and pancreatic cancer risk.
| Author, Year | PC in CD * | CD * | PC in Patients without CD | Patients without CD | Total Number of Patients | % | RR (95% CI) |
OR (95% CI) |
p Value * |
|---|---|---|---|---|---|---|---|---|---|
| Askling et al., 2002 [32] | 9 | 240 | 4 | 131 | 384 | 0.8 | 1.22 (0.38–3.89) |
1.23 (0.37–4.06) |
0.736 |
| Goldacre et al., 2008 [34] | 2 | 572 | 89 | 14,758 | 15,421 | 32.2 | 0.58 (0.14–2.35) |
0.58 (0.14–2.36) |
0.441 |
| Landgren et al., 2011 [35] | 13 | 50 | 535 | 7852 | 8450 | 17.6 | 3.23 (1.98–5.29) |
3.82 (2.06–7.07) |
0 |
| Ilus et al., 2014 [36] | 45 | 1581 | 62 | 1673 | 3361 | 7.0 | 0.775 (0.53–1.13) |
0.77 (0.52–1.13) |
0.218 |
| Lebwohl et al., 2022 [30] | 152 | 3735 | 302 | 16,136 | 20,325 | 42.4 | 2.17 (1.76–2.58) |
2.17 (1.78–2.65) |
0 |
| Overall | 221 | 6178 | 992 | 40,550 | 47,941 | 100.0 | 1.4 (1.25–1.67) |
1.46 (1.26–1.7) |
0 |
PC, pancreatic cancer; CD, celiac disease ; RR relative risk; OR, odds ratio; CI, confidence interval; * Mantel-Haenszel test. *-patients with CD and malignancies.
Figure 2.
Forest plot for the association between celiac disease and pancreatic cancer [30,32,34,35,36].
4. Discussion
CD is associated with an increased risk of mortality [45] and several types of malignancies [30]. The results of available studies on the risk of PC in CD are inconsistent and vary from the studies demonstrating the increased risk of PC in CD patients [30,31,32,35] to research showing that PC risk and mortality were not increased [33,34,37] and one study in which the risk of PC was decreased [35]. The results of our meta-analysis of five eligible studies suggest, that the risk of PC in CD patients with other cancers might be increased. However, this finding should be interpreted with caution, primarily because of the lack of homogeneity in the pooled data.
All of the available studies, with one exception, have been performed in Europe. All European studies have been carried out in the northern part of the continent and most of the studies (five out eight) are in Scandinavian countries (Finland, Sweden). In turn, the only study carried out outside Europe involved a cohort restricted to males [35]. This might be seen as its limitation, as PC is more common in men [17], whereas CD is more common in women [3]. Considering the geographic diversity in the prevalence of both CD [5] and PC [17], the results of studies conducted in one world region cannot be directly extrapolated to a global extension.
The length of the observation influenced the risk of malignancies in CD patients. However, here also the results of the studies could have been more consistent. For instance, the study by Elfstrom et al. [31] demonstrated the increased risk only in the first year after diagnosis. In the study by Askling et al. [32], risk was reduced with time. Authors found out that adult patients with CD had an increased overall risk of cancer (SIR = 1.3; 95% CI, 1.2–1.5); however, the RR declined with duration of follow-up and was only slightly or even insignificantly increased after 10 years, reaching SIR of 1.1 [33]. Furthermore, Lebwohl et al. observed the association between CD and cancer only in the first year after diagnosis (HR, 2.47; 95% CI, 2.22–2.74). This might be partially explained by the adherence to GFD and its protective effect on cancer in patients with CD. On the contrary, it may be due to the increased surveillance and medical care among CD patients. The strongest association between CD and cancer was noted in hematologic cancers (HR, 1.90; 95% CI, 1.70–2.13), lymphoproliferative cancers (HR, 2.20; 95% CI, 1.94–2.49) and GI cancers (HR, 1.34; 95%CI, 1.24–1.45). Among the last subtype of cancer, the risk of PC (HR, 2.30; 95% CI, 1.87–2.82) and hepatobiliary cancer (HR, 1.80; 95% CI, 1.44–2.25) were increased. Interestingly, the elevated risk of PC (HR, 1.66; 95% CI, 1.32–2.10) and hepatobiliary cancer (HR, 1.61; 95% CI, 1.26–2.05) persisted in long-term observation [30]. To avoid bias resulting from inclusion of the cases being a cause for the work-up leading to the diagnosis of CD, or having been recognized during the work-up for CD, authors excluded the first year after CD diagnosis [32] or calculated the risk including and excluding the first year after diagnosis [34]. In turn, in a population-based, nationwide case-control study by van Gils et al. [46], authors took a different approach and assessed the risk of lymphomas and GI adenocarcinomas in newly diagnosed CD patients. They did not report data on PC, but found out, that enteropathy-associated T-cell lymphoma, lymphoma and CD were often synchronously diagnosed. In turn, in the study by Ilus et al. patients were stratified according to the duration of follow-up (<2 years (21,508), 2–4.9 years (21,556) and ≥5 years (7794). 1626 cancers occurred among CD patients, while 1735 were expected (SIR 0.94; 95% CI 0.89–0.98). Compared with the general population, the overall risk of malignancy was not increased, yet SIR was increased after 5 years from CD diagnosis. 45 cases of PC were observed, whereas 62 were expected, resulting in decreased SIR for PC. The risk was decreased, especially in females (Table 2). [36]. This may reflect smaller exposure among females to the other risk factors for PC, such as obesity or smoking; however, it was not directly assessed by the study. In turn, Lebwohl et al. observed, that cancer risk in CD was elevated in men but not women [30]. The other significant risk factor for cancers is age. This is crucial as there is a rise in CD cases in older groups of patients [47]. It was shown that the increased risk of cancer in CD patients was mostly limited to patients above the age of 40 [30]. Furthermore, the highest risk for T-cell lymphoma was observed in males between the ages of 50 and 80 when CD was diagnosed at age 50 [46]. Authors noticed, that risk increase was confined to patients diagnosed with CD after age 40 and is primarily present within the first year of diagnosis [37], which corresponds with the findings of Lebwohl et al. [30]. In this study, the overall risk was highest in individuals diagnosed with CD after the age of 60 years (HR, 1.22; 95% CI, 1.16–1.29). Such an observation regarding the risk of PC was not demonstrated in the available studies.
Furthermore, as CD often remains undiagnosed, studies have practically no chance of establishing a cohort covering all patients with CD. Most often, studies identify CD patients from the registries of hospital discharges. Therefore, they may include CD patients with more severe diseases or complications. On the other hand, the prevalence of CD among patients with cancer is unknown and, similar to in the general population, it can be underestimated [48]. It remains challenging to determine if cancer was developing in unrecognized CD or CD developed after cancer diagnosis or cancer therapy exposure.
Summarizing, the available data on the risk of PC in patients with CD are inconsistent and do not allow the derivation of a true correlation between CD and PC.
Limitations
Our study has several limitations. We searched two databases, Web of Science and Scopus and, although there is literature supporting the choice of these databases [49], this may be considered as a potential limitation. Furthermore, the search was limited to papers published since 1 January 2000 and, while this in our opinion was justified by the available literature demonstrating that there are no earlier papers on the risk of PC in CD patients, it may be interpreted as a limitation. Furthermore, the meta-analysis revealed a lack of homogeneity in the pooled data, which reduces confidence in the results. Therefore, the results should be interpreted with caution. Additionally, there are also limitations related to the analyzed studies demonstrated in the quality assessment. No study could prove the ascertainment of exposure. Two out of five studies showed no outcome of interest at the start of the study. Only one study showed the adequacy of a follow-up cohort. In one of the studies [32] reporting increased risk for PC, a wide CI for SIR of PC leaves considerable doubt about the true SIR and may indicate an unstable statistic. Furthermore, the majority were performed in northern Europe and, considering the geographic variation in the occurrence of both PC and CD, their results cannot be extrapolated to the global population. Studies incorporated into our review differ in methodology and studied populations (prospective and retrospective design, general population-based, hospital-based, different duration of observation). The other issue which has to be considered is the small number of PC cases in CD patients reported in three studies. There were two cases of PC in two studies [33,34] and nine cases in one study [32]. A small sample of participants with PC may cause difficulties in the interpretation of results. With three exceptions [30,32,35], comorbidities were not reported. Concomitant diseases such as diabetes, pancreatitis, obesity and non-alcoholic fatty liver disease may impact PC risk. Finally, a significant number of CD cases remain undiagnosed. Therefore the studies have no prospect of covering all CD patients as the number of patients with an established diagnosis is lower than the serological prevalence of the disease. Moreover, patients, while being diagnosed with cancer or with a diagnosis of cancer, may undergo biopsies that would reveal CD, thus inducing a bias.
5. Conclusions
The available data on the risk of PC in patients with CD remain inconsistent and do not allow the deduction of a real correlation between CD and PC. The meta-analysis performed may suggest an increased risk of PC in CD patients with malignancies. However, cautious interpretation of the results is warranted because of the lack of homogeneity of the pooled data. Yet a recent large cohort study suggested the increased risk of PC in CD patients. Further prospective studies, for instance, designed as international projects, are requested to clarify and better understand the complex and still unclear relationship between PC and CD.
Author Contributions
Conceptualization, I.G. and K.N.; methodology, I.G. and K.N.; writing—original draft preparation, I.G. and K.N.; writing—review and editing, I.G. and K.N.; visualization, I.G. and K.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Trynka G., Wijmenga C., van Heel D.A. A genetic perspective on coeliac disease. Trends Mol. Med. 2010;16:537–550. doi: 10.1016/j.molmed.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Parzanese I., Qehajaj D., Patrinicola F., Aralica M., Chiriva-Internati M., Stifter S., Elli L., Grizzi F. Celiac disease: From pathophysiology to treatment. World J. Gastrointest. Pathophysiol. 2017;8:27. doi: 10.4291/wjgp.v8.i2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King J.A., Jeong J., Underwood F.E., Quan J., Panaccione N., Windsor J.W., Coward S., deBruyn J., Ronksley P.E., Shaheen A.-A., et al. Incidence of Celiac Disease Is Increasing Over Time: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2020;115:507–525. doi: 10.14309/ajg.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 4.Catassi C., Verdu E.F., Bai J.C., Lionetti E. Coeliac disease. Lancet. 2022;399:2413–2426. doi: 10.1016/S0140-6736(22)00794-2. [DOI] [PubMed] [Google Scholar]
- 5.Singh P., Arora A., Strand T.A., Leffler D.A., Catassi C., Green P.H., Kelly C.P., Ahuja V., Makharia G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018;16:823–836.e2. doi: 10.1016/j.cgh.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Lebwohl B., Rubio-Tapia A. Epidemiology, Presentation, and Diagnosis of Celiac Disease. Gastroenterology. 2021;160:63–75. doi: 10.1053/j.gastro.2020.06.098. [DOI] [PubMed] [Google Scholar]
- 7.Pinto-Sanchez M.I., Silvester J.A., Lebwohl B., Leffler D.A., Anderson R.P., Therrien A., Kelly C.P., Verdu E.F. Society for the Study of Celiac Disease position statement on gaps and opportunities in coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 2021;18:875–884. doi: 10.1038/s41575-021-00511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raiteri A., Granito A., Giamperoli A., Catenaro T., Negrini G., Tovoli F. Current guidelines for the management of celiac disease: A systematic review with comparative analysis. World J. Gastroenterol. 2022;28:154–176. doi: 10.3748/wjg.v28.i1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kivelä L., Caminero A., Leffler D.A., Pinto-Sanchez M.I., Tye-Din J.A., Lindfors K. Current and emerging therapies for coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 2021;18:181–195. doi: 10.1038/s41575-020-00378-1. [DOI] [PubMed] [Google Scholar]
- 10.Catassi C. Risk of Non-Hodgkin Lymphoma in Celiac Disease. JAMA. 2002;287:1413. doi: 10.1001/jama.287.11.1413. [DOI] [PubMed] [Google Scholar]
- 11.Freeman H.J. Malignancy in adult celiac disease. World J. Gastroenterol. 2009;15:1581. doi: 10.3748/wjg.15.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marafini I., Monteleone G., Stolfi C. Association Between Celiac Disease and Cancer. Int. J. Mol. Sci. 2020;21:4155. doi: 10.3390/ijms21114155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. [(accessed on 4 January 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer.
- 14.UN. [(accessed on 4 January 2023)]. Available online: https://www.un.org/en/chronicle/article/losing-25000-hunger-every-day.
- 15.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 16.Mizrahi J.D., Surana R., Valle J.W., Shroff R.T. Pancreatic cancer. Lancet. 2020;395:2008–2020. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 17.Rawla P., Sunkara T., Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019;10:10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGuigan A., Kelly P., Turkington R.C., Jones C., Coleman H.G., McCain R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018;24:4846–4861. doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sulser T.B., Beach R.H., Wiebe K.D., Dunston S., Fukagawa N.K. Disability-adjusted life years due to chronic and hidden hunger under food system evolution with climate change and adaptation to 2050. Am. J. Clin. Nutr. 2021;114:550–563. doi: 10.1093/ajcn/nqab101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wudil A.H., Usman M., Rosak-Szyrocka J., Pilař L., Boye M. Reversing Years for Global Food Security: A Review of the Food Security Situation in Sub-Saharan Africa (SSA) Int. J. Environ. Res. Public Health. 2022;19:14836. doi: 10.3390/ijerph192214836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michaud D.S., Skinner H.G., Wu K., Hu F., Giovannucci E., Willett W.C., Colditz G.A., Fuchs C.S. Dietary Patterns and Pancreatic Cancer Risk in Men and Women. JNCI J. Natl. Cancer Inst. 2005;97:518–524. doi: 10.1093/jnci/dji094. [DOI] [PubMed] [Google Scholar]
- 22.Maisonneuve P., Lowenfels A.B. Risk factors for pancreatic cancer: A summary review of meta-analytical studies. Int. J. Epidemiol. 2015;44:186–198. doi: 10.1093/ije/dyu240. [DOI] [PubMed] [Google Scholar]
- 23.Vincent A., Herman J., Schulick R., Hruban R.H., Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He M., Lo C.-H., Wang K., Polychronidis G., Wang L., Zhong R., Knudsen M.D., Fang Z., Song M. Immune-Mediated Diseases Associated with Cancer Risks. JAMA Oncol. 2022;8:209. doi: 10.1001/jamaoncol.2021.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bascuñán K.A., Vespa M.C., Araya M. Celiac disease: Understanding the gluten-free diet. Eur. J. Nutr. 2017;56:449–459. doi: 10.1007/s00394-016-1238-5. [DOI] [PubMed] [Google Scholar]
- 26.Ciccone A., Gabrieli D., Cardinale R., Di Ruscio M., Vernia F., Stefanelli G., Necozione S., Melideo D., Viscido A., Frieri G., et al. Metabolic Alterations in Celiac Disease Occurring after Following a Gluten-Free Diet. Digestion. 2019;100:262–268. doi: 10.1159/000495749. [DOI] [PubMed] [Google Scholar]
- 27.Ludvigsson J.F., Elfström P., BroomÉ U., Ekbom A., Montgomery S.M. Celiac Disease and Risk of Liver Disease: A General Population-Based Study. Clin. Gastroenterol. Hepatol. 2007;5:63–69.e1. doi: 10.1016/j.cgh.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 28.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [(accessed on 26 May 2022)]. Available online: http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf.
- 30.Lebwohl B., Green P.H.R., Emilsson L., Mårild K., Söderling J., Roelstraete B., Ludvigsson J.F. Cancer Risk in 47,241 Individuals with Celiac Disease: A Nationwide Cohort Study. Clin. Gastroenterol. Hepatol. 2022;20:e111–e131. doi: 10.1016/j.cgh.2021.05.034. [DOI] [PubMed] [Google Scholar]
- 31.Elfström P., Granath F., Ye W., Ludvigsson J.F. Low Risk of Gastrointestinal Cancer Among Patients with Celiac Disease, Inflammation, or Latent Celiac Disease. Clin. Gastroenterol. Hepatol. 2012;10:30–36. doi: 10.1016/j.cgh.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 32.Askling J., Linet M., Gridley G., Halstensen T.S., Ekström K., Ekbom A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology. 2002;123:1428–1435. doi: 10.1053/gast.2002.36585. [DOI] [PubMed] [Google Scholar]
- 33.Card T.R., West J., Holmes G.K.T. Risk of malignancy in diagnosed coeliac disease: A 24-year prospective, population-based, cohort study: COELIAC DISEASE AND RISK OF CANCER. Aliment. Pharmacol. Ther. 2004;20:769–775. doi: 10.1111/j.1365-2036.2004.02177.x. [DOI] [PubMed] [Google Scholar]
- 34.Goldacre M.J., Wotton C.J., Yeates D., Seagroatt V., Jewell D. Cancer in patients with ulcerative colitis, Crohn′s disease and coeliac disease: Record linkage study. Eur. J. Gastroenterol. Hepatol. 2008;20:297–304. doi: 10.1097/MEG.0b013e3282f2a5e2. [DOI] [PubMed] [Google Scholar]
- 35.Landgren A.M., Landgren O., Gridley G., Dores G.M., Linet M.S., Morton L.M. Autoimmune disease and subsequent risk of developing alimentary tract cancers among 4.5 million US male veterans. Cancer. 2011;117:1163–1171. doi: 10.1002/cncr.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ilus T., Kaukinen K., Virta L.J., Pukkala E., Collin P. Incidence of Malignancies in Diagnosed Celiac Patients: A Population-based Estimate. Am. J. Gastroenterol. 2014;109:1471–1477. doi: 10.1038/ajg.2014.194. [DOI] [PubMed] [Google Scholar]
- 37.Koskinen I., Virta L.J., Huhtala H., Ilus T., Kaukinen K., Collin P. Overall and Cause-Specific Mortality in Adult Celiac Disease and Dermatitis Herpetiformis Diagnosed in the 21st Century. Am. J. Gastroenterol. 2020;115:1117–1124. doi: 10.14309/ajg.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 38.Grainge M.J., West J., Solaymani-Dodaran M., Card T.R., Logan R.F.A. The long-term risk of malignancy following a diagnosis of coeliac disease or dermatitis herpetiformis: A cohort study. Aliment. Pharmacol. Ther. 2012;35:730–739. doi: 10.1111/j.1365-2036.2012.04998.x. [DOI] [PubMed] [Google Scholar]
- 39.Green P.H.R., Fleischauer A.T., Bhagat G., Goyal R., Jabri B., Neugut A.I. Risk of malignancy in patients with celiac disease. Am. J. Med. 2003;115:191–195. doi: 10.1016/S0002-9343(03)00302-4. [DOI] [PubMed] [Google Scholar]
- 40.Lohi S., Maki M., Montonen J., Knekt P., Pukkala E., Reunanen A., Kaukinen K. Malignancies in cases with screening-identified evidence of coeliac disease: A long-term population-based cohort study. Gut. 2009;58:643–647. doi: 10.1136/gut.2007.140970. [DOI] [PubMed] [Google Scholar]
- 41.West J., Logan R.F.A., Smith C.J., Hubbard R.B., Card T.R. Malignancy and mortality in people with coeliac disease: Population based cohort study. BMJ. 2004;329:716–719. doi: 10.1136/bmj.38169.486701.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viljamaa M., Kaukinen K., Pukkala E., Hervonen K., Reunala T., Collin P. Malignancies and mortality in patients with coeliac disease and dermatitis herpetiformis: 30-year population-based study. Dig. Liver Dis. 2006;38:374–380. doi: 10.1016/j.dld.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Anderson L. Malignancy and mortality in a population-based cohort of patients with coeliac disease or ‘gluten sensitivity’. World J. Gastroenterol. 2007;13:146. doi: 10.3748/wjg.v13.i1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The Collaborating Centers of the Italian Registry of the Complications of Coeliac Disease. Silano M., Volta U., Mecchia A.M., Dessì M., Di Benedetto R., De Vincenzi M. Delayed diagnosis of coeliac disease increases cancer risk. BMC Gastroenterol. 2007;7:8. doi: 10.1186/1471-230X-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lebwohl B., Green P.H.R., Söderling J., Roelstraete B., Ludvigsson J.F. Association between Celiac Disease and Mortality Risk in a Swedish Population. JAMA. 2020;323:1277. doi: 10.1001/jama.2020.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gils T., Nijeboer P., Overbeek L.I., Hauptmann M., Castelijn D.A., Bouma G., Mulder C.J., Leeuwen F.E., Jong D. Risks for lymphoma and gastrointestinal carcinoma in patients with newly diagnosed adult-onset celiac disease: Consequences for follow-up: Celiac disease, lymphoma and GI carcinoma. United Eur. Gastroenterol. J. 2018;6:1485–1495. doi: 10.1177/2050640618800540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vilppula A., Kaukinen K., Luostarinen L., Krekelä I., Patrikainen H., Valve R., Mäki M., Collin P. Increasing prevalence and high incidence of celiac disease in elderly people: A population-based study. BMC Gastroenterol. 2009;9:49. doi: 10.1186/1471-230X-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dutra B.E.S., Wei D., Tan D., Alasadi M., Zhang H.C., Thomas A., Thomas A.S., Richards D.M., Wang Y. Clinical Characteristics and Outcomes of Patients with Positive Celiac Serology and Cancer Therapy Exposure. J. Cancer. 2022;13:815–822. doi: 10.7150/jca.63837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gusenbauer M., Haddaway N.R. Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google Scholar, PubMed, and 26 other resources. Res. Synth. Methods. 2020;11:181–217. doi: 10.1002/jrsm.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.