Abstract
Sequencing of the entire genome of Mycobacterium tuberculosis identified a novel multigene family composed of two closely related subfamilies designated PE and PE_PGRS. The major difference between these two families is the presence of a domain containing numerous Gly-Ala repeats extending to the C terminus of the PE_PGRS genes. We have used a representative PE_PGRS gene from M. tuberculosis, Rv1818c (1818PE_PGRS), and its amino-terminal PE region (1818PE), to investigate the immunological response to these proteins during experimental tuberculosis and following immunization with DNA constructs. During infection of mice with M. tuberculosis, a significant humoral immune response was observed against recombinant 1818PE_PGRS but not toward the 1818PE protein. Similarly, immunization with a 1818PE_PGRS DNA construct induced antibodies directed against 1818PE_PGRS but not against 1818PE proteins, and no humoral response was induced by 1818PE DNA. These results suggest that certain PE_PGRS genes are expressed during infection of the host with M. tuberculosis and that an antibody response is directed solely against the Gly-Ala-rich PGRS domain. Conversely, splenocytes from 1818PE-vaccinated mice but not mice immunized with 1818PE_PGRS secreted gamma interferon following in vitro restimulation and demonstrated protection in the mouse tuberculosis challenge model. These results suggest that the PE vaccine can elicit an effective cellular immune response and that immune recognition of the PE antigen is influenced by the Gly-Ala-rich PGRS domain.
The elucidation of the complete genome sequence of Mycobacterium tuberculosis (5) has provided critical information crucial to an understanding of the biology of M. tuberculosis and the pathogenesis of tuberculosis. The use of genomics, together with the newly developed microarray technology, should accelerate our understanding of the regulation of gene expression in M. tuberculosis and help identify new targets for prophylactic and therapeutic treatments (3). Genomic analysis has already provided a more comprehensive understanding of the metabolic pathways of these bacilli and, as a result a new approach to drug development has been postulated and is under investigation (2). One of the major challenges, however, will be to analyze the properties of proteins expressed by genes that are unique to the M. tuberculosis genome.
One interesting outcome of the M. tuberculosis genome sequencing was the discovery of the multigene family designated PE. These genes account for about 5% of the whole M. tuberculosis genome and consist of 38 homologous PE genes and 61 homologous PE_PGRS genes scattered throughout the genome (5, 27). The high degree of homology of the PE domain located at the N terminus of PE_PGRS genes with the 38 PE genes indicates that these genes are closely related. To date, homology with nonmycobacterial genes is restricted to similarities with glycine-rich proteins, including the EBNA-1 antigen of Epstein-Barr virus (EBV) (16, 17). Recent evidence suggests that the expression of two PE_PGRS genes by M. marinum is associated with replication in macrophages and persistence in infected frogs (24). Therefore, it is tempting to postulate that members of the PE multigene family play an important role in the virulence of tuberculosis and related diseases. It has also been suggested that multiple PE_PGRS genes could function as a source of antigenic variability for M. tuberculosis in order to evade the host immune response (4, 5). In addition, similarities between the PGRS region of the mycobacterial genes and the EBNA-1 antigen of EBV, suggests that the PE_PGRS proteins could have a role in inhibition of antigen presentation as postulated for EBNA-1 (16, 17).
We have recently found that a PE_PGRS protein with a sequence identical to the protein encoded by the M. tuberculosis gene Rv1818c is located on the surface of M. bovis BCG (M. J. Brennan, G. Delogu, Y. Chen, S. Bardarov, M. Alavi, and W. R. Jacobs, unpublished results). This protein is typical of members of the PE_PGRS family in that it is composed of 41% glycine and 20% alanine, the gene encodes a protein with 499 amino acids (the median size of the proteins encoded by the PE_PGRS family is approximately 550 amino acids), and its amino-terminal PE region shows a very high homology with members of the PE family (5). In the studies described here, the PE_PGRS gene Rv1818c was used as a prototype to construct recombinant PE and PE_PGRS proteins and their respective DNA vaccine vectors to compare the antigenic properties of a PE and a PE_PGRS protein.
MATERIALS AND METHODS
Microorganisms.
M. tuberculosis Erdman (TMC#107), M. tuberculosis strains H37Rv and H37Ra, and M. bovis BCG Pasteur (TMC#1011) were obtained from the Trudeau Mycobacterial Culture Collection, Saranac Lake, N.Y. Escherichia coli JM109 and Top 10 strains (Invitrogen, San Diego, Calif.) were used for cloning. For expression of histidine-tagged antigens, the E. coli BL21(DE3)pLysS strain (Invitrogen) was used for transformation with pET15b expression constructs. L-929 cells were a gift from Catherine Bosio, Center for Biologics Evaluation and Research, Food and Drug Administration (CBER, FDA).
Animals.
Specific-pathogen-free C57BL/6 female mice were obtained from Jackson Laboratories (Bar Harbor, Mame). The mice were 10 weeks of age at the time of aerogenic challenge and 8 weeks of age when immunizations were initiated. Mice were maintained under barrier conditions and fed commercial mouse chow and water ad libitum.
Molecular methods and recombinant protein purification.
The gene encoding Rv1818c was amplified using three different “forward” primers, each bearing a different restriction enzyme adapter (HindIII, XbaI, and NdeI as indicated with an X), in order to clone the fragment into different plasmids (primer 5′-ACXXXXXXATGTCATTTGTGGTCACGATC-3′). The oligonucleotide 5′-TAGCGAGGATCCCTACGGTAACCCGTTCATCCC-3′, bearing the BamHI site and the stop codon, was used as the “reverse primer.” The amino-terminal fragment containing the PE region of the protein was amplified using the forward primers used for Rv1818c, while 5′-ACGGATCCCTAGTTGCCGATCAAGATTCCGCCGTC-3′ (ending at position 423 in the nucleotide sequence) was used as the reverse primer. The genes were amplified from M. tuberculosis H37Rv DNA and cloned into pCRBlunt (Invitrogen, San Diego, Calif.). For DNA vaccine constructs, Rv1818c and its PE fragment were cloned into the vector pJW4303 (8) using HindIII and BamHI sites. The genes were also cloned into the pET15b expression vector (Novagen Inc., Madison, Wis.) fused to a histidine tag. Histidine-tagged proteins were expressed in E. coli and purified by nickel chromatography using the X-Press system (Invitrogen), as previously described (7). The histidine-tagged 1818PE_PGRS protein was purified under denaturating conditions, while 1818PE was purified using native conditions. Final preparations were dialyzed against 0.01 M Tris-buffered saline at pH 8.
Immunization with DNA vaccines and tuberculosis challenge studies.
Endotoxin-free plasmid DNA was prepared and purified using the Qiagen EndoFree Plasmid Maxi Kit (Qiagen, Chatsworth, Calif.) as previously described (8, 18). Groups of C57BL/6 mice were vaccinated intramuscularly in both hind limbs on days 1, 21, and 42 using 100 μg of plasmid DNA in a total volume of 0.1 ml. For challenge studies, mice were infected aerogenically with approximately 500 CFU of M. tuberculosis Erdman per mouse 5 weeks after the final immunization, using a Middlebrook chamber (GlasCol, Terre Haute, Ind.). The number of CFU per mouse organ were determined as described earlier (6). As controls for the efficacy studies, mice were vaccinated subcutaneously with 5 × 105 CFU BCG Pasteur on day 1. The lungs and spleens were aseptically removed at days 28, 63, and 112 days following challenge and homogenized separately in 5 ml of sterile 0.05% Tween 80-saline (PBST). The homogenates were diluted 10-fold in PBST, and 50-μl aliquots were plated on Middlebrook 7H11 agar (Difco, Detroit, Mich.) containing oleic acid-albumin-dextrose-catalase (OADC) enrichment medium (Becton Dickinson, Cockeysville, Md.), as well as 2 μg of thiophenecarboxylic acid hydrazide (Sigma) per ml for mice immunized with BCG (6). For histopathology, lung tissues were perfused immediately after sacrifice with 10% phosphate-buffered formalin. The fixed tissues were then embedded in paraffin, sectioned, and stained with hematoxylin and eosin or, for acid-fast bacilli, by using the Kinyoun method (Histopath of America, Millersville, Md.). Unpaired, two tailed, t test statistical analysis was performed to compare the CFU determinations among the groups of mice vaccinated with DNA or BCG.
Determination of the humoral immune response to mycobacterial antigens.
At various time points following aerogenic challenge with M. tuberculosis or after immunization with DNA vaccines, sera from infected mice were analyzed by enzyme-linked immunosorbent assay (ELISA). Immunlon-1 plates (Dynatech, Chantilly, Va.) were coated overnight at 4°C with 0.1 ml of purified recombinant antigen (5 μg/ml) in Coating Solution (KPL, Gaithersburg, Md.) and then blocked with bovine serum albumin (BSA) (Sigma Inc., St. Louis, Mo.). Sera from each infected mouse were applied in 0.1-ml volume at a 1:100 dilution in saline or, for the DNA vaccinated groups, serum samples from mice of the same group were pooled and applied in 0.1 ml of serial twofold dilutions, starting at a 1:25 dilution. Anti-mouse immunoglobulin G (IgG) whole-molecule alkaline phosphatase conjugate (Sigma) was used as the second antibody to measure total immunoglobulin IgG response. For color development, the pNPP phosphatase system was used according to the directions supplied by the manufacture (KPL, Gaithersburg, Md.) and the optical density at 405 nm (OD405) was read on a Microplate ELISA reader (BioTech Instruments). The endpoint was defined as the highest dilution of serum that gave a OD405 value greater than 0.050 and that was two-fold greater than that of the matched dilution of normal mouse sera.
For immunoblots, 4 to 20% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Laemmli (15), and proteins were transferred to nitrocellulose membranes as described by Harlow and Lane (12). The nitrocellulose blots were incubated with pooled mouse sera at a dilution of 1:500, and reactive bands were visualized using alkaline phosphatase-conjugated anti-mouse whole-molecule IgG (Sigma) and the NPP/BCIP System (Life Technology, Gaithersburg, Md.).
Cytokine assay.
At 30 days after the third immunization with DNA, splenocytes were obtained from vaccinated and control mice and restimulated in vitro with primed mouse bone marrow macrophages (BMMφ). Murine BMMφ were established as previously described (25) by flushing the femurs of C57BL6/J mice and then culturing the cells in Dulbecco Modified Eagle Medium media containing 10% fetal calf serum (Hy-Clone, Logan, Utah), 2 mM glutamine, 10 mM HEPES, 0.1 mM nonessential amino acids, 50 μg of gentamicin per ml, and 10% L-929 conditioned medium. BMMφ were infected with M. tuberculosis (Erdman strain) at a multiplicity of infection (MOI) of 5:1 or primed with 1 μg of purified protein derivative (PPD) per ml (28) 24 h prior to the incubation with splenocytes. Supernatants were collected 72 h later, and the amount of gamma interferon (IFN-γ) secreted was analyzed by a cytokine-specific ELISA using immunoglobulin specific for IFN-γ (Pharmingen, San Diego, Calif.) (8, 25).
RESULTS
A humoral immune response to a PE_PGRS protein is observed during experimental tuberculosis.
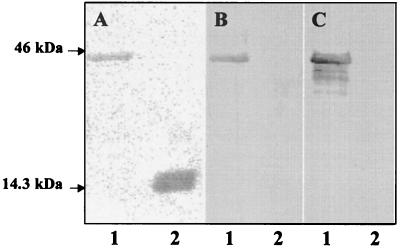
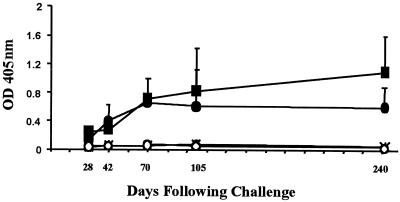
Since little is known about the expression and antigenic properties of proteins encoded by the PE and PE_PGRS genes of M. tuberculosis, the immune response to a representative PE_PGRS protein encoded by the M. tuberculosis gene Rv1818c was investigated. The M. tuberculosis gene Rv1818c was cloned, and a recombinant PE_PGRS protein was constructed and designated 1818PE_PGRS (Fig. 1). To compare the immunogenicity of a protein encoded by a PE gene (5), the PE domain of the Rv1818c gene was also cloned and designated 1818PE. Histidine-tagged recombinant proteins were purified from E. coli lysates using nickel-affinity chromatography and migrated in SDS-PAGE at the predicted molecular mass of approximately 43 kDa for 1818PE_PGRS and as a diffuse 17-kDa band for 1818PE (Fig. 2A). The purified recombinant proteins were used to monitor the production of a humoral immune response directed against the PE or PE_PGRS proteins following an aerosol infection of mice with the virulent Erdman strain of M. tuberculosis in order to determine if these proteins are expressed during infection. We observed a robust humoral immune response to purified recombinant 1818PE_PGRS by ELISA, using the sera pooled from five challenged mice, which continued over an eight-month period (Fig. 3). The antibody response elicited against the 1818PE_PGRS protein was similar to that observed using the well-characterized mycobacterial antigen Ag85b (29). In contrast, no humoral immune response was observed to purified recombinant 1818PE, which lacks the Gly-Ala-rich PGRS domain. In agreement with the ELISA results, the pooled sera obtained from the mice following M. tuberculosis challenge specifically recognized 1818PE_PGRS but not 1818PE protein in Western blots containing the purified recombinant antigens separated by SDS-PAGE (Fig. 2B). These results provide evidence that M. tuberculosis expresses at least some PE_PGRS proteins during infection of challenged animals and, since there was no response to the PE domain, that the Gly-Ala-rich PGRS domain likely represents the major target of the host humoral response to PE_PGRS proteins.
FIG. 1.
Schematic showing the domains of the Rv1818c gene from M. tuberculosis H37Rv used to clone the intact gene, 1818PE_PGRS, and its N-terminal PE region, 1818PE, in expression plasmids. In the sequence of Rv1818c, the amino acids found in the 1818PE construct are shown in italics, while the signature PE sequence and the 26 -GGAGG- repeats found in the PGRS domain are underlined.
FIG. 2.
Immunodetection of PE_PGRS protein using sera from infected mice and mice immunized with the 1818PE_PGRS DNA vaccine. Purified recombinant 1818PE_PGRS (lanes 1) and 1818PE (lanes 2) proteins were separated by 4 to 20% gradient SDS-PAGE and transferred to nitrocellulose. A gel was stained with Coomassie brilliant blue (A), or similar nitrocellulose blots were probed using pooled sera from M. tuberculosis-infected mice (B) or mice immunized with the 1818PE_PGRS DNA vaccine (C). Arrows show the position of the 14.3- and 46-kDa molecular mass standards.
FIG. 3.
Humoral immune response to 1818PE_PGRS and 1818PE proteins following infection of mice with M. tuberculosis. Mice (C57BL/6) were challenged aerogenically with 500 CFU of the virulent Erdman strain of M. tuberculosis, and sera were collected at different time points from five mice. The humoral response to 1818PE_PGRS (●) 1818PE (○), the mycobacterial antigen Ag85B (■), and the control protein BSA (∗) was evaluated by ELISA. Plates were coated with purified histidine-tagged recombinant proteins, incubated with each sera at a dilution of 1:100, and detected using anti-mouse IgG whole-molecule alkaline phosphatase conjugate as previously described. Datum points are the averaged readings (with the indicated standard deviations) from the individual sera from five infected mice.
Immune responses induced by PE and PE_PGRS DNA vaccines.
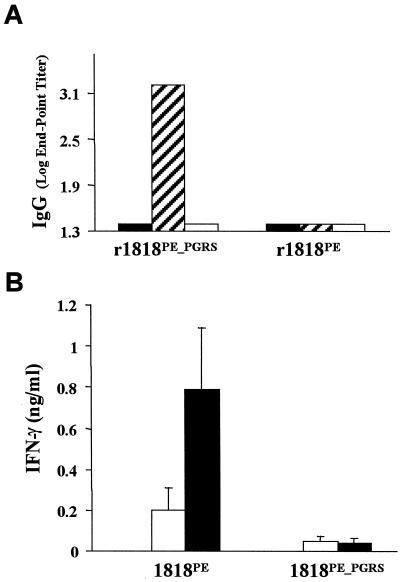
In order to investigate the immunological response elicited by specific PE and PE_PGRS immunogens, mice were immunized with DNA vaccine constructs encoding the 1818PE_PGRS and 1818PE proteins using an immunization schedule that has proven successful for investigating other DNA vaccines for tuberculosis (8, 18). In Western blots, pooled sera from five mice immunized with 1818PE_PGRS DNA reacted strongly with purified recombinant 1818PE_PGRS but not with purified 1818PE (Fig. 2C). This sera also recognized a number of bands in immunoblots containing cell extracts of M. tuberculosis, suggesting that M. tuberculosis may express certain PE-PGRS genes in culture (data not shown). In contrast, no reaction with either 1818PE_PGRS or 1818PE was observed when pooled sera from mice immunized with 1818PE DNA was used in immunoblotting (data not shown). In addition, as shown by ELISA (Fig. 4A), individual sera from mice vaccinated with 1818PE_PGRS DNA elicited a hardy humoral immune response against the 1818PE_PGRS protein but not the 1818PE protein. Conversely, mice immunized with 1818PE DNA did not produce a detectable humoral immune response to either 1818PE protein or 1818PE_PGRS protein. This is similar to what was observed in the infection studies (Fig. 3) and indicates that only the 1818PE_PGRS immunogen elicits a humoral response and that it is directed toward the PGRS domain which contains the Gly-Ala repeats.
FIG. 4.
Immunity to tuberculosis induced by DNA vaccines constructed from 1818PE_PGRS and 1818PE. Mice were immunized with endotoxin-free plasmid DNA purified as previously indicated (8). Groups of C57BL/6 mice were immunized intramuscularly in the hind limbs on days 1, 21, and 42 using 100 μg of plasmid DNA in a total volume of 0.1 ml. (A) The humoral response induced in mice vaccinated with vector only (solid bars), 1818PE_PGRS (hatched bars), or 1818PE (open bars) was analyzed by ELISA using sera from mice (five mice each) collected 30 days following the third DNA vaccination. Plates were coated with purified histidine-tagged recombinant proteins r1818PE_PGRS or r1818PE as indicated, and results are expressed as the IgG log10 endpoint titer. The dispersion around the mean value for the five mice, for each response was not significant. (B) Comparison of the IFN-γ response generated by immunization of mice with 1818cPE and 1818cPE_PGRS DNA vaccines and assessed by cytokine ELISA, following in vitro restimulation of splenocytes with activated macrophages. Murine BMMφ were infected with M. tuberculosis (solid bars) at an MOI of 5:1 or were primed with 1 μg of PPD (open bars) per ml 24 h prior to incubation with splenocytes obtained from vaccinated mice 30 days after the final immunization. Supernatants were collected 72 h later, and the amount of IFN-γ was measured using a cytokine-specific ELISA. The data represent the pooled response from five mice and show the standard deviation from the mean.
The cellular immune response generated by the DNA vaccines was evaluated in ex vivo studies, where splenocytes from the vaccinated mice were tested for their ability to secrete IFN-γ following in vitro restimulation (21, 22). In order to mimic the response to in vivo infection, bone marrow-derived macrophages were infected with live M. tuberculosis (25) and, for comparison, also primed with PPD (28). As shown in Fig. 4B, only splenocytes from 1818PE-vaccinated mice secreted IFN-γ following in vitro restimulation, while no IFN-γ response could be detected in cells from the 1818PE_PGRS group. These results suggest that mice immunized with 1818PE may develop a cellular immune response in the absence of a specific humoral response.
Efficacy of PE and PE_PGRS DNA vaccines in the mouse aerosol challenge model for tuberculosis.
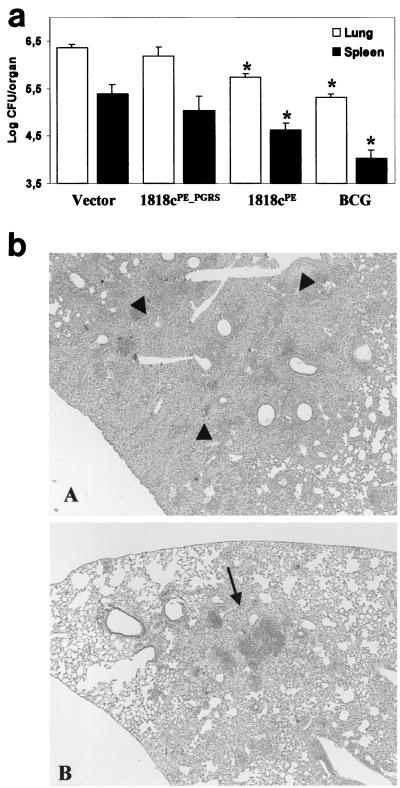
Noting the difference in the immunological response of the host to the PE and PE_PGRS immunogens, we investigated whether 1818PE_PGRS and 1818PE DNA constructs can protect mice against an aerogenic challenge with virulent M. tuberculosis. Mice were vaccinated with three doses of 100 μg of DNA per mouse as previously described (8). Thirty days after the third vaccination, mice were challenged aerogenically with the virulent Erdman strain of M. tuberculosis (6). The ability of a vaccine to induce a protective immune response was measured by the reduction in bacterial colonization of the lungs and spleens at different time points following challenge and compared with protection afforded by BCG. The numbers of viable counts in the lungs, as well as in the spleens, in mice immunized with 1818PE_PGRS, at 28 days after challenge, were not significantly different from those found in mice vaccinated with the DNA vector only (Fig. 5a). However, mice immunized with 1818PE DNA showed a statistically significant reduction in CFU in the lungs (0.61-log reduction) and in the spleens (0.77-log reduction) compared to immunization with the vector only. Vaccination with 1818PE DNA was not as efficacious as BCG vaccine, which resulted in a 1.04-log reduction in the lung and 1.37-log reduction in the spleen compared to immunization with the vector. However, the 1818cPE vaccine was as effective as some of the most promising DNA vaccine candidates, which commonly provide about 50% of the protective activity afforded by BCG (8, 13, 18, 26).
FIG. 5.
Efficacy of DNA vaccines encoding 1818PE_PGRS and 1818PE compared with vector only and BCG vaccine. (a) Thirty days following the third vaccination with DNA, C57BL/6 mice were aerogenically challenged with approximately 500 CFU of Erdman M. tuberculosis per mouse, and the numbers of viable bacilli in the lung (open bars) and in the spleen (solid bars) were determined at 28 days as described earlier (6). At least five mice per group were used, and the results from one of two experiments in which similar results were obtained are shown. The asterisks indicate groups of vaccinated mice that were significantly reduced in bacterial counts compared to the vector control using the t test (P < 0.05). (b) Histopathological examination of lung tissue from mice 112 days after challenge with M. tuberculosis, stained with hematoxylin and eosin. Lungs from mice vaccinated with 1818PE_PGRS prior to challenge contain a number of extensively consolidated granulomas (arrowheads) (A), while mice vaccinated with 1818PE exhibit a less severe pathology characterized by interstitial pneumonia (arrow) and mild alveolitis (B). Magnification, ×400.
The reduction in bacterial load in the lung tissues of 1818PE-vaccinated mice was also observed at 63 and 112 days (data not shown). At 112 days, the histopathological analysis of sections of lung tissue from mice vaccinated with the vector only or with 1818PE_PGRS demonstrated that these mice developed an extensive granulomatous pneumonia involving about 50 to 60% of the lung (Fig. 5b, panel A). The lung tissues were infiltrated with large numbers of epithelioid cells and histiocytes, resulting in a severe loss of alveolar space. Conversely, 1818PE-vaccinated mice were more like the BCG-immunized mice and exhibited significantly fewer areas of cellular infiltration (20 to 30%), with centrally located lymphocytes and smaller numbers of macrophages, with most of the alveolar spaces still intact or showing signs of mild alveolitis (Fig. 5b, panel B). Analysis of the AFB staining of these lung sections indicated that mice belonging to the 1818PE_PGRS group had significantly more bacilli in the lung tissue compared to the 1818PE group, in agreement with the colonization data (data not shown). These results indicate that mice vaccinated with the PE DNA vaccine develop an immune response that controls the bacterial growth and limits the tissue damage associated with M. tuberculosis infection.
DISCUSSION
In this study, we have focused on the immunological characterization of the PE_PGRS gene Rv1818c found in the genome of the M. tuberculosis strain H37Rv, as a representative member of the PE multigene family. The Rv1818c gene is typical of the PE_PGRS family in a number of ways. It is highly homologous with many of the average size PE_PGRS genes; as an example, Rv1818c shows about 60% identity with the PE_PGRS gene Rv1756c (1). Also, like many other PE_PGRS genes, Rv1818c encodes for a protein with a very high content of glycine and alanine (41% Gly and 20% Ala) found mostly as multiple (polymorphic) Gly-Ala-rich repeats (i.e., the PGRS domain) which extend to the C terminus (5). In addition, the N-terminal PE region of Rv1818c shows a very high degree of homology with those genes in the PE family, which encode only a PE polypeptide (5). For this reason, we used the PE sequence of Rv1818c to construct a recombinant PE protein (1818PE) and a PE DNA vaccine for comparison with the full-length PE_PGRS immunogen (1818PE_PGRS).
An important finding of this investigation is the observation that one or more of the PE_PGRS genes present in M. tuberculosis are expressed during infection of mice with the virulent Erdman strain of M. tuberculosis. In our studies, we found antibodies in sera shortly after aerosol infection of mice with M. tuberculosis that recognized recombinant 1818PE_PGRS by immunoblotting and ELISA. IgG reactive with 1818PE_PGRS was present in sera up through 8 months (the end of the test period). This result is similar to the immune response that we (see Fig. 3) and others have found using the protein of the Ag85 complex, Ag85b (29). Preliminary isotyping studies suggest that the majority of reactive IgG was of the IgG2a isotype, with no significant IgG1 response. There was no significant antibody response to the 1818PE protein as assayed by either Western blotting or ELISA. Although these studies do not prove that the Rv1818c gene itself is expressed in vivo, they indicate that certain PE_PGRS genes expressing the immunogenic PGRS domain are produced during M. tuberculosis infection and support two recent investigations. One study, carried out with M. marinum, demonstrated that two PE_PGRS genes are expressed within macrophages and in infected frog tissues (24). Also, a report by Espitia et al. has indicated that the fibronectin-binding PE_PGRS protein encoded by Rv1759c is expressed during tuberculosis infection (11). Moreover, in recent studies using transposon mutagenesis, we have evidence that the BCG homologue of Rv1818c is expressed and that the protein is localized to the cell surface (Brennan et al., unpublished).
To investigate the role of 1818PE_PGRS and 1818PE as effective immunogens, DNA constructs expressing the native forms of the PE_PGRS and PE proteins were evaluated for their ability to induce an immune response in mice as well as an effective immunity against M. tuberculosis challenge. In agreement with the M. tuberculosis infection studies, we observed that the 1818PE_PGRS DNA vaccine construct elicited a significant antibody response against recombinant 1818PE_PGRS protein but did not recognize the purified recombinant 1818PE protein. Also, no humoral immune response against either 1818PE_PGRS or 1818PE proteins was observed following vaccination with the 1818PE DNA construct. These results confirm that the major antibody response is directed toward epitopes located in the PGRS domain of the 1818PE_PGRS protein which contains the Gly-Ala repeats. It should be noted that the Gly-Ala-rich region of the Epstein-Barr virus protein EBNA1, a protein which shows significant homology with the PE_PGRS proteins (5), is the major target of the humoral immune response in the human host (9).
In contrast to the antibody response induced by 1818PE_PGRS, when splenocytes from mice immunized with the two DNA vaccines were tested for their ability to secrete IFN-γ, following in vitro restimulation with M. tuberculosis-infected BMMφ, only splenocytes from 1818PE-vaccinated mice secreted significant amounts of IFN-γ. This suggests that, like certain protective mycobacterial antigens such as Mtb39A (10). ESAT6 (20), and MPT64 (19), 1818PE elicits mainly a Th1-type immune response (21, 23). Moreover, we found that immunization with the 1818PE DNA vaccine resulted in significant protection in the mouse aerosol tuberculosis challenge model as shown by the reduction in bacterial colonization in the lungs as well as in the spleen. Reduced bacterial loads were evident in 1818PE-vaccinated mice up to 120 days following challenge, and histopathological examination of infected mouse lungs demonstrated that the 1818PE-vaccinated mice developed less tissue pathology compared to the controls. The ability of 1818PE-vaccinated mice to control infection with M. tuberculosis was similar to that observed for other tuberculosis DNA vaccines (10, 13, 14, 18, 26), although it was not equivalent to the BCG vaccine.
Our results indicate that a PE DNA vaccine, but not a PE_PGRS DNA construct, elicits a cellular immune response and induces an effective immunity against tuberculosis. This suggests that the presence of the Gly-Ala-rich PGRS region in the PE_PGRS construct influences the presentation of the PE region of the host's immune system and may prevent the development of an effective cellular immune response. It is interesting that the domain of the homologous EBNA-1 of EBV, which also contains repetitive Gly-Ala sequences, has been shown to inhibit EBNA-1 antigen processing and presentation through the major histocompatibility complex class I (MHC-I) pathway by interfering with proteosome-dependent antigen processing (16, 17). This raises the possibility that the Gly-Ala-rich PGRS domain, by a similar mechanism of immune interference, inhibits antigen presentation of the PE polypeptide through the MHC-I pathway. In fact, preliminary results from our laboratory indicate that 1818PE_PGRS is relatively resistant to proteasome-dependent intracellular degradation compared with other mycobacterial antigens, including 1818PE (G. Delogu and M. J. Brennan, unpublished results).
The presence of the numerous highly conserved family of PE and PE_PGRS genes found in the M. tuberculosis genome implies that these genes have been maintained under evolutionary pressure by the organism for some purpose. The reason for their existence remains an intriguing subject for scientific investigation. Our studies suggest that it will be important to determine if the PGRS domain containing the multiple Gly-Ala-rich repeats regulates the function and immunogenicity of the linked PE region. Also, it will be important to determine if M. tuberculosis expresses genes that encode only for PE polypeptides and to investigate the function and immunogenicity of these proteins. Our results suggest that the PE family of proteins may also be of interest for more practical applications, as immunological markers of infection or for the development of vaccines against tuberculosis.
ACKNOWLEDGMENTS
We thank the following colleagues at CBER, FDA: Yiping Chen for the gift of purified recombinant histidine-tagged 85b antigen, Amy Li for technical assistance on this project Frank Collins and Sheldon Morris for helpful advice on the study of vaccines using the tuberculosis mouse model, and Catherine Bosio for assistance with the histopathology studies and isolation of the murine BMMφ.
REFERENCES
- 1.Abou-Zeid C, Garbe T, Lathigra R, Wiker H G, Harboe M, Rook G A, Young D B. Genetic and immunological analysis of Mycobacterium tuberculosis fibronectin-binding proteins. Infect Immun. 1991;59:2712–2718. doi: 10.1128/iai.59.8.2712-2718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry C E, III, Slayden R A, Sampson A E, Lee R E. Use of genomics and combinatorial chemistry in the development of new antimycobacterial drugs. Biochem Pharmacol. 2000;59:221–231. doi: 10.1016/s0006-2952(99)00253-1. [DOI] [PubMed] [Google Scholar]
- 3.Behr M A, Wilson M A, Gill W P, Salamon H, Schoolnik G K, Rane S, Small P M. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–1523. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 4.Cole S T. Learning from the genome sequence of Mycobacterium tuberculosis H37Rv. FEBS Lett. 1999;452:7–10. doi: 10.1016/s0014-5793(99)00536-0. [DOI] [PubMed] [Google Scholar]
- 5.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Genties S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. . (Erratum, 396:190). [DOI] [PubMed] [Google Scholar]
- 6.Collins F M. Protection to mice afforded by BCG vaccines against an aerogenic challenge by three mycobacteria of decreasing virulence. Tubercle. 1985;66:267–276. doi: 10.1016/0041-3879(85)90064-9. [DOI] [PubMed] [Google Scholar]
- 7.Delogu G, Brennan M J. Functional domains present in the mycobacterial hemagglutinin, HBHA. J Bacteriol. 1999;181:7464–7469. doi: 10.1128/jb.181.24.7464-7469.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delogu G, Howard A, Collins F M, Morris S L. DNA vaccination against tuberculosis: expression of a ubiquitin-conjugated tuberculosis protein enhances antimycobacterial immunity. Infect Immun. 2000;68:3097–3102. doi: 10.1128/iai.68.6.3097-3102.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dillner J, Sternas L, Kallin B, Alexander H, Ehlin-Henriksson B, Jornvall H, Klein G, Lerner R. Antibodies against a synthetic peptide identify the Epstein-Barr virus-determined nuclear antigen. Proc Natl Acad Sci USA. 1984;81:4652–4656. doi: 10.1073/pnas.81.15.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dillon D C, Alderson M R, Day C H, Lewinsohn D M, Coler R, Bement T, Campos-Neto A, Skeiky Y A, Orme I M, Roberts A, Steen S, Dalemans W, Badaro R, Reed S G. Molecular characterization and human T-cell responses to a member of a novel Mycobacterium tuberculosis mtb39 gene family. Infect Immun. 1999;67:2941–2950. doi: 10.1128/iai.67.6.2941-2950.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espitia C, Laclette J P, Mondragon-Palomino M, Amador A, Campuzano J, Martens A, Singh M, Cicero R, Zhang Y, Moreno C. The PE-PGRS glycine-rich proteins of Mycobacterium tuberculosis: a new family of fibronectin-binding proteins? Microbiology. 1999;145:3487–3495. doi: 10.1099/00221287-145-12-3487. [DOI] [PubMed] [Google Scholar]
- 12.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 13.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 14.Kamath A T, Feng C G, Macdonald M, Briscoe H, Britton W J. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun. 1999;67:1702–1707. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Levitskaya J, Coram M, Levitsky V, Imreh S, Steigerwald-Mullen P M, Klein G, Kurilla M G, Masucci M G. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature. 1995;375:685–688. doi: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- 17.Levitskaya J, Sharipo A, Leonchiks A, Clechanover A, Masucci M G. Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein-Barr virus nuclear antigen 1. Proc Natl Acad Sci USA. 1997;94:12616–12621. doi: 10.1073/pnas.94.23.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Howard A, Kelley C, Delogu G, Collins F, Morris S. Immunogenicity of DNA vaccines expressing tuberculosis proteins fused to tissue plasminogen activator signal sequences. Infect Immun. 1999;67:4780–4786. doi: 10.1128/iai.67.9.4780-4786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oettinger T, Holm A, Mtoni I M, Andersen A B, Hasloov K. Mapping of the delayed-type hypersensitivity-inducing epitope of secreted protein MPT64 from Mycobacterium tuberculosis. Infect Immun. 1995;63:4613–4618. doi: 10.1128/iai.63.12.4613-4618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen A W, Hansen P R, Holm A, Andersen P. Efficient protection against Mycobacterium tuberculosis by vaccination with a single subdominant epitope from the ESAT-6 antigen. Eur J Immunol. 2000;30:1724–1732. doi: 10.1002/1521-4141(200006)30:6<1724::AID-IMMU1724>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 21.Orme I M, Andersen P, Boom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 22.Pais T F, Silva R A, Smedegaard B, Appelberg R, Andersen P. Analysis of T cells recruited during delayed-type hypersensitivity to purified protein derivative (PPD) versus challenge with tuberculosis infection. Immunology. 1998;95:69–75. doi: 10.1046/j.1365-2567.1998.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramachandra L, Chu R S, Askew D, Noss E H, Canaday D H, Potter N S, Johnsen A, Krieg A M, Nedrud J G, Boom W H, Harding C V. Phagocytic antigen processing and effects of microbial products on antigen processing and T-cell responses. Immunol Rev. 1999;168:217–239. doi: 10.1111/j.1600-065x.1999.tb01295.x. [DOI] [PubMed] [Google Scholar]
- 24.Ramakrishnan L, Federspiel N A, Falkow S. Granuloma-specific expression of mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science. 2000;288:1436–1439. doi: 10.1126/science.288.5470.1436. [DOI] [PubMed] [Google Scholar]
- 25.Rhoades E R, Orme I M. Susceptibility of a panel of virulent strains of Mycobacterium tuberculosis to reactive nitrogen intermediates. Infect Immun. 1997;65:1189–1195. doi: 10.1128/iai.65.4.1189-1195.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 27.Tekaia F, Gordon S V, Garnier T, Brosch R, Barrell B G, Cole S T. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tuberc Lung Dis. 1999;79:329–342. doi: 10.1054/tuld.1999.0220. [DOI] [PubMed] [Google Scholar]
- 28.Villarino M E, Brennan M J, Nolan C N, Catanzaro A, Lundergan L L, Bock N N, Jones C L, Wang Y-C, Burman W J. Comparison testing of current (PPD-S1) and proposed (PPD-S2) reference tuberculin standards. Am J Respir Crit Care Med. 2000;161:1167–1171. doi: 10.1164/ajrccm.161.4.9906050. [DOI] [PubMed] [Google Scholar]
- 29.Wiker H G, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]