Abstract
The extraction and commercialization of palm hearts is the most profitable activity involving the peach palm (Bactris gasipaes), while consumption of its fruits is limited to Amazonian communities. The excessive attention paid to the implementation of germplasm banks contributed to the lack of development of high-performance varieties, limiting the production and consumption of peach palm fruits and by-products. In addition, with the fragmentation of the Amazonian rainforest, wild populations are in danger of extinction. The species domestication, initiated by Native Amazonians, generated a large variety of peach palm populations, as evidenced by the diversity in fruit sizes and quality. Some advances in agronomic traits also took place. However, more research needs to be conducted to understand the implications of climatic changes on plant physiological performance. Indeed, the key point is that the exploitation of the full potential of B. gasipaes has not been completely exploited. Therefore, understanding the state-of-the-art research on the peach palm with a focus on its underutilized resources is essential for expanding plantations and, consequently, promoting the market expansion of the peach palm as a fruit crop.
Keywords: agronomic traits, biotechnological applications, chemical composition, crop fruit, genetic resources, germplasm banks, peach palm plantation
1. Introduction
Pupunha (Brazil), pejibaye (Costa Rica and Nicaragua), pijuayo (Peru), perapon (Guyana), chontaduro (Colombia and Ecuador), and peach palm (in English-speaking countries) are some of the common names of Bactris gasipaes Kunth, a caespitose palm considered to be “multi-purpose”. This is the reason for its great importance to Native Amazonian populations, who started its domestication process [1,2]. The species is naturally distributed between Bolivia, Brazil (North and West-Central), Colombia, Costa Rica, Ecuador, French Guiana, Honduras, Nicaragua, Panama, Peru, and Venezuela [1,3]. In addition, the peach palm has also been cultivated in Hawaii, Indonesia, Malaysia, and Réunion Island [4].
Modern populations of cultivated peach palms have high heterozygosity, resulting in a vast variety of fruit morphology and, therefore, in fruit quality. This phenotypic richness could contribute to obtaining genetically improved individuals, but it also implies obtaining various by-products with different qualities. However, even with its multiple potentials, the peach palm has been considered an economically underutilized species [1].
The excessive focus on market prospection directed R&D efforts to the implementation of germplasm banks, the prospection of an ideotype for palm heart production, and failed attempts at creating new niches in the market for fruit by-products [1]. High maintenance costs, susceptibility to diseases caused by high-density plantations, and other problems of an infrastructural and political nature led most of the projects involving germplasm banks to fail [1,5]. The lack of success in developing high-quality varieties restricts the expansion of peach palm production. Moreover, the seed selection of limited matrices contributes to genetic erosion [1,6]. In addition, the increasing fragmentation of the Amazon rainforest due to deforestation, illegal fires, livestock production, and agro-industrial expansion affect the wild populations of B. gasipaes, which also leads to genetic erosion. The loss of genetic variability may affect potential genetic breeding programs [6,7]. In contrast, recent advances in the establishment of in vitro culture protocols for the species may contribute to both the conservation of threatened genotypes and advances in the genetic transformation of the species.
To promote peach palm as a fruit crop, it is important to understand the current state of the species. Therefore, this review focuses on updating technical knowledge, existing limitations, agronomic aspects, and advances in the field of biotechnology with peach palms. We first describe the general aspects of peach palm biology, its regional use, and biotechnological applications, followed by an overview of agronomic practices and genetic resources of this species. Finally, we discuss how the current knowledge may be integrated, with the aim of identifying profitable activities involved in the peach palm market and discovering novel products with biotechnological applications.
2. Peach Palm Is a Domesticated Palm in Neotropics
Naturally distributed throughout northern South America and southern Central America, the peach palm is considered the only domesticated palm in the Neotropics [8,9]. The domestication of Bactris gasipaes was guided by centuries of cultivation by Native Americans due to its multiple uses. In addition to the fruits that were later diversified and adapted to each population’s traditions, wood was also used for manufacturing tools or as construction material [8,10].
Until the end of the 20th century, the peach palm was considered a cultigen [11]. Considering the cultivated populations, Mora-Urpí and Clement [12] proposed a classification based on fruit morphometric characteristics. The populations were classified into three groups: microcarpa, for fruits with a weight below 20 g; mesocarpa, for fruits between 20 and 70 g; and macrocarpa, for fruits with a weight above 70 g. Considering the suggestions of Mora-Urpí and Clement [12], as well as of Harlan and De Wet’s [13] gene pool approach, Henderson [3] proposed a revision of Bactris based on morphology, cytotaxonomy, and palynology. The peach palm was recognized as Bactris gasipaes with two varieties: var. chichagui (types one, two, and three) and var. gasipaes. The var. chichagui comprised wild populations with small and oilier fruits, and the var. gasipaes comprised domesticated populations with large and starchier fruits.
Molecular analyses involving peach palms began at the end of the 1990s. Clement et al. [14] used allozymes to investigate the relationship between three peach palm populations from San Carlos (Costa Rica), Yurimaguas (Peru), and Benjamin Constant (Brazil). High intraspecific heterozygosity was found, a result that was reinforced later with the use of RAPD markers [15], demonstrating the proximity between Benjamin Constant and Yurimaguas, Iquitos and Pará, and finally, San Carlos.
Rodrigues et al. [16] also investigated the genetic variability of seven morphologically distinct populations using RAPD markers. Contrasting results were found compared to those presented by Clement et al. [14] regarding heterozygosity between Benjamin Constant (Putumayo landrace), Yurimaguas (Pampa Hermosa landrace), and San Carlos (Guatuso landrace). In the same study, the formation of distinct evolutionary groups was visualized, reinforced posteriorly by Araújo et al. [17]. The group referring to Central America would be composed of representatives of Utilis (ex-Tuíra) and Guatuso landraces. The Western Amazon group would be composed of populations from Putumayo (former Solimões, including Benjamin Constant) and Pampa Hermosa landraces. Finally, the group referring to the Southeastern Amazon would be composed of Pará landrace and Acre populations (var. chichagui). These results support the hypothesis of landraces (locally improved, domesticated cultivars), which had been pointed out, until then, by studies based on the morphometric differences between populations [12,16]. In addition, it is argued that the morphometric and phylogenetic relationship of the landraces indicates at least two migratory routes for peach palms: one through the Eastern Amazon and the other through the Northwest, corroborating the idea of multiple domestication events.
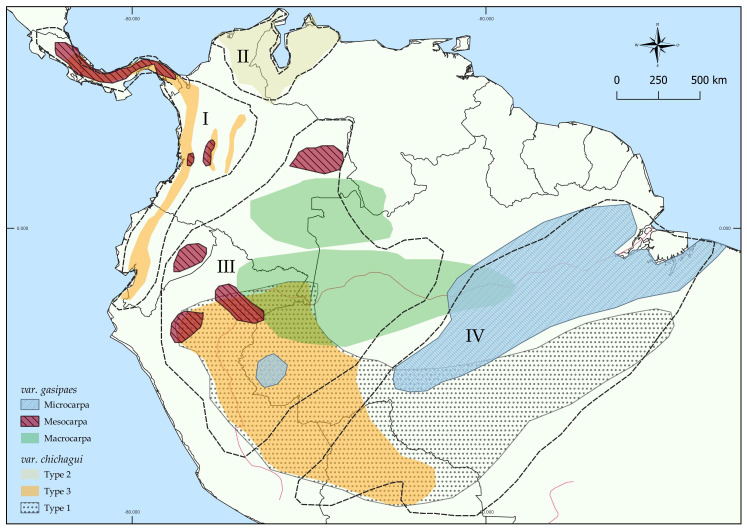
This hypothesis was contradicted by other studies using cpDNA and nDNA analyses through the recognition of an incipiently domesticated genotype: the var. chichagui type three [18,19]. Hernández-Ugalde et al. [20] supported the idea of at least three domestication sites, providing a basis for what was pointed out previously [16]. Galluzzi et al. [19] and Clement et al. [18] defended the idea of multiple domestication events, based on two major distributions: across southwest Amazonia to the west (and beyond) and the second across the Madeira River region, to eastern Amazonia (Figure 1). It was also argued that the distribution of peach palms was not only correlated with indigenous management but also with the geological events that occurred in the South American continent between the Miocene and the Pliocene [18,19,20]. In addition, it was hypothesized that there were wild and cultivated populations that had not yet been recognized [20].
Figure 1.
Distribution of B. gasipaes through Central and South America (adapted from Clement et al. [18] and Hernández-Ugalde et al. [20]). The dashed lines comprehend the four main complexes: I. Occidental group: composed of representants of var. chichagui (Upala, Azuero, Daríen, Chontila, and Chinamato) and var. gasipaes (Utilis landrace); II. Maracaibo group: comprehending B. caribaea and B. macana (outgroups); III. Upper Amazonia: composed of Caquetá, Putumayo (Yurimaguas population), Vaupés, Inirida, Ayacucho, Pastaza, Tigre, and Juruá landraces; IV. Eastern Amazonia: Pará and Tembé landraces, and var. chichagui Acre and Xingú.
A recent complete plastome characterization of var. gasipaes and var. chichagui demonstrated that the differences between these two varieties are also visualized at the molecular level [21,22]. With the advances of molecular tools and new findings regarding the evolutionary processes, further studies are still required to better understand the infra-specific relationships among the landraces, as well as the revision of B. gasipaes nomenclature [18].
2.1. General Morphology of Peach Palm
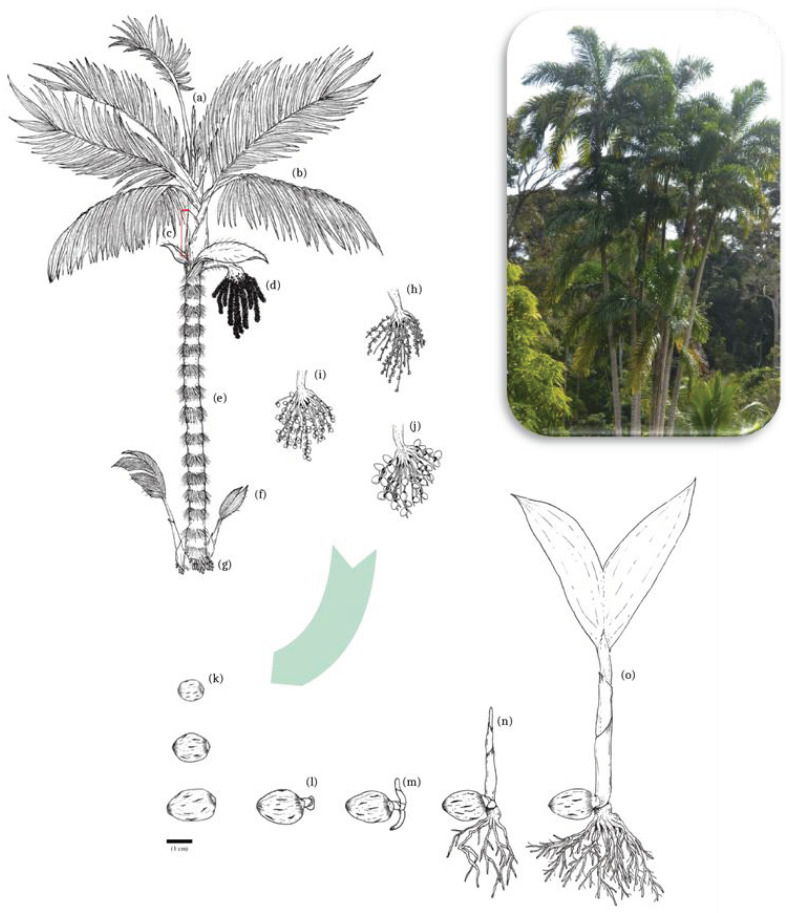
The peach palm has a slender trunk that can reach up to 25 m in height [3,23]. The trunk sustains a crown with up to 20 pinnate leaves that may vary from two to four meters in length. The monoecious inflorescences in the form of racemes occur in the axils of senescent leaves and comport small yellowish flowers. The staminate flower is deciduous, diclamide, and has six stamens. The pistillate flowers have an annular calyx and tubular corolla, and the presence of staminoids with a protogynous development (Figure 2) [3].
Figure 2.
General morphology of B. gasipaes and germination. On the right, a Peach palm near to an ombrophilous dense forest in Altamira, Para, Brazil, followed by a representation of an adult individual and different fruits sizes. The arrow indicates different seed sizes and the germination morphology, adapted from Silva et al. [24]. Legends: (a) apical gem; (b) pinnate leaves; (c) palm heart; (d) inflorescences; (e) internode with spines; (f) shoots; (g) roots; (h–j) different sizes of fruits; (k) seeds; (l) non-differentiated cell mass; (m) radicular and caulinar primordium; (n) eophyll elongation; (o) bifid primary leaf.
After maturation, the inflorescence becomes a bunch containing about 100 fruits, rarely up to 1000. The fruit can be starchy or oily and vary between 10 and 250 g in weight for var. gasipaes and between 0.5 and 5 g in weight for var. chichagui [3]. The fruits have either a rounded or ovoid form, and the fibrous epicarp may vary from a yellow ocher to a reddish color [3,8]. A natural segregant that produces white fruit is now also documented [25].
The seed has a lignified endocarp with a germination pore, an oily-fibrous, and homogeneous endosperm with a light color (almost white), a cotyledon, and a peripheric conic embryo [24,26]. Germination occurs between 38 and 133 days or up to 150 or more in non-ideal conditions [24,27]. The long time of germination is related to physiological dormancy, but this characteristic also presents a great variation in duration, not being well understood [28]. The germination is classified as cryptocotyledonar-hypogynous [24].
The peach palm can sprout shoots at the trunk base, characterizing it as a caespitose. According to Edelman and Richards [29], there are five types of vegetative branching in Arecaceae, with lateral axillary branching similar to that observed in the peach palm. The roots rise from the base of the trunk, creating a fasciculate system that penetrates two meters into the ground, and is distributed five meters around the plant, each containing absorbent hairs. It also forms adventitious roots on the base, forming a clump where the shoots aggregate, which may represent an adaptation to flooded environments [24,30,31].
Most peach palm genotypes present dark or yellowish-brown spines on the trunk internode, on the petiole and the leaves’ abaxial side, on inflorescence peduncles, and also on the shoots [3,8]. The occurrence of spines is a form of mechanical protection against plagues and herbivory but is also related to water caption and absorption [32]. However, the importance of spines for fruit and palm heart production was reviewed, and no direct correlation between the spines and higher yield was found [33].
2.2. Ecological Relationships
The peach palm is widely distributed in the Amazon Basin and has a hole in forest systems. A great diversity of mammals and birds (e.g., deers, agoutis, Penelope obscura or jacuaçu, toucans) consume the fruits in the bunch or that fall under the tree. These animals are also extremely important in aiding the seed dispersal of wild populations [23]. Trichomes are also consumed by some insects. The B. gasipaes trichomes are modified and present brachysclereids (sclerenchyma) [34]. They have a high percentage of lignin and some proteins, glucose, saccharose, palmitic, oleic, and linoleic fatty acids. This structure is consumed before pollen by Diptera, Coleoptera (mostly Curculionidae), Hymenoptera, Hemiptera, Neuroptera, Lepidoptera, Orthoptera, and Dictyoptera, and has a similar function to gastroliths [34,35].
It has been demonstrated that the peach palm can be colonized by different morphotypes of the Acaulospora, Glomus, and Scutellospora genera [36]. The arbuscular mycorrhizal fungi (AMF) develop internal and external mycelium with arbuscules on the root cortex and can also present some vesicles that are external to the roots [37]. They produce a glycoprotein known as glomalin that involves the glomospore and hyphae. This aids plant carbon absorption/fixation and contributes to natural soil fertilization and colloid formation, helping to provide resistance against pathogens and abiotic stress [36,37,38]. Besides the permanence of AMF on peach palm roots all year [36], the symbiotic relationship between AMF and B. gasipaes differs among the progenies, as observed by Clement and Habte [39]. In progenies of Pampa Hermosa, Putumayo, and Guatuso landraces, differences were observed in their dry matter accumulation when associated with AMF, e.g., 17%, 54%, and 64%, respectively. No correlation among more primitive landraces or dependency on AMF was observed, although there may be different physiological requirements among these landraces.
Chalita et al. [40] identified Pseudomonas putida, Burkholderia ambifaria, and Burkholderia sp. in agroforestry systems containing peach palms. The Pseudomonas genus is widely reported to be a solubilizing bacteria and is related to auxin (AIA) and the precursor of ethylene synthase (ACC deaminase) production. The Burkholderia genus is recognized for fixing N2 and can be associated with mycorrhizal fungi, as well as having the potential to biologically combat the oomycete Phytophthora palmivora. Moreover, Burkholderia is considered to be a plant growth-promoting rhizobacteria [41]. In addition, the aforementioned genera are considered biofilm formers, which have been of interest to the agroindustry due to their capacity to enhance soil fertility and crop production [42].
3. Agronomic and Physiological Aspects of Peach Palm
3.1. Practices in B. gasipaes Cultivation
The most effective way to propagate the peach palm is via seeds; however, this type of propagation has been one of the limiting factors for its agricultural expansion [43]. This is related to the lack of improved peach palm seed production and phytosanitary control, which can contribute to a reduction in the material quality, germination rates, and irregular yield [44,45].
After matrix selection, fruits should be harvested almost fully ripened to conserve the material against soil pathogens and increase germination rates. The pulp must be removed to prevent fermentation. After the manual removal of the tegument, the seeds must be soaked in water for one or two days, with a daily change of water. It is also recommended to undertake a cleaning step with commercial hypochlorite and water (100 mL NaClO to 1 L of water) for 15 min, followed by a wash in running water [46]. Fungicides can also be used for seed asepsis [43].
Peach palm seeds are recalcitrant and the embryo viability is compromised by water content loss, with no germination in water content below 17% [27]. High humidity associated with warmth can favor fungal contamination, therefore, sowing right after the cleaning step is recommended. The seeds can also be conserved for short periods with the manutention of water content (to maintain the embryo viability), temperature (between 15 and 18 °C), and packaging [43].
For sowing, nursery bags or beds are used with a substrate of light material (such as sand or sawdust). Mature manure can be incorporated into the substrate in a proportion of 1:1. The seeds must be sowed at a 2 cm depth in the substrate. The use of shade cloths is highly recommended to protect the seedling from direct sunlight. The germination occurs between 30 and 120 days in ideal conditions. Daily irrigation (or every 2 days depending on the type of soil and the season) is recommended [43,46].
Peach palm is known as a rustic plant; however, for better performance, soil preparation and fertilization are necessary. Before transplantation, ploughing and harrowing are essential for compacted soils and/or heavy structures (e.g., Ultisol). Fertilization for production is also commented on. For small-scale production, the use of plant residues (e.g., dead leaves and dropped fruits) or manure may be sufficient. For a larger scale, the use of chemical fertilizers (for N, P2O2, K2O, S e B, especially) must be initiated six months after transplant. Biofertilizer alternatives are currently available in Brazil’s market and may be a more sustainable way to increase production. In both scale categories, regular management of weeds is required [46,47].
The first fruit harvest starts in the third year after the transplant. The main difficulties related to bunch harvest are the height and presence of spines. When the plant has spines, the harvest can be performed with the aid of a mesh (to catch the bunch when it falls after cutting) and a long stick with a knife similar to a scythe (or a long scythe as well). The remotion of spines (or plants without spines) allows climbing and direct bunch harvest [48]. The harvesting of the palm hearts can be conducted when the first internode is visible (18th month) or when the trunk achieves 1.5 m [46,47].
Additionally, the use of shoots (asexual propagation) could help maintain the preferred characteristics of the parent plant i.e., fruit production, precocity, and absence of spines. Due to its caespitose characteristic, B. gasipaes can be propagated by separating the shoots from the mother plant. To propagate it asexually, it is more appropriate to use shoots that are between 30 and 60 cm. The presence of a primary root system could help with adaptation when removed from the clump. This method is divided into two phases, i.e., the field phase, consisting of the selection of the shoots and separation of the clump, and maintenance in the greenhouse for 30 days, and then transfer to nursery bags with the use of shade cloths in 50% of the sunlight [49]. However, this propagation method still has a lower success ratio when compared to the use of seeds. The actual protocols of peach palm macropropagation require methodological improvement, and the use of growth regulators must be better understood.
The peach palm is commonly associated with monocultural practices (for palm heart production), but due to forest multi-strata adaptation, it also has a good yield in agroforestry systems. Theobroma cacao, Theobroma grandiflorum, Manihot sp., and palms such as Euterpe spp. are some of the species that can be associated with peach palm [50,51]. The possibility of culture with other plants, such as Coffea canephora and Coffea arabica, peanut, rice, and sorghum, has also been studied. Some of these combinations with B. gasipaes showed an increase in physic-hydraulic quality, soil water content, and microporosity, which improves the product quality [52,53]. This activity is not only more profitable, but it can also be a way to recover degraded areas [54].
Peach palm culture has great potential for the expansion of fruit and palm heart production; however, efforts to better understand the management necessities are still needed. The selection of high-performance genotypes and the development of improved seeds through genetic transformation may be a way to maintain a large genetic base and improve fruit and palm heart yield. In the same way, asexual propagation is promising; however, it is necessary to investigate efficient ways of rooting, the physiological impacts of this type of propagation, and how much they interfere with productivity. In addition, agroforestry systems may represent a union between productivity and conservation. Although promising, it is still necessary to expand experiments with different crop combinations and compare the impacts on production and ecological services provided by these systems.
3.2. Abiotic Stress
As a tropical crop, the peach palm has a high-water demand. Water stress is more visible in subtropical cultures since there is expressive seasonal variation [55]. This type of stress induces stomatal closure and the dynamic photoinhibition of the secondary photosystem (PSII) as a protective mechanism against evapotranspiration water loss, indicating peach palm resistance to drought [55,56]. Aiming to investigate the response of peach palm plantlets to waterlogged soil, a study revealed that hypoxic conditions reduce N and K absorption, chlorophyll content, and stomatic conductance. In addition, they increase the content of soluble sugars in leaves and roots. Although the presence of the adventitious roots does not seem to promote long-term survival, it may help with increased tolerance to hypoxia conditions [30].
The major nutrient dependency is nitrogen, whose deficiency can cause chlorosis followed by necrosis in senescent leaf margins [57,58]. It is also associated with the low production of chlorophyll due to chloroplast modification and a reduction in the AMF symbiotic interaction [59]. The peach palm is also affected by potassium deficiency, which causes chlorosis and necrosis on the leaf blade. A lack of calcium causes the uneven development of leaves, associated with putrescine accumulation on the lesions. Other macronutrient deficiency symptoms can be listed, i.e., a reduction in plant growth (P), internerval chlorosis (Mg), or, on newer leaves, a substitution of green to a green-yellowish color (S) [57,58].
Micronutrient deficiency is also reported; Fe, B, and Zn omission promotes chlorotic leaves, the death of the terminal meristem and reduction in root proliferation, and narrow leaves with necrosis on the tip, respectively [57]. In addition, the omission of Na is related to chlorosis and necrosis in the leaf tips [58]. In another study, Fernandes et al. [60] analyzed the peach palm seedlings’ resistance to salinity. The best treatment was with Na at 1 mmol L−1 and Cl at 0.5 mmol L−1, which increased plant development. The worst treatment was with NaCl at 15 mmol L−1, indicating that peach palm is sensitive to saline soils (considering Abrol et al. [61] soil salinity classes).
Considered a rustic plant, the knowledge about peach palm performance in abiotic stresses must be expanded. In addition, it is necessary to investigate potential differences between genotype performance under abiotic stress, in which information could help the selection of stress-tolerant genotypes for seed improvement programs.
3.3. Pests and Diseases
The mite Retracrus johnstoni is known to promote chlorotic and necrotic spots which can affect peach palm productivity [62]. Peach palm is also affected by Coleoptera, e.g., Rhynchophorus palmarum, and Dynamis borassi [63,64]. In addition, D. borassi is a vector of the nematoid Bursaphelenchus cocophilus, which can represent a threat to coconut intercropped with peach palm [64]. The peach palm can also be attacked by caterpillars from Lepidoptera of the Noctuidae family [65]. The triatomine Leptoglossus conchoides is related to early fruit fall [66]. For these pests, better irrigation management to avoid the ideal conditions for pest establishment is appropriate, as well as the use of pesticides for species whose life cycle is known. In addition, the application of synthetic pheromones, such as rhynchophorol, is also recommended for the ethological control of D. borassi [67].
Fungal infection is the most common cause of a reduction in production and plant death in the peach palm. The seeds and fruits can be infected by common soil fungi, i.e., Aspergillus spp., Trichoderma spp., and Fusarium spp. reducing the germination rate [43]. Fruits are also affected by Thielaviopsis spp.: a promoter of black rot [68]. The infection by Colletotrichum gloeosporioides causes anthracnose in different parts of the plant. The leaves can be infected by Bipolaris bicolor and Curvularia spp., which cause leaf spot disease and leaf blight, respectively. Cercospora sp. and Alternaria sp. are also related to leaf spots. Moreover, Fusarium sp. is related to systemic yellowing, wilting, and death in plants [68,69,70]. In addition, infection by the bacteria Pantoea stewartii, associated with Fusarium sp., is also related to leaf necrosis. M. hemipterus and R. palmarum are reported as vectors of pathogenic bacteria [71,72].
There is a growing concern about the oomycete Phytophthora palmivora, which causes rot in the base of the stem, principally at low temperatures, to represents a notable risk to plantations in subtropical climates. This disease can cause the loss of more than 80% of the production of fruits and palm hearts. Its identification in the field is difficult, and there are no known ways to combat the disease [46,73].
In general, the management of humidity, water, and heat in order to reduce plantation density, along with the application of copper or boron solutions, are recommended for disease control. The use of insecticides or other biocides can be an alternative but may not assure the control of contamination and contribute to increasing microorganism resistance [70]. Good nutrition and association with symbiotic microorganisms could reinforce the peach palm’s resistance to these diseases and pests [37,38,40,70], however, more studies on the peach palm that focus on biological control are still needed.
4. Peach Palm Products: Diversity in Consumption, Chemical Composition and Biotechnological Application
Patiño [10] emphasizes that the use of peach palms by native Amerindians was encouraged by the properties of the wood and later by the fruit and other parts. The multipurpose uses of peach palms translate into the literal use of all parts of the plant [9]. For example, the dried leaves are used as the font of straw to manufacture materials such as baskets, mats, and body adornments; the palm heart is consumed in natura; the inflorescences, dried and macerated, can be used as condiment/flavoring; the wood is used to manufacture instruments, tools (such as knives, arrows, and bows), and as a construction material; and the roots are used as a large-spectrum vermicide, while other medicinal uses are also documented [2,10,74,75]. Besides its manufacturing application and use as a food source, the peach palm parts are also related to traditional folklore [2,10,75].
In Brazil, the peach palm is now mostly limited as a source of palm heart, being popularized mainly by the trade in brines and by the fruit that has its consumption limited to the regions where the species naturally occurs [1]. In this topic, some aspects of the peach palm market will be discussed, whose economic potential has not been fully exploited. Furthermore, the chemical composition of the fruits and their biotechnological applications will be described.
4.1. Palm Heart Is Present in International Market, but Fruits and By-Products Consumption Is Associated with Basal Market
In fact, the most profitable activity of peach palms is palm heart commercialization. The low activity of polyphenol oxidases and peroxidases confers the stability of the taste and color, allowing its commercialization in natura or in brines [47,76]. It is estimated that the international market for processed palm hearts (regardless of the species) is around US$ 500 million. Ecuador is the main exporter of canned palm hearts, totaling around US$ 65.6 million and 28.9 thousand tons in 2021, followed by Bolivia [77,78].
Over the last 15 years, Brazil has been losing space in the international market; in 2006, it was considered the third largest exporter, totaling around US$10 million and 1.7 thousand tons. However, in 2017 it was considered the eighth largest exporter, totaling US$1.47 million with exports of only 265.4 tons. Nowadays, Brazil still remains as the eighth largest exporter of processed palm hearts, totaling around US$1.77 million, exporting 284.6 tons in 2021 [79,80]. This has been justified by the lower quality of the processed palm heart, extractives’ practices, and high internal consumption [81].
In Brazil, palm heart comes from different species (Bactris gasipaes, Euterpe edulis, Euterpe oleracea, and the invasive palm Archontophoenix cunninghaniana) natural populations and exploitation of plantations [82]. According to the Brazilian Institute of Geography and Statistics (IBGE), in 2006, palm heart obtention was mostly carried out by extractive activity, amounting to about 53% of the total production [83,84]. In 2017, around 90.8% (approximately 93 thousand tons) came from plantations in the states of Santa Catarina, São Paulo, and Paraná [85,86]. However, the lack of objective information regarding the species used for palm heart production makes it difficult to understand the impact of peach palms on the national and international markets. Likewise, there is little information about the distinct types of commercialized palm hearts (in natura and canned) as well as national consumption patterns.
As commented previously, the fruit is limited to the domestic market. In 2006, gross production reached around 6.7 thousand tons, amounting to approximately US$3.13 million [83,84]. An increase in production between 2006 and 2017 was visualized, hovering around 10.3 thousand tons and adding up to US$4.12 million [83,84,85,86]. However, this scenario may not reflect the direct consumption of the population. It is estimated that almost 60% of fruit production is wasted and/or not consumed by the local population. This is associated with the fact that the fruit is a drupe, which hinders its storage and longevity/quality of the fruit. It is also estimated that the population prefers the fruit in natura rather than processed [1].
Besides direct consumption after cooking, fruit pulp can also be added to lunch dishes. In Peru and Bolivia, the use of starchier fruits is related to the creation of a fermented drink known as chicha. In Brazil, caiçuma fermented drink consumption is limited to ethnic communities [23,47]. The fruits can also be used as raw material for gluten-free flour production. Peach palm flour can be used in bakeries; however, human consumption is still not widespread. Indeed, the use of this by-product as a base for animal feed has also been studied, but its economic potential is still not well understood [1]. The peels also pursue potential as a font of fibers and metabolites, such as carotenoids [87]. The seeds also represent economic potential: an oily endosperm proves to be a rich source of vegetable oil. However, the removal of the endosperms can be laboring due to the hard and woody consistency of the seed’s exocarp [88,89]. In Brazil, the fruits and by-products did not achieve a place in supermarkets but instead were found in street markets though they are difficult to obtain in the offseason [1]. In addition, the use of other parts of peach palms is even more limited, and little is known about the current production demand and market for these by-products.
Clement et al. [1] discussed the creation of market niches for peach palm fruit by-products in Brazil; however, the engagement of R&D has failed in the popularization of these items in recent decades. Bearing in mind the potential of the fruit and by-products, as well as the expansion of palm heart cultivation, authors have, therefore, classified peach palms as an economically underutilized species [1,48,75].
From this perspective, an increasing interest in this species was observed in the last 10 years, resulting in a rise in the knowledge about its chemical properties and biotechnological applications. The technological investigation of this species may represent a change in perspective to the point where the species presents its economic potential.
4.2. Fruit Cheminal Composition
The peach palm fruit, in comparison to other Amazonian fruits, such as buriti (Mauritia flexuosa), can be considered a reliable source of energy (Table 1). Furthermore, peach palm fruits present relatively low quantities of macroelements, providing minor contributions to daily allowances [90]. A study using flame atomic absorption spectrometry [91] detected lower quantities of these minerals in peach palm fruits than reported in the literature [90,92,93], a factor that may be directly associated with the material quality of the investigated genotype. Regarding micronutrients, the presence of selenium and chromium adds to their nutritional value since these elements are associated with blood sugar regulation, cholesterol control, and the strengthening of the immune system [90,94].
Table 1.
Nutritional aspects of peach palm fruit pulp.
| Bactris gasipaes | Mauritia flexuosa | References | |
|---|---|---|---|
| Moisture (%) | 45–65.1 | 50.5–79.35 | [90,95,96,97] |
| Starch (%) | 27–59.5 | 7.28–36.3 | [89,95,98] |
| Protein (%) | 2.12–14.7 | 1.8–3.7 | [89,92,95,99] |
| Oils (%) | 5.57–27 | 11.20–19.0 | [89,95,96,99] |
| Total fiber (%) | 1.25–6.6 | 7.9–22.8 | [90,92,95,99] |
| Ashes (%) | 0.6–0.9 | 0.6–0.8 | |
| Energy (Kcal 100 g−1) | 351.4 | 189.6–1006 | |
| Minerals (100 g) | |||
| K (mg) | 206.4–289.3 | 183.55–919.6 | [90,93,97,100] |
| Ca (mg) | 10.2–24.7 | 35.4–132 | |
| Mg (mg) | 16.9–17.6 | 14.29–60.2 | |
| Na (mg) | 0.2–12.6 | 134.4 | [90,93,100] |
| Fe (mg) | 0.47–0.74 | 0.69–4 | [90,97,100] |
| Cu (mg) | - | 0.61 | [100] |
| Zn (mg) | 0.26–0.28 | 1.08 | [90,93,100] |
| Mn (mg) | 0.08–0.11 | 8.72 | |
| Cl (µg) | 7.6–30.7 | - | [90] |
| Cr (µg) | 8.2–13.9 | - | |
| Se (µg) | 3.3–11.4 | - | |
| Rb (µg) | 491.4–924.1 | - | |
| Br (µg) | 34.3–189.4 | - | |
| Ba (µg) | 103.9–164.5 | - | |
| Pa (µg) | 56.4–60.9 | - | |
| Ce (µg) | 1.3–2.1 | - | |
| La (ng) | 70.5–521.8 | - | |
| Sb (ng) | 31.0–99.0 | - | |
| Au (ng) | 30.3–57.8 | - | |
| Sc (ng) | 7–10.4 | - | |
| Fatty acids (%) | |||
| Palmitic (16:0) | 24.1–39.6 | 18.9 | [90,99] |
| Palmitoleic (16:1) | 5.2–7.4 | 0.3 | |
| Margaric (17:0) | - | - | |
| Stearic (18:0) | 0.8–1.7 | 1.3 | |
| Oleic (18:1) | 42.8–60.8 | 75.7 | |
| Linoleic (18:2) | 1.2–1.4 | 2.1 | |
| Linolenic (18:3) | 0–1.8 | - | |
| Arachidonic (20:0) | - | 1.7 | |
| Amino acids | |||
| Bactris gasipaes | FAO | ||
| Essential (mg g−1) | |||
| Histidine | 0.09 | 16 | [90,101,102] |
| Isoleucine | 0.16–1.70 | 13 | |
| Leucine | 0.28–3.14 | 19 | |
| Lysine | 0.21–1.67 | 16 | |
| Methionine | 0.08–0.8 | 17 a | |
| Phenylalanine | 0.14–2.04 | 19 b | |
| Threonine | 0.18–2.71 | 9 | |
| Valine | 0.19–2.83 | 13 | |
| Tryptophan | 0.45 | 5 | |
| Non-essential (ug g−1) | |||
| Alanine | 3.51 | - | [90,101] |
| Arginine | 0.29 | - | |
| Aspartate | 4.33 | - | |
| Serine | 2.72 | - | |
| Glutamate | 4.98 | - | |
| Glycine | 0.27–2.87 | - | |
| Tyrosine | 0.14 | - | |
| Proline | 2.57 | - | |
Associated with cysteine a or tyrosine b.
The peach palm fruit is considered a good source of fiber as it is mainly composed of pectic polysaccharides that create a linear, highly methyl-esterified homogalacturonan structure with minor portions of xylogalacturonan and type I rhamnogalacturonan [103]. Amylose represents 18.2% of total carbohydrates [104]. Yuyama et al. [90] commented that the protein value of peach palm fruit is low due to the total protein content and also to the reduced amounts of essential amino acids. Although it is not a robust source of these nutrients, it is possible to state that its consumption can be supplemental to other protein sources.
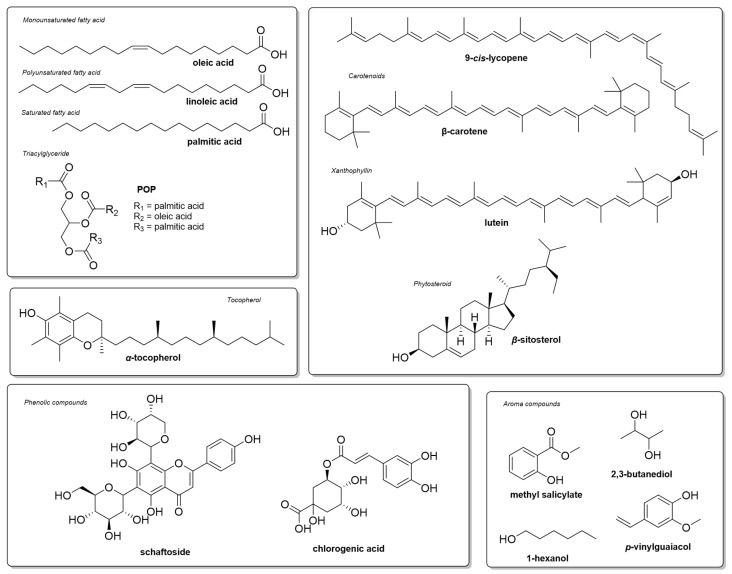
In addition, fruits have a high content of unsaturated fatty acids, especially oleic acid (Table 1). The consumption of long-chain unsaturated fatty acids is essential for physiological processes, whether through energy generation or maintenance at the cellular level. Moreover, in balanced amounts, their consumption plays a significant role in reducing the risk of developing vascular and neurodegenerative diseases [105]. The seed also presents itself as a major source of oil. The composition of the peach palm seed endosperm contains high quantities of lauric and oleic acids (33.3 and 24.3%, respectively) and minor percentages of sterols (Figure 3) [88,89].
Figure 3.
Chemical structures of the major metabolites described for the peach palm.
It should also be noted that the consumption of the fruit should be undertaken after cooking due to anti-nutritional factors [89,93]. Therefore, despite the low content of essential amino acids, peach palms can be considered a fruit of high nutritional value. This is mostly due to its energy supply, oil quality, essential and non-essential mineral content in the human diet, and high availability of vitamins, and thus its consumption is strongly encouraged.
Specialized Metabolites
The peach palm fruit can be considered a complex pool of bioactive compounds (Figure 3). Among the terpenoids that occur in the lipid fraction of peach palm fruit, carotenoids are found in greater abundance than xanthophylls. De Rosso and Mercadante [106] compared the carotenoid content of the peach palm mesocarp to other species and revealed the high availability of these compounds in peach palms, surpassing palm oil (Elaeis guineensis). However, it presents an inferior content of carotenoids when compared to buriti (Mauritia flexuosa) (Table 2).
Table 2.
Specialized compounds in peach palm pulp.
| Terpenoids (μg/g) | Bactris gasipaes | Mauritia flexuosa | References |
|---|---|---|---|
| cis-γ-Carotene 1 | 3.2 | - | [106] |
| cis-γ-Carotene 2 | 2.3 | 2.33 | |
| cis-γ-Carotene 3 | 2.1 | 9.88 | |
| cis-γ-Carotene 4 | 28.3 | - | |
| cis-γ-Carotene 5 | 0.13 | - | |
| cis-δ-Carotene 1 | 5.2 | 5.46 | |
| cis-δ-Carotene 2 | 2.1 | 3.67 | |
| cis-δ-Carotene 3 | 0.86 | 2.42 | |
| cis-β-Zeacarotene 1 | - | - | |
| cis-β-Zeacarotene 2 | - | - | |
| cis-Violaxanthin | - | - | |
| cis-Neoxanthin | - | - | |
| cis-Lutein | - | - | |
| 9-cis-Lycopene | 8.4 | - | |
| 9-cis-β-Carotene | 2.2 | 18.57 | |
| 13-cis-β-Carotene | 4.02 | 59.23 | |
| 15-cis-β-Carotene | 0.08 | 8.87 | |
| all-trans-α-Carotene | 1.8 | 3.23 | |
| all-trans-α-Cryptoxanthin | 0.12 | 1.28 | |
| all-trans-β-Carotene | 55.5 | 372.32 | |
| all-trans-β-Cryptoxanthin | - | - | |
| all-trans-β-Zeacarotene | - | - | |
| all-trans-δ-Carotene | 45.8 | 2.09 | |
| all-trans-γ-Carotene | 35.4 | 14.76 | |
| all-trans-ζ-Carotene | - | 0.08 | |
| all-trans-Neoxanthin | - | - | |
| all-trans-Zeaxanthin | - | - | |
| 5,6-epoxy-β-Carotene | - | 0.41 | |
| 5,6-epoxy-β-Cryptoxanthin | - | 0.1 | |
| 5,8-epoxy-β-carotene | 0.03 | 7.44 | |
| Phytoene | - | 0.34 | |
| Zeaxanthin | - | - | |
| all-trans-Lutein | - | 0.03 | |
| di-cis-α-Carotene | - | 1.25 | |
| Vitamins (100 g) | |||
| Thiamine (μg) | - | - | [92] |
| Riboflavin (μg) | - | - | |
| Niacin (mg) | 0.13 | - | |
| Ascorbic acid (mg) | 0.9–14 | 13 | [92,107] |
| α-Tocopherol (mg) | 11.7 | 110–197 | [108,109] |
| β + γ-Tocopherol (mg) | - | 476 | |
| δ-Tocopherol (mg) | - | 44.1 | |
| Phenolic compounds (μg g−1) | |||
| Apigenin | 0.002 | 102.48 | [110,111] |
| Caffeic acid | - | 895.53 | |
| Chlorogenic acid | 0.02 | 1154.15 | |
| Ferulic acid | 0,16 | 184.66 | |
| Kaempferol | - | 41.54 | |
| Luteolin | - | 1060.9 | |
| Myricetin | 0.02 | 145.11 | |
| Protocatechuic acid | 0.03 | 2175.93 | |
| p-Coumaric acid | 0.01 | 277.74 | |
| Quercetin | - | 83.27 | |
| Quinic acid (mg g−1) | - | 230.71 | |
| (+)-Catechin | - | 961.21 | |
| (−)-Epicatechin | - | 1109.93 | |
| Phytosterols (mg 100 g−1) | |||
| β-Sitosterol | 8.22 | 7.66 | [108] |
| Campesterol | 1.09 | 1.39 | |
| Stigmasterol | 0.42 | 0.81 | |
| Δ5-Avenasterol | 0.27 | 0.14 | |
The high quantity of carotenoids in the peach palm fruit adds to its nutritional value. The consumption of bioactive compounds, such as carotenoids, helps in the fight against degenerative diseases and in the maintenance of human health [112]. In this case, the presence of long-chain unsaturated fatty acids in the pulp also favors the delivery of lipophilic compounds to the body. Furthermore, the bioavailability of carotenoids in the peach palm does not appear to be affected by cooking; therefore, it is a safe and highly bioavailable source of these compounds [113]. Additionally, peels are considered the greatest font of fiber and carotenoids [87].
Chisté et al. [113] observed that orange fruits have a greater number of bioavailable carotenoids than yellow fruits. On the other hand, lutein was more available in the yellow variety, accounting for 14% of the total carotenoid content. These discrepancies may explain the variety of colors found in peach palm fruits. In addition, peach palm fruits are also considered a good source of sterols (Table 2), with β-sitosterol and campesterol in major quantities.
Regarding vitamins (fat and water soluble), peach palm fruit is seen as a good source of vitamins A, B, and C, in addition to the mesocarp being rich in α-tocopherol, similar to other species in the Amazon region [107,114,115]. Yuyama et al. [115] evaluated the bioavailability of vitamin A from the peach palm using the preventive method in rats. They found that the peach palm possesses a high bioavailability of this vitamin, with a relative efficiency of 250.8% when compared with the respective control groups (100%).
The identification of polyphenols in peach palms is recent, and several compounds belonging to different classes have been identified. Simple phenolic compounds, such as protocatechuic acid phenylpropanoids, were identified in the mesocarp [110]. Trace amounts of the flavonoids apigenin and myricetin, in addition to flavonoid di-C-glycosyl flavones, were identified in the cooked pulps of yellow and orange varieties. Among these, schaftoside (Figure 3) was the major compound in both, while vicenin-2 was detected at higher concentrations (21% of total phenolics) in the pulp of orange peach palm fruits, contrasting with the 6% found in yellow fruits. Other identified compounds were vitexin, isovitexin, isovitexin sulfate, vicenin-1, vicenin-3, isoschaftoside, and neoschaftoside [113]. Phenolic compounds act as antioxidants, and the long-term use of these compounds is encouraged for the prevention of cardiovascular and degenerative diseases [116].
The aroma compounds of the fresh fruit pulps were recently described by Faria et al. [110]. Different classes of compounds were detected, with esters (methyl salicylate) and alcohols (1-hexanol) as main components (Figure 3). Peach palm aroma composition has a contrasting profile when compared to other Amazonian fruits, which were dominated by terpenes, mainly mono- and sesquiterpenes. The consumption of this product is endorsed by its composition and high benefits to human health.
Despite the current knowledge regarding the chemical composition of fruit tissues, further efforts on metabolome analyses and the visualization of large-scale metabolite profile data should be performed and associated with metabolic pathways. This procedure, coupled with new tools for metabolite analyses, may provide useful information about the global biochemical regulation of fruit ripening. Understanding the biosynthetic pathways and their regulation is essential for the crucial biotechnological significance of the commercial production of specialized metabolites in fruit tissues that might be produced or explored in this to-date neglected peach palm fruit tissues.
4.3. Beside the Fruit, Peach Palm Agro-Industrial Residues Has Been Focus of Research
It is speculated that peach palms attracted the attention of Native Americans by the stem mechanical properties used for manufacturing house decorations, musical instruments, and tools as arrows and bows for hunting [2,10,74]. This is related to the high lignocellulosic material found in peach palm wood. The peach palm stem’s flexural property varies from the hardness of maçaranduba wood which has the flexibility [117]. Another property of peach palm wood is abrasion resistance, which allows the material to be manipulated without loss of quality, and resistance to water-based composts [118].
The increased interest in replacing synthetic fibers in favor of agro-industrial residue exploitation, aiming at cheaper raw materials with diverse applications, encourages the investigation of the peach palm’s potential as a supplier of natural fibers [75,119]. The haustorium residues can be used for crafting agglomerate panels for interior use. The best results in “finishing” and durability are seen when these fibers are combined with coir (Cocos nucifera) [120]. In addition, cellulose nanofibrils can also be used as the basis for biodegradable packaging or for stable emulsions, which can be applied in medicinal and cosmetic industries [121,122]. Agro-industrial waste can also be used as substrate metabolites prospection. Some examples are the association with Ganoderma lucidum for the production of a biosorbent, used to remove dyes present in wastewater from the textile industry [123], and as a substrate for xylanase production (for the food industry) when fermented using Trichoderma stromaticum [124], or for obtaining xylooligosaccharides with a higher antioxidant capacity [125]. These initiatives present a new perspective on this species’ exploitation and support the valorization of peach palm waste.
5. Genetic Resources: Conservation, Breeding and In Vitro Culture
5.1. Breeding and Implications for Genetic Erosion and Conservation
The peach palm gained international attention due to its potential as food insurance for a hungry world and as an economic resource, aspects which were highlighted in 1970 by Camacho and Soria [8,44]. Studies with B. gasipaes started in the same decade with the acquisition of foreign seeds, the first results of which were published in 1978 by the Agronomic Institute of Campinas (IAC), National Institute for Amazonian Research (INPA), and The Executive Commission for Cocoa Cultivation Planning (CEPLAC). The first main area of peach palm plantations (for extraction of palm hearts) was in association with the BONAL farm (Rio Branco, Acre) [44].
With the establishment of an ideotype for palm heart and fruit production, the modern genetic improvement of peach palm started in the 80s with the establishment of germplasm banks, focusing the hybridization and production of high-quality seeds. However, this model of selection is considered difficult since the peach palm is a perennial crop and the selected characters have low heritability [126]. In addition, due to institutional stagnation and germplasm banks high-costs manutention, over time, many breeding programs have been paralyzed or discontinued [1,127].
On the other hand, landrace populations that fit into the ideotype’s physical characteristics were found; the Pampa Hermosa landrace, from Yurimaguas (Peru), with between 60 and 80% of spineless plants; the Guatuso landrace from San Carlos (Costa Rica), with between 15 and 30% of spineless plants; the Putumayo genotype from Benjamin Constant (Brazil) with between 15 and 25% of spineless plants [44,126,127]. With the decrease in the natural populations of Euterpe spp. and lack of enhanced seeds, the producers sought the spineless seeds by themselves, introducing germplasm with no phytosanitary and quality controls [44].
Genetic manipulation implies two main factors: the conservation of germplasm and genetic erosion. Cornelius et al. [6] commented that the intensive selection of a breeding population and the use of seeds from limited germplasm are ways in which breeding programs could lead to inbreeding depression and a loss of genetic diversity. With the discovery of spineless populations, a good part of the plantations was established from these seeds. In addition to no care for genetic management, this activity puts the cultivated genotypes at risk of genetic erosion. Furthermore, wild populations are also suffering from genetic erosion due to the expansion of the Amazonian arc of fire. In some populations, it is no longer possible to guarantee the seed bank, which can lead to the extinction of the population [7,48].
High-density germplasm banks with wild and cultivated genotypes were established to support the early genetic programs. The atual active germplasm banks are Embrapa Acre; Embrapa Amapá, INPA and Embrapa Oriental (Brazil); Coorpica (Colombia); INIAP (Ecuador); INIA/ICRAF (Peru) and INIA (Venezuela) [128]. The success of genetic enhancement programs is associated with some factors; the complete characterization of germplasm banks, prospection of landraces, characterization of products chemical composition, adaptation of propagation methodologies, determination of physiologic parameters, and enlargement of phytosanitary research are some examples [126]. Today, most of remaining germplasm banks have not been fully characterized, and this creates a huge delay in prospecting selected progenies in comparison to the date of creation in these programs. Even with the selection of some high-quality progenies for both fruit and palm heart, the production of improved seeds continues to be small [48].
Germplasm banks play an important role in germplasm conservation for the genetic transformation of food crops [129]. However, associating different germplasms with on farm or in situ conservation seems to be an alternative to the coexistence of conservation and genetic improvement and for a reduction in maintenance costs [6,130]. A small in situ conservation project was successfully established at the Brazilian Agricultural Research Corporation (EMBRAPA/Acre) and associated with the RECA project in 1997, which is still responsible for agroforestry systems that focus on the recuperation of degraded areas, the conservation of biodiversity, and sustainable development of local communities. The peach palm germplasm conserved on RECA’s land is from Benjamin Constant spineless plants, and the matrix selection is based on this population with attention to spineless seeds and the commerce of the palm hearts [126]. Additionally, the World Agroforestry Centre (ICRAF) and the Peruvian National Institute for Agricultural Research and Extension (INIEA) developed a participatory improvement program in Peru (1997). With a focus on agroforestry systems and smallholders’ necessities, fruit and palm heart production, and the development of local markets were stimulated [6].
In this context, with previous efforts in germplasm banks establishment, Clement et al. [1] endorsed the idea that the expansion of peach palm germplasm banks in the tropics is not necessary. Working with genotypes of the current germplasm banks and with the molecular analyses of wild varieties, whether associated or not with agroecological systems seems, this seemed a more practical and sure way of the genetic enhancement of peach palms.
5.2. In Vitro Culture as a Perspective for Advances in Breeding
Different protocols for the in vitro propagation of peach palms have already been developed. In vitro cultures consist of the multiplication of cells, tissues, and organs in a controlled environment to obtain pathogen-free plants and assist in genetic enhancement [131]. Morphogenesis occurred through organogenesis, which is considered to be a natural regenerative strategy of plants and relies on the pluripotent acquisition of somatic cells, thus regenerating the organ or the plant (de novo organogenesis) [132], or by somatic embryogenesis (SE), which consists of the recovery of the cells’ totipotency through dedifferentiation, without a direct vascular connection with the explant [133,134]. Both morphogenic pathways can occur in the direct or indirect (through callogenesis) regeneration induced by growth regulators.
Indirect organogenesis was first documented by Arias and Huete [135], who pointed out the stimulatory effect of 2,4-dichlorophenoxyacetic acid (auxin analog) in callus formation. The same growth regulator was used by Stein and Stephens [136] for SE induction. Valverde et al. [137] demonstrated the influence of picloram (also an auxin analog) in callogenesis and SE induction. Almeida and Kerbauy [138] documented the direct organogenesis with the use of flower buds. However, these protocols often had random results when replicated and low rates of plantlet conversion or regeneration. A high level of explant oxidation was also observed, demonstrating the need for protocol optimization.
Steinmacher et al. [139,140,141] developed protocols for SE induction through zygotic embryo cultures, immature inflorescences, and the use of techniques known as a thin cell layer (TCL) [142]. This consists of using small slices of meristematic tissue from basal, medial, and apical plantlet meristem. These results reaffirmed the positive interaction between picloram and different peach palm explants for SE induction and also validated the applicability of indirect SE for this plant.
Steinmacher et al. [4] mentioned that the use of the temporary immersion system and cyclic cultures could shorten and scale in vitro multiplication. The use of the RITA system helps to enhance the embryogenic capacity, protein, and starch content, lower the DNA global methylation rate, and lower alcohol dehydrogenase activity [143]. Padilha et al. [144] also applied a temporary immersion system (TIS) to promote plantlet rooting, creating a successfully improved protocol for plantlet conversion.
In a recent study, Campos-Boza et al. [145] applied protocols using shoots while also testing different concentrations of picloram and different positions of TCL meristems in the culture medium. The study concluded that there were no significant differences in the picloram concentrations and meristem position in embryonic competence acquisition, contrasting observations by Steinmacher et al. [141]. These results may be related to the phenological phase of the tissue. Other experiments used plant material from a juvenile or immature phase (seedlings, immature inflorescences, zygotic embryos), unlike the shoots used by Campos-Boza et al. [145]. The material provided from shoots showed less contamination and browning in comparison with cryopreserved zygotic embryos and TCLs from seedlings. The induction phase was precocious (<70 days), and the conversion phase generated 12 plantlets with additional lateral shoots and roots, with a rate of 60% successful conversion. Although the plantlet yield was low, this study attested shoot viability as vegetative material for adult peach palm propagation.
Therefore, it is possible to state that the in vitro cultivation of peach palms, aiming at the regeneration of seedlings by somatic embryogenesis, is possible. It is important to point out that further studies should seek to reduce the level of contamination of explants, as well as to increase the number of embryogenic calluses and the conversion rate, in order to create more effective protocols. In addition, the application of immersion systems is seen as an alternative due to the increase in seedling production in less time. Finally, advances in the in vitro cultivation of this species open space for the genetic improvement of the species, as well as being an alternative for the conservation of threatened genotypes.
6. Conclusions and Future Directions
Centuries of domestication have provided humanity with an immense variety of peach palm genotypes whose economic and production potential has not yet been fully exploited. In addition to the relative knowledge of peach palm cultivation traits, the lack of enhanced seeds and inefficiency of macropropagation configure a barrier to peach palm cultivation expansion.
Still, the most profitable activities are palm heart and fruit commercialization. Peach palm fruits present themselves as nutritious food, with a good quantity of specialized metabolites that benefit human health but are also desired by the pharmaceutical and food industries. In addition to direct consumption, the fruit can also provide by-products of high nutritional value. Finally, not only does the fruit have economic potential, but also other possibilities, such as agro-industrial residues, which are considered a cheap source of natural fibers. The manufacturing of fiber panels, the obtention of cellulose nanofibrils for biofilms reinforcement, and the use of lignocellulose as a substrate for the prospection of prebiotics and other molecules are good examples of peach palm agro-industrial waste biotechnological applications.
The lack of complete molecular characterization of germplasm banks associated with the inexistence of an efficient in vitro protocol was pointed out as the main limitation in peach palm genetic transformation. These barriers have been surpassed in the last few years, providing a clear view of the landraces diversity that is available for conservation and genetic enhancement. However, genomic information and transcriptome analyses are still limited to peach palm fruits. The production of such a large amount of nucleotide sequence data using next-generation technologies is fundamental for biotechnological applications of distinct aspects of B. gasipaes. Thus, with the recent plastome sequencing of both varieties of B. gasipaes, advances in the establishment of efficient in vitro cultivation protocol, and better recognition of the plant’s exploitable biological aspects, means that doors for genetic engineering with peach palms are opening.
In summary, here we present an illustration of the combined use of the current knowledge (technical-scientific) about the species and genetic tools coupled with adaptive fitness-related quantitative markers to enhance our understanding of underutilized tropical species in the framework of a biotechnological program involving Bactris gasipaes. Further studies investigating whether epigenomic and phenotypic variations are present in tropical species may explain their responses to environmental stress conditions. Indeed, B. gasipaes presents itself as a useful non-model species for understanding the biotechnological significance (or lack thereof) of neglected and underutilized plants with virtually no genetic improvement in a changing climate.
Acknowledgments
The authors are grateful to the National Institute for Amazonian Research (MCTI-INPA), the Amazonas State Research Support Foundation (FAPEAM), the Coordination for the Improvement of Higher Education Personnel (CAPES—Brazil), and the National Council for Scientific and Technological Development (CNPq, Brazil) for their financial support of the research. Gonçalves JF de C, Clement C.R., Koolen, HHF; Araújo, WL, and Nunes-Nesi, A are CNPq—PQ scientific research fellows.
Author Contributions
Conceptualization: Y.V.K., C.R.C. and J.F.d.C.G. All authors contributed to the writing, review, and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Amazonas State Research Support Foundation (FAPEAM, Brazil) grand POSGRAD 2021/2022, the National Council for Scientific and Technological Development (CNPq, Brazil, No 308373/2019-7), project Pro-Amazonia (grant No 52/2012), and the Coordination for the Improvement of Higher-Level Personnel (CAPES, Brazil—finance code 001).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Clement C.R., Weber J.C., van Leeuwen J., Astorga Domian C., Cole D.M., Arévalo Lopez L.A., Argüello H. Why Extensive Research and Development Did Not Promote Use of Peach Palm Fruit in Latin America. Agrofor. Syst. 2004;61–62:195–206. doi: 10.1023/B:AGFO.0000028999.84655.17. [DOI] [Google Scholar]
- 2.Patiño V.M. An Ethnobotanical Sketch of the Palm Bactris (Guilielma) Gasipaes. Principes. 1992;35:143–147. [Google Scholar]
- 3.Henderson A. Bactris (Palmae) Flora Neotrop. 2000;79:181. [Google Scholar]
- 4.Steinmacher D.A., Guerra M.P., Saare-Surminski K., Lieberei R. A Temporary Immersion System Improves in Vitro Regeneration of Peach Palm through Secondary Somatic Embryogenesis. Ann. Bot. 2011;108:1463–1475. doi: 10.1093/aob/mcr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Leeuwen J., Pérez L., Clement C.R. Field Genebanks May Impede Instead of Promote Crop Development: Lessons of Failed Genebanks of “Promising” Brazilian Palms. Agrociencia. 2005;9:61–66. [Google Scholar]
- 6.Cornelius J.P., Clement C.R., Weber J.C., Sotelo-Montes C., van Leeuwen J., Ugarte-Guerra L.J., Ricse-Tembladera A., Arévalo-López L. The Trade off between Genetic Gain and Conservation in a Participatory Improvement Programme: The Case of Peach Palm (Bactris gasipaes Kunth) For. Trees Livelihoods. 2006;16:17–34. doi: 10.1080/14728028.2006.9752543. [DOI] [Google Scholar]
- 7.Clement C.R., Santos R.P., Desmouliere S.J.M., Ferreira E.J.L., Neto J.T.F. Ecological Adaptation of Wild Peach Palm, Its In Situ Conservation and Deforestation-Mediated Extinction in Southern Brazilian Amazonia. PLoS ONE. 2009;4:e4564. doi: 10.1371/journal.pone.0004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clement C.R. Domestication of the Pejibaye Palm (Bactris gasipaes): Past and Present. Adv. Econ. Bot. 1988;6:155–174. [Google Scholar]
- 9.Couvreur T.L.P., Hahn W.J., de Granville J.-J., Pham J.-L., Ludeña B., Pintaud J.-C. Phylogenetic Relationships of the Cultivated Neotropical Palm Bactris gasipaes (Arecaceae) with Its Wild Relatives Inferred from Chloroplast and Nuclear DNA Polymorphisms. Syst. Bot. 2007;32:519–530. doi: 10.1600/036364407782250526. [DOI] [Google Scholar]
- 10.Patiño V.M. Datos Etnobotánicos Adicionales Sobre El Cachipay o Pijibay (Bactris gasipaes Kunth), Arecaceae, y Especies Afines En América Intertropical. Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 1995;19:661–671. [Google Scholar]
- 11.Silva J.B.F., Clement C.R. Wild Pejibaye (Bactris gasipaes Kunth var. chichagui) in Southeastern Amazonia. Acta Bot. Bras. 2005;19:281–284. doi: 10.1590/S0102-33062005000200010. [DOI] [Google Scholar]
- 12.Mora-Urpí J., Clement C.R. Races and Populations of Peach Palm Found in the Amazon Bacin. In: Clement C.R., Coradin L., editors. Final Report (revised): Peach Palm (Bactris gasipaes H.B.K.) Germplasm Bank. Instituto Nacional de Pesquisas da Amazônia/Centro Nacional de Recursos Genéticos; Manaus, Brasil: 1988. pp. 78–94. [Google Scholar]
- 13.Harlan J.R., Wet J.M.J. Toward a Rational Classification of Cultivated Plants. Taxon. 1971;20:509–517. doi: 10.2307/1218252. [DOI] [Google Scholar]
- 14.Clement C.R., Aradhya M.K., Manshardt R.M. Allozyme Variation in Spineless Pejibaye (Bactris gasipaes Palmae) Econ. Bot. 1997;51:149–157. doi: 10.1007/BF02893108. [DOI] [Google Scholar]
- 15.Sawazaki H.E., Bovi M.L.A., Sodek L., Colombo C.A. Diversidade Genética em Palmeiras Através de Isoenzimas e RAPD. Rev. Bras. Biol. 1998;58:681–691. doi: 10.1590/S0034-71081998000400016. [DOI] [Google Scholar]
- 16.Rodrigues D.P., Astolfi Filho S., Clement C.R. Molecular Marker-Mediated Validation of Morphologically Defined Landraces of Pejibaye (Bactris gasipaes) and Their Phylogenetic Relationships. Genet. Resour. Crop Evol. 2005;51:871–882. doi: 10.1007/s10722-005-0774-2. [DOI] [Google Scholar]
- 17.Araújo M.C., Rodrigues D.P., Astolfi Filho S., Clement C.R. Genetic Variability in the Peach Palm Genebank with RAPD Markers. Crop Breed. Appl. Biotechnol. 2010;10:211–217. doi: 10.1590/S1984-70332010000300005. [DOI] [Google Scholar]
- 18.Clement C.R., de Cristo-Araújo M., Coppens d’Eeckenbrugge G., dos Reis V.M., Lehnebach R., Picanço-Rodrigues D. Origin and Dispersal of Domesticated Peach Palm. Front. Ecol. Evol. 2017;5:148. doi: 10.3389/fevo.2017.00148. [DOI] [Google Scholar]
- 19.Galluzzi G., Dufour D., Thomas E., van Zonneveld M., Escobar Salamanca A.F., Giraldo Toro A., Rivera A., Salazar Duque H., Suárez Baron H., Gallego G., et al. An Integrated Hypothesis on the Domestication of Bactris gasipaes. PLoS ONE. 2015;10:e0144644. doi: 10.1371/journal.pone.0144644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernández-Ugalde J.A., Mora-Urpí J., Rocha O.J. Genetic Relationships among Wild and Cultivated Populations of Peach Palm (Bactris gasipaes Kunth, Palmae): Evidence for Multiple Independent Domestication Events. Genet. Resour. Crop Evol. 2011;58:571–583. doi: 10.1007/s10722-010-9600-6. [DOI] [Google Scholar]
- 21.Buitrago Acosta M.C., Montúfar R., Guyot R., Mariac C., Tranbarger T.J., Restrepo S., Couvreur T.L.P. Bactris gasipaes Kunth var. gasipaes Complete Plastome and Phylogenetic Analysis. Mitochondrial DNA Part B. 2022;7:1540–1544. doi: 10.1080/23802359.2022.2109437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva R.S., Clement C.R., Balsanelli E., de Baura V.A., Souza E.M., Fraga H.P.F., Vieira L.N. The Plastome Sequence of Bactris gasipaes and Evolutionary Analysis in Tribe Cocoseae (Arecaceae) PLoS ONE. 2021;16:e0256373. doi: 10.1371/journal.pone.0256373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cymerys M., Clement C.R. Frutíferas e Plantas Úteis na Vida Amazônica. Imazon; Belém, Brazil: 2005. Bactris gasipaes Kunth; pp. 203–208. [Google Scholar]
- 24.Silva V.L., Môro F.V., Damião Filho C.F., Môro J.R., Silva B.M.d.S.e., Charlo H.C.d.O. Morfologia e Avaliação do Crescimento Inicial de Plântulas de Bactris gasipaes Kunth. (Arecaceae) em Diferentes Substratos. Rev. Bras. Frutic. 2006;28:477–480. doi: 10.1590/S0100-29452006000300030. [DOI] [Google Scholar]
- 25.Dos Santos O.V., Soares S.D., Dias P.C.S., Nascimento F.C.A., Conceição L.R.V., da Costa R.S., Pena R.S. White Peach Palm (Pupunha) a New Bactris gasipaes Kunth. Variety from the Amazon: Nutritional Composition, Bioactive Lipid Profile, Thermogravimetric and Morphological Characteristics. J. Food Compos. Anal. 2022;112:104684. doi: 10.1016/j.jfca.2022.104684. [DOI] [Google Scholar]
- 26.Nazário P., Ferreira S.A.d.N., Borges E.E.d.L., Genovese-Marcomini P.R., Mendonça M.S.d. Anatomical and Histochemical Aspects of the Peach Palm (Bactris gasipaes Kunth) Seed. J. Seed Sci. 2013;35:171–178. doi: 10.1590/S2317-15372013000200005. [DOI] [Google Scholar]
- 27.Ferreira S.A.d.N., dos Santos L.A. Viabilidade de Sementes de Pupunha (Bactris gasipaes Kunth.) Acta Amaz. 1992;22:303–307. doi: 10.1590/1809-43921992223307. [DOI] [Google Scholar]
- 28.Nazário P., Ferreira S.A.d.N., Borges E.E.d.L. Embryonic Dormancy in Seeds of Bactris gasipaes Kunth (Peach-Palm) J. Seed Sci. 2017;39:106–113. doi: 10.1590/2317-1545v39n2163507. [DOI] [Google Scholar]
- 29.Edelman S.M., Richards J.H. Review of Vegetative Branching in the Palms (Arecaceae) Bot. Rev. 2019;85:40–77. doi: 10.1007/s12229-018-9200-2. [DOI] [Google Scholar]
- 30.De Carvalho C.J.R., Ishida F.Y. Respostas de Pupunheiras (Bactris gasipaes Kunth) Jovens ao Alagamento. Pesqui. Agropecuária Bras. 2002;37:1231–1238. doi: 10.1590/S0100-204X2002000900005. [DOI] [Google Scholar]
- 31.Ferreira S.A.d.Ν., Clement C.R., Ranzani G., Costa S.d.S. Contribuição ao Conhecimento do Sistema Radicular da Pupunheira (Bactris gasipaes Kunth, Palmae). II. Solo Latossolo Amarelo, Textura Argilosa. Acta Amaz. 1995;25:161–170. doi: 10.1590/1809-43921995253170. [DOI] [Google Scholar]
- 32.Armani M., Charles-Dominique T., Barton K.E., Tomlinson K.W. Developmental Constraints and Resource Environment Shape Early Emergence and Investment in Spines in Saplings. Ann. Bot. 2019;124:1133–1142. doi: 10.1093/aob/mcz152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clement C.R., Manshardt R.M. A Review of the Importance of Spines for Pejibaye Heart-of-Palm Production. Sci. Hortic. 2000;83:11–23. doi: 10.1016/S0304-4238(99)00066-7. [DOI] [Google Scholar]
- 34.Rickson F.R., Cresti M., Beach J.H. Plant Cells Which Aid in Pollen Digestion within a Beetle’s Gut. Oecologia. 1990;82:424–426. doi: 10.1007/BF00317493. [DOI] [PubMed] [Google Scholar]
- 35.Garcia V.A., Soliman E.P., Pavarini R., Zorzenon F.J., Nomura E.S., Rodrigues D.S. A Survey of the Entomofauna Associated with the Inflorescences of Pejibaye (Arecaceae: Bactris gasipaes Kunth) in the Ribeira Valley, SP, Brazil. Arq. Inst. Biológico. 2013;80:111–115. doi: 10.1590/S1808-16572013000100017. [DOI] [Google Scholar]
- 36.Hurtado F.H.M., Mosquera-Espinosa A.T., Gómez-Carabalí A., Otero J.T. Temporal Variation in Arbuscular Mycorrhizal Fungi Colonization of Bactris gasipaes Kunth in Buenaventura, Colombia. Acta Agron. 2013;62:344–351. [Google Scholar]
- 37.Begum N., Qin C., Ahanger M.A., Raza S., Khan M.I., Ashraf M., Ahmed N., Zhang L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019;10:1068. doi: 10.3389/fpls.2019.01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diagne N., Ngom M., Djighaly P.I., Fall D., Hocher V., Svistoonoff S. Roles of Arbuscular Mycorrhizal Fungi on Plant Growth and Performance: Importance in Biotic and Abiotic Stressed Regulation. Diversity. 2020;12:370. doi: 10.3390/d12100370. [DOI] [Google Scholar]
- 39.Clement C.R., Habte M. Genotypic Variation in Vesicular-arbuscular Mycorrhizal Dependence of the Pejibaye Palm. J. Plant Nutr. 1995;18:1907–1916. doi: 10.1080/01904169509365032. [DOI] [Google Scholar]
- 40.Chalita P.B., Farias E.d.N.C., da Costa I.B., Sousa B.F., dos Santos M.A.O., de Albuquerque T.C.S., Vital M.J.S., da Silva K. Characterization of Bacterial Endophytes from the Roots of Native and Cultivated Brazil Nut Trees (Bertholletia excelsa) Acta Amaz. 2019;49:257–267. doi: 10.1590/1809-4392201804831. [DOI] [Google Scholar]
- 41.Tang A., Haruna A.O., Majid N.M.A., Jalloh M.B. Potential PGPR Properties of Cellulolytic, Nitrogen-Fixing, Phosphate-Solubilizing Bacteria in Rehabilitated Tropical Forest Soil. Microorganisms. 2020;8:442. doi: 10.3390/microorganisms8030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rana K.L., Kour D., Yadav A.N., Yadav N., Saxena A.K. Agriculturally Important Microbial Biofilms: Biodiversity, Ecological Significances, and Biotechnological Applications. In: Yadav M.K., Singh B.P., editors. New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Biofilms. Elsevier; Amsterdam, The Netherlands: 2020. pp. 221–265. [DOI] [Google Scholar]
- 43.Belniaki A.C., Panobianco M. Sementes de Pupunha: Da Colheita Ao Armazenamento. EMBRAPA; Colombo, Brasil: 2020. p. 11. Comunicado Técnico. [Google Scholar]
- 44.Bovi M.L.A. Expansão do Cultivo da Pupunheira Para Palmito no Brasil. Vol. 15. Horticultura Brasileira; Brasília, Brazil: 1997. pp. 183–185. Palestra. Suplemento. [Google Scholar]
- 45.Ellis R.H. Seed and seedling vigour in relation to crop growth and yield. Plant Growth Regul. 1992;11:249–255. doi: 10.1007/BF00024563. [DOI] [Google Scholar]
- 46.Modolo V.A. Instruções Agrícolas para as Principais Culturas Econômicas. Instituto Agronômico de Campinas; Campinas, Brasil: 2014. Palmito (Pupunha) pp. 329–333. [Google Scholar]
- 47.Clement C.R., UrpÍ J.E.M. Pejibaye Palm (Bactris gasipaes, Arecaceae): Multi-Use Potential for the Lowland Humid Tropics. Econ. Bot. 1987;41:302–311. doi: 10.1007/BF02858977. [DOI] [Google Scholar]
- 48.Graefe S., Dufour D., van Zonneveld M., Rodriguez F., Gonzalez A. Peach Palm (Bactris gasipaes) in Tropical Latin America: Implications for Biodiversity Conservation, Natural Resource Management and Human Nutrition. Biodivers. Conserv. 2013;22:269–300. doi: 10.1007/s10531-012-0402-3. [DOI] [Google Scholar]
- 49.Flores W.B., Yuyama K., da Silva R.G. Asexual Propagation of Peach Palm by Division of the Clump and Extraction of the Off-Shoots. Hortic. Bras. 2012;30:151–154. doi: 10.1590/S0102-05362012000100025. [DOI] [Google Scholar]
- 50.Vieira T.A., Rosa L.S., Vasconcelos P.C.S., dos Santos M.M., Modesto R.d.S. Sistemas Agroflorestais em Áreas de Agricultores Familiares em Igarapé-Açu, Pará: Caracterização Florística, Implantação e Manejo. Acta Amaz. 2007;37:549–557. doi: 10.1590/S0044-59672007000400010. [DOI] [Google Scholar]
- 51.Moreira A., Moraes L.A.C., Fageria N.K. Nutritional Limitations in Multi-Strata Agroforestry System with Native Amazonian Plants. J. Plant Nutr. 2012;35:1791–1805. doi: 10.1080/01904167.2012.706676. [DOI] [Google Scholar]
- 52.Yuyama K., Silva F.M.S. Desenvolvimento Inicial da Pupunheira em Monocultivo e Intercalado com Culturas Anuais. Hortic. Bras. 2003;21:15–19. doi: 10.1590/S0102-05362003000100003. [DOI] [Google Scholar]
- 53.De Souza G.S., Alves D.I., Dan M.L., Lima J.S.d.S., da Fonseca A.L.C.C., Araújo J.B.S., Guimarães L.A.d.O.P. Soil Physico-Hydraulic Properties Under Organic Conilon Coffee Intercropped with Tree and Fruit Species. Pesqui. Agropecuária Bras. 2017;52:539–547. doi: 10.1590/s0100-204x2017000700008. [DOI] [Google Scholar]
- 54.Alegre J., Lao C., Silva C., Schrevens E. Recovering Degraded Lands in the Peruvian Amazon by Cover Crops and Sustainable Agroforestry Systems. Peruvian J. Agron. 2017;1:7. doi: 10.21704/pja.v1i1.1005. [DOI] [Google Scholar]
- 55.Tucci M.L.S., Erismann N.M., Machado E.C., Ribeiro R.V. Diurnal and Seasonal Variation in Photosynthesis of Peach Palms Grown under Subtropical Conditions. Photosynthetica. 2010;48:421–429. doi: 10.1007/s11099-010-0055-y. [DOI] [Google Scholar]
- 56.Tucci M.L.S., Machado E.C., Modolo V.A., de Magalhães Erismann N. Photosynthesis and Water Relations of Peach Palms (Bactris gasipaes Kunth) under Soil Water Deficit. Theor. Exp. Plant Physiol. 2018;30:29–39. doi: 10.1007/s40626-018-0099-0. [DOI] [Google Scholar]
- 57.Da Silva J.R.A., Falcão N.P.d.S. Caracterização de Sintomas de Carências Nutricionais em Mudas de Pupunheira Cultivadas Em Solução Nutritiva. Acta Amaz. 2002;32:529. doi: 10.1590/1809-43922002324539. [DOI] [Google Scholar]
- 58.Fernandes A.R., de Matos G.S.B., de Carvalho J.G. Deficiências Nutricionais de Macronutrientes e Sódio em Mudas de Pupunheira. Rev. Bras. Frutic. 2013;35:1178–1189. doi: 10.1590/S0100-29452013000400029. [DOI] [Google Scholar]
- 59.Bovi M.L.A., Tucci M.L.S., Spiering S.H., Godoy G., Jr., Lambais M.R. Biomass Accumulation and Arbuscular Mycorrhizal Colonization in Pejibaye (Bactris gasipaes Kunth) as a Function of NPK Fertilization. Acta Hortic. 1998;513:153–168. doi: 10.17660/ActaHortic.1998.513.18. [DOI] [Google Scholar]
- 60.Fernandes A.R., de Carvalho J.G., Curi N., Pinto J.E.B.P., Guimarães P.d.T.G. Nutrição Mineral de Mudas de Pupunheira Sob Diferentes Níveis de Salinidade. Pesqui. Agropecuária Bras. 2002;37:1613–1619. doi: 10.1590/S0100-204X2002001100013. [DOI] [Google Scholar]
- 61.Abrol I.P., Yadav J.S.P., Massoud F.I. Salt-Affected Soils and Their Management. Food and Agriculture Organization of The United Nations; Rome, Italy: 1988. pp. 153–168. FAO Soils Bulletin. [Google Scholar]
- 62.Gondim M.G.C., Jr., de Moraes G.J. Life Cycle of Retracrus johnstoni Keifer (Acari: Phytoptidae) Neotrop. Entomol. 2003;32:197–201. doi: 10.1590/S1519-566X2003000200002. [DOI] [Google Scholar]
- 63.Vásquez-Ordóñez A.A., Löhr B.L., Marvaldi A.E. Comparative Morphology of the Larvae of the Palm Weevils Dynamis borassi (Fabricius) and Rhynchophorus palmarum (Linnaeus) (Curculionidae: Dryophthorinae): Two Major Pests of Peach Palms in the Neotropics. Papéis Avulsos Zool. 2020;60:14. doi: 10.11606/1807-0205/2020.60.special-issue.27. [DOI] [Google Scholar]
- 64.Cuellar-Palacios C.M., Gaviria-Vega J., Montoya-Lerma J. Life Cycle and Larval Growth of Dynamis borassi (Coleoptera: Dryophthoridae), an Emerging Pest to the Peach Palm. Ann. Agric. Sci. 2020;65:218–224. doi: 10.1016/j.aoas.2020.12.002. [DOI] [Google Scholar]
- 65.Thomazini M.J. Ocorrência de Herminodes sp. (Lepidoptera: Noctuidae) em Pupunheira nos Estados do Acre e Rondônia, Brasil. Acta Amaz. 2004;34:505–506. doi: 10.1590/S0044-59672004000300017. [DOI] [Google Scholar]
- 66.Couturier G. Conocimiento y Manejo de Los Insectos y Plagas de Los Frutales de La Amazonia. Folia Amaz. 2006;4:31. doi: 10.24841/fa.v4i1.176. [DOI] [Google Scholar]
- 67.Gaviria J., Montoya-Lerma J., Armbrecht I., Löhr B., Vásquez-Ordóñez A.A. Dynamis Borassi (Coleoptera: Curculionidae), a New Potential Pest to the Palms (Arecaceae): An Early Warning for the Palm Producers. Fla. Entomol. 2021;104:107–116. doi: 10.1653/024.104.0206. [DOI] [Google Scholar]
- 68.Pava J., Gonzáles A., Patiño H. Aspectos de Interés Fitosanitario de La Palma de Chontaduro Bactris gasipaes H.B.K. En Algunas Regiones Del Valle Y Choco. Acta Agron. 1983;33:25–35. [Google Scholar]
- 69.Santos Á.F., Tessmann D.J., Nunes W.M.C., Vida J.B., Jaccoud Filho D.S. Doenças Foliares da Pupunheira (Bactris gasipaes) No Estado Do Pará. Bol. Pesqui. Florest. 2001;42:125–129. [Google Scholar]
- 70.Mafacioli R., Santos A.F., Tessmann D.J., Vida J.B. Etiologia e Manejo das Doenças da Pupunheira no Brasil. Pesq. Flor. Bras. 2009;58:61–68. doi: 10.4336/2009.pfb.58.61. [DOI] [Google Scholar]
- 71.Arroyo-Oquendo C., Bogantes-Arias A., Mora-Urpí J. La Deshoja En El Manejo de La Bacteriosis del Palmito de Pejibaye (Bactris gasipaes) Agron. Mesoam. 2005;18:129. doi: 10.15517/am.v18i1.5043. [DOI] [Google Scholar]
- 72.Mora-Urpi J., Arroyo-Oquendo C., Mexzón-Vargas R., Bogantes-Arias A. Diseminación de La “Bacteriosis del Palmito” de Pejibaye (Bactris gasipaes Kunth) Agron. Mesoam. 2007;19:155. doi: 10.15517/am.v19i2.4998. [DOI] [Google Scholar]
- 73.Lopes H.V., dos Santos Á.F., Luz E.D.M.N., Tessmann D.J. Phytophthora Palmivora: Agente Causal da Podridão da Base do Estipe da Pupunheira no Brasil. Summa Phytopathol. 2019;45:164–171. doi: 10.1590/0100-5405/189782. [DOI] [Google Scholar]
- 74.Patiño V.M. Plantas Cultivadas y Animales Domésticos En América Equinoccial I: Frutales. Imprenta Departamental; Cali, Colombia: 1963. p. 379. [Google Scholar]
- 75.González-Jaramillo N., Bailon-Moscoso N., Duarte-Casar R., Romero-Benavides J.C. Peach Palm (Bactris gasipaes Kunth.): Ancestral Tropical Staple with Future Potential. Plants. 2022;11:3134. doi: 10.3390/plants11223134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stevanato N., Ribeiro T.H., Giombelli C., Cardoso T., Wojeicchowski J.P., Danesi E.D.G., Bolanho Barros B.C. Effect of Canning on the Antioxidant Activity, Fiber Content, and Mechanical Properties of Different Parts of Peach Palm Heart. J. Food Process. Preserv. 2020;44:e14554. doi: 10.1111/jfpp.14554. [DOI] [Google Scholar]
- 77.De Cássia Spacki K.d.C., Corrêa R.C.G., Uber T.M., Barros L., Ferreira I.C.F.R., Peralta R.A., de Fátima Peralta Muniz Moreira R., Helm C.V., de Lima E.A., Bracht A., et al. Full Exploitation of Peach Palm (Bactris gasipaes Kunth): State of the Art and Perspectives. Plants. 2022;11:3175. doi: 10.3390/plants11223175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.World Bank World Trade Organization Palm Hearts; Prepared or Preserved, Whether or Not Containing Added Sugar, Other Sweetening Matter or Spirit Exports by Country in 2021. [(accessed on 25 December 2022)]. Available online: https://wits.worldbank.org/trade/comtrade/en/country/ALL/year/2021/tradeflow/Exports/partner/WLD/product/200891#.
- 79.World Bank World Trade Organization Palm Hearts; Prepared or Preserved, Whether or Not Containing Added Sugar, Other Sweetening Matter or Spirit Exports by Country in 2006. [(accessed on 25 December 2022)]. Available online: https://wits.worldbank.org/trade/comtrade/en/country/ALL/year/2006/tradeflow/Exports/partner/WLD/product/200891.
- 80.World Bank World Trade Organization Palm Hearts; Prepared or Preserved, Whether or Not Containing Added Sugar, Other Sweetening Matter or Spirit Exports by Country in 2017. [(accessed on 25 December 2022)]. Available online: https://wits.worldbank.org/trade/comtrade/en/country/ALL/year/2017/tradeflow/Exports/partner/WLD/product/200891.
- 81.Hernández I., Cely N., González F., Muñoz E., Prieto I. The Discovery of New Export Products in Ecuador. SSRN Electron. J. 2010;47:122. doi: 10.2139/ssrn.1817280. [DOI] [Google Scholar]
- 82.Santos Á.F., Corrêa Júnior C., Neves E.J.M., editors. Palmeiras para Produção de Palmito: Juçara, Pupunheira e Palmeira Real. Embrapa Florestas; Colombo, Brasil: 2008. p. 190. [Google Scholar]
- 83.Instituto Brasileiro de Geografia e Estatística (IBGE) Tabela 816—Produção, Venda e Valores Da Produção e Da Venda Na Extração Vegetal Por Produtos Da Extração Vegetal, Condição Do Produtor Em Relação Às Terras, Destino Da Produção, Grupos de Atividade Econômica e Grupos de Área Total. [(accessed on 26 December 2022)]; Available online: https://sidra.ibge.gov.br/tabela/816#/n1/all/n2/all/n3/all/v/1981,1983/p/all/c229/111573,111575/c218/0/c12763/0/c12764/0/c12517/113601/c220/0/d/v1981%200,v1983%200/l/v,p+c229+c218+c12763,t+c12764+c12517+c220/resultado.
- 84.Instituto Brasileiro de Geografia e Estatística (IBGE) Tabela 1689—Produção, Valor da Produção, Venda, Valor da Venda, Colheita, Área Plantada e Efetivos das Plantações da Lavoura Permanente nos Estabelecimentos Agropecuários com Mais de 50 Pés Existentes por Produtos da Lavoura Permanente, Grupos de Atividade Econômica e Grupos de Área Total. [(accessed on 26 December 2022)]; Available online: https://sidra.ibge.gov.br/tabela/1689#/n1/all/n2/all/n3/all/v/2382,2390/p/all/c227/4976,111653/c12517/0/c220/0/d/v2382%200,v2390%200/l/v,p+c227+c12517,t+c220/resultado.
- 85.Instituto Brasileiro de Geografia e Estatística (IBGE) Tabela 6950—Número de Estabelecimentos Agropecuários com Produtos da Extração Vegetal, Quantidade Produzida na Extração Vegetal, Quantidade Vendida de Produtos da Extração Vegetal, Valor da Produção na Extração Vegetal e Valor da Venda de Produtos da Extração Vegetal, por Tipologia, Produtos da Extração Vegetal e Grupos de Área Total. [(accessed on 26 December 2022)]; Available online: https://sidra.ibge.gov.br/tabela/6950#/n1/all/n2/all/n3/all/v/144,10073/p/all/c829/46302/c229/111573,111575/c220/110085/d/v144%200,v10073%200/l/v,p+c829+c229,t+c220/resultado.
- 86.Instituto Brasileiro de Geografia e Estatística (IBGE) Tabela 6956—Produção, Valor da Produção, Venda, Valor da Venda, Colheita, Área Plantada e Efetivos das Plantações da Lavoura Permanente nos Estabelecimentos Agropecuários, por Tipologia, Produtos Da Lavoura Permanente e Grupos de Área Total. [(accessed on 26 December 2022)]; Available online: https://sidra.ibge.gov.br/tabela/6956#/n1/all/n2/all/n3/all/v/9506,10077/p/all/c829/46302/c227/4976,111653/c220/110085/d/v9506%200,v10077%200/l/v,p+c829+c227,t+c220/resultado.
- 87.Noronha Matos K.A., Praia Lima D., Pereira Barbosa A.P., Zerlotti Mercadante A., Campos Chisté R. Peels of Tucumã (Astrocaryum Vulgare) and Peach Palm (Bactris gasipaes) Are by-Products Classified as Very High Carotenoid Sources. Food Chem. 2019;272:216–221. doi: 10.1016/j.foodchem.2018.08.053. [DOI] [PubMed] [Google Scholar]
- 88.Radice M., Viafara D., Neill D., Asanza M., Sacchetti G., Guerrini A., Maietti S. Chemical Characterization and Antioxidant Activity of Amazonian (Ecuador) Caryodendron orinocense Karst. and Bactris gasipaes Kunth Seed Oils. J. Oleo Sci. 2014;63:1243–1250. doi: 10.5650/jos.ess14007. [DOI] [PubMed] [Google Scholar]
- 89.Arkcoll D.B., Aguiar J.P.L. Peach Palm (Bactris gasipaes H.B.K.), a New Source of Vegetable Oil from the Wet Tropics. J. Sci. Food Agric. 1984;35:520–526. doi: 10.1002/jsfa.2740350508. [DOI] [Google Scholar]
- 90.Yuyama L.K.O., Aguiar J.P.L., Yuyama K., Clement C.R., Macedo S.H.M., Fávaro D.I.T., Afonso C., Vasconcellos M.B.A., Pimentel S.A., Badolato E.S.G., et al. Chemical Composition of the Fruit Mesocarp of Three Peach Palm (Bactris gasipaes) Populations Grown in Central Amazonia, Brazil. Int. J. Food Sci. Nutr. 2003;54:49–56. doi: 10.1080/0963748031000061994. [DOI] [PubMed] [Google Scholar]
- 91.Alves B.S.F., Pereira Junior J.B., Carvalho F.I.M., Dantas Filho H.A., Fernandes Dantas K.G. Mineral Composition of Amazonian Fruits by Flame Atomic Absorption Spectrometry Using Multivariate Analysis. Biol. Trace Elem. Res. 2019;189:259–266. doi: 10.1007/s12011-018-1451-6. [DOI] [PubMed] [Google Scholar]
- 92.Johannessen C.L. Pejibaye Palm: Physical and Chemical Analysis of the Fruit. Econ. Bot. 1967;21:371–378. doi: 10.1007/BF02863164. [DOI] [Google Scholar]
- 93.Yuyama L.K.O., Aguiar J.P.L., Macedo S.H.M., Gioia T., Yuyama K., Fávaro D.I.T., Afonso C., Vasconcellos M.B.A., Cozzolino S.M.F. Determinação dos Teores de Elementos Minerais em Alimentos Convencionais e Não Convencionais da Região Amazônica pela Técnica de Análise por Ativação com Nêutrons Instrumental. Acta Amaz. 1997;27:183–195. doi: 10.1590/1809-43921997273196. [DOI] [Google Scholar]
- 94.Panchal S.K., Wanyonyi S., Brown L. Selenium, Vanadium, and Chromium as Micronutrients to Improve Metabolic Syndrome. Curr. Hypertens. Rep. 2017;19:10. doi: 10.1007/s11906-017-0701-x. [DOI] [PubMed] [Google Scholar]
- 95.Aguiar J.P.L. Tabela de Composição de Alimentos Da Amazônia. Acta Amaz. 1996;26:121–126. doi: 10.1590/1809-43921996261126. [DOI] [Google Scholar]
- 96.Santos M.F.G., Marmesat S., Brito E.S., Alves R.E., Dobarganes M.C. Major Components in Oils Obtained from Amazonian Palm Fruits. Grasas Aceites. 2013;64:328–334. doi: 10.3989/gya.023513. [DOI] [Google Scholar]
- 97.Schiassi M.C.E.V., de Souza V.R., Lago A.M.T., Campos L.G., Queiroz F. Fruits from the Brazilian Cerrado Region: Physico-Chemical Characterization, Bioactive Compounds, Antioxidant Activities, and Sensory Evaluation. Food Chem. 2018;245:305–311. doi: 10.1016/j.foodchem.2017.10.104. [DOI] [PubMed] [Google Scholar]
- 98.Neri-Numa I.A., Soriano Sancho R.A., Pereira A.P.A., Pastore G.M. Small Brazilian Wild Fruits: Nutrients, Bioactive Compounds, Health-Promotion Properties and Commercial Interest. Food Res. Int. 2018;103:345–360. doi: 10.1016/j.foodres.2017.10.053. [DOI] [PubMed] [Google Scholar]
- 99.Darnet S.H., da Silva L.H.M., Rodrigues A.M.d.C., Lins R.T. Nutritional Composition, Fatty Acid and Tocopherol Contents of Buriti (Mauritia flexuosa) and Patawa (Oenocarpus bataua) Fruit Pulp from the Amazon Region. Ciênc. E Tecnol. Aliment. 2011;31:488–491. doi: 10.1590/S0101-20612011000200032. [DOI] [Google Scholar]
- 100.Vásquez-Ocmín P.G., Freitas Alvarado L., Sotero Solís V., Paván Torres R., Mancini-Filho J. Chemical Characterization and Oxidative Stability of the Oils from Three Morphotypes of Mauritia flexuosa L.f., from the Peruvian Amazon. Grasas Aceites. 2010;61:390–397. doi: 10.3989/gya.010110. [DOI] [Google Scholar]
- 101.Zumbado M.E., Murillo M.G. Composition and Nutritive Value of Pejibaye (Bactris gasipaes) in Animal Feeds. Rev. Biol. Trop. 1984;32:51–56. [PubMed] [Google Scholar]
- 102.Food and Agriculture Organization of the United Nations. World Health Organization. United Nations University . Energy and Protein Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation. World Health Organization; Rome, Italy: 1985. [Google Scholar]
- 103.Cantu-Jungles T.M., Cipriani T.R., Iacomini M., Hamaker B.R., Cordeiro L.M.C. A Pectic Polysaccharide from Peach Palm Fruits (Bactris gasipaes) and Its Fermentation Profile by the Human Gut Microbiota In Vitro. Bioact. Carbohydr. Diet. Fibre. 2017;9:1–6. doi: 10.1016/j.bcdf.2016.11.005. [DOI] [Google Scholar]
- 104.Ferrari Felisberto M.H., Costa M.S., Villas Boas F., Leivas C.L., Franco C.M.L., Souza S.M., Clerici M.T.P.S., Cordeiro L.M.C. Characterization and Technological Properties of Peach Palm (Bactris gasipaes var. gasipaes) Fruit Starch. Food Res. Int. 2020;136:109569. doi: 10.1016/j.foodres.2020.109569. [DOI] [PubMed] [Google Scholar]
- 105.Zárate R., Jaber-Vazdekis N., Tejera N., Pérez J.A., Rodríguez C. Significance of Long Chain Polyunsaturated Fatty Acids in Human Health. Clin. Transl. Med. 2017;6:25. doi: 10.1186/s40169-017-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.De Rosso V.V., Mercadante A.Z. Identification and Quantification of Carotenoids, By HPLC-PDA-MS/MS, from Amazonian Fruits. J. Agric. Food Chem. 2007;55:5062–5072. doi: 10.1021/jf0705421. [DOI] [PubMed] [Google Scholar]
- 107.Dos Santos M., Mamede R., Rufino M., de Brito E., Alves R. Amazonian Native Palm Fruits as Sources of Antioxidant Bioactive Compounds. Antioxidants. 2015;4:591–602. doi: 10.3390/antiox4030591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Santos M.F.G., Alves R.E., Ruíz-Méndez M.V. Minor Components in Oils Obtained from Amazonian Palm Fruits. Grasas Aceites. 2013;64:531–536. doi: 10.3989/gya.048913. [DOI] [Google Scholar]
- 109.Rodrigues A.M.d.C., Darnet S., Silva L.H.M. Fatty Acid Profiles and Tocopherol Contents of Buriti (Mauritia flexuosa), Patawa (Oenocarpus bataua), Tucuma (Astrocaryum vulgare), Mari (Poraqueiba paraensis) and Inaja (Maximiliana maripa) Fruits. J. Braz. Chem. Soc. 2010;21:2000–2004. doi: 10.1590/S0103-50532010001000028. [DOI] [Google Scholar]
- 110.Faria J.V., Valido I.H., Paz W.H.P., da Silva F.M.A., de Souza A.D.L., Acho L.R.D., Lima E.S., Boleti A.P.A., Marinho J.V.N., Salvador M.J., et al. Comparative Evaluation of Chemical Composition and Biological Activities of Tropical Fruits Consumed in Manaus, Central Amazonia, Brazil. Food Res. Int. 2021;139:109836. doi: 10.1016/j.foodres.2020.109836. [DOI] [PubMed] [Google Scholar]
- 111.Bataglion G.A., da Silva F.M.A., Eberlin M.N., Koolen H.H.F. Simultaneous Quantification of Phenolic Compounds in Buriti Fruit (Mauritia flexuosa L.f.) by Ultra-High Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Food Res. Int. 2014;66:396–400. doi: 10.1016/j.foodres.2014.09.035. [DOI] [Google Scholar]
- 112.Saini R.K., Nile S.H., Park S.W. Carotenoids from Fruits and Vegetables: Chemistry, Analysis, Occurrence, Bioavailability and Biological Activities. Food Res. Int. 2015;76:735–750. doi: 10.1016/j.foodres.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 113.Chisté R.C., Costa E.L.N., Monteiro S.F., Mercadante A.Z. Carotenoid and Phenolic Compound Profiles of Cooked Pulps of Orange and Yellow Peach Palm Fruits (Bactris gasipaes) from the Brazilian Amazonia. J. Food Compos. Anal. 2021;99:103873. doi: 10.1016/j.jfca.2021.103873. [DOI] [Google Scholar]
- 114.Santos M.F.G., Alves R.E., Roca M. Carotenoid Composition in Oils Obtained from Palm Fruits from the Brazilian Amazon. Grasas Aceites. 2015;66:e086. doi: 10.3989/gya.1062142. [DOI] [Google Scholar]
- 115.Yuyama L.K.O., Yonekura L., Aguiar J.P.L., Sousa R.F.S. Biodisponibilidade de Vitamina A da Pupunha (Bactris gasipaes Kunth) Em Ratos. Acta Amaz. 1999;29:497. doi: 10.1590/1809-43921999293500. [DOI] [Google Scholar]
- 116.Del Rio D., Rodriguez-Mateos A., Spencer J.P.E., Tognolini M., Borges G., Crozier A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013;18:1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bacellar R.S., d’Almeida J.R.M. Microstructural Characterization and Evaluation of Thermal, Mechanical and Wear Properties of Pupunha (Bactris gasipaes) Pseudostem. Polym. Renew. Resour. 2010;1:123–142. doi: 10.1177/204124791000100301. [DOI] [Google Scholar]
- 118.Pinho Pinheiro A.P., Moraes d’Almeida J.R. Peach Palm: Pseudo-Wood for Sustainable Jewelry Design. Mater. Today Proc. 2020;33:1869–1873. doi: 10.1016/j.matpr.2020.05.228. [DOI] [Google Scholar]
- 119.Alhijazi M., Zeeshan Q., Safaei B., Asmael M., Qin Z. Recent Developments in Palm Fibers Composites: A Review. J. Polym. Environ. 2020;28:3029–3054. doi: 10.1007/s10924-020-01842-4. [DOI] [Google Scholar]
- 120.Quinaya D.C.P., da Silva E.S., d’Almeida J.R.M. On the Use of Residues from the Sustainable Extraction of Heart of Palm in Agglomerated Panels. J. Nat. Fibers. 2016;13:172–177. doi: 10.1080/15440478.2014.1004009. [DOI] [Google Scholar]
- 121.Franco T.S., Potulski D.C., Viana L.C., Forville E., de Andrade A.S., de Muniz G.I.B. Nanocellulose Obtained from Residues of Peach Palm Extraction (Bactris gasipaes) Carbohydr. Polym. 2019;218:8–19. doi: 10.1016/j.carbpol.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 122.Martins M.P., Dagostin J.L.A., Franco T.S., de Muñiz G.I.B., Masson M.L. Application of Cellulose Nanofibrils Isolated from an Agroindustrial Residue of Peach Palm in Cassava Starch Films. Food Biophys. 2020;15:323–334. doi: 10.1007/s11483-020-09626-y. [DOI] [Google Scholar]
- 123.Chicatto J.A., Rainert K.T., Gonçalves M.J., Helm C.V., Altmajer-Vaz D., Tavares L.B.B. Decolorization of Textile Industry Wastewater in Solid State Fermentation with Peach-Palm (Bactris gasipaes) Residue. Braz. J. Biol. 2018;78:718–727. doi: 10.1590/1519-6984.175074. [DOI] [PubMed] [Google Scholar]
- 124.Carvalho E.A., Nunes L.V., Goes L.M.d.S., Da Silva E.G.P., Franco M., Gross E., Uetanabaro A.P.T., da Costa A.M. Peach-Palm (Bactris gasipaes Kunth.) Waste as Substrate for Xylanase Production by Trichoderma stromaticum AM7. Chem. Eng. Commun. 2018;205:975–985. doi: 10.1080/00986445.2018.1425208. [DOI] [Google Scholar]
- 125.Vieira T.F., Corrêa R.C.G., de Fatima Peralta Muniz Moreira R., Peralta R.A., de Lima E.A., Helm C.V., Garcia J.A.A., Bracht A., Peralta R.M. Valorization of Peach Palm (Bactris gasipaes Kunth) Waste: Production of Antioxidant Xylooligosaccharides. Waste Biomass Valorization. 2021;12:6727–6740. doi: 10.1007/s12649-021-01457-3. [DOI] [Google Scholar]
- 126.Clement C.R., Bovi M.L.A. Melhoramento Genético da Pupunheira: Conhecimentos Atuais e Necessidades. Embrapa Rondônia; Porto Velho, Brazil: 1999. pp. 57–70. [Google Scholar]
- 127.Clement C.R. Pupunha: Recursos genéticos para a produção de palmito. Hort. Bras. 1997;15:186–191. [Google Scholar]
- 128.Scheldeman X., Kanashiro M., Porro R., Dantas Medeiros R. Amazon Initiative Workshop on Conservation and Use of Amazonian Fruits. IPGRI, EMBRAPA and the Amazon initiative; Boa Vista, Brasil: 2006. [Google Scholar]
- 129.Engels J.M.M., Ebert A.W. A Critical Review of the Current Global Ex Situ Conservation System for Plant Agrobiodiversity. I. History of the Development of the Global System in the Context of the Political/Legal Framework and Its Major Conservation Components. Plants. 2021;10:1557. doi: 10.3390/plants10081557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bellon M.R., Dulloo E., Sardos J., Thormann I., Burdon J.J. In Situ Conservation-Harnessing Natural and Human-Derived Evolutionary Forces to Ensure Future Crop Adaptation. Evol. Appl. 2017;10:965–977. doi: 10.1111/eva.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Phillips G.C., Garda M. Plant Tissue Culture Media and Practices: An Overview. In Vitro Cell. Dev. Biol. Plant. 2019;55:242–257. doi: 10.1007/s11627-019-09983-5. [DOI] [Google Scholar]
- 132.Sang Y.L., Cheng Z.J., Zhang X.S. Plant Stem Cells and De Novo Organogenesis. New Phytol. 2018;218:1334–1339. doi: 10.1111/nph.15106. [DOI] [PubMed] [Google Scholar]
- 133.De Almeida M., Graner É.M., Brondani G.E., Artioli A., Almeida L.V., Leone G.F., Baccarin F.J.B., de Oliveira Antonelli P., Cordeiro G.M., Oberschelp G.P.J., et al. Plant Morphogenesis: Theorical Bases. Adv. For. Sci. 2015;2:13–22. [Google Scholar]
- 134.Fehér A. Callus, Dedifferentiation, Totipotency, Somatic Embryogenesis: What These Terms Mean in the Era of Molecular Plant Biology? Front. Plant Sci. 2019;10:536. doi: 10.3389/fpls.2019.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Arias O., Huete F. Propagacion Vegetativa In Vitro del Pejibaye (Bactris gasipaes H.B.K.) Turrialba. 1983;33:103–108. [Google Scholar]
- 136.Stein M., Stephens C. Effect of 2,4-Dichlorophenoxyacetic Acid and Activated-Charcoal on Somatic Embryogenesis of Bactris gasipaes H.B.K. Turrialba. 1991;41:196–201. [Google Scholar]
- 137.Valverde R., Arias O., Thorpe T.A. Picloram-Induced Somatic Embryogenesis in Pejibaye Palm (Bactris gasipaes H.B.K.) Plant Cell Tissue Organ Cult. 1987;10:149–156. doi: 10.1007/BF00035913. [DOI] [Google Scholar]
- 138.De Almeida M., Kerbauy G.B. Micropropagation of Bactris gasipaes (Palmae) through Flower Bud Culture. Rev. Bras. Fisiol. Veg. 1996;8:215–217. [Google Scholar]
- 139.Steinmacher D.A., Clement C.R., Guerra M.P. Somatic Embryogenesis from Immature Peach Palm Inflorescence Explants: Towards Development of an Efficient Protocol. Plant Cell Tissue Organ Cult. 2007;89:15–22. doi: 10.1007/s11240-007-9207-6. [DOI] [Google Scholar]
- 140.Steinmacher D.A., Cangahuala-Inocente G.C., Clement C.R., Guerra M.P. Somatic Embryogenesis from Peach Palm Zygotic Embryos. Vitro Cell. Dev. Biol. Plant. 2007;43:124–132. doi: 10.1007/s11627-007-9032-y. [DOI] [Google Scholar]
- 141.Steinmacher D.A., Krohn N.G., Dantas A.C.M., Stefenon V.M., Clement C.R., Guerra M.P. Somatic Embryogenesis in Peach Palm Using the Thin Cell Layer Technique: Induction, Morpho-Histological Aspects and AFLP Analysis of Somaclonal Variation. Ann. Bot. 2007;100:699–709. doi: 10.1093/aob/mcm153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tran Thanh Van K., Richard L., Gendy C.A. An Experimental Model for the Analysis of Plant/Cell Differentiation: Thin Cell Layer Concept, Strategy, Methods, Records and Potential. In: Rodríguez R., Tamés R.S., Durzan D.J., editors. Plant Aging. Springer; Boston, MA, USA: 1990. pp. 215–224. [Google Scholar]
- 143.Heringer A.S., Steinmacher D.A., Fraga H.P.F., Vieira L.N., Montagna T., Quinga L.A.P., Quoirin M.G.G., Jiménez V.M., Guerra M.P. Improved High-Efficiency Protocol for Somatic Embryogenesis in Peach Palm (Bactris gasipaes Kunth) Using RITA® Temporary Immersion System. Sci. Hortic. 2014;179:284–292. doi: 10.1016/j.scienta.2014.09.041. [DOI] [Google Scholar]
- 144.Padilha J.H.D., Steinmacher D., Quoirin M. Peach Palm Plantlet Growth in Different Culture Media in a Temporary Immersion System. Ciênc. Rural. 2021;51:e20190075. doi: 10.1590/0103-8478cr20190075. [DOI] [Google Scholar]
- 145.Campos-Boza S., Vinas M., Solórzano-Cascante P., Holst A., Steinmacher D.A., Guerra M.P., Jiménez V.M. Somatic Embryogenesis and Plant Regeneration from Transverse Thin Cell Layers of Adult Peach Palm (Bactris gasipaes) Lateral Offshoots. Front. Plant Sci. 2022;13:995307. doi: 10.3389/fpls.2022.995307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.