Abstract
BACKGROUND
Anaemia is frequently recorded during preoperative screening and has been suggested to affect outcomes after surgery negatively.
OBJECTIVES
The objectives were to assess the frequency of moderate to severe anaemia and its association with length of hospital stay.
DESIGN
Post hoc analysis of the international observational prospective ‘Local ASsessment of VEntilatory management during General Anaesthesia for Surgery’ (LAS VEGAS) study.
PATIENTS AND SETTING
The current analysis included adult patients requiring general anaesthesia for non-cardiac surgery. Preoperative anaemia was defined as a haemoglobin concentration of 11 g dl−1 or lower, thus including moderate and severe anaemia according to World Health Organisation criteria.
MAIN OUTCOME MEASURES
The primary outcome was length of hospital stay. Secondary outcomes included hospital mortality, intra-operative adverse events and postoperative pulmonary complications (PPCs).
RESULTS
Haemoglobin concentrations were available for 8264 of 9864 patients. Preoperative moderate to severe anaemia was present in 7.7% of patients. Multivariable analysis showed that preoperative moderate to severe anaemia was associated with an increased length of hospital stay with a mean difference of 1.3 ((95% CI 0.8 to 1.8) days; P < .001). In the propensity-matched analysis, this association remained present, median 4.0 [IQR 1.0 to 5.0] vs. 2.0 [IQR 0.0 to 5.0] days, P = .001. Multivariable analysis showed an increased in-hospital mortality (OR 2.9 (95% CI 1.1 to 7.5); P = .029), and higher incidences of intra-operative hypotension (36.3 vs. 25.3%; P < .001) and PPCs (17.1 vs. 10.5%; P = .001) in moderately to severely anaemic patients. However, this was not confirmed in the propensity score-matched analysis.
CONCLUSIONS
In this international cohort of non-cardiac surgical patients, preoperative moderate to severe anaemia was associated with a longer duration of hospital stay but not increased intra-operative complications, PPCs or in-hospital mortality.
TRIAL REGISTRATION
The LAS VEGAS study was registered at Clinicaltrials.gov, NCT01601223.
Introduction
The global prevalence of anaemia in the general population is high, around one in every three individuals.1 Consequently, anaemia is recorded frequently during preoperative screening. In orthopaedic patients, reported incidences of anaemia vary between 14% and 34%.2–6 In patients scheduled for major surgical interventions at increased risk of cardiac complications, incidences of 31% and 58% have been reported.7,8 Around 40% of elderly9 and oncology patients have anaemia before surgery.10–12
Regardless of its cause, it has been suggested that preoperative anaemia in non-cardiac surgical patients has a negative impact on postoperative morbidity and mortality.9,13,14 Of note, previous investigations focusing on preoperative anaemia were either retrospective, prospective–cohort, or database studies in specific patient groups and focused mainly on in-hospital mortality. Also, in the majority of studies, patient groups remained unmatched.15 Propensity score-matching could provide additional insight into the multifactorial association between anaemia and outcome. Finally, in this analysis we focused on the most clinically relevant levels of anaemia by excluding mildly anaemic patients.
The aim of this investigation, therefore, was to investigate the association between preoperative moderate to severe anaemia and relevant patient-centred postoperative endpoints. For this purpose, the conveniently sized prospective ‘Local ASsessment of VEntilatory management during General Anaesthesia for Surgery’ (LAS VEGAS) study was re-analysed.16 Preoperative anaemia was defined as a haemoglobin concentration <11.0 g dl−1, thus including both moderate and severe anaemia according to the World Health Organisation (WHO).17 The primary hypothesis tested was that preoperative moderate to severe anaemia increases length of hospital stay. In addition to simple descriptive analyses comparing patients with preoperative moderate to severe anaemia with patients who were not anaemic at preoperative screening, a propensity score-matched analysis was performed to correct for other factors known to impact postoperative outcomes.
Methods
For this post hoc analysis, data were retrieved from the database of the LAS VEGAS study. The LAS VEGAS study was an international, multicentre, prospective study aimed at determining the incidence of patients at risk of postoperative pulmonary complications (PPCs) and to compare ventilation strategies. The study protocol was first approved by the ethics committee of the Academic Medical Centre, Amsterdam, the Netherlands (W12_190#12.17.0227). Each site was requested to seek approval to implement the study protocol from their respective institutional review boards and, if required, to obtain written informed consent from patients. Surgical patients were enrolled over a period of seven consecutive days in the calendar year 2013. National coordinators selected the exact period during which data were collected for the study in their respective countries.
Adult patients receiving invasive ventilation via either an endotracheal tube or supraglottic device during general anaesthesia for elective or non-elective surgery were eligible. Patients were excluded if aged less than 18 years, or scheduled for pregnancy-related surgery. Procedures outside the operating room and patients who required cardiopulmonary bypass were also excluded. The database contains granular data of 9864 adult patients from 146 centres in 29 countries scheduled for invasive ventilation during general anaesthesia for elective and non-elective non-cardiac surgery.
From the database of the LAS VEGAS study, all patients with available preoperative haemoglobin concentrations were identified and included in the current analysis. According to the WHO, anaemia can be divided into different categories.17 For women, a preoperative haemoglobin concentration between 11.0 and 11.9 g dl−1 and for men between 11.0 and 12.9 g dl−1 is defined as mild anaemia. For both sexes, a preoperative haemoglobin concentration <11.0 g dl−1 has been suggested as a cutoff for moderate anaemia and a concentration <8.0 g dl−1 for severe anaemia. For the purpose of the current post hoc analysis, the focus was on a sex neutral and clinically relevant haemoglobin concentration of 11.0 g dl−1, thus including moderate and severe anaemia, as defined by the WHO definitions.
The primary endpoint was the association between preoperative anaemia and length of hospital stay (LOS). Secondary endpoints included the associations between preoperative anaemia and the occurrence of intra-operative adverse events (AEs), PPCs, and in-hospital mortality. All endpoints were examined at postoperative day 28.
Definitions
Intra-operative AEs included the following: hypotension (systolic arterial blood pressure < 90 mm Hg for 3 min or longer); vasopressor use (any vasoactive drug given to correct hypotension); and arrhythmias (new onset of atrial fibrillation, sustained ventricular tachycardia, supraventricular tachycardia or ventricular fibrillation).
PPCs in the LAS VEGAS study were defined as the unplanned administration of supplementary oxygen (PaO2 < 8 kPa or SpO2 < 90%), respiratory failure (PaO2 < 8 kPa or SpO2 < 90% despite oxygen therapy) or the need for non-invasive positive pressure ventilation, unplanned or prolonged invasive mechanical ventilation, acute respiratory distress syndrome (ARDS), pneumonia and pneumothorax developed in the first five postoperative days.
Other reported data included patient characteristics, American Society of Anesthesiologists’ (ASA) physical status classification, comorbidities, preoperative oxygen saturation, type of surgery, (open, laparoscopic or peripheral), condition of surgery, (elective, urgent or emergency) and durations of anaesthesia and the surgical procedure.
Statistical analysis
Distribution of variables was tested using the Kolmogorov-Smirnov test. Parametric data are reported as mean ± SD or median [IQR], as appropriate. Categorical variables were compared using the χ2 or Fisher exact test. The t-test or Wilcoxon rank–sum test was used for continuous variables, as appropriate.
Mixed–effect multivariable models were built for LOS (Gaussian distribution) and mortality (binomial distribution) including the centres as random effect and using the following preoperative variables: age, gender, body mass index (BMI), ASA physical status, preoperative SpO2, smoking, chronic obstructive pulmonary disease (COPD), chronic kidney disease, heart failure, obstructive sleep apnoea, respiratory infection, laparoscopic or not laparoscopic surgery, elective, urgent or emergency surgery, duration of anaesthesia, epidural anaesthesia, antibiotic prophylaxis, total fluid during surgery, intraoperative red blood cell (RBC) transfusion (yes or no) and presence of anaemia (yes or no). Effects were expressed as mean difference and odds ratio (OR) (95% CI) as appropriate.
The time until hospital discharge was compared using Kaplan–Meier curves, and hazard ratios with a 95% confidence interval were calculated with (shared–frailty) Cox proportional hazard models adjusted for the same covariates described above and with centres as frailty. The Schoenfeld residuals against the transformed time were used to test the proportional hazard assumptions.
To assess consistency of the findings, a covariate balancing propensity score (CBPS) matched analysis was performed. A calliper of 0.01 was set to constrain the differences between pairs. Similar variables were included for the multivariable models. However, in order to match both groups more precisely, additional variables were included in the propensity score: Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) risk score without anaemia, functional status, cancer and preoperative RBC transfusion.
The effect of anaemia on hospital length of stay in the matched cohort was assessed through an unadjusted mixed-effect model. To assess the consistency of the findings, the impact of anaemia on hospital length of stay was re-assessed according to different corrections by the CBPS, namely: a mixed-effect model including the CBPS as a linear term; a mixed-effect model including the CBPS as decile categories; an inverse-probability-of-treatment-weighted mixed-effect linear regression model with the weights equal to 1/CBPS for anaemic individuals and 1/(1 – CBPS) for non-anaemic individuals; and a standardised mortality ratio-weighted mixed-effect linear regression model with weights of 1 for anaemic and CBPS/(1 – CBPS) for non-anaemic individuals.
Finally, one sensitivity analysis was performed to assess the extent to which our results are affected by the exclusion of patients with mild anaemia (haemoglobin 11.0 to 11.9 g dl−1 for women; 11.0 to 12.9 g dl−1 for men). The above-mentioned mixed effect multivariable models and CBPS matched analyses were repeated, including also all mildly anaemic men and women. Covariates and outcome parameters were similar.
All analyses were performed using R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria). A P value < .05 was considered statistically significant.
Results
Unmatched cohort
Figure 1 illustrates the flow of included patients. After exclusion of 1600 patients for whom there was no preoperative haemoglobin concentration recorded during preoperative screening, a total of 8264 patients remained for the final analysis. Baseline and intra-operative characteristics are shown in Table 1. Preoperative moderate to severe anaemia was recorded in 638 patients (7.7%). Baseline characteristics between anaemic and non-anaemic patients differed importantly. Patients with anaemia were older, and more likely to have cancer, chronic kidney disease and heart failure. Anaemic patients underwent emergency surgery or non-laparoscopic procedures more often. Anaemic patients had epidural anaesthesia in addition to general anaesthesia more frequently, and anaesthesia and surgical procedures lasted longer in anaemic patients. Finally, anaemic patients received red blood cell (RBC) transfusions more often peri-operatively and larger volumes of intravenous fluids during anaesthesia were administered.
Fig. 1.
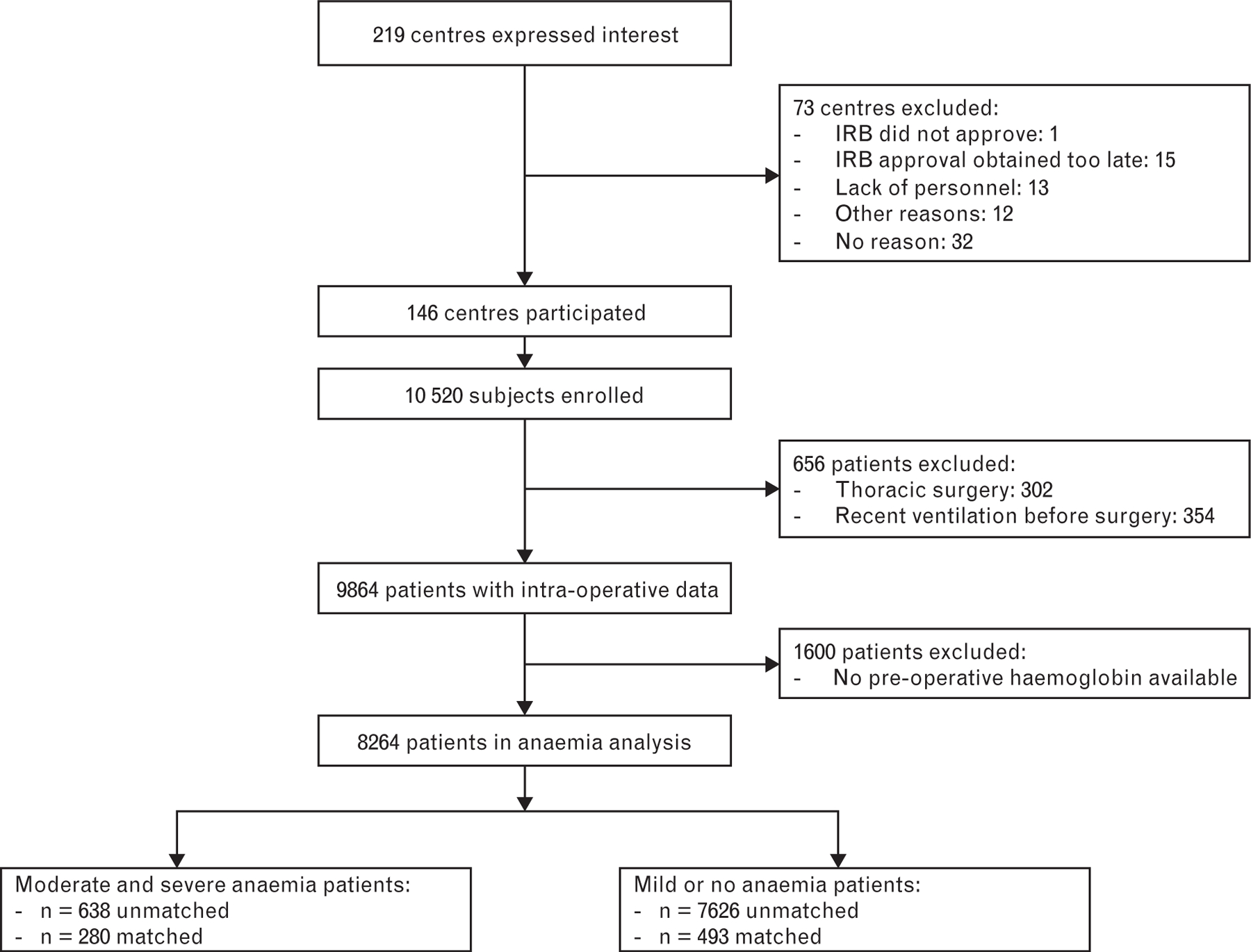
Flow chart of included patients
Table 1.
Baseline and intra-operative characteristics in unmatched cohort
| Moderate to severe anaemia (Hb < 11) (n = 638) | No or mild anaemia (Hb ≥ 11) (n = 7626) | P | |
|---|---|---|---|
| Age, years | 60 [45 to 73] | 54 [40 to 66] | <0.001 |
| Female sex | 402 (63.0) | 4169 (54.7) | <0.001 |
| Body mass index, kg m−2 | 24.9 [22.0 to 28.7] | 26.4 [23.4 to 30.0] | <0.001 |
| ASA physical status | 2 [2 to 3] | 2 [1 to 2] | <0.001 |
| Anaemia category | |||
| No anaemia (Hb >12a; >13b) | 6617 (80.1) | ||
| Mild anaemia (Hb 11 to 11.9a; 11 to 12.9b) | 1009 (12.2) | ||
| Moderate anaemia (Hb 8 to 10.9) | 608 (7.3) | ||
| Severe anaemia (Hb<8) | 30 (0.4) | ||
| ARISCAT | 32 [26 to 48] | 16 [3 to 26] | <0.001 |
| Functional status | <0.001 | ||
| Independent | 490 (76.9) | 7100 (93.1) | |
| Partially dependent | 122 (19.2) | 437 (5.7) | |
| Totally dependent | 25 (3.9) | 86 (1.1) | |
| COPD | 44 (6.9) | 479 (6.3) | 0.597 |
| Cancer | 91 (14.3) | 283 (3.7) | <0.001 |
| Chronic kidney failure | 70 (11.0) | 218 (2.9) | <0.001 |
| Heart failure | 70 (11.0) | 481 (6.3) | <0.001 |
| Preoperative red blood cell transfusion | 61 (9.6) | 14 (0.2) | <0.001 |
| Preoperative haemoglobin, g dl−1 | 10.0 [9.3 to 10.6] | 13.9 [12.9 to 14.9] | <0.001 |
| Condition surgery | <0.001 | ||
| Elective | 490 (76.8) | 6766 (88.7) | |
| Urgency | 110 (17.2) | 668 (8.8) | |
| Emergency | 38 (6.0) | 190 (2.5) | |
| Surgical technique | |||
| Open | 259 (40.6) | 1393 (18.3) | <0.001 |
| Laparoscopic | 86 (13.5) | 1403 (18.4) | 0.002 |
| Laparo-assisted | 9 (1.4) | 146 (1.9) | 0.454 |
| Peripheral | 103 (16.1) | 1343 (17.6) | 0.378 |
| Other | 191 (29.9) | 3389 (44.4) | <0.001 |
| Use of epidural anaesthesia | 67 (10.5) | 384 (5.0) | <0.001 |
| Antibiotic prophylaxis | 503 (79.0) | 5413 (71.0) | <0.001 |
| Duration of anaesthesia, min | 130 [85 to 206] | 105 [70 to 165] | <0.001 |
| Duration of surgery, min | 95 [55 to 165] | 75 [45 to 125] | <0.001 |
| Total fluid intake, l | 1.5 [1.0 to 2.0] | 1.0 [0.9 to 1.8] | <0.001 |
| Crystalloids, l | 1.2 [0.9 to 2.0] | 1.0 [0.7 to 1.5] | <0.001 |
| Colloids, l | 0.5 [0.4 to 0.5] | 0.5 [0.0 to 0.5] | 0.003 |
| Red blood cell transfusion during surgery | 109 (17.1) | 208 (2.7) | <0.001 |
Values are median [IQR] or n (%). ARISCAT, Assess Respiratory Risk in Surgical Patients in Catalonia score; ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; Hb, haemoglobin concentration in g dl−1.
Female patients.
Male patients.
Anaemic patients had a longer median length of hospital stay than non-anaemic patients: 9.0 [6.0 to 12.0] vs. 7.0 [5.0 to 9.0] days, P < 0.001, (Table 2). When corrected for confounding variables, preoperative anaemia independently increased length of hospital stay: mean difference 1.3 (95% CI 0.8 to 1.8), P < 0.001, (Table 3; see eFigure 1, Supplementary Digital Content, presenting probability of hospital discharge, http://links.lww.com/EJA/A452). Of note, RBC transfusion during surgery also increased LOS. There was an interaction between the presence of anaemia and transfusion during surgery with a LOS mean difference of 2.3 (95% CI 1.0 to 3.6) days, (P < 0.001), indicating that transfusion in an anaemic patient additionally increased hospital stay.
Table 2.
Patient outcomes and adverse events in unmatched cohort
| Moderate to severe anaemia (Hb < 11) (n = 638) | No or mild anaemia (Hb ≥ 11) (n = 7626) | P | |
|---|---|---|---|
| Hospital length of stay, days | 9.0 [6.0 to 12.0] | 7.0 [5.0 to 9.0] | <0.001 |
| In-hospital mortality | 14 (2.4) | 39 (0.6) | <0.001 |
| Intra-operative AEsa | 278 (43.7) | 2537 (33.3) | <0.001 |
| Hypotension | 231 (36.3) | 1929 (25.3) | <0.001 |
| Need for vasopressor | 191 (30.0) | 1615 (21.2) | <0.001 |
| Arrhythmia | 6 (0.9) | 47 (0.6) | 0.464 |
| PPCsb | 110 (17.7) | 787 (10.5) | <0.001 |
| Need for oxygen | 84 (13.5) | 639 (8.5) | <0.001 |
| Respiratory failure | 19 (3.1) | 133 (1.8) | 0.032 |
| Need for mechanical ventilation | 17 (2.7) | 84 (1.1) | 0.001 |
| ARDS | 1 (0.2) | 8 (0.1) | 1.000 |
| Pneumonia | 8 (1.3) | 28 (0.4) | 0.003 |
| Barotrauma | 1 (0.2) | 10 (0.1) | 1.000 |
Values are median [IQR] or number n (%).
Total intra-operative AEs, number and proportion of patients who developed at least one adverse event.
Total PPCs, number and proportion of patients who developed at least one postoperative pulmonary complication.ARDS, adult respiratory distress syndrome; Hb, haemoglobin concentration in g dl−1; PPC, postoperative pulmonary complication.
Table 3.
Multivariable analysis using hospital length of stay as outcome in unmatched moderate and severe anaemia cohort
| Mean difference (95%CI) | P | |
|---|---|---|
| Presence of anaemia | 1.30 (0.83 to 1.77) | <0.001 |
| Transfusion during surgery | 0.84 (0.21 to 1.48) | 0.009 |
| Age, years | 0.17 (0.02 to 0.32) | 0.028 |
| Female sex | 0.05 (−0.18 to 0.29) | 0.656 |
| Body mass index, kg m−2 | 0.00 (−0.12 to 0.12) | 0.982 |
| ASA physical status | 0.18 (0.02 to 0.34) | 0.028 |
| SpO2 | −0.12 (−0.27 to 0.03) | 0.125 |
| Smoker | −0.22 (−0.49 to 0.06) | 0.130 |
| COPD | −0.04 (−0.53 to 0.45) | 0.880 |
| Chronic kidney failure | 0.31 (−0.32 to 0.95) | 0.334 |
| Heart failure | −0.34 (−0.84 to 0.15) | 0.175 |
| Respiratory infection | 1.06 (0.49 to 1.63) | <0.001 |
| Type of surgery | ||
| Open | 1 (Reference) | |
| Laparoscopic | −0.57 (−0.87 to −0.28) | <0.001 |
| Condition of surgery | ||
| Elective | 1 (Reference) | |
| Urgency | 0.46 (0.02 to 0.90) | 0.040 |
| Emergency | 0.67 (-0.15 to 1.49) | 0.109 |
| Duration of anaesthesia | 0.65 (0.48 to 0.83) | <0.001 |
| Epidural anaesthesia | 0.98 (0.45 to 1.50) | <0.001 |
| Antibiotic prophylaxis | 0.39 (0.10 to 0.68) | 0.008 |
| Total fluid intake | 0.25 (0.08 to 0.42) | 0.004 |
ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease. Coefficient of the continuous variables were calculated after standardisation. Mixed-effect multivariable model considering centres as random-effects and using a Gaussian distribution.
Anaemic patients had intra-operative AEs more frequently, which included hypotension and need for use of vasopressors (Table 2). PPCs also occurred more often in anaemic patients, in particular the unplanned administration of supplemental oxygen, respiratory failure with a need for mechanical ventilation, and pneumonia.
More in-hospital deaths occurred in anaemic than non-anaemic or mildly anaemic patients (Table 2), and the presence of moderate to severe anaemia was an independent risk factor for mortality: OR 2.9 (95% CI 1.1 to 7.50), P = 0.029, (Table 4). However, the survival analysis did not confirm the association between preoperative anaemia and in-hospital mortality: OR 2.7 (95% CI 0.9 to 7.7), P = 0.071, (see eFigure 2, Supplementary Digital Content, showing probability of survival, http://links.lww.com/EJA/A452).
Table 4.
Multivariable analysis using mortality as outcome in unmatched moderate and severe anaemia cohort
| Odds Ratio (95% CI) | P | |
|---|---|---|
| Presence of anaemia | 2.88 (1.11 to 7.45) | 0.029 |
| Transfusion during surgery | 0.62 (0.18 to 2.07) | 0.436 |
| Age, years | 1.48 (0.89 to 2.48) | 0.134 |
| Female sex | 0.86 (0.4 to 1.86) | 0.707 |
| BMI, kg m−2 | 0.54 (0.33 to 0.88) | 0.014 |
| ASA physical status | 2 (1.29 to 3.11) | 0.002 |
| SpO2 | 0.72 (0.54 to 0.97) | 0.030 |
| Smoker | 0.59 (0.19 to 1.86) | 0.371 |
| COPD | 0.36 (0.07 to 1.88) | 0.228 |
| Chronic kidney failure | 0.73 (0.15 to 3.41) | 0.686 |
| Heart failure | 1.59 (0.58 to 4.36) | 0.366 |
| Respiratory infection | 1.8 (0.48 to 6.71) | 0.382 |
| Type of surgery | 1 (Reference) | |
| Open | ||
| Laparoscopic | 0.22 (0.03 to 1.7) | 0.146 |
| Condition of surgery | 1 (Reference) | |
| Elective | ||
| Urgency | 2.82 (1.11 to 7.14) | 0.029 |
| Emergency | 3.08 (0.84 to 11.34) | 0.091 |
| Duration of anaesthesia | 1.2 (0.84 to 1.73) | 0.313 |
| Epidural anaesthesia | 0.54 (0.11 to 2.58) | 0.442 |
| Antibiotic prophylaxis | 0.89 (0.33 to 2.43) | 0.824 |
| Total fluid intake | 1.31 (1.02 to 1.68) | 0.037 |
Coefficients of the continuous variables were calculated after standardisation. Mixed-effect multivariable model considering centres as random effects. ASA, American Society of Anesthesiologists; BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Propensity score-matched analysis
After matching, 280 moderate or severe anaemia patients and 493 no anaemia or mild anaemia patients could be compared. In terms of baseline characteristics, anaemic patients had a higher BMI and ASA score, a lower functional status, lower preoperative SpO2, and more respiratory infections. Intra-operative characteristics were comparable between groups, except for a higher intra-operative rate of transfusion of RBC in anaemic patients (Table 5). In accordance with the multivariable analysis, preoperative anaemia was associated with an increased LOS (Table 6, Fig. 2). In contrast with findings of the unmatched analysis, occurrences of intra-operative hypotension, PPCs and in-hospital mortality were not different between moderate or severe anaemia and mild or no anaemia patients.
Table 5.
Baseline and intra-operative characteristics in matched cohort with moderate and severe anaemia
| Moderate to severe anaemia (Hb < 11) (n = 280) | No or mild anaemia (Hb > 11) (n = 493) | SMD | P | |
|---|---|---|---|---|
| Age, years | 62 [47 to 73] | 57 [43 to 70] | 0.157 | 0.066 |
| Female gender | 170 (60.7) | 315 (63.9) | 0.066 | 0.423 |
| Body mass index, kg m−2 | 24.8 [21.9 to 28.4] | 26.1 [23.1 to 29.7] | 0.241 | 0.001 |
| ASA physical status | 2 [2 to 3] | 2 [2 to 3] | 0.168 | 0.029 |
| ARISCAT (with anaemia) | 29 [14 to 45] | 18 [15 to 38] | 0.511 | <0.001 |
| ARISCAT (without anaemia) | 26 [14 to 35] | 18 [15 to 38] | 0.106 | 0.428 |
| Functional status | 0.295 | <0.001 | ||
| Independent | 220 (78.6) | 440 (89.2) | ||
| Partially dependent | 52 (18.6) | 47 (9.5) | ||
| Totally dependent | 8 (2.9) | 6 (1.2) | ||
| SpO2 | 98 [96 to 99] | 98 [96 to 100] | 0.156 | 0.006 |
| Smoker | 55 (19.6) | 99 (20.1) | 0.011 | 0.958 |
| COPD | 21 (7.5) | 43 (8.7) | 0.045 | 0.648 |
| Cancer | 35 (12.5) | 57 (11.6) | 0.029 | 0.786 |
| Chronic kidney disease | 25 (8.9) | 32 (6.5) | 0.091 | 0.270 |
| Heart failure | 31 (11.1) | 39 (7.9) | 0.108 | 0.180 |
| Obstructive sleep apnoea | 1 (0.4) | 10 (2.0) | 0.154 | 0.116 |
| Respiratory infection | 18 (6.4) | 15 (3.0) | 0.160 | 0.040 |
| Haemoglobin, g dl−1 | 9.90 [9.2 to 10.5] | 13.40 [12.5 to 14.5] | 2.881 | <0.001 |
| Condition surgery | 0.160 | 0.076 | ||
| Elective | 230 (82.1) | 419 (85.0) | ||
| Urgency | 37 (13.2) | 65 (13.2) | ||
| Emergency | 13 (4.6) | 9 (1.8) | ||
| Surgical technique | ||||
| Open | 99 (35.4) | 177 (35.9) | 0.011 | 0.941 |
| Laparoscopic | 37 (13.2) | 87 (17.6) | 0.123 | 0.130 |
| Laparo-assisted | 5 (1.8) | 10 (2.0) | 0.018 | 1.000 |
| Peripheral | 53 (18.9) | 83 (16.8) | 0.055 | 0.525 |
| Other | 91 (32.5) | 138 (28.0) | 0.098 | 0.216 |
| Use of epidural anaesthesia | 26 (9.3) | 53 (10.8) | 0.049 | 0.601 |
| Antibiotic prophylaxis | 219 (78.2) | 359 (72.8) | 0.126 | 0.116 |
| Total fluid, ml | 1500 [1000 to 2000] | 1400 [1000 to 2000] | 0.037 | 0.633 |
| Crystalloid, ml | 1300 [886 to 2000] | 1181 [1000 to 2000] | 0.006 | 0.873 |
| Colloid, ml | 500 [250 to 750] | 500 [250 to 500] | 0.124 | 0.776 |
| Intra-operative RBC transfusion | 45 (16.1) | 46 (9.3) | 0.203 | 0.007 |
| Use of NMBA | 247 (88.2) | 454 (92.1) | 0.130 | 0.098 |
| Use of NMBA antagonist | 100 (35.7) | 197 (40.0) | 0.088 | 0.276 |
| Duration of anaesthesia, min | 125 [80 to 200] | 130 [80 to 200] | 0.001 | 0.711 |
| Duration of surgery, min | 95 [55 to 150] | 97 [55 to 160] | 0.036 | 0.588 |
Values are median [IQR] or n (%). ARISCAT, Assess Respiratory Risk in Surgical Patients in Catalonia score; ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; Hb, haemoglobin concentration; NMBA, neuromuscular blocking agent; RBC, red blood cell; SMD, standardised mean difference.
Table 6.
Patient outcomes and adverse events in matched cohort with moderate and severe anaemia
| Moderate to severe anaemia (Hb < 11) (n = 280) | No or mild anaemia (Hb > 11) (n = 493) | SMD | P | |
|---|---|---|---|---|
| Hospital length of stay, days | 4.0 [1.0 to 5.0] | 2.0 [0.0 to 5.0] | 0.139 | 0.001 |
| In-hospital mortality | 5 (1.9) | 3 (0.7) | 0.111 | 0.245 |
| Intra-operative complicationsa | 126 (45.0) | 206 (41.8) | 0.065 | 0.428 |
| Desaturation | 13 (4.6) | 22 (4.5) | 0.009 | 1.000 |
| Unplanned recruitment manoeuvre | 16 (5.7) | 24 (4.9) | 0.037 | 0.742 |
| Need for pressure reduction | 8 (2.9) | 21 (4.3) | 0.076 | 0.434 |
| Flow limitation | 2 (0.7) | 2 (0.4) | 0.042 | 0.955 |
| Hypotension | 106 (37.9) | 155 (31.4) | 0.135 | 0.083 |
| Need for vasopressor | 87 (31.1) | 134 (27.2) | 0.086 | 0.286 |
| Arrhythmia | 2 (0.7) | 6 (1.2) | 0.051 | 0.772 |
| Postoperative pulmonary complicationsb | 53 (19.0) | 70 (14.2) | 0.129 | 0.099 |
| Need for oxygen | 39 (14.0) | 57 (11.6) | 0.072 | 0.388 |
| Respiratory failure | 7 (2.5) | 10 (2.0) | 0.032 | 0.856 |
| Need for mechanical ventilation | 6 (2.2) | 11 (2.2) | 0.006 | 1.000 |
| ARDS | 0 (0.0) | 1 (0.2) | 0.064 | 1.000 |
| Pneumonia | 5 (1.8) | 3 (0.6) | 0.109 | 0.234 |
| Barotrauma | 1 (0.4) | 1 (0.2) | 0.029 | 1.000 |
Values are median [IQR] or number n (%).
Total intra-operative adverse events: number and proportion of patients who developed at least one adverse event.
Total postoperative pulmonary complications: number and proportion of patients who developed at least one postoperative pulmonary complication.ARDS, adult respiratory distress syndrome; SMD, standardised mean difference.
Fig. 2.
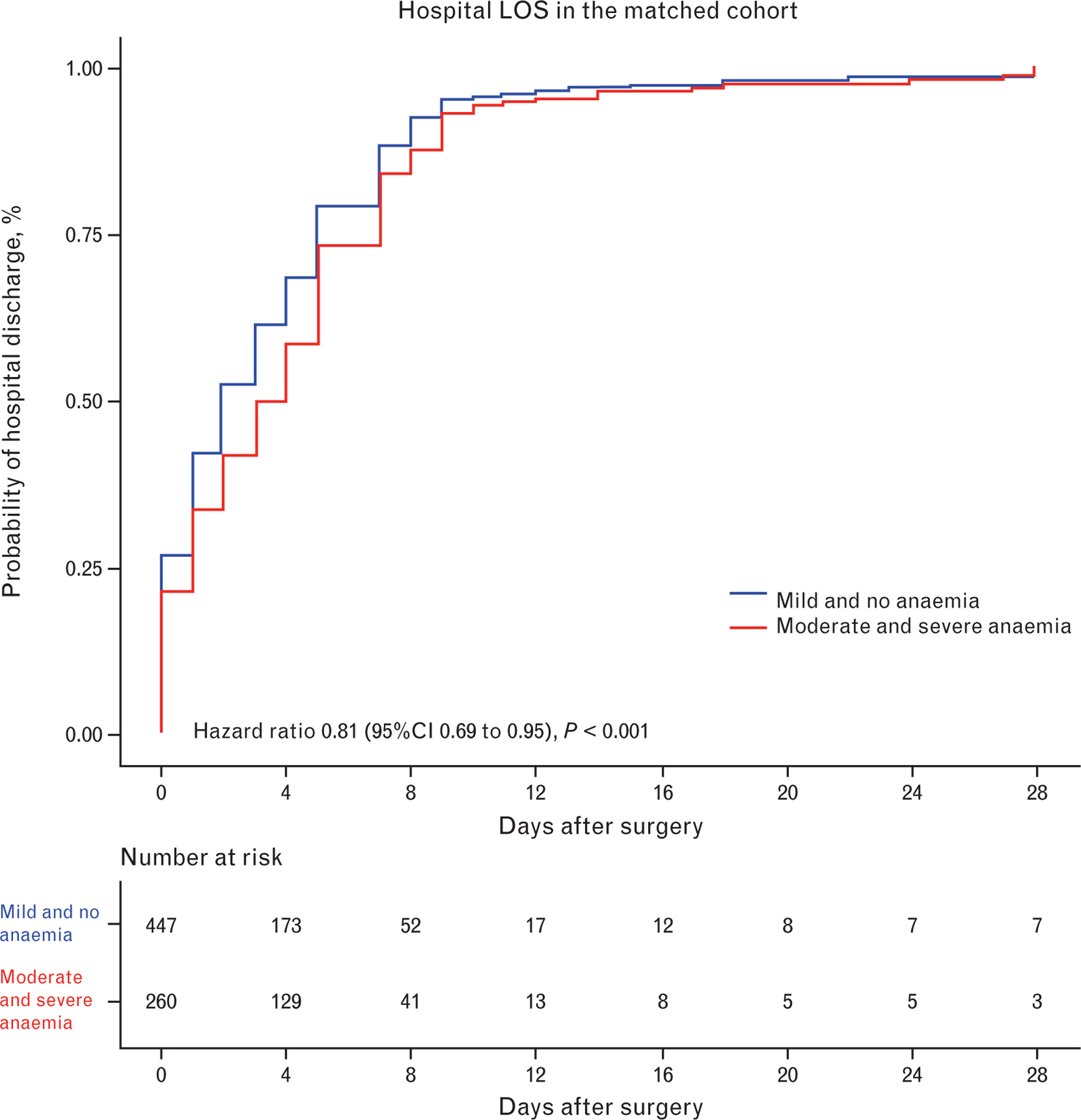
Probability of hospital discharge in matched patients with moderate to severe anaemia and patients with mild or no anaemia
The inclusion of patients with mild anaemia resulted in a total of 1644 patients with anaemia vs. 6617 non-anaemic patients (24.8%). The baseline and intra-operative characteristics of both groups are presented in eTable 1 (Supplementary Digital Content, http://links.lww.com/EJA/A452). Multivariable analysis showed an increase in LOS in anaemic patients: mean difference 0.9 (95% CI 0.6 to 1.2) days, P < 0.001, (Table 7; see eFigure 3, Supplementary Digital Content, presenting probability of hospital discharge, http://links.lww.com/EJA/A452). In contrast with the results of the main analysis, the mortality rate was not different between anaemic and non-anaemic patients: OR 1.9 (95% CI 0.9 to 4.3) days, P = 0.114, (Table 8; see eFigure 4, Supplementary Digital Content, presenting probability of survival, http://links.lww.com/EJA/A452).
Table 7.
Multivariable analysis using hospital length of stay as outcome in unmatched cohort (sensitivity analysis)
| Mean difference (95% CI) | P | |
|---|---|---|
| Presence of anaemia | 0.88 (0.56 to 1.19) | <0.001 |
| Transfusion during surgery | 0.95 (0.32 to 1.58) | 0.003 |
| Age, years | 0.16 (0.01 to 0.31) | 0.036 |
| Female gender | 0.07 (−0.16 to 0.31) | 0.537 |
| Body mass index, kg m−2 | 0.01 (−0.11 to 0.13) | 0.819 |
| ASA | 0.17 (0.01 to 0.33) | 0.033 |
| SpO2 | − 0.11 (−0.25 to 0.03) | 0.118 |
| Smoker | − 0.20 (−0.47 to 0.08) | 0.168 |
| COPD | − 0.05 (−0.55 to 0.44) | 0.830 |
| Chronic kidney failure | 0.24 (−0.39 to 0.88) | 0.453 |
| Heart failure | − 0.38 (−0.88 to 0.11) | 0.129 |
| Respiratory infection | 1.07 (0.50 to 1.64) | <0.001 |
| Type of surgery | ||
| Open | 1 (Reference) | |
| Laparoscopic | − 0.58 (−0.87 to −0.28) | <0.001 |
| Condition of surgery | ||
| Elective | 1 (Reference) | |
| Urgency | 0.41 (−0.04 to 0.85) | 0.071 |
| Emergency | 0.65 (−0.18 to 1.47) | 0.123 |
| Duration of anaesthesia | 0.63 (0.46 to 0.80) | <0.001 |
| Epidural anaesthesia | 0.95 (0.42 to 1.47) | <0.001 |
| Antibiotic prophylaxis | 0.38 (0.09 to 0.67) | 0.010 |
| Total fluid intake | 0.25 (0.08 to 0.42) | 0.004 |
Coefficients of the continuous variables were calculated after standardisation. Mixed-effect multivariable model considering centres as random effects and using a Gaussian distribution. ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease.
Table 8.
Multivariable analysis using mortality as outcome in unmatched cohort (sensitivity analysis)
| Odds Ratio (95% CI) | P | |
|---|---|---|
| Presence of anaemia | 1.92 (0.85 to 4.32) | 0.114 |
| Transfusion during surgery | 0.74 (0.24 to 2.29) | 0.606 |
| Age, years | 1.36 (0.81 to 2.28) | 0.240 |
| Female gender | 0.91 (0.43 to 1.96) | 0.817 |
| Body mass index, kg m−2 | 0.58 (0.36 to 0.94) | 0.026 |
| ASA | 2.06 (1.34 to 3.18) | 0.001 |
| SpO2 | 0.74 (0.57 to 0.96) | 0.021 |
| Smoker | 0.61 (0.19 to 1.89) | 0.388 |
| COPD | 0.35 (0.07 to 1.76) | 0.203 |
| Chronic kidney failure | 0.67 (0.15 to 3.05) | 0.601 |
| Heart failure | 1.63 (0.57 to 4.66) | 0.366 |
| Respiratory infection | 1.78 (0.48 to 6.65) | 0.391 |
| Type of surgery | ||
| Open | 1 (Reference) | |
| Laparoscopic | 0.19 (0.02 to 1.64) | 0.132 |
| Condition of surgery | ||
| Elective | 1 (Reference) | |
| Urgency | 2.53 (1.02 to 6.27) | 0.046 |
| Emergency | 2.85 (0.76 to 10.68) | 0.120 |
| Duration of anaesthesia | 1.13 (0.79 to 1.61) | 0.502 |
| Epidural anaesthesia | 0.51 (0.11 to 2.46) | 0.404 |
| Antibiotic prophylaxis | 0.85 (0.32 to 2.25) | 0.743 |
| Total fluid intake | 1.31 (1.02 to 1.68) | 0.032 |
Coefficients of the continuous variables were calculated after standardisation. Mixed-effect multivariable model considering centres as random-effects. ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease.
In the cohort used for the sensitivity analysis, 900 anaemic patients and 1636 non-anaemic patients could be matched. Anaemic patients were older, had a lower BMI, and a higher incidence of chronic kidney failure; intraoperative characteristics were comparable (see eTable 2, Supplementary Digital Content, http://links.lww.com/EJA/A452). Similar to the results in moderate and severe anaemia patients, in this matched cohort preoperative anaemia was associated with an increased LOS (Table 8; see eFigure 5, Supplementary Digital Content, presenting probability of hospital discharge, http://links.lww.com/EJA/A452). In-hospital mortality was not different between all anaemic and non-anaemic patients (see eFigure 6, Supplementary Digital Content, presenting probability of survival, http://links.lww.com/EJA/A452). Differences between intra-operative AEs and PPCs in the unmatched and matched cohort can be found in the Supplementary Digital Content (see eTables 3 and 4, http://links.lww.com/EJA/A452).
Discussion
The findings of this post hoc analysis in a large international cohort of patients undergoing different types of non-cardiac surgery can be summarised as follows: in unmatched and matched analysis, preoperative moderate to severe anaemia was associated with an increased length of hospital stay; in an unmatched analysis, preoperative moderate to severe anaemia was associated with increased occurrence of intra-operative hypotension, PPCs and in-hospital mortality, although this was not confirmed in a matched analysis; when the analysis included mildly anaemic patients, the association between anaemia and length of hospital stay remained, but there was no longer an association with in-hospital mortality.
The strength of this post hoc analysis is that it included data from a large international cohort from 146 centres in 29 countries across five continents (LAS VEGAS study).16 Results from our analysis therefore represent diverse aetiologies of, and approaches to, preoperative patients for non-cardiac surgical procedures. The 1-week design of the LAS VEGAS study avoids effects of changes over time as the data were collected within a narrow time window. In addition to mixed-effect multivariable models, propensity score-matched analyses were used to assess the consistency of the findings.
The number of patients with moderate to severe anaemia in our population was lower than in previous reports. In comparison, a European study showed preoperative anaemia in 31.1% of men and 26.5% of women.18 More recently, an international study in 27 low-, middle- and high-income countries worldwide reported a prevalence of 30.1%.19 The variance can be explained partly by the difference in study populations. The LAS VEGAS database included relatively young and healthy patients compared with other studies. Also, a different cut-off was used for anaemia in the present analysis. For the present analysis, anaemia was defined as a haemoglobin concentration <11.0 g dl−1, which includes moderate and severe anaemia according to the currently used WHO classification. This cut-off level was chosen because it comprises a clinically relevant level of anaemia in both women and men. In a selection of other studies, mildly anaemic patients were also included in the cohorts analysed (Hb ≥ 11 g dl−1 and < 12 g dl−1 in women, Hb ≥ 11 g dl−1 1 and < 13 g dl−1 in men according to WHO). The difference in cut-off levels hampers comparison between studies.
In the 1980s, the first report was published on a possible association between preoperative haemoglobin concentration and outcomes after surgery.20 The current finding that preoperative anaemia is associated with an increased length of hospital stay confirms the findings of several previous studies.14,18,19,21 The propensity score-matched analysis confirms the association between anaemia and prolonged in-hospital stay, even when different statistical models were used (see eTable 5, Supplementary Digital Content, http://links.lww.com/EJA/A452).
The association between preoperative moderate to severe anaemia and intra-operative AEs or PPCs has been less well studied so far. Similar to the current investigation, anaemia does not increase the chance of arrhythmias but the major morbidity rate is higher in anaemic patients.22 Studies on adverse cardiac events in anaemic patients showed contrasting results; both positive23,24 and negative associations14,19,25 have been found. Specific definitions for adverse cardiac events differ among publications, but none include intra-operative hypotension or vasopressor use. An association between moderate to severe anaemia and PPCs was established previously with the introduction of the ARISCAT risk score for PPC.26 Several studies could be identified reporting increased respiratory morbidity in patients with preoperative anaemia.24,27,28
The results of the current analysis regarding an association between preoperative moderate to severe anaemia and 30-day mortality are not unequivocal. The findings of the multivariable model could not be confirmed in the survival or propensity score-matched analysis. The absence of a distinct association between preoperative anaemia and mortality contrasts with other data. One of the largest studies in non-cardiac surgery showed an increased risk of mortality for preoperative haematocrit levels < 39%.9 Others reported an increased mortality in their studies in anaemic patients.13,14,23,24,29 In 2015, a meta-analysis showed that preoperative anaemia is associated with increased 30-day mortality.15 This meta-analysis was limited by a high level of heterogeneity between studies, due to inclusion of both cardiac and non-cardiac studies, use of different definitions of anaemia and availability of data. A lack of power, particularly in the CBPS matched analysis could be responsible for the difference compared with our findings. Last, but not least, patients in the LAS VEGAS cohort were relatively young in age, which might also contribute to finding no association.
In the current analysis, patients with moderate and severe anaemia more frequently received a RBC transfusion during surgery. Furthermore, if these patients received an intra-operative transfusion, LOS was additionally increased. Whether RBC transfusion in anaemic patients is actually beneficial was previously studied retrospectively.8,30 Patients at increased cardiac risk for non-cardiac surgery were more likely to have preoperative anaemia and receive a postoperative transfusion. Interestingly, RBC transfusion lowered mortality only when the haemoglobin concentration was below 8.0 g dl−1. Similarly, in major vascular surgery patients a liberal peri-operative transfusion regimen in anaemic patients was associated with more postoperative adverse events.31 In the present cohort, intra-operative RBC transfusion in anaemic patients was negatively associated with the primary outcome, suggesting an additional burden for these patients. In order to prevent allogeneic RBC transfusion in anaemic surgical patients, several algorithms for evaluation and treatment of preoperative anaemia including iron therapy have been published recently.32–34 Also, a tool for prediction of peri-operative risk of transfusion was introduced.35 Knowledge about the aetiology of anaemia and the peri-operative risk of transfusion may guide individual strategy in anaemic patients.
The focus of this analysis was on patients with the clinically most relevant forms of anaemia, that is moderate and severe anaemia. A sensitivity analysis also including patients with mild anaemia revealed that the prevalence of preoperative anaemia increased to 24.8%, which is more in line with other studies. In this group, the association between the presence of anaemia and an increased LOS was confirmed. Interestingly, mortality was no longer associated with any form of anaemia, either in the multivariable analysis, or in the matched analysis. This may indicate that in-hospital mortality is associated mainly with the most severe forms of preoperative anaemia.
This analysis has several limitations. This was a post hoc analysis of the LAS VEGAS study, and the results should be interpreted with caution. Haemoglobin values were not reported in 16% of the LAS VEGAS population, and removal of these patients from the current analyses may have introduced a selection bias. Our propensity score-matched cohort was considerably smaller than the original cohort. The number of non-anaemic subjects in the original cohort was significantly larger than the number of anaemic patients. As a consequence, a large proportion of non-anaemic patients remained unmatched and were excluded, leading to a smaller distribution of variables in the matched subpopulation. This effect of propensity matching might explain why secondary patient outcomes such as hypotension and postoperative pulmonary complications were no longer associated with preoperative anaemia. It should be stressed that the design of the LAS VEGAS study excludes the possibility of determining cause-effect relationships and does not allow the defining of any pathophysiological associations with the outcome measures. Finally, we observed that anaemic patients more often had preoperative blood transfusions. The exact number and timing of transfusions and whether these transfusions were part of a patient blood management programme (PBM) is unknown, as this information was not collected in the LAS VEGAS study. Of note, although PBM was mentioned as early as in 2005 and adopted by the WHO in 2011, it is not until recently that major reviews and consensus statements on implementation of PBM programmes and the effect on outcome have been published. Because the data of the present study were collected several years ago, only a minority of participating centres had a fully functioning PBM programme at the time of collection of data.
In conclusion, in this international non-cardiac surgical cohort, preoperative anaemia as defined by haemoglobin concentration < 11.0 g dl−1 was associated with an increased length of hospital stay but not with increased occurrences of intra-operative hypotension, number of PPCs and in-hospital mortality. Future studies focusing on preoperative optimisation of anaemia are necessary to determine whether hospital stay in anaemic patients can be improved.
Supplementary Material
Acknowledgements relating to this article
Assistance with the study: the LAS VEGAS study Collaborators collected the original data for the LAS VEGAS trial. A full list of Collaborators can be found in the Supplementary Digital Content, http://links.lww.com/EJA/A452.
Financial support and sponsorship:
the LAS VEGAS study was endorsed and partly funded by a research grant from the European Society of Anaesthesiology through the Clinical Trial Network, and by the Academic Medical Center, Amsterdam, The Netherlands. For the current posthoc analysis there was no additional funding. MF-VM was funded by NIH-NHLBI UH3 HL140177.
Footnotes
Conflicts of interest: none.
Presentation: none.
Contributor Information
Carolien S.E. Bulte, Department of Anaesthesiology, Amsterdam UMC, VU University Amsterdam, Amsterdam, The Netherlands.
Christa Boer, Department of Anaesthesiology, Amsterdam UMC, VU University Amsterdam, Amsterdam, The Netherlands.
Sabrine N.T. Hemmes, Department of Intensive Care and Laboratory of Experimental Intensive Care and Anaesthesia (L·E·I·C·A); Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Department of Anaesthesiology, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands
Ary Serpa Neto, Department of Intensive Care and Laboratory of Experimental Intensive Care and Anaesthesia (L·E·I·C·A); Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Department of Critical Care Medicine, Hospital Israelita Albert Einstein, São Paolo, Brazil.
Jan M. Binnekade, Department of Intensive Care and Laboratory of Experimental Intensive Care and Anaesthesia (L·E·I·C·A); Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands
Goran Hedenstierna, Hedenstierna Laboratory, Department of Surgical Sciences, Uppsala University, Uppsala, Sweden.
Samir Jaber, Montpellier University Hospital, Saint Eloi Intensive Care Unit and PhyMedExp, University of Montpellier, INSERM, CNRS, Montpellier, France.
Michael Hiesmayr, Division of Cardiac, Thoracic, Vascular Anaesthesia and Intensive Care, Medical University of Vienna, Vienna, Austria.
Markus W. Hollmann, Department of Anaesthesiology, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands
Gary H. Mills, Operating Services, Critical Care and Anaesthesia, Sheffield Teaching Hospitals, Sheffield and University of Sheffield, Sheffield, UK
Marcos F. Vidal Melo, Department of Anaesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Bostan, MA, USA.
Rupert M. Pearse, Queen Mary University of London, London, UK
Christian Putensen, Department of Anaesthesiology and Intensive Care Medicine, University Hospital Bonn, Bonn, Germany.
Werner Schmid, Division of Cardiac, Thoracic, Vascular Anaesthesia and Intensive Care, Medical University of Vienna, Vienna, Austria.
Paolo Severgnini, Department of Biotechnology and Sciences of Life, ASST Sette Laghi Ospedale di Circolo e Fondazione Macchi, University of Insubria, Varese, Italy.
Hermann Wrigge, Department of Anaesthesiology and Intensive Care Medicine, University of Leipzig, Leipzig, Germany.
Marcelo Gama de Abreu, Department of Anaesthesiology and Intensive Care Medicine, Pulmonary Engineering Group, University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany.
Paolo Pelosi, Dipartimento di Scienze Chirurgiche e Diagnostiche Integrate, Universitá degli Studi di Genova, Genova, Italy, IRCCS Ospedale Policlinico San Martino, Genova, Italy.
Marcus J. Schultz, Department of Intensive Care and Laboratory of Experimental Intensive Care and Anaesthesia (L·E·I·C·A); Mahidol Oxford Tropical Medicine Research Unit (MORU), Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands, Mahidol University, Bangkok, Thailand, Nuffield Department of Medicine, University of Oxford, Oxford, UK
References
- 1.Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014; 123:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bierbaum BE, Callaghan JJ, Galante JO, et al. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am 1999; 81:2–10. [DOI] [PubMed] [Google Scholar]
- 3.Goodnough LT, Vizmeg K, Sobecks R, et al. Prevalence and classification of anemia in elective orthopedic surgery patients: implications for blood conservation programs. Vox Sang 1992; 63:90–95. [DOI] [PubMed] [Google Scholar]
- 4.Greenky M, Gandhi K, Pulido L, et al. Preoperative anemia in total joint arthroplasty: is it associated with periprosthetic joint infection? Clin Orthop Relat Res 2012; 470:2695–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heschl M, Gombotz H, Haslinger-Eisterer B, et al. The efficacy of preoperative preparation with intravenous iron and/or erythropoietin in anaemic patients undergoing orthopaedic surgery: an observational study. Eur J Anaesthesiol 2018; 35:289–297. [DOI] [PubMed] [Google Scholar]
- 6.Lasocki S, Krauspe R, Heymann von C, et al. PREPARE: the prevalence of perioperative anaemia and need for patient blood management in elective orthopaedic surgery. A multicentre, observational study. Eur J Anaesthesiol 2015; 32:160–167. [DOI] [PubMed] [Google Scholar]
- 7.Klein AA, Collier TJ, Brar MS, et al. The incidence and importance of anaemia in patients undergoing cardiac surgery in the UK - the first Association of Cardiothoracic Anaesthetists’ national audit. Anaesthesia 2016; 71:627–635. [DOI] [PubMed] [Google Scholar]
- 8.Feng S, Machina M, Beattie WS. Influence of anaemia and red blood cell transfusion on mortality in high cardiac risk patients undergoing major noncardiac surgery: a retrospective cohort study. Br J Anaesth 2017; 118:843–851. [DOI] [PubMed] [Google Scholar]
- 9.Wu W-C, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA 2007; 297:2481–2488. [DOI] [PubMed] [Google Scholar]
- 10.Ludwig H, Van Belle S, Barrett-Lee P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 2004; 40:2293–2306. [DOI] [PubMed] [Google Scholar]
- 11.Wilson MJ, Dekker JWT, Buettner S, et al. The effect of intravenous iron therapy on long-term survival in anaemic colorectal cancer patients: results from a matched cohort study. Surg Oncol 2018; 27:192–199. [DOI] [PubMed] [Google Scholar]
- 12.Melis M, McLoughlin JM, Dean EM, et al. Correlations between neoadjuvant treatment, anemia, and perioperative complications in patients undergoing esophagectomy for cancer. J Surg Res 2009; 153:114–120. [DOI] [PubMed] [Google Scholar]
- 13.Beattie WS, Karkouti K, Wijeysundera DN, Tait G. Risk associated with preoperative anemia in noncardiac surgery: a single-center cohort study. Anesthesiology 2009; 110:574–581. [DOI] [PubMed] [Google Scholar]
- 14.Saager L, Turan A, Reynolds LF, et al. The association between preoperative anemia and 30-day mortality and morbidity in noncardiac surgical patients. Anesth Analg 2013; 117:909–915. [DOI] [PubMed] [Google Scholar]
- 15.Fowler AJ, Ahmad T, Phull MK, et al. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br J Surg 2015; 102:1314–1324. [DOI] [PubMed] [Google Scholar]
- 16.LAS VEGAS investigators. Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS - an observational study in 29 countries. Eur J Anaesthesiol 2017; 34:492–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System 2011; http://www.who.int/vmnis/indicators/haemoglobin.pdf [Accessed 18 November 2020]. [Google Scholar]
- 18.Baron DM, Hochrieser H, Posch M, et al. Preoperative anaemia is associated with poor clinical outcome in noncardiac surgery patients. Br J Anaesth 2014; 113:416–423. [DOI] [PubMed] [Google Scholar]
- 19.Fowler AJ, Ahmad T, Abbott TEF, et al. Association of preoperative anaemia with postoperative morbidity and mortality: an observational cohort study in low-, middle-, and high-income countries. Br J Anaesth 2018; 121:1227–1235. [DOI] [PubMed] [Google Scholar]
- 20.Carson JL, Poses RM, Spence RK, Bonavita G. Severity of anaemia and operative mortality and morbidity. Lancet 1988; 1:727–729. [DOI] [PubMed] [Google Scholar]
- 21.Tee MC, Shubert CR, Ubl DS, et al. Preoperative anemia is associated with increased use of hospital resources in patients undergoing elective hepatectomy. Surgery 2015; 158:1027–1038. [DOI] [PubMed] [Google Scholar]
- 22.Ranucci M, Di Dedda U, Castelvecchio S, et al. Impact of preoperative anemia on outcome in adult cardiac surgery: a propensity-matched analysis. Ann Thorac Surg 2012; 94:1134–1141. [DOI] [PubMed] [Google Scholar]
- 23.Gupta PK, Sundaram A, Mactaggart JN, et al. Preoperative anemia is an independent predictor of postoperative mortality and adverse cardiac events in elderly patients undergoing elective vascular operations. Ann Surg 2013; 258:1096–1102. [DOI] [PubMed] [Google Scholar]
- 24.Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in noncardiac surgery: a retrospective cohort study. Lancet 2011; 378:1396–1407. [DOI] [PubMed] [Google Scholar]
- 25.Kulier A, Levin J, Moser R, et al. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation 2007; 116:471–479. [DOI] [PubMed] [Google Scholar]
- 26.Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010; 113:1338–1350. [DOI] [PubMed] [Google Scholar]
- 27.Tohme S, Varley PR, Landsittel DP, et al. Preoperative anemia and postoperative outcomes after hepatectomy. HPB 2016; 18: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oshin OA, Torella F. Low hemoglobin concentration is associated with poor outcome after peripheral arterial surgery. Vasc Endovascular Surg 2013; 47:449–453. [DOI] [PubMed] [Google Scholar]
- 29.Velescu A, Clará A, Cladellas M, et al. Anemia increases mortality after open or endovascular treatment in patients with critical limb ischemia: a retrospective analysis. Eur J Vasc Endovasc Surg 2016; 51:543–549. [DOI] [PubMed] [Google Scholar]
- 30.Gabriel RA, Clark AI, Nguyen AP, et al. The association of preoperative hematocrit and transfusion with mortality in patients undergoing elective noncardiac surgery. World J Surg 2018; 42:1939–1948. [DOI] [PubMed] [Google Scholar]
- 31.Bursi F, Barbieri A, Politi L, et al. Perioperative red blood cell transfusion and outcome in stable patients after elective major vascular surgery. Eur J Vasc Endovasc Surg 2009; 37:311–318. [DOI] [PubMed] [Google Scholar]
- 32.Munting KE, Klein AA. Optimisation of preoperative anaemia in patients before elective major surgery - why, who, when and how? Anaesthesia 2019; 74 Suppl 1:49–57. [DOI] [PubMed] [Google Scholar]
- 33.Muñoz M, Acheson AG, Auerbach M, et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2016; 72:233–247. [DOI] [PubMed] [Google Scholar]
- 34.Mueller MM, Van Remoortel H, Meybohm P, et al. Patient blood management: recommendations from the 2018 Frankfurt consensus conference. JAMA 2019; 321:983–997. [DOI] [PubMed] [Google Scholar]
- 35.Klein AA, Collier T, Yeates J, et al. The ACTA PORT-score for predicting perioperative risk of blood transfusion for adult cardiac surgery. Br J Anaesth 2017; 119:394–401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


