Abstract
Background
Prevention and timely treatment of gestational diabetes mellitus (GDM) are important to the prognosis of pregnant women and neonates. We aimed to conduct a meta-analysis to evaluate the effects and safety of vitamin D supplementation on GDM patients and neonates, to provide insights into clinical GDM treatment.
Methods
Two authors searched the Medline, PubMed, Cochrane Library, Web of Science, Embase, CNKI, and Wanfang databases for randomized controlled trials (RCTs) on the effects and safety of vitamin D supplementation in GDM patients. The quality of the included RCTs was evaluated according to Cochrane handbook. RevMan 5.3 software was used for statistical analysis.
Results
A total of 20 RCTs involving 1682 GDM patients were finally included, of whom 837 received vitamin D supplementation. Vitamin D supplementation in GDM patients increased the serum 25(OH)D level (SMD = 4.07, 95% CI: (2.73, 5.41)) and HDL level (SMD = 0.41, 95% CI: (0.23, 0.58)) and reduced serum LDL (SMD = −0.49, 95% CI: (−0.68, −0.29)), TG (SMD = −0.59, 95% CI: (−1.01, −0.17)), and TC (SMD = −0.67, 95% CI: (−1.19, −0.14)) levels in GDM patients (all P < 0.05). Besides, vitamin D supplementation reduced the risk of premature birth (OR = 0.37, 95% CI: (0.22, 0.62)), hyperbilirubinemia (OR = 0.38, 95% CI: (0.25, 0.58)), and neonatal hospitalization (OR = 0.38, 95% CI: (0.25, 0.58)) of neonates (all P < 0.05). No significant publication bias in synthesized results was found (all P > 0.05).
Conclusions
Vitamin D supplementation improves the blood lipid level in GDM patients and reduces adverse neonatal outcomes. The dose and duration of vitamin D supplementation for safety need to be further investigated in future high-quality studies.
1. Background
Gestational diabetes mellitus (GDM) is a metabolic disorder, in which glucose tolerance is normal before pregnancy and abnormality occurs for the first time during pregnancy [1]. Previous studies [2–4] have shown that GDM can increase the risk of various perinatal complications, such as hypertensive disorders of pregnancy, polyhydramnios, fetal distress, and preterm birth. Besides, GDM is closely associated with the long-term health impairment of patients and offspring [5]. For example, the incidence of postpartum type 2 diabetes in GDM women is significantly higher than that of normal pregnant women, and the risk of metabolic syndrome in their offspring increases [6]. Previous studies [7, 8] have pointed out that the incidence of GDM has increased significantly in recent years with changes in lifestyle and increasing maternal age. Some studies [9, 10] have pointed out that the incidence of GDM in China is at the middle level, whereas it is at the upper level in the world, and the incidence of GDM is as high as 15.08%. Therefore, the prevention and treatment of GDM are important to the prognosis of pregnant women and newborns.
Studies [11, 12] have shown that vitamin D, as a micronutrient, has a certain correlation with GDM and various adverse maternal and infant outcomes. Studies [13, 14] have shown that in order to maintain the growth of fetal bones during pregnancy, the consumption of vitamin D in pregnant women increases significantly, which can lead to general insufficiency or even deficiency of vitamin D in women after pregnancy. Among them, pregnant women with GDM are a high-risk group of vitamin D deficiency. Supplementation with vitamin D preparations is an important way to prevent vitamin D deficiency during pregnancy. At present, the effect of vitamin D supplementation on GDM is the focus of research by many scholars. There are many domestic and foreign studies on the effects and safety of vitamin D supplementation, but sample sizes are small, and results are not inconsistent. Therefore, this study assessed the effect of vitamin D supplementation on blood lipid levels in GDM patients by conducting a meta-analysis of published randomized controlled trials (RCTs) on the efficacy and safety of vitamin D supplementation in pregnant women with GDM, to evaluate the effects and safety of vitamin D supplementation in GDM women, thereby providing reliable evidence for the treatment of GDM.
2. Methods
This meta-analysis and systematic review was conducted and performed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [15].
2.1. Retrieval Strategy
We searched the Medline, PubMed, Cochrane Library, Web of Science, Embase, CNKI, and Wanfang databases for RCTs on the effects and safety of vitamin D supplementation in pregnant women with GDM. The search date limit of databases was from the establishment of the database to May 15, 2022. The search formula used in this meta-analysis was (vitamin D OR 25-(OH)D OR 1.25(OH)2D) AND (gestational diabetes OR GDM OR diabetes and pregnancy). In addition, we performed additional searches for the references of the included RCTs and relevant reviews to make literature research more comprehensive.
2.2. Inclusion and Exclusion Criteria for RCTs
The inclusion criteria for this meta-analysis were as follows: (1) RCT study design, the published language was Chinese or English; (2) patients diagnosed with GDM according to clear diagnostic criteria; (3) the intervention group was supplemented with vitamin D, and the control group was supplemented with placebos or without vitamin D supplementation; (4) relevant data could be extracted. The exclusion criteria for this meta-analysis were as follows: (1) the types of literature studies were case reports and reviews; (2) articles with repeated reports; and (3) the data on outcomes could not be extracted.
2.3. Literature Quality Evaluation
Two researchers independently completed quality evaluation and data extraction, then cross-checked the work, and discussed and resolved any disagreements. The quality of the included studies was evaluated in accordance with the evaluation criteria recommended by the Cochrane Systematic Review Guidebook [16]. Evaluation contents mainly included the following seven aspects: generation of random sequences, concealment of assignment sequence, blinding of all study participants and personnel, blinding of outcome assessments, completeness of outcome data, selective outcome reporting, and other sources of bias. Every item could be rated “yes,” “no,” or “unclear” accordingly.
2.4. Data Extraction
Two authors screened the identified articles and extracted data accordingly. The data extraction content of this meta-analysis included first author, publication time, region, age, GDM diagnostic criteria, vitamin D testing method, sample size, intervention details of intervention and control groups, and study outcome indicators. Outcomes extracted from this meta-analysis were serum 25-hydroxyvitamin D levels, total cholesterol (TC), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), triglycerides (TG), incidence of hyperbilirubinemia, premature birth, and neonatal hospitalization.
2.5. Statistical Analysis
This meta-analysis used RevMan 5.3 software for statistical analysis. We tried to transform and uniform the units of vitamin D measurement to make results consistent. The standardized mean difference (SMD) and the odds ratio (OR) were used to calculate effect statistics and the 95% confidence interval (CI), and the chi-square test (test level was 0.1) was used to evaluate heterogeneity. When the homogeneity of the research results was good (P > 0.1, when I2 < 50%), a fixed-effect model was used; otherwise (P ≤ 0.1, when I2 ≥ 50%), a random-effect model was used. In addition, pooled effect sizes were re-estimated after excluding individual studies in turn, and data were reanalyzed using different statistical methods to test the sensitivity of the results. We used funnel plot symmetry to judge whether there was publication bias, and we performed Egger regression analysis to evaluate the publication bias of the literature. In this meta-analysis, P < 0.05 was considered to be statistically significant between groups.
3. Results
3.1. Study Selection and Characteristics
As indicated in Figure 1, 232 studies were initially identified, and after filtering layer by layer, a total of 20 RCTs [17–36] were included. Of the 20 included RCTs, a total of 1682 patients were involved, of whom 837 received vitamin D supplementation. The characteristics of the included RCTs are presented in Table 1.
Figure 1.
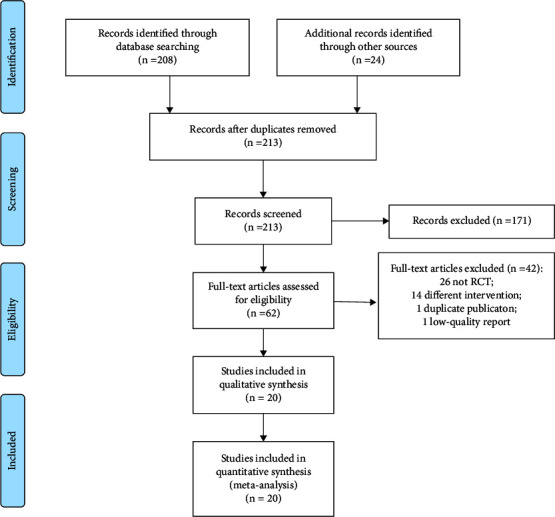
PRISMA flow diagram of study selection.
Table 1.
Characteristics of the included RCTs.
| RCT | Country | Age (y) | Sample size | Detection method | Intervention | Duration of follow-up (weeks) | ||
|---|---|---|---|---|---|---|---|---|
| Vitamin D group | Control group | Vitamin D group | Control group | |||||
| Asemi 2013 | Iran | 31.5 ± 6.1 | 27 | 27 | ELISA | 50,000 U VD3/21 d, 2 times/day | Placebo | 6 |
| Asemi 2014 | Iran | 18∼40 | 28 | 28 | ELISA | VD3 50,000 U/2 week | Placebo | 6 |
| Asemi 2015 | Iran | 30.9 ± 5.8 | 22 | 23 | ELISA | 50,000 U VD3/21 d, 2 times/day | Placebo | 6 |
| Deng 2020 | China | 18∼35 | 30 | 30 | ELISA | 400 U VD3/d | Placebo | 8 |
| Jamilian 2016 | Iran | 28.4 ± 6.2 | 30 | 30 | ELISA | 1000 U VD3/d | Placebo | 6 |
| Jamilian 2017 | Iran | 18∼40 | 35 | 35 | ELISA | VD3 50,000 U/2 week | Placebo | 6 |
| Jamilian 2018 | Iran | 18∼35 | 30 | 28 | ELISA | VD3 50,000 U/2 week | Placebo | 6 |
| Jamilian 2019 | Iran | 18∼35 | 30 | 30 | ELISA | 200 U VD3/d | Placebo | 6 |
| Jin 2017 | China | 18∼35 | 29 | 30 | ELISA | 2000 U VD3/d | Black control | 6 |
| Karamali 2015 | Iran | 18∼40 | 30 | 30 | ELISA | 50,000 U VD3/21 d, 2 times/day | Placebo | 6 |
| Karamali 2018 | Iran | 18∼40 | 30 | 31 | ELISA | 200 U VD3/d, 2 times/day | Placebo | 6 |
| Li 2016 | China | 28.0 ± 4.0 | 48 | 49 | ELISA | 500 U VD3/d | Placebo | 16 |
| Li 2019 | China | 35.2 ± 5.2 | 45 | 45 | ECL | 400 U VD3/d | Black control | 6 |
| Li 2020 | China | 20∼40 | 52 | 52 | ELISA | 400 U VD3/d | Placebo | 6 |
| Mao 2019 | Iran | 18∼35 | 59 | 59 | ELISA | 400 U VD3/d | Placebo | 8 |
| Valizadeh 2016 | Iran | 32.0 ± 5.0 | 48 | 48 | ELISA | 700,000 U VD3 in total | Placebo | 5 |
| Yazdchi 2016 | Iran | 31.9 ± 4.0 | 36 | 36 | ECL | VD3 50,000 U/2 week | Placebo | 8 |
| Yue 2019 | China | 18∼35 | 116 | 122 | ECL | 1200 U VD3/d | Placebo | 16 |
| Zhang 2020 | China | 25∼30 | 52 | 52 | ELISA | 400 U VD3/d | Placebo | 2 |
| Zhou 2018 | China | 18∼35 | 60 | 60 | ECL | 400 U VD3/d | Black control | 16 |
3.2. Quality of Included RCTs
The quality assessment of the literature included in this meta-analysis is shown in Supplementary Figures 1 and 2. All the included RCTs adopted the principle of randomized control, data integrity was good, and there was no other bias. However, some RCTs [27, 28, 30, 34, 35] did not explain the concealment of the allocation sequence and the use of the blinding method. All RCTs used internationally certified standard methods to measure outcome indicators, and some studies were lost to follow-up.
3.3. Meta-Analysis
3.3.1. Serum 25(OH)D Level
Nine RCTs [17, 18, 20, 23, 24, 26, 27, 35, 36] reported the serum 25(OH)D level. There was statistical heterogeneity among the analyzed data (I2 = 97%, P < 0.001), so a random-effect model was used for the meta-analysis, and the results showed that vitamin D supplementation intervention could significantly increase serum 25(OH)D levels in GDM patients (SMD = 4.07, 95% CI: (2.73, 5.41), P < 0.001, Figure 2(a)).
Figure 2.
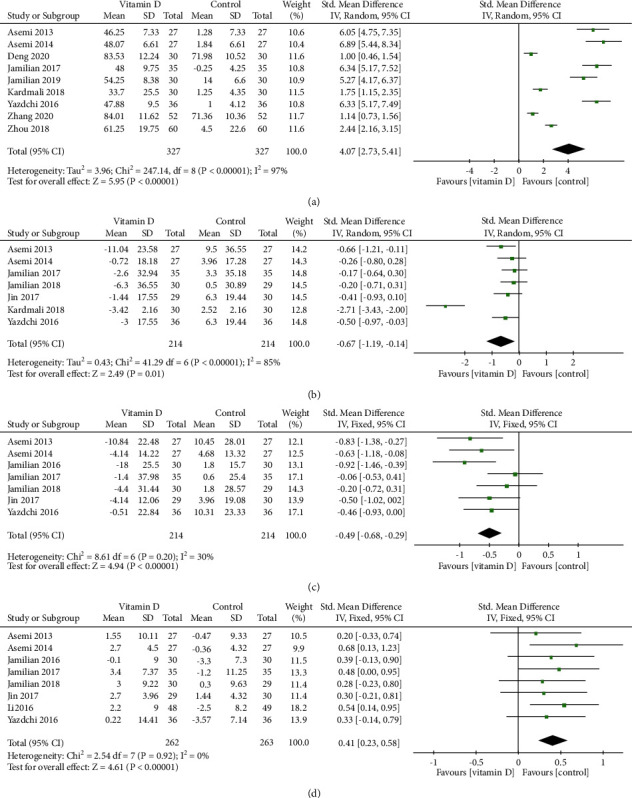
Forest plots for (a) the serum 25(OH)D level, (b) the serum TC level, (c) the serum LDL level, and (d) the serum HDL level.
3.3.2. Serum TC Level
Seven RCTs [17, 18, 21, 24, 26, 29, 35] reported the serum TC level. There was statistical heterogeneity among the analyzed data (I2 = 85%, P < 0.001), so a random-effect model was used for the meta-analysis, and the results showed that vitamin D supplementation intervention could significantly reduce the TC levels in GDM patients (SMD = −0.67, 95% CI: (−1.19, −0.14), P=0.01, Figure 2(b)).
3.3.3. Serum LDL Level
Seven RCTs [17, 18, 21, 22, 24, 29, 35] reported the serum LDL level. There was no statistical heterogeneity among the analyzed data (I2 = 30%, P=0.20), so a fixed-effect model was used for the meta-analysis, and the results showed that vitamin D supplementation intervention could significantly reduce the LDL levels in GDM patients (SMD = −0.49, 95% CI: (−0.68, −0.29), P < 0.001, Figure 2(c)).
3.3.4. Serum HDL Level
Eight RCTs [17, 18, 22, 24, 26, 29, 30, 35] reported the serum HDL level. There was no statistical heterogeneity among the analyzed data (I2 = 0%, P=0.92), so a fixed-effect model was used for the meta-analysis, and the results showed that vitamin D supplementation intervention could significantly increase the HDL levels in GDM patients (SMD = 0.41, 95% CI: (0.23, 0.58), P < 0.001, Figure 2(d)).
3.3.5. Serum TG Level
Six RCTs [17, 24, 26, 29, 30, 35] reported the serum TG level. There was no statistical heterogeneity among the analyzed data (I2 = 77%, P < 0.01), so a random-effect model was used for the meta-analysis, and the results showed that vitamin D supplementation intervention could significantly reduce the TG levels in GDM patients (SMD = −0.59, 95% CI: (−1.01, −0.17), P=0.006, Figure 3(a)).
Figure 3.
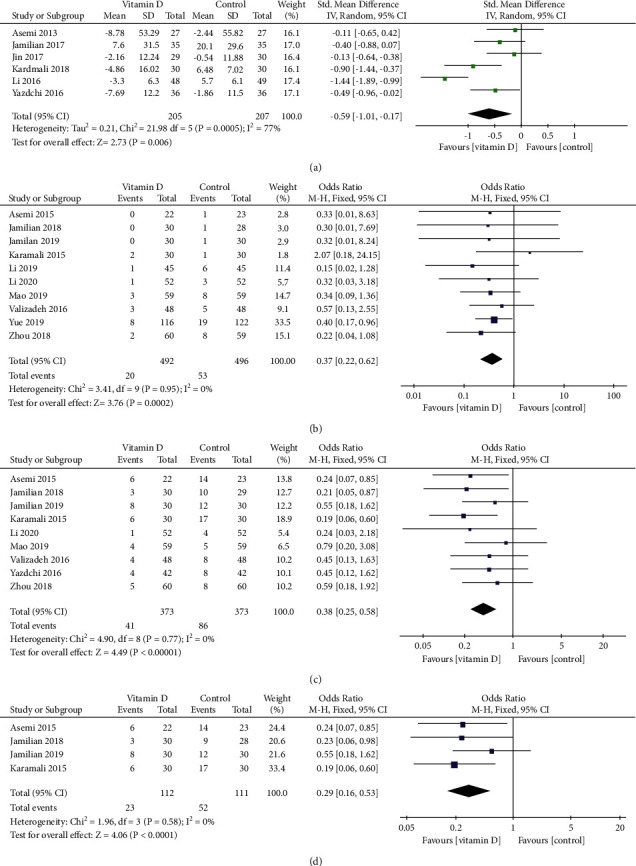
Forest plots for (a) the serum TG level and the incidence of (b) premature birth, (c) hyperbilirubinemia, and (d) neonatal hospitalization.
3.3.6. Incidence of Premature Birth
Nine RCTs [19–21, 23, 31–33, 35] reported the incidence of premature birth. There was no statistical heterogeneity among the analyzed data (I2 = 0%, P=0.77), so a fixed-effect model was used for the meta-analysis, and the results showed that vitamin D supplementation intervention could significantly reduce the incidence of premature birth in neonates (OR = 0.38, 95% CI: (0.25, 0.58), P < 0.001, Figure 3(b)).
3.3.7. Incidence of Hyperbilirubinemia
Ten RCTs [19–21, 23, 28, 31–34] reported the incidence of hyperbilirubinemia. There was no statistical heterogeneity among the analyzed data (I2 = 0%, P=0.95), so a fixed-effect model was used for the meta-analysis, and the results showed that vitamin D supplementation intervention could significantly reduce the incidence of hyperbilirubinemia in neonates (OR = 0.37, 95% CI: (0.22, 0.62), P < 0.001, Figure 3(c)).
3.3.8. Incidence of Neonatal Hospitalization
Four RCTs [19, 21, 23, 25] reported the incidence of neonatal hospitalization. There was no statistical heterogeneity among the analyzed data (I2 = 0%, P=0.58), so a fixed-effect model was used for the meta-analysis, and the results showed that vitamin D supplementation intervention could significantly reduce the incidence of neonatal hospitalization (OR = 0.29, 95% CI: (0.16, 0.53), P < 0.001, Figure 3(d)).
3.4. Sensitivity Analysis and Publication Bias
We sequentially excluded individual studies for sensitive analysis to evaluate the stability of the results. The results showed that combined effect values before and after the exclusion of any study were relatively close, and the study results did not change significantly, suggesting that the results of each meta-analysis were stable.
The distribution of points on the funnel plot of each variable was symmetrical (Figures 4 and 5). The results of Egger regression analysis indicated that there was no significant publication bias in the results of each meta-analysis (all P > 0.05).
Figure 4.
Funnel plots for (a) the serum 25(OH)D level, (b) the serum TG level, (c) the serum LDL level, and (d) the serum HDL level.
Figure 5.
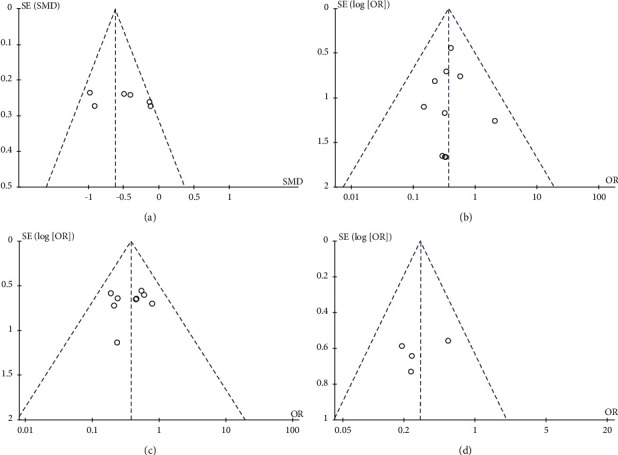
Funnel plots for (a) the serum TG level and the incidence of (b) premature birth, (c) hyperbilirubinemia, and (d) neonatal hospitalization.
4. Discussion
GDM is the most common complication of pregnant women during pregnancy, and prevalence has gradually increased in recent years. The probability of type 2 diabetes, metabolic syndrome, and obesity in GDM patients and their offspring can be as high as 60.16% [37, 38]. The pathogenesis of GDM has not yet been elucidated. Some studies [39, 40] suggest that the occurrence and development of GDM are closely related to dietary structure, family history of diabetes, obesity, chronic inflammatory response, genetic differences, insulin resistance, pancreatic β-cell damage, and immune dysfunction. In recent years, in order to prevent the occurrence of gestational diabetes mellitus, clinical blood glucose monitoring is usually carried out according to the pregnancy cycle of pregnant women. However, it is mostly detected at 24 to 28 weeks of pregnancy. The treatment of GDM at this stage is more difficult and may have caused harm to health of mothers and babies [41]. Therefore, clinical diagnosis of gestational diabetes mellitus should be performed as soon as possible, and targeted treatment should be given to avoid adverse pregnancy outcomes. The results of this present meta-analysis have shown that vitamin D supplementation is beneficial to increasing the serum 25(OH)D and HDL levels and is helpful for reducing the serum TC and LDL levels of GDM patients and maternal hyperbilirubinemia as well as neonatal hyperbilirubinemia and hospitalization risk. There are some discrepancies between the results of this meta-analysis and other previous meta-analyses [42, 43]. Previous meta-analyses [42] have found that vitamin D can improve LDL levels, but they did not find its effects on TG, TC, and HDL. The possible reason for this is that most of the included RCTs have an intervention time of less than 6 weeks, and there is a lack of long-term follow-up studies. Multiple studies [44–46] have shown that when GDM patients have abnormal lipid metabolism, their risk of pregnancy complications increases. Therefore, vitamin D supplementation is of great significance for improving the prognosis of GDM patients and neonates, and it is worthy of clinical promotion and use for GDM treatment.
Vitamin D is a hormone-like substance, which can promote the secretion of insulin in the human body under normal physiological conditions and promote normal glucose tolerance in the body, and it can effectively regulate the content of calcium ions in the body [47]. The deficiency of vitamin D is closely related to the occurrence of gestational diabetes mellitus. By detecting the content of vitamin D in pregnant women with gestational diabetes mellitus, the degree of deficiency can be clarified and a reasonable supplementation plan can be formulated as soon as possible. For pregnant women with GDM who are overweight or obese before pregnancy, diet and weight should be strictly controlled and blood sugar management should be strengthened. Previous studies [48–50] have pointed out that vitamin D can regulate insulin secretion through the following pathways: First, vitamin D affects the function of pancreatic islet B cells by directly activating VD receptors or by interfering with VD response elements in the insulin receptor-initiating gene region; second, vitamin D improves insulin sensitivity and glucose transport by enhancing the response of insulin receptors to insulin; third, vitamin D increases the conversion of proinsulin to insulin. In addition, it has been reported that active vitamin D can reduce food intake, reduce body weight, and improve glucose tolerance and insulin sensitivity through vitamin D receptors in the paraventricular nucleus of the hypothalamus.
Vitamin D is a fat-soluble vitamin that plays an important role during pregnancy. In recent years, many studies [51, 52] have suggested that vitamin D is closely related to GDM. Animals with vitamin D deficiency (especially in early life) have an increased incidence of diabetes, and supplementation of vitamin D and its analogs can reduce or delay the occurrence of diabetes [53]. Studies [54, 55] have shown that vitamin D deficiency is associated with an increased incidence of type 2 diabetes, and vitamin D supplementation can significantly increase insulin sensitivity in people with insulin resistance and vitamin D deficiency. Insulin resistance and insufficient secretion are one of the pathogeneses of GDM [56]. Vitamin D levels are negatively correlated with blood sugar, and they are positively associated with insulin resistance. Vitamin deficiency in pregnant women with GDM increases the risk of insulin resistance and metabolic syndrome [57, 58]. At present, an international consensus has not been reached on the dosage of VD supplementation during pregnancy. The dietary nutrient reference amount for Chinese residents recommends a routine vitamin D supplementation of 400 U/d during pregnancy, and the maximum tolerated dose is 2000 U/d. At present, most experts believe that 1000–2000 U/d can be supplemented for pregnant women with vitamin D deficiency during pregnancy, and the maximum safe dose is 4000 U/d. However, the dose and safety of vitamin D supplementation during pregnancy remain to be further studied in the future.
Neonatal hyperbilirubinemia is a common yet serious clinical disease, which damages the nervous system of infants and young children, resulting in sequelae such as involuntary movements of hands and feet, deafness, and even cerebral palsy with serious long-term damage [59–61].
This meta-analysis has found that vitamin D supplementation during pregnancy in mothers with GDM reduces the incidence of hyperbilirubinemia, preterm birth, and neonatal hospitalization. The possible reason is that vitamin D deficiency is prevalent in pregnant women, and vitamin D supplementation can increase the formation of antimicrobial peptides in the body, inhibit the production of inflammatory cytokines, and play an important role in immune regulation [62]. In addition, studies [63, 64] have shown that vitamin D deficiency during pregnancy is associated with preterm birth and hospitalization rates of neonates. Some studies [65–67] have pointed out that vitamin D supplementation can improve the maternal vitamin D status during pregnancy. The improvement of maternal vitamin D status may directly affect the fetal vitamin D supply and neonatal vitamin D level, thereby reducing the risk of preeclampsia and premature birth [68]. Therefore, vitamin D supplementation during pregnancy is very important and necessary for the prognosis of pregnant women and neonates.
There are some limitations in this meta-analysis worth considering. First, most of the included RCTs are from China and Iran, which may have certain geographical or population bias. Second, the study design of group concealment and outcome blinding in some included RCTs is not rigorous, and there can be certain biases in outcomes. Third, the weight gain during pregnancy may be a confounding factor for our results, yet we failed to conduct subgroup analysis based on the weight gain during pregnancy as limited by the collected data. Finally, the heterogeneity of the synthesized results of some outcome indicators is high, which may be related to the differences in the dose and treatment duration of vitamin D included in RCTs. Therefore, the therapeutic effect and safety of vitamin D in GDM patients still need to be further explored in future large-sample, strictly designed, high-quality studies.
5. Conclusions
In conclusion, the results of this meta-analysis have found that vitamin D supplementation during pregnancy in GDM patients can reduce serum LDL, TG, and TC levels and increase the serum 25(OH)D level and HDL level in GDM patients. Besides, vitamin D supplementation is beneficial to reducing maternal hyperbilirubinemia, as well as neonatal hyperbilirubinemia and hospitalization risk. Vitamin D supplementation can effectively improve the prognosis of pregnant women with GDM and reduce the incidence of adverse pregnancy outcomes. It is worth noting that the dose and duration of vitamin D supplementation still need to be further analyzed and investigated in future high-quality studies to provide evidence for the prevention and quality of GDM.
Data Availability
All data generated or analyzed during this study are included in this published article.
Ethical Approval
In this study, all methods were performed in accordance with the relevant guidelines and regulations. Ethical approval is not necessary since this study is a meta-analysis and systematic review.
Consent
Consent is not applicable to this study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
CW designed the research; CW, YS, and XW conducted the research; CW and Y S analyzed the data; CW and YS wrote the first draft of the manuscript; CW had primary responsibility for final content. All authors read and approved the final manuscript.
Supplementary Materials
Supplementary Figure 1: risk of the bias graph. Supplementary Figure 2: risk of bias summary.
References
- 1.Moon J. H., Jang H. C. Gestational diabetes mellitus: diagnostic approaches and maternal-offspring complications. Diabetes & Metabolism J . 2022;46(1):3–14. doi: 10.4093/dmj.2021.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fritsche L., Heni M., Eckstein S. S., et al. Incretin hypersecretion in gestational diabetes mellitus. Journal of Clinical Endocrinology and Metabolism . 2022;107(6):e2425–e2430. doi: 10.1210/clinem/dgac095. [DOI] [PubMed] [Google Scholar]
- 3.Faal Siahkal S., Javadifar N., Najafian M., Iravani M., Zakerkish M., Heshmati R. The psychosocial challenges associated with gestational diabetes mellitus: a systematic review of qualitative studies. Primary Care Diabetes . 2022;16(1):11–26. doi: 10.1016/j.pcd.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Diaz-Santana M. V., O’Brien K. M., Park Y. M. M., Sandler D. P., Weinberg C. R. Persistence of risk for type 2 diabetes after gestational diabetes mellitus. Diabetes Care . 2022;45(4):864–870. doi: 10.2337/dc21-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang S., Magny-Normilus C., McMahon E., Whittemore R. Systematic review of lifestyle interventions for gestational diabetes mellitus in pregnancy and the postpartum period. Journal of Obstetric, Gynecologic, and Neonatal Nursing . 2022;51(2):115–125. doi: 10.1016/j.jogn.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johns E. C., Denison F. C., Norman J. E., Reynolds R. M. Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends in Endocrinology and Metabolism . 2018;29(11):743–754. doi: 10.1016/j.tem.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Chiou Y. L., Hung C. H., Yu C. Y., Chan T. F., Liu M. G. Risk factors for women with gestational diabetes mellitus developing type 2 diabetes and the impact on children’s health. Journal of Clinical Nursing . 2022;31(7-8):1005–1015. doi: 10.1111/jocn.15959. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Z. H., Wei Y. M., Li H. T., Yu H. Z., Liu J. M., Zhou Y. B. Gestational diabetes mellitus as an effect modifier of the association of gestational weight gain with perinatal outcomes: a prospective cohort study in China. International Journal of Environmental Research and Public Health . 2022;19(9):p. 5615. doi: 10.3390/ijerph19095615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kainan Z., Xiaoxiao L., Hui W. The relationship between changes in intestinal flora structure and gestational diabetes mellitus. China General Medicine . 2020;23(21):5–7. [Google Scholar]
- 10.Wenqian L., Xiangtian Y., Mingjuan L. Combined analysis of risk factors and metabolomics for adverse pregnancy outcomes in gestational diabetes mellitus. Chinese Journal of Diabetes . 2021;13(6):6–10. [Google Scholar]
- 11.Zeng Q., Zou D., Wei Y., Ouyang Y., Lao Z., Guo R. Association of vitamin D receptor gene rs739837 polymorphism with type 2 diabetes and gestational diabetes mellitus susceptibility: a systematic review and meta-analysis. European Journal of Medical Research . 2022;27(1):p. 65. doi: 10.1186/s40001-022-00688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo C., Li Z., Lu Y., et al. Association of serum vitamin D status with gestational diabetes mellitus and other laboratory parameters in early pregnant women. BMC Pregnancy and Childbirth . 2022;22(1):p. 400. doi: 10.1186/s12884-022-04725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yong H. Y., Mohd Shariff Z., Palaniveloo L., et al. High early pregnancy serum 25-hydroxy vitamin D level, within a sub-optimal range, is associated with gestational diabetes mellitus: a prospective cohort study. Nutr Res Pract . 2022;16(1):120–131. doi: 10.4162/nrp.2022.16.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma J., Han L., Zhou X., Li Z. Clinical significance of Vitamin-D and other bone turnover markers on bone mineral density in patients with gestational diabetes mellitus. Pakistan Journal of Medical Sciences . 2022;38(1):23–27. doi: 10.12669/pjms.38.1.4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberati A., Altman D. G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ . 2009;339 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng X., Zhang Y., Kwong J. S., et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. Journal of Evidence-Based Medicine . 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 17.Asemi Z., Hashemi T., Karamali M., Samimi M., Esmaillzadeh A. Retracted: effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: a double-blind randomized controlled clinical trial. The American Journal of Clinical Nutrition . 2013;98(6):1425–1432. doi: 10.3945/ajcn.113.072785. [DOI] [PubMed] [Google Scholar]
- 18.Asemi Z., Karamali M., Esmaillzadeh A. Effects of calcium-vitamin D co-supplementation on glycaemic control, inflammation and oxidative stress in gestational diabetes: a randomised placebo-controlled trial. Diabetologia . 2014;57(9):1798–1806. doi: 10.1007/s00125-014-3293-x. [DOI] [PubMed] [Google Scholar]
- 19.Asemi Z., Karamali M., Esmaillzadeh A. Favorable effects of vitamin D supplementation on pregnancy outcomes in gestational diabetes: a double blind randomized controlled clinical trial. Hormone and Metabolic Research . 2015;47(08):565–570. doi: 10.1055/s-0034-1394414. [DOI] [PubMed] [Google Scholar]
- 20.Haixian Z., Huibiao L., Qun X. The effect of vitamin D on prevention and treatment of gestational diabetes in obese pregnant women and the effect of pregnancy outcome. Modern Chinese Doctor . 2018;56(6):45–48. [Google Scholar]
- 21.Jamilian M., Amirani E., Asemi Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: a randomized, double-blind, placebo-controlled trial. Clinical Nutrition . 2019;38(5):2098–2105. doi: 10.1016/j.clnu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Jamilian M., Karamali M., Taghizadeh M., et al. Vitamin D and evening primrose oil administration improve glycemia and lipid profiles in women with gestational diabetes. Lipids . 2016;51(3):349–356. doi: 10.1007/s11745-016-4123-3. [DOI] [PubMed] [Google Scholar]
- 23.Jamilian M., Mirhosseini N., Eslahi M., et al. The effects of magnesium-zinc-calcium-vitamin D co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. BMC Pregnancy and Childbirth . 2019;19(1):p. 107. doi: 10.1186/s12884-019-2258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jamilian M., Samimi M., Ebrahimi F. A., et al. The effects of vitamin D and omega-3 fatty acid co-supplementation on glycemic control and lipid concentrations in patients with gestational diabetes. Journal of Clinical Lipidology . 2017;11(2):459–468. doi: 10.1016/j.jacl.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Karamali M., Asemi Z., Dastjerdi M. A., Esmaillzadeh A. Calcium plus vitamin D supplementation affects pregnancy outcomes in gestational diabetes: randomized, double-blind, placebo-controlled trial. Public Health Nutrition . 2015;19(1):156–163. doi: 10.1017/s1368980015000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karamali M., Bahramimoghadam S., Sharifzadeh F., Asemi Z. Magnesium-zinc-calcium-vitamin D co-supplementation improves glycemic control and markers of cardiometabolic risk in gestational diabetes: a randomized, double-blind, placebo-controlled trial. Applied Physiology Nutrition and Metabolism . 2018;43(6):565–570. doi: 10.1139/apnm-2017-0521. [DOI] [PubMed] [Google Scholar]
- 27.Li D., Yan H., Lingling H. Effect of vitamin D_3 supplementation during pregnancy on T lymphocyte subsets in gestational diabetes mellitus. Laboratory Medicine and Clinic . 2020;17(16):5–7. [Google Scholar]
- 28.Li L., Cailiang M. Effect of vitamin D supplementation in pregnant women with gestational diabetes and its impact on pregnancy outcomes. Diabetes New World . 2019;22(17):42–43. [Google Scholar]
- 29.Liping J., Jiezhi Z., Fang W. Effects of vitamin D on insulin resistance and lipid metabolism in patients with gestational diabetes mellitus. Journal of Changzhi Medical College . 2017;31(5):361–363. [Google Scholar]
- 30.Li Q., Xing B. Vitamin d<sub>3</sub>-supplemented yogurt drink improves insulin resistance and lipid profiles in women with gestational diabetes mellitus: a randomized double blinded clinical trial. Annals of Nutrition & Metabolism . 2016;68(4):285–290. doi: 10.1159/000447433. [DOI] [PubMed] [Google Scholar]
- 31.Shuhui M., Na W. Effect of vitamin D supplementation in pregnant women with gestational diabetes mellitus and its impact on pregnancy outcomes. China Modern Doctor . 2019;57(2):54–57. [Google Scholar]
- 32.Valizadeh M., Piri Z., Mohammadian F., Kamali K., Amir Moghadami H. R. The impact of vitamin D supplementation on post-partum glucose tolerance and insulin resistance in gestational diabetes: a randomized controlled trial. International Journal of Endocrinology and Metabolism . 2016;14(2) doi: 10.5812/ijem.34312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiling L., Tingting L., Likun C. Effect of vitamin D supplementation in pregnant women with gestational diabetes and its impact on pregnancy outcomes. Diabetes New World . 2020;23(18):3–5. [Google Scholar]
- 34.Xiaoling Y., Fang Z., Qian Z. Effects of vitamin D on blood sugar control and pregnancy outcomes in pregnant women with normal body mass index gestational diabetes mellitus. Contemporary Chinese Medicine . 2019;26(8):99–102. [Google Scholar]
- 35.Yazdchi R., Gargari B. P., Asghari-Jafarabadi M., Sahhaf F. Effects of vitamin D supplementation on metabolic indices and hs-CRP levels in gestational diabetes mellitus patients: a randomized, double-blinded, placebo-controlled clinical trial. Nutr Res Pract . 2016;10(3):328–335. doi: 10.4162/nrp.2016.10.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ying Z., Zhuang Z. Effects of vitamin D3 supplementation on T lymphocyte subsets in patients with gestational diabetes mellitus. Diabetes New World . 2020;9(19):36–40. [Google Scholar]
- 37.Tammo O., Yıldız S. Vitamin D deficiency and its clinical results in preeclamptic mothers and their babies. Cureus . 2022;14(3) doi: 10.7759/cureus.23519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao R., Zhou L., Wang S., Xiong G., Hao L. Association between maternal vitamin D levels and risk of adverse pregnancy outcomes: a systematic review and dose-response meta-analysis. Food & Function . 2022;13(1):14–37. doi: 10.1039/d1fo03033g. [DOI] [PubMed] [Google Scholar]
- 39.Ismail N. A., Mohamed Ismail N. A., Bador K. M. Vitamin D in gestational diabetes mellitus and its association with hyperglycaemia, insulin sensitivity and other factors. Journal of Obstetrics and Gynaecology (Basingstoke) . 2021;41(6):899–903. doi: 10.1080/01443615.2020.1820462. [DOI] [PubMed] [Google Scholar]
- 40.Magnusdottir K. S., Tryggvadottir E. A., Magnusdottir O. K., et al. Vitamin D status and association with gestational diabetes mellitus in a pregnant cohort in Iceland. Food & Nutrition Research . 2021;65 doi: 10.29219/fnr.v65.5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pham T. T. M., Huang Y. L., Chao J. C. J., et al. Plasma 25(OH)D concentrations and gestational diabetes mellitus among pregnant women in taiwan. Nutrients . 2021;13(8):p. 2538. doi: 10.3390/nu13082538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akbari M., Moosazadeh M., Lankarani K. B., et al. Correction: the effects of vitamin D supplementation on glucose metabolism and lipid profiles in patients with gestational diabetes: a systematic review and meta-analysis of randomized controlled trials. Hormone and Metabolic Research . 2017;49(09):p. e3. doi: 10.1055/s-0037-1600933. [DOI] [PubMed] [Google Scholar]
- 43.Wang H., Xia N., Yang Y., Peng D. Q. Influence of vitamin D supplementation on plasma lipid profiles: a meta-analysis of randomized controlled trials. Lipids in Health and Disease . 2012;11(1):p. 42. doi: 10.1186/1476-511x-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kron-Rodrigues M. R., Rudge M. V. C., Lima S. A. M. Supplementation of vitamin D in the postdelivery period of women with previous gestational diabetes mellitus: systematic review and meta-analysis of randomized trials. Revista Brasileira de Ginecologia e Obstetrícia . 2021;43(09):699–709. doi: 10.1055/s-0041-1734000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saha S., Saha S. Changes in anthropometric and blood 25-hydroxyvitamin D measurements in antenatal vitamin supplemented gestational diabetes mellitus patients: a systematic review and meta-analysis of randomized controlled trials. Journal of the Turkish-German Gynecological Association . 2021;22(3):217–234. doi: 10.4274/jtgga.galenos.2021.2020.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang M. M., Chen Z. J., Wang Y., Xu X. R., Li H. J., Yang J. [Effects of vitamin D supplementation on serum lipid profiles and neonatal outcomes in gestational diabetes mellitus:a meta-analysis] Zhongguo Yi Xue Ke Xue Yuan Xue Bao . 2021;43(1):82–91. doi: 10.3881/j.issn.1000-503X.12940. [DOI] [PubMed] [Google Scholar]
- 47.Aguero-Domenech N., Jover S., Sarrion A., et al. Vitamin D deficiency and gestational diabetes mellitus in relation to body mass index. Nutrients . 2021;14(1):p. 102. doi: 10.3390/nu14010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mo M., Shao B., Xin X., et al. The association of gene variants in the vitamin D metabolic pathway and its interaction with vitamin D on gestational diabetes mellitus: a prospective cohort study. Nutrients . 2021;13(12):p. 4220. doi: 10.3390/nu13124220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang M., Chen Z., Hu Y., et al. The effects of vitamin D supplementation on glycemic control and maternal-neonatal outcomes in women with established gestational diabetes mellitus: a systematic review and meta-analysis. Clinical Nutrition . 2021;40(5):3148–3157. doi: 10.1016/j.clnu.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 50.Huang S., Fu J., Zhao R., et al. The effect of combined supplementation with vitamin D and omega-3 fatty acids on blood glucose and blood lipid levels in patients with gestational diabetes. Annals of Palliative Medicine . 2021;10(5):5652–5658. doi: 10.21037/apm-21-1018. [DOI] [PubMed] [Google Scholar]
- 51.Karamali M., Asemi Z., Ahmadi-Dastjerdi M., Esmaillzadeh A. Calcium plus vitamin D supplementation affects pregnancy outcomes in gestational diabetes: randomized, double-blind, placebo-controlled trial - EXPRESSION OF CONCERN. Public Health Nutrition . 2021;24(13):p. 4369. doi: 10.1017/s1368980021002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Immanuel J., Simmons D., Harreiter J., et al. Metabolic phenotypes of early gestational diabetes mellitus and their association with adverse pregnancy outcomes. Diabetic Medicine . 2021;38(2) doi: 10.1111/dme.14413. [DOI] [PubMed] [Google Scholar]
- 53.Ciebiera M., Wojtyla C., Lukaszuk K., et al. The role of vitamin D in perinatology. An up-to-date review. Archives of Medical Science . 2021;17(4):992–1005. doi: 10.5114/aoms.2019.81747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang C., Jing W., Ge S., Sun W. Vitamin D status and vitamin D deficiency risk factors among pregnancy of Shanghai in China. BMC Pregnancy and Childbirth . 2021;21(1):p. 431. doi: 10.1186/s12884-021-03889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chatzakis C., Sotiriadis A., Tsakmaki E., et al. The effect of dietary supplements on oxidative stress in pregnant women with gestational diabetes mellitus: a network meta-analysis. Nutrients . 2021;13(7):p. 2284. doi: 10.3390/nu13072284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang L., Zhang C., Song Y., Zhang Z. Serum vitamin D deficiency and risk of gestational diabetes mellitus: a meta-analysis. Archives of Medical Science . 2020;16(4):742–751. doi: 10.5114/aoms.2020.94433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abouzid M., Glowka A. K., Karazniewicz-Lada M. Trend research of vitamin D receptor: bibliometric analysis. Health Informatics Journal . 2021;27(4) doi: 10.1177/14604582211043158.146045822110431 [DOI] [PubMed] [Google Scholar]
- 58.Mahendra A., Fall C. H. D. Maternal vitamin D deficiency and GDM risk: evidence for the case of investing more attention in antenatal clinics. Proceedings of the Nutrition Society . 2021:1–7. doi: 10.1017/s0029665121003840. [DOI] [PubMed] [Google Scholar]
- 59.Feleke B. E., Feleke T. E., Adane W. G., et al. Maternal and newborn effects of gestational diabetes mellitus: a prospective cohort study. Primary Care Diabetes . 2022;16(1):89–95. doi: 10.1016/j.pcd.2021.09.007. [DOI] [PubMed] [Google Scholar]
- 60.Fernando M., Ellery S. J., Marquina C., Lim S., Naderpoor N., Mousa A. Vitamin D-binding protein in pregnancy and reproductive health. Nutrients . 2020;12(5):p. 1489. doi: 10.3390/nu12051489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aljanahi A., Hadhiah H., Al-Nasr W., et al. The effect of dietary intake of vitamin D on gestational diabetes mellitus. Nutrition and Metabolic Insights . 2020;13 doi: 10.1177/1178638820932164.1178638820932164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y., Wang T., Huo Y., et al. Placenta expression of vitamin D and related genes in pregnant women with gestational diabetes mellitus. The Journal of Steroid Biochemistry and Molecular Biology . 2020;204 doi: 10.1016/j.jsbmb.2020.105754.105754 [DOI] [PubMed] [Google Scholar]
- 63.Yin W. J., Tao R. X., Hu H. L., et al. The association of vitamin D status and supplementation during pregnancy with gestational diabetes mellitus: a Chinese prospective birth cohort study. American Journal of Clinical Nutrition . 2020;111(1):122–130. doi: 10.1093/ajcn/nqz260. [DOI] [PubMed] [Google Scholar]
- 64.Kim K. S., Park S. W., Cho Y. W., Kim S. K. Vitamin D deficiency at mid-pregnancy is associated with a higher risk of postpartum glucose intolerance in women with gestational diabetes mellitus. Endocrinol Metab (Seoul) . 2020;35(1):97–105. doi: 10.3803/enm.2020.35.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elsori D. H., Hammoud M. S. Vitamin D deficiency in mothers, neonates and children. The Journal of Steroid Biochemistry and Molecular Biology . 2018;175:195–199. doi: 10.1016/j.jsbmb.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 66.Agarwal S., Kovilam O., Agrawal D. K. Vitamin D and its impact on maternal-fetal outcomes in pregnancy: a critical review. Critical Reviews in Food Science and Nutrition . 2018;58(5):755–769. doi: 10.1080/10408398.2016.1220915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pilz S., Zittermann A., Obeid R., et al. The role of vitamin D in fertility and during pregnancy and lactation: a review of clinical data. International Journal of Environmental Research and Public Health . 2018;15(10):p. 2241. doi: 10.3390/ijerph15102241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De-Regil L. M., Palacios C., Lombardo L. K., Pena-Rosas J. P. Vitamin D supplementation for women during pregnancy. Cochrane Database of Systematic Reviews . 2016;(1) doi: 10.1002/14651858.CD008873.pub3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: risk of the bias graph. Supplementary Figure 2: risk of bias summary.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


