Abstract
A patient with a history of Crohn’s disease on infliximab presented to the hospital with sepsis and a new heart murmur. He was found to have native aortic valve infective endocarditis from a rare species of group D Streptococcusin his blood. The patient was also noted to be in an acute flare of Crohn’s disease. The hospital course was complicated by florid heart failure from acute aortic insufficiency. He eventually improved after source control and appropriate antibiotic therapy. S. pasteuranis bacteremia and endocarditis are attributable to the patient’s immunocompromised state as a result of infliximab treatment. While S. pasteuranis is infrequently grown in blood cultures, it is commonly found in normal gut flora. We hypothesize that it gained access to the bloodstream through the epithelium in the terminal ileum, which was inflamed due to an acute flare of Crohn’s disease.
Keywords: immunocompromised status, crohn’s disease (cd), immunotherapy, aortic valve endocarditis, infective endocarditis
Introduction
Infective endocarditis (IE) is a significant cause of mortality and morbidity in patients despite treatment [1]. Timely diagnosis and prompt medical and surgical treatment are crucial for ensuring survival and mitigating problems associated with IE. IE usually presents with fevers, chills, night sweats, fatigue, and a new cardiac murmur on physical examination; however, it can be vague at times which can pose a challenge for clinicians to diagnose. We are presenting an interesting case of IE in a patient with Crohn’s disease by a rare pathogen, Streptococcus gallolyticus subspecies pasteuranis.
Case presentation
A 53-year-old male with a history of Crohn’s disease on infliximab presented with complaints of nausea, vomiting, decreased appetite, and generalized body aches for two to three weeks. On presentation, vital signs were significant for a temperature of 101.5°F. Initial blood workup showed leukocytosis and elevated inflammatory markers (Table 1).
Table 1. Laboratory values.
| Laboratory tests | Results | Reference value |
| White blood cell count | 13.8 × 103/mm3 | 3,300–8,700/mm3 |
| Hemoglobin | 8.6 g/dL | 13.8–17.2 g/dL |
| Serum creatinine | 1.1 mg/dL | 0.8–1.0 mg/dL |
| C-reactive protein | 7.9 mg/L | <3 mg/L |
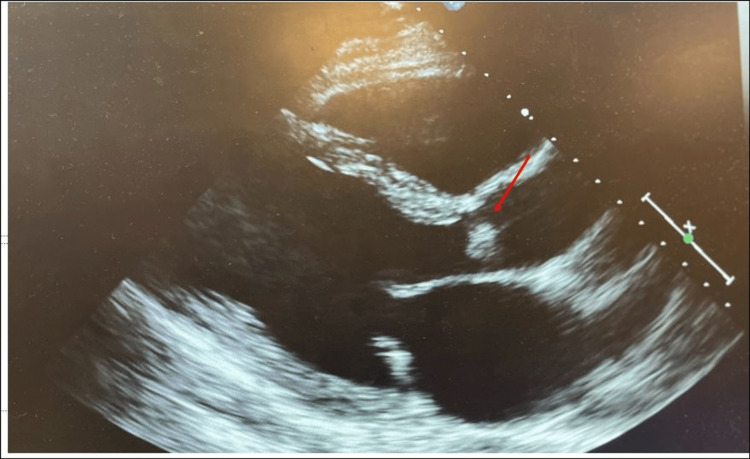
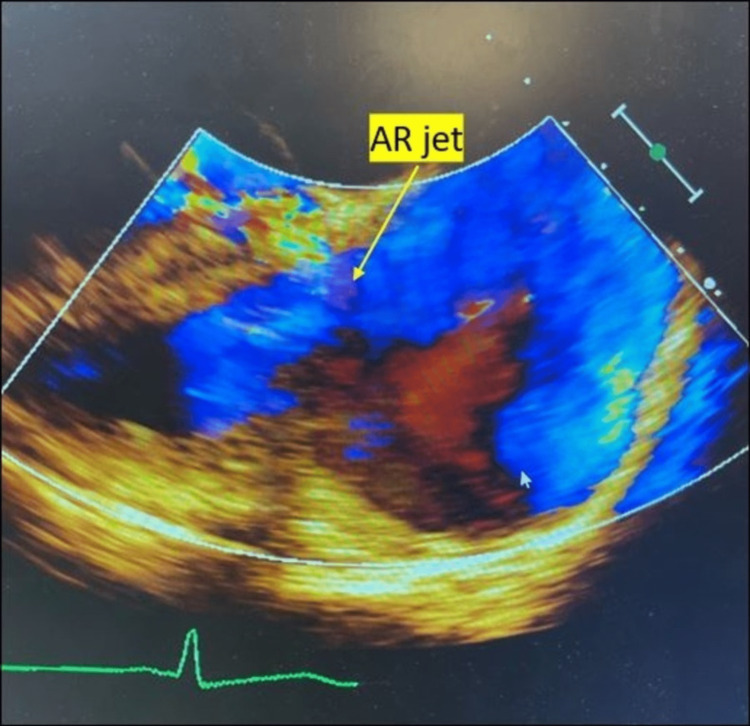
Blood cultures were drawn, and he was empirically started on levofloxacin 750 mg Q24hour for colitis concerns due to the patient’s history of Crohn’s disease. On examination, there was a murmur heard along the left sternal border. Due to the presence of murmur and fever, IE was high in the differential. Hence, a transthoracic echocardiogram was obtained, which showed an ejection fraction of 60-65% and an echo density in the right coronary cusp of the aortic valve (AV) concerning vegetation (Figure 1) and moderate-to-severe AV insufficiency (Figure 2) directed toward the anterior mitral leaflet.
Figure 1. Transthoracic echocardiographic view: transverse long-axis view showing the aortic valve with a mobile echo density.
Transthoracic echocardiogram depicting a mobile echo density (red arrow) attached to the aortic valve in the transverse long-axis view.
Figure 2. Transthoracic echocardiography showing aortic insufficiency.
The echocardiogram in the parasternal long-axis view shows aortic insufficiency and aortic regurgitation (AR) jet (yellow arrow), the jet is noted to fill the left ventricular outflow tract. The flow toward the transducer is red in color, and flow away from the transducer is blue in color.
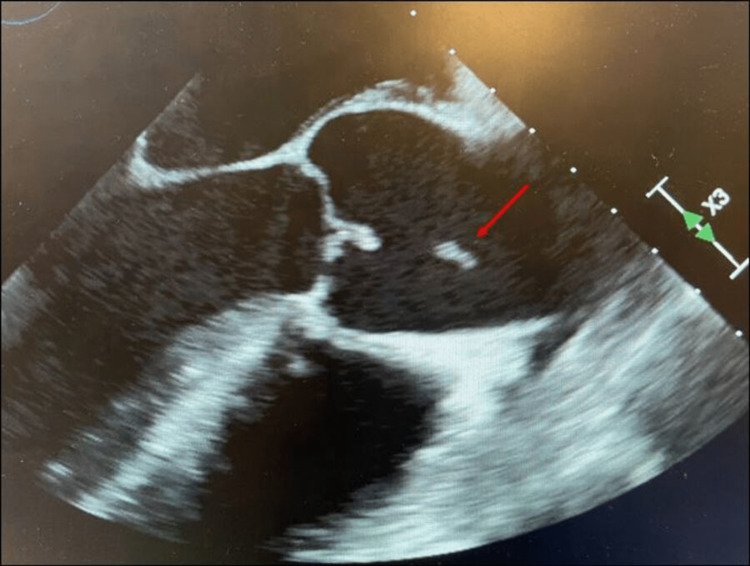
The findings were confirmed with a transesophageal echocardiogram (TEE) which also revealed 0.7 × 1.0 cm mass echo density on the right coronary cusp of the AV (Figure 3) with mild-to-moderate eccentric aortic regurgitation/aortic insufficiency (AR/AI), AI maximum velocity (MV) of 122.0 cm/second, and AI maximum peak gradient (MPG) of 50.5 mmHg.
Figure 3. Transesophageal echocardiographic view showing the aortic valve with a mobile echo density (red arrow).
Blood cultures grew S. gallolyticus subsp. pasteuranis which was resistant to penicillin. Levofloxacin was switched to intravenous (IV) ampicillin and IV gentamicin by the infectious disease service. The diagnosis of AV endocarditis caused by S. gallolyticus bacteremia with vegetation on the AV was confirmed.
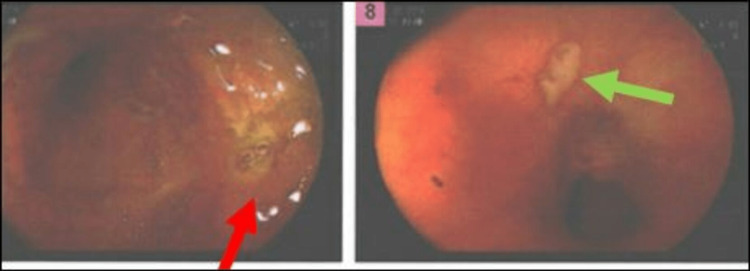
The hospital course was complicated by new-onset heart failure with pedal edema and dyspnea in the setting of moderate-to-severe AR; however, surgical repair of the AV was deferred as source control was challenging in this patient because IE occurred secondary to underlying Crohn’s disease. Consequently, the patient was managed medically, and he responded well to diuretics (IV furosemide 40 mg daily) and fluid restriction. The patient underwent a colonoscopy which revealed terminal ileum inflammation and ulceration (Figure 4).
Figure 4. Colonoscopy images showing inflammation and ulcer in the terminal ileum.
Colonoscopy depicting erythema and ulceration in the terminal ileum (red and green arrows).
There were no polyps or discrete masses suspicious of malignancy; nevertheless, a biopsy of the terminal ileum was obtained, which showed mild active chronic inflammation of lamina propria with associated hyperplastic changes and edema. The patient was on infliximab for Crohn’s disease, which was held due to bacteremia and IE. Gastroenterology was consulted for Crohn’s management and recommended treatment with oral prednisone 50 mg daily with a gradual taper and sulfasalazine 3 g daily.
Differential diagnosis
Because the patient presented to the hospital with two weeks of generalized malaise, abdominal pain, nausea, and vomiting, and he had a history of Crohn’s disease, he was thought to be having an acute flare of Crohn’s disease. An elevated C-reactive protein was suggestive of an acute inflammatory disorder. Colitis, diverticulitis, and cholecystitis were other differential diagnoses. However, the patient had a temperature of 101.5°F and, on the physical examination, was found to have a heart murmur. IE was at the top of the differentials. Anemia is a common consequence of IE. The fever, sepsis, and bacteremia were other differentials. Once the blood cultures grew S. pasteuranis, all three differential diagnoses could be linked to one another, as discussed below.
Treatment
Sepsis was managed in a standard manner with fluids and antibiotics. Levofloxacin was chosen because the initial working diagnosis was an acute flare of Crohn’s disease. Subsequently, this was changed to ampicillin and gentamicin. Because the IE was complicated by aortic insufficiency and florid heart failure, surgical correction was indicated. However, surgical correction or replacement would be in vain without controlling the source of bacteremia, which was the inflamed gut. This was achieved with the help of sulfasalazine and prednisone. Meanwhile, heart failure was managed with diuretics and fluid restriction.
Outcome and follow-up
The patient was discharged to a skilled nursing facility for completion of a six-week course of IV antibiotics. The patient underwent a repeat TEE six weeks after the initial diagnosis of IE, which revealed persistence of 0.7 × 1.0 cm echo density on the ventricular surface of the AV; however, compared to the previous TEE, the echo density appeared calcified. The AR appeared moderate but based on pressure half-time measurements; the AR was mild in nature, AI MV was 333.4 cm/second, and AI MPG was 44.5 mmHg. There was an eccentric AR jet converging on the anterior mitral valve leaflet. He also underwent a repeat colonoscopy after six weeks which showed significant improvement in the inflammation and ulceration of the ileum (Figure 5).
Figure 5. Colonoscopy images during follow-up showing the reduction in inflammation in the terminal ileum.
Significant reduction in the erythema and resolution of the ulceration (blue arrow) of the terminal ileum on repeat follow-up colonoscopy.
Discussion
IE is an important cause of morbidity and mortality with a significant impact on healthcare. A systematic review done in 2009 suggested that despite optimal medical therapy, mortality rates from IE are around 25% [2]. Microbiologic analysis of culture-positive IE in one study showed prevalence culture positivity for Staphylococcus aureus in 31% of cases, viridians group streptococci in 17%, enterococci in 11%, Streptococcus bovis in 7%, and only 5% were other streptococci [3]. A systematic review conducted a decade later showed that the incidence of staphylococcal IE increased while that of streptococcal viridians and culture-negative IE decreased significantly in the United States [2]. Recently published studies show the emergence of other rare subspecies causes of IE including Streptococcus acidominimus, and Aggregatibacter paraphrophilus [4,5]. S. pasteuranis (S. gallolyticus subspecies pastueranis) is a non-enterococcal group D streptococcus commonly present as part of the gut flora [6]. It has been known to cause a variety of infectious diseases ranging from IE, neonatal sepsis, adult meningitis, peritonitis, biliary tract infections, and septicemia [7,8].
Depending upon risk factors, the microbiological profile of IE also varies [9,10]. S. pasteuranis and its associations with diseases in humans are largely underreported, and an increasing number of studies have been published in recent years. It has been shown to be associated with gastrointestinal malignancies, including pancreatic, gastric, hepatobiliary, and colorectal cancer with bacteremia [11,12]. As with the more commonly associated IE causative organisms, further testing and antibiotic susceptibility remain an issue with the less frequently isolated organisms. Another important aspect with regard to the underreported cases of S. pasteuranis is the potential drug resistance, as reported in multiple studies [13]. A retrospective study done in 2016 to assess the accuracy of the identification of S. pasteuranis and antibiotic resistance showed Bruker Biotyper to be the most accurate modality, 31.8% of strains were resistant to both erythromycin and clindamycin with positivity for resistance-conferring ermB gene [14].
An interesting aspect of the resistance pattern was based on geographic distribution [15]. A study done in China revealed higher resistance to macrolides and clindamycin [14,16,17]. This type of resistance pattern is alarming, especially in conjunction with the potential to cause serious diseases such as IE. Our patient was found to have penicillin-resistant species and his antibiotic regimen was tailored accordingly. Region-based resistance patterns will help guide the treatment for bacteremia caused by S. pasteuranis in the future. Another important aspect of S. pasteuranis IE is the screening for malignancies as it is noted to be associated with gastrointestinal malignancies, as mentioned above. Our patient underwent a colonoscopy, and terminal ileum biopsies were obtained which fortunately were negative for malignancy. As S. pasteuranis infections usually originate from the gastrointestinal tract, our patient’s underlying Crohn’s disease and immunocompromised status (being on infliximab) could have predisposed him to bacteremia and IE. Despite advances in medical therapy including antibiotics and early surgical intervention in patients with acute IE, mortality continues to be high due to the increasing burden of IE in healthcare and the increasing presence of comorbidities. Mortality has been noted to be higher for IE cases presenting to tertiary care facilities [18].
Conclusions
Streptococcus pasteuranis (group D streptococci) are underreported causes of IE. S. pasteuranis usually disseminate from the gastrointestinal tract. Because S. pasteuranis IE has been associated with gastrointestinal tract cancers, screening for gastrointestinal malignancies is pivotal. However, other gastrointestinal diseases could also predispose to S. pasteuranis bacteremia. Immunosuppressive medications, such as biological agents, should be discontinued promptly in instances of bacteremia to prevent infection propagation. Specialty services should be contacted immediately for the treatment of underlying predisposing conditions (in our case, Crohn’s disease) because source control is imperative.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Global and regional burden of infective endocarditis, 1990-2010: a systematic review of the literature. Bin Abdulhak AA, Baddour LM, Erwin PJ, Hoen B, Chu VH, Mensah GA, Tleyjeh IM. Glob Heart. 2014;9:131–143. doi: 10.1016/j.gheart.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011: the devil is in the details. DeSimone DC, Wilson WR, Baddour LM. https://www.jacc.org/doi/10.1016/j.jacc.2015.05.079. J Am Coll Cardiol. 2015;66:1201–1202. doi: 10.1016/j.jacc.2015.05.079. [DOI] [PubMed] [Google Scholar]
- 3.Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Murdoch DR, Corey GR, Hoen B, et al. Arch Intern Med. 2009;169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Infective endocarditis by Aggregatibacter paraphrophilus: case report and literature review. Sood S. J Clin Diagn Res. 2013;7:2577–2578. doi: 10.7860/JCDR/2013/5794.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Infective endocarditis caused by Streptococcus acidominimus. Kabbara WK, Azar-Atallah S. Am J Health Syst Pharm. 2019;76:1926–1929. doi: 10.1093/ajhp/zxz234. [DOI] [PubMed] [Google Scholar]
- 6.The erm(T) gene is flanked by IS1216V in inducible erythromycin-resistant Streptococcus gallolyticus subsp. pasteurianus. Tsai JC, Hsueh PR, Chen HJ, Tseng SP, Chen PY, Teng LJ. Antimicrob Agents Chemother. 2005;49:4347–4350. doi: 10.1128/AAC.49.10.4347-4350.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Streptococcus bovis group and biliary tract infections: an analysis of 51 cases. Corredoira J, Alonso MP, García-Garrote F, et al. Clin Microbiol Infect. 2014;20:405–409. doi: 10.1111/1469-0691.12333. [DOI] [PubMed] [Google Scholar]
- 8.Splenic abscess caused by Streptococcus gallolyticus subsp. pasteurianus as presentation of a pancreatic cancer. Su Y, Miao B, Wang H, Wang C, Zhang S. J Clin Microbiol. 2013;51:4249–4251. doi: 10.1128/JCM.01709-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Infective endocarditis. Holland TL, Baddour LM, Bayer AS, Hoen B, Miro JM, Fowler VG Jr. Nat Rev Dis Primers. 2016;2:16059. doi: 10.1038/nrdp.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An interesting case of Serratia endocarditis in a patient with chronic myeloid leukemia. Singh A, Tappeta K, Chellapuram N, Singh D. Cureus. 2022;14:0. doi: 10.7759/cureus.21238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Infective endocarditis caused by Streptococcus gallolyticus subspecies pasteurianus and colon cancer. Takamura N, Kenzaka T, Minami K, Matsumura M. BMJ Case Rep. 2014;2014:0. doi: 10.1136/bcr-2013-203476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The association of Streptococcus gallolyticus subspecies pasteurianus bacteremia with the detection of premalignant and malignant colonic lesions. Chand G, Shamban L, Forman A, Sinha P. https://www.hindawi.com/journals/crigm/2016/7815843/ Case Rep Gastrointest Med. 2016;2016:7815843. doi: 10.1155/2016/7815843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phenotypic and genetic characterization of resistance against macrolides and lincosamides in Streptococcus gallolyticus strains isolated from pigeons and humans. Kimpe A, Decostere A, Martel A, Devriese LA, Haesebrouck F. Microb Drug Resist. 2003;9 Suppl 1:0–8. doi: 10.1089/107662903322541874. [DOI] [PubMed] [Google Scholar]
- 14.Identification, antimicrobial resistance and molecular characterization of the human emerging pathogen Streptococcus gallolyticus subsp. pasteurianus. Gherardi G, Palmieri C, Marini E, et al. Diagn Microbiol Infect Dis. 2016;86:329–335. doi: 10.1016/j.diagmicrobio.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Characterization of erythromycin and tetracycline resistance genes of Streptococcus gallolyticus subspecies pasteurianus strains isolated from patients with septicemia and bacteremia in Thailand. Takarn P, Tharavichitkul P, Malasao R, Boonthum A. Clin Lab. 2019;65 doi: 10.7754/Clin.Lab.2018.180902. [DOI] [PubMed] [Google Scholar]
- 16.Microbiological and clinical characteristics of Streptococcus gallolyticus subsp. pasteurianus infection in China. Li Y, Chen X, Zhang Z, et al. BMC Infect Dis. 2019;19:791. doi: 10.1186/s12879-019-4413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reidentification of Streptococcus bovis isolates causing bacteremia according to the new taxonomy criteria: still an issue? Romero B, Morosini MI, Loza E, Rodríguez-Baños M, Navas E, Cantón R, Campo RD. J Clin Microbiol. 2011;49:3228–3233. doi: 10.1128/JCM.00524-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.[Predictors of hospital mortality in 186 cases of active infective endocarditis treated in a tertiary medical center (1992-2001)] Horacio Casabé J, Deschle H, Cortés C, et al. Rev Esp Cardiol. 2003;56:578–585. doi: 10.1016/s0300-8932(03)76919-x. [DOI] [PubMed] [Google Scholar]