Abstract
Background and Objectives:
Hashimoto's thyroiditis is a chronic inflammation and an autoimmune disease of the thyroid gland that causes hypothyroidism. Genetic, internal, and environmental factors are the causes of this disease. Because human herpes viruses such as herpesvirus type 6 (HHV-6) are involved in some autoimmune disorders, they may also play a role in causing this disease. This study aimed to evaluate the association between human herpes virus 6 (HHV-6) with Hashimoto's thyroiditis.
Materials and Methods:
In the present study, 64 samples of thyroid paraffin tissue including 32 samples of thyroid paraffin tissue of healthy individuals as control, and 32 samples of thyroid paraffin tissue of Hashimoto's thyroiditis patients were taken from the pathology department of Loghman Hakim Hospital in Tehran. A questionnaire collected demographic information of patients. After DNA extraction from the samples, the nested-PCR technique was performed using specific primers for HHV-6.
Results:
Totally, the HHV6-DNA was found in 34.4% of thyroid tissues of healthy individuals (81.8% female and 18.2% male) and 46.9% of patients with Hashimoto's thyroiditis (73.3% female and 26.7% male). It was found that this difference in virus frequency between the two groups was not statistically significant (P value=0.309). There was also no statistically significant relationship between the prevalence of human herpesvirus type 6 and age or sex.
Conclusion:
Based on the present study, the number of HHV-6-infected individuals in Hashimoto's patients and controls did not differ significantly; therefore, HHV-6 appears not to be associated with Hashimoto's thyroiditis.
Keywords: Hashimoto disease, Autoimmune diseases, Herpesvirus 6, Polymerase chain reaction
INTRODUCTION
The word thyroiditis contains a diverse group of diseases defined by thyroid inflammation (1). The autoimmune disease can involve the thyroid tissues more than other targets. The incidence of autoimmune thyroiditis (AIT) has dramatically increased over time worldwide (2). AIT affects up to 10% of the world population and is more prevalent in women than men (3). This disease is described by lymphocytic infiltrate of the thyroid gland, with T-helper lymphocytes of the Th1 type, cytotoxic T-lymphocytes (CTL), natural killer cells (NK), monocytes, plasma cells, and lymphoid centers (4). Hashimoto's thyroiditis (HT) is an autoimmune disease known as chronic autoimmune thyroiditis and chronic lymphocytic thyroiditis that damages thyroid cells by cell and antibody-mediated immune processes. It is the most common cause of hypothyroidism, especially in developed countries (5). HT almost begins between the third to fifth decades of life and, nearly 5% of the individuals would be affected in their life (3). The pathogenesis of HT is associated with both cellular and humoral immune responses (4). Although HT has been controversial until now, several studies have shown that both genetic and environmental factors play a role in the etiology of the disease (3). Viral infections have been suggested as potential causes of this disease (6). The progression of TH disease may be associated with human herpesvirus 6 (HHV-6) according to some studies (2).
The HHV-6 is a double-stranded DNA virus belonging to the Herpesvirinae subfamily of the Herpesviridae family, and it includes two distinct species HHV-6A and HHV-6B (7, 8). HHV-6B is the ubiquitous virus with over 90% worldwide infection rate. HHV-6B has been identified as a principal etiological agent of the exanthema subitem (roseola infantum), but HHV-6A causes a more life-threatening infection especially in immunocompromised patients, including hematopoietic stem-cell transplant (HSCT), solid-organ transplant recipients, and acquired immunodeficiency syndrome (AIDS) patients (2, 7, 9, 10). Several studies have identified saliva as the main route of HHV-6 transmission, but the exact mechanism of viral transmission yet has not been determined (10).
HLA class II molecules in thyrocytes behave as functional antigen-presenting cells for CD4+ T lymphocytes. There was a significant increase in CD4+ T lymphocytes that recognize HHV-6A/B antigens in HT patients, especially in the subset of polyfunctional CD4+ T cells that secrete both IFN-γ and IL-2. Thus, IFN-α may act as non-specific triggers for the development of autoimmunity (11). HHV-6A/B infection upregulates HLA-II expression on thyrocytes and induces an immune response to virus antigens in HT patients (12). Nevertheless, further studies are necessary to fully explain this association and the mechanisms leading to the possible triggering of HT by HHV-6. Therefore, the present study aimed to evaluate the association between human herpes virus 6 (HHV-6) with Hashimoto's thyroiditis.
MATERIALS AND METHODS
Clinical specimens.
This study was approved by research ethics committees of Islamic Azad Tehran Medical Sciences (IR.IAU.PS.REC.1398.226). Formalin-fixed paraffin-embedded (FFPE) tissue blocks from 32 patients with Hashimoto's thyroiditis (HT) and 32 healthy people without HT (control) were collected.by the Pathology Department of Loghman Hakim Hospital in Tehran. At least two experienced pathologists reviewed all thyroid tissue samples to confirm the diagnosis of HT. Hashimoto's thyroiditis was diagnosed based on its characteristic pathological appearance. The main characteristic is infiltration with lymphocytes, organized in lymphoid follicles with prominent germinal centers. It was accompanied by the transformation of normal thyrocytes into Hürthle cells in some areas, destruction and atrophy of thyrocytes in other areas, and the formation of interstitial fibrosis in others. Cases of normal thyroid tissue obtained from adjacent adenoma normal tissues of patients with thyroid adenoma. Demographic information of patients was collected by a questionnaire. Total DNA from tissues was extracted using the QIAamp DNA FFPE Tissue Kit (QIAGEN, Germany) according to the manufacture protocol. The integrity of DNA was checked by electrophoresis on a 1% agarose gel stained with DNA safe stain. The quantity of DNA was measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Rockford, IL, USA) from 2-μL aliquots of each sample. When OD260/ OD280 was 1.8 ± 0.1. If the concentration of DNA was more than 10 ng/μL, the total DNA extracted was eligible.
HHV-6 DNA detection.
A nested-PCR (nPCR) was performed for the HHV-6 major capsid protein (MCP) gene, which recognizes both variants (A and B) of the virus. The forward primer 5'-GCGTTTTCAGTGTGTAGTTCGGCAG-3' and the reverse primer 5'-TGGCCGCATTCGTACAGATACGGAGG-3' were used to perform the first-round of PCR with amplicon size of 520 bp (13). The second-round of PCR was performed using the forward primer 5'-GCTAGAACGTATTTGCTGCAGAACG-3' and the reverse primer 5'-ATCCGAAACAACTGTCTGACTGGCA-3' (13) with amplicon size of 258 bp. The reaction mix contained 6 ng of template DNA or controls, 10 μL of master mix (Ampliqon, Denmark), 2 μL of primers mix (F1+R1), and 2 μL of sterilized DW in a total volume of 20 μL for the first reaction. The second-round of PCR performed using 3 ng of the first round of PCR product and the reaction mix similar to the previous step. PCR protocol was included 5 min at 95°C (for the first round) and 94°C (for the second round), 35 cycles of the 30s at 95°C (for the first round) and 94°C (for the second round), 30s at 55°C, 30s at 72°C and one final extension step at 72°C for 5 min. We used positive and negative controls in every run of PCR. We used human β-globin gene as an internal control. PCR products were run on a 2% agarose gel and visualized using a UV transilluminator after staining with SYBR Safe DNA gel stain.
Statistical analysis.
Student's t-test and chi-square test used for statistical analysis through SPSS software version 21 (SPSS Inc., Chicago, IL, USA).
RESULTS
Thirty-two Hashimoto's thyroiditis patients and 32 healthy controls were participated in the study. In the group of patients, there were 5 (15.6%) males and 27 (84.4%) females with the total mean age of 48 ± 13 years. In the group of control, there were 7 (21.9%) males and 25 (78.1%) females with the total mean age of 50 ± 13 years. There was no significant difference in mean age between two groups (p= 0.804) (Table 1).
Table 1.
Distribution of participants according to age and sex
| Characteristic | Hashimoto’s thyroiditis (n=32) | Non-Hashimoto’s thyroiditis (n=32) | P value | |
|---|---|---|---|---|
| Age (mean ± SD) | 48 ± 13 | 50 ± 13 | 0.804 | |
| Sex | Men | 5 (15.6%) | 7 (21.9%) | 0.775 |
| Women | 27 (84.4%) | 25 (78.1%) | ||
In total, 64 thyroid specimens were subjected to nested-PCR amplification, and the HHV-6 genome was detected in 15 of 32 (46.9%) Hashimoto's patients and 11 of 32 (34.4%) non-Hashimoto's control group. The difference in virus frequency between the two groups was not statistically significant (P value=0.309) (Table 2).
Table 2.
Number and percentage of HHV-6 DNA positive cases in both group
| Sex | Hashimoto’s thyroiditis | Non-Hashimoto’s thyroiditis | P value |
|---|---|---|---|
| Men | 4 (26.7%) | 2 (18.2%) | |
| Women | 11 (73.3%) | 9 (81.8%) | 0.309 |
| Total | 15 (100%) | 11 (100%) |
HHV-6 DNA was detected in thyroid tissues from 11 of 27 women in Hashimoto’s thyroiditis group and from 9 of 25 women in non-Hashimoto’s thyroiditis (Table 2). There was no statistically significant relationship between the prevalence of human herpesvirus type 6 and age and or sex.
DISCUSSION
Several studies have noted the roles of genetic and environmental factors in etiology of AIT disease. Viral infections have been suggested as possible environmental triggers, but no conclusive evidence has been found. In the current study, the prevalence of HHV-6 was compared between patients with Hashimoto's thyroiditis and healthy individuals. HHV-6 was detected in 15 of 32 patients (46.9%) and in 11 of 32 controls (34.4%). The difference in HHV6 positive rates between the two groups was not statistically significant (P=0.309). Statistics showed no significant association between age (p=0.098), gender (P=0.463), and HHV-6 positivity in both groups. The female-to-male ratio suggests that women are more often affected (5), which could be defined by differentiation in hormonal profiles (2).
Caselli's observations recommend a possible role for HHV-6 (probably variant A) in the progression or triggering of HT. They were analyzed the presence and transcriptional state of HHV-6 in thyroid fine needle aspirates (FNA) and peripheral blood mononuclear cells (PBMCs) from 34 HT patients and 28 controls. HHV-6 DNA prevalence (82% vs. 10%, p≤0.001) and viral load were significantly increased in FNA from HT patients, and thyrocytes from HT FNA displayed a 100-fold higher HHV-6 DNA load compared to infiltrating lymphocytes A (6). According to our study, no link between the virus and HT was found, perhaps because our study was located in a different location, and used a different sample type. In addition, we used from a nested-PCR for virus detection but Caselli et al. was performed a real time qPCR. They have reported the presence of HHV-6 DNA in PBMCs. However, Detection of HHV-6 DNA in PBMCs, where latent infection is established, does not distinguish between active and latent/chronic infection.
Seyyedi et al. have found HHV-6A DNA in serum samples of 57 of 151 patients (38%) with HT, which was significantly more than in patients with non-autoimmune thyroid disorders (P = 0.001) (14). Contrary to their study, we did not use serum samples. Detection of HHV-6 DNA in the serum sample shows an active infection, but viral genome is not always present in serum when the virus is active in a distant lymphoid tissue or organ.
Fig. 1.
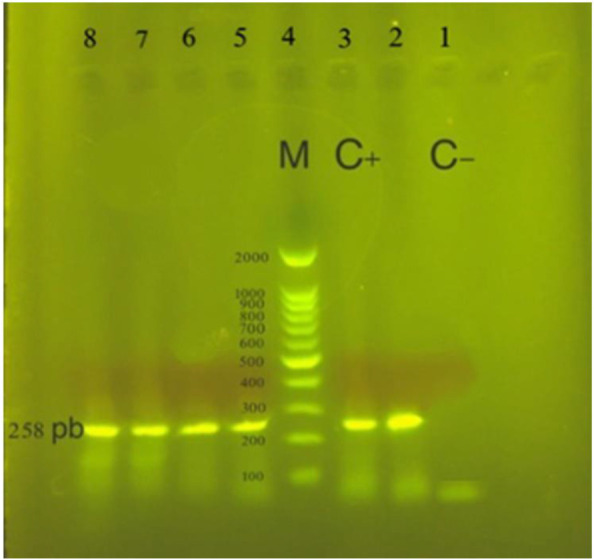
Electrophoresis gel showing the products of HHV-6 second round of nested-PCR amplification in Hashimoto’s thyroiditis patients. In positive specimens a fragment of 258 base pairs (bp) was amplified. Lane 1: negative control, Lane 3: positive control, Lane 4: ladder, Lane 2, 5, 6, 7, 8: positive samples
In Sultanova's study, HHV-6 infection was detected in 98% (44 out of 45) of patients with AIT (autoimmune thyroiditis), which was significantly higher (P= 0.0058) than in the control group (77%) (15). Thomas et al. have isolated the HHV-6 genome from 2 of 15 (13.3%) of Hashimoto's thyroiditis patients (16). They have not found an association between HHV-6 and HD similar to our study. In their study, they also detected HHV-6 in paraffin embedded tissue sections by PCR, but their sample size was smaller than ours. The study of AL-Zarzour et al. showed no evidence that human herpesviruses play a role in the pathogenesis of AIT. They examined the presence of HHV-6, EBV, CMV, HSV-1, and HSV-2 in 100 samples of thyroid tissue. They extracted DNA from freshly frozen tissue which are better digested and yield good quality DNA which is different from our procedure, we used FFPE. Their finding is similar to our study although their samples included not only Hashimoto’s thyroiditis but also Grave’s disease, atrophic autoimmune hypothyroidism, postpartum thyroiditis, and silent thyroiditis. However, their samples were obtained from one site of a biopsy which does not represent whole thyroid (17). Small sample size and low quality of DNA extracted from some FFPE samples were limitations in the study.Further studies are required to fully explain a possible role for HHV-6 in triggering of HT.
CONCLUSION
The results of the present study showed that although the frequency of human herpesvirus 6 in patients with Hashimoto's thyroiditis is higher than the control group, there is no significant relationship between the prevalence of human herpesvirus-6 and Hashimoto's thyroiditis. Therefore, this study does not confirm the potential role of human herpesvirus-6 in the pathogenesis of Hashimoto's thyroiditis and future studies are required.
ACKNOWLEDGEMENTS
The authors would like to acknowledge members of Microbiology Department, Faculty of Advanced Science and Technology, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran for their support.
REFERENCES
- 1.Desailloud R, Hober D. Viruses and thyroiditis: An update. Virol J 2009; 6: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sultanova A, Cistjakovs M, Sokolovska L, Todorova K, Cunskis E, Murovska M. HHV-6 infection and chemokine RANTES signaling pathway disturbance in patients with autoimmune thyroiditis. Viruses 2020; 12: 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assaad SN, Meheissen MA, Elsayed ET, Alnakhal SN, Salem TM. Study of Epstein–Barr virus serological profile in Egyptian patients with Hashimoto’s thyroiditis: A case-control study. J Clin Transl Endocrinol 2020; 20: 100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Crescenzo V, D'Antonio A, Tonacchera M, Carlomagno C, Vitale M. Human herpes virus associated with Hashimoto's thyroiditis. Infez Med 2013; 21: 224–228. [PubMed] [Google Scholar]
- 5.Ralli M, Angeletti D, Fiore M, D'Aguanno V, Lambiase A, Artico M, et al. Hashimoto's thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun Rev 2020; 19: 102649. [DOI] [PubMed] [Google Scholar]
- 6.Caselli E, Zatelli MC, Rizzo R, Benedetti S, Martorelli D, Trasforini G, et al. Virologic and immunologic evidence supporting an association between HHV-6 and Hashimoto's thyroiditis. PLoS Pathog 2012; 8(10): e1002951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agut H, Bonnafous P, Gautheret-Dejean A. Human Herpesviruses 6A, 6B, and 7. Microbiol Spectr 2016; 4: 10.1128/microbiolspec.DMIH2-0007-2015. [DOI] [PubMed] [Google Scholar]
- 8.Caselli E, D'Accolti M, Caccuri F, Soffritti I, Gentili V, Bortolotti D, et al. The U94 gene of human herpesvirus 6: a narrative review of its role and potential functions. Cells 2020; 9: 2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ablashi D, Agut H, Alvarez-Lafuente R, Clark DA, Dewhurst S, DiLuca D, et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch Virol 2014; 159: 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer BT, Krantz EM, Wald A, Corey L, Casper C, Gantt S, et al. Estimating the risk of human herpesvirus 6 and cytomegalovirus transmission to Ugandan infants from viral shedding in saliva by household contacts. Viruses 2020; 12: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prummel MF, Strieder T, Wiersinga WM. The environment and autoimmune thyroid diseases. Eur J Endocrinol 2004; 150: 605–618. [DOI] [PubMed] [Google Scholar]
- 12.Broccolo F, Fusetti L, Ceccherini-Nelli L. Possible role of human herpesvirus 6 as a trigger of autoimmune disease. ScientificWorldJournal 2013; 2013: 867389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaklikkaya I, Kaklikkaya N, Birincioglu I, Buruk K, Turan N. Detection of human herpesvirus 6 DNA but not human herpesvirus 7 or 8 DNA in atherosclerotic and nonatherosclerotic vascular tissues. Heart Surg Forum 2010; 13(5): E345–349. [DOI] [PubMed] [Google Scholar]
- 14.Seyyedi N, Dehbidi GR, Karimi M, Asgari A, Esmaeili B, Zare F, et al. Human herpesvirus 6A active infection in patients with autoimmune Hashimoto's thyroiditis. Braz J Infect Dis 2019; 23: 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sultanova A, Cistjakovs M, Gravelsina S, Chapenko S, Roga S, Cunskis E, et al. Association of active human herpesvirus-6 (HHV-6) infection with autoimmune thyroid gland diseases. Clin Microbiol Infect 2017; 23(1): 50.e1–50.e5. [DOI] [PubMed] [Google Scholar]
- 16.Thomas D, Liakos V, Michou V, Kapranos N, Kaltsas G, Tsilivakos V, et al. Detection of herpes virus DNA in post-operative thyroid tissue specimens of patients with autoimmune thyroid disease. Exp Clin Endocrinol Diabetes 2008;116: 35–39. [DOI] [PubMed] [Google Scholar]
- 17.AL-Zarzour N, Monem F. Are human herpes viruses associated with autoimmune thyroid disease? J Infect Dev Ctries 2011; 5: 890–892. [DOI] [PubMed] [Google Scholar]


