Abstract
Background
Autosomal dominant polycystic kidney disease (ADPKD) is a common hereditary disorder, characterized by kidney cyst formation. A major pathological feature of ADPKD is the development of interstitial inflammation. Due to its role in inflammation and oxidative stress, tryptophan metabolism and related kynurenines may have relevance in ADPKD.
Methods
Data were collected from a well-characterized longitudinal cohort of pediatric and adult patients with ADPKD and compared to age-matched healthy subjects. To evaluate the role of kynurenines in ADPKD severity and progression, we investigated their association with height-corrected total kidney volume (HtTKV) and kidney function (estimated glomerular filtration rate (eGFR)). Key tryptophan metabolites were measured in plasma using a validated liquid chromatography-mass spectrometry assay.
Results
There was a significant accumulation of kynurenine and kynurenic acid (KYNA) in children and adults with ADPKD as compared to healthy subjects. Downstream kynurenines continued to accumulate in adults with ADPKD concurrent with the increase of inflammatory markers IL-6 and MCP-1. Both markers remained unchanged in ADPKD as compared to healthy children, suggesting alternate pathways responsible for the observed rise in kynurenine and KYNA. KYNA and kynurenine/tryptophan positively associated with disease severity (HtTKV or eGFR) in patients with ADPKD. After Bonferroni adjustment, baseline kynurenines did not associate with disease progression (yearly %change in HtTKV or yearly change in eGFR) in this limited number of patients with ADPKD.
Conclusion
Kynurenine metabolism seems dysregulated in ADPKD as compared to healthy subjects. Inhibition of kynurenine production by inhibition of main pathway enzymes could present a novel way to reduce the progression of ADPKD.
Keywords: ADPKD, Kynurenines, Tryptophan
Graphical Abstract
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disease. It is caused mainly by mutations in the polycystin-1 and polycystin-2 genes [1] and is characterized by uncontrolled cyst formation and expansion [2]. ADPKD affects an estimated 1:500 to 1:1000 people and is responsible for approximately 4% of kidney failure in the United States alone [3]. In ADPKD, inflammation develops early and is linked to cyst progression. An accumulation of inflammatory macrophages and many pro-inflammatory cytokines has been reported in human and animal models of PKD. The degree of macrophage infiltrate was associated with disease progression [4, 5].
The essential amino acid tryptophan contributes to the synthesis of nicotinamide adenine dinucleotide (NAD(H)), a coenzyme necessary for cellular energy production [6]. The majority of free tryptophan undergoes oxidative metabolism along the kynurenine pathway via two key enzymes: tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase. Tryptophan 2,3-dioxygenase is almost exclusively expressed in the liver, while indoleamine 2,3-dioxygenase can be found in various cells including tubular epithelial and immune cells [7]. Indoleamine 2,3-dioxygenase induction is often accompanied by increased levels of proinflammatory cytokines such as interferon γ, interleukin-6 or monocyte chemoattractant protein-1 [8, 9]. In chronic states of inflammation, enhanced indoleamine 2,3-dioxygenase activation creates local immune suppression via cellular stress response pathways [10].
Many kynurenine pathway intermediates, the so-called kynurenines, have been shown to induce and potentiate oxidative stress, dysregulate calcium homeostasis and induce mitochondrial dysfunction. This causes severe disruption of cellular metabolism, leads to cell damage and triggers further inflammatory processes [11–13].
Furthermore, kynurenine, 3-hydroxykynurenine (3-OH kynurenine) and quinolinic acid have been shown to induce glomerular fibrosis in kidney tissues [14]. They also independently associated with cardiovascular disease in uremic patients [15, 16] and with atherosclerosis-inducing factors in chronic kidney disease patients [14, 17–19].
Based on the established role of kynurenines in mediating inflammation in chronic kidney disease patients, we aimed to investigate their role in patients with ADPKD. We hypothesized that an increase in kynurenines (mainly kynurenine, kynurenic acid and quinolinic acid) will associate a) with an increase in inflammation as assessed by elevated interleukin-6 and monocyte chemoattractant protein-1 cytokines and b) with the severity and progression of ADPKD as calculated by the increase in height-corrected total kidney volume and/or decline of kidney function in pediatric and adult patients with ADPKD.
Materials and methods
Pediatric ADPKD trial design
The present study was based upon plasma samples collected during the referenced clinical trial [20, 21]. Eligible participants with ADPKD were aged 8–22 years and had a Schwartz estimated glomerular filtration rate (eGFR) > 80 ml/min per 1.73 m2 [20, 21].
All participants underwent a 2-day inpatient clinical evaluation at the Children’s Hospital of Colorado (Aurora, CO). During the first night of admission, participants fasted for a 10-h period and then blood was drawn for various analyses, including for the present analysis. From the baseline visit, we had access to 64 samples. Abdominal magnetic resonance imaging (MRI) was used for assessment of total kidney volume (Analyze 9.0; Mayo Foundation, Biomedical Imaging Resource Core, Rochester, MN) [20, 21]. Total kidney volume was corrected for height to account for normal growth in children.
Adult ADPKD trial design
Here, we utilized baseline plasma samples from 80 subjects from the HALT-PKD trials. Of these, 38 patients were from HALT study group A and 42 patients from HALT study group B. Study A enrolled individuals aged 15–49 diagnosed with ADPKD and a GFR of > 60 mL/min/1.73 m2. Study B enrolled individuals aged 18 to 64 diagnosed with ADPKD and a GFR in the range of 25–60 mL/min/1.73m2. In addition, all participants had high-normal blood pressure or were hypertensive, as defined by having blood pressure ≥ 130/80 or receiving treatment for hypertension. Major exclusion criteria included renovascular disease, diabetes, and history of severe heart failure [22, 23]. Patients in study group A underwent abdominal MRI measurements of total kidney volume and heart MRI for left ventricular mass index determination [22, 23].
Both clinical trial study protocols were approved and routinely reviewed by the Colorado Multiple Institutional Review Board (COMIRB, Aurora, CO) and registered at ClinicalTrials.gov (identifier NCT00456365 (pediatric trial) and NCT00283686 (HALT-PKD trial)). The studies were in full compliance with the principles of the Declaration of Helsinki and its amendments. All patients and/or guardians gave written informed assent or consent as applicable.
Healthy subjects
Plasma samples from 50 healthy pediatric subjects (aged 6–18 years) were collected onsite based upon a COMIRB approved protocol. Plasma samples from 66 healthy adults were purchased from BioIVT (Westbury, NY). None of the healthy subjects were on blood pressure control medication and did not have a history of kidney or heart disease.
Measurement of kynurenines by liquid chromatography/tandem mass spectrometry (LC/MS–MS) in plasma
Kynurenines were analyzed using a modification of the assay described by Zhu et al. [24]. Briefly, 50 μL of plasma samples were enriched with 10 μL of an isotope-labeled internal standard mix (to a final concentration of 60 nM for d4-serotonin, d5-kynureninic acid and 13C6-antralinic acid; 240 nM for 13C3,15 N-3OH kynurenine and d4-picolinic acid; and 600 nM for 13C6-kynurenine, 13C4,15 N-quinolinic acid and d5-tryptophan). Then 240 μL of methanol were added and samples were vortexed and centrifuged at 13,000xg for 10 min. The supernatant was evaporated to dryness and the residue was reconstituted in 50 μL of water containing 0.1% formic acid. Calibrator standards were prepared in 0.1% formic acid in water as surrogate matrix. Final concentration ranges for calibrators were as follows: tryptophan: 62.5–125,000 ng/mL; kynurenine, kynurenic acid and anthranilic acid: 1–2000 ng/mL; 3OH kynurenine, picolinic acid, quinolinic acid, 3OH anthranilic acid and serotonin: 0.25–500 ng/mL. Quality control samples were prepared by enriching blank plasma samples with appropriate volumes of kynurenines to reach low quality control levels of 5 × lowest calibrator, mid quality control levels of 37.5% of the highest calibrator and high quality control levels of 75% of the highest calibrator. All calibrators and quality control samples contained the same final concentrations of internal standards and were handled in the same way as the patient study samples. LC–MS/MS was performed using an Agilent Technologies (Santa Clara, CA) 1100 HPLC system connected to a SCIEX (Concord, ON, Canada) 5500 QTRAP mass spectrometer equipped with a turbo ion spray source operated in electrospray mode. Ten (10) μL of sample was injected onto an Atlantis T3 3 μm (2.1 × 50 mm) column (with attached 2.1 × 5 mm guard column, both from Waters, Milford, MA). Mobile phase consisted of 0.1% formic acid in water (Solvent A) and acetonitrile (Solvent B). The following 8-min-long gradient was run: from 0 to 0.5 min: 98% Solvent A, 0.5–4.2 min: 98% to 76% Solvent A, 4.2–4.5 min: 76–10% Solvent A, from 4.5 to 5.5 min the gradient was held at 10% Solvent A. Hereafter, the column was re-equilibrated to initial conditions for an additional 2.5 min. The flow was 0.6 mL/min. All analytes were detected in positive ion multiple reaction monitoring (MRM) mode. The following quantifier ion-transitions were monitored: tryptophan (m/z = 205 → 118), kynurenine (m/z = 209 → 192), kynurenic acid (m/z = 190 → 144), 3OH kynurenine (m/z = 225 → 208), anthranilic acid (m/z = 138 → 120), picolinic acid (m/z = 124 → 78), quinolinic acid (m/z = 168 → 78), 3-OH anthranilic acid (m/z = 154 → 80), and serotonin (m/z = 177 → 115). The following ion transitions were used for the isotope-labeled internal standards: d5-tryptophan (m/z = 210 → 147), 13C3,15 N-3OH kynurenine (m/z = 229 → 110), d5-kynurenic acid (m/z = 195 → 149), 13C4,15 N-quinolinic acid (m/z = 173 → 81), 13C6-kynurenine (m/z = 215 → 152), 13C6-anthranilic acid (m/z = 144 → 98), d4-picolinic acid (m/z = 128 → 82), and d4-serotonin (m/z = 181 → 164).
Measurement of interleukin-6 and monocyte chemoattractant protein-1 (MCP-1) by enzyme-linked immunosorbent assay (ELISA)
Due to sample volume limitations, plasma samples from 50 healthy children and 50 healthy adults (instead of 66 used for kynurenines analysis), 44 children with ADPKD (instead of 64 used for kynurenines) and 80 adult ADPKD patients were assayed for interleukin-6 and MCP-1 (ELISA R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The intra- and inter-assay variability for the interleukin-6 HS-ELISA was 5% and 11%, respectively. For MCP-1 these were 8% and 7%, respectively (as stated by the manufacturer). The lower limit of detection for interleukin-6 was 0.27 pg/mL, and sensitivity of the assay was 0.09 pg/mL. For MCP-1, the lower limit of detection was 31.2 pg/mL, and sensitivity was 10 pg/mL (as stated by the manufacturer).
Statistical analyses
Descriptive statistics were calculated for the study participants including means (± standard deviation (SD)) for continuous variables, and medians (with 25th–75th percentile interquartile ranges for skewed variables). The Shapiro–Wilk test was used for testing for normal distribution. Variables that were not normally distributed were log-transformed prior to statistical analysis. Correlation analysis and stepwise linear regression was used to evaluate the relationship of kynurenines and MCP-1 with clinical parameters (height-corrected total kidney volume and eGFR). Furthermore, to evaluate the association of kynurenines with disease progression, we used linear regression analysis between baseline kynurenines and clinical parameters. For eGFR, we used absolute changes (delta, Δ(parameter) = value4 years − valuebaseline). For height-corrected total kidney volume, we used a yearly relative growth rate (percent change as delta (parameter) normalized to the baseline). Adjustments for age, gender, ethnicity, systolic blood pressure, body mass index and kidney function/eGFR were used whenever appropriate. After Bonferroni adjustment, p < 0.0056 was considered statistically significant. For statistical analysis, interleukin-6 concentrations that were below the lower limit of detection were assigned a value of 30% of the reported lower limit of detection (= 0.027 pg/mL). One-way ANOVA with Tukey’s post-hoc analysis was used to compare differences between ADPKD patients and healthy subjects. All statistical analyses were carried out using SPSS version 27.0 (IBM, Armonk, NY).
Results
Plasma kynurenines in ADPKD patients (at baseline) versus healthy subjects
Plasma samples from 64 pediatric patients (at the initiation of the pravastatin trial) and 90 adult ADPKD patients (at the initiation of the HALT-PKD trials) were used for the cross-sectional analysis of kynurenines (Table 1). All pediatric patients had almost normal kidney function (eGFR above 80 mL/min/1.73m2). Among the 80 adult ADPKD patients, 21 from the HALT study A had normal (eGFR above 80 mL/min/1.73m2) and all other patients had reduced eGFRs. Furthermore, plasma samples from healthy children (N = 50) and healthy adults (N = 66) were utilized to establish control levels. Demographic data in the different study cohorts are presented in Table 1.
Table 1.
Subject characteristics: healthy children and adults versus patients with ADPKD
| Healthy children (N = 50) | ADPKD children (N = 64) | Healthy adults (N = 66) | ADPKD adults (N = 38) Study A | ADPKD adults (N = 42) Study B | |
|---|---|---|---|---|---|
| Gender | 21 M, 29F | 26 M, 38F | 50 M, 16F | 26 M, 12F | 26 M, 18F |
| Age [years] | 11.8 ± 4.2 | 15.5 ± 3.6 | 51.0 [41.0, 57.3] | 36.4*** [30.0, 45.1] | 47.8*** [42.4, 56.8] |
| BMI [kg/m2] | – | 22.7 ± 4.6 | – | 26.1 [23.3, 30.0] | 25.4 [23.4, 29.8] |
| eGFR [mL/min/1.73m2] | Above 90 | 142 ± 38.1 | Above 90 | 94.0 [75.4, 106] | 52.5### [44.6, 58.6] |
| HtTKV [mL/m] | – | 280 [214, 373] | – | 530 [403, 871] | – |
| UAE [mg/24 h] | – | 26.9 [14.8, 46.5] | – | 22.4 [14.8, 33.7] | 25.6 [15.6, 42.8] |
| SBP [mm Hg] | – | 122 ± 10.7 | – | 123 [117, 132] | 126 [121, 136] |
| LVMI [g/m2] | – | 51.1 [45.7, 57.2] | – | 57.6 [49.1, 65.5] | – |
Data are presented as means ± standard deviations for normally distributed variables and as medians with 25th and 75th percentiles (interquartile ranges) for skewed variables. Study A enrolled individuals aged 15 to 49 diagnosed with ADPKD and a GFR of > 60 mL/min/1.73 m2. Study B enrolled individuals aged 18 to 64 diagnosed with ADPKD and a GFR in the range of 25–60 mL/min/1.73 m2
Significance levels were determined by one-way ANOVA with Tukey’s post-hoc: *p < 0.05, **p < 0.01, ***p < 0.001, and ###p < 0.001 for ADPKD patients with eGFR above or below 60 mL/min/1.73m2, respectively
BMI body mass index; eGFR estimated glomerular filtration rate, HtTKV height-corrected total kidney volume, LVMI left-ventricular mass index; UAE urinary albumin excretion; SBP systolic blood pressure
Children with ADPKD had similar levels of tryptophan and slightly increased levels of kynurenine and kynurenic acid as compared to healthy children (Fig. 1A and C). Concentrations of quinolinic acid were higher in pediatric ADPKD patients, whereas picolinic acid concentrations were lower than in the healthy controls (Fig. 1B). More pronounced differences were observed in adults with ADPKD. Here the following kynurenine pathway metabolites were higher than in the corresponding healthy controls: kynurenine, kynurenic acid, 3-OH kynurenine, 3-OH anthranilic acid, quinolinic acid and the kynurenine/tryptophan-ratio (as an indicator of indoleamine 2,3-dioxygenase activity) (Fig. 1).
Fig. 1.
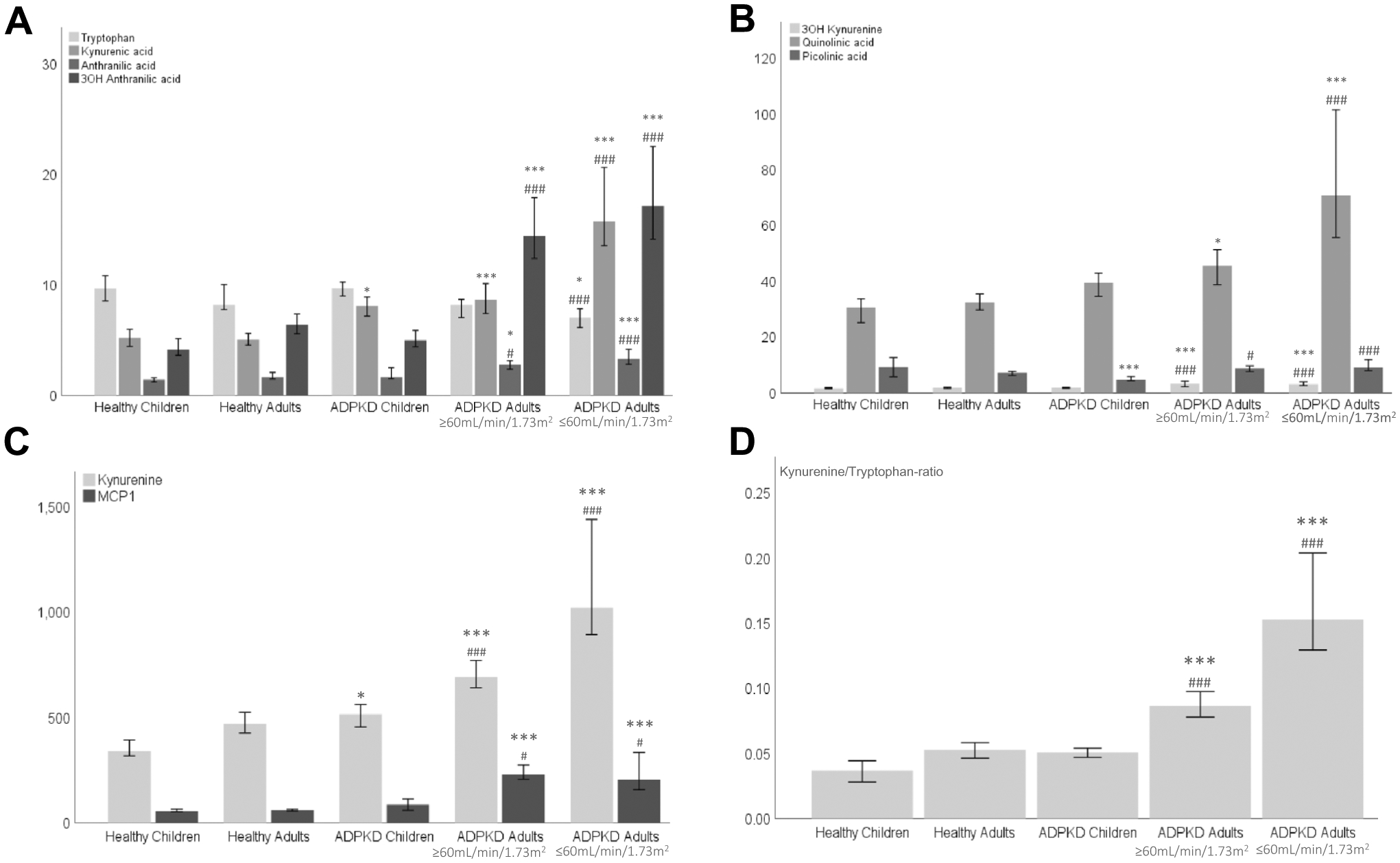
Changes in plasma concentrations of kynurenine pathway intermediates (A–D) across the investigated study groups: healthy children, children with ADPKD (normal eGFR), healthy adults and adults with ADPKD (eGFR above or below 60 mL/min/1.73m2, respectively). All concentrations are presented as ng/mL, except for tryptophan (calculated in μg/mL) and MCP-1 (calculated in pg/mL). Significance levels (after Bonferroni adjustment): healthy subjects versus ADPKD patients (children and adults, respectively): *p < 0.0056, ***p < 0.0001 and children versus adults with ADPKD: #p < 0.0056, ###p < 0.0001
Inflammation markers (interleukin-6 and MCP-1) in ADPKD patients (at baseline) versus healthy subjects
Interleukin-6 was detectable in plasma from 11 out of 50 evaluated healthy children [median = 0.03 pg/mL (0.03–0.47 pg/mL)]. In children with ADPKD, interleukin-6 was also only detected in 12 out of 44 patients [median = 0.03 pg/mL (0.03–0.65 pg/mL)]. Due to many missing values, we did not perform any further statistical analysis in this data set. Similarly, in healthy adults, interleukin-6 was detectable in 18 out of 50 subjects [median = 0.03 pg/mL (0.03–0.71 pg/mL)].
In adult patients with ADPKD at baseline, interleukin-6 was higher as compared to healthy subjects and detectable in 70 out of 80 patients [median = 0.25 pg/mL (0.12–0.45 pg/mL)] in patients from the HALT study A cohort. In patients with lower eGFR from the HALT study B cohort, the median was 0.37 pg/mL [0.19–0.87 pg/mL]. After controlling for age, gender, race, systolic blood pressure and body mass index (and Bonferroni correction), we did not observe any significant correlations between interleukin-6 and kynurenine levels.
MCP-1 was detectable in all samples. However, there was no significant difference between MCP-1 levels in plasma from the corresponding healthy controls and children with ADPKD (Fig. 1C). Adults with ADPKD showed higher levels of MCP-1 as compared to healthy adult subjects (Fig. 1C). No difference between the patients with ADPKD and with eGFRs above 60 or below 60 mL/min/1.73m2 were observed (Fig. 1C). MCP-1 did not correlate or associate with any of the clinical outcome parameters in patients with ADPKD (after controlling for age, gender, race, systolic blood pressure, body mass index and Bonferroni correction). MCP-1, after all adjustments, only correlated with quinolinic acid concentrations in plasma (Pearson r = 0.404, p < 0.005).
Association of plasma kynurenines with ADPKD severity and progression
In baseline samples collected in children and young adults with ADPKD at the initiation of the pravastatin trial (N = 64), we observed significant associations between height-corrected total kidney volume and plasma concentrations of kynurenic acid (beta = 0.332, p < 0.005) (with correction for gender, race, age, systolic blood pressure, body mass index and eGFR). 3-OH kynurenine and kynurenine/tryptophan ratio associated with eGFR (p < 0.05) after correction for gender, race, age, systolic blood pressure and body mass index), but not after Bonferroni adjustment.
The adult patients in the HALT-PKD study A trial were the only patients that had MRIs performed. In samples collected from said patients at baseline, stepwise linear regression analysis revealed significant associations between height-corrected total kidney volume and kynurenine/tryptophan ratio (β = 0.575, p < 0.001, N = 38) (after adjustment for gender, age, race, systolic blood pressure, body mass index and eGFR). eGFR itself associated with kynurenic acid (β = − 0.328, p < 0.001, N = 80; as before, adjusted for gender, age, race, systolic blood pressure and body mass index).
In children and young adults with ADPKD, baseline picolinic acid concentrations associated negatively with disease progression as expressed by the yearly percent change in height-corrected total kidney volume (calculated as: ((height-corrected total kidney volume4years − height-corrected total kidney volumebaseline)/height-corrected total kidney volumebaseline)/time4years). Baseline 3-OH kynurenine concentrations associated positively with the yearly change in eGFR (eGFR4years − eGFRbaseline)/time4 years) in children with ADPKD (p < 0.05 after adjustment for gender, age, race, systolic blood pressure and body mass index). However, both markers did not remain significant after Bonferroni adjustment (Table 2). In adults, similar observations were made for 3-OH kynurenine and anthranilic acid concentrations and yearly percent change in height-corrected total kidney volume, but again no kynurenines remained associated with the disease progression after Bonferroni adjustment (Table 2).
Table 2.
Stepwise linear regression analysis of baseline levels of kynurenines versus corresponding yearly change in clinical parameters (ΔeGFR and percent change of HtTKV) in ADPKD patients
| Children and young adults with ADPKD | ||
| Baseline 3-OH KYN | beta = 0.324, p = 0.046 | Yearly change in eGFR |
| Baseline PA | beta = − 0.345, p = 0.013 | Yearly % change in HtTKV |
| Adults with ADPKD | ||
| Baseline AA | beta = 0.427, p = 0.012 | Yearly % change in HtTKV |
| Baseline 3-OH KYN | beta = 0.550, p = 0.006 | Yearly % change in HtTKV |
Samples collected at the initiation of the pravastatin interventional trial in children and young adults (N = 64) and at invitation of the HALT-PKD trial (N = 80) were used for analysis. Yearly absolute change (delta) in eGFR was calculated as the difference between the levels at 4 years as compared to the baseline in individual patients, whereas for yearly percent change in HtTKV we normalized the yearly change Δ to the corresponding baseline value and so calculated the percent change in the respective patient. Adjustments for gender, age, race, SBP, BMI and study group were made. p < 0.0056 is considered significant (after Bonferroni correction)
3-OH KYN 3-hydroxykynurenine; AA anthranilic acid; PA picolinic acid
Discussion
Inflammation begins early in ADPKD [25, 26]. We and others have shown that significant oxidative stress and inflammation are present in early stage ADPKD prior to deterioration in kidney function and development of hypertension [25–30]. Kynurenine and downstream metabolites are believed to play an important role in systemic inflammation and oxidative stress by promoting lipid peroxidation and generation of free radicals [15]. Hence, accumulation of kynurenines has been linked to the development of atherosclerosis and cardiovascular disease [31], particularly in patients with impaired kidney function [14–19].
The first synthesis step of kynurenine from tryptophan is oxidation of the latter by indoleamine 2,3-dioxygenase (Fig. 1). In a large cohort study of the general population (n = 921, 46–76 years old), indoleamine 2,3-dioxygenase activity, as indicated by kynurenine/tryptophan ratio, was positively correlated with increased carotid artery intima-media thickness. Based on these results, indoleamine 2,3-dioxygenase was proposed as a risk marker of atherosclerosis [32]. Moreover, an increased kynurenine/tryptophan ratio was found in subjects with angiographically verified coronary heart disease as compared to healthy controls [33].
In a prospective blinded endpoint analysis of patients with chronic kidney disease, the serum levels of kynurenine, kynurenic acid and quinolinic acid increased with chronic kidney disease severity, while tryptophan levels remained unchanged [34]. The kynurenine/tryptophan ratio was significantly higher in patients with chronic kidney disease. It correlated with disease severity and key inflammatory markers such as hypersensitive C reactive protein and soluble tumor necrosis factor receptor-1 independent of serum creatinine, age, and body weight [34]. Metabolomics analysis of plasma from 1,434 adult participants of the Framingham Heart Study also found kynurenic acid concentrations to be significantly increased in patients with faster progressing chronic kidney disease [35].
Furthermore, in a recent study in a cohort of type 2 diabetic individuals across different stages of chronic kidney disease, kynurenine, kynurenic acid and quinolinic acid concentrations were positively correlated with the severity of kidney disease [13]. Based on these and many other results, kynurenines are currently classified as uremic toxins. Overall, our results revealed that children and young adults with ADPKD and normal kidney function (above 60 mL/min/1.73m2) present with higher levels of kynurenine and kynurenic acid as well as with unchanged kynurenine/tryptophan-ratios (after Bonferroni adjustment) when compared to healthy children (Fig. 2). In the same patients, interleukin-6 and MCP-1 levels remained unchanged suggesting that pathways different than those involved in the production of proinflammatory interleukin-6 and MCP-1 are responsible for this initial increase in kynurenines. With progressing disease as indicated by higher height-corrected total kidney volume or lower eGFR, adults with ADPKD diverged further from the healthy subjects. Thus, adult patients with ADPKD with normal kidney function presented with higher levels of interleukin-6 and MCP-1 as well as of kynurenine, kynurenic acid, 3-OH kynurenine, 3-OH anthranilic acid, quinolinic acid and a higher kynurenine/tryptophan-ratio (Fig. 2).
Fig. 2.
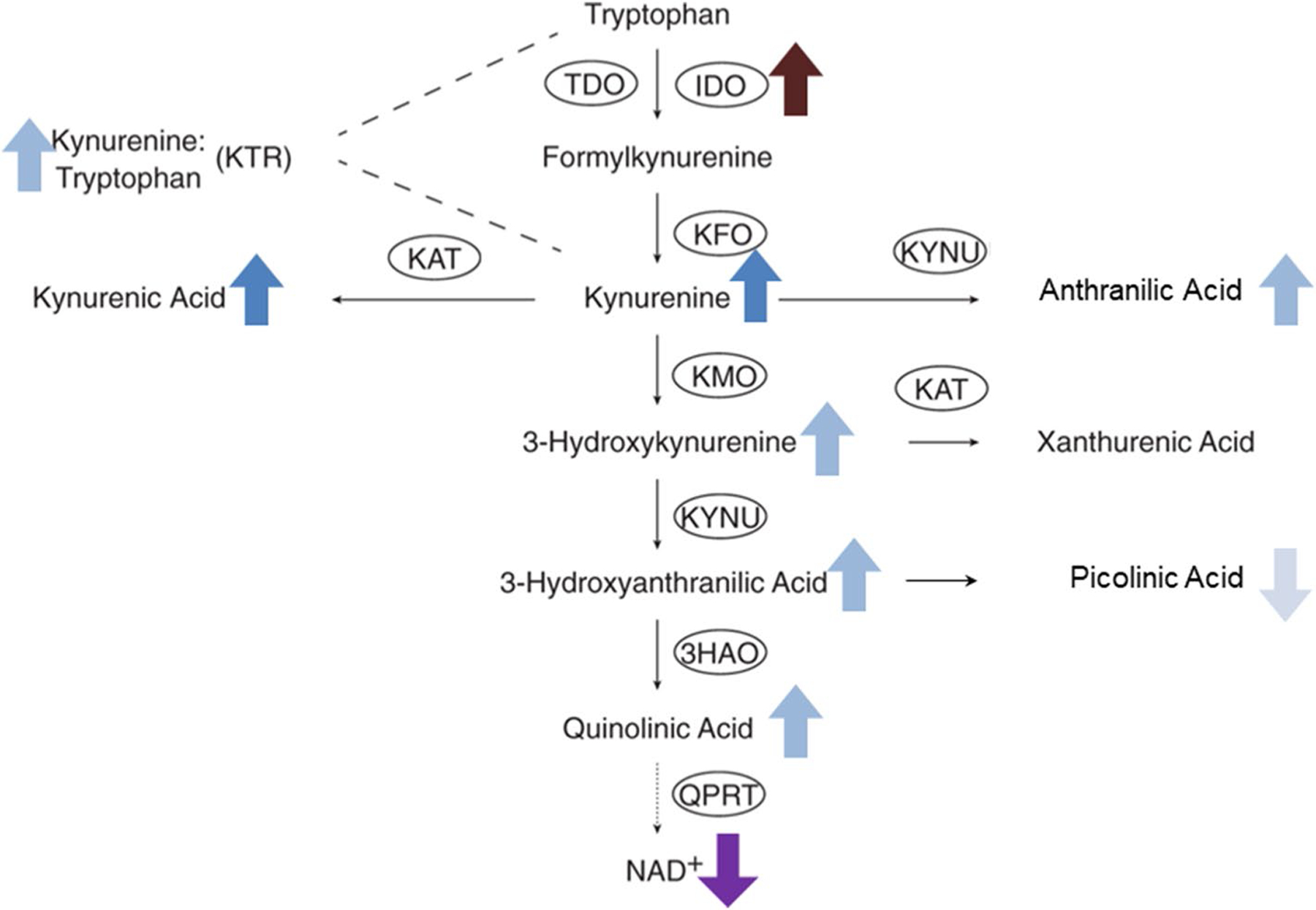
Tryptophan/kynurenine pathway and changes in plasma concentrations of kynurenines in ADPKD patients versus healthy subjects. Dark blue arrows (up or down for concentration increase or decrease as compared to healthy age-matched subjects) represent changes observed in children and adults with ADPKD; light blue arrows represent change only seen in adults and light grey only in children; other colors such as for the increase of IDO1 (indoleamine 2,3-dioxygenase) expression and the decline of nicotinamide indicate hypothetical changes. Abbreviations: 3HAO 3-hydroxyanthranilate dioxygenase; IDO indoleamine 2,3-dioxygenase; KAT kynurenine transaminase; KFO kynurenine formylase; KMO kynurenine monooxygenase; KYNU kynureninase; NAD+ nicotinamide adenine dinucleotide; QPRT quinolinate phosphoribosyl transferase; TDO tryptophan 2,3-dioxygenase
At baseline, kynurenic acid and kynurenine/tryptophan ratio associated with the disease severity in patients with ADPKD. This included kynurenic acid with height-corrected total kidney volume in children and with kidney function in adults as well as kynurenine/tryptophan ratio with height-corrected total kidney volume in adults.
We previously observed a positive correlation between the percent change in kynurenic acid and the percent change in height-corrected total kidney volume over 3 years in pediatric patients with ADPKD. In said study, the same patients as studied here were evaluated at baseline and at 3 years using a targeted metabolomics approach (N = 51) [36]. However, baseline kynurenines did not predict the rate of ADPKD progression in a stepwise linear regression model after Bonferroni and confounders adjustments in children or adults with ADPKD. Baseline picolinic acid and 3-OH kynurenine in children, and additionally anthranilic acid in adults with ADPKD, came close to associate with yearly disease progression (% change in height-corrected total kidney volume) but lost the significance after Bonferroni adjustment.
Our study is in agreement with the study performed in adult participants of the Modification of Diet in Renal Disease Study where higher levels of kynurenic acid were observed in patients with ADPKD as compared to patients with other causes of chronic kidney disease [37]. In contrast, another recent study showed that there were no significant differences in the kynurenate levels between healthy subjects (n = 25, serum) and ADPKD patients with preserved kidney function (n = 31, plasma), but confirmed increased plasma kynurenic acid levels in ADPKD patients with reduced kidney function (n = 95, eGFR < 90 ml/min per 1.73m2) [38]. Interestingly, the plasma kynurenic acid levels that we observed in ADPKD patients were similar between both studies. These were 8.2 ng/mL in our study versus 8.8 ng/mL reported by Wang et al. in patients with normal or slightly reduced eGFR and 13.9 ng/mL versus 15.2 ng/mL in patients with ADPKD and with reduced eGFR [38]. The only difference seems to be in the lower kynurenic acid levels we observed in plasma of healthy subjects whereas Wang et al. used serum for their comparisons [38].
In summary, our study showed that pediatric and adult patients with ADPKD exhibit an overactive tryptophan/kynurenine metabolism as compared to healthy subjects. Kynurenine, kynurenic acid, and quinolinic acid concentrations in plasma were higher in children and adults with ADPKD as compared to the corresponding healthy control subjects. Baseline kynurenic acid concentrations and kynurenine/tryptophan ratios associated with disease severity as indicated by baseline height-corrected total kidney volume or eGFR. While interleukin-6 and MCP-1 plasma levels remained unchanged in pediatric patients, both were elevated in adults with ADPKD. Baseline kynurenines did not associate with the disease progression as calculated by the yearly percent change in height-corrected total kidney volume or yearly change in eGFR in either patient population. Still, inhibition of the observed activated kynurenine pathway may present a novel opportunity to slow progression of ADPKD.
Study Limitation. Sample volume in pediatric ADPKD patients was limited and we unfortunately did not have sufficient sample volume as necessary to evaluate additional inflammatory pathways (apart from interleukin-6 and MCP-1) that could have contributed to the increased kynurenine and kynurenic acid levels in children and young adults with ADPKD with unchanged interleukin-6 and MCP-1 levels as compared to healthy subjects.
Furthermore, healthy subjects were on average 4 years younger than the patients with ADPKD at baseline. Despite the adjustment for age as a confounding variable in our analyses, it should still be noted that some of the differences between the healthy children and children with ADPKD might arise from puberty-related metabolic changes. Analysis of a larger number of clinical samples is warranted, since several of the baseline kynurenines associated with disease severity and progression but lost their significance after Bonferroni adjustment (for multiple testing).
Funding
This research was supported by the National Institutes of Health NIH U01DK062404, R01 DK058793 and R01DK114424.
Footnotes
Conflict of interest The Authors declare that there is no conflict of interest.
Ethical statement Approval was obtained from the ethics committee of University of Colorado. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
References
- 1.Ong AC, Wheatley DN (2003) Polycystic kidney disease–the ciliary connection. Lancet 361(9359):774–776 [DOI] [PubMed] [Google Scholar]
- 2.Wilson PD (2004) Polycystic kidney disease. N Engl J Med 350(2):151–164 [DOI] [PubMed] [Google Scholar]
- 3.Schrier RW (2009) Renal volume, renin-angiotensin-aldosterone system, hypertension, and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 20(9):1888–1893 [DOI] [PubMed] [Google Scholar]
- 4.Karihaloo A, Koraishy F, Huen SC, Lee Y, Merrick D, Caplan MJ et al. (2011) Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 22(10):1809–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swenson-Fields KI, Vivian CJ, Salah SM, Peda JD, Davis BM, van Rooijen N et al. (2013) Macrophages promote polycystic kidney disease progression. Kidney Int 83(5):855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poyan Mehr A, Tran MT, Ralto KM, Leaf DE, Washco V, Messmer J et al. (2018) De novo NAD(+) biosynthetic impairment in acute kidney injury in humans. Nat Med 24(9):1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minhas PS, Liu L, Moon PK, Joshi AU, Dove C, Mhatre S et al. (2019) Macrophage de novo NAD(+) synthesis specifies immune function in aging and inflammation. Nat Immunol 20(1):50–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munn DH (2006) Indoleamine 2,3-dioxygenase, tumor-induced tolerance and counter-regulation. Curr Opin Immunol 18(2):220–225 [DOI] [PubMed] [Google Scholar]
- 9.Zhai L, Spranger S, Binder DC, Gritsina G, Lauing KL, Giles FJ et al. (2015) Molecular pathways: targeting IDO1 and other tryptophan dioxygenases for cancer immunotherapy. Clin Cancer Res 21(24):5427–5433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munn DH, Mellor AL (2013) Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 34(3):137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munipally PK, Agraharm SG, Valavala VK, Gundae S, Turlapati NR (2011) Evaluation of indoleamine 2,3-dioxygenase expression and kynurenine pathway metabolites levels in serum samples of diabetic retinopathy patients. Arch Physiol Biochem 117(5):254–258 [DOI] [PubMed] [Google Scholar]
- 12.Prendergast GC (2011) Cancer: why tumours eat tryptophan. Nature 478(7368):192–194 [DOI] [PubMed] [Google Scholar]
- 13.Debnath S, Velagapudi C, Redus L, Thameem F, Kasinath B, Hura CE et al. (2017) Tryptophan metabolism in patients with chronic kidney disease secondary to type 2 diabetes: relationship to inflammatory markers. Int J Tryptophan Res 10:1178646917694600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pawlak K, Domaniewski T, Mysliwiec M, Pawlak D (2009) Kynurenines and oxidative status are independently associated with thrombomodulin and von Willebrand factor levels in patients with end-stage renal disease. Thromb Res 124(4):452–457 [DOI] [PubMed] [Google Scholar]
- 15.Pawlak K, Domaniewski T, Mysliwiec M, Pawlak D (2009) The kynurenines are associated with oxidative stress, inflammation and the prevalence of cardiovascular disease in patients with end-stage renal disease. Atherosclerosis 204(1):309–314 [DOI] [PubMed] [Google Scholar]
- 16.Pawlak K, Buraczewska-Buczko A, Mysliwiec M, Pawlak D (2010) Hyperfibrinolysis, uPA/suPAR system, kynurenines, and the prevalence of cardiovascular disease in patients with chronic renal failure on conservative treatment. Am J Med Sci 339(1):5–9 [DOI] [PubMed] [Google Scholar]
- 17.Schefold JC, Zeden JP, Fotopoulou C, von Haehling S, Pschowski R, Hasper D et al. (2009) Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: a possible link between chronic inflammation and uraemic symptoms. Nephrol Dial Transpl 24(6):1901–1908 [DOI] [PubMed] [Google Scholar]
- 18.Sallee M, Dou L, Cerini C, Poitevin S, Brunet P, Burtey S (2014) The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: a new concept to understand cardiovascular complications of chronic kidney disease. Toxins (Basel) 6(3):934–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolachalama VB, Shashar M, Alousi F, Shivanna S, Rijal K, Belghasem ME et al. (2018) Uremic solute-aryl hydrocarbon receptor-tissue factor axis associates with thrombosis after vascular injury in humans. J Am Soc Nephrol 29(3):1063–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cadnapaphornchai MA, George DM, Masoumi A, McFann K, Strain JD, Schrier RW (2011) Effect of statin therapy on disease progression in pediatric ADPKD: design and baseline characteristics of participants. Contemp Clin Trials 32(3):437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cadnapaphornchai MA, George DM, McFann K, Wang W, Gitomer B, Strain JD et al. (2014) Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 10.2215/CJN.08350813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schrier RW, Abebe KZ, Perrone RD, Torres VE, Braun WE, Steinman TI et al. (2014) Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med 371(24):2255–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres VE, Abebe KZ, Chapman AB, Schrier RW, Braun WE, Steinman TI et al. (2014) Angiotensin blockade in late autosomal dominant polycystic kidney disease. N Engl J Med 371(24):2267–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu W, Stevens AP, Dettmer K, Gottfried E, Hoves S, Kreutz M et al. (2011) Quantitative profiling of tryptophan metabolites in serum, urine, and cell culture supernatants by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 401(10):3249–3261 [DOI] [PubMed] [Google Scholar]
- 25.Klawitter J, Klawitter J, McFann K, Pennington AT, Abebe KZ, Brosnahan G et al. (2014) Bioactive lipid mediators in polycystic kidney disease. J Lipid Res 55(6):1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klawitter J, Reed-Gitomer BY, McFann K, Pennington A, Klawitter J, Abebe KZ et al. (2014) Endothelial dysfunction and oxidative stress in polycystic kidney disease. Am J Physiol Renal Physiol 307(11):F1198–F1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Strandgaard S, Borresen ML, Luo Z, Connors SG, Yan Q et al. (2008) Asymmetric dimethylarginine and lipid peroxidation products in early autosomal dominant polycystic kidney disease. Am J Kidney Dis 51(2):184–191 [DOI] [PubMed] [Google Scholar]
- 28.Wang D, Strandgaard S, Iversen J, Wilcox CS (2009) Asymmetric dimethylarginine, oxidative stress, and vascular nitric oxide synthase in essential hypertension. Am J Physiol Regul Integr Comp Physiol 296(2):R195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menon V, Rudym D, Chandra P, Miskulin D, Perrone R, Sarnak M (2010) Inflammation, oxidative stress, and insulin resistance in polycystic kidney disease. Clin J Am Soc Nephrol 6(1):7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klawitter J, McFann K, Pennington AT, Wang W, Klawitter J, Christians U et al. (2015) Pravastatin Therapy and Bio-marker Changes in Children and Young Adults with Autosomal Dominant Polycystic Kidney Disease. Clin J Am Soc Nephrol 10(9):1534–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song P, Ramprasath T, Wang H, Zou MH (2017) Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases. Cell Mol Life Sci 74:2899–2916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niinisalo P, Raitala A, Pertovaara M, Oja SS, Lehtimaki T, Kahonen M et al. (2008) Indoleamine 2,3-dioxygenase activity associates with cardiovascular risk factors: the Health 2000 study. Scand J Clin Lab Invest 68(8):767–770 [DOI] [PubMed] [Google Scholar]
- 33.Sulo G, Vollset SE, Nygard O, Midttun O, Ueland PM, Eussen SJ et al. (2013) Neopterin and kynurenine-tryptophan ratio as predictors of coronary events in older adults, the Hordaland Health Study. Int J Cardiol 168(2):1435–1440 [DOI] [PubMed] [Google Scholar]
- 34.Zinellu A, Sotgia S, Mangoni AA, Sanna M, Satta AE, Carru C (2015) Impact of cholesterol lowering treatment on plasma kynurenine and tryptophan concentrations in chronic kidney disease: relationship with oxidative stress improvement. Nutr Metab Cardiovasc Dis 25(2):153–159 [DOI] [PubMed] [Google Scholar]
- 35.Rhee EP, Clish CB, Ghorbani A, Larson MG, Elmariah S, McCabe E et al. (2013) A Combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol 24(8):1330–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baliga MM, Klawitter J, Christians U, Hopp K, Chonchol M, Gitomer BY et al. (2021) Metabolic profiling in children and young adults with autosomal dominant polycystic kidney disease. Sci Rep 11(1):6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grams ME, Tin A, Rebholz CM, Shafi T, Kottgen A, Perrone RD et al. (2017) Metabolomic alterations associated with cause of CKD. Clin J Am Soc Nephrol 12(11):1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang K, Zelnick LR, Chen Y, Hoofnagle AN, Watnick T, Seliger S et al. (2020) Alterations of proximal tubular secretion in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 15(1):80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]


