Abstract
Haemophilus ducreyi expresses a soluble cytolethal distending toxin (CDT) that is encoded by the cdtABC gene cluster and can be detected in culture supernatant fluid by its ability to kill HeLa cells. The cdtA, cdtB, and cdtC genes of H. ducreyi were cloned independently into plasmid vectors, and their encoded proteins expressed singly or in various combinations in an Escherichia coli background. All three gene products had to be expressed in order for E. coli-derived culture supernatant fluids to demonstrate cytotoxicity for HeLa cells. Isogenic H. ducreyi cdtA and cdtB mutants were constructed and used in combination with the wild-type parent strain and a previously described H. ducreyi cdtC mutant (M. K. Stevens, J. L. Latimer, S. R. Lumbley, C. K. Ward, L. D. Cope, T. Lagergard, and E. J. Hansen, Infect. Immun. 67:3900–3908, 1999) to determine the relative contributions of the CdtA, CdtB, and CdtC proteins to CDT activity. Expression of CdtA, CdtB, and CdtC appeared necessary for H. ducreyi-derived culture supernatant fluid to exhibit cytotoxicity for HeLa cells. Whole-cell sonicates and periplasmic extracts from the cdtB and cdtC mutants had no effect on HeLa cells, whereas these same fractions from a cdtA mutant had a very modest cytotoxic effect on these same human cells. CdtA appeared to be primarily associated with the H. ducreyi cell envelope, whereas both CdtB and CdtC were present primarily in the soluble fraction from sonicated cells. Both the cdtA mutant and the cdtB mutant were found to be fully virulent in the temperature-dependent rabbit model for experimental chancroid.
The genital ulcer disease chancroid, caused by the bacterium Haemophilus ducreyi, is one of the most prevalent sexually transmitted diseases and is a major cause of morbidity in the resource-poor countries of Africa, Asia, and Latin America (50). In the pre-antibiotic era, chancroid ulcers were noted to heal at slow rates and often resolution was incomplete (38, 50). Bacterial factors, acting either directly or indirectly, may be responsible for this observed chronicity of H. ducreyi genital ulceration. In particular, it is possible that one or both of the two toxins known to be elaborated by H. ducreyi could be involved in this effect. The hemolysin HhdA, which has been described as being bacterial cell-associated, kills human foreskin fibroblasts (HFF) in vitro (1, 28). The other H. ducreyi cytotoxin, a member of the family of toxins known as cytolethal distending toxins (CDTs) (33), has little effect on HFF cells but readily kills other human cells, including HeLa cells in vitro (37, 44), and has been shown to cause Jurkat T cells to undergo apoptosis (14).
The vast majority of H. ducreyi strains (35) produce CDT that can be readily detected in culture supernatant fluid. The H. ducreyi CDT, first described phenotypically by Purven and Lagergard (37), is chromosomally encoded by three adjacent genes, cdtA, cdtB, and cdtC, which appear to be transcriptionally linked (8). Similar CDTs are expressed by a number of different enteric organisms, including Escherichia coli (18, 31, 40), Shigella species (17, 27), and Campylobacter species (19, 32), as well as by Actinobacillus actinomycetemcomitans (25, 46). CDT activity was originally characterized as causing relatively slow morphological changes in cultured epithelial cells, including progressive cellular distention and death within 96 to 120 h (18). CDT exerts its effect through cell cycle arrest in the G2 phase of growth (7, 9, 30, 41, 46, 52). Most recently, it was reported that CDT has intrinsic DNAse activity associated with the CdtB polypeptide (11, 12, 22).
The exact composition of the active form of CDT is as yet unknown. All three gene products (i.e., CdtA, CdtB, and CdtC) appear to be necessary for the expression of E. coli CDT activity in culture supernatant fluid (31, 40). There is also evidence to support the involvement of the H. ducreyi cdtC gene product in the expression of cytotoxic activity because a monoclonal antibody (MAb) to the H. ducreyi CdtC protein has been shown to neutralize H. ducreyi CDT activity in vitro (8, 25), although it is unlikely that CdtC itself has toxic activity like that attributed to CdtB (41).
In the present study, we report the construction and characterization of isogenic cdtA and cdtB mutants of H. ducreyi 35000. The phenotypes of these two new mutants and that of a H. ducreyi cdtC mutant (44) were assessed through the use of in vitro cytotoxicity assays with HeLa cells and in the temperature-dependent rabbit model for experimental chancroid. Cytotoxic activity assays of whole-cell sonicates and periplasmic extracts prepared from these three isogenic mutants revealed that these preparations from the cdtA mutant still possessed some CDT activity, whereas the same preparations from the cdtB and cdtC mutants were devoid of CDT activity.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. All H. ducreyi strains were routinely cultivated on chocolate agar (CA) plates containing 1% (vol/vol) Iso VitaleX (BBL Microbiological Systems, Cockeysville, Md.) in a humidified atmosphere of 95% air–5% CO2 at 33°C as previously described (23). Broth cultures of H. ducreyi were grown overnight (16 h) in modified Columbia broth (51) for the preparation of whole-cell sonicates, periplasmic extracts, and culture supernatant fluids. Cells of E. coli strains were grown on Luria-Bertani medium (39) at 37°C. For antimicrobial supplementation, kanamycin was used at 30 μg/ml (for both E. coli and H. ducreyi), tetracycline was used at 15 μg/ml (for E. coli), and chloramphenicol was used at 0.5 μg/ml (for H. ducreyi) and 15 μg/ml (for E. coli).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | Host strain for cloning experiments | 39 |
| DH5α(pDL20-A pDL10-B pJL300-C) | Contains the H. ducreyi cdtA, cdtB, and cdtC genes on three separate plasmids; expresses CDT activity detectable in culture supernatant fluid | This study |
| DH5α(pDL19-2 pDL16-1 pLS88) | Contains three different vectors; expresses no detectable CDT activity in culture supernatant fluid | This study |
| DH5α(pJL300) | Contains H. ducreyi 35000 cdtABC gene cluster; expresses H. ducreyi CDT activity detectable in culture supernatant fluid | 44 |
| DH5α(pJL301) | Contains H. ducreyi 35000 cdtABC gene cluster with mutated cdtA gene; expresses no detectable CDT activity in culture supernatant fluid | This study |
| DH5α(pJL302) | Contains H. ducreyi 35000 cdtABC gene cluster with mutated cdtB gene; expresses no detectable CDT activity in culture supernatant fluid | This study |
| DH5α(pJL303) | Contains H. ducreyi 35000 cdtABC gene cluster with mutated cdtC gene; expresses no detectable CDT activity in culture supernatant fluid | 44 |
| HB101 | Host strain essential for propagating plasmids carrying mutated H. ducreyi DNA inserts used in H. ducreyi mutant construction | 39 |
| H. ducreyi | ||
| 35000 | Wild-type strain isolated in Winnipeg, Manitoba, Canada; produces CDT | 15 |
| 35000.301 | Isogenic cdtA mutant with kan1 cartridge inserted into cdtA gene; does not express CDT activity detectable in culture supernatant fluid | This study |
| 35000.302 | Isogenic cdtB mutant with kan1 cartridge inserted into cdtB gene; does not express CDT activity detectable in culture supernatant fluid | This study |
| 35000.303 | Isogenic cdtC mutant with cat cartridge inserted into cdtC gene; does not express CDT activity detectable in culture supernatant fluid | 44 |
| Plasmids | ||
| pBR322 | Cloning vector, Ampr Tetr | 39 |
| pUC18K | pUC18 with kan1 gene ligated into multi-cloning site | 26 |
| pACYC184 | Cloning vector capable of replication in H. ducreyi; Chlorr, Tetr | 6 |
| pLS88 | Cloning vector capable of replication in H. ducreyi; Kanr, Smr, Sulr | 10 |
| pJL300 | pBR322 with 3-kb H. ducreyi 35000 DNA insert containing cdtABC gene cluster | 44 |
| pJL301 | pJL300 with kan1 cartridge inserted into RsrII site within cdtA gene | This study |
| pJL302 | pJL300 with kan1 cartridge inserted into MscI site within cdtB gene | This study |
| pJL303 | pJL300 with cat cartridge inserted into BseRI site within cdtC gene | 44 |
| pDL19-2 | Plasmid obtained by blunt-ending and self-ligating 3.6-kb PstI-EcoRI fragment from pBR322; vector control for pDL20-A | This study |
| pDL20-A | pDL19-2 with 1.1-kb PCR-derived PstI-EcoRI DNA fragment containing H. ducreyi cdtA gene | This study |
| pDL16-1 | Plasmid obtained by blunt-ending and self-ligating 4-kb SalI-BamHI fragment from pACYC184; vector control for pDL10-B | This study |
| pDL10-B | pDL16-1 with 1.2-kb PCR-derived SalI-BamHI DNA fragment containing H. ducreyi cdtB gene | This study |
| pJL300-C | pLS88 with 813-bp PCR-derived insert containing H. ducreyi cdtC gene | 44 |
MAbs.
The H. ducreyi CdtA-reactive MAb 1G8, the CdtB-reactive MAb 20B2, and the CdtC-reactive MAb 8C9 have been described (44).
Recombinant DNA techniques.
Standard techniques, including restriction enzyme digests, ligation, transformation, and plasmid purification, have been described elsewhere (4, 39). An aphA-3 gene (kan1) lacking both its own promoter and transcriptional terminators was utilized for the construction of nonpolar insertion mutations in the H. ducreyi cdtA and cdtB genes. This aphA-3 gene is preceded by translational stop codons in all three reading frames and is immediately followed by a consensus ribosome binding site and a start codon. The use of appropriate restriction sites allows placement of the start codon (at the 3′ end of this cassette) in the open reading frame of the stop codon of the mutated gene so that translation of the remaining 3′ portion of the mutated gene will allow translational coupling, if any, with a downstream gene(s). This aphA-3 gene was excised from pUC18K by digestion with SmaI (26). PCR was performed according to manufacturers' instructions with either Taq DNA polymerase (Promega, Madison, Wis.) or Pfu DNA polymerase (Stratagene, La Jolla, Calif.). Boiled bacterial cell preparations (16) or purified H. ducreyi chromosomal DNA (5) were used as templates for PCR.
Nucleotide sequence analysis.
DNA sequencing was performed using a model 373A automated DNA sequencer (Applied Biosystems Inc., Foster City, Calif.). All PCR products used for complementation analyses were sequenced to confirm the absence of PCR-derived mutations.
RT-PCR detection of H. ducreyi mRNA from rabbit lesions.
A lesion from a rabbit injected intradermally 48 h previously with 105 CFU of H. ducreyi 35000 was excised and macerated in 1 ml of Ultraspec RNA isolation reagent (BiotecX Laboratories, Inc., Houston, Tex.) by using a glass homogenizer and then stored at −70°C until processed. After suspension of the purified RNA pellet in 50 μl of diethyl pyrocarbonate-H2O, the RNA sample was treated with 10 U of DNase at 37°C for 30 min to degrade any residual DNA using the Message CleanR Kit (GenHunter Corporation, Nashville, Tenn.).
Reverse transcriptase (RT) PCR was performed using the Titan One Tube RT-PCR system (Boehringer-Mannheim Biochemicals, Indianapolis, Ind.) according to the manufacturer's instructions. Test sample (lesion RNA), positive control (H. ducreyi 35000 chromosomal DNA), no-RT control (reactions containing lesion RNA placed in the thermocycler at step 2), and negative control (no template) reactions were run simultaneously. The oligonucleotide primers P1 (5′-GCACCAGCATTTGTATTAACGGC-3′) and P2 (5′-CGGTTGAAACTTGGCTAACGC-3′) were used in RT-PCR to obtain a predicted 355-bp product derived from the pal gene, which encodes a H. ducreyi lipoprotein (positive control) (43). The following oligonucleotide primers were used in RT-PCR to obtain the corresponding products derived from the cdtABC genes: a 132-bp product from the cdtA gene using P3 (5′-TTCGTAATTGGAAGATGG-3′) and P4 (5′-CGTGCTAATTTGTCCAGAC-3′), a 460-bp product from the cdtB gene using P5 (5′-GGTTCCTTACCAAGTTCGGCAG-3′) and P6 (5′-TATTTTCACTCACTGCGG-3′), and a 271-bp product from the cdtC gene using P7 (5′-ATGTTTTGCTTTCCTGGG-3′) and P8 (5′-ACCCTGATTTCTTCGCAC-3′). The RT-PCR cycle contained seven steps: step 1, 50°C (30 min); step 2, 94°C (2 min); step 3, 94°C (1 min); step 4, 52°C (1 min); step 5, 68°C (1 min); step 6, 29 cycles through steps 3 to 5; step 7, 68°C for 10 min.
Construction of isogenic H. ducreyi cdtA and cdtB mutants.
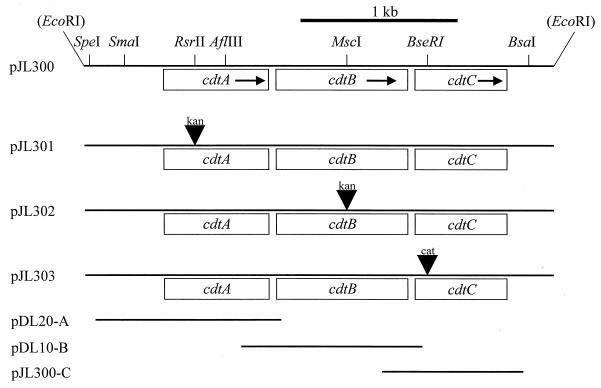
The cdtA gene in the cdtABC gene cluster in pJL300 (44) was inactivated by insertion of the kan1 cartridge into the RsrII site (Fig. 1) (26) to produce pJL301. Inactivation of the cdtB gene was accomplished by inserting the same kan1 cartridge into the MscI site within the cdtB gene in pJL300; the resultant construct was designated pJL302. Selection for kanamycin resistance followed by nucleotide sequence analysis confirmed that the cartridge was inserted in the proper orientation in each open reading frame. Plasmids pJL301 and pJL302 were purified from E. coli HB101 cells by using the Wizard Plus minipreps purification system (Promega), linearized by digestion with AatII, and used to electroporate H. ducreyi 35000. Transformants were selected on GC-heme plates supplemented with kanamycin (45). H. ducreyi transformants with potential mutations in the cdtA gene were identified by PCR-based amplification of chromosomal DNA from single colonies by using the oligonucleotide primers P9 (5′-CCTTGTAGATTATTCACCGT-3′) and P10 (5′-AAAGTTTGATGGTTCAGACGG-3′) and by the loss of reactivity with the H. ducreyi CdtA-reactive MAb 1G8 using Western blot analysis. Similarly, H. ducreyi transformants with potential mutations in the cdtB gene were identified by PCR-based amplification of chromosomal DNA from single colonies using oligonucleotide primers P11 (5′-GCAAACCGAGTGAACTTAG-3′) and P12 (5′-TATTTTCACTCACTGCGG-3′) and by loss of reactivity with the H. ducreyi CdtB-reactive MAb 20B2. The isogenic H. ducreyi 35000 cdtC mutant used in this study has been described (44).
FIG. 1.
Partial restriction map of the H. ducreyi 35000 chromosomal DNA insert in pJL300 and related plasmids. Restriction sites in parentheses indicate vector cloning sites. The arrows indicate the direction of transcription. Plasmid pJL300 was derived from the pBR322 vector; both it and pJL303 have been described (44). The kan1 cartridge was blunt-ended and ligated into the similarly treated RsrII site in cdtA to construct pJL301 and into the similarly treated MscI site of cdtB to produce pJL302. Plasmid pDL20-A is a modified pBR322 vector containing the 1.1-kb PstI- and EcoRI-digested PCR product with the cdtA gene. Plasmid pDL10-B is a modified pACYC184 vector containing the 1.2-kb SalI- and BamHI-digested PCR product with the cdtB gene. Plasmid pJL300-C is pLS88 containing the cdtC gene (44).
Construction of a plasmid containing the H. ducreyi cdtA gene for complementation studies in E. coli.
A 1.1-kb DNA fragment containing the cdtA gene was amplified from H. ducreyi 35000 chromosomal DNA by PCR using Pfu DNA polymerase and the oligonucleotide primers P13 (5′-TACTGCAGTTCCCATACGCCAGGATAG-3′) and P14 (5′-TAGAATTCATAACATCACACAGAAAACCACAC-5′); the underlined sequences indicate PstI and EcoRI sites, respectively. The PCR product was digested with PstI and EcoRI before ligation into the 3.6-kb PstI-EcoRI fragment of the plasmid vector pBR322. The ligation mixture was used to transform E. coli DH5α; tetracycline-resistant transformants were screened for expression of CdtA by Western blot analysis using MAb 1G8. Plasmid pDL20-A containing the H. ducreyi cdtA gene was obtained from one of these transformants. In addition, E. coli DH5α was transformed with the 3.6-kb pBR322-derived vector pDL19-2 (Table 1).
Construction of a plasmid containing the H. ducreyi cdtB gene for complementation studies in E. coli.
A 1.2-kb DNA fragment containing the cdtB gene was amplified from H. ducreyi 35000 chromosomal DNA as described above using the oligonucleotide primers P15 (5′-TAGGATCCTGGTGCGGTTGTCATTAAAAG-3′) and P16 (5′-ATTAGTCGACGAGGAGGTGATAACTCTACATCAGG-3′); the underlined sequences indicate BamHI and SalI sites, respectively. The PCR product was digested with BamHI and SalI before ligation into the 4-kb BamHI-SalI fragment of the plasmid vector pACYC184 (6). The ligation mixture was used to transform E. coli DH5α; chloramphenicol-resistant transformants were screened for expression of CdtB by Western blot analysis using MAb 20B2. Plasmid pDL10-B was obtained from one of these transformants (Table 1). Plasmid pACYC184 was digested with both BamHI and SalI and, after blunt-ending, was self-ligated to form the vector pDL16-1.
Plasmid pDL20-A containing the cdtA gene (Table 1), pDL10-B containing the cdtB gene (Table 1), and pJL300-C containing the cdtC gene (reference 44 and Table 1), in addition to their respective vector controls (pDL19-2 for pDL20-A, pDL16-1 for pDL10-B, and pLS88 for pJL300-C), were used in complementation studies with E. coli cells (Table 1). E. coli DH5α cells were electroporated with pDL20-A, pDL10-B, or pJL300-C alone or in various combinations in order to examine the contributions of CdtA, CdtB, and CdtC to CDT activity as assayed in the HeLa cell cytotoxicity assay.
Preparation of bacterial extracts for cytotoxicity testing.
E. coli culture supernatant fluid preparations were prepared as previously described (44). To obtain H. ducreyi culture supernatant fluid preparations, 16-h overnight broth cultures were subjected to centrifugation at 7,600 × g for 20 min to collect the bacterial cells which were then used to prepare whole-cell sonicates and periplasmic extracts as described below. The resultant supernatant was sterilized by filtration through a cellulose acetate filter (0.2-μm pore size; Nalgene, Rochester, N.Y.) and then subjected to centrifugation at 219,000 × g for 1.5 h. The final supernatant fluid from this last centrifugation step was filter sterilized again and used as the culture supernatant fluid for cytotoxicity testing. To prepare whole-cell sonicates, the bacterial cells collected by centrifugation from the broth culture were washed once with pH 7.3 phosphate-buffered saline and collected again by centrifugation. The cell pellet was suspended in 20 mM Tris-HCl (pH 8.0) containing 20% (wt/vol) sucrose, using 5 ml of this solution per gram (wet weight) of cells. This suspension was sonicated five times (1-min sonication followed by a 1-min cooling period each time) using a Branson model 450 sonifier (Branson Sonic Power Co., Danbury, Conn.) and a tapered microtip on power setting no. 10 and at 60% duty. The whole-cell sonicate was subjected to centrifugation at 27,000 × g for 20 min to remove unbroken cells and debris. The resultant whole-cell sonicate was sterilized by filtration through a cellulose acetate filter (0.2-μm pore size) and stored at 4°C overnight prior to performing the cytotoxicity assay. Periplasmic extracts were prepared as previously described (23), filter sterilized, and stored at 4°C until used to perform cytotoxicity assays. To confirm the presence of periplasmic proteins in the periplasmic extract, MAb 3F1, which is specific for the H. ducreyi periplasmic ZnuA protein (23), was used in Western blot analysis. In addition, periplasmic extracts and culture supernatant fluids were tested for the presence of cell envelopes by using MAb 3F12, specific for the H. ducreyi major outer membrane protein (MOMP) (21). These preparations were also tested for the presence of cytosolic contamination by assaying the cytoplasmic enzyme glucose-6-phosphate dehydrogenase (24).
Cytotoxicity assays.
Cytotoxicity assays for CDT activity used HeLa cells (ATCC CCL-2) grown in supplemented Dulbecco's modified Eagle's medium (GIBCO-BRL, Gaithersburg, Md.) in 24-well plates as previously described (44). After incubation of freshly seeded HeLa cells (3 × 104 cells/well) in 0.5 ml of tissue culture medium overnight, either a sample of bacterial culture supernatant fluid (0.5 ml) or a sample of H. ducreyi periplasmic extract or whole-cell sonicate (50 μg of protein in 0.5 ml of tissue culture medium) was added to the HeLa cells for 3 h before removal of the fluid and replacement with fresh tissue culture medium. Serial twofold dilutions of these three preparations were made in tissue culture medium to determine CDT titers. The plates were incubated for 96 h at 37°C in an atmosphere of 95% air–5% CO2. At the 96 h time point, the wells were either stained with Giemsa and photographed or the CellTiter 96Aqueous One Solution Cell Proliferation Assay (Promega) was used to determine the extent of killing (i.e., which wells still had viable cells). The CDT titer was defined as the greatest dilution which did not produce detectable killing of the HeLa cells.
Preparation of soluble cell proteins and Sarkosyl-insoluble cell fractions.
CA plate-grown H. ducreyi cells were subjected to sonication as described above. The sonicate was centrifuged at 3,000 × g for 10 min to remove whole cells and gross debris; the supernatant fluid from this centrifugation step was then subjected to centrifugation at 40,000 × g for 1 h to collect cell envelopes. The supernatant fluid from this second centrifugation step was subjected to ultracentrifugation at 156,000 × g for 2 h to remove any membrane fragments; the final supernatant fluid was designated as the soluble cell fraction. The cell envelopes were washed twice with phosphate-buffered saline and then treated with Sarkosyl as previously described (13) to obtain the Sarkosyl-insoluble fraction.
Sodium dodecylsulfate-polyacrylamide gel electrophoresis and Western blot (immunoblot) methods.
Proteins present in various cell fractions and concentrated culture supernatant fluids were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose paper for Western blot analysis with MAbs as previously described (44).
Virulence testing.
The relative virulence of the three H. ducreyi cdt mutants used in this study was determined in a blinded manner using the temperature-dependent rabbit model for experimental chancroid (34). Lesion characteristics on days 2, 4, and 7 postinfection were scored with the following numeric values: 0 = no change, 1 = erythema, 2 = induration, 3 = nodule, 4 = pustule or necrosis. On day 7 postinfection, material excised from lesions caused by injection of 105 CFU was cultured on CA plates. Statistical analyses were performed as previously described (2, 45).
RESULTS
Complementation studies with E. coli.
In a preliminary effort to evaluate the contribution of the CdtA, CdtB, and CdtC proteins to the cytotoxic activity of H. ducreyi CDT, E. coli was used as the host for various combinations of the H. ducreyi cdtA, cdtB, and cdtC genes carried individually on plasmids. Two new plasmids with H. ducreyi cdtA (i.e., pDL20-A) and cdtB (i.e., pDL10-B) gene inserts were constructed for this purpose (Table 1 and Fig. 1). Plasmid pJL300-C containing a functional H. ducreyi cdtC gene (44) was also used (Table 1). Western blot analysis of whole-cell lysates of the various recombinant E. coli strains with CdtA-reactive, CdtB-reactive, and CdtC-reactive MAbs confirmed that each construct expressed the expected cdt gene product (data not shown). Assays for CDT activity were performed using culture supernatant fluids obtained from overnight cultures of strains possessing each gene individually as well as all possible double or triple combinations of the three cdt genes and their relevant vector controls. In addition, E. coli strains DH5α(pBR322) and DH5α(pJL300) (Table 1) were used as negative and positive controls, respectively, in the cytotoxicity assays.
HeLa cell cytotoxicity was observed only with culture supernatant fluids from E. coli DH5α(pJL300), which contained the cdtABC gene cluster, and from E. coli DH5α(pDL20-A pDL10-B pJL300-C), which contained the individual cdtA, cdtB, and cdtC genes on separate plasmids (data not shown). Culture supernatant fluids derived from the corresponding transformants DH5α(pBR322) and DH5α(pDL19-2 pDL16-1 pLS88) (Table 1), which contained only vector, were not cytotoxic to HeLa cells. Culture supernatant fluids from E. coli strains containing plasmids expressing either one or two of the CdtA, CdtB, and CdtC proteins failed to exhibit cytotoxicity for HeLa cells (data not shown).
Construction of isogenic H. ducreyi cdtA and cdtB mutants.
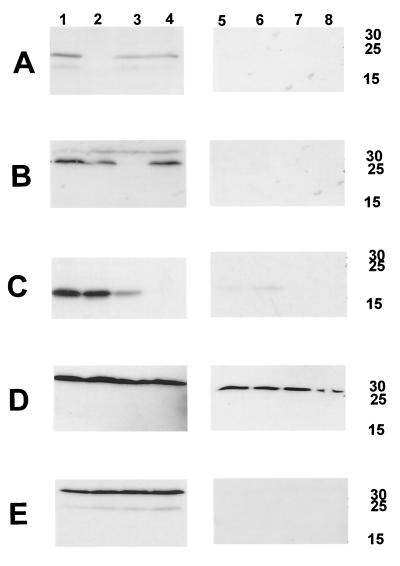
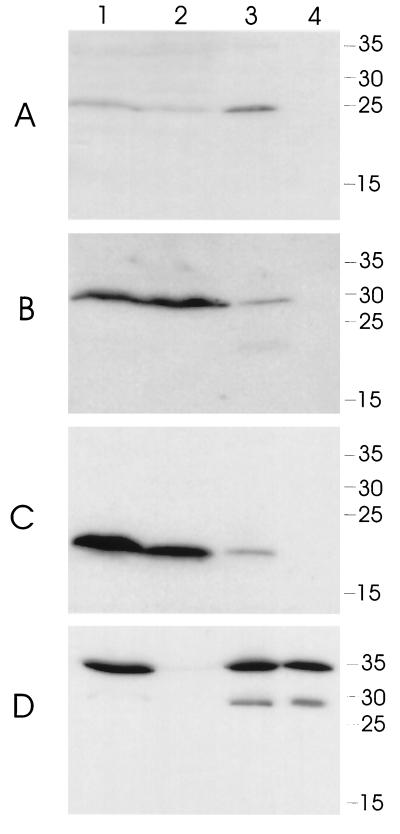
To construct an isogenic H. ducreyi cdtA mutant, pJL301 (Fig. 1) was linearized and used to electroporate H. ducreyi 35000. A kanamycin-resistant transformant which failed to react with the H. ducreyi CdtA-reactive MAb 1G8 was selected for further study and designated strain 35000.301. Western blot analysis revealed that this cdtA mutant expressed no detectable CdtA protein (Fig. 2A, lane 2) and readily detectable levels of both CdtB (Fig. 2B, lane 2) and CdtC (Fig. 2C, lane 2).
FIG. 2.
Western blot-based detection of H. ducreyi CdtA, CdtB, and CdtC proteins expressed by wild-type and mutant H. ducreyi strains. Whole-cell sonicates (lanes 1 to 4) and periplasmic extracts (lanes 5 to 8) of these four strains were probed with the H. ducreyi CdtA-reactive MAb 1G8 (A), the H. ducreyi CdtB-reactive MAb 20B2 (B), the H. ducreyi CdtC-reactive MAb 8C9 (C), the ZnuA-reactive MAb 3F1 (D), and the MOMP-reactive MAb 3F12 (E). Equivalent amounts of whole-cell sonicate and periplasmic extract were loaded into each lane. Lanes: 1 and 5, wild-type H. ducreyi 35000; 2 and 6, H. ducreyi cdtA mutant 35000.301; 3 and 7, H. ducreyi cdtB mutant 35000.302; 4 and 8, H. ducreyi cdtC mutant 35000.303. Molecular size markers (in kilodaltons) are indicated on the right.
An isogenic cdtB mutant was constructed in H. ducreyi 35000 by the method described above except that linearized pJL302 (Fig. 1) was used to electroporate H. ducreyi 35000 cells. A kanamycin-resistant H. ducreyi transformant that failed to react with the H. ducreyi CdtB-reactive MAb 20B2 was designated 35000.302. Western blot analysis showed that this cdtB mutant expressed no detectable CdtB protein (Fig. 2B, lane 3) but did express both CdtA (Fig. 2A, lane 3) and CdtC (Fig. 2C, lane 3). The level of CdtC expressed by this cdtB mutant was lower than that expressed by the wild-type parent strain. PCR and Southern blot analyses confirmed that the desired allelic exchanges had occurred in both strains 35000.301 and 35000.302 (data not shown).
Cytotoxic activity of the wild-type and mutant H. ducreyi strains.
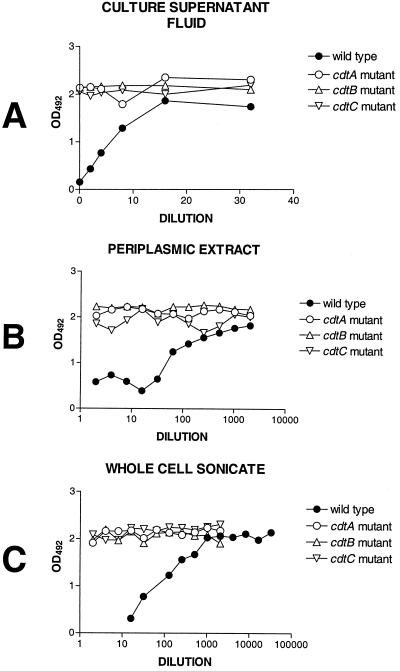
Initially, culture supernatant fluids from the wild-type strain 35000, the cdtA mutant 35000.301, the cdtB mutant 35000.302, and the cdtC mutant 35000.303 (44) were tested for HeLa cell cytotoxicity. These culture supernatant fluids were free of cytosolic contamination as determined by the absence of detectable glucose-6-phosphate dehydrogenase activity (data not shown) and did not contain either periplasmic proteins or outer membranes, as evidenced by their lack of reactivity with the ZnuA-specific MAb 3F1 and the MOMP-specific MAb 3F12, respectively (data not shown). Cytotoxicity was observed with culture supernatant fluids from the wild-type strain only (Fig. 3A); culture supernatant fluids from the three isogenic mutants, 35000.301, 35000.302, and 35000.303, all failed to exhibit cytotoxic activity. When CdtA-, CdtB-, and CdtC-directed MAbs were used in Western blot analysis to probe culture supernatant fluid obtained from the wild-type parent strain, none of the Cdt proteins could be detected (data not shown). Fortyfold concentrations of these supernatant fluids still did not allow detection of Cdt proteins by Western blot analysis (data not shown).
FIG. 3.
Killing of HeLa cells by CDT in subcellular fractions and culture supernatant fluids from wild-type and mutant strains of H. ducreyi. Cytotoxic activity was assessed in culture supernatant fluids (A), periplasmic extracts (B), and whole-cell sonicates (C) obtained from the wild-type H. ducreyi strain 35000, the cdtA mutant 35000.301, the cdtB mutant 35000.302, and the cdtC mutant 35000.303. HeLa cells were exposed to filter-sterilized culture supernatant fluids, periplasmic extracts, or whole-cell sonicates as described in Materials and Methods. After 96 h, wells in which viable cells were present were detected by the use of the tetrazolium reduction-based assay described in Materials and Methods. An optical density at 492 nm (OD492) greater than 1.8 indicates wells in which all of the HeLa cells were viable.
Cytotoxicity of subcellular fractions from wild-type and mutant strains.
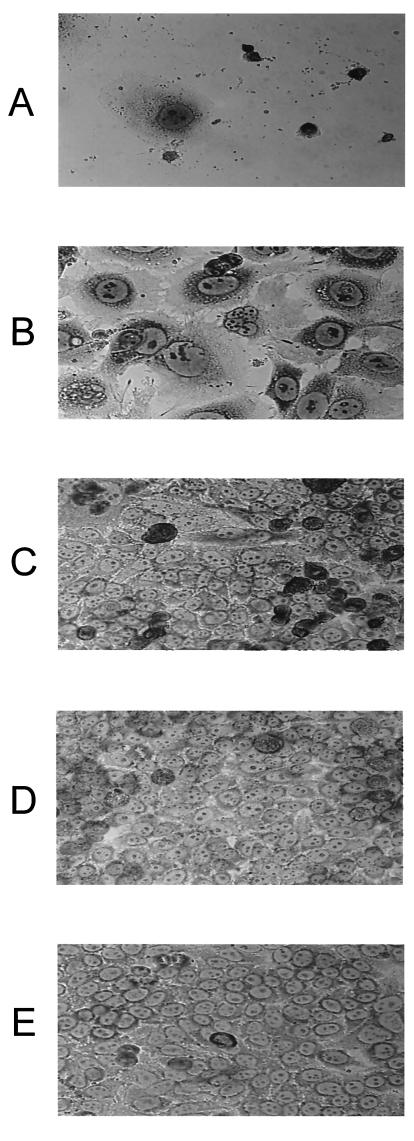
Both periplasmic extracts and whole-cell sonicates from the wild-type strain and the cdtA, cdtB, and cdtC mutants were tested for their ability to kill HeLa cells. The periplasmic extracts were free of cytosolic contamination (data not shown) and outer membrane fragments (Fig. 2E, lanes 5 to 8) as determined above and did contain the periplasmic protein ZnuA (Fig. 2D, lanes 5 to 8). The relevant Cdt proteins could be detected readily in the whole-cell sonicates (Fig. 2A to C, lanes 1 to 4). However, in most cases, Cdt proteins could not be detected by Western blot analysis in the periplasmic extracts (Fig. 2A to C, lanes 5 to 8), even with the wild-type strain. Both whole-cell sonicates (Fig. 3C) and periplasmic extracts (Fig. 3B) from wild-type strain 35000 caused marked killing of the HeLa cells, whereas the same preparations from the cdtA mutant 35000.301, the cdtB mutant 35000.302, and the cdtC mutant 35000.303 appeared to lack killing activity as determined by a tetrazolium dye reduction test (described in Materials and Methods). However, microscopy of the HeLa monolayers exposed to the most concentrated samples (i.e., 50 μg of protein in 0.5 ml) of whole-cell sonicate from the cdtA mutant 35000.301 (Fig. 4B) revealed the presence of many distended or rounded HeLa cells. These distended cells were not present in the monolayers treated with whole-cell sonicates from the cdtB mutant (Fig. 4C) and the cdtC mutant (Fig. 4D). No viable HeLa cells were present in monolayers treated with the whole-cell sonicate from the wild-type parent strain (Fig. 4A). The periplasmic extract from the cdtA mutant also caused HeLa cell rounding and distention whereas the periplasmic extracts from the cdtB and cdtC mutants did not affect these cells (data not shown).
FIG. 4.
Cytopathic effect of CDT from wild-type and mutant strains of H. ducreyi. HeLa cells were exposed to whole-cell sonicates from the wild-type strain 35000 (A), the H. ducreyi cdtA mutant 35000.301 (B), the H. ducreyi cdtB mutant 35000.302 (C), and the H. ducreyi cdtC mutant 35000.303 (D), and 96 h later the wells were photographed at an original magnification of ×40. (E) HeLa cells exposed to the buffer used for the preparation of the whole-cell sonicates were used as the negative control.
In a preliminary effort to localize the Cdt proteins in H. ducreyi, we prepared a whole-cell sonicate (Fig. 5A to D, lanes 1) from the wild-type strain 35000 and separated the soluble cell contents (Fig. 5, lanes 2) from the cell envelopes (Fig. 5, lanes 3) and then treated the cell envelopes with Sarkosyl to obtain the Sarkosyl-insoluble fraction of the cell envelopes (Fig. 5, lanes 4). The amounts of soluble cell contents, cell envelopes, and Sarkosyl-insoluble material were equivalent to the number of cells used to prepare the sample contained in Fig. 5, lane 1. The bulk of the CdtB (Fig. 5B, lane 2) and CdtC (Fig. 5C, lane 2) proteins appeared to be soluble, whereas most of the CdtA protein (Fig. 5A, lane 3) was associated with the cell envelopes. These cell envelopes did contain the MOMP (Fig. 5D, lane 3). When these envelopes were extracted with Sarkosyl, the CdtA protein was not detectable in the Sarkosyl-insoluble fraction (Fig. 5A, lane 4), which did contain the MOMP (Fig. 5D, lane 4).
FIG. 5.
Detection of Cdt proteins in soluble and insoluble fractions from H. ducreyi cells. Whole-cell sonicates were prepared from the wild-type strain 35000 as described in Materials and Methods by using centrifugation at 3,000 × g to remove whole cells and gross debris. Equivalent amounts of whole-cell sonicate (lane 1), soluble cell contents (lane 2), cell envelopes (lane 3), and Sarkosyl-insoluble material (lane 4) were probed by Western blot analysis using the CdtA-reactive MAb 1G8 (A), the CdtB-reactive MAb 20B2 (B), the CdtC-reactive MAb 8C9 (C), and the MOMP-reactive MAb 3F12 (D). Molecular size position markers (in kilodaltons) are on the right.
Virulence testing.
After confirming that the three isogenic mutants 35000.301, 35000.302, and 35000.303 each grew at a rate indistinguishable from that of the wild-type strain 35000 in broth (data not shown), all four strains were tested in the temperature-dependent rabbit model. The cdtA and cdtB mutants both proved to be as virulent as the wild-type parent strain with regard to lesion production (Table 2). As seen previously (44), the cdtC mutant 35000.303 also was no less virulent than the wild-type strain (Table 2). In addition, viable H. ducreyi organisms were isolated from the lesions resulting from an injection of 105 CFU for all four strains (data not shown). The numbers of CFUs of each of the four strains recovered from these lesions were similar (data not shown).
TABLE 2.
Lesion formation by wild-type and mutant H. ducreyi strains in the temperature-dependent rabbit modela
| Strain | Inoculum size (CFU) | Mean (±SD) lesion scoreb
|
P valuec | ||
|---|---|---|---|---|---|
| Day 2 | Day 4 | Day 7 | |||
| 35000 (wild type) | 105 | 4.00 (0) | 4.00 (0) | 4.00 (0) | |
| 35000.301 (cdtA mutant) | 105 | 4.00 (0) | 4.00 (0) | 4.00 (0) | |
| 35000.302 (cdtB mutant) | 105 | 3.88 (0.35) | 4.00 (0) | 4.00 (0) | |
| 35000.303 (cdtC mutant) | 105 | 4.00 (0) | 4.00 (0) | 4.00 (0) | |
| 35000 (wild type) | 104 | 3.50 (0.53) | 4.00 (0) | 4.00 (0) | |
| 35000.301 (cdtA mutant) | 104 | 3.38 (0.52) | 3.75 (0.46) | 4.00 (0) | 0.0796 |
| 35000.302 (cdtB mutant) | 104 | 3.25 (0.46) | 3.38 (0.52) | 3.75 (0.46) | 0.0256 |
| 35000.303 (cdtC mutant) | 104 | 3.63 (0.52) | 3.75 (0.46) | 4.00 (0) | 0.6845 |
Eight rabbits were used in this experiment.
Lesion characteristics were scored as follows: 0, no change; 1, erythema; 2, induration; 3, nodule; 4, pustule or necrosis.
P values were calculated for the difference between wild-type and mutant strain lesion scores. P values were calculated using the lesion scores from the 104-CFU inoculum size on 3 days. Using the Bonferroni correction necessitated by the number of comparisons between strains in this analysis, the P value needed for significance is 0.0083. Therefore, there was no significant difference in lesion scores produced by the three isogenic mutants when they were compared in a pairwise manner.
Detection of cdtA, cdtB, and cdtC mRNA produced in vivo.
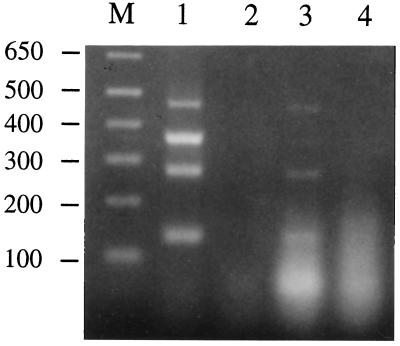
The fact that the mutants lacking CDT activity were as virulent as the wild-type strain in the rabbit model prompted us to confirm that the cdtABC genes were actually transcribed in the cells of the wild-type parent strain growing in vivo. RNA was prepared from pustular lesion material obtained 48 h after infection of rabbits with the wild-type strain, 35000. RT-PCR confirmed the presence of mRNA transcribed from the cdtA, cdtB, and cdtC genes. Specific primers amplified a 132-bp cdtA-encoded product, a 460-bp cdtB-encoded product, and a 271-bp cdtC-encoded product from the RNA (Fig. 6, lane 3). A 355-bp pal-encoded product was amplified from the RNA as a positive control (Fig. 6, lane 3); the H. ducreyi pal gene encodes an unprocessed 18-kDa lipoprotein (43). H. ducreyi 35000 chromosomal DNA was used as a template positive control (Fig. 6, lane 1). No PCR product was seen in a reaction devoid of template (Fig. 6, lane 2) or in a reaction in which the RT step was omitted (Fig. 6, lane 4).
FIG. 6.
Agarose gel electrophoresis of H. ducreyi multiplex RT-PCR products. The sizes of the predicted RT-PCR products were as follows: pal, 355 bp; cdtA, 132 bp; cdtB, 460 bp; cdtC, 271 bp. Templates included the following: lane 1, 100 ng of H. ducreyi 35000 genomic DNA (positive control); lane 2, no template (negative control); lanes 3 and 4, 2 μg of RNA isolated from H. ducreyi-infected rabbit lesions. The reaction mixture loaded in lane 4 was not subjected to the reverse transcription step of the RT-PCR protocol and served as a control to detect DNA contamination of the RNA template. Lane M, DNA size markers (in base pairs).
DISCUSSION
Johnson and Lior (18) originally described CDT activity as a toxic factor produced by certain E. coli strains which would distend and kill CHO cells in vitro. CDT production in several unrelated pathogenic bacteria was subsequently demonstrated (33). CDT can affect a number of different cell types in vitro, including keratinocytes (9, 44), HeLa cells, HEp-2 cells, Vero cells (20), Caco-2 cells (52), hamster lung fibroblasts (9), and both CD4+ and CD8+ human T cells (42). While new data have appeared recently concerning the mechanism of action of CDT (7, 9, 30, 46, 52), there is little information about the composition of the CDT holotoxin.
To date, there have been only two reports describing the purification of a CDT. Working with H. ducreyi, Lagergard and colleagues (36) used a MAb specific for CdtC in an immunoaffinity-based purification method and concluded that H. ducreyi CDT consisted of only CdtC. In contrast, Shenker et al. (42) purified an immunosuppressive factor from A. actinomycetemcomitans cells that killed human T cells and HeLa cells and indicated that this purified toxin was comprised of CdtB. (The protein products of the A. actinomycetemcomitans cdtABC genes are 92 to 97% identical to those of the H. ducreyi cdtABC genes [25].) In neither case was the purity of the protein preparation demonstrated conclusively; in particular, the absence of the other two cdt gene products was not proven.
Data derived from the use of individually cloned H. ducreyi cdtA, cdtB, and cdtC genes in an E. coli background demonstrated that CDT activity in culture supernatant fluid was detectable only when all three cdt gene products were expressed. This result reinforces results obtained in earlier studies of the cdtABC gene clusters from two different E. coli strains (31, 40). Similarly, culture supernatant fluids from isogenic H. ducreyi cdtA, cdtB, and cdtC mutants all failed to kill HeLa cells, whereas culture supernatant fluid from the wild-type parent strain caused extensive cell killing (Fig. 3). In contrast to the results obtained with culture supernatant fluids, both whole-cell sonicates and periplasmic extracts prepared from the H. ducreyi cdtA mutant produced visible cytopathic effects in HeLa cells, with cell distention being most prominent. It should be noted that cell distention caused by CDT precedes killing (18, 20), such that the cell distention effect of the whole-cell sonicate (Fig. 4B) and periplasmic extract of the cdtA mutant likely reflects the presence of either a small quantity of active CDT or a partially inactive CDT. More importantly, the whole-cell sonicates and periplasmic extracts prepared from the H. ducreyi cdtB and cdtC mutants showed no evidence of cytotoxic activity in the HeLa cell assay.
The composition of active H. ducreyi CDT remains to be determined. The results described above indicate that mutations in cdtB and cdtC eliminate CDT activity completely, whereas some very limited CDT activity is expressed by the cdtA mutant. These results, taken together with the facts that MAbs to CdtC can neutralize CDT activity (8, 25, 36) and that the CdtB protein has cytotoxic DNAse activity (11, 12, 22), suggest that both CdtB and CdtC are likely present in the CDT holotoxin. Our finding that a cdtA mutant still expressed detectable CDT activity does not preclude the presence of CdtA in the CDT holotoxin. The very weak cytotoxic effect detectable in the cdtA mutant would be consistent with CdtA being a structural component essential for fully active toxin. Alternatively, CdtA could function to posttranslationally modify either CdtB or CdtC or both proteins to enable them to function optimally.
This study also confirmed our previous observation that a H. ducreyi cdtC mutant was as virulent as its wild-type parent strain in the temperature-dependent rabbit model (44); this mutant was also recently shown to form pustules in human volunteers at a rate similar to that obtained with its wild-type parent strain (53). In addition, we were unable to demonstrate any decrease in virulence associated with either a cdtA or cdtB mutation (Table 2). RT-PCR analysis of RNA prepared from rabbit lesion material confirmed that all three genes of the H. ducreyi cdtABC cluster were transcribed in rabbit skin during infection. In vivo transcription of at least cdtB was also shown to occur in human volunteers infected with wild-type H. ducreyi (48). These findings suggest that the failure to observe diminished virulence with the H. ducreyi cdtA, cdtB, and cdtC mutants in rabbits and with the cdtC mutant in humans is unlikely to be due to a lack of expression of the relevant gene products in vivo.
The apparent lack of effect of mutations in the cdtABC gene cluster on virulence expression by H. ducreyi in both human and animal models of infection indicates that CDT does not play a role in the development of the pustule that forms in the early stages of chancroid. The determination of whether CDT might be involved in either ulcer development or the retardation of healing of the chancroidal ulcer cannot be accomplished in these model systems. In the former, safety concerns preclude studies involving ulcer development while in the latter model, the lack of ulcer production by the wild-type strain in rabbits will not allow either issue to be addressed. However, data from recent in vitro experiments suggest that CDT could affect the immune response to this pathogen. It has been shown that the spectrum of H. ducreyi CDT activity also includes human T cells (i.e., the Jurkat T-cell line) (14), and this finding is reinforced by a recent study in which H. ducreyi CDT was shown to inhibit proliferation of human T cells and B cells in vitro (47). These results are complemented by studies which indicated that the immunosuppressive factor produced by A. actinomycetemcomitans is a CDT that causes G2 arrest in human T cells in vitro (42). Therefore, it is conceivable that H. ducreyi CDT might be responsible at some level for the delayed healing of chancroidal ulcers observed in the pre-antibiotic era (38, 50) and for the apparent lack of protective immunity after chancroid (3). Whether H. ducreyi CDT can exert immunosuppressive activity in the human host remains a matter of speculation at this time.
ACKNOWLEDGMENTS
This study was supported by U.S. Public Health Service grant AI32011 to E.J.H. and by a Wellcome Training Fellowship in Clinical Tropical Medicine (reference number 049246/Z/96) to D.A.L. under the joint sponsorship of J. N. Weber (Department of Genitourinary Medicine and Communicable Disease) and D. B. Young (Department of Microbiology) at Imperial College School of Medicine, St. Mary's Campus, London, United Kingdom. C.K.W. was supported by National Research Service Award F32-AI09845.
The kan1 cartridge used in this study was kindly provided by James B. Kaper.
REFERENCES
- 1.Alfa M J, Degagne P, Totten P A. Haemophilus ducreyi hemolysin acts as a contact cytotoxin and damages human foreskin fibroblasts in cell culture. Infect Immun. 1996;64:2349–2352. doi: 10.1128/iai.64.6.2349-2352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfa M J, Stevens M K, Degagne P, Klesney-Tait J, Radolf J D, Hansen E J. Use of tissue culture and animal models to identify virulence-associated traits of Haemophilus ducreyi. Infect Immun. 1995;63:1754–1761. doi: 10.1128/iai.63.5.1754-1761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Tawfiq J A, Palmer K L, Chen C-Y, Haley J C, Katz B P, Hood A F, Spinola S M. Experimental infection of human volunteers with Haemophilus ducreyi does not confer protection against subsequent challenge. J Infect Dis. 1999;179:1283–1287. doi: 10.1086/314732. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1990. [Google Scholar]
- 5.Bauer B A, Stevens M K, Hansen E J. Involvement of the Haemophilus ducreyi gmhA gene product in lipooligosaccharide expression and virulence. Infect Immun. 1998;66:4290–4298. doi: 10.1128/iai.66.9.4290-4298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comayras C, Tasca C, Peres S Y, Ducommun B, Oswald E, De Rycke J. Escherichia coli cytolethal distending toxin blocks HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect Immun. 1997;65:5088–5095. doi: 10.1128/iai.65.12.5088-5095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cope L D, Lumbley S R, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson R S, Jr, Lagergard T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortes-Bratti X, Chaves-Olarte E, Lagergard T, Thelestam M. The cytolethal distending toxin from chancroid bacterium Haemophilus ducreyi induces cell-cycle arrest in the G2 phase. J Clin Investig. 1999;103:107–115. doi: 10.1172/JCI3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon L G, Albritton W L, Willson P J. An analysis of the complete nucleotide sequence of the Haemophilus ducreyi broad-host-range plasmid pLS88. Plasmid. 1994;32:228–232. doi: 10.1006/plas.1994.1060. [DOI] [PubMed] [Google Scholar]
- 11.Elwell C, Chao K, Patel K, Dreyfus L. Escherichia coli CdtB mediates cytolethal distending toxin cell cycle arrest. Infect Immun. 2001;69:3418–3422. doi: 10.1128/IAI.69.5.3418-3422.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elwell C A, Dreyfus L A. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol Microbiol. 2000;37:952–963. doi: 10.1046/j.1365-2958.2000.02070.x. [DOI] [PubMed] [Google Scholar]
- 13.Filip C, Fletcher G, Wulff J L, Earhart C F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gelfanova V, Hansen E J, Spinola S M. Cytolethal distending toxin of Haemophilus ducreyi induces apoptotic death of Jurkat T cells. Infect Immun. 1999;67:6394–6402. doi: 10.1128/iai.67.12.6394-6402.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond G W, Lian C J, Wilt J C, Ronald A R. Comparison of specimen collection and laboratory techniques for isolation of Haemophilus ducreyi. J Clin Microbiol. 1978;7:39–43. doi: 10.1128/jcm.7.1.39-43.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hennessy K J, Iandolo J J, Fenwick B W. Serotype identification of Actinobacillus pleuropneumoniae by arbitrarily primed polymerase chain reaction. J Clin Microbiol. 1993;31:1155–1159. doi: 10.1128/jcm.31.5.1155-1159.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson W M, Lior H. Production of Shiga toxin and a cytolethal distending toxin (CLDT) by serogroups of Shigella ssp. FEMS Microbiol Lett. 1987;48:235–238. [Google Scholar]
- 18.Johnson W M, Lior H. Response of Chinese hamster ovary cells to a cytolethal toxin (CDT) of Escherichia coli and possible misinterpretation as heat-labile (LT) enterotoxin. FEMS Microbiol Lett. 1987;43:19–23. [Google Scholar]
- 19.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter ssp. Microb Pathog. 1988;4:115–126. doi: 10.1016/0882-4010(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 20.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Escherichia coli isolates from clinical material. Microb Pathog. 1988;4:103–113. doi: 10.1016/0882-4010(88)90052-6. [DOI] [PubMed] [Google Scholar]
- 21.Klesney-Tait J, Hiltke T J, Maciver I, Spinola S M, Radolf J D, Hansen E J. The major outer membrane protein of Haemophilus ducreyi consists of two OmpA homologs. J Bacteriol. 1997;179:1764–1773. doi: 10.1128/jb.179.5.1764-1773.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lara-Tejero M, Galan J E. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science. 2000;290:354–357. doi: 10.1126/science.290.5490.354. [DOI] [PubMed] [Google Scholar]
- 23.Lewis D A, Klesney-Tait J, Lumbley S R, Ward C K, Latimer J L, Ison C A, Hansen E J. Identification of the znuA-encoded periplasmic zinc transport protein of Haemophilus ducreyi. Infect Immun. 1999;67:5060–5068. doi: 10.1128/iai.67.10.5060-5068.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malamy M H, Horecker B L. Release of alkaline phosphates from cells of Escherichia coli upon alyssum spherules formation. Biochemistry. 1964;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- 25.Mayer M P A, Bueno L C, Hansen E J, DiRienzo J M. Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans. Infect Immun. 1999;67:1227–1237. doi: 10.1128/iai.67.3.1227-1237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okuda J, Kurazono H, Takeda Y. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb Pathog. 1995;18:167–172. doi: 10.1016/s0882-4010(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 28.Palmer K L, Goldman W E, Munson R S., Jr An isogenic haemolysin-deficient mutant of Haemophilus ducreyi lacks the ability to produce cytopathic effects on human foreskin fibroblasts. Mol Microbiol. 1996;21:13–19. doi: 10.1046/j.1365-2958.1996.00615.x. [DOI] [PubMed] [Google Scholar]
- 29.Palmer K L, Munson R S., Jr Cloning and characterization of the genes encoding the haemolysin of Haemophilus ducreyi. Mol Microbiol. 1995;18:821–830. doi: 10.1111/j.1365-2958.1995.18050821.x. [DOI] [PubMed] [Google Scholar]
- 30.Peres S Y, Marches O, Daigle F, Nougayrede J-P, Herault F, Tasca C, De Rycke J, Oswald E. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol. 1997;24:1095–1107. doi: 10.1046/j.1365-2958.1997.4181785.x. [DOI] [PubMed] [Google Scholar]
- 31.Pickett C L, Cottle D L, Pesci E C, Bikah G. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect Immun. 1994;62:1046–1051. doi: 10.1128/iai.62.3.1046-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pickett C L, Pesci E C, Cottle D L, Russell G, Erdem A N, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB genes. Infect Immun. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickett C L, Whitehouse C A. The cytolethal distending toxin family. Trends Microbiol. 1999;7:292–297. doi: 10.1016/s0966-842x(99)01537-1. [DOI] [PubMed] [Google Scholar]
- 34.Purcell B K, Richardson J A, Radolf J D, Hansen E J. A temperature-dependent rabbit model for production of dermal lesions by Haemophilus ducreyi. J Infect Dis. 1991;164:359–367. doi: 10.1093/infdis/164.2.359. [DOI] [PubMed] [Google Scholar]
- 35.Purven M, Falsen E, Lagergard T. Cytotoxin production in 100 strains of Haemophilus ducreyi from different geographic locations. FEMS Microbiol Lett. 1995;129:221–224. doi: 10.1111/j.1574-6968.1995.tb07583.x. [DOI] [PubMed] [Google Scholar]
- 36.Purven M, Frisk A, Lonnroth I, Lagergard T. Purification and identification of Haemophilus ducreyi cytotoxin by use of a neutralizing monoclonal antibody. Infect Immun. 1997;65:3496–3499. doi: 10.1128/iai.65.8.3496-3499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purven M, Lagergard T. Haemophilus ducreyi, a cytotoxin-producing bacterium. Infect Immun. 1992;60:1156–1162. doi: 10.1128/iai.60.3.1156-1162.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rauschkolb J E. Circumcision in treatment of chancroidal lesions of male genitalia. Arch Dermatol Syphilol. 1939;39:319–328. [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning, a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 40.Scott D A, Kaper J B. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect Immun. 1994;62:244–251. doi: 10.1128/iai.62.1.244-251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shenker B J, Hoffmaster R H, McKay T L, Demuth D R. Expression of the cytolethal distending toxin (Cdt) operon in Actinobacillus actinomycetemcomitans: evidence that the CdtB protein is responsible for G2 arrest of the cell cycle in human T cells. J Immunol. 2000;165:2612–2618. doi: 10.4049/jimmunol.165.5.2612. [DOI] [PubMed] [Google Scholar]
- 42.Shenker B J, McKay T, Datar S, Miller M, Chowhan R, Demuth D. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J Immunol. 1999;162:4773–4780. [PubMed] [Google Scholar]
- 43.Spinola S M, Hiltke T J, Fortney K R, Shanks K L. The conserved 18,000-molecular-weight outer membrane protein of Haemophilus ducreyi has homology to PAL Infect. Immun. 1996;64:1950–1955. doi: 10.1128/iai.64.6.1950-1955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevens M K, Latimer J L, Lumbley S R, Ward C K, Cope L D, Lagergard T, Hansen E J. Characterization of a Haemophilus ducreyi mutant deficient in expression of cytolethal distending toxin. Infect Immun. 1999;67:3900–3908. doi: 10.1128/iai.67.8.3900-3908.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stevens M K, Porcella S, Klesney-Tait J, Lumbley S R, Thomas S E, Norgard M V, Radolf J D, Hansen E J. A hemoglobin-binding outer membrane protein is involved in virulence expression by Haemophilus ducreyi in an animal model. Infect Immun. 1996;64:1724–1735. doi: 10.1128/iai.64.5.1724-1735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugai M, Kawamoto T, Peres S, Ueno Y, Komatsuzawa H, Fujiwara T, Kurihara H, Suginaka H, Oswald E. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun. 1998;66:5008–5019. doi: 10.1128/iai.66.10.5008-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Svensson L A, Tarkowski A, Thelestam M, Lagergard T. The impact of Haemophilus ducreyi cytolethal distending toxin on cells involved in immune response. Microb Pathog. 2001;30:157–166. doi: 10.1006/mpat.2000.0422. [DOI] [PubMed] [Google Scholar]
- 48.Throm R E, Spinola S M. Transcription of candidate virulence genes of Haemophilus ducreyi during infection of human volunteers. Infect Immun. 2001;69:1483–1487. doi: 10.1128/IAI.69.3.1483-1487.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Totten P A, Norn D V, Stamm W E. Characterization of the hemolytic activity of Haemophilus ducreyi. Infect Immun. 1995;63:4409–4416. doi: 10.1128/iai.63.11.4409-4416.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trees D L, Morse S A. Chancroid and Haemophilus ducreyi: an update. Clin Microbiol Rev. 1995;8:357–375. doi: 10.1128/cmr.8.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward C K, Lumbley S R, Latimer J L, Cope L D, Hansen E J. Haemophilus ducreyi secretes a filamentous hemagglutinin-like protein. J Bacteriol. 1998;180:6013–6022. doi: 10.1128/jb.180.22.6013-6022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitehouse C A, Balbo P B, Pesci E C, Cottle D L, Mirabito P M, Pickett C L. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66:1934–1940. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young R S, Fortney K R, Gelfanova V, Phillips C L, Katz B P, Hood A F, Latimer J L, Munson R S, Hansen E J, Spinola S M. Expression of cytolethal distending toxin and hemolysin is not required for pustule formation by Haemophilus ducreyi in human volunteers. Infect Immun. 2001;69:1938–1942. doi: 10.1128/IAI.69.3.1938-1942.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]