Dear editor,
Recently, Moussaoui's study has assessed the effectiveness of the third dose of SARS-CoV-2 vaccine among people living with HIV (PLWH) and healthy controls (HC).1 Booster vaccination elicited robust humoral and cellular responses which were similar in both groups. In contrast with prominent increment of anti-wild type (WT) neutralizing antibodies (NAbs), anti-Omicron NAbs were 8-fold lower in PLWH.
It was presented in WHO Global Tuberculosis (TB) report2 that, the COVID-19 pandemic has generated a disrupted accessibility on TB services in hospital, which, as a result, brought a complete upswing of TB diagnosis in 2021. Dire global situation of tuberculosis causes leading deaths which is second only to SARS-CoV-2, especially in PLWH coinfected with tuberculosis.3 Despite the fact that decreasing seroconversion rate and blunting vaccine response has been reported in immunocompromised patients than immunocompetent ones,4 , 5 scarce data on the safety and immunogenicity of SARS-CoV-2 vaccine among people with TB has hindered the further exploration in optimizing vaccine schedule to enhance protection against SARS-CoV-2.
To investigate the immunological responses among TB patients who have already received at least two doses of inactivated SARS-CoV-2 vaccine, we performed a cross-sectional study containing 72 adults with TB from outpatient of the Fifth Hospital of Shijiazhuang (Table 1 ), as well as 39 age-, sex- and vaccination time-matched HC from department of health medicine in Peking Union Medical College Hospital (Table S1). We divided TB patients into four groups according to the vaccination dose after excluding those with previous infection of SARS-CoV-2, including TB inoculated with third dose of inactivated vaccine including Sinopharm and Sinovac (n = 23), those received full vaccination regimen of recombinant protein vaccine (Zhifei Longcom, China) (n = 17), those only inoculated with second dose of inactivated vaccine (n = 16), and unimmunized TB individuals (n = 16). Total antibodies against SARS-CoV-2 (Beijing Wantai Biological Pharmacy Enterprise, Beijing, China), NAbs towards SARS-CoV-2 WT and Omicron BA.4/5 subvariant (utilizing SARS-CoV-2 Surrogate Virus Neutralization Test (sVNT) by GenScript cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit), as well as IgG anti-SARS-CoV-2 Spike receptor binding domain (RBD) antibodies (PROPRIUM Co., Ltd.) were detected using ELISA kits as previously described.6 Moreover, we simultaneously conducted an online survey powered by www.wjx.cn with 246 TB patients get involved, where side-effect of COVID-19 vaccination and willingness for vaccination were evaluated. We compared SARS-CoV-2 specific antibodies among TB patients and HCs using Mann-Whitney U test and analyzed the associations of these humoral immune parameters using Spearman's rank/Pearson's correlation test, meanwhile, χ2 test was employed to identify reasons for COVID-19 vaccine hesitancy among TB patients. A P-value of <0.05 was considered statistically significant.
Table 1.
Demographics and clinical characterization of adults with TB.
| Category | Secondary Tuberculosis |
|---|---|
| Numbers | 72 |
| Age | 34[23,51.5] |
| Gender (F/M) | 28/44 |
| Vaccination status | |
| 3rd dose of inactivated vaccine | 23(31.94%) |
| 3rd dose of recombinant protein vaccine | 17(23.61%) |
| 2nd dose of inactivated vaccine | 16(22.22%) |
| Unvaccinated | 16(22.22%) |
| Disease stage* | |
| Active | 48(66.67%) |
| Improved | 12(16.67%) |
| Stable | 2(2.78%) |
| Tuberculosis infection site | |
| Unilateral lung | 23(31.94%) |
| Bilateral lung | 32(44.44%) |
| Bronchus | 19(26.39%) |
| Laryngeal | 2(2.78%) |
| Pleurisy | 6(8.33%) |
| Peritonitis | 1(1.38%) |
| Intestinal | 1(1.38%) |
| Comorbidity | |
| Drug-induced liver injury | 5(6.94%) |
| Type 2 diabetes mellitus | 11(15.28%) |
| Hypertension | 6(8.33%) |
| Hepatapostema | 2(2.78%) |
| Pulmonary emphysema | 3(4.17%) |
| Cerebral infarction | 2(2.78%) |
| Others a | 26(36.11%) |
| None | 22(30.56%) |
| Therapeutic regimen | |
| Isoniazid | 52(72.22%) |
| Rifampicin | 51(70.83%) |
| Rifapentine | 6(8.33%) |
| Ethambutol | 53(73.61%) |
| Pyrazinamide | 54(75.00%) |
| Levofloxacin | 23(31.94%) |
| Sodium Deoxyribonucleotide | 5(6.94%) |
| Bozhi Glycopeptide | 8(11.11%) |
| Kangfuxin Liquid | 17(23.61%) |
| Others b | 18(25.00%) |
| Positivity for purified protein derivative (PDD) test | 40(55.56%) |
| ESR(mm/h) | 14.5[6.75,43] |
| CRP(mg/L) | 5.05[1.65,21.88] |
Ten TB patients from outpatient have missed their complete medical history.
including: non-alcoholic fatty liver disease, anemia, carotid artery stenosis, hyponatremia, hypoproteinemia, fatty liver disease, coronary arteriosclerosis, lower limb vein thrombus, intestinal perforation, bronchiectasis, intestinal obstruction and chronic hepatitis.
including: amikacin, aztreonam, bedaquiline, ceftazidime, ceftriaxone, clofazimine, cycloserine, linezolid, moxifloxacin, pasiniazide, piperacillin, protionamide, tazobactam.
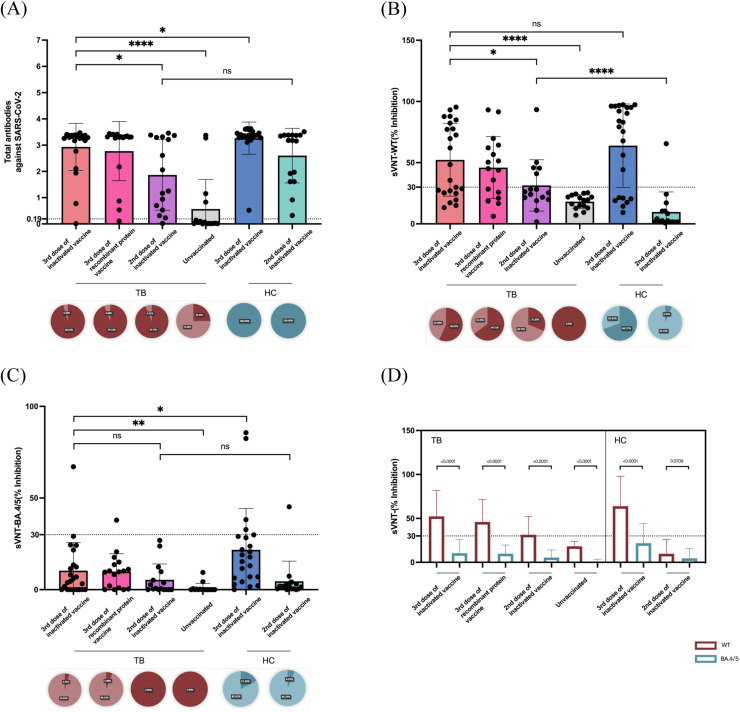
After triple doses of inactivated vaccination, TB patients held a significantly lower level of total antibodies against SARS-CoV-2 compared with HCs (3.298[3.17,3.371] vs. 3.364[3.282,3.461], P = 0.0299) (shown in Fig. 1 A and Table S2). The effect of booster inoculation on neutralizing Omicron subvariant BA.4/5 also decreased in TB than HCs (4.629[0,13.52] vs. 17.79[6.34,29.03], P = 0.0118) (Fig. 1C). No statistical discrepancies of inhibition rate (%) were found in NAbs towards SARS-CoV-2 WT (48.5[25.33,82.27] vs. 79.25[21.11,95.13], P = 0.1950) among TB and HCs receiving booster dose of inactivated vaccination (Fig. 1B). Among TB patients, third dose of inactivated vaccine could not only enhance total humoral responses against SARS-CoV-2 (3rd dose of inactivated vaccine: 3.298[3.17,3.371] vs. 2nd dose of inactivated vaccine: 1.657[0.5708,3.31], P = 0.0233; 3rd dose of inactivated vaccine: 3.298[3.17,3.371] vs. unvaccinated: 0.0375[0.01125,0.6608], P < 0.0001) but also intensify protection rate (%) of NAbs towards both WT (3rd dose of inactivated vaccine: 48.5[25.33,82.27] vs. 2nd dose of inactivated vaccine: 25.75[20.29,44.37], P = 0.0463; 3rd dose of inactivated vaccine: 48.5[25.33,82.27] vs. unvaccinated: 18.16±5.7, P < 0.0001) and Omicron BA.4/5 (3rd dose of inactivated vaccine: 4.629[0,13.52] vs. 2nd dose of inactivated vaccine: 0.6187[0,8.65], P = 0.3347; 3rd dose of inactivated vaccine: 4.629[0,13.52] vs. unvaccinated: 0[0,0.3093], P = 0.0057). No prominent differences were identified in antibody responses triggered by different platforms of COVID-19 vaccines among TB patients who received third dose of inactivated vaccine or recombinant protein vaccine (Table S2). The intergroup discrepancies of IgG anti-RBD antibodies were as similar as that of total antibodies against SARS-CoV-2 among patients with TB and HCs (Fig. S1A), additionally, the magnitude of IgG anti-RBD antibodies was closely correlated with inhibition rate of NAbs against WT and BA.4/5 (Fig. S1B and Table S3). By contrasting the effect of NAbs in blocking infection toward SARS-CoV-2 WT and Omicron lineage BA.4/5, we observed attenuated inhibition rates of BA.4/5 than that of WT in both TB and HC cohort who have inoculated at least second dose of COVID-19 vaccination (Fig. 1D and Table S2). The positivity of NAbs towards WT among TB rankings: TB after 3rd dose of recombinant protein vaccine (64.71%) > TB after 3rd dose of inactivated vaccine (56.52%) > TB after 2nd dose of inactivated vaccine (31.25%) > unvaccinated TB (0.00%) (Fig. 1B), whereas positive rates of NAbs towards BA.4/5 were extremely lower even in TB who received third dose of COVID-19 vaccination (4.35% for those received inactivated ones and 5.88% for those injected recombinant protein vaccines) (Fig. 1C).
Fig. 1.
Levels, positivity and neutralizing effect on wild type (WT) and Omicron subvariant BA.4/5 of SARS-CoV-2 specific antibodies among adults with TB. (A-C) Levels (OD value) of total antibodies against SARS-CoV-2, inhibition rates (%) of neutralizing antibodies (NAbs) against WT and Omicron subvariant BA.4/5 evaluated by SARS-CoV-2 surrogate virus neutralization test (sVNT) among adults with TB received third (booster) dose of inactivated COVID-19 vaccine, third dose (complete regime) of recombinant protein vaccine, second dose of inactivated vaccine and those unvaccinated TB patients. HCs were age-, gender- and vaccination time-matched with TB patients who have inoculated second and third (booster) dose of inactivated vaccine. Antibody positivity was shown each panel below the figures where positive rate was presented as dark red for adults with TB and as dark blue for HCs. Optical density (OD) values above 0.19 were regarded as positive for total antibodies against SARS-CoV-2. Inhibition rate above 30% were regarded as positive. (D) Inhibition ability of NAbs against BA.4/5 (purple bar) and against WT (dark blue bar) among adults with TB and HCs. Seropositivity of NAbs against BA.4/5 remained below positive threshold (30%) at all sampling time after vaccination.
Results from online survey among TB patients revealed that 66% of them received triple doses of COVID-19 vaccine whilst 12% of them were unimmunized (Fig. S2A). When it comes to adverse effects happened in TB patients who ever received vaccine (n = 217), SARS-CoV-2 vaccines exerted rather good safety with 79.7% (n = 173) of them gained no side-effect reactions. Pain (36.4%, 40.9% and 20.5%, respectively, from first to third dose of COVID-19 vaccination) in vaccination site became the most common local adverse effect, while fever (9.1%, 9.1% and 9.1%, respectively, from first to third dose of COVID-19 vaccination) was the first prevalent systemic adverse effect (Fig. S2B). Regarding with the vaccination willingness among adults with TB, we identified a high acceptance (91%) of SARS-CoV-2 vaccine, only 6% of them held uncertainty of being injected and 3% were unwilling for the vaccination. Among all the TB patients involved in our survey, “Worry about adverse effects of COVID-19 vaccines” and “Worry about influencing TB treatment or accelerating disease progression” were the major reasons for vaccination hesitancy (Fig. S2C). TB participants were then categorized into willingness, unwillingness and uncertainty subgroups depending on vaccination attitudes, we found that belief in the effectiveness and the safety of COVID-19 vaccine, and careers as professional or technical staffs (such as teachers, doctors, engineers and technicians, writers and others) significantly increased the vaccine compliance (Fig. S2D and Table S4). However, belief in the adverse effect of COVID-19 vaccination on TB, patients with comorbidity and poor health conditions dominantly attenuated the willingness of COVID-19 inoculation. Moreover, we also queried whether TB patients would like to receive the fourth dose of COVID-19 vaccine, the willingness to get fourth injection was presented as 72%, whereas the most substantial reason of the fourth vaccination unwillingness was being afraid of adverse effect of an additional dose (Fig. S3).
Our observations have suggested that a booster inoculation enables broad humoral immunity among TB patients when compared with the second dose of inactivated vaccine, despite lower than that of HCs. However, we also identified that serum samples from both TB and HC who already received third injection of COVID-19 vaccine have presented a lower-than-threshold neutralizing ability against Omicron BA.4/5 subvariant than WT. Mutations in spike protein RBD of Omicron subvariant give incentive to breakthrough infections and evasions from immunological “shield” constructed by COVID-19 vaccines which contain only ancestral strains from early 2020 Wuhan SARS-CoV-2 virus.7 , 8 Hence, a tailored vaccine designed for Omicron variant is indispensable. Since Chinese authorities have planned to downgrade its management of COVID-19 from Category A to Category B on January 8th, policies are now reopening in an orderly manner, it is inevitable that such strategy would bring burdens to vulnerable populations. SARS-CoV-2 vaccination is the most effective strategy in constructing herd immunity and reducing critical disease progression, heterologous additional vaccine doses are highly recommended in most-at-risk individuals such as those with lung injuries or immunocompromised ones.9 , 10 Understanding factors influencing the willingness of getting the fourth vaccination (second booster dose) and taking targeted propaganda to alleviate the situation of vaccine hesitancy could assist to improve the vaccination rate and protective efficacy among patients with TB.
Our study has several limitations. We were not able to provide prospective data after each dose of SARS-CoV-2 vaccination among individuals with TB due to inherent cross-sectional study design. Larger TB cohort should be recruited to assess both humoral and cellular responses after booster dose of COVID-19 vaccine.
In conclusion, our research has filled the vacancy of immunological assessment among TB patients with/without SARS-CoV-2 vaccine injection. We should notice that although booster shot of COVID-19 vaccines bolstered both SARS-CoV-2 specific antibody levels and positivity among TB patients, poor humoral responses were found in TB subjects than HCs. Therefore, dynamic serologic antibody surveillance could help to extrapolate the optimal occasion of additional injection, exploit its best advantage and provide healthcare recommendations as well as public health implications, especially for patients with suboptimal antibody responses.
Declaration of Competing Interest
The authors have no conflict of interest declared and declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Funding statement
This work was supported by the National Key Research and Development Program of China (2018YFE0207300), Beijing Municipal Science & Technology Commission (Z211100002521021) and Key R&D project of Hebei Province (22377744D).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contribution statement
Professor YZL and EHD conceived and designed the research. PhD. HTZ extracted data, performed software analyses, and visualized graphs and tables. PhD. HTZ wrote and revised the paper. HTZ, YML, XML and HLL performed the experiments. LY and XRG provided the clinical samples and data of participants. Clinical specialists HBW, JFC and SHK diagnosed patients with tuberculosis and provided professional consultant to this paper. All authors are accountable for all aspects of the study, and attest to the accuracy and integrity of the results. All authors have read and approved the final manuscript as submitted.
Ethics statement
This study was approved by the Medical Ethics Committee of Peking Union Medical College Hospital (JS-2156 and I-22PJ147) and the Fifth Hospital of Shijiazhuang (2022-018-1).
Acknowledgements
Thanks for the patients and healthy individuals participated in this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2023.01.030.
Appendix. Supplementary materials
References
- 1.Moussaoui ME, Desmecht S, Tashkeev A, Lambert N, Maes N, Braghini J, et al. Reduced T-cell response following a third dose of SARS-CoV-2 vaccine in infection-naïve people living with HIV. J Infect. 2022;85(6):702–769. doi: 10.1016/j.jinf.2022.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Global tuberculosis report 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022/covid-19-and-tb.
- 3.Furin J, Cox H, Pai M. Tuberculosis. Lancet. 2019;393(10181):1642–1656. doi: 10.1016/S0140-6736(19)30308-3. [DOI] [PubMed] [Google Scholar]
- 4.Deepak P, Kim W, Paley MA, Yang M, Carvidi AB, Demissie EG, et al. Effect of Immunosuppression on the Immunogenicity of mRNA vaccines to SARS-CoV-2 : a prospective cohort study. Ann Intern Med. 2021;174(11):1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker EPK, Desai S, Marti M, Nohynek H, Kaslow DC, Kochhar S, et al. Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. Lancet Glob Health. 2022;10(3):e326–e3e8. doi: 10.1016/S2214-109X(21)00593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhan H, Gao H, Liu Y, Zhang X, Li H, Li X, et al. Booster shot of inactivated SARS-CoV-2 vaccine induces potent immune responses in people living with HIV. J Med Virol. 2023;95(1) doi: 10.1002/jmv.28428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuekprakhon A, Nutalai R, Dijokaite-Guraliuc A, Zhou D, Ginn HM, Selvaraj M, et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185(14):2422–2433. doi: 10.1016/j.cell.2022.06.005. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connolly CM, Paik JJ. SARS-CoV-2 vaccination in the immunocompromised host. J Allergy Clin Immunol. 2022;150(1):56–58. doi: 10.1016/j.jaci.2022.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong J, Liu S, Cui T, Li J, Zhu F, Zhong N, et al. Heterologous booster with inhaled adenovirus vector COVID-19 vaccine generated more neutralizing antibodies against different SARS-CoV-2 variants. Emerg Microbes Infect. 2022;11(1):2689–2697. doi: 10.1080/22221751.2022.2132881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.