Abstract
Context
Intermedin (IMD) is the member of calcitonin gene-related peptide family, and tightly associated with type 2 diabetes mellitus (T2DM). The change of plasma IMD levels in T2DM is still unknown.
Objective
We aimed to investigate the plasma levels of IMD in patients with T2DM.
Design
Fortyone patients with T2DM who were hospitalized in the endocrinology department of Civil Aviation General Hospital from January 2012 to June 2015 were enrolled, and 44 volunteers were selected as the control group.
Subjects and Methods
Plasma level of IMD was detected by ELISA. Diagnostic value of IMD was analyzed by area under the receiver operating characteristic (ROC) curve (AUC).
Results
The plasma level of IMD in T2DM group was higher than that in the healthy control group, whereas smoking or cardiovascular complications did no influence the IMD levels. IMD levels were correlated with BMI, DBP, triglyceride, uric acid, urea nitrogen, fasting and 2 hours postprandial blood glucose, and HbA1C. The greatest value of AUC for IMD was only 58.73%.
Conclusions
Although plasma levels of IMD were increased in patients with T2DM, the very low diagnostic value of IMD for T2DM might not be used for the disease diagnosis.
Keywords: Intermedin, type 2 diabetes mellitus, diagnosis
INTRODUCTION
Diabetes Mellitus (DM) is a complex metabolic disorder featured by hyperglycemia during a sustained time. The prevalence of DM is boosting globally with an estimated 425 million patients in 2017 (1). DM leaded an estimated 4 million deaths and a global economic health associated expenditure of 730 billion US$ per year (1). If untreated, DM may result in serious of chronic complications involving microvascular and macrovascular complications. Type 2 DM (T2DM) is the most common type (about 90%) and is featured by insulin resistance that may cause reduction of insulin production. Recently, large amount of blood and urine-based biomarkers have been identified for DM and its complications (2-4). However, discovering biomarkers for T2DM and its complications keeps challenging as the heterogeneous character of T2DM. The heterogeneity associates not only with glycemic control or treatment response, but also with clinical phenotypes, biochemical characteristics, and differences in environmental exposures (5-7).
Intermedin (IMD), also called adrenomedullin (ADM) 2, was a new member of calcitonin gene-related peptide (CGRP) family (8, 9), which also includes αCGRP, βCGRP, calcitonin receptor-stimulating peptide, amylin, ADM and ADM5 (10). The human IMD gene encodes a prepropeptide composed of 148 amino acids, which has a signal peptide for secretion at its N-terminus. The main active fragments of IMD includes IMD1-40, IMD1-47 and IMD1-53. IMD exerts its biological effects by selectively combining with calcitonin receptor-like receptor and receptor activity modifying protein 1/2/3. IMD is highly conserved in various orthologs suggested the significant and unique function of IMD in the body. Increasing amounts of proofs have demonstrated the crucial role of IMD in maintains of cardiovascular and metabolic homeostasis. IMD exerts protective effect against myocardial ischemia/reperfusion injury, atherosclerosis, vascular calcification, and immunoglobulin A nephropathy (11-13). IMD has been confirmed to improve dyslipidemia, high-fat diet-induced obesity, and insulin resistance (14, 15). In a DM model of rats induced by streptozotocin, IMD ameliorates diabetic ischemic heart injury (16). These studies suggest the tight association between IMD and DM.
ADM, another widely known peptide hormone of CGRP family, controls insulin balance and may take part in the progression of DM (17). In people with DM including type 1, type 2 and gestational diabetes, the plasma levels of ADM are all increased, and are further increased in patients with diabetic complications (18-20). Plasma ADM level is associated with renal failure and retinopathy in type 1 DM, while its level is correlated with a more extensive range of complications in T2DM (21-24). However, the plasm levels of IMD in patients with T2DM remains unclear. Based on the previous studies, we hypothesized that plasma levels of IMD would be elevated in patients with T2DM, and the increased levels of IMD might be helpful for diagnosis of diabetic patients.
MATERIALS AND METHODS
Subjects
In this study, patients with T2DM diagnostic were enrolled who were hospitalized in the endocrinology department of Civil Aviation General Hospital from January 2012 to June 2015. Diagnostic criteria were conformed with the WHO T2DM diagnosis standard in 1997. Exclusion criteria for patients included diabetic ketoacidosis, ketosis hypertonic state, acute infection, severe liver and kidney function damage, and malignant tumors.
Inclusion criteria for control group was normal blood glucose, blood lipid, blood pressure and blood uric acid, and exclusion criteria was abnormality of fasting glucose and glucose tolerance after 75g glucose tolerance test, acute infection, severe liver and kidney function damage and malignant tumors. This study was conducted in accordance with the Helsinki Declaration (revised in 2013), and was approved by the ethics committee of Civil aviation general hospital (2012004). Informed consent was obtained from subjects before their inclusion.
Sample collection
Blood samples from all subjects were placed in tubes containing ethylenediamine tetra-acetic acid and aprotinin (5×105 IU/mL) that were centrifuged immediately at 3500 rpm for 15 minutes at 4 ℃. Plasma was collected and stored at – 80 ℃.
Enzyme linked immunosorbent assay
Plasma samples extracted through a sep-pak C18 cartridge were assayed by use of an Enzyme linked immunosorbent assay (ELISA) kit (Phoneix pharmaceuticals, Belmont, CA, USA). The IC50 of human IMD was 31 pg per tube, and cross-reactivity with human IMD was 100%. No cross-reactivity was found with human CGRP and ADM. The within- and between-assay coefficients of variation for the immunoreactive IMD assay were less than 5% and 10%, respectively.
Measurement of other variables
The levels of fasting blood glucose (FBG), 2-hour blood glucose, triglycerides, total cholesterol, urea nitrogen, creatinine and uric acid were tested by enzyme. Low density lipoprotein (LDL) and high-density lipoprotein (HDL) were tested using clearance method. C-reactive protein (CRP) was detected by immunoturbidimetry. The above tests were performed by Hitachi 7600 instruments. The electrochemiluminescence method was used to detect the fasting and post-meal C peptide, which was completed by Roche 601. Hemoglobin A1c (HbA1c) is detected by high performance liquid chromatography (G8 instruments). All the above tests were completed in our clinical laboratory.
Statistical analysis
Continuous data such as serum IMD levels were expressed as mean ± standard deviation because of normally distribution. The comparison between IMD in T2DM group and healthy control group used t test for independent samples. The expression of IMD in T2DM group was conducted by using independent samples of t test to perform subgroup analysis based on whether or not to merge cardiovascular disease or smoking. In addition, correlations between variables were tested by Pearson correlation analysis. The diagnostic value of IMD was analyzed by area under the receiver operating characteristic (ROC) curve (AUC). All tests were 2-sided and P < 0.05 was considered statistically significant.
RESULTS
Subject profiles
There were 41 patients with T2DM (26 males, 15 females, mean age 51.41 ± 8.28 years) and 44 control volunteers (31 males and 13 females, mean age 46.36±6.55 years) recruited in the study. Among all of patients, 11 persons associated hypertension, 3 persons associated coronary atherosclerotic heart disease, 4 persons associated hypertension and coronary atherosclerotic heart disease, 3 persons associated cerebrovascular disease. Body mass index (BMI), blood pressure, total cholesterol, triglyceride, uric acid, HbA1c, CRP, FBG, 2 hours postprandial blood glucose and C peptide among T2DM group were higher than healthy controls. However, low density lipoprotein (LDL), high-density lipoprotein (HDL), creatinine and fasting C peptide were no significant difference between the two groups (Table 1 and Fig 1).
Table 1.
Compared with general characteristics and biochemical data between T2DM group and control group
| Control group(n=44) | T2DM group(n=41) | P | |
|---|---|---|---|
| Gender (male/female) | 31/13 | 26/15 | 0.6446 |
| Age (year) | 46.36±6.55 | 51.41±8.28 | < 0.0001 |
| Smoking (yes/no) | 5/39 | 22/19 | <0.0001 |
| Body mass index (kg/m2) | 23.02±2.45 | 24.82±3.77 | 0.0117 |
| Systolic blood pressure (mmHg) | 122.16±11.19 | 130.17±16.83 | 0.0125 |
| Diastolic blood pressure (mmHg) | 70.57±9.54 | 75.39±10.82 | 0.0164 |
| Total cholesterol (mmol/L) | 4.5±0.69 | 5.13±1.42 | 0.0064 |
| Triglycerides (mmol/L) | 1.01±0.4 | 2.18±1.74 | < 0.0001 |
| High density lipoprotein (mmol/L) | 1.55±0.33 | 1.43±1.55 | 0.3149 |
| Low density lipoprotein (mmol/L) | 2.64±0.55 | 2.7±0.88 | 0.3549 |
| Creatinine (mmol/L) | 67.09±9.6 | 66.35±16.04 | 0.3995 |
| Uric acid (umol/L) | 273.13±63.73 | 301.03±68.47 | <0.0001 |
Data are expressed as mean ± SD.
Figure 1.
Levels of fasting blood glucose (A), 2 hours postprandial blood glucose (B), fasting C peptide (C), 2 hours postprandial C peptide (D), Hemoglobin A1c (HbA1c) (E) and C reactive protein (F) in plasma. *, P < 0.05 vs. control group.
Plasma levels of IMD
The plasma level of IMD in T2DM group was higher than that in the healthy control group (Fig. 2A). For subgroup of T2DM patients, there is no difference for IMD levels between T2DM with cardiovascular complications, including coronary heart disease, cerebrovascular disease and hypertension (21 subjects), and without complications (Fig. 2B). The plasma levels of IMD in T2DM patients with smoking were similar with that in T2DM patients without smoking (Fig. 2C).
Figure 2.
Plasma levels of IMD. A, comparison of IMD levels between control subjects and T2DM patients; B, comparison of IMD levels between T2DM patients with and without cardiovascular complications; C, comparison of IMD levels between T2DM patients with and without smoking. *, P < 0.05 vs. control group.
Correlation analysis of IMD levels with the subjects
Used by Pearson analysis, IMD plasma levels was positively associated with BMI, diastolic blood pressure (DBP), triglyceride, uric acid, urea nitrogen, fasting and 2 hours postprandial blood glucose, and HbA1C (Fig. 3). There was not significantly correlation between IMD and other values, including age, systolic blood pressure (SBP), cholesterol, LDL, HDL, creatinine, fasting and 2 hours postprandial C peptide, and CRP.
Figure 3.
Regression analysis of IMD with body mass index (BMI) (A), diastolic blood pressure (DBP) (B), triglyceride (C), uric acid (D), urea nitrogen (E), fasting blood glucose (F), 2 hours postprandial blood glucose (G), and HbA1C (H).
The deficient diagnostic value of IMD analyzed by ROC curve
ROC curve was plotted for data of all T2DM and control patients. The greatest value of AUC for IMD was 58.73% (P = 0.166, Std. Error 0.0640, 95% confidence interval 0.4620 to 0.7127, Fig. 4).
Figure 4.
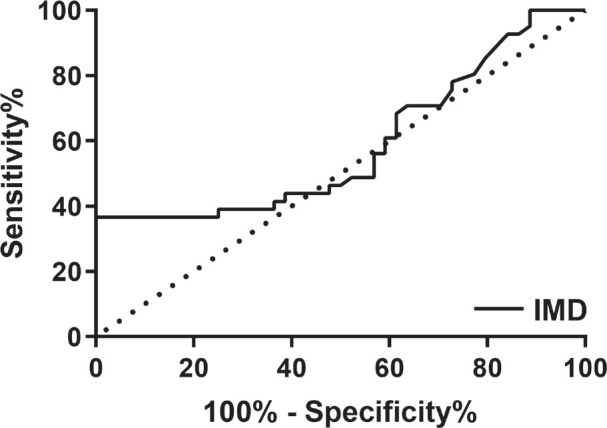
ROC curve for IMD.
DISCUSSION
Here, we show that plasma levels of IMD in patients with T2DM were significantly increased compared with control subjects. There was no difference between the subgroup of T2DM patients, including smoking or cardiovascular complications. IMD levels were correlated with BMI, DBP, triglyceride, uric acid, urea nitrogen, fasting and 2 hours postprandial blood glucose, and HbA1C. However, there was no diagnostic value of IMD for T2DM.
IMD extensively distributed throughout the body, and is a potent endogenous regulated peptide in the heart, vascular, kidney and so on (25). A series of studies have shown that IMD not only inhibits cardiovascular disease directly but also indirectly has a protective effect against metabolic syndrome, improving the risk factors for cardiovascular diseases (25). IMD has been considered as an attractive drug candidate for the treatment of cardiometabolic disease, and its plasma level may be used as putative biomarkers for cardiometabolic disease. Plasma levels of IMD were increased in many diseases, including acute coronary syndrome (26, 27), heart failure (28), and breast cancer (29). In this article, we show the increased plasma levels of IMD in T2DM patients, but the IMD levels in plasma could not be used for diagnosis of T2DM due to the very low diagnostic value. Considered the ameliorative effect of IMD on insulin resistance in mice (14, 15) and myocardial ischemia-reperfusion injury in diabetic rats (16), the increased IMD levels might be an endogenous compensatory mechanism attempted to block the progression of the disease and avoid the complications. The higher levels of IMD might present the better prognosis. The prognostic value of IMD for T2DM should be investigated in the future.
It was very less known about the regulation of IMD plasma levels, and the association of the levels and diseases. In a study in healthy human volunteers and patients with heart failure, IMD levels were also positively correlated with BMI (28). In the same article, IMD levels were greater in heart failure patients with concomitant renal impairment than those without. Expression of IMD increased in kidney of rat with unilateral ureteral obstruction. IMD dramatically attenuated unilateral ureteral obstruction-induced renal fibrosis (30), IgA nephropathy (31) and ischemia/reperfusion injury (32). In our study, IMD levels were positively correlated with uric acid and urea nitrogen. These results suggested that IMD levels might exert crucial role in physiology and pathophysiology of kidney. Although anti-inflammatory effect of IMD involved in the renoprotective effect (33), our data did not show a significant correlation between IMD and CRP, a general marker of inflammation.
The association between levels of IMD and either obesity, or insulin resistance was also controversial. One team reported that IMD mRNA in fat depots was higher in obese patients, whether diabetic or not. However, serum IMD levels were significantly lower in obese patients (34). The decreased levels of IMD in obese patients were also reported by another team (14, 35), and the expression of IMD in adipocytes was same decreased in mice with obese or hyperhomocysteinemia-induced insulin resistance (15, 36). However, in our study we found that plasma IMD increased in patients with T2DM, and positively associated with BMI. This might be caused by the complexity of T2DM. The role of IMD in T2DM should be thoroughly studied in the future.
Although our study firstly observed the increased plasma levels of IMD in patients with T2DM, there are several limitations in our study. The small numbers of patients enrolled in this study is one of major limitations, and the poor diagnostic value of IMD for T2DM determined by AUC of ROC is another limitation. Of course, plasma levels of IMD might be used for predicting prognosis of T2DM, and provide more information to guide the clinical practice. Otherwise, it will be interesting to assess the relationship between IMD and further diabetic complications, especially diabetic retinopathy, or neuropathy (37). All of those could be investigated in the future.
In conclusion, plasma levels of IMD were increased in patients with T2DM. This data suggested that IMD might be tightly associated with the pathogenesis of diabetes.
Acknowledgment
This study was funded by the project of Civil Aviation General Hospital (2012004).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.International Diabetes Federation . 8th ed. Brussels: International Diabetes Federation; 2017. IDF Diabetes Atlas. [PubMed] [Google Scholar]
- 2.Winther SA, Øllgaard JC, Tofte N, Tarnow L, Wang Z, Ahluwalia TS, Jorsal A, Theilade S, Parving HH, Hansen TW, Hazen SL, Pedersen O, Rossing P. Utility of Plasma Concentration of Trimethylamine N-Oxide in Predicting Cardiovascular and Renal Complications in Individuals With Type 1 Diabetes. Diabetes Care. 2019;42(8):1512–1520. doi: 10.2337/dc19-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilemann-Lyberg S, Hansen TW, Tofte N, Winther SA, Theilade S, Ahluwalia TS, Rossing P. Uric Acid Is an Independent Risk Factor for Decline in Kidney Function, Cardiovascular Events, and Mortality in Patients With Type 1 Diabetes. Diabetes Care. 2019;42(6):1088–1094. doi: 10.2337/dc18-2173. [DOI] [PubMed] [Google Scholar]
- 4.Rotbain Curovic V, Hansen TW, Eickhoff MK, von Scholten BJ, Reinhard H, Jacobsen PK, Persson F, Parving HH, Rossing P. Urinary tubular biomarkers as predictors of kidney function decline, cardiovascular events and mortality in microalbuminuric type 2 diabetic patients. Acta Diabetol. 2018;55(11):1143–1150. doi: 10.1007/s00592-018-1205-0. [DOI] [PubMed] [Google Scholar]
- 5.Faerch K, Hulmán A, Solomon TP. Heterogeneity of Pre-diabetes and Type 2 Diabetes: Implications for Prediction, Prevention and Treatment Responsiveness. Curr Diabetes Rev. 2016;12(1):30–41. doi: 10.2174/1573399811666150416122903. [DOI] [PubMed] [Google Scholar]
- 6.Ahlqvist E, van Zuydam NR, Groop LC, McCarthy MI. The genetics of diabetic complications. Nat Rev Nephrol. 2015;11(5):277–287. doi: 10.1038/nrneph.2015.37. [DOI] [PubMed] [Google Scholar]
- 7.Ahluwalia TS, Kilpeläinen TO, Singh S, Rossing P. Front Endocrinol (Lausanne) 2019. Editorial: Novel Biomarkers for Type 2 Diabetes; pp. 10–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roh J, Chang CL, Bhalla A, Klein C, Hsu SY. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J Biol Chem. 2004;279(8):7264–7274. doi: 10.1074/jbc.M305332200. [DOI] [PubMed] [Google Scholar]
- 9.Takei Y, Hyodo S, Katafuchi T, Minamino N. Novel fish-derived adrenomedullin in mammals: structure and possible function. Peptides. 2004;25(10):1643–1656. doi: 10.1016/j.peptides.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Hong Y, Hay DL, Quirion R, Poyner DR. The pharmacology of adrenomedullin 2/intermedin. Br J Pharmacol. 2012;166(1):110–120. doi: 10.1111/j.1476-5381.2011.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang JR, Duan XH, Zhang BH, Teng X, Zhou YB, Liu Y, Yu YR, Zhu Y, Tang CS, Qi YF. Intermedin1-53 attenuates vascular smooth muscle cell calcification by inhibiting endoplasmic reticulum stress via cyclic adenosine monophosphate/protein kinase A pathway. Exp Biol Med (Maywood) 2013;238(10):1136–1146. doi: 10.1177/1535370213502619. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Tian J, Guo H, Mi Y, Zhang R, Li R. Intermedin ameliorates IgA nephropathy by inhibition of oxidative stress and inflammation. Clin Exp Med. 2016;16(2):183–192. doi: 10.1007/s10238-015-0351-8. [DOI] [PubMed] [Google Scholar]
- 13.Teng X, Song J, Zhang G, Cai Y, Yuan F, Du J, Tang C, Qi Y. Inhibition of endoplasmic reticulum stress by intermedin(1-53) protects against myocardial injury through a PI3 kinase-Akt signaling pathway. J Mol Med (Berl) 2011;89(12):1195–1205. doi: 10.1007/s00109-011-0808-5. [DOI] [PubMed] [Google Scholar]
- 14.Zhang SY, Lv Y, Zhang H, Gao S, Wang T, Feng J, Wang Y, Liu G, Xu MJ, Wang X, Jiang C. Adrenomedullin 2 Improves Early Obesity-Induced Adipose Insulin Resistance by Inhibiting the Class II MHC in Adipocytes. Diabetes. 2016;65(8):2342–2355. doi: 10.2337/db15-1626. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Zhang SY, Jiang C, Li Y, Xu G, Xu MJ, Wang X. Intermedin/adrenomedullin 2 polypeptide promotes adipose tissue browning and reduces high-fat diet-induced obesity and insulin resistance in mice. Int J Obes (Lond) 2016;40(5):852–860. doi: 10.1038/ijo.2016.2. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Bian Y, Zhang N, Guo J, Wang C, Lau WB, Xiao C. Intermedin protects against myocardial ischemia-reperfusion injury in diabetic rats. Cardiovasc Diabetol. 2013;12:91. doi: 10.1186/1475-2840-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong HK, Tang F, Cheung TT, Cheung BM. Adrenomedullin and diabetes. World J Diabetes. 2014;5(3):364–371. doi: 10.4239/wjd.v5.i3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Unzueta MT, Montalbán C, Pesquera C, Berrazueta JR, Amado JA. Plasma adrenomedullin levels in type 1 diabetes. Relationship with clinical parameters. Diabetes Care. 1998;21(6):999–1003. doi: 10.2337/diacare.21.6.999. [DOI] [PubMed] [Google Scholar]
- 19.Lim SC, Morgenthaler NG, Subramaniam T, Wu YS, Goh SK, Sum CF. The relationship between adrenomedullin, metabolic factors, and vascular function in individuals with type 2 diabetes. Diabetes Care. 2007;30(6):1513–1519. doi: 10.2337/dc06-1899. [DOI] [PubMed] [Google Scholar]
- 20.Dong Y, Banadakoppa M, Chauhan M, Balakrishnan M, Belfort M, Yallampalli C. Circulating adrenomedullin is elevated in gestational diabetes and its role in impaired insulin production by β-Cells. J Clin Endocrinol Metab. 2019;104(3):697–706. doi: 10.1210/jc.2018-01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pecková M, Charvat J, Schuck O, Zamrazil V, Bilek R, Hill M, Svab P, Horackova M. Plasma adrenomedullin and subclinical cardiorenal syndrome in patients with type 2 diabetes mellitus. J Int Med Res. 2012;40(4):1552–1559. doi: 10.1177/147323001204000435. [DOI] [PubMed] [Google Scholar]
- 22.Landman GW, van Dijk PR, Drion I, van Hateren KJ, Struck J, Groenier KH, Gans RO, Bilo HJ, Bakker SJ, Kleefstra N. Midregional fragment of proadrenomedullin, new-onset albuminuria, and cardiovascular and all-cause mortality in patients with type 2 diabetes (ZODIAC-30) Diabetes Care. 2014;37(3):839–845. doi: 10.2337/dc13-1852. [DOI] [PubMed] [Google Scholar]
- 23.Charvat J, Svab P, Havlin J, Bilek R, Zamrazil V. The significance of plasma adrenomedullin and calcitonin gene-related peptide concentration in patients with Type 2 diabetes mellitus who are treated for cardiovascular risk factors. Neuro Endocrinol Lett. 2014;35(2):154–158. [PubMed] [Google Scholar]
- 24.Fraty M, Velho G, Gand E, Fumeron F, Ragot S, Sosner P, Mohammedi K, Gellen B, Saulnier PJ, Halimi JM, Montaigne D, Ducrocq G, Rehman M, Marre M, Roussel R, Hadjadj S, SURDIAGENE Study Group Prognostic value of plasma MR-proADM vs. NT-proBNP for heart failure in people with type 2 diabetes: the SURDIAGENE prospective study. Diabetologia. 2018;61(12):2643–2653. doi: 10.1007/s00125-018-4727-7. [DOI] [PubMed] [Google Scholar]
- 25.Zhang SY, Xu MJ, Wang X. Adrenomedullin 2/intermedin: a putative drug candidate for treatment of cardiometabolic diseases. Br J Pharmacol. 2018;175(8):1230–1240. doi: 10.1111/bph.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin YW, Teng X, He JQ, Du J, Tang CS, Qi YF. Increased plasma levels of intermedin and brain natriuretic peptide associated with severity of coronary stenosis in acute coronary syndrome. Peptides. 2013;42:84–88. doi: 10.1016/j.peptides.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Li P, Shi L, Han Y, Zhao Y, Qi Y, Wang B. Prognostic Value of Plasma Intermedin Level in Patients With Non-ST-Segment Elevation Acute Coronary Syndrome. Medicine (Baltimore) 2016;95(16):e3422. doi: 10.1097/MD.0000000000003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell D, Gordon BJ, Lavery A, Megaw K, Kinney MO, Harbinson MT. Plasma levels of intermedin (adrenomedullin-2) in healthy human volunteers and patients with heart failure. Peptides. 2016;76:19–29. doi: 10.1016/j.peptides.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Lu YM, Zhong JB, Wang HY, Yu XF, Li ZQ. The prognostic value of intermedin in patients with breast cancer. Dis Markers. 2015;2015:862158. doi: 10.1155/2015/862158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao X, Wang L, Wang Y, Zhao N, Zhang R, Han W, Peng Z. Intermedin is upregulated and attenuates renal fibrosis by inhibition of oxidative stress in rats with unilateral ureteral obstruction. Nephrology (Carlton) 2015;20(11):820–831. doi: 10.1111/nep.12520. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Tian J, Guo H, Mi Y, Zhang R, Li R. Intermedin ameliorates IgA nephropathy by inhibition of oxidative stress and inflammation. Clin Exp Med. 2016;16(2):183–192. doi: 10.1007/s10238-015-0351-8. [DOI] [PubMed] [Google Scholar]
- 32.Qiao X, Li RS, Li H, Zhu GZ, Huang XG, Shao S, Bai B. Intermedin protects against renal ischemia-reperfusion injury by inhibition of oxidative stress. Am J Physiol Renal Physiol. 2013;304(1):F112–F119. doi: 10.1152/ajprenal.00054.2012. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Tian J, Mi Y, Ren X, Lian S, Kang J, Wang J, Zang H, Wu Z, Yang J, Qiao X, Zhou X, Wang G, Zhou Y, Li R. Experimental study on renoprotective effect of intermedin on diabetic nephropathy. Mol Cell Endocrinol. 2021;528:111224. doi: 10.1016/j.mce.2021.111224. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Lee SK, Kim D, Choe H, Jang YJ, Park HS, Kim JH, Hong JP, Lee YJ, Heo Y. Altered Expression of Adrenomedullin 2 and its Receptor in the Adipose Tissue of Obese Patients. J Clin Endocrinol Metab. 2020;105(1):dgz066. doi: 10.1210/clinem/dgz066. [DOI] [PubMed] [Google Scholar]
- 35.Lv Y, Zhang SY, Liang X, Zhang H, Xu Z, Liu B, Xu MJ, Jiang C, Shang J, Wang X. Adrenomedullin 2 Enhances Beiging in White Adipose Tissue Directly in an Adipocyte-autonomous Manner and Indirectly through Activation of M2 Macrophages. J Biol Chem. 2016;291(45):23390–23402. doi: 10.1074/jbc.M116.735563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pang Y, Li Y, Lv Y, Sun L, Zhang S, Li Y, Wang Y, Liu G, Xu MJ, Wang X, Jiang C. Intermedin Restores Hyperhomocysteinemia-induced Macrophage Polarization and Improves Insulin Resistance in Mice. J Biol Chem. 2016;291(23):12336–12345. doi: 10.1074/jbc.M115.702654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Opatrilova R, Kubatka P, Caprnda M, Büsselberg D, Krasnik V, Vesely P, Saxena S, Ruia S, Mozos I, Rodrigo L, Kruzliak P, Dos Santos KG. Nitric oxide in the pathophysiology of retinopathy: evidences from preclinical and clinical researches. Acta Ophthalmol. 2018;96(3):222–231. doi: 10.1111/aos.13384. [DOI] [PubMed] [Google Scholar]





