Abstract
Background
Increasing socioeconomic distress has been associated with worse cardiac surgery outcomes. The extent to which the pandemic affected cardiac surgical access and outcomes remains unknown. We sought to examine the relationship between the COVID-19 pandemic and outcomes after cardiac surgery by socioeconomic status.
Methods
All patients undergoing a Society of Thoracic Surgeons (STS) index operation in a regional collaborative, the Virginia Cardiac Services Quality Initiative (2011-2022), were analyzed. Patients were stratified by timing of surgery before vs during the COVID-19 pandemic (March 13, 2020). Hierarchic logistic regression assessed the relationship between the pandemic and operative mortality, major morbidity, and cost, adjusting for the Distressed Communities Index (DCI), STS predicted risk of mortality, intraoperative characteristics, and hospital random effect.
Results
A total of 37,769 patients across 17 centers were included. Of these, 7269 patients (19.7%) underwent surgery during the pandemic. On average, patients during the pandemic were less socioeconomically distressed (DCI 37.4 vs DCI 41.9; P < .001) and had a lower STS predicted risk of mortality (2.16% vs 2.53%, P < .001). After risk adjustment, the pandemic was significantly associated with increased mortality (odds ratio 1.398; 95% CI, 1.179-1.657; P < .001), cost (+$4823, P < .001), and STS failure to rescue (odds ratio 1.37; 95% CI, 1.10-1.70; P = .005). The negative impact of the pandemic on mortality and cost was similar regardless of DCI.
Conclusions
Across all socioeconomic statuses, the pandemic is associated with higher cost and greater risk-adjusted mortality, perhaps related to a resource-constrained health care system. More patients during the pandemic were from less distressed communities, raising concern for access to care in distressed communities.
Visual Abstract

Cardiothoracic Anesthesiology:
The Annals of Thoracic Surgery CME Program is located online at http://www.annalsthoracicsurgery.org/cme/home. To take the CME activity related to this article, you must have either an STS member or an individual non-member subscription to the journal.
The Supplemental Tables can be viewed in the online version of this article [10.1016/j.athoracsur.2022.12.042] on http://www.annalsthoracicsurgery.org.
The relationship between socioeconomic status and poor cardiac surgery outcomes as well as socioeconomic status and COVID-19 case fatality rates have both been well studied.1, 2, 3, 4 Patients identified as members of socioeconomically distressed communities have higher risk-adjusted odds of postprocedural mortality and failure to rescue. In addition, decreasing socioeconomic status has been significantly associated with increasing COVID-19 mortality rates and susceptibility to the deleterious financial and health impacts of the pandemic.5
The impact of the COVID-19 pandemic on the association between socioeconomic status and cardiac surgery outcomes is unknown. Although recent work has demonstrated that the onset of the COVID-19 pandemic was associated with increased rates of postoperative mortality and complications among cardiac surgery patients, no study of cardiac surgery outcomes during the pandemic has included a comprehensive metric for socioeconomic determinants of health, such as the Distressed Communities Index (DCI), nor evaluated how the pandemic may have differentially influenced postoperative outcomes by socioeconomic status.6 , 7 It has been well documented that the pandemic has placed extreme strain on the US health care system capacity and has led to substantial workforce losses. In mid January of 2022, one of every five hospitals in the United States was having critical staffing shortages.8 In addition to contributing to documented increases in costs to the health care system, it is possible that these staffing shortages have contributed to decreased access to cardiac surgical care (ie, forcing surgical practices to cancel or delay necessary care), resulting in increased risk-adjusted morbidity and mortality.9, 10, 11 This effect may be especially pronounced among patients from socioeconomically distressed communities, as they may have limited ability to navigate the health care system to find alternative, timely access to cardiac surgical care. Similarly, as health care resources become increasingly scarce, they may be increasingly allocated to patients who are not socioeconomically distressed owing to implicit or explicit bias.12
The objective of this study was to evaluate the effect of the COVID-19 pandemic on cardiac surgery outcomes by socioeconomic status, measured using the DCI. We hypothesized that the pandemic would disproportionately drive higher postoperative morbidity and mortality among patients who underwent cardiac surgery who reside in more distressed communities.
Patients and Methods
The Virginia Cardiac Services Quality Initiative (VCSQI) includes 17 hospitals and surgical practices in Virginia and contains 99% of all adult cardiac surgeries in the region. Clinical data and cost methodology have been described previously.13 , 14 Cost data were available through December 2020. Owing to the deidentified nature of the quality database, this study was exempt from review by the University of Virginia's Institutional Review Board (Protocol #23305, deemed exempt July 14, 2021).
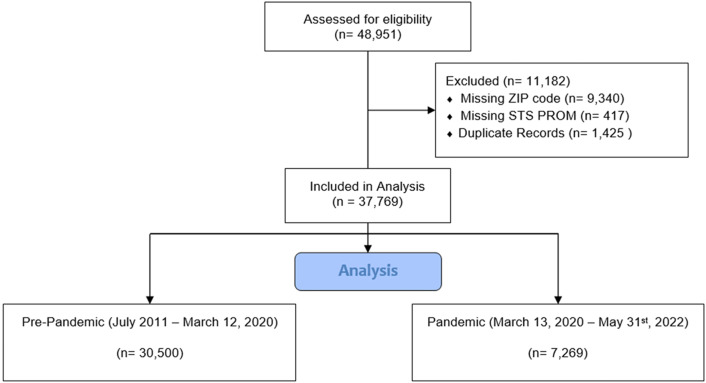
All patients undergoing The Society of Thoracic Surgeons (STS) index operation (coronary artery bypass graft surgery [CABG], aortic valve replacement, mitral valve replacement/repair) between July 2011 and May 2022 were extracted from the VCSQI database. These data were linked with the Economic Innovation Group DCI data file. Use of the Economic Innovation Group DCI in cardiac surgery has been described in earlier studies.1 , 2 The DCI estimates the level of socioeconomic distress by zip code, with a score of zero corresponding to a nondistressed community and a score of 100 corresponding to a maximally distressed community. The DCI is based on seven key indicators: percent of residents with a high school degree, housing vacancy rate, unemployment rate, poverty rate, median income ratio, change in employment, and change in business establishments. Patients were excluded if they were missing a zip code (required for linkage with the Economic Innovation Group DCI file) or were missing STS predicted risk of mortality (PROM [Figure 1 ]).
Figure 1.
Consolidated Standards of Reporting Trials diagram. (STS PROM, The Society of Thoracic Surgeons predicted risk of mortality.)
Standard STS definitions were used for all variables.15 Operative mortality is defined as inhospital mortality or death within 30 days of surgery. Failure to rescue is defined as operative mortality after an STS-defined failure to rescue complication (prolonged ventilation, postoperative renal failure, reoperation, and stroke). Patients were stratified by whether they underwent surgery before vs during the COVID-19 pandemic. March 13, 2020, was chosen as the date signifying the onset of the pandemic for our study population as it is the date on which the COVID-19 outbreak was declared a national emergency in the United States.16
Categoric variables are presented as count (percentage) and continuous variables are presented as median (interquartile range) owing to skewed distributions. The Wilcoxon rank sum test was used for nonnormal distributed continuous variables, and the χ2 test was used for all categoric variables. Hierarchic logistic regression was used to assess the relationship between the onset of the pandemic and operative mortality, major morbidity, STS-defined failure to rescue, and cost, adjusting for DCI score, STS-PROM, intraoperative characteristics, and hospital random effect (to account for center-level differences). Median (continuous) or mode (categoric) imputation was used for missingness (all missingness was less than 5%). Variables included in the final multivariable model were selected based on clinical importance and statistical significance in univariate analyses. Effect modification analyses for DCI, sex, age, race, and insurance were performed, as were sensitivity analyses among procedure-specific subgroups and with the pandemic era subdivided into an early period (March 13, 2020 to March 30, 2021) and late period (April 1, 2021 to July 1, 2022). All statistical analyses were carried out using SAS 9.4 (SAS Institute Inc).
Results
Baseline Characteristics Before vs During Pandemic
A total of 37,769 patients across 17 centers were identified during the study period. Of these, 7269 patients (19.70%) underwent surgery during the pandemic. On average, patients undergoing cardiac surgery during the pandemic were more often male (74.36% vs 71.13%, P < .001) and non-White (21.70% vs 17.84%, P < .001), were less socioeconomically distressed (DCI 37.4 vs DCI 41.9, P < .001), and had a lower STS-PROM (2.16% vs 2.53%, P < .001; Table 1 ). Furthermore, compared with prepandemic patients, patients undergoing cardiac surgery during the pandemic had a greater burden of immunodeficiencies (4.32% vs 3.57%, P = .002), end-stage renal disease (3.02% vs 2.60%, P = .044), preoperative arrhythmias (11.04% vs 10.01%, P < .001), congestive heart failure (36.83% vs 33.27%, P < .001), prior myocardial infarction (46.89% vs 44.18%, P < .001), and tobacco use (57.74% vs 47.89%, P < .001). However, pandemic era patients had a comparatively lesser burden of reoperative surgery (2.81% vs 5.10%, P < .001) More procedures during the pandemic were nonelective (54.45% vs 51.03%, P < .001) and isolated CABG (75.59% vs 67.12%, P < .001).
Table 1.
Baseline Characteristics by Pandemic
|
Characteristics |
Before Pandemic (n = 30,500 [80.30]) | During Pandemic (n = 7269 [19.70]) | P Value |
|---|---|---|---|
| Age, y | 66 (58-73) | 66 (59-73) | .418 |
| DCI score | 41.90 (20.94-63.13) | 37.40 (15.72-61.92) | <.001 |
| Immunocompromised | 1080 (3.57) | 323 (4.32) | .002 |
| Peripheral arterial disease | 3851 (12.72) | 937 (12.52) | .648 |
| Hypertension | 25,534 (84.31) | 6451 (86.20) | <.001 |
| Diabetes mellitus | 12,811 (42.30) | 3357 (44.86) | <.001 |
| Prior stroke | 2614 (8.63) | 665 (8.89) | .484 |
| Cerebrovascular disease | 6083 (20.09) | 1683 (22.49) | <.001 |
| Race | <.001 | ||
| White | 24,881 (82.16) | 5860 (78.30) | |
| Black | 3643 (12.03) | 1023 (13.67) | |
| American Indian | 40 (0.13) | 14 (0.19) | |
| Asian | 930 (3.07) | 270 (3.61) | |
| Other | 791 (2.61) | 317 (4.24) | |
| Female | 8744 (28.87) | 1919 (25.64) | <.001 |
| Liver disease | 1250 (4.13) | 267 (3.57) | .027 |
| MELD score | 7.47 (6.40-8.92) | 7.47 (6.40-9.06) | .639 |
| Preop serum creatinine, mg/dL | 1.00 (0.80-1.20) | 0.99 (0.80-1.20) | .088 |
| Preop serum hemoglobin, mg/dL | 13.23 (11.97-14.30) | 13.53 (12.23-14.60) | <.001 |
| Preop serum WBC, ×109/L | 7.50 (6.10-9.10) | 7.53 (6.20-9.22) | .003 |
| Preop serum albumin, g/L | 3.80 (3.50-4.10) | 3.80 (3.50-4.20) | .071 |
| Body surface area, m2 | 2.88 (2.75-3.00) | 2.88 (2.78-3.01) | <.001 |
| End-stage renal disease | 787 (2.60) | 226 (3.02) | .044 |
| Current tobacco use | 6020 (19.88) | 1515 (20.24) | <.001 |
| Oxygen-dependent lung disease | 115 (0.38) | 50 (0.67) | <.001 |
| Chronic lung disease | 8810 (29.09) | 2129 (28.45) | .272 |
| Sleep apnea | 5299 (17.50) | 1397 (18.67) | .018 |
| Preop arrhythmia, within 30 days | 3032 (10.01) | 826 (11.04) | <.001 |
| Congestive heart failure | 10,077 (33.27) | 2756 (36.83) | <.001 |
| Previous PCI | 7263 (23.98) | 1838 (24.56) | .296 |
| Previous CABG | 825 (2.72) | 60 (0.80) | <.001 |
| Previous valve surgery | 807 (2.66) | 193 (2.58) | .679 |
| Prior myocardial infarction | 13,379 (44.18) | 3509 (46.89) | <.001 |
| Reoperative surgery | 1544 (5.10) | 210 (2.81) | <.001 |
| Aortic stenosis | 5793 (19.13) | 971 (12.97) | <.001 |
| Aortic regurgitation | 9835 (32.5) | 2371 (31.7) | .189 |
| Mitral stenosis | 876 (2.89) | 229 (3.06) | .442 |
| Mitral regurgitation | 20,666 (68.2) | 5763 (77.0) | <.001 |
| Tricuspid stenosis | 52 (0.17) | 9 (0.12) | .321 |
| Tricuspid regurgitation | 18,173 (60.01) | 5390 (72.02) | <.001 |
| Preop ejection fraction, % | 55 (48-60) | 58 (48-61) | .391 |
| Status | <.001 | ||
| Elective | 14,830 (48.97) | 3409 (45.55) | |
| Urgent | 14,555 (48.06) | 3907 (52.20) | |
| Emergent | 856 (2.83) | 157 (2.10) | |
| Emergent salvage | 44 (0.15) | 11 (0.15) | |
| Intraaortic balloon pump | 2060 (6.80) | 545 (7.28) | .142 |
| Procedure type | <.001 | ||
| AV replacement | 3513 (11.60) | 483 (6.45) | |
| AV replacement/CABG | 2183 (7.21) | 370 (4.94) | |
| Isolated CABG | 20,327 (67.12) | 5657 (75.59) | |
| MV repair | 1960 (6.47) | 368 (4.92) | |
| MV repair/CABG | 680 (2.25) | 125 (1.67) | |
| MV replacement/CABG | 353 (1.17) | 104 (1.39) | |
| MV replacement only | 1269 (4.19) | 377 (5.04) | |
| Cross-clamp time, min | 75 (58-94) | 76 (59-99) | <.001 |
| CPB time, min | 102 (79-127) | 101 (79-129) | .968 |
| Intraoperative blood products, units | 0 (0-2) | 0 (0-2) | <.001 |
| STS PROM, % | 2.53 (0.06-2.73) | 2.16 (0.06-2.21) | <.001 |
Values are median (interquartile range) or n (%).
AV, aortic valve; CABG, coronary artery bypass graft surgery; CPB, cardiopulmonary bypass; DCI, Distressed Communities Index; MELD, model for end-stage liver disease; MV, mitral valve; PCI, percutaneous coronary intervention; Preop, preoperative; PROM, predicted risk of mortality; STS, The Society of Thoracic Surgeons; WBC, white blood cells.
Additional data concerning acuity and severity of presentation can be found in Supplemental Tables 1 to 3. The incidence of acute papillary muscle rupture among patients undergoing mitral valve surgery was not significantly increased during the pandemic (Supplemental Table 1). However, patients undergoing CABG during the pandemic were significantly more likely to present with unstable angina, non-ST-segment elevation myocardial infarction, or ST-segment elevation myocardial infarction, relative to patients before the pandemic (Supplemental Table 2). No patients in this study had ischemic ventricular septal defect nor required left ventricle aneurysm repair.
Unadjusted Outcomes Before Vs During Pandemic
Unadjusted postoperative outcomes before vs during the pandemic are presented in Table 2 . Before adjustment, the onset of the pandemic was significantly associated with increased mortality (2.87% vs 2.18%, P < .001) and cost (median cost of $40,236 vs $35,209; P < .001), but not with increased major morbidity (10.92% vs 11.20%, P = .485; Table 2). STS-defined failure to rescue was significantly increased during the pandemic (18% vs 14.4%, P = .011), as were postoperative complications including atrial fibrillation (25.84% vs 22.78%, P < .001) and pneumonia (2.97% vs 2.23%, P < .001).
Table 2.
Unadjusted Outcomes by Pandemic
| Characteristic | Before Pandemic | During Pandemic | P Value |
|---|---|---|---|
| Operative mortality | 659 (2.18) | 215 (2.87) | <.001 |
| Major morbiditya | 3392 (11.20) | 817 (10.92) | .485 |
| STS failure to rescueb | 479 (14.4) | 144 (18) | .011 |
| Prolonged ventilation | 2673 (8.83) | 648 (8.66) | .647 |
| Postoperative renal failure | 752 (2.48) | 240 (3.21) | <.001 |
| Reoperation, any cause | 867 (2.86) | 216 (2.89) | .914 |
| Permanent stroke | 386 (1.27) | 90 (1.20) | .617 |
| Postoperative dialysis | 506 (1.67) | 184 (2.46) | <.001 |
| Readmission | 2932 (9.68) | 659 (8.81) | .021 |
| Atrial fibrillation | 6899 (22.78) | 1934 (25.84) | <.001 |
| Cardiac arrest | 536 (1.77) | 147 (1.96) | .259 |
| Deep sternal wound infection | 98 (0.32) | 24 (0.32) | .968 |
| Surgical site infectionc | 428 (1.41) | 113 (1.51) | .529 |
| Deep vein thrombus/PE | 376 (1.24) | 79 (1.06) | .187 |
| Pneumonia | 676 (2.23) | 222 (2.97) | <.001 |
| Non-home discharge | 7092 (23.42) | 1161 (15.51) | <.001 |
| Total intensive care unit stay, h | 48 (26-86.9) | 66.53 (35.5-102) | <.001 |
| Length of stay,d d | 6 (4-8) | 6 (5-8) | <.001 |
| Total cost, USD | 35,209 (27,742-46,652) | 40,236 (32,077-53,538) | <.001 |
Values are n (%) or median (interquartile range).
PE, pulmonary embolism.
Major morbidity defined as permanent stroke, postoperative renal failure, prolonged ventilation, deep sternal wound infection, or reoperation.
The Society of Thoracic Surgeons (STS) failure to rescue is death after prolonged ventilation, permanent stroke, reoperation, or renal failure.
Non–deep sternal wound infection.
Surgery to discharge.
Multivariable Model of Impact of Pandemic on Operative Outcomes
After risk adjustment, the pandemic remained significantly associated with increased mortality (odds ratio 1.398; 95% CI, 1.179-1.657; P < .001; Table 3 , Figure 2 ), failure to rescue (odds ratio 1.37; 95% CI, 1.10-1.70; P = .005; Table 4 ), and cost (+$4823, P < .001; Table 5 ), but not with major morbidity (odds ratio 0.962; 95% CI, 0.879-1.052; P = .398; Supplemental Table 4). Effect modification analyses revealed that the effect of the pandemic on mortality and cost was similar regardless of DCI, age, sex, race, and insurance status (P values of interaction terms all greater than .05; Supplemental Table 5). Sensitivity analyses were performed among procedure-specific subgroups. Among the isolated CABG subgroup, the pandemic remained significantly associated with increased mortality, failure to rescue, and cost (Supplemental Tables 6-8); as in the main analysis, major morbidity was not associated with the pandemic (Supplemental Table 9). Among the aortic valve replacement subgroup, the pandemic was qualitatively associated with increased mortality, failure to rescue and cost—however, only the association between the pandemic and cost was statistically significant (Supplemental Tables 10-13). Among the mitral valve replacement/repair subgroup, mortality, failure to rescue, and cost were all qualitatively increased during the pandemic but this association was not statistically significant (Supplemental Tables 14-17). Lastly, a sensitivity analysis in which the pandemic was subdivided into early and late periods demonstrated significantly elevated risk-adjusted odds of operative mortality throughout the pandemic. Risk-adjusted odds of failure to rescue were significantly higher in the early pandemic but not in the late pandemic. Risk-adjusted odds of major morbidity were significantly lower in the late pandemic (Supplemental Tables 18-20).
Table 3.
Multivariable Model of Impact of Pandemic on Operative Mortality After Cardiac Surgery
| Characteristics | OR | 95% CI | P Value |
|---|---|---|---|
| Pandemic | 1.398 | 1.179-1.657 | <.001 |
| Predicted risk of mortality | 1.093 | 1.084-1.102 | <.001 |
| Cardiopulmonary bypass time, min | 1.007 | 1.006-1.008 | <.001 |
| Intraoperative transfusion, units | 1.168 | 1.136-1.201 | <.001 |
| Distress score | 1.005 | 1.002-1.008 | <.001 |
OR, odds ratio.
Figure 2.
Multivariable model of the impact of the pandemic on operative mortality after cardiac surgery. Horizontal lines indicate 95% CI. The orange circle highlights the variable of interest: the pandemic. (CPB, cardiopulmonary bypass.)
Table 4.
Multivariable Model of Impact of Pandemic on The Society of Thoracic Surgeons-Defined Failure to Rescue After Cardiac Surgery
| Characteristics | Estimate | 95% CI | P Value |
|---|---|---|---|
| Pandemic | 1.367 | 1.10-1.698 | .005 |
| Predicted risk of mortality | 1.043 | 1.034-1.052 | <.001 |
| Cardiopulmonary bypass time, min | 1.003 | 1.001-1.004 | <.001 |
| Intraoperative transfusion, units | 1.056 | 1.027-1.086 | <.001 |
| Distress score | 1.003 | 1.000-1.007 | .084 |
Table 5.
Multivariable Model of Impact of Pandemic on Total Cost After Cardiac Surgery
| Characteristics | Estimate | SE | P Value |
|---|---|---|---|
| Pandemic | $4823 | $979 | <.001 |
| Predicted risk of mortality | $2012 | $51.1 | <.001 |
| Cardiopulmonary bypass time, min | $137 | $5.16 | <.001 |
| Intraoperative transfusion, units | $3905 | $164 | <.001 |
| Distress score | $39.1 | $8.93 | <.001 |
COVID-19 Status of Patients During Pandemic
Data concerning laboratory-confirmed diagnosis of COVID-19 was available in the STS Adult Cardiac Surgery Database (Temporary Field Code 7230). Of patients who underwent surgery during the pandemic, 425 (5.68%) had a laboratory-confirmed diagnosis of COVID-19 before, during, or within 30 days of their procedure. Of these, the vast majority (359 [84.5%]) had been diagnosed with COVID-19 before hospitalization for their procedure. Only 38 patients (8.9%) were diagnosed with COVID-19 during the same hospitalization, before surgery. A small minority (28 [6.5%]) had laboratory-confirmed COVID-19 after their procedure. History of laboratory-confirmed COVID-19 was not significantly associated with operative mortality, major morbidity, nor cost during the pandemic era (all P values greater than .05).
Comment
In this regional retrospective cohort study of patients undergoing cardiac surgery, the pandemic was significantly associated with increased risk-adjusted mortality and cost, but not major morbidity, across all socioeconomic statuses. Patients who underwent surgery during the pandemic were members of significantly less distressed communities and had lower STS-PROM. Before adjustment, pandemic-era patients had significantly increased mortality, cost, and failure to rescue. After adjustment—which included the DCI, STS-PROM, intraoperative characteristics, and hospital random effect—the pandemic remained significantly associated with increased mortality and cost, apparently primarily due to higher failure to rescue rates. History of laboratory-confirmed COVID-19 infection was not associated with outcomes during the pandemic era, similar to other studies.17
Failure to rescue has been proposed as a measurement of a center’s ability to successfully prevent patient mortality after complications, and previous studies have reported a significant association between failure to rescue status and preventability adjudication.18, 19, 20 A potential cause of increased rates of failure to rescue (and thereby, operative mortality) is resource strain (eg, staffing shortages, health care worker burnout) leading to suboptimal triage decisions and patient care. Therefore, our analysis adds to the growing body of research demonstrating that the COVID-19 pandemic stressed hospital systems and caused resource and capacity limitations that negatively affected patient outcomes.21 , 22
Our research is unique in demonstrating that the COVID-19 pandemic did not differentially affect surgical outcomes in patients from more distressed communities once they established care. We initially hypothesized that the COVID-19 pandemic would disproportionately drive higher morbidity and mortality in more distressed communities. Our rationale for this hypothesis is multifold. First, low-income workers bore the brunt of the adverse economic consequences of the pandemic, and prior research shows that socioeconomic distress is a significant predictor of worse surgical outcomes.1 , 2 , 4 , 23 Second, essential workers, who often come from distressed communities, were subjected to extreme daily stress at the height of the pandemic. In a meta-analysis, Rosenberger and associates24 suggest that psychosocial factors such as attitude and mood are strong predictors of surgical outcomes. Finally, COVID-19 infection and mortality rates increased with decreasing socioeconomic status, and perioperative COVID-19 infection may negatively influence surgical outcomes.3 Despite these proven trends, a differential impact of DCI on outcomes after cardiac surgery was not observed.
Our research also supports previous studies indicating that the volume of cardiac surgery performed decreased precipitously during the pandemic.25 Notably, however, we found that pandemic-era cardiac surgery patients had a higher average socioeconomic status (ie, lower DCI score) as compared with patients treated before the pandemic. This finding suggests that the pandemic may have precluded poorer and marginalized patients from making it to hospitals in the first place, possibly resulting in their death at home secondary to acute coronary syndrome, critical aortic stenosis, or other treatable cardiovascular conditions. If that is true, these patients reflect the disproportionate impact of the pandemic on distressed communities. This hypothesis is substantiated by Centers for Disease Control and Prevention data that demonstrated that the age-adjusted mortality rate for heart disease (which importantly includes both inhospital and out-of-hospital deaths) increased in 2020—the largest such increase since 2012—as well as our sensitivity analysis, which demonstrated that the proportion of patients undergoing CABG for unstable angina and non-ST-segment elevation or ST-segment elevation myocardial infarction increased during the pandemic.26
Given the increase in postoperative failure to rescue and mortality and decrease in patients from distressed communities seen during the COVID-19 pandemic, there clearly exists a need for evidence-based cardiac surgery protocols for resource constrained settings, and particularly, infectious disease outbreaks. An international consortium of cardiac surgeons published guidance based on institutional experiences during the height of the COVID-19 pandemic that may serve as a good starting point. Their recommendations encompassed the resumption of cardiac surgical care, delivery of information to the community, and care provision itself.27 In short, they called for flexible center-level triggers for scaling cardiac surgery volume up or down in response to hospital capacity and disease burden, triaging of cases by a multidisciplinary heart team, proactive and timely guidance to the community on the availability of cardiovascular services and avenues to access them, a regional response to the delivery of elective cardiac surgeries, preoperative screening of all patients for infection, and virtual care after discharge. In the future, this framework should be modified by hospital systems according to evolving scientific understanding and government guidance to ultimately reduce failure to rescue, mortality, and cost during periods of resource strain, and ensure distressed pockets of the community continue to receive access to care.
Study Limitations
First, as in all retrospective studies, our results may be affected by unmeasured confounding, although this has been minimized by extensive risk adjustment. Second, our results may not be generalizable outside of our regional collaborative. This possibility, although not negligible, is significantly reduced by the demographically and socioeconomically diverse composition of the population studied. Third, the results of the analysis could change depending on the date selected as the breakpoint signifying the onset of the COVID-19 pandemic. The first COVID-19 case in the United States was confirmed in January 2020; however, detected cases did not rise until early to mid March 2020. March 13, 2020, therefore, seems to appropriately represent the beginning of the COVID-19 pandemic in the United States.
Fourth, this analysis is limited, as all analyses of the STS Adult Cardiac Surgery Database are, by short follow-up. It is possible that the pandemic may have a differential impact based on DCI score but that this impact can only be observed with longer follow-up than is available in our societal database. Fifth, cost data were only available through December 2020, limiting inference regarding resource use to the early stage of the pandemic. Sixth, we are unable to completely adjust or account for all systemic level changes affecting outcomes after cardiac surgery during the pre-pandemic era, which may bias our estimate of the pandemic’s effect. However, no significant trend in operative mortality, major complications, or failure to rescue was observed in the 5 years leading up to the pandemic (2016-2020).
Last, it is possible that our study underestimates the impact of the COVID-19 pandemic on the association of DCI on outcomes (ie, type II error) after cardiac surgery, as patients from more distressed communities may have been unable to access life-saving cardiac surgical care owing to pandemic-related barriers and are therefore not included in our study. Similarly, it is possible that some patients who normally would have undergone cardiac surgery instead received percutaneous therapy owing to limited inpatient capacity during the pandemic. A study of outcomes among all patients with cardiovascular disease is necessary to fully address the question of access for low DCI patients during the pandemic.
Conclusion
Across all socioeconomic statuses, the pandemic was associated with higher cost and greater risk-adjusted mortality driven by a higher rate of failure to rescue, perhaps related to a resource-constrained health care system. Patients undergoing cardiac surgery during the pandemic were from less distressed communities, raising concern for access to care in distressed communities.
Acknowledgments
Funding Sources
This work was funded in part by a grant under Award Number 2UM HL088925, as well as by the National Heart, Lung, and Blood Institute (grant T32 HL007849).
Disclosures
The authors have no conflicts of interest to disclose.
Supplementary Data
References
- 1.Strobel RJ, Kaplan EF, Young AM, et al; Investigators for the Virginia Cardiac Services Quality Initiative. Socioeconomic distress is associated with failure to rescue in cardiac surgery. J Thorac Cardiovasc Surg. Published online July 20, 2022. https://doi.org/10.1016/j.jtcvs.2022.07.013 [DOI] [PMC free article] [PubMed]
- 2.Charles E.J., Mehaffey J.H., Hawkins R.B., et al. Socioeconomic Distressed Communities Index predicts risk-adjusted mortality after cardiac surgery. Ann Thorac Surg. 2019;107:1706–1712. doi: 10.1016/j.athoracsur.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawkins R.B., Charles E.J., Mehaffey J.H. Socio-economic status and COVID-19-related cases and fatalities. Public Health. 2020;189:129–134. doi: 10.1016/j.puhe.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strobel RJ, Charles EJ, Mehaffey JH, et al. Effect of socioeconomic distress on risk-adjusted mortality after valve surgery for infective endocarditis. Semin Thorac Cardiovasc Surg. Published online May 16, 2022. https://doi.org/10.1053/j.semtcvs.2022.05.007 [DOI] [PubMed]
- 5.Chen J.T., Krieger N. Revealing the unequal burden of COVID-19 by income, race/ethnicity, and household crowding: US county vs zip code analyses. J Public Health Manag Pract. 2021;27(suppl 1):S43–S56. doi: 10.1097/PHH.0000000000001263. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen T.C., Thourani V.H., Nissen A.P., et al. The effect of COVID-19 on adult cardiac surgery in the United States in 717 103 patients. Ann Thorac Surg. 2022;113:738–746. doi: 10.1016/j.athoracsur.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Economic Innovation Group Introduction to the Distressed Communities Index. https://eig.org/dci
- 8.Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services Impact of the COVID-19 pandemic on the hospital and outpatient clinician workforce: challenges and policy responses (Issue Brief No. HP-2022-13) May 2022. https://aspe.hhs.gov/reports/covid-19-health-care-workforce
- 9.American Hospital Association Data brief: health care workforce challenges threaten hospitals’ ability to care for patients. https://www.aha.org/fact-sheets/2021-11-01-data-brief-health-care-workforce-challenges-threaten-hospitals-ability-care
- 10.Griffiths P., Maruotti A., Recio Saucedo A., et al. Nurse staffing, nursing assistants and hospital mortality: retrospective longitudinal cohort study. BMJ Qual Saf. 2019;28:609–617. doi: 10.1136/bmjqs-2018-008043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aiken L.H., Clarke S.P., Sloane D.M., Sochalski J., Silber J.H. Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA. 2002;288:1987–1993. doi: 10.1001/jama.288.16.1987. [DOI] [PubMed] [Google Scholar]
- 12.Arpey N.C., Gaglioti A.H., Rosenbaum M.E. How socioeconomic status affects patient perceptions of health care: a qualitative study. J Prim Care Community Health. 2017;8:169–175. doi: 10.1177/2150131917697439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkins R.B., Downs E.A., Johnston L.E., et al. Impact of transcatheter technology on surgical aortic valve replacement volume, outcomes, and cost. Ann Thorac Surg. 2017;103:1815–1823. doi: 10.1016/j.athoracsur.2017.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osnabrugge R.L.J., Speir A.M., Head S.J., et al. Costs for surgical aortic valve replacement according to preoperative risk categories. Ann Thorac Surg. 2013;96:500–506. doi: 10.1016/j.athoracsur.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 15.Adult Cardiac Surgery Database. The Society of Thoracic Surgeons. https://www.sts.org/registries-research-center/sts-nationaldatabase/adult-cardiac-surgery-database/data-collection
- 16.Proclamation on Declaring a National Emergency Concerning the Novel Coronavirus Disease (COVID-19) Outbreak. Trump White House. https://trumpwhitehouse.archives.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/
- 17.Lopez-Marco A., Harky A., Verdichizzo D., et al. Early experience of aortic surgery during the COVID-19 pandemic in the UK: a multicentre study. J Card Surg. 2021;36:848–856. doi: 10.1111/jocs.15307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Likosky D.S., Strobel R.J., Wu X., et al. Interhospital failure to rescue after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2023;165:134–143.e3. doi: 10.1016/j.jtcvs.2021.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy H.G., Shih T., Englesbe M.J., et al. Analyzing “failure to rescue”: is this an opportunity for outcome improvement in cardiac surgery? Ann Thorac Surg. 2013;95:1976–1981. doi: 10.1016/j.athoracsur.2013.03.027. [discussion: 1981]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo L.E., Kaufman E., Hoffman R.L., et al. Failure-to-rescue after injury is associated with preventability: the results of mortality panel review of failure-to-rescue cases in trauma. Surgery. 2017;161:782–790. doi: 10.1016/j.surg.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anesi G.L., Kerlin M.P. The impact of resource limitations on care delivery and outcomes: routine variation, the coronavirus disease 2019 pandemic, and persistent shortage. Curr Opin Crit Care. 2021;27:513–519. doi: 10.1097/MCC.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.French G., Hulse M., Nguyen D., et al. Impact of hospital strain on excess deaths during the COVID-19 pandemic—United States, July 2020-July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1613–1616. doi: 10.15585/mmwr.mm7046a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kantamneni N. The impact of the COVID-19 pandemic on marginalized populations in the United States: a research agenda. J Vocat Behav. 2020;119 doi: 10.1016/j.jvb.2020.103439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberger P.H., Jokl P., Ickovics J. Psychosocial factors and surgical outcomes: an evidence-based literature review. J Am Acad Orthop Surg. 2006;14:397–405. doi: 10.5435/00124635-200607000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Rubino A.S., De Santo L.S., Pisano A., et al. Cardiac surgery practice during the COVID-19 outbreak: a multicentre national survey. Eur J Cardiothorac Surg. 2021;59:901–907. doi: 10.1093/ejcts/ezaa436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy SL, Kochanek KD, Xu J, Arias E. Mortality in the United States, 2020. Centers for Disease Control and Prevention 2021. Accessed October 26, 2022. https://doi.org/10.15620/cdc:112079.
- 27.Chikwe J., Gaudino M., Hameed I., et al. Committee recommendations for resuming cardiac surgery activity in the SARS-CoV-2 era: guidance from an international cardiac surgery consortium. Ann Thorac Surg. 2020;110:725–732. doi: 10.1016/j.athoracsur.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.