Abstract
Introduction
The advent of the coronavirus disease 2019 (COVID-19) pandemic has led to the development of vaccines against severe acute respiratory syndrome coronavirus 2. Prospective evidence regarding safety for pregnant people and their developing fetuses is lacking. The aim of the COVID-19 Vaccines International Pregnancy Exposure Registry (C-VIPER) is to estimate the relative risk of obstetric, neonatal, and infant outcomes by comparing participants vaccinated against COVID-19 during pregnancy to a reference group of people enrolled in the Pregistry International Pregnancy Exposure Registry (PIPER) who remained unvaccinated during pregnancy.
Methods
The C-VIPER and the PIPER are international, non-interventional, real-world cohort studies. Participants receiving a COVID-19 vaccine during pregnancy will be matched in the analyses by country and gestational age at enrollment to unvaccinated individuals. Self-enrolled and self-consented participants complete online questionnaires at enrollment, during pregnancy, and for 12 months after the delivery of a live infant. Where possible, outcomes are verified by medical records. The study aims to recruit at least 500 pregnancies for each approved or authorized vaccine and will last for 5 years for each product.
Conclusions
By collecting data for each vaccine brand, the C-VIPER will be able to determine individual safety profiles. The study design allows for analysis of the effects of exposure to COVID-19 vaccines during specific etiologically relevant periods of gestation. Although the sample size may be too small to detect associations with rare outcomes, the study will be used to generate hypotheses for future research. Ultimately, the C-VIPER should provide data that will allow pregnant people and their healthcare providers to make informed decisions about COVID-19 vaccination.
Clinical Trial Registration
ClinicalTrials.gov NCT04705116. Registered on 12 January, 2021. EU PAS EUPAS39096. Registered on 20 January, 2021.
Key Points
| Comprehensive safety data regarding coronavirus disease 2019 (COVID-19) vaccine use during pregnancy have not yet been collected. |
| COVID-19 Vaccines International Pregnancy Exposure Registry (C-VIPER) is an ongoing, large, international, prospective, observational study that will allow the generation of hypotheses for future research. |
| It is designed to rapidly identify strong safety signals and flexibly enroll participants over geography and time. |
Introduction
Background and Rationale
To date, pregnant people have been excluded from all the phase II/III pivotal clinical trials used to gain authorization for the currently available coronavirus disease 2019 (COVID-19) vaccines [1, 2]. Therefore, the available safety data in this population have come exclusively from post-marketing administration of the vaccines [3–6]. Data are now available on over 185,000 people vaccinated during pregnancy [7]; however, many of the individual studies from which these data are drawn involve only small numbers of participants and consider only a limited number of outcomes. Study populations are drawn exclusively from high-income countries and often lack a comparable reference group. In addition, most studies recorded vaccination relatively late in pregnancy. Only one study appears to have followed up infants, and then only for 6 months [8]. Data on the use of different vaccine brands for subsequent vaccinations are also lacking.
Because the first countries to offer vaccines to pregnant populations used BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna), early safety data were only available for these brands. As a result, subsequent countries tended to preferentially offer mRNA vaccines to pregnant people; therefore, the majority of safety data come from these vaccines [7]. No concerning safety signals have been reported, with the rates of outcomes such as pregnancy loss, preterm birth, small for gestational age, and congenital anomalies being similar to published background rates [7, 9]. In the one study focusing on exposure to an mRNA vaccine during early pregnancy, the age-standardized cumulative risk of miscarriage among the 2456 participants was 12.8%, which is similar to that expected in the general obstetric population [5]. Early data from developmental and reproductive toxicity studies for Jcovden (Janssen) also have suggested this vaccine to be safe [10]. Similar safety data have been reported for Vaxzevria (AstraZeneca) [11]. However, there have been many anecdotal reports of menstruation-related adverse events (AEs) [12] and fears about effects on fertility [13] following vaccination with any of the currently available vaccines. Although mild premenstrual and menstrual changes have now been reported in formal studies [14, 15], fertility problems have not been confirmed in either men or women [16, 17]. As the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus is likely to become endemic, vaccine use will likely be ongoing and it is, therefore, imperative to collect and analyze more comprehensive safety data [9].
Along with poor communication from governments and other trusted sources, the influence of the “anti-vax” movement, and issues with the engagement of disenfranchised populations, the methodological limitations of the available studies have led to the proliferation of myths and misconceptions about COVID-19 vaccines [7, 9, 18]. These have increased safety fears and vaccine hesitancy among pregnant people and, in many countries, rates of vaccination in this population remain very low [7]. In South Africa, lack of vaccination has become the single biggest risk factor for severe disease and death in pregnant people [17].
Study Objective
The objective of the COVID-19 Vaccines International Pregnancy Exposure Registry (C-VIPER) is to estimate the risk of obstetric, neonatal, and infant outcomes among pregnant people and their offspring who were exposed to the single (homologous) or mixed (heterologous) brand of COVID-19 vaccines during pregnancy relative to a country- and gestational-age-matched reference group of people who received no COVID-19 vaccines during pregnancy.
Methods
Study Design
The C-VIPER is an international, non-interventional, post-marketing, cohort study designed to collect prospective safety and birth outcome data among people vaccinated with a COVID-19 vaccine during pregnancy or within 30 days prior to the first day of their last menstrual period (LMP). As of November 2022, ethics committee approval to conduct the C-VIPER has been obtained in Australia, Austria, Colombia, Cyprus, Germany, Liberia, Malta, Mexico, Philippines, Portugal, USA, and Zambia. In addition, confirmation that approval is not required has been obtained in Bulgaria, Canada, Croatia, Denmark, Greece, Hong Kong, Italy, Malaysia, New Zealand, Nigeria, Poland, Romania, Singapore, Slovakia, Slovenia, South Africa, South Korea, and the UK. As vaccines become available to pregnant people in additional countries, the study may expand. Countries contribute information when specific vaccines are authorized in-country and have been recommended for use in this population.
Recruitment
Recruitment into the study takes place via the dedicated website (https://c-viper.pregistry.com), links to which are included on other relevant websites worldwide. Awareness of the study is increased by advertising on social media channels frequented by pregnant people and by disseminating C-VIPER data to a wider scientific audience, including attendees at relevant meetings and the readership of peer-reviewed medical journals. Particular efforts are made to enroll participants from minority communities from social media and parenting platforms with high minority engagement, as well as through targeting geographical areas with high minority population densities. The study is sponsored by Pregistry and administered online via a web-based data collection tool. Participants can withdraw from the C-VIPER at any time. Although participants receive automatic reminders from the web-based system and from C-VIPER staff to complete modules, losses to follow-up are anticipated to occur. These losses will be censored from the cohort at the last time of contact. An attempt will be made to collect a minimum set of de-identified demographic information from all participants who are lost to follow-up in order to assess data representativeness in final analyses relative to the initial population. It is also anticipated that some participants in the unexposed group will subsequently receive a vaccination dose, at which point they will be censored from the study.
Participant retention is facilitated with short easy-to-complete questionnaire modules. Participants can also sign up for optional e-mails, texts, and phone calls during the study. All study materials are available in the languages spoken in the countries covered by the C-VIPER.
Study Population
The target study population consists of pregnant people who are at least 18 years of age. The exposed group includes pregnant people who have received at least one dose of a COVID-19 vaccine during the time period from 30 days prior to the first day of the LMP until the end of the pregnancy. The study is intended to include both individuals receiving a primary vaccination course as well as those receiving booster vaccinations. Participants are asked to upload documentary proof of vaccination, and reminders are sent to those who fail to provide it.
The exposed group will be compared to a second cohort of pregnant people who are participants in the Pregistry International Pregnancy Exposure Registry (PIPER, ClinicalTrials.gov NCT05352256, EUPAS46841). These subjects have not received a COVID-19 vaccine during pregnancy (although they may have received it more than 30 days prior to the first day of the LMP). The PIPER, which is also international in scope, follows the same methods of recruitment and data collection as the C-VIPER.
Study subjects self-enroll using the C-VIPER website and can enroll at any time during their pregnancy. Participants who enroll before an outcome of interest is known are considered prospective enrollees for that outcome. Conversely, participants who enroll after an outcome is recognized or suspected are considered retrospective enrollees for that outcome, with the exception of pregnancy loss before 20 weeks gestation.
Enrollment occurs during the first 2 years of the study, with follow-up of pregnancies and infants continuing in years 3 and 4. Data analysis and preparation of publications will take place during the final year. The C-VIPER may be discontinued if other methods of gathering appropriate information become achievable or are deemed preferable, or if the feasibility of collecting sufficient information diminishes to unacceptable levels because of poor enrollment or a lack of funding.
Sample Size
The C-VIPER aims to recruit a minimum of 500 pregnancies for each COVID-19 vaccine brand. As vaccine exposure in early pregnancy is of particular interest, the aim is to enroll at least 150 subjects vaccinated during the first trimester of gestation. The exposed to unexposed ratio is 1:2. Therefore, for each vaccine brand, the unexposed group will consist of approximately 1000 pregnancies. These sample sizes give sufficient power to detect relative risks of 3.0 or higher for outcomes originating in early pregnancy with frequencies of around 3%. Including a larger proportion of participants exposed later in pregnancy allows smaller effects to be detectable for outcomes originating in late pregnancy, assuming a loss to follow-up of around 50%. For a common outcome such as spontaneous abortion (i.e., the spontaneous loss of a pregnancy prior to 20 weeks of gestation), which occurs in around 15–20% of pregnancies [19], a two-fold increase in risk could be detected, based on a minimum of 150 exposed pregnancies.
Data Collection
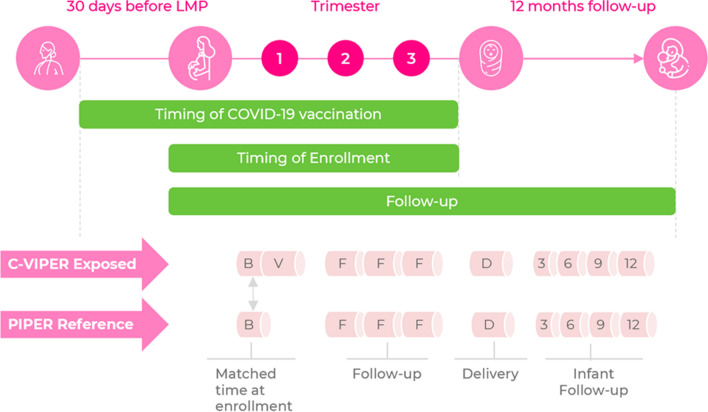
Details of the timing of data collection are presented in Fig. 1. Data are collected mainly through a series of structured web-based questionnaires (available from the corresponding author upon request). Every effort is made to ensure participant confidentiality, and personal identifying data are available to few C-VIPER personnel on a need basis. These data are kept securely in a separate file from the exposure and outcome data and are linked to each participant by a six-digit alphanumeric code. At enrollment, all participants must complete an initial COVID-19 vaccination module. Updates are then collected monthly until pregnancy loss or delivery, including exposure to additional vaccine doses. At this point, the delivery module records the pregnancy outcome. In the case of a live birth, data are collected at 3-monthly intervals during the first 12 months of the infant’s life, or until the infant’s death, should this occur during the first 12 months after delivery.
Fig. 1.
Timing of modules for collection of key variables. B baseline module, C-VIPER COVID-19 Vaccines International Pregnancy Registry, COVID-19 coronavirus disease 2019, D delivery module, F follow-up modules during pregnancy, LMP last menstrual period, PIPER Pregistry International Pregnancy Exposure Registry, V Vaccination module; 3, 6, 9, 12: maternal and infant outcomes modules at 3, 6, 9, and 12 months of age
In order to verify the self-reported data collected by the study and reduce potential exposure misclassification, participants are encouraged to upload copies of relevant supporting documents. A photograph of the vaccination certificate is requested, as well as medical records for the mother and the infant, from which all identifying information has been redacted. These records can include the results of SARS-CoV-2 tests, vaccination certificates, reports from healthcare professionals, photographic evidence of medication use, the delivery hospital discharge report, pediatric reports if applicable, and any other healthcare records the participant considers relevant. In case of a report of a serious AE (i.e., death, a life-threatening AE, inpatient hospitalization or prolongation of existing hospitalization, a persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions, or a congenital anomaly/birth defect), the study participant may receive a Medical Records Release Authorization Form and, if signed, the relevant healthcare provider is contacted to request medical records. These records will be used to validate maternally reported diagnoses. They also allow for adjudication of outcomes by the study dysmorphologist. In the event of discordance between the medical and maternal reports of COVID-19 vaccine administration, the subject will be classified as exposed to vaccination.
The semi-structured questionnaires have been thoroughly tested in Pregistry’s previous International Registry of Coronavirus Exposure in Pregnancy (IRCEP) study (ClinicalTrials.gov NCT04366986, EUPAS37360), which used a similar data collection system [20]. Following a pilot study with non-study volunteers, and comments from IRCEP participants, improvements were made, particularly to the wording of questions or to clarify instructions before the questionnaires were used in the C-VIPER and PIPER studies. The answers at baseline and the questions in the subsequent modules are interconnected to provide a personalized experience.
Details of the exposures measured by the C-VIPER and PIPER studies are provided in Table 1. The main exposure of interest in the C-VIPER is COVID-19 vaccination. Participants are asked to provide the number of vaccinations received, name of the vaccine or manufacturer, and date of vaccinations(s). Co-administration of another vaccine and AEs experienced during the 48 h following vaccination are also recorded. The specific gestational timing of vaccination is also of interest, as the etiologically relevant period for each outcome measure will vary.
Table 1.
Key variables collected by the C-VIPER and the PIPER
| Baseline module | COVID-19 vaccine modulea | Pregnancy follow-up and birth outcome modules | Infant follow-up modules |
|---|---|---|---|
| Date of study enrollment | Date of vaccination | Single/multiple pregnancy | Sex of infant |
| Gestational age at time of enrollment, as measured in weeks from first day of last menstrual period | Gestational age at time of vaccination by dose | New or recurrent medical conditions at any time during pregnancy, except adverse events following vaccination | Neonatal/infant death |
| Maternal age in years | Number of COVID-19 vaccination doses received and whether during pregnancy | Start and end dates of medical conditions | Birthweight |
| Country and continent of residence | Manufacturer of each COVID-19 vaccine dose | Outcome of medical conditions | Admission to neonatal intensive care unit; length of stay |
| Maternal race/ethnicity | Batch/lot number of each COVID-19 vaccine dose | Severity of medical conditions | Major congenital malformation |
| Maternal education level | Adverse events within 48 h of vaccination | Pregnancy-related medical conditions | Genetic/inherited disorder |
| Maternal employment status | Start and end dates of adverse events | Pregnancy outcome | Other infant outcomes |
| Healthcare coverage at onset of pregnancy | Outcome of adverse events | Other obstetric outcomes | Feeding method |
| Number of previous pregnancies | Severity of adverse events | Gestational age at delivery | Duration of breastfeeding |
| Number of previous live births | Pre-existing conditions affecting immune response | Preterm labor; reason | Infant vaccinations |
| Number of previous stillbirths | Other non-COVID-19 vaccines received in 4 weeks prior to COVID-19 vaccine | Mode of delivery | Variables repeated at 3, 6, 9, and 12 months: |
| Number of previous ectopic or tubal pregnancies | Intensive care unit admission during/after delivery | Infant’s weight | |
| Number of previous miscarriages | Medication use during pregnancy | Infant’s length | |
| Number of previous elective terminations | Adverse events in mother and baby | Infant medical conditions | |
| Self-reported pre-pregnancy health status | Adverse events in mother and baby | ||
| High-risk pregnancy as assessed by healthcare professional | Variables repeated at 6, 9, and 12 months: | ||
| Pre-existing medical conditions | Standardized motor development score | ||
| History of COVID-19 infection | Standardized cognitive development score | ||
| Tobacco use | Standardized language development score | ||
| Use of e-cigarettes/other vaping products | Standardized social/emotional development score | ||
| Recreational drug use | Standardized total development score | ||
| Alcohol use during first 3 months of pregnancy | |||
| Vitamin use during first 3 months of pregnancy | |||
| Medication use at enrollment | |||
| Maternal exposure to known human teratogen during pregnancy | |||
| Antenatal screening at enrollment | |||
| Intended pregnancy | |||
| Use of fertility procedures | |||
| Maternal height | |||
| Maternal pre-pregnancy weight | |||
| Maternal pre-pregnancy body mass index | |||
| Due date |
The bold values indicate that the variables listed below are collected at various points in time (e.g., at 3, 6, 9, and 12 months of the baby's age). The variables not listed under the bold values are collected once
COVID-19 coronavirus disease 2019, C-VIPER COVID-19 Vaccines International Pregnancy Registry, PIPER Pregistry International Pregnancy Exposure Registry
aThis module was administered to the C-VIPER cohort only
The C-VIPER and the PIPER collect data on a wide range of outcomes, full details of which are given in Table 2. The selection of obstetric and neonatal outcomes of interest and their definitions are based on guidelines published by the Global Alignment of Immunization safety Assessment in pregnancy (GAIA) network [21, 22]. Information on maternal and infant medical conditions, new COVID-19 vaccination doses, use of medications, environmental exposures, and results of SARS-CoV-2 tests are collected during pregnancy and until 12 months after delivery for all live births. In addition, infant motor, cognitive, language, social-emotional, and mental health milestones are assessed at 6, 9, and 12 months of age using the Caregiver-Reported Early Development Instrument [23]. This instrument has been adapted to allow parents to report their child’s developmental milestones using a brief set of yes/no questions. It was designed to be used within large-sample studies and contains items that are not affected by culturally specific contexts. It has been validated in more than 25 countries [24, 25] and is available in the languages used by the C-VIPER study. The Caregiver-Reported Early Development Instrument Short Form, which takes approximately 5 min to complete, is used in the 6- and 9-month modules, while the Long Form, which takes 15–20 min to complete, is used in the 12-month module.
Table 2.
Obstetric, neonatal, and infant outcomes assessed by the C-VIPER and the PIPER
| Obstetrica | Neonatal (< 29 days of age)a | Infant (29 days–12 months of age) |
|---|---|---|
| Spontaneous abortion | Major congenital malformations | Developmental milestones (motor, cognitive, language, social-emotional, and mental health skills) at 6, 9, and 12 months of ageb |
| Antenatal bleeding | Low birth weight | Height |
| Gestational diabetes | Neonatal death | Weight |
| Gestational hypertension | Neonatal encephalopathy | Failure to thrive |
| Intrauterine growth restriction | Neonatal infections | Medical conditions during the first 12 months of life |
| Postpartum hemorrhage | Neonatal acute kidney injury | COVID-19 |
| Fetal distress | Preterm birth | |
| Uterine rupture | Respiratory distress in the newborn | |
| Placenta previa | Small for gestational age | |
| Chorioamnionitis | Stillbirth | |
| Caesarean delivery | COVID-19 | |
| COVID-19 |
COVID-19 coronavirus disease 2019, C-VIPER COVID-19 Vaccines International Pregnancy Registry, PIPER Pregistry International Pregnancy Exposure Registry
aBased on case definitions developed by the Global Alignment of Immunization safety Assessment in pregnancy network [21], except COVID-19
bAs assessed by the Caregiver-Reported Early Development Instrument[23]
The baseline modules collect maternal demographic information, data on reproductive history, health-related behaviors, and pre-pregnancy health. Information collected by the pregnancy and birth outcome modules includes, among other variables, the number of fetuses present, health status throughout pregnancy, concomitant medications, pregnancy outcome (livebirth, miscarriage, stillbirth, elective termination), gestational length, and mode of delivery. Variables included in the delivery module concern infant demographics and health complications, feeding (breastmilk only; mostly breastmilk, some formula; mostly formula, some breastmilk; bank/donated breastmilk only; some bank/donated breastmilk, some formula; formula only; neither breastmilk nor formula), and vaccination history. Infants’ weight, length, and medical history are recorded at 3, 6, 9, and 12 months of age, and mothers are requested to provide redacted medical records for their infant, if available (medical records will also be solicited from healthcare professionals after maternal consent is obtained). This length of follow-up will allow the capture of structural and functional malformations that do not become clinically apparent until several months after birth as well as the ascertainment of developmental milestones.
Statistical Methods
Unexposed controls will be matched 2:1 to the vaccinated on country and gestational age at enrollment (time zero of follow-up). As the C-VIPER and PIPER studies may not cover the exact same time period, matching by calendar time of enrollment is not always possible. The frequency of outcomes of interest will be estimated for exposed and unexposed participants, using absolute risks, risk differences and unadjusted relative risks with 95% confidence intervals. Kaplan–Meier survival curves will present cumulative risk for outcomes that are dependent on gestational age. Adjusted analyses will be conducted using propensity score stratification, and relative risks will be calculated from generalized linear models when appropriate.
For each outcome, a directed acyclic graph, informed by expert knowledge and available literature, will be used to identify relevant confounders, competing events, and censoring. The baseline characteristics of the two study groups will be compared using the standardized mean difference, with an absolute difference >0.2 being considered an indicator of substantial imbalance. Unbalanced risk factors will be controlled through the use of propensity score; only characteristics collected at baseline will be considered as potential confounders. The baseline module collects information on characteristics that existed before vaccination, thus avoiding adjustment for factors in the causal pathway [26].
The optimal group for analysis for each specific outcome is participants enrolled before the outcome is known. Early enrollment reduces potential selection bias and allows the collection of reliable prospective information on vaccine exposure and other characteristics unaffected by potential outcomes. Analyses of malformations will be restricted to participants who enroll before the results from informative tests, such as ultrasound screening after 12 weeks of gestation, are known. Analysis of early pregnancy losses will be restricted to participants who enrolled before 20 weeks of gestation, while those for preterm delivery will be restricted to participants enrolled before 37 weeks of gestation.
In most cases, enrollment in the study and information on covariables will be collected before the outcome occurs, to avoid selection and recording bias [27, 28]. However, as retrospective enrollment is permitted, enrollment and recording of information on exposure and baseline covariates may occur after some outcomes have been identified. To assess the impact of retrospective enrollment, the primary analysis for each outcome will be restricted to prospectively enrolled participants.
Some participants will receive more than one dose of vaccine during the pregnancy being studied. Secondary analyses will explore the effect of two or more doses. The effects of exposure to the first dose, compared to subsequent doses, will also be examined. The effects of homologous or heterologous vaccination will be examined in analyses on an intention-to-treat, per protocol, or combined approach. In the intention-to-treat approach, exposure is defined by the first vaccine brand used, regardless of the brand for any subsequent doses. In the per-protocol approach, participants will be censored from the study when they receive a second vaccination dose with a brand that is different from the first dose received. In the combined approach, a participant is considered to be exposed if at least one dose of any approved or authorized vaccine is received during pregnancy. Therefore, participants receiving both homologous and heterologous vaccine regimens will be included in analyses.
Participants who enroll into the unexposed group and subsequently receive a COVID-19 vaccination during pregnancy will be censored from the study on the vaccination date. An intention-to-treat approach that would keep such participants in the unexposed group is inappropriate for this study, which focusses on vaccine safety, as it may bias results towards the null. Weights will be used to adjust for potential informative censoring.
Data Monitoring
The C-VIPER is overseen by a scientific advisory committee, made up of experts in maternal-fetal medicine, infectious diseases, epidemiology, and biostatistics. Members of the scientific advisory committee receive interim reports from the C-VIPER coordinator. The responsibilities of the scientific advisory committee include decisions regarding emerging safety issues, resolution of operational issues, and development of strategies to increase awareness of the study.
Several marketing authorization holder companies have contracted with Pregistry to fund analyses of the C-VIPER in order to fulfill their post-authorization safety study requirements. These companies have access to a dedicated online portal that allows their staff to see all available de-identified case data for pregnancies exposed to their COVID-19 vaccine in near real time. Serious AEs occurring during the study period in exposed participants or their offspring are reported expeditely to the relevant marketing authorization holder company (see “Funding”) within agreed timelines in accordance to country-specific regulations via an electronic gateway. Non-serious AEs are reported in a non-expedited way. Safety analyses of data occurring in participants vaccinated with products of marketing authorization holder companies not financially contributing to the C-VIPER will be published in the medical literature.
Discussion
Pregnancy is known to be a risk factor for increased illness from both pandemic and seasonal influenza. Data from the 1918 influenza pandemic show that mortality rates among pregnant people were abnormally high and even during interpandemic periods, higher rates of influenza complications have been reported [29, 30]. Infection control measures may also impact pregnant people [29, 31]. Interventions such as voluntary quarantine may lead to conflicting advice about routine prenatal care and delivery, and pregnant patients may be reluctant to take antiviral medications out of concern for the health of their fetus. These issues highlight the need to provide suitable interventions for this high-risk population [29].
Three years into the COVID-19 pandemic, the management of pregnant people infected with the SARS-CoV-2 virus continues to pose challenges for healthcare systems and practitioners. Pregnancy leads to physiological changes in the respiratory, cardiovascular, and immune systems, which are necessary to accommodate the growing fetus [9, 17]. The decrease in lung volume as the fetus grows, along with immunological changes, may make pregnant people more susceptible to respiratory pathogens [17]. Although higher rates of COVID-19 infection have been reported in pregnant populations, it is difficult to compare these rates to other populations as universal screening is rare [9].
The physiological changes associated with pregnancy may also impact the outcome of concurrent infection [32]. A growing body of evidence suggests that pregnant people are at a higher risk of morbidity and mortality from COVID-19, compared with age-matched individuals who are not pregnant. Rates of admission to an intensive care unit, invasive ventilation, extra-corporeal membrane oxygenation, and death from COVID-19 are higher in pregnant patients [33, 34]. COVID-19 has been associated with an increased risk of adverse maternal and neonatal outcomes, including preeclampsia, HELLP (hemolytic anemia, elevated liver enzymes, low platelets) syndrome, gestational diabetes, cesarean delivery, low birthweight, and stillbirth [9, 17, 35–37]. Although preterm birth, cesarean delivery, and low birthweight are associated with COVID-19 infection, these complications are not always a direct result of the virus, but may be iatrogenic, reflecting a decision to deliver the baby in order to save the critically ill mother [37]. There is currently no clear evidence of COVID-19 transmission in utero [9, 17], and most cases identified among infants after birth are due to exposure to infected caregivers [9]. Conversely, placental infection has been reported and can lead to placentitis, which is a risk factor for fetal hypoxia or death in cases of severe placental injury [7].
A significant proportion of symptomatic patients have reported health issues that persist for weeks, or sometimes months, after the initial COVID-19 infection. This condition, commonly known as “long COVID” or “post-COVID-19 syndrome”, has not been consistently defined, but it appears to be more common in female individuals and in patients with a high body mass index [38]. However, there is little correlation between the risk of persistent symptoms and objective measures of disease severity. The mechanism behind long COVID is not yet fully understood, but it may arise directly from infection with the virus, or indirectly as the result of immune responses to infection. The long-term management of individuals with long COVID remains a challenge [39], and information regarding its effects during pregnancy is lacking. For instance, it is not clear whether pregnancy is associated with a longer disease course [9].
The literature has focused on symptomatic patients with confirmed infection, and the maternal and neonatal risks associated with COVID-19 infection are likely to be underestimated as many individuals are asymptomatic [40]. Furthermore, much of the data on obstetric outcomes were collected during early waves of the COVID-19 pandemic, when the predominant variants were different from those currently circulating. The effects of current and future variants may not be the same. There is already evidence that maternal and neonatal outcomes were worse during the Delta wave than in preceding waves [7]. Consequently, the pregnant person should be considered a priority recipient for candidate vaccines and immunotherapies [7, 41]. A safe and effective vaccine will not only protect maternal health during pregnancy and delivery but could minimize the risk of fetal and neonatal infection by potentially inducing transplacental antibodies that may help prevent infection from household and community exposures [42].
The C-VIPER aims to evaluate the possible effects of single- or mixed-vaccine administration to prevent COVID-19 on obstetric, neonatal, and infant outcomes. These outcomes will be compared in participants exposed to vaccination during pregnancy and those who remain unvaccinated, or who receive a vaccination more than 30 days prior to the first day of the LMP.
One major strength of the C-VIPER is its international coverage, including low- and middle-income countries typically excluded from pregnancy safety post-authorization surveillance. This should also allow for rapid enrollment, which has previously often been an issue in pregnancy registries [43, 44]. Experience with our previous study shows that it is possible to recruit large numbers of pregnant people in a relatively short period of time. In the first 9 months of the IRCEP study, over 17,000 participants from more than 70 countries were enrolled [45]. The data collection tool is available in a variety of languages, allowing pregnant people from many countries, of differing socioeconomic status, to enroll. The use of social media to recruit participants should be especially effective at targeting young people of child-bearing age. It is anticipated that the C-VIPER participants will represent a wide range of maternal age, ethnic background, and health status categories, given the ubiquitous presence of smartphones and internet connectivity. Furthermore, data collection is standardized across countries and vaccine brands and can easily be modified for implementation with new products as they are released onto the market. In an evolving pandemic, this is an important benefit.
Since the advent of the COVID-19 pandemic, several other pregnancy registries have been established to examine the effects of vaccination for COVID-19 during pregnancy [3, 6, 8, 46–56]. However, many of these only include a small number of participants, are based in one high-income country, do not include many, if any, participants vaccinated early in pregnancy, or only examine the acute effects of vaccination on maternal health. Although the COVI-PREG registry does cover numerous countries, to date, the association between vaccination during pregnancy and birth outcomes has only been reported for Swiss participants [57]. A further issue with most pregnancy registries is the lack of a suitable comparator cohort [58]. With its broader geographical scope, inclusion of a comparable unexposed cohort, and focus on the association between vaccination during pregnancy, particularly during the first trimester, and obstetric and neonatal outcomes, we believe that the C-VIPER will generate a unique dataset.
Participants in the C-VIPER are not prevented from joining another registry study if they choose to do so, nor is participation in another registry a barrier to the C-VIPER. It is therefore possible that results from some participants may be reported in more than one registry. It is also possible that participation in the C-VIPER will affect recruitment for other registries. It is beyond the scope of this paper to accurately assess the impact of these issues, but we believe that the number of participants taking part in more than one study is likely to be low.
The longitudinal nature of the C-VIPER facilitates the estimation of both absolute and relative risks. Patient registries are an efficient means of assessing rare exposure and detecting strong effects. However, they often lack the statistical power to evaluate rare outcomes and modest effects. The statistical power of a study to detect an effect at or above a certain level is affected by the sample size and the baseline risk of the outcome in the population [59]. The sample size of the C-VIPER should give sufficient power to detect common outcomes, such as the total prevalence of all major congenital malformations, and very large increases in rare individual defects. It will also be used to generate hypotheses that could form the basis for further investigation using complementary approaches, study designs, and data sources. However, if insufficient numbers of participants are recruited, an underpowered sample size will result, and real effects may be missed.
Other limitations of the C-VIPER study include the potential for spurious associations due to multiple comparisons, biases due to self-selection and survivor cohort effects, and residual confounding. Furthermore, some adverse outcomes, such as neurodevelopmental delays and some major congenital malformations, may not become apparent until after 12 months of age and so may be missed. An additional limitation of pregnancy registries is the length of time typically required to enroll a high enough number of exposed participants to generate statistically robust estimates of pregnancy outcomes. However, the scope, methodology, and anticipated sample size of the C-VIPER should address many of these issues.
Retention of participants may also be a potential limitation of this study. Therefore, the technology used to collect the information and the timing of the modules have been tailored to maximize participant engagement. There is no financial incentive to enroll or participate in the C-VIPER. Instead, the study appeals to the altruism of participants by stressing the importance of their continued participation for the benefit of others [45].
Conclusions
A large number of governmental and non- governmental organizations currently recommend that pregnant people, in conjunction with their clinical care team, should be free to make their own decision regarding the use of COVID-19 vaccines [60]. Given the very limited amount of safety information available for currently marketed COVID-19 vaccines when used in pregnancy, the C-VIPER is expected to generate data that may ultimately enable healthcare professionals and pregnant people to make evidence-based choices.
Acknowledgments
Editorial assistance for this manuscript was provided by Alison Thornton.
Declarations
Funding
The C-VIPER receives funding from AstraZeneca, Janssen, Novavax, Sanofi, and Valneva.
Conflicts of Interest/Competing Interests
Diego F. Wyszynski, Mondira Bhattacharya, Oscar Martínez-Pérez, Anthony R. Scialli, Melissa Tassinari, Naor Bar-Zeev, Cheryl Renz, and Sonia Hernández-Díaz have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The C-VIPER study protocol may be made available by the corresponding author on reasonable request, following applicable regulations.
Code Availability
Not applicable.
Authors’ Contributions
The original study protocol was designed by DFW and SH-D. DFW planned the study, designed the study methodology, supervised data collection and data verification, and drafted the first version of the article. MB, OM, AS, MT, NB-Z, CR, and SH-D provided advice on the study methodology and edited the first and final versions of the article. All authors have read and approved the final version of the manuscript.
References
- 1.Rubin R. Pregnant people's paradox: excluded from vaccine trials despite having a higher risk of COVID-19 complications. JAMA. 2021;325(11):1027–1028. doi: 10.1001/jama.2021.2264. [DOI] [PubMed] [Google Scholar]
- 2.Klein SL, Creisher PS, Burd I. COVID-19 vaccine testing in pregnant females is necessary. J Clin Invest. 2021;131(5):1–3. [DOI] [PMC free article] [PubMed]
- 3.Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant people. N Engl J Med. 2021;384(24):2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbone L, Trinchillo MG, Di Girolamo R, Raffone A, Saccone G, Iorio GG, et al. COVID-19 vaccine and pregnancy outcomes: a systematic review and meta-analysis. Int J Gynaecol Obstet. 2022;159:651–661. doi: 10.1002/ijgo.14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zauche LH, Wallace B, Smoots AN, Olson CK, Oduyebo T, Kim SY, et al. Receipt of mRNA Covid-19 vaccines and risk of spontaneous abortion. N Engl J Med. 2021;385(16):1533–1535. doi: 10.1056/NEJMc2113891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moro PL, Panagiotakopoulos L, Oduyebo T, Olson CK, Myers T. Monitoring the safety of COVID-19 vaccines in pregnancy in the US. Hum Vaccin Immunother. 2021;17(12):4705–4713. doi: 10.1080/21645515.2021.1984132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Male V. SARS-CoV-2 infection and COVID-19 vaccination in pregnancy. Nat Rev Immunol. 2022;22(5):277–282. doi: 10.1038/s41577-022-00703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldshtein I, Steinberg DM, Kuint J, Chodick G, Segal Y, Shapiro Ben David S, et al. Association of BNT162b2 COVID-19 vaccination during pregnancy with neonatal and early infant outcomes. JAMA Pediatr. 2022;176(5):470–477. doi: 10.1001/jamapediatrics.2022.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jamieson DJ, Rasmussen SA. An update on COVID-19 and pregnancy. Am J Obstet Gynecol. 2022;226(2):177–186. doi: 10.1016/j.ajog.2021.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipkind HS, Vazquez-Benitez G, DeSilva M, Vesco KK, Ackerman-Banks C, Zhu J, et al. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth: eight integrated health care organizations, United States, December 15, 2020-July 22, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(1):26–30. doi: 10.15585/mmwr.mm7101e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillson K, Clemens SC, Madhi SA, Voysey M, Pollard AJ, Minassian AM, et al. Fertility rates and birth outcomes after ChAdOx1 nCoV-19 (AZD1222) vaccination. Lancet. 2021;398(10312):1683–1684. doi: 10.1016/S0140-6736(21)02282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nazir M, Asghar S, Rathore MA, Shahzad A, Shahid A, Khan AA, et al. Menstrual abnormalities after COVID-19 vaccines: a systematic review. Vacunas. 2022;23(2):S77–S87. doi: 10.1016/j.vacun.2022.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz P, Zizzo J, Balaji NC, Reddy R, Khodamoradi K, Ory J, et al. Fear about adverse effect on fertility is a major cause of COVID-19 vaccine hesitancy in the United States. Andrologia. 2022;54(4):e14361. doi: 10.1111/and.14361. [DOI] [PubMed] [Google Scholar]
- 14.Baena-García L, Aparicio VA, Molina-López A, Aranda P, Cámara-Roca L, Ocón-Hernández O. Premenstrual and menstrual changes reported after COVID-19 vaccination: the EVA project. Womens Health (Lond). 2022;18:17455057221112237. doi: 10.1177/17455057221112237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong KK, Heilig CM, Hause A, Myers TR, Olson CK, Gee J, et al. Menstrual irregularities and vaginal bleeding after COVID-19 vaccination reported to v-safe active surveillance, USA in December, 2020-January, 2022: an observational cohort study. Lancet Digit Health. 2022;4(9):e667–e675. doi: 10.1016/S2589-7500(22)00125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen F, Zhu S, Dai Z, Hao L, Luan C, Guo Q, et al. Effects of COVID-19 and mRNA vaccines on human fertility. Hum Reprod. 2021;37(1):5–13. doi: 10.1093/humrep/deab238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adam S, Pheiffer C, Dias S, Hlongwane T, Vannevel V, Soma-Pillay P, et al. Coronavirus and pregnancy: the challenges of the 21(st) century: a review. Front Microbiol. 2022;13:923546. doi: 10.3389/fmicb.2022.923546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Löffler P. Review: vaccine myth-buster: cleaning up with prejudices and dangerous misinformation. Front Immunol. 2021;12:663280. doi: 10.3389/fimmu.2021.663280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smart CJ, Smith BL. A transdisciplinary team approach to perinatal loss. MCN Am J Matern Child Nurs. 2013;38(2):110–114. doi: 10.1097/NMC.0b013e318270db45. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Diaz S, Smith LH, Dollinger C, Rasmussen SA, Schisterman EF, Bellocco R, et al. International Registry of Coronavirus Exposure in Pregnancy (IRCEP): cohort description and methodological considerations. Am J Epidemiol. 2022;191(6):967–979. doi: 10.1093/aje/kwac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonhoeffer J, Kochhar S, Hirschfeld S, Heath PT, Jones CE, Bauwens J, et al. Global alignment of immunization safety assessment in pregnancy: the GAIA project. Vaccine. 2016;34(49):5993–5997. doi: 10.1016/j.vaccine.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Jones CE, Munoz FM, Spiegel HM, Heininger U, Zuber PL, Edwards KM, et al. Guideline for collection, analysis and presentation of safety data in clinical trials of vaccines in pregnant women. Vaccine. 2016;34(49):5998–6006. doi: 10.1016/j.vaccine.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCoy DC, Sudfeld CR, Bellinger DC, Muhihi A, Ashery G, Weary TE, et al. Development and validation of an early childhood development scale for use in low-resourced settings. Popul Health Metr. 2017;15(1):3. doi: 10.1186/s12963-017-0122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altafim ERP, McCoy DC, Brentani A, Escobar AMU, Grisi S, Fink G. Measuring early childhood development in Brazil: validation of the Caregiver Reported Early Development Instruments (CREDI) J Pediatr (Rio J) 2020;96(1):66–75. doi: 10.1016/j.jped.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Tang L, Bai Y, Zhao S, Shi Y. Reliability and validity of the Caregiver Reported Early Development Instruments (CREDI) in impoverished regions of China. BMC Pediatr. 2020;20(1):475. doi: 10.1186/s12887-020-02367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155(2):176–184. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez-Diaz S, Schisterman EF, Hernan MA. The birth weight "paradox" uncovered? Am J Epidemiol. 2006;164(11):1115–1120. doi: 10.1093/aje/kwj275. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald SC, Hernan MA, McElrath TF, Hernandez-Diaz S. Assessment of recording bias in pregnancy studies using health care databases: an application to neurologic conditions. Paediatr Perinat Epidemiol. 2018;32(3):281–286. doi: 10.1111/ppe.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerg Infect Dis. 2008;14(1):95–100. doi: 10.3201/eid1401.070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dodds L, McNeil SA, Fell DB, Allen VM, Coombs A, Scott J, et al. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ. 2007;176(4):463–468. doi: 10.1503/cmaj.061435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wastnedge EAN, Reynolds RM, van Boeckel SR, Stock SJ, Denison FC, Maybin JA, et al. Pregnancy and COVID-19. Physiol Rev. 2021;101(1):303–318. doi: 10.1152/physrev.00024.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mertz D, Geraci J, Winkup J, Gessner BD, Ortiz JR, Loeb M. Pregnancy as a risk factor for severe outcomes from influenza virus infection: a systematic review and meta-analysis of observational studies. Vaccine. 2017;35(4):521–528. doi: 10.1016/j.vaccine.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status: United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):769–775. doi: 10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collin J, Bystrom E, Carnahan A, Ahrne M. Public Health Agency of Sweden's brief report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99(7):819–822. doi: 10.1111/aogs.13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O'Brien P, Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA. 2020;324(7):705–706. doi: 10.1001/jama.2020.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moran Antolin E, Broullon Molanes JR, de la Cruz Conty ML, Encinas Pardilla MB, Guadix Martin MDP, Sainz Bueno JA, et al. SARS-CoV-2 infection and C-section: a prospective observational study. Viruses. 2021;13(11):2330. doi: 10.3390/v13112330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Perez O, Prats Rodriguez P, Muner Hernandez M, Encinas Pardilla MB, Perez Perez N, Vila Hernandez MR, et al. The association between SARS-CoV-2 infection and preterm delivery: a prospective study with a multivariable analysis. BMC Pregnancy Childbirth. 2021;21(1):273. doi: 10.1186/s12884-021-03742-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreira JC, Moreira TCL, de Araujo AL, Imamura M, Damiano RF, Garcia ML, et al. Clinical, sociodemographic and environmental factors impact post-COVID-19 syndrome. J Glob Health. 2022;12:05029. doi: 10.7189/jogh.12.05029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bliddal S, Banasik K, Pedersen OB, Nissen J, Cantwell L, Schwinn M, et al. Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Sci Rep. 2021;11(1):13153. doi: 10.1038/s41598-021-92045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutton D, Fuchs K, D'Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382(22):2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heath PT, Le Doare K, Khalil A. Inclusion of pregnant women in COVID-19 vaccine development. Lancet Infect Dis. 2020;20(9):1007–1008. doi: 10.1016/S1473-3099(20)30638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muldoon KM, Fowler KB, Pesch MH, Schleiss MR. SARS-CoV-2: is it the newest spark in the TORCH? J Clin Virol. 2020;127:104372. doi: 10.1016/j.jcv.2020.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bird ST, Gelperin K, Taylor L, Sahin L, Hammad H, Andrade SE, et al. Enrollment and retention in 34 United States pregnancy registries contrasted with the manufacturer's capture of spontaneous reports for exposed pregnancies. Drug Saf. 2018;41(1):87–94. doi: 10.1007/s40264-017-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinclair S, Cunnington M, Messenheimer J, Weil J, Cragan J, Lowensohn R, et al. Advantages and problems with pregnancy registries: observations and surprises throughout the life of the International Lamotrigine Pregnancy Registry. Pharmacoepidemiol Drug Saf. 2014;23(8):779–786. doi: 10.1002/pds.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hernandez-Diaz S, Smith LH, Dollinger C, Rasmussen SA, Schisterman EF, Bellocco R, et al. International Registry of Coronavirus Exposure in Pregnancy (IRCEP): cohort description and methodological considerations. Am J Epidemiol. 2022;191(6):967–979. doi: 10.1093/aje/kwac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panchaud A, Favre G, Pomar L, Vouga M, Aebi-Popp K, Baud D. An international registry for emergent pathogens and pregnancy. Lancet. 2020;395(10235):1483–1484. doi: 10.1016/S0140-6736(20)30981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zauche LH, Wallace B, Smoots AN, Olson CK, Oduyebo T, Kim SY, et al. Receipt of mRNA COVID-19 vaccines preconception and during pregnancy and risk of self-reported spontaneous abortions, CDC v-safe COVID-19 Vaccine Pregnancy Registry 2020-21. Res Sq. 2021. 10.21203/rs.3.rs-798175/v1.
- 48.Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM. 2021;3(6):100467. doi: 10.1016/j.ajogmf.2021.100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pratama NR, Wafa IA, Budi DS, Putra M, Wardhana MP, Wungu CDK. mRNA Covid-19 vaccines in pregnancy: a systematic review. PLoS ONE. 2022;17(2):e0261350. doi: 10.1371/journal.pone.0261350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fell DB, Dhinsa T, Alton GD, Török E, Dimanlig-Cruz S, Regan AK, et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA. 2022;327(15):1478–1487. doi: 10.1001/jama.2022.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magnus MC, Örtqvist AK, Dahlqwist E, Ljung R, Skår F, Oakley L, et al. Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA. 2022;327(15):1469–1477. doi: 10.1001/jama.2022.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fell DB, Dimanlig-Cruz S, Regan AK, Håberg SE, Gravel CA, Oakley L, et al. Risk of preterm birth, small for gestational age at birth, and stillbirth after covid-19 vaccination during pregnancy: population based retrospective cohort study. BMJ. 2022;378:e071416. doi: 10.1136/bmj-2022-071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maguire S, Al-Emadi S, Alba P, Aguiar MC, Lawati TA, Alle G, et al. Obstetric outcomes in women with rheumatic disease and COVID-19 in the context of vaccination status. Rheumatology (Oxford). 2022. 10.1093/rheumatology/keac534. [DOI] [PMC free article] [PubMed]
- 54.Brinkley E, Mack CD, Albert L, Knuth K, Reynolds MW, Toovey S, et al. COVID-19 vaccinations in pregnancy: comparative evaluation of acute side effects and self-reported impact on quality of life between pregnant and non-pregnant women in the United States. Am J Perinatol. 2022;39(16):1750–1753. doi: 10.1055/s-0042-1748158. [DOI] [PubMed] [Google Scholar]
- 55.McClymont E, Abenhaim H, Albert A, Boucoiran I, Cassell K, Castillo E, et al. Canadian Surveillance of COVID-19 in Pregnancy (CANCOVID-Preg): a rapidly coordinated national response using established regional infrastructures. J Obstet Gynaecol Can. 2021;43(2):165–166. doi: 10.1016/j.jogc.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cottin J, Benevent J, Khettar S, Lacroix I. COVID-19 vaccines and pregnancy: what do we know? Therapie. 2021;76(4):373–374. doi: 10.1016/j.therap.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Favre G, Maisonneuve E, Pomar L, Winterfeld U, Daire C, Martinez Tejada B, et al. COVID-19 mRNA vaccine in pregnancy: results of the Swiss COVI-PREG registry, an observational prospective cohort study. Lancet Reg Health Eur. 2022;18:100410. doi: 10.1016/j.lanepe.2022.100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dolk H, Damase-Michel C, Morris JK, Loane M. COVID-19 in pregnancy-what study designs can we use to assess the risk of congenital anomalies in relation to COVID-19 disease, treatment and vaccination? Paediatr Perinat Epidemiol. 2022;36(4):493–507. doi: 10.1111/ppe.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gelperin K, Hammad H, Leishear K, Bird ST, Taylor L, Hampp C, et al. A systematic review of pregnancy exposure registries: examination of protocol-specified pregnancy outcomes, target sample size, and comparator selection. Pharmacoepidemiol Drug Saf. 2017;26(2):208–214. doi: 10.1002/pds.4150. [DOI] [PubMed] [Google Scholar]
- 60.American College of Obstetricians and Gynecologists. Vaccinating pregnant and lactating patients against COVID-19. 2022. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19. Accessed 17 Jan 2023.