Abstract
A broad range of in vitro test methods have been developed given their numerous potential advantages over in vivo tests. We describe here key resources and tools to increase the reliability and reproducibility of in vitro toxicological test methods.
Widespread research efforts and resources have been dedicated to developing in vitro toxicological test methods due to their potential for higher throughput, lower cost, and ability to generate more human relevant mechanistic data than animal tests. In vitro methods are being used to test a wide range of biological endpoints and substances, including chemicals, particles (e.g., nanomaterials or microplastics), biomaterials (e.g., dental composites), and mixtures—in various physical states (e.g., liquids, creams, and aerosols). To meet this broad scope, there is great diversity among in vitro test methods in terms of the biological test system (i.e., the species and organ the cells originated from) and complexity (e.g., two-dimensional, or three-dimensional tissue models).
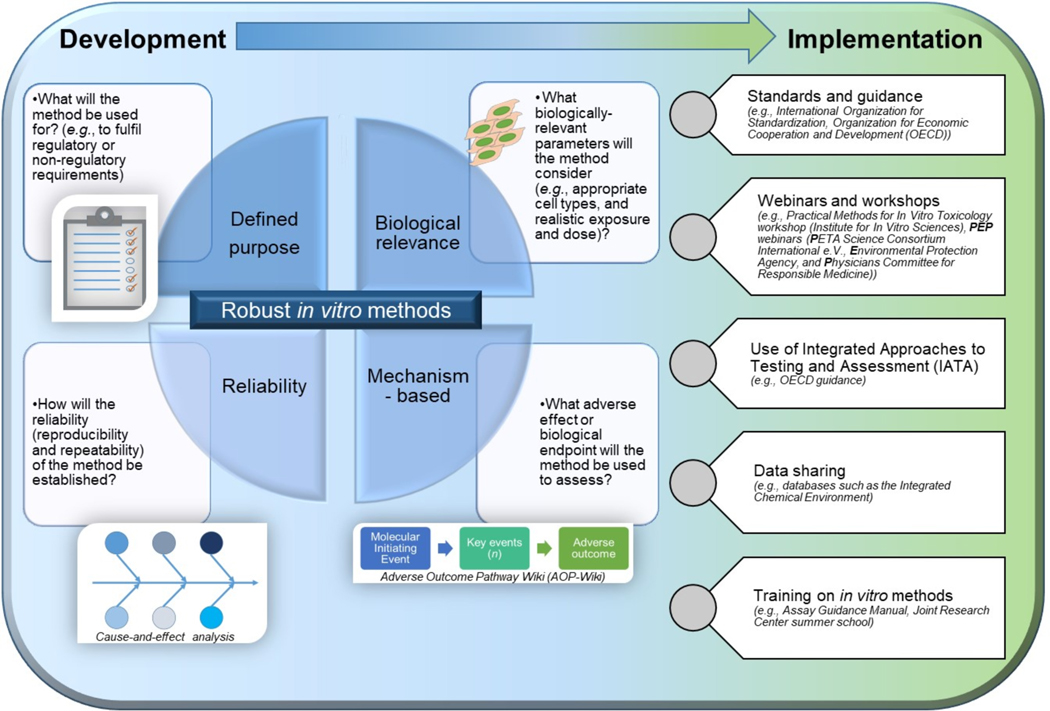
The choice of an appropriate in vitro test system to develop and use depends on several factors (Figure 1). In vitro methods should have a defined purpose, and be reliable, biologically relevant, and governed by a known mechanism of action.
Figure 1:
Key factors in the development and implementation of in vitro test methods
We focus here on identifying resources to help guide the development of reliable in vitro toxicological test methods to enable the generation of consistent results within and among laboratories across time. The Guidance Document on Good In Vitro Method Practices (GIVIMP) is a general tool for the development and implementation of in vitro methods for regulatory use in safety assessment (available at https://www.oecd-ilibrary.org/environment/guidance-document-on-good-in-vitro-method-practices-givimp_9789264304796-en). GIVIMP covers a range of topics, and those most relevant to this paper pertain to guidance on the key elements for developing reliable in vitro methods, including test systems and their performance, equipment and materials, appropriate controls, standard operating procedures, and reporting and storage of results and records. In addition, the U.S. National Center for Advancing Translational Sciences (NCATS) Assay Guidance Manual (AGM; available at https://www.ncbi.nlm.nih.gov/books/NBK53196/) provides researchers with guidelines for robust assay development and validation with a focus on high-throughput in vitro assays.1 This information was commonly used in the US Toxicology in the 21st Century program (Tox21) to develop, validate, and screen a battery of in vitro assays (e.g. pathway-, target- or phenotypic-based assays) in a quantitative high throughput screening (qHTS) platform to evaluate tens of thousands of environmental chemicals (https://tox21.gov/overview/).
Tools developed to improve the reliability of measurements in manufacturing and other research areas can be adopted for in vitro method development. For example, cause-and-effect (C&E) analysis is a conceptual systematic approach to identifying potential sources of variability that may impact assay results.2,3 The results of C&E analysis can guide robustness testing to evaluate and quantify the impact of assay protocol variations. Based on this information, the major sources of variability can be identified and monitored each time the assay is performed through in-process control measurements or through preliminary one-time experiments.2,3 Results from the in-process control measurements can be used to set specifications for a method to support consistent inter- and intra-laboratory performance of the test method.4 Control charts can be used to monitor these in-process control measurements to evaluate their mean response and its variability over time. If systematic changes in the mean response or its day-to-day variability are observed within a laboratory (e.g., an increase in the mean response across time), it may be important to assess if changes in performing the assay, such as using a different manufacturer for reagents, could have impacted the result. One key in-process control measurement is the positive chemical control. This control may provide insight about the assay’s dynamic range by assessing the maximum potential response (e.g., 100 % cell death) and also about the sensitivity of the assay by, for example, measuring the response to different concentrations of the positive chemical control and showing a concentration-dependent response. Guidance on choosing an optimal positive chemical control was recently described including ten key characteristics of the positive chemical control such as its stability, chemical purity, and ease of preparation.5 Evaluating a method’s applicability domain across the physicochemical property space will allow exclusion of classes of substances incompatible with the particular assay (e.g., creams in assays using submerged cell models). Inter-laboratory testing may be needed to determine the transferability of an assay among laboratories and can reveal the steps of a protocol that are most vulnerable to interpretation differences among laboratories.4 Another tool for ensuring the transferability of a method is to specify proficiency test chemicals that new laboratories can use to evaluate their performance on substances that yield a range of assay responses. Transferability of these methods is also supported by hands on trainings for new operators such as the Institute for In Vitro Sciences (IIVS) Practical Methods for In Vitro Toxicology Workshop (https://iivs.org/education-outreach/). Similarly, NCATS has held AGM workshops to support newcomers with high throughput assay development.
Reliable and reproducible in vitro methods have the potential to yield a scientifically sound strategy to test the ever-growing list of chemicals and substances. Publications, webinars, workshops, and sharing results (e.g., via public databases) are promising approaches to raise awareness of the methods and obtain feedback from the scientific community. The development of standard methods and guidance documents—by organizations such as the International Organization for Standardization (ISO) (e.g., technical committee 212), American Society for Testing and Materials (ASTM) International, and the Organization for Economic Cooperation and Development (OECD)—further establishes confidence in the use of in vitro methods and could guide their application in areas such as the development of integrated approaches to testing and assessment (IATA) based on adverse outcome pathways (AOPs). Overall, improving the reliability and reproducibility of in vitro methods using the resources described throughout this manuscript promises to support increased usage of these methods.
Acknowledgements:
This study was supported in part by the Intramural Research Program of the National Center for Advancing Translational Sciences (NCATS). The views expressed in this paper are those of the authors and do not necessarily reflect the statements, opinions, views, conclusions, or policies of the National Center for Advancing Translational Sciences or the National Institutes of Health. Certain instruments or materials are identified in this paper to adequately specify experimental details. In no case does it imply endorsement by NIST or imply that it is necessarily the best product for the experimental procedure.
References
- 1.Coussens NP; Sittampalam GS; Guha R; Brimacombe K; Grossman A; Chung TDY; Weidner JR; Riss T; Trask OJ; Auld D; Dahlin JL; Devanaryan V; Foley TL; McGee J; Kahl SD; Kales SC; Arkin M; Baell J; Bejcek B; Gal-Edd N; Glicksman M; Haas JV; Iversen PW; Hoeppner M; Lathrop S; Sayers E; Liu H; Trawick B; McVey J; Lemmon VP; Li Z; McManus O; Minor L; Napper A; Wildey MJ; Pacifici R; Chin WW; Xia M; Xu X; Lal-Nag M; Hall MD; Michael S; Inglese J; Simeonov A; Austin CP, Assay Guidance Manual: Quantitative Biology and Pharmacology in Preclinical Drug Discovery. Clinical and translational science 2018, 11 (5), 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen EJ; Hirsch C; Elliott JT; Krug HF; Aengenheister L; Arif AT; Bogni A; Kinsner-Ovaskainen A; May S; Walser T; Wick P; Roesslein M, Cause-and-Effect Analysis as a Tool To Improve the Reproducibility of Nanobioassays: Four Case Studies. Chem. Res. Toxicol. 2020, 33 (5), 1039–1054. [DOI] [PubMed] [Google Scholar]
- 3.Leibrock L; Jungnickel H; Tentschert J; Katz A; Toman B; Petersen EJ; Bierkandt FS; Singh AV; Laux P; Luch A, Parametric optimization of an air-liquid interface system for flow-through inhalation exposure to nanoparticles: assessing dosimetry and intracellular uptake of CeO2 nanoparticles. Nanomaterials 2020, 10, article number 2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott JT; Rosslein M; Song NW; Toman B; Kinsner-Ovaskainen A; Maniratanachote R; Salit ML; Petersen EJ; Sequeira F; Romsos EL; Kim SJ; Lee J; von Moos NR; Rossi F; Hirsch C; Krug HF; Suchaoin W; Wick P, Toward Achieving Harmonization in a Nanocytotoxicity Assay Measurement Through an Interlaboratory Comparison Study. Altex-Alt. Anim. Exper. 2017, 34 (2), 201–218. [DOI] [PubMed] [Google Scholar]
- 5.Petersen EJ; Nguyen AD; Brown J; Elliott JT; Clippinger AJ; Gordon J; Kleinstreuer N; Roesslein M, Characteristics to consider when selecting a positive control material for an in vitro assay. ALTEX - Alternatives to animal experimentation 2021, in press. [DOI] [PubMed] [Google Scholar]