Abstract
Acute kidney injury (AKI) is a major renal disease characterized by a sudden decrease in kidney function. After AKI, the kidney has the ability to repair, but if the initial injury is severe the repair may be incomplete or maladaptive and result in chronic kidney problems. Autophagy is a highly conserved pathway to deliver intracellular contents to lysosomes for degradation. Autophagy plays an important role in maintaining renal function and is involved in the pathogenesis of renal diseases. Autophagy is activated in various forms of AKI and acts as a defense mechanism against kidney cell injury and death. After AKI, autophagy is maintained at a relatively high level in kidney tubule cells during maladaptive kidney repair but the role of autophagy in maladaptive kidney repair has been controversial. Nonetheless, recent studies have demonstrated that autophagy may contribute to maladaptive kidney repair after AKI by inducing tubular degeneration and promoting a profibrotic phenotype in renal tubule cells. In this review, we analyze the role and regulation of autophagy in kidney injury and repair and discuss the therapeutic strategies by targeting autophagy.
Keywords: Autophagy, Acute kidney injury, Maladaptive repair, Fibrosis, Chronic kidney disease
Highlights.
Autophagy is activated rapidly in renal tubular cells during AKI as an intrinsic protective mechanism.
During AKI, autophagy protects renal tubules by maintaining cellular homeostasis including the clearance of damaged mitochondria via mitophagy.
After AKI, autophagy remains high in stressed renal tubules and contributes to maladaptive kidney repair and renal fibrogenesis.
During maladaptive kidney repair, persistent autophagy promotes a pro-fibrotic phenotype in renal tubular cells leading to the production and secretion of pro-fibrotic cytokines for renal interstitial fibrosis.
Background
Acute kidney injury (AKI) is a medical syndrome defined by a sudden loss of kidney function, which has become a common public health problem worldwide [1]. The common causes of AKI include ischemic AKI, sepsis and nephrotoxins. A characteristic pathological feature of AKI is renal tubular injury and cell death [2,3]. After AKI, surviving tubular cells have the capacity to regenerate and repair injured kidney tubules. Complete kidney repair would result in fully recovered renal tubules and renal function. However, if the initial injury is too severe, the repair may be incomplete or maladaptive. Maladaptive kidney repair is characterized by tubular atrophy and degeneration, chronic inflammation, interstitial fibrosis and possible progressive decrease of renal function (chronic kidney disease) [4–10]. The development of AKI and maladaptive kidney repair is a multifactorial process involving virtually all parenchymal and mesenchymal cell types in the kidney and various molecular mediators and signaling pathways.
Autophagy is a highly conserved pathway for the delivery of cytoplasmic contents to lysosomes for degradation. Autophagy degradation substrates include damaged organelles, protein aggregates and other macromolecules within cells. According to the pathway of degradation, autophagy is broadly classified into three categories: macroautophagy (hereafter termed ‘autophagy’), microautophagy and chaperone-mediated autophagy. Autophagy is a dynamic and continuous process involving initiation, nucleation, expansion, fusion and degradation. Although autophagy was originally thought to be regulated by nutrient/energy-sensing pathways, including mammalian target of rapamycin (mTOR), AMP-activated protein kinases (AMPK) and sirtuins (SIRTs), now autophagy is well known to be induced by a variety of cellular stresses such as intracellular reactive oxygen species (ROS), endoplasmic reticulum (ER) stress, hypoxia, DNA damage and immune signaling [11–17].
Since the first reports about autophagy in AKI [18,19], numerous studies have shown that autophagy is activated in AKI as an intrinsic protective mechanism. After AKI, autophagy also plays a key role in kidney repair. This review analyzes recent understanding of the role and regulation of autophagy in kidney injury and repair.
Review
Autophagy in AKI
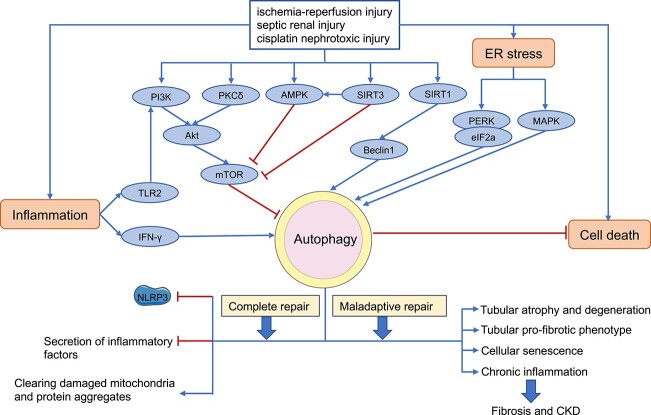
The first evidence of autophagy activation in AKI was reported in 2008 in a model of cisplatin-induced acute nephrotoxocity [18,19]. Subsequent research further demonstrated autophagy activation in renal tubule cells in other types of AKI including renal ischemia/reperfusion injury and septic or endotoxic AKI [20–22]. Despite initial debate regarding the role of autophagy in AKI, three studies published in 2012 proved unequivocally that autophagy plays a protective role against tubular damage in AKI [21–23]. Consistently, these studies demonstrated that autophagy deficiency in kidney tubules exaggerates both ischemic and nephrotoxic AKI in mice. During the acute phase of injury, autophagy is activated to remove damaged mitochondria and toxic DNA/protein aggregates to protect cells by cooperating with other stress response mechanisms, such as oxidative stress, inflammatory responses and ER stress. The role and regulation of autophagy in different etiologies of AKI are presented in detail below (Figure 1).
Figure 1.
Overview of autophagy in acute kidney injury and subsequent kidney repair Akt AKT serine/threonine kinase, AMPK AMP-activated protein kinases, ER endoplasmic reticulum, mTOR mammalian target of rapamycin, PI3K phosphoinositide 3-kinase, SIRT3 sirtuin-3, TLR2 toll-like receptors 2, IFN- γ interferon-γ, PKC δ protein kinase C δ, PERK endoplasmic reticulum kinase, CKD chronic kidney disease
Autophagy in renal ischemic AKI
Ischemic AKI is a leading cause of AKI and delayed recovery of renal function after kidney transplantation [24,25]. The changes in autophagy in ischemic AKI may vary depending on the models or experimental conditions. Autophagy was shown to be inhibited during ischemic AKI in a couple of studies [26,27], but the majority of studies demonstrated autophagy activation in ischemic AKI [20,23,28]. It has been suggested that the severity of ischemic AKI may determine whether autophagy is suppressed or activated. In rats, decreased autophagy and activation of mTOR may be associated with impairment of renal function in mild ischemic AKI, whereas autophagy was activated in severe ischemic AKI [27]. Functionally, specific knockout of autophagy-related genes (ATG) from kidney tubules led to autophagy deficiency in kidneys, which exaggerated ischemic AKI [21,23]. Moreover, pharmacological inhibitors of autophagy, including 3-methylpurine and chloroquine, enhanced kidney damage in ischemic AKI, whereas rapamycin (an mTOR inhibitor) activated autophagy and attenuated ischemic AKI [23,29]. Together, these observations indicate that autophagy acts as a cellular defense or protective mechanism against ischemic AKI, a conclusion that is consistent with the initial studies reporting autophagy in AKI [18,19]. These findings support the therapeutic potential of autophagy activation for the treatment of ischemic AKI.
In ischemic AKI, autophagy can be activated through various pathways, including the activation of autophagy regulatory pathways such as AMPK, SIRT1 and Beclin1 [30–32]. In addition, autophagy may interact with ER stress, hypoxia-inducible factor-1α (HIF-1α) and inflammatory responses in ischemic AKI. During the treatment of renal tubular cells with oxidants and chemical hypoxia, ER stress can promote the activation of autophagy, and inhibition of autophagy with chloroquine can aggravate cell death [33]. Lipoprotein-2 (Lcn2)-deficient mice have impaired renal function and attenuated autophagy in ischemic AKI. Recombinant Lcn2 attenuated hypoxia-induced apoptosis of renal proximal tubular cells and activation of NF-κB subunit p65 in vitro, and down-regulation of HIF-1α attenuated autophagy activation and inhibition of apoptosis by Lcn2 [34]. These studies suggest that ischemia-hypoxic injury may activate autophagy in kidneys through direct and indirect effects, thereby maintaining cellular homeostasis under the stress condition.
Autophagy in cisplatin-induced nephrotoxicity
Cisplatin is a broad-spectrum chemotherapy drug commonly used for the treatment of solid tumors but it has obvious toxic effects on the kidneys and often leads to AKI. Clinically, ~20–35% of the cancer patients receiving cisplatin develop AKI within days, which is one of the main reasons for limiting the use of cisplatin. The main damage site of cisplatin in the kidney is the proximal tubules, involving a variety of molecules and mechanisms, such as ROS-mediated signal transduction pathways, DNA damage, mitochondrial dysfunction, inflammation, ER stress and autophagy [23,35–39]. Multiple pathways mediate cellular damage by acting individually or intersecting with each other. Among them, autophagy plays an important regulatory role. In 2008, we reported that cisplatin treatment induced a rapid activation of autophagy in cultured rat kidney proximal tubular cells [18]. Similar results were shown by Kaushal and colleagues [19]. We further detected the formation of autophagosomes in mouse kidneys after acute cisplatin nephrotoxicity [18]. Importantly, inhibition of autophagy pharmacologically or genetically enhanced rat kidney proximal tubular cell apoptosis during cisplatin treatment [18,19], suggesting a protective role of autophagy in cisplatin-induced AKI. To further prove the role of autophagy in vivo, we and others established autophagy-deficient mouse models in which autophagy-related genes were knocked out from kidney tubule cells and found that these mice suffered more severe AKI after cisplatin treatment [22,23]. Mechanistically, we showed these autophagy-deficient mice had significantly more damaged mitochondria, ROS in the cytoplasm, p53 and activation of stress kinases such as c-Jun N-terminal kinases [23]. These results suggest that autophagy is activated in renal tubular cells as an intrinsic protective mechanism to counteract AKI by clearing damaged mitochondria and toxic DNA/protein aggregates.
The mechanism of autophagy activation during cisplatin-induced AKI remains poorly understood. It has been reported that cisplatin increases intrarenal interferon-γ (IFN-γ) in mice, which may accelerate autophagic flux to combat kidney injury [40]. In this regard, CD3(+) T cells and Ly-6G neutrophils were shown to be the primary sources of IFN-γ. Toll-like receptors are an important part of the innate immune response. Cisplatin treatment may activate toll-like receptors 2 in renal tubular cells, which further activates autophagy via the phosphorylate phosphoinositide 3-kinase (PI3K)/AKT serine/threonine kinase (Akt) signaling pathway to protect against cisplatin-induced AKI [41]. Cisplatin-induced intrarenal inflammation can also participate in the response through autophagy. In addition, the major regulators of autophagy, especially AMPK and mTOR, have been implicated in autophagy activation during cisplatin-induced AKI [42]. Interestingly, cisplatin treatment may also activate autophagy suppressive signaling pathways. For example, protein kinase C δ (PKC δ) is activated during cisplatin treatment of renal tubular cells as well as mice, and upon activation PKC δ can phosphorylate and activate Akt, which further activates mTOR to suppress autophagy. Therefore, multiple regulatory mechanisms are involved in autophagy activation during cisplatin-induced AKI.
Autophagy in septic AKI
Sepsis is a syndrome characterized by a robust systemic inflammatory response to infection accompanied by organ failure, and the kidney is a vital target organ of sepsis. AKI occurs in ~50% of sepsis patients and is associated with 40% of intensive care unit mortality [43,44]. Sepsis causes renal vasoconstriction and ischemia or decreased renal tissue perfusion, and decreased glomerular filtration rate, leading to a rapid decline in renal function. In addition, it has been suggested that during sepsis, the whole body’s blood is full of cytokines, chemokines and complement fragments. During renal filtration, these molecules act in the ultrafiltrate on the surface of the tubule lumen, causing toxic effects on tubular cells [45]. Autophagy has been reported to have marked changes in sepsis-induced AKI. There is a report that autophagy was inhibited at the early time-point of 4 h in lipopolysaccharide (LPS)-induced endotoxic AKI in rats [46]. However, our 2016 study demonstrated a dynamic change of autophagy in LPS-induced AKI in mice. Specifically, autophagy increased at 8 h, peaked after 24 h and then gradually decreased to basal levels in kidney tubule cells after LPS injection in mice [47]. Also LPS may affect autophagy dose-dependently. For example, in mouse heart cells, LPS induced autophagy at low concentrations but inhibited autophagy at high levels [48]. The inhibition of autophagy by a high concentration of LPS may be related to the activation of mTOR signaling [48]. After the cells are severely injured, the anabolism in the cytoplasm may be mobilized, accompanied by mTOR activation, which inhibits autophagy. Autophagy has also been examined in the sepsis model of cecal ligation and puncture (CLP) in rats, where autophagy was significantly activated 6–8 h after CLP, but the level of autophagy was also significantly decreased at 24 h [49], which is consistent with our observation of the dynamic changes in autophagy during LPS treatment [47].
We showed that pharmacological inhibition of autophagy worsened LPS-induced AKI in mice. Moreover, LPS induced more severe AKI in renal tubule autophagy-deficient mice [47], suggesting a protective role of tubular autophagy in septic AKI. Consistently, rapamycin activated autophagy and attenuated renal injury in CLP-treated rats [49]. Also dexmedetomidine can activate autophagy through the PI3K/AKT/mTOR pathway to protect against sepsis-induced kidney injury [46]. SIRT3 can activate autophagy and protect CLP by up-regulating p-AMPK- and down-regulating p-mTOR-induced AKI, while SIRT1 exerts renal protection by upregulating autophagy through deacetylating Beclin1 [50,51]. In conclusion, there is compelling evidence for the protective role of autophagy in septic AKI.
Mitophagy as a major mechanism in the protective effect of autophagy in AKI
Autophagy can engulf a portion of cytoplasm randomly in a ‘non-selective’ fashion, but it can also specifically recognize and take up a particular cargo for degradation in a way called selective autophagy [52]. Mitophagy is an important form of selective autophagy that selectively removes excess and defective mitochondria. Research in recent years has proved mitophagy to be a major mechanism underlying the protective effect of autophagy in AKI [53,54]. Mitophagy is mainly mediated by LC3-associated autophagy receptors via ubiquitin (Ub)-dependent and Ub-independent pathways. In the Ub-dependent pathway, PTEN-induced kinase 1 (PINK1) recruits the E3 ubiquitin ligase Parkin to mitochondria, which ubiquitylates mitochondrial proteins for recognition by mitophagy receptors. In the Ub-independent pathway, mitophagy receptors, such as BCL2-interacting protein 3 (BNIP3) and FUN14 domain-containing protein 1 (FUNDC1), are induced on the mitochondrial outer membrane to link the target mitochondria to autophagosomes.
We and others detected significant mitophagy activation in AKI from various etiologies, including ischemia, sepsis and nephrotoxicity, by cisplatin and contrast agents [55–58]. Functionally, activation of mitophagy in AKI was partially abrogated in PINK1 and/or Parkin knockout mice, and the lack of PINK1/Parkin RBR E3 ubiquitin protein ligase (PARK2)-dependent mitophagy led to aggravation of kidney injury [55,56,59]. In cisplatin-treated HK-2 human renal proximal tubular cells in vitro, overexpression of PINK1 and PARK2 attenuated cell apoptosis by inducing mitophagy, providing further support for the protective role of PINK/PARK2-dependent mitophagy [60]. Ub-independent mitophagy has also been shown in AKI. In this regard, we reported that Bnip3-deficincey reduced mitophagy in both in vivo and in vitro models of ischemic AKI, which resulted in the accumulation of damaged mitochondria and increased production of ROS, accompanied by exaggerated kidney damage [61]. Conversely, adenovirus-mediated overexpression of BNIP3 significantly enhanced mitophagy and prevented kidney injury [62]. Ischemic preconditioning (IPC) is known to have protective effects against subsequent injury, which was recently shown to involve the clearance of damaged mitochondria via mitophagy [63]. Subsequent work showed that IPC-mediated protection was diminished in proximal tubule-specific FUNDC1 knockdown mice, suggesting a critical role of Fundc1-mediated mitophagy in the protective effect of IPC [64]. Together, these studies indicate that mitophagy contributes critically to the protective effects of autophagy in AKI.
Damaged mitochondria are not only low in function but release cell death promoting factors such as cytochrome c, which induce the formation of apoptosomes for caspase activation with subsequent apoptosis. In addition, damaged mitochondria generate excessive, cytotoxic ROS. Consistently, in sepsis-induced AKI, mitophagy protected against kidney injury by inhibiting mitochondrial dysfunction and activating the NLRP3 inflammasome [58]. In contrast-induced AKI, Pink1/Parkin-mediated mitophagy prevented tubular apoptosis and tissue injury by reducing mitochondrial ROS and subsequent NLRP3 inflammasome activation [58]. In addition, mitophagy may contribute to the protective effects of inhibiting the NLRP3 inflammasome in contrast-induced AKI [65]. In conclusion, mitophagy protects by removing damaged mitochondria and the associated release or production of toxic molecules and inflammation.
Autophagy in kidney repair
After AKI, the kidney has the capacity to repair the injured tubules. However, if the initial damage is too severe or persistent, tubular repair may be maladaptive and characterized by cell cycle arrest, cell death, senescence and continuous inflammatory response, leading to tubular atrophy and degradation, renal interstitial fibrosis and a decline in kidney function. Autophagy has been implicated in maladaptive kidney repair, but it has been controversial as to whether autophagy prevents or promotes maladaptive repair. Several studies support that autophagy still serves as a protective mechanism to scavenge overproduced collagens, reduce oxidative stress and inhibit endothelial-to-mesenchymal transition, and as a result, to prevent renal fibrosis [66–68]. However, other studies have found that sustained activation of autophagy during kidney repair promotes a pro-fibrotic phenotype in kidney tubular cells including senescence and the secretion of pro-inflammatory and pro-fibrotic cytokines [69–71]. Therefore, the role and regulation of autophagy in maladaptive kidney repair may vary in different animal models and disease courses (Figure 1).
Autophagy in kidney repair after ischemic AKI
The role of autophagy in kidney repair after ischemic AKI has been hotly debated. On the one hand, autophagy has been shown to reduce oxidative stress and accumulation of Collagen I to suppress renal fibrosis [72,73]. After ischemic AKI, HIF-1 is significantly activated and can act as a transcription factor to activate factor forkhead box O-3, which was suggested to reduce fibrosis by maintaining autophagy in tubule cells [72]. It has also been shown that αKlotho-overexpressing mice have higher autophagic flux after ischemic AKI than heterozygous αKlotho-hypomorphic mice, which protects kidney cells from oxidative stress damage and Collagen I accumulation by upregulating autophagy, thereby reducing the degree of renal fibrosis [73]. On the other hand, continuous activation of autophagy after ischemic AKI can promote the process of renal fibrosis by promoting a secretory, pro-fibrotic phenotype in kidney tubule cells. Mice with autophagy-related gene 5 (Atg5) knockout from proximal tubules had significantly lower levels of tubular senescence and interstitial fibrosis 30 days after ischemic AKI than wild type mice [74]. Our recent work [70] further verified the role of autophagy in maladaptive kidney repair by using an inducible kidney tubule Atg7-knockout mouse model. In this model, tubular autophagy deficiency after initial ischemic AKI prevented the development of the pro-fibrotic phenotype in kidney tubule cells, including tubular degeneration, senescence and G2/M cell cycle arrest, and as a result, suppressed renal fibrosis. Importantly, persistent autophagy in kidney tubules was shown to specifically induce the expression and secretion of fibroblast growth factor 2, which acts as a paracrine factor to stimulate interstitial fibroblasts for renal fibrogenesis in post-AKI kidneys [70]. In line with these findings, Canaud et al. demonstrated the formation of mTOR-autophagy spatial coupling compartment in post-ischemic kidneys, which led to the production of various pro-fibrotic factors to promote renal fibrosis and maladaptive kidney repair [69]. In addition, Kim et al. showed that at 28 days after ischemic AKI, farnesoid X receptor knockout mice exhibited increased renal autophagic flux and higher levels of fibrosis-related proteins and ROS than wild-type mice, which may result from kidney cell death due to persistent excessive autophagy [75]. In conclusion, despite some controversies, recent studies demonstrate that persistent autophagy in post-ischemic kidneys promotes maladaptive kidney repair and renal fibrosis mainly by inducing tubular degeneration and a pro-fibrotic secretory phenotype in renal tubule cells.
Autophagy in kidney repair after cisplatin nephrotoxicity
One injection of a high dose of cisplatin has been used to study acute nephrotoxicity of cisplatin, however it induces significant animal death, preventing the investigation of long-term effects or maladaptive repair in kidneys. In 2011, we established a model of repeated low-dose cisplatin treatment (RLDC) in mice [76], which allowed long-term animal survival. This model was adopted or modified for investigating maladaptive kidney repair after cisplatin nephrotoxicity [77–80]. We and the Siskind lab investigated the role of autophagy in maladaptive kidney repair after RLDC treatment in mice [71,81]. In both studies, pharmacological inhibitors of autophagy given after RLDC treatment improved kidney function and reduced renal fibrosis. We further showed that autophagy inhibition attenuated RLDC-induced secretion of pro-fibrotic factors in cultured renal tubular cells in vitro [71]. Shi et al. recently examined the effects of Beclin1 (Atg 6) and Beclin1 peptide on AKI and subsequent kidney repair. In their study, the mice with high Beclin1 had higher autophagic flux and less renal interstitial fibrosis during the repair stage of cisplatin nephrotoxicity, suggesting that Beclin1 and related autophagy may suppress renal fibrosis and improve kidney repair [82]. It is noteworthy that Beclin1 had notable protective effects against initial kidney injury in this study, which was likely to account for the observed effects on kidney repair. In addition, Beclin1 is a B-cell lymphoma 2 family protein that affects cell death in AKI and kidney repair and therefore the observed effects of Beclin1 or its mimic peptide may not be autophagy-dependent. Regardless, it is currently appreciated that autophagy contributes to maladaptive kidney repair and fibrogenesis after cisplatin nephrotoxicity, leading to the development of chronic kidney problems.
Conclusions
Autophagy is activated during AKI as an adaptive response in renal tubule cells. Activated autophagy helps the cells remove damaged organelles and toxic protein aggregates to maintain cellular homeostasis, viability and function. Therapeutically, enhancing the activity of autophagy may protect kidneys against AKI. The beneficial effects of autophagy inducers, such as rapamycin, trehalose and resveratrol, have been demonstrated in AKI models. After AKI, autophagy decreases, but if the initial injury is severe, autophagy may be maintained at a relatively high level during maladaptive kidney repair. The role of autophagy in maladaptive kidney repair has been debated for some time. However, several recent studies have provided evidence that persistent autophagy after AKI may promote maladaptive kidney repair by inducing tubular degeneration and a pro-fibrotic phenotype in tubular cells for renal fibrosis, associated with the decline of renal function. Currently, neither autophagy activators nor its inhibitors have been tested for clinical application for AKI treatment or improving kidney repair. The main reason is that it is hard to determine when to activate autophagy. We wish to activate autophagy before AKI or during the early stage of AKI, but clinically, injury and repair phases are not easy to separate. In addition, autophagy is a dynamic process, and the initiation, progression and completion of autophagy require the coordination of multiple signaling pathways in cells. Using an activator or inhibitor of a pathway alone may have off-target effects on other biological processes. For example, rapamycin can activate autophagy and protect against AKI but it may also prevent tubular cell proliferation and kidney repair. Future work should identify specific activators and inhibitors of autophagy for therapeutic use.
Abbreviations
AKI: Acute kidney injury; Akt: AKT serine/threonine kinase; AMPK: AMP-activated protein kinases; ATG: Autophagy-related protein; BNIP3: BCL2-interacting protein 3; CLP: Cecal ligation and puncture; ER: Endoplasmic reticulum; Fundc1: FUN14 domain-containing protein 1; HIF-1α: Hypoxia-inducible factor-1α; IPC: Ischemic preconditioning; Lcn2: Lipoprotein-2; LPS: Lipopolysaccharide; mTOR: Mammalian target of rapamycin; PARK2: Parkin RBR E3 ubiquitin protein ligase; PINK: PTEN-induced kinase 1; PI3K: Phosphoinositide 3-kinase; RLDC: Repeated low-dose cisplatin-induced fibrosis; ROS: Reactive oxygen species; SIRT1: Sirtuin-1. LC3: Microtubule-associated protein light chain 3; PTEN: phosphatase and tensin homolog; NF-κB: the nuclear factor ĸB.
Funding
The work was supported partly by grants from the National Natural Science Foundation of China (81720108008, 82090024), the National Key R&D Program of China (2020YFC2005000).
Authors’ contributions
YX analyzed the literature and prepared the initial draft of this review. YF, WW and CT participated in literature collection and analysis. ZD guided the writing and revised the review. All authors read and approved the final manuscript.
Conflicts of interest
The authors declare that they have no competing interests.
Contributor Information
Yu Xiang, Department of Nephrology, Hunan Key Laboratory of Kidney Disease and Blood Purification, The Second Xiangya Hospital at Central South University, Changsha 410000, Hunan Province, China.
Ying Fu, Department of Nephrology, Hunan Key Laboratory of Kidney Disease and Blood Purification, The Second Xiangya Hospital at Central South University, Changsha 410000, Hunan Province, China.
Wenwen Wu, Department of Nephrology, Hunan Key Laboratory of Kidney Disease and Blood Purification, The Second Xiangya Hospital at Central South University, Changsha 410000, Hunan Province, China.
Chengyuan Tang, Department of Nephrology, Hunan Key Laboratory of Kidney Disease and Blood Purification, The Second Xiangya Hospital at Central South University, Changsha 410000, Hunan Province, China.
Zheng Dong, Department of Nephrology, Hunan Key Laboratory of Kidney Disease and Blood Purification, The Second Xiangya Hospital at Central South University, Changsha 410000, Hunan Province, China; Department of Cellular Biology and Anatomy, Medical College of Georgia at Augusta University and Charlie Norwood VA Medical Center, Augusta, GA 30912, USA.
References
- 1. Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders H-J. Acute kidney injury. Nat Rev Dis Primers. 2021;7:52. 10.1038/s41572-021-00284-z. [DOI] [PubMed] [Google Scholar]
- 2. Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z. Regulated cell death in AKI. J Am Soc Nephrol. 2014;25:2689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maremonti F, Meyer C, Linkermann A. Mechanisms and models of kidney tubular necrosis and nephron loss. J Am Soc Nephrol. 2022;33:472–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu SMW, Bonventre JV. Acute kidney injury and maladaptive tubular repair leading to renal fibrosis. Curr Opin Nephrol Hypertens. 2020;29:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Djudjaj S, Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol Asp Med. 2019;65:16–36. [DOI] [PubMed] [Google Scholar]
- 7. Basile DP, Bonventre JV, Mehta R, Nangaku M, Unwin R, Rosner MH, et al. Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol. 2016;27:687–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Humphreys BD. Mechanisms of renal fibrosis. Annu Rev Physiol. 2018;80:309–26. [DOI] [PubMed] [Google Scholar]
- 9. Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol. 2015;26:1765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li L, Fu H, Liu Y. The fibrogenic niche in kidney fibrosis: components and mechanisms. Nat Rev Nephrol. 2022;18:545–57. [DOI] [PubMed] [Google Scholar]
- 11. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–41. [DOI] [PubMed] [Google Scholar]
- 12. Abada A, Elazar Z. Getting ready for building: signaling and autophagosome biogenesis. EMBO Rep. 2014;15:839–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol. 2013;14:759–74. [DOI] [PubMed] [Google Scholar]
- 14. Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21:183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441:523–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ravanan P, Srikumar IF, Talwar P. Autophagy: the spotlight for cellular stress responses. Life Sci. 2017;188:53–67. [DOI] [PubMed] [Google Scholar]
- 17. Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Periyasamy-Thandavan S, Jiang M, Wei Q, Smith R, Yin XM, Dong Z. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int. 2008;74:631–40. [DOI] [PubMed] [Google Scholar]
- 19. Yang C, Kaushal V, Shah SV, Kaushal GP. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol. 2008;294:F777–87. [DOI] [PubMed] [Google Scholar]
- 20. Jiang M, Liu K, Luo J, Dong Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol. 2010;176:1181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu S, Hartleben B, Kretz O, Wiech T, Igarashi P, Mizushima N, et al. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy. 2012;8:826–37. [DOI] [PubMed] [Google Scholar]
- 22. Takahashi A, Kimura T, Takabatake Y, Namba T, Kaimori J, Kitamura H, et al. Autophagy guards against cisplatin-induced acute kidney injury. Am J Pathol. 2012;180:517–25. [DOI] [PubMed] [Google Scholar]
- 23. Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82:1271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shiva N, Sharma N, Kulkarni YA, Mulay SR, Gaikwad AB. Renal ischemia/reperfusion injury: An insight on in vitro and in vivo models. Life Sci. 2020;256:117860. [DOI] [PubMed] [Google Scholar]
- 25. Zhao H, Alam A, Soo AP, George AJT, Ma D. Ischemia-reperfusion injury reduces long term renal graft survival: mechanism and beyond. EBioMedicine. 2018;28:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lempiäinen J, Finckenberg P, Mervaala EE, Sankari S, Levijoki J, Mervaala EM. Caloric restriction ameliorates kidney ischaemia/reperfusion injury through PGC-1α-eNOS pathway and enhanced autophagy. Acta Physiol (Oxf). 2013;208:410–21. [DOI] [PubMed] [Google Scholar]
- 27. Decuypere J-P, Hutchinson S, Monbaliu D, Martinet W, Pirenne J, Jochmans I. Autophagy dynamics and modulation in a rat model of renal ischemia-reperfusion injury. Int J Mol Sci. 2020;21:7185. 10.3390/ijms21197185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kimura T, Takabatake Y, Takahashi A, Kaimori J-y, Matsui I, Namba T, et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol. 2011;22:902–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Y-L, Zhang J, Cui L-Y, Yang S. Autophagy activation attenuates renal ischemia-reperfusion injury in rats. Exp Biol Med (Maywood). 2015;240:1590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu T, Guo J, Wei M, Wang J, Yang K, Pan C, et al. Aldehyde dehydrogenase 2 protects against acute kidney injury by regulating autophagy via the Beclin-1 pathway. JCI. Insight. 2021;6:e138183. 10.1172/jci.insight.138183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gong L, He J, Sun X, Li L, Zhang X, Gan H. Activation of sirtuin1 protects against ischemia/reperfusion-induced acute kidney injury. Biomed Pharmacother. 2020;125:110021. [DOI] [PubMed] [Google Scholar]
- 32. Wang L-T, Chen B-L, Wu C-T, Huang K-H, Chiang C-K, Hwa Liu S. Protective role of AMP-activated protein kinase-evoked autophagy on an in vitro model of ischemia/reperfusion-induced renal tubular cell injury. PLoS One. 2013;8:e79814. 10.1371/journal.pone.0079814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chandrika BB, Yang C, Ou Y, Feng X, Muhoza D, Holmes AF, et al. Endoplasmic reticulum stress-induced autophagy provides Cytoprotection from chemical hypoxia and oxidant injury and ameliorates renal ischemia-reperfusion injury. PLoS One. 2015;10:e0140025. 10.1371/journal.pone.0140025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qiu S, Chen X, Pang Y, Zhang Z. Lipocalin-2 protects against renal ischemia/reperfusion injury in mice through autophagy activation mediated by HIF1α and NF-κb crosstalk. Biomed Pharmacother. 2018;108:244–53. [DOI] [PubMed] [Google Scholar]
- 35. Mapuskar KA, Wen H, Holanda DG, Rastogi P, Steinbach E, Han R, et al. Persistent increase in mitochondrial superoxide mediates cisplatin-induced chronic kidney disease. Redox Biol. 2019;20:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pabla N, Dong Z. Dong Z: cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. [DOI] [PubMed] [Google Scholar]
- 37. Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int. 2014;2014:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Volarevic V, Djokovic B, Jankovic MG, Harrell CR, Fellabaum C, Djonov V, et al. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J Biomed Sci. 2019;26:25. 10.1371/journal.pone.0140025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Y, Liu K, Xie X, Song B. Contrast-associated acute kidney injury: An update of risk factors, risk factor scores, and preventive measures. Clin Imaging. 2021;69:354–62. [DOI] [PubMed] [Google Scholar]
- 40. Kimura A, Ishida Y, Inagaki M, Nakamura Y, Sanke T, Mukaida N, et al. Interferon-γ is protective in cisplatin-induced renal injury by enhancing autophagic flux. Kidney Int. 2012;82:1093–104. [DOI] [PubMed] [Google Scholar]
- 41. Shen Q, Zhang X, Li Q, Zhang J, Lai H, Gan H, et al. TLR2 protects cisplatin-induced acute kidney injury associated with autophagy via PI3K/Akt signaling pathway. J Cell Biochem. 2019;120:4366–74. [DOI] [PubMed] [Google Scholar]
- 42. Wang Y, Liu Z, Shu S, Cai J, Tang C, Dong Z. AMPK/mTOR Signaling in autophagy regulation during cisplatin-induced acute kidney injury. Front Physiol. 2020;11:619730. 10.3389/fphys.2020.619730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Poukkanen M, Vaara ST, Pettilä V, Kaukonen KM, Korhonen AM, Hovilehto S, et al. Acute kidney injury in patients with severe sepsis in Finnish intensive care units. Acta Anaesthesiol Scand. 2013;57:863–72. [DOI] [PubMed] [Google Scholar]
- 44. Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43:816–28. [DOI] [PubMed] [Google Scholar]
- 45. Gómez H, Kellum JA. Sepsis-induced acute kidney injury. Curr Opin Crit Care. 2016;22:546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao Y, Feng X, Li B, Sha J, Wang C, Yang T, et al. Dexmedetomidine protects against lipopolysaccharide-induced acute kidney injury by enhancing autophagy through inhibition of the PI3K/AKT/mTOR pathway. Front Pharmacol. 2020;11:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mei S, Livingston M, Hao J, Li L, Mei C, Dong Z. Autophagy is activated to protect against endotoxic acute kidney injury. Sci Rep. 2016;6:22171. 10.1038/srep22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sun Y, Yao X, Zhang Q-J, Zhu M, Liu Z-P, Ci B, et al. Beclin-1-dependent autophagy protects the heart during sepsis. Circulation. 2018;138:2247–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sunahara S, Watanabe E, Hatano M, Swanson PE, Oami T, Fujimura L, et al. Influence of autophagy on acute kidney injury in a murine cecal ligation and puncture sepsis model. Sci Rep. 2018;8:1050. 10.1038/s41598-018-19350-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhao W, Zhang L, Chen R, Lu H, Sui M, Zhu Y, et al. SIRT3 protects against acute kidney injury via AMPK/mTOR-regulated autophagy. Front Physiol. 2018;9:1526. 10.3389/fphys.2018.01526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Deng Z, Sun M, Wu J, Fang H, Cai S, An S, et al. SIRT1 attenuates sepsis-induced acute kidney injury via Beclin1 deacetylation-mediated autophagy activation. Cell Death Dis. 2021;12:217. 10.1038/s41419-021-03508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gatica D, Lahiri V, Klionsky DJ. Cargo recognition and degradation by selective autophagy. Nat Cell Biol. 2018;20:233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tang C, Cai J, Yin XM, Weinberg JM, Venkatachalam MA, Dong Z. Mitochondrial quality control in kidney injury and repair. Nat Rev Nephrol. 2021;17:299–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Su L, Zhang J, Gomez H, Kellum JA, Peng Z. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy. 2022;1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang Y, Tang C, Cai J, Chen G, Zhang D, Zhang Z, et al. PINK1/parkin-mediated mitophagy is activated in cisplatin nephrotoxicity to protect against kidney injury. Cell Death Dis. 2018;9:1113. 10.1038/s41419-018-1152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tang C, Han H, Yan M, Zhu S, Liu J, Liu Z, et al. PINK1-PRKN/PARK2 pathway of mitophagy is activated to protect against renal ischemia-reperfusion injury. Autophagy. 2018;14:880–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang Y, Zhu J, Liu Z, Shu S, Fu Y, Liu Y, et al. The PINK1/PARK2/optineurin pathway of mitophagy is activated for protection in septic acute kidney injury. Redox Biol. 2021;38:101767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lin Q, Li S, Jiang N, Shao X, Zhang M, Jin H, et al. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 2019;26:101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gao Y, Dai X, Li Y, Li G, Lin X, Ai C, et al. Role of parkin-mediated mitophagy in the protective effect of polydatin in sepsis-induced acute kidney injury. J Transl Med. 2020;18:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhao C, Chen Z, Xu X, An X, Duan S, Huang Z, et al. Pink1/parkin-mediated mitophagy play a protective role in cisplatin induced renal tubular epithelial cells injury. Exp Cell Res. 2017;350:390–7. [DOI] [PubMed] [Google Scholar]
- 61. Tang C, Han H, Liu Z, Liu Y, Yin L, Cai J, et al. Activation of BNIP3-mediated mitophagy protects against renal ischemia-reperfusion injury. Cell Death Dis. 2019;10:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fu Z-J, Wang Z-Y, Xu L, Chen X-H, Li X-X, Liao W-T, et al. HIF-1α-BNIP3-mediated mitophagy in tubular cells protects against renal ischemia/reperfusion injury. Redox Biol. 2020;36:101671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Livingston MJ, Wang J, Zhou J, Wu G, Ganley IG, Hill JA, et al. Clearance of damaged mitochondria via mitophagy is important to the protective effect of ischemic preconditioning in kidneys. Autophagy. 2019;15:2142–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang J, Zhu P, Li R, Ren J, Zhou H. Fundc1-dependent mitophagy is obligatory to ischemic preconditioning-conferred renoprotection in ischemic AKI via suppression of Drp1-mediated mitochondrial fission. Redox Biol. 2020;30:101415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lin Q, Li S, Jiang N, Jin H, Shao X, Zhu X, et al. Inhibiting NLRP3 inflammasome attenuates apoptosis in contrast-induced acute kidney injury through the upregulation of HIF1A and BNIP3-mediated mitophagy. Autophagy. 2021;17:2975–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li H, Peng X, Wang Y, Cao S, Xiong L, Fan J, et al. Atg5-mediated autophagy deficiency in proximal tubules promotes cell cycle G2/M arrest and renal fibrosis. Autophagy. 2016;12:1472–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Takagaki Y, Lee SM, Dongqing Z, Kitada M, Kanasaki K, Koya D. Endothelial autophagy deficiency induces IL6 - dependent endothelial mesenchymal transition and organ fibrosis. Autophagy. 2020;16:1905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gui Z, Suo C, Wang Z, Zheng M, Fei S, Chen H, et al. Impaired ATG16L-dependent autophagy promotes renal interstitial fibrosis in chronic renal graft dysfunction through inducing EndMT by NF-κB signal pathway. Front Immunol. 2021;12:650424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Canaud G, Brooks CR, Kishi S, Taguchi K, Nishimura K, Magassa S, et al. Cyclin G1 and TASCC regulate kidney epithelial cell G2-M arrest and fibrotic maladaptive repair. Sci Transl Med. 2019;11(476):eaav4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Livingston MJ, Shu S, Fan Y, Li Z, Jiao Q, Yin X-M, et al. Tubular cells produce FGF2 via autophagy after acute kidney injury leading to fibroblast activation and renal fibrosis. Autophagy. 2023;19(1):256–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fu Y, Xiang Y, Wu W, Cai J, Tang C, Dong Z. Persistent activation of autophagy after cisplatin nephrotoxicity promotes renal fibrosis and chronic kidney disease. Front Pharmacol. 2022;13:918732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li L, Kang H, Zhang Q, D'Agati VD, Al-Awqati Q, Lin F. FoxO3 activation in hypoxic tubules prevents chronic kidney disease. J Clin Invest. 2019;129:2374–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shi M, Flores B, Gillings N, Bian A, Cho HJ, Yan S, et al. αKlotho mitigates progression of AKI to CKD through activation of autophagy. J Am Soc Nephrol. 2016;27:2331–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Baisantry A, Bhayana S, Rong S, Ermeling E, Wrede C, Hegermann J, et al. Autophagy induces Prosenescent changes in proximal tubular S3 segments. J Am Soc Nephrol. 2016;27:1609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kim D-H, Park JS, Choi H-I, Kim CS, Bae EH, Ma SK, et al. The critical role of FXR is associated with the regulation of autophagy and apoptosis in the progression of AKI to CKD. Cell Death Dis. 2021;12:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pabla N, Dong G, Jiang M, Huang S, Kumar MV, Messing RO, et al. Inhibition of PKCdelta reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J Clin Invest. 2011;121:2709–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Landau SI, Guo X, Velazquez H, Torres R, Olson E, Garcia-Milian R, et al. Regulated necrosis and failed repair in cisplatin-induced chronic kidney disease. Kidney Int. 2019;95:797–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Katagiri D, Hamasaki Y, Doi K, Negishi K, Sugaya T, Nangaku M, et al. Interstitial renal fibrosis due to multiple cisplatin treatments is ameliorated by semicarbazide-sensitive amine oxidase inhibition. Kidney Int. 2016;89:374–85. [DOI] [PubMed] [Google Scholar]
- 79. Fu Y, Cai J, Li F, Liu Z, Shu S, Wang Y, et al. Chronic effects of repeated low-dose cisplatin treatment in mouse kidneys and renal tubular cells. Am J Physiol Renal Physiol. 2019;317:F1582–92. [DOI] [PubMed] [Google Scholar]
- 80. Fu Y, Tang C, Cai J, Chen G, Zhang D, Dong Z. Rodent models of AKI-CKD transition. Am J Physiol Renal Physiol. 2018;315:F1098–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sears SM, Feng JL, Orwick A, Vega AA, Krueger AM, Shah PP, et al. Pharmacological inhibitors of autophagy have opposite effects in acute and chronic cisplatin-induced kidney injury. Am J Physiol Renal Physiol. 2022;323:F288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shi M, Maique J, Shepard S, Li P, Seli O, Moe OW, et al. In vivo evidence for therapeutic applications of beclin 1 to promote recovery and inhibit fibrosis after acute kidney injury. Kidney Int. 2022;101:63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]