Abstract
Covid-19 is transmitted mainly by respiratory droplets and as the upper airway mucosa is the first innate immune barrier, it is crucial to understand the effects of SARS-CoV-2 on this system. In the current study, we aimed to evaluate the nasal mucociliary clearance in patients with SARS-CoV-2 infection and their symptom development. Observational cross-sectional study. The nasal mucociliary clearance (NMC) time was evaluated by the saccharin test and the results were compared between patients with SARS-CoV-2 infection (group 1) and controls (group 2, asymptomatic patients with a negative polymerase chain reaction test). We also compared the NMC time for each specific symptom suffered by participants in group 1 with the NMC time of the control group as well as with the patients in group 1 who were asymptomatic. There was a significant increase in NMC time in group 1 with dyspnea when compared to the control group (p = 0.032) and also when compared to patients who were infected were not dyspneic (p = 0.04). There were no differences in the clearance times when considering other symptoms. COVID-19 patients with dyspnea present with altered nasal mucociliary clearance.
Keywords: COVID-19, SARS-CoV-2, Mucociliary clearance, Anosmia, Saccharin
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in 2019 as the cause of coronavirus disease (COVID-19). The disease has shown an important impact on global health due to its high degree of transmissibility, which has led to rapid worldwide dispersion. SARS-CoV-2 is transmitted mainly by respiratory droplets and the upper airway mucosa is the first innate immune barrier and the main route of viral entry [1].
It is known that this virus targets cells that express angiotensin-converting enzyme 2 (ACE2) receptors. ACE2 receptors are predominantly expressed by epithelial cells of the lungs, gastrointestinal tract, kidney, heart, and blood vessels; the former being the main organ affected by SARS-CoV-2. In addition to pneumonia, loss of smell (anosmia) and/or taste (hypogeusia) have been reported as common complaints in patients with SARS-CoV-2, often without concomitant nasopharyngeal symptoms. Due to their high prevalence, changes in smell and taste are potential early, and sometimes the only signs of SARS-CoV-2 infection [2]. These deficiencies modify the ability to smell odors in food and the environment, affecting quality of life, social interactions, and general well-being. Recent studies have tried to explain the main cell stages that occur in the olfactory epithelium, leading to the dysosmia initiated by SARS-CoV-2 infection [3–5]. The current theory of the mechanism of olfaction postulates that numerous odor receptors, which accumulate in the sensory cilia of the olfactory epithelium, perceive the corresponding odorants and transduce the signal to the olfactory cortex in the brain [4]. Primary cilia are organelles based on microtubules projected from the cell surface, acting as the cell’s antenna to perceive various environmental stimuli [3]. Despite olfaction having a major impact on quality of life, pulmonary involvement is, by far, the main cause for morbidity and mortality in patients infected by SARS-CoV-2 and the first innate barrier that the virus faces before reaching the lung is the mucosal covering the upper airway (nasal and oral cavity), the nasal mucociliary function of the mucosa and the salivary barrier. It has been demonstrated that the salivary immune barrier is impaired in SARS-CoV-2 patients, with altered composition of lactoferrin, IgM, and IgA [6].
Ciliary dysfunction leads to more than 35 types of diseases, which are called ciliopathies [3]. The sense of smell of humans and many other species requires the standard structure and function of olfactory cilia, and anosmia turns out to be a common feature of some types of ciliopathies. While virus-induced inflammation may disturb the Central Nervous System (CNS) and cause loss of smell, viral infection could disturb the ciliary structure [7], abolishing the ciliary location of olfactory receptors, and preventing the perception of odorous molecules. Therefore, some studies suggest that coronavirus infects hair cells on human nasal epithelium [8], resulting in deciliation or alteration in ciliary functioning [7]. In addition, recent studies on human SARS-CoV-2-protein–protein interactions [9, 10] suggest that the non-structural viral protein Nsp13 (a highly conserved protein in the coronavirus family with 100% aminoacid sequence identity of Nsp13 in SARS-CoV-2 and SARS-CoV) interacts with up to 12 components of the centrosome, providing a potential molecular bond. Another possibility is that Nsp13 is actively located in the centriole and competes with endogenous binding partners of the centrosome proteins, abolishing physiological interactions within the structure. Therefore, ectopic interactions of SARS-CoV-2 protein with the host proteins could interrupt the centriole structure or the interaction between the centriole and the cilia, which would eventually lead to deciliation.
Once mucociliary beating removes inhaled pathogenic particles and acts as the first line of protection mechanism against viral infection in human airways, understanding the effects of SARS-Cov-2 on the upper airway mucosa is essential, not only because it is the first host barrier that the virus encounters, but it may also reflect the function of the lower airway mucosa given the upper and lower airway share the same pseudostratified ciliated columnar epithelium [11].
The study’s purpose was to determine the mucociliary function using the saccharin test in patients infected with SARS-CoV-2 in order to evaluate the relationship between SARS-CoV-19 infection, the development of symptoms and mucociliary function.
Materials and Methods
This is a cross-sectional comparative study carried out between April and July, 2020, during the COVID 19 pandemic in Brazil. The study was approved by the Research Ethics Committee of the Federal University of São Paulo, under registration number 34983220.4.0000.5505. All patients included agreed to participate voluntarily by signing an informed consent form, as well as consenting to the publication of their data. This study was conducted in accordance with the ethical standards set in the Declaration of Helsinki and its subsequent amendments or comparable ethical standards.
Patients were included if they met the following criteria: firefighters with and without COVID-19 between 18 and 60 years old and who had not received a previous SARS-CoV-19 vaccine. The study was divided into two groups: group 1 (SARS-CoV-2+ patients) and group 2 (controls). Group1 consisted of patients infected by SARS-CoV-2 confirmed by a polymerase chain reaction (PCR) test. For group 2, we selected individuals asymptomatic for the last 3 months and with a negative PCR test for SARS-Cov-2 (collected 1 day before the saccharin test).
Patients were excluded from the study if they met any of the following criteria: chronically impaired ability to taste flavors, chronic diseases of the nasal mucosa (chronic rhinosinusitis with and without nasal polyposis), disorders of ciliary motility (e.g.: Cystic Fibrosis, Kartagener’s syndrome), use of systemic immunosuppressant, and severe systemic diseases.
During the study period the ancestral strain, without accumulated mutations in the Spike (S) protein was found in Brazil.
Demographic Data
The following data were collected: age, smoking status, presence of rhinitis, presence or absence of symptomatic disease, and symptoms (cough, sore throat, nasal obstruction, runny nose, impaired taste or dysgeusia, loss of smell, dyspnea and headache). All individuals, except those who had dysgeusia, performed the saccharin test to assess the efficiency of mucociliary clearance [12].
Saccharin Test
The saccharin test was carried out to assess the nasal mucociliary clearance (NMC) time. Participants were instructed to avoid eating immediately before the test was performed. Patients were instructed to sit on a chair with back support and backrest for the upper limbs, maintaining a slight cervical extension, looking at the horizon line. At the time of placing the saccharin, without using anesthetic, patients were instructed to remain apneic. A small amount of saccharin (about 25 µg) was gently introduced to the surface close to the edge of the right lower turbinate, using a small spatula. After placement, the patients’ head was repositioned and they were instructed to return to normal breathing, avoiding hyperventilation, coughing, sneezing, blowing, or hyper-inhalation. It is important to note that at the time the saccharin test was performed, no patient with dyspnea had any manipulation or intensive treatment such as nasal tubes, intubation, or oxygen therapy that could change the physiology of mucociliary transport.
NMC time, in minutes, was analyzed based on the difference between the time of saccharin placement and the time of initial perception of a sweet taste in the pharynx. The reference time for healthy individuals is approximately 10 ± 2 min. If the perception of taste did not occur after 60 min, the test was interrupted and a small amount of saccharin was placed directly on the tongue, to verify the ability to taste flavors. The taste was not informed to the participant to generate reliability of the test.
The NMC time was compared between groups 1 and 2. Within group 1, we analyzed the NMC time among specific symptoms between subjects who presented and did not present with symptoms.
Statistical Analysis
The normality of continuous variables was verified by the Shapiro–Wilk test. When comparing the measures of central tendency of NMC time according to the variables tested, the Student's T or Mann–Whitney test were used according to the normality of continuous variables, and p values < 0.05 were considered to be statistically significant.
Results
A total of 99 individuals were included: 64 patients with SARS-CoV-2+ and 35 healthy controls. In the SARS-CoV-2 group, 14 patients (21.8%) were asymptomatic and 50 (78.2%) reported symptoms, including headache (n = 42, 84%), cough (n = 34, 68%), runny nose (n = 32, 64%), loss of smell (n = 29, 58%), loss of taste (n = 25, 50%), nasal obstruction (n = 23, 46%), sore throat (n = 21, 42%), and dyspnea (n = 13, 26%). For the analysis, 25 patients in group 1 were excluded due to impaired taste leaving 39 patients (25 with symptoms, 14 without symptoms).
The samples of the two groups were comparable (39 SARS-CoV-2+ and 35 healthy subjects). There was a significant age difference between the groups, p < 0.001; the mean age of subjects with SARS-CoV-2 was 32 years old (from 20 to 48) and of the control group was 39 years old (from 18 to 50). There were no significant differences in gender, smoking, or the presence of rhinitis between the groups p = 0.7, p = 0.49, and p = 0.78, respectively.
There was no statistical difference in the NMC time between group 1 (SARS-CoV+) and group 2 (control), p = 0.81. When comparing the asymptomatic patients in group 1 (14 patients) with the control group (35 subjects), we did not find a statistical difference (p = 0.34). When we analyzed each symptom separately, we found no statistical differences between patients from group 1 and group 2 for headache (p = 0.7), cough (p = 0.15), runny nose (p = 0.2), loss of smell (p = 0.11), sore throat (p = 0.21), and nasal obstruction (p = 0.38).
There was a statistical difference between patients with dyspnea in group 1 and controls, (p = 0.032, Fig. 1), and also when comparing SARS-CoV-2+ patients with and without dyspnea, regardless of other symptoms (p = 0.04, Fig. 2).
Fig. 1.

Nasal mucociliary clearance comparison between SARS-CoV-2+ patients with dyspnea and non-infected subjects (control group)
Fig. 2.
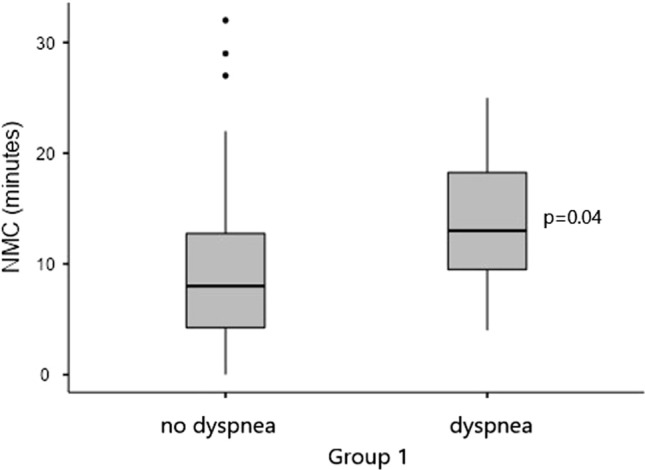
Nasal mucociliary clearance comparison between SARS-CoV-2+ patients with and without dyspnea
Discussion
The results of our study confirmed that patients with dyspnea had an increased time of NMC when compared to the control group. This suggests involvement of the first innate airway protection barrier affecting more severe cases of COVID-19. A previous study demonstrated an increased time of NMC in patients who were hospitalized in the pandemic ward due to Covid-19 [13]. In our study, the patients with SARS-CoV-2 were not hospitalized, demonstrating mild to moderate symptoms. Worsening NMC time has been previously reported to be associated with smoking and SARS-CoV-2 virus infection [14].
The saccharin test is a useful method for scientific research, since it is easily reproducible, simple to perform, non-invasive, and low cost, making it an interesting alternative to other methods that depend on more complex equipment, with greater technical requirements and aptitude of the examiners [14–16]. It is important to note that in all evaluations the air humidity was above 50%. Mucociliary transport could be influenced by several other conditions such as age, level of physical activity, use of medications, and exposure to cigarettes, caffeine, and alcohol. Smoking and the presence of rhinitis did not differ between the groups in our study. However, there was a statistical difference in age between the study groups but we feel this difference (32–39 years old) likely does not have clinical relevance.
It has previously been reported that young adults with deviated nasal septum and chronic nasal obstruction showed compromised mucociliary clearance in both nostrils when compared to controls [17]. In the present study, nasal obstruction as a SARS-CoV-2 symptom did not significantly compromise the nasal mucociliary clearance compared to the control group. We found similar results when we compared anosmia between groups. Patients with loss of their sense of smell did not show impairment in NMC. This evidence corroborates the hypothesis of neurotropism and an affected olfactory nerve and receptors instead of mucosal inflammation as a cause for anosmia development [18, 19].
The suspicion that SARS-CoV-2 is able to reach cells of the lower respiratory tract and nervous system from the upper mucosa epithelium is still not completely clarified. There is evidence that the expression of ACE2 receptors has a direct relationship with the age group, justifying that children have less symptoms and morbidity as they express less of these receptors [20]. The presence of ACE2 receptors in epithelial cells of the nasal mucosa is well established in adults, however, the correlation between this and the development of symptoms in COVID-19, including anosmia or dyspnea, is not clear. Considering the results of our study, we can suggest that cases of dyspnea in COVID-19 might be related to greater aggressiveness with the airway mucosa, promoting worsening of the disease and resulting in pulmonary impairment and dyspnea. There is still no evidence on whether aggressiveness of the infection in the mucosa of the upper airway could be associated with the concentration of membrane receptors like ACE2 and TMPRSS2.
The importance of the finding in this research is that patients presenting dyspnea, a potentially severe symptom of COVID-19, could present compromised innate defenses for protection of the respiratory system. In addition, the NMC could be used as a predictive factor for pulmonary involvement and a worse prognosis. In summary, our study highlights that the presence of dyspnea, a potentially severe symptom in COVID-19, is related to a compromised mucoepithelial barrier and its ciliary beat system.
Funding
the authors declare that they received no funding for this study.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xu K, Lai XQ, Liu Z. Suggestions for prevention of 2019 novel coronavírus infection in otolaryngology head and necksurgery medical staff. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020;55:313–315. doi: 10.3760/cma.j.cn115330-20200201-00240. [DOI] [PubMed] [Google Scholar]
- 2.Joffily L, Ungierowicz A, David AG, Melo B, Brito CLT, Mello L, Santos PSCD, Pezato R. The close relationship between sudden loss of smell and Covid-19. Braz J Otorhinolaryngol. 2020;86:632–638. doi: 10.1016/j.bjorl.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiter JF, Leroux MR. Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol. 2017;18:533–547. doi: 10.1038/nrm.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glezer I, Malnic B. Olfactory receptor function. Handb Clin Neurol. 2019;164:67–78. doi: 10.1016/B978-0-444-63855-7.00005-8. [DOI] [PubMed] [Google Scholar]
- 5.Pallanti S. Importance of SARs-CoV-2 anosmia: from phenomenology to neurobiology. Compr Psychiatry. 2020;100:152184. doi: 10.1016/j.comppsych.2020.152184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santos JGO, Migueis DP, Amaral JBD, Bachi ALL, Boggi AC, Thamboo A, Voegels RL, Pezato R. Impact of SARS-CoV-2 on saliva: TNF-⍺, IL-6, IL-10, lactoferrin, lysozyme, IgG, IgA, and IgM. J Oral Biosci. 2022 doi: 10.1016/j.job.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W, Li M, Ou G. COVID-19, cilia, and smell. FEBS J. 2020;287:3672–3676. doi: 10.1111/febs.15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.L Jakobsen, K Vanselow, M Skogs, et al. Novel asymmetrically localizing components of human centrosomes identified by complementary proteomics methods. EMBO J. 2011;30:1520–1535. doi: 10.1038/emboj.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Rajendran V, Sethumadhavan R, Purohit R. CEP proteins: the knights of centrosome dynasty. Protoplasma. 2013;250:965–983. doi: 10.1007/s00709-013-0488-9. [DOI] [PubMed] [Google Scholar]
- 11.Pezato R, Voegels R. Why do we not find polyps in the lungs? Bronchial mucosa as a model in the treatment of polyposis. Med Hypotheses. 2012;78:468–470. doi: 10.1016/j.mehy.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Caponnetto P, Emma R, Benfatto F, et al. Saccharin test: methodological validation and systematic review of the literature. Ear Nose Throat J. 2021 doi: 10.1177/01455613211064044. [DOI] [PubMed] [Google Scholar]
- 13.Kahraman ME, Yüksel F, Özbuğday Y. The relationship between Covid-19 and mucociliary clearance. Acta Otolaryngol. 2021;141:989–993. doi: 10.1080/00016489.2021.1991592. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues F, Freire AP, Uzeloto J, et al. Particularities and clinical applicability of saccharin transit time test. Int Arch Otorhinolaryngol. 2019;2:229–240. doi: 10.1055/s-0038-1676116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanley P, MacWillian L, Greenstone M, Mackay I, Cole P. Efficacy of a saccharin test for screening to detect abnormal mucociliary clearance. Br J Dis Chest. 1984;78(1):62–65. doi: 10.1016/0007-0971(84)90098-6. [DOI] [PubMed] [Google Scholar]
- 16.Cordeiro TG, do Amaral JB, Pavao V, et al. Fire simulator exposure alters the innate epithelial response and inflammatory status in the airways of firefighters. Rhinology. 2021;59(3):267–276. doi: 10.4193/Rhin21.002. [DOI] [PubMed] [Google Scholar]
- 17.Kamani T, Yilmaz T, Surucu S, Turan E, Brent KA. Scanning electron microscopy of ciliae and saccharine test for ciliary function in septal deviations. Laryngoscope. 2006;116:586–590. doi: 10.1097/01.MLG.0000205608.50526.28. [DOI] [PubMed] [Google Scholar]
- 18.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 19.Mao L, Jin H, Wang M, et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin converting enzyme 2 in children and adults. JAMA. 2020;323:2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]


