Abstract
Despite increasing emphasis on emergent brain-behavior patterns supporting language, cognitive, and socio-emotional development in toddlerhood, methodologic challenges impede their characterization. Toddlers are notoriously difficult to engage in brain research, leaving a developmental window in which neural processes are understudied. Further, EEG and ERP paradigms at this age typically employ structured, experimental tasks that rarely reflect formative naturalistic interactions with caregivers. Here, we introduce and provide proof of concept for a new “Social EEG” paradigm, in which parent-toddler dyads interact naturally during EEG recording. Parents and toddlers sit at a table together and engage in different activities, such as book sharing or watching a movie. EEG is time-locked to the video recording of their interaction. Offline, behavioral data are microcoded with mutually exclusive engagement state codes. From 216 sessions to date with 2- and 3-year-old toddlers and their parents, 72% of dyads successfully completed the full Social EEG paradigm, suggesting that it is possible to collect dual EEG from parents and toddlers during naturalistic interactions. In addition to providing naturalistic information about child neural development within the caregiving context, this paradigm holds promise for examination of emerging constructs such as brain-to-brain synchrony in parents and children.
Keywords: EEG, parent-child interaction, synchrony, hyperscanning, neurodevelopment
Young children develop cognitive, language, and socio-emotional skills through rich, reciprocal interactions with caregivers, yet neuroscience methods typically study the neural correlates of these developmental processes in solitary, rather unnatural experimental settings. Electroencephalography (EEG) allows researchers to examine the moment-by-moment natural electrical activity of the brain while a child is in a given state such as rest, or in response to various types of stimuli or conditions. Because it provides a temporally precise signal, can be portable, and is rather tolerant of motion, EEG has been widely used to examine development and brain-behavior associations in infants and children and has provided key insights into a variety of developmental processes (reviewed in Anderson & Perone, 2018; Bell & Cuevas, 2012). Despite these benefits and advances, however, the vast majority of EEG research does not reflect the naturalistic social interactions with a caregiver that facilitate development. Identifying the neural correlates that underlie specific social and communicative behaviors that occur during parent-child interactions holds great promise for providing more ecologically valid scientific findings regarding the neurobiological processes of development (Rolison et al., 2015; Suveg et al., 2016). Further, the opportunity to study brain-to-brain neural synchrony during naturalistic social interaction could provide new insights, particularly into social development, given the importance of behavioral and physiological synchrony for child development (DePasquale, 2020; Konvalinka & Roepstorff, 2012; Lunkenheimer et al., 2020; Quiñones‐Camacho et al., 2020).
In this paper, we first provide background on current “social” neuroscience methods for studying development along a continuum of naturalness with commentary on the opportunities and challenges that the field currently faces. We then describe how studying the brain during parent-child interactions can be uniquely informative, especially when examining naturally occurring behaviors among the dyad. Additionally, we review some of the benefits of studying interpersonal neural synchrony and its contributions to our understanding of child development. We introduce a paradigm that we have developed called Social EEG, designed to assess parent and child neural signals as well as their synchrony, during naturalistic interaction. Social EEG uses state-based behavioral coding to identify naturally occurring behaviors in true-to-life social interaction, in order to examine their neural correlates. Finally, we provide data on the rates of success for the paradigm and initial usability in order to evaluate its utility.
Use of EEG in Developmental Research
EEG is a brain measure commonly used to study infants and children; in contrast to magnetic resonance imaging (MRI), it is more tolerant of motion, can be done in a child-friendly room with the parent or researchers present for comfort, and is relatively low-cost (Bell & Cuevas, 2012; Gilmore et al., 2018). Functional near-infrared spectroscopy (fNIRS) is another noninvasive brain measure used with young children because of similar advantages (Gervain et al., 2011), but its temporal resolution is poor relative to EEG. Both because of its temporal resolution and the well-established understanding of correlates of certain EEG power bands (e.g., Koenig et al., 2002; Marshall et al., 2002), EEG lends the opportunity to meaningfully examine time-sensitive brain-behavior associations in populations that are typically difficult to study with MRI, including infants, toddlers, and young children who experience difficulty with communication and language (McWeeny & Norton, 2020). In this way, EEG has the potential to help close the gap that currently exists in which infants and older preschoolers are characterized in brain studies, but toddler age is often skipped, because it can be difficult to obtain MRI either during natural sleep or while the child is awake (Zhang et al., 2019).
Resting state is among the most common methods employed in EEG research with young children. Resting state or “baseline” paradigms are designed to assess intrinsic neural activity without a specific task or stimulus. In classic resting state paradigms with adults, a participant sits quietly with their eyes closed in order to capture neural activity in a state as close to true resting as possible. For toddlers and young children, resting paradigms have been adapted to record continuous EEG while children are presented with minimally stimulating objects or events, such as watching a neutral, child-friendly video (e.g., McEvoy et al., 2015; Simon et al., 2017), a spinning bingo wheel (e.g., Marshall et al., 2004), or bubbles blown by an experimenter (e.g., Gabard-Durnam et al., 2019; Tierney et al., 2012).
Measures from resting or near-resting state in children can be used to inform how intrinsic brain function relates to social development and related skills including language and communication. Resting state studies have revealed neural correlates that affect infant and toddler development in terms of social behaviors and socioemotional outcomes (Liu et al., 2021; Paulus et al., 2013), the influence of early maternal behavior during parent-child interactions on later child brain activity (Bernier et al., 2016), and neural mechanisms that contribute to variation in language and cognitive abilities (Bell & Fox, 1992; Benasich et al., 2008; Gou et al., 2011; Tarullo et al., 2017). Further, studies have examined differential brain activation among children with communication difficulties such as autism spectrum disorder (Bernier et al., 2007; Murias et al., 2007; Wang et al., 2013) and language and learning disorders (Schiavone et al., 2014).
In order to study social development in children, some studies have investigated differences in neural activity between distinct passive-viewing conditions, such as those with versus without social stimuli. For example, studies have presented video recordings of age-matched peers performing a task (van Elk et al., 2008) or videos of an experimenter demonstrating social actions (Jones et al., 2015) during EEG recording. Including both baseline/rest and passive-viewing conditions offers added informative value in that these studies can capture children’s baseline brain response relative to manipulated social contexts in order to parse out baseline-state neural mechanisms from the neural mechanisms that may be uniquely involved in processing socially relevant information.
Toward Naturalistic EEG Paradigms with Children in Developmental Context
Studying social development using structured stimuli, during passive viewing, or at rest, although valuable, does not allow for examining the child’s naturally occurring behavior. Some researchers have developed approaches to bringing more true-to-life, dynamic qualities of the social world to the laboratory during EEG in part by expanding beyond presenting repeated stimuli via a computer. These include having an in-person interaction with the participant in real-time by playing peek-a-boo or directly speaking or singing to the child (Goodman et al., 2021; Jones et al., 2015; Leong et al., 2017; Orekhova et al., 1999; 2006; Ruysschaert et al., 2013; Shimada & Hiraki, 2006; St. John et al., 2016; Stroganova et al., 1997). Yet even with these methodological adaptations that aim to move toward stimuli more relevant to the child’s own social context, the socialization being captured is still somewhat restricted in that it does not give children the opportunity to freely navigate interactions as they normally would in real-world contexts outside of the laboratory. By examining changes in EEG brain activity with paradigms that are more closely aligned with dyadic, naturalistic interaction, research is likely getting closer to pinpointing the neural mechanisms that underlie more complex, live social communication that children experience in their daily lives.
Studying the Brain During Parent-Child Interaction
The parent-child context is considered the most formative experience of early childhood for shaping neurodevelopment and has long been examined in developmental science behaviorally (Kochanska & Aksan, 2004; Wakschlag & Hans, 1999). However, characterization of the brain during this process has largely been lacking. Though scientific advancements toward naturalistic neuroscience continue to provide meaningful information, we, and others (Bell, 2020; Dumas et al., 2010; Hari & Kujala, 2009; Konvalinka & Roepstorff, 2012; Markova et al., 2019; Nyugen et al., 2020; Rolison et al., 2015; Schilbach et al., 2013; Wass et al., 2020), recognize an important gap in our understanding of the interacting developing brain, especially interaction between parents and children, complementary to typical single-participant research paradigms. Parents and their children must draw on additional skills when actively engaging in reciprocal interactions by coordinating their own internalized social cognition with the rapidly unfolding responses and expectations of their communication partners in real-time (De Jaegher et al., 2010; Konvalinka & Roepstorff, 2012).
One method for increasing naturalness of EEG paradigms is to directly involve parents as participants together with their children. Neuroscience paradigms incorporate parent involvement in studies by instructing parent-child dyads to jointly attend to the same videos or stimuli (Azhari et al., 2019; Krzeczkowski et al., 2020), work together to assemble a tangram or puzzle (Atzaba-Poria et al., 2017; Nguyen et al., 2020; Quiñones‐Camacho et al., 2020), or play a computer game (Liao et al., 2015; Reindl et al., 2018). Paradigms have also been designed to simultaneously collect brain data from both the parent and child with less restrictive study protocols that allow dyads to have greater autonomy and flexibility in the interaction. This includes giving parent-child dyads one or two toys to freely play with (Hoyniak et al., 2021; Quiñones‐Camacho et al., 2020) or being instructed to play silently (Wass et al., 2018), in addition to simply having parent-child dyads freely engage in unguided verbal conversation with each other for ten minutes (Nguyen et al., 2021). Giving flexibility to these opportunities of interaction between parents and their children while collecting measurements of the brain permits children to naturally elicit behaviors and responses related to the transactions of communication. From there, researchers are better equipped to then characterize these social and communicative behaviors in the context of the underlying brain activation patterns that are simultaneously being observed.
Studying Naturally Occurring Child Social Behaviors
Another consideration in naturalistic EEG paradigms is how best to capture social constructs of interest. Most previous EEG work has examined child social processing using experimentally presented stimuli, which has the advantage of strict standardization. This can be helpful in differentiating neural mechanisms required for social processing from endogenic factors. Social processing during experimental tasks, however, varies in similarity to true-to-life social interaction. Additionally, in measuring a construct like joint engagement, it is difficult to control whether a child truly engages with a social partner or does not. By behavioral coding the interaction, researchers are able to identify behaviors of interest as they occur naturally without strict experimental protocols that constrain how the interaction unfolds. This is especially advantageous for young children and children with disabilities who may be less able to follow directions or watch a computer screen for long periods of time.
For example, social behaviors of interest coded during studies of interpersonal neural synchrony include looking behavior (such as mutual gaze or visual attention to an object, Leong et al., 2017; Piazza et al., 2020; Wass et al., 2018), turn-taking or other aspects of conversational quality (Nguyen et al., 2020; 2021; Quiñones‐Camacho et al., 2021), and participant affect (Atzaba-Poria et al., 2017; Nguyen et al., 2020; Piazza et al., 2020). Interactions have also been coded second-by-second more generally as synchronous or asynchronous, taking into account several types of social actions at once (Hoyniak et al., 2021; Quiñones‐Camacho et al., 2020). However, current work primarily focuses on discrete, child markers of engagement, where the constructs observed are aggregated into one composite data point per dyad that summarizes the level of engagement for that interaction session. This is in contrast to the design of the coding approach in the current paradigm, where the ongoing state of the parent and child is assessed. This is an important added methodological consideration to complement current work in this area as moments of joint engagement, which consider the timing and reciprocal nature of parent-child interaction, are uniquely related to language development above discrete measures of child attention alone (Adamson et al., 2019).
Inter-Brain Synchrony as a Method to Study Child Social Development
A promising avenue for studying child social development is to characterize parent-child attunement during interactions at the level of behavior, physiological processes, and, especially, neural entrainment (Feldman, 2007, 2012). For example, adult participants who align the rhythm of a joint finger-tapping action (Heggli et al., 2021; Hove & Risen, 2009; Konvalinka et al., 2014) or students attending to the same lesson in the same classroom (Dikker et al., 2017) demonstrate coordination or similarity in brain activation patterns with each other. Part of what supports interpersonal coordination among interaction partners is the concept of synchrony. Social synchrony is a “dynamic process by which hormonal, physiological, and behavioral cues are exchanged” to coordinate the timing of social behaviors between partners (Feldman, 2012). Synchrony among communication partners can be observed in terms of behaviors such as joint engagement in a task, mutual gaze, and turn-taking. Greater behavioral synchrony during parent-toddler interactions has been associated with communicative competence and self-control (Lindsey et al., 2009) and emotion-related socialization behaviors (Levy et al., 2017; Levy & Feldman, 2019; Kochanska & Aksan, 2004; Lunkenheimer et al., 2020). Synchrony can also be assessed via physiological measures such as alignment in levels of cortisol (e.g., Laurent et al., 2017, Pratt et al., 2017), heartbeat rhythms (Suveg et al., 2016), and respiratory rhythms (e.g., MacNeill et al., 2021). Synchrony is generally considered to be an important factor in child development, but our interest in how inter-brain synchrony between children and parents relates to important outcomes is relatively new.
Investigation of the neural mechanisms underpinning these synchronous behavioral and physiological changes during parent-child interactions have only recently begun to be empirically studied in a developmental context, especially with regard to toddlerhood. Toddlers are transitioning into an increasingly active role in dialogue as they expand on their linguistic, cognitive, and socio-emotional skills, to communicate more complex needs and intentions to their social partners (Bloom, 1993; Harrist & Waugh, 2002). As such, it is of growing interest to investigate not only young children’s neural activity when actively engaged in interactions, as opposed to studying the brain during isolated and passive processing of social information, but to investigate dyadic brain-to-brain synchrony during rapid social and language development in toddlerhood, particularly within the caregiving context (Schilbach, 2010; Schilbach et al., 2013). Specifically, “hyperscanning” research, or the method of collecting brain data from two or more individuals simultaneously, permits researchers to analyze the directionality of synchronization between parents and their children, with special focus on the concurrent brain activity during interactions and play.
Moreover, single participant neuroscience essentially assumes that the stimuli presented uniquely give rise to the measured brain states, whereas stimuli that naturally arise from communication in hyperscanning studies are not fixed; rather, they ecologically adapt with every social or linguistic transaction in a bidirectional manner. In fact, hyperscanning research has illuminated novel brain activation patterns that are otherwise distinct from findings in single-participant paradigms (Redcay & Schilbach, 2019; Redcay & Warnell, 2018; Schilbach et al., 2013; Wass et al., 2020). Although this is still a growing field, these methods and analyses have already been successfully translated for use with parents and their infants (Krzeczkowski et al., 2020; Wass et al., 2018), young toddlers (Atzaba-Poria et al., 2017; Liao et al., 2015), and preschool-aged children (Azhari et al., 2019; Hoyniak et al., 2021; Nguyen et al., 2020; Nguyen et al., 2021; Quiñones‐Camacho et al., 2020) across fNIRS and EEG measures. Importantly, these studies have also been able to include children with disabilities, for example children with severe physical disabilities who are nonverbal (Samadani et al., 2021).
However, to our knowledge, no published research to date has investigated parent-child EEG hyperscanning with young children to examine brain activation during naturally unfolding complex social behaviors such as joint engagement. Joint engagement is of particular interest in relation to brain-to-brain synchrony during parent-child interaction because it has been established as a key correlate and predictor of language development (Adamson et al., 2019; Conway et al., 2018; Trautman & Rollins, 2006). Further, our approach, described below, is the first to apply a coding scheme to the entirety of the parent-child interaction, allowing us to analyze EEG during a variety of different behavioral states between the parent and the child. The field has yet to fully explore dyadic brain activity during unrestricted play with toddlers, especially, and their parents as it naturally unfolds, allowing for even closer ecological validity with behaviors naturally elicited during parent-toddler interactions. In the next section, we describe our “Social EEG” paradigm.
The Social EEG Paradigm
Here, we introduce the Social EEG paradigm, which was designed to examine neural activity from toddlers and their parents during face-to-face, naturalistic interaction. Unlike previous experiments which have presented social stimuli through video clips or discrete actions performed by experimenters, we aimed to measure neural activity in the child and parent as it occurs during interaction. We designed contexts to elicit frequent moments of social interaction, and for contrast, baselines without interaction. Acknowledging the fact that it is impossible to control whether a dyad truly interacts or does not interact, a crucial feature of this paradigm is the use of microcoding of the dyad’s interaction to identify engagement states offline, after data collection. This approach has several key advantages, including allowing for the analysis of data during truly naturally occurring interaction, examining neural activity during joint engagement as it is defined in the behavioral literature (e.g., Adamson et al., 2004), and recording from the parent and child simultaneously, allowing for additional analysis of inter-brain synchrony. Further, this approach allows separation of salient states and actions rather than collapsing across all interactions in a given time period. This is important, as it allows examinations of variations in synchrony that may be specific to parent-child interaction (Suveg et al., 2016).
As an overview, the Social EEG paradigm involves the toddler and parent dyad sitting together, while wearing EEG caps. The researchers instruct the parent and provide different materials to facilitate the dyad engaging in different contexts, which were designed to elicit varying levels and types of interaction. The entire session is video-recorded and the video is linked to the EEG recording. Offline after the session, the video is microcoded and the codes are applied back to the EEG data for analysis.
Social EEG Setup Procedure
The lab space for the Social EEG paradigm includes two adjoining rooms, one room where the dyad sits and the other room where the EEG acquisition computer is located. Before the parent and child arrive, materials are set up to facilitate completing the EEG capping process as quickly as possible. For a typical session, one research assistant is solely responsible for controlling the EEG acquisition software from an adjoining room. Another one to two researchers are present for the session, depending on the experience-level of the researchers relative to capping young children (van der Velde & Junge, 2020), and consideration of the additional supports that some children may need during EEG data collection (e.g., due to individual differences in temperament or restlessness). The research assistant(s) in this role set up the caps, provide instructions to the parent, and ensure that the child does not pull on or remove the EEG cap. Regardless of the participant’s level of comfort with the cap, at least one researcher stays with the child for the entirety of the session, sitting behind them once the EEG recording begins.
During the Social EEG session with 2- and 3-year-old children, the child sits in a booster chair at a table and the parent sits at a 90-degree angle, so each are at the corner of the table (Figure 1). This setup allows a single camera angle to capture both people’s faces (more so than if they were sitting directly facing each other), and for the dyad to easily interact face to face (more so than if they were sitting side by side). For the EEG cap setup, the child is given the option of watching a movie on a laptop or playing with toys. Next, a researcher explains the EEG setup to the parent and the parent is encouraged to offer suggestions that might ensure success and child comfort during the capping process. The child is familiarized with the EEG caps, gel, and plastic syringes and the process is narrated in child-friendly language. The parent is capped first, in order to model the process for the child. The child is encouraged to touch the cap, plastic syringe, and gel to familiarize themselves with the process and is enlisted to “help” the researcher place the cap on their parent’s head or on a teddy bear. Once parent capping is complete, the researchers work to fit the child’s cap and gel the electrodes. One researcher keeps the child engaged during the capping process and offers engaging toys if the child becomes restless. The setup process is similar between 2-year-old and 3-year-old visits; however, 3-year-old children who complete the Social EEG a second time as part of the longitudinal aspect of our larger study are typically more comfortable wearing the cap, facilitating the setup process thereby decreasing setup time.
Figure 1.
Picture of Social EEG paradigm setup.
As the cap is set up, the EEG signal is observed on the acquisition computer and offset values (indicators of quality of the connection) are lowered if needed. During the entirety of the paradigm, one researcher sits in the room behind the child to monitor the child’s hands and keep the child engaged during transitions between the contexts, in order to prevent the child from touching or removing the EEG cap. There is also consideration of the potential influence of the researcher’s presence in the room on the dyad’s social responses. At the start of the session, the parent is instructed to pay as little attention as possible to the researcher in the room. This is feasible in most, if not all, cases because the researcher is typically seated on the floor in the corner of the room out of the parent’s view. The other researcher remains in the adjacent room to monitor data quality. Once setup is complete, the dyad begins the four contexts and EEG is recorded during each context, in addition to the video recording of the dyad interacting. We record from two identical, interconnected EEG systems and record both the parent and child’s data into a single file, ensuring precise timing alignment between their data.
Social EEG Paradigm Contexts
Various contexts were designed to elicit frequent, spontaneous moments of interaction between the toddler and their parent, as well as moments without interaction (including shared and separate foci of attention for the parent and child) for comparison. The length of contexts and thus the time of EEG data collection differs, as contexts involving more social interaction elicit more speaking and movement, thereby requiring more time to collect enough usable data, compared to contexts that tend to yield less movement and engagement (see description of contexts, below). All materials, such as puzzles and books, are placed on the table in front of the dyad within easy reach.
The four contexts are as follows:
Movie (6 minutes): The dyad watches a child-friendly movie of their choice together. The parent is instructed to interact as little as possible with the child; however, the parent is allowed to respond if their child needs assistance or to redirect them to keep watching the movie if necessary. The goal of this context is to capture moments where the dyad attends to the same stimulus but are not interacting with each other. Because the child often watches a movie during cap setup, this is a more seamless transition from the setup and allows the child some additional time to warm up to the EEG session.
Puzzles (8 minutes): The dyad is given 3–4 age-appropriate puzzles. The parent is instructed to interact with their child as much as possible, like they would at home.
Books (8 minutes): The dyad is given a set of books that encourage interaction (e.g., peek-a-boo books, books with flaps to look under, etc.). The parent is again instructed to interact as much as possible, like they would at home.
Movie and Forms (6 minutes): For this context, the child again watches a movie of their choice while the parent fills out a form on a clipboard. The parent is again instructed to interact as little as possible. This context is designed to elicit moments where the parent and child are primarily attending to different stimuli and not to each other.
For the movie contexts, the parent helps select a movie or show that the child will enjoy (from Netflix or YouTube) in order to maximize the child’s engagement with the movie, yielding a sufficient number of epochs when the child is engaged with the movie. Some dyads choose to continue watching the same movie used during setup and some chose to watch a different movie. Giving children and parents the option to choose the movie was partly a methodological consideration for increasing child compliance, particularly after having completed 2–3 hours of behavioral assessments prior to the EEG as part of a larger study. In parallel, the variety of movies chosen by the child mirror the variety of naturally occurring differences in social interaction that arise during the Books and Puzzles contexts. This design consideration is covered again in the discussion.
Each dyad completes the four contexts in succession, unless child fatigue or distress warrants a context to be repeated or discontinued. For example, if a child is very active and/or not attending while watching the movie (reducing available data for the relevant code), the researcher would try to repeat the movie context once more either immediately at the end of the paradigm or at the end of the session to ensure sufficient usable data. If the child is distressed or fatigued, both of which are undesirable for the family’s enjoyment, but also often result in poor EEG data quality, the researcher discontinues the context and consults with the parent to decide the best course of action, which could include attempting a different context (with the goal of repeating the discontinued context later) or discontinuing the entire paradigm. For children with extreme difficulties transitioning between the Movie and interaction contexts (i.e., Puzzles and Books), the order of contexts can be modified accordingly.
Microcoding of Social EEG Session
As described above, the dyads complete various contexts so as to elicit frequent, spontaneous moments of interaction between toddlers and their parents, as well as moments without interaction. Because of the naturalistic design of the paradigm, there are times that children may have been distracted or otherwise off-task during any context. We thus chose to adapt and apply a state-based joint engagement coding system that was originally designed to capture naturalistic social interaction between parents and children. The coding system is based on the state-based joint engagement coding scheme developed by Bakeman, Adamson, and colleagues (Adamson et al., 2004; Bakeman & Adamson, 1984). This coding scheme was designed to characterize a child’s attention to people and objects, with a focus on parent-child joint engagement. Whereas measures of joint attention traditionally focus on discrete child skills such as initiating bids, joint engagement is a state in which a child and parent are involved with the same object or activity and reflects both partners contribution to the shared interaction (Adamson et al., 2019). Additional codes were developed specific to this paradigm, such as separate and parallel object engagement, to describe each person’s attention when the dyad was not primarily interacting with each other. Because the Movie and the Movie and Forms contexts were designed to elicit the child’s and parent’s attention to the movie (and then elicit the parent’s attention to the forms in that context), the object engagement code was most used to note separate or parallel attention to these objects. Additionally, unlike the original Adamson and Bakeman team’s coding scheme which did not focus on the parent’s activity when not interacting with the child, our coding scheme reflects both partners even when they are not interacting (for example, the child may be watching a movie while the parent is observing the child). The specific codes and corresponding definitions and examples are found in Table 1.
Table 1.
Joint Engagement Coding Scheme (Adapted from Adamson et al., 2004).
| Code | Description | Example |
|---|---|---|
| Coordinated Joint Engagement | The parent and child were actively engaged with the same object and the child was actively and repeatedly acknowledging the parent’s participation, including with sustained visual interest or directed language. | The parent and child were jointly engaged in play with a puzzle, taking turns, and directing eye gaze and language towards each other. |
| Supported Joint Engagement | The parent and child were engaged with the same object, but the child’s engagement was asymmetrical and nearly exclusively on the object rather than the parent. | The parent and child were playing with a ball; the child took turns rolling the ball but was focused on the movement of the ball rather than the parent. |
| Person Engagement | The parent and child were mutually and exclusively engaged with each other, without any objects. | The parent and child were playing peek-a-boo. |
| Parallel Object Engagement | The parent and child were actively involved with the same object or activity, but without any social interaction. | The parent and child were both looking at the computer and watching a movie but were not interacting with each other. |
| Separate Object Engagement | The parent and child were actively involved with different objects or activities without any social interaction. | The parent was filling out forms while the child watched a movie. |
| Onlooking | One partner watched the other partner’s activity without engaging. | The parent was observing the child as the child watched a movie or the child watched the parent fill out a form. |
| Unengaged | One partner was uninvolved with any objects, people, or activities. | The parent looked around the room distractedly. |
| Interruption | The study was interrupted for any reason. | The experimenter entered the room to add gel to the child’s cap. |
Offline, after the EEG session, each interaction is microcoded using INTERACT software (Mangold, 2020). The coding system allows for extraction of moments of interest (e.g., joint engagement, object engagement) and separation into respective conditions for analysis. Using this event-based coding scheme, research assistants coded the video of the entire Social EEG recording session into mutually exclusive and exhaustive engagement states; that is, every moment of the session is assigned to a code. Coders watched the videos and identified the frame that the transition to an engagement state began and when that engagement state ended. In order to be coded, engagement states need to last at least 2 seconds. If the state lasts fewer than 2 seconds, a new state is not coded. After coding is complete, the list of the behavioral states and their corresponding time (in milliseconds) is exported in order to be linked to the EEG data.
Before beginning coding, each research assistant is trained and oriented to a codebook and examples of states. Each coder must demonstrate at least 80% fidelity (i.e., that the correct codes were used within 1 second of the beginning of the engagement state) across 3 consecutive videos coded by the criterion coder (author B.L.M.). 20 contexts were also double-coded to examine ongoing inter-rater reliability, with agreement mean = 92.2% of total time (SD = 7.7%). For any context with <80% agreement (1 out of 20 contexts), the two coders met to establish agreement.
Integrating Behavior Codes and EEG Data for Analysis
An important consideration for this work is time-locking the EEG data and behavioral codes. Stimulus presentation software is coordinated with the EEG acquisition computer; the software sends port codes to the EEG recording computer that is coordinated with stimulus presentation on a computer screen behind the participants that is visible in the recording. The screen displays a mark for the onset of EEG recording for that context, as well as a time mark after every elapsed minute of the context. Coders watch the video and identify the moment the word “begin” appears on the monitor, at which time they begin behavioral coding. Port codes (i.e. event codes) from the EEG files are merged with behavioral codes from the INTERACT files, and the beginning port code is used to time-lock the files. Although the refresh rate of the display screen and the frame rate of the video recording will influence how closely time locked the EEG and behavioral codes will be, data analysis centers on continuous EEG over seconds-long states, rather than event-related analysis as is common in ERP work.
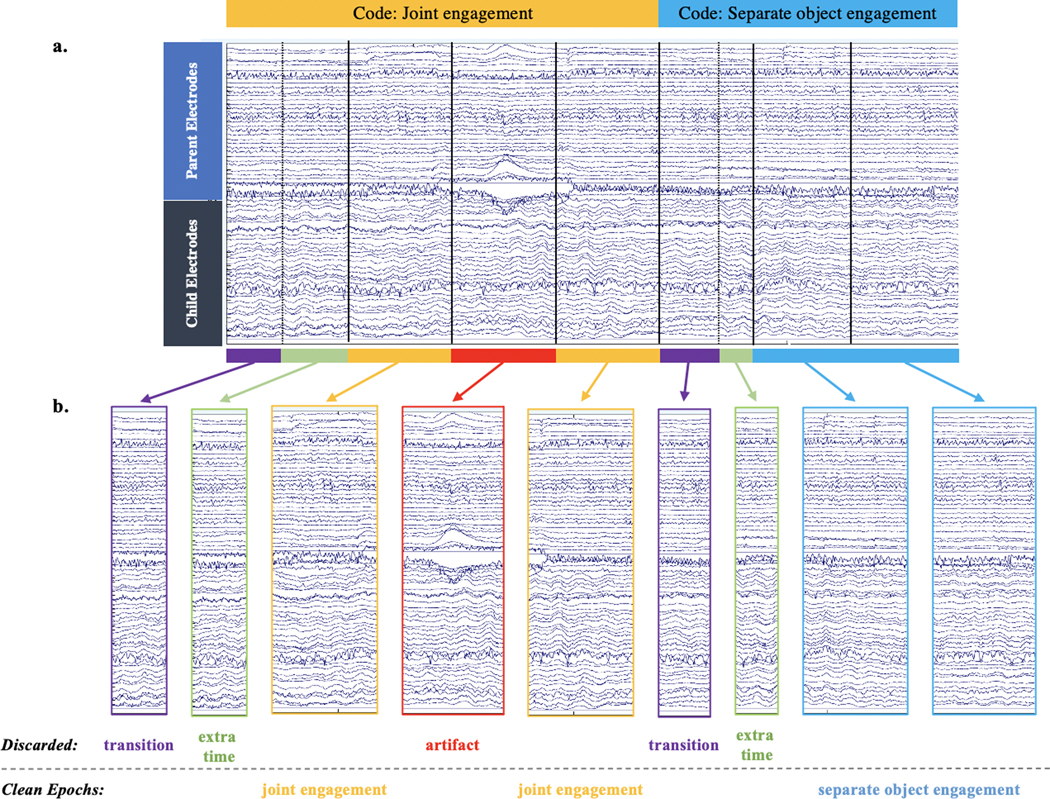
In order to analyze EEG data, the behavioral codes are applied as events to the EEG file that contains both participants’ data. Artifacts are first identified and corrected using independent components analysis (ICA) (separately for each person) and remaining artifacts are detected and rejected. EEG data are marked with non-overlapping 1-second events for epoching, and with events corresponding to the behavioral codes (see Figure 2). Some data are discarded, including the first 500ms of all codes in order to remove the time when the dyad may not yet be fully in the state, and additional time that does not fit in the 1-sec epochs. (For example, for a coded state that lasted 3.83 seconds, the first 500ms would be discarded as transition time, three 1-second epochs would be included in analysis if data were artifact free, then the remaining .33 seconds that do not fit into an epoch would be discarded.)
Figure 2.
This figure depicts the process of creating epochs of EEG data corresponding to the behavioral microcoding. The file begins as continuous EEG with parent data on top and child on the bottom. Event codes are added to the EEG and separated into 1-second epochs corresponding to behavioral state (solid lines). A 500ms transition period (dotted line) is excluded as well as the extra time that does not fit cleanly into 1-second epochs. Epochs with remaining artifacts are then rejected, leaving only artifact-free epochs.
After this event applying and epoching process, EEG data are processed using a relatively typical approach. Epochs corresponding to each code of interest can be collapsed for analysis. (Codes like “Interruption” are included so that all moments fit within one of the mutually exclusive codes, but are not included in analysis.) For example, child baseline data can be examined from the object engagement epochs in both the Movie and the Movie and Form contexts. Parent-child synchrony can also be analyzed during various types of behavior as indicated by the codes. For example, we plan to compare child and parent EEG synchrony during moments of joint engagement versus parallel object engagement (no interaction but shared object engagement, such as in the Movie context) and separate object engagement (no interaction and different object engagement, such as in the Movie and Forms context). Crucially, this approach will allow us to disentangle what degree of brain-to-brain synchrony occurs because of shared sensory input versus the contribution of naturalistic, social interaction. By comparing across codes rather than entire contexts, we can extract the moments of true engagement and combine them for analysis, rather than averaging across a variety of behaviors within a longer time interval, like an entire context.
Feasibility Study
To illustrate the feasibility of the Social EEG paradigm, we present data from an ongoing study of young children’s language and socio-emotional development. First, we report the percent of successful sessions, meaning that the child and parent were successfully capped and completed all 4 contexts without significant movement or fatigue precluding collection of usable data as per visual inspection and session notes. For dyads without successful completion of all contexts, the reasons for missing data are provided (Table 3). Secondly, for a subset of 145 contexts for which data processing is currently complete, the number and percent of clean epochs after artifact rejection are reported across timepoint and context (Table 4). Finally, for these 145 contexts, the number of epochs in each engagement state of interest after artifact rejection is reported (Table 5).
Table 3.
EEG Data Usability and Reasons for Data Loss for All Dyads (n = 216)
|
|
||
|---|---|---|
| n | % | |
| Session usable | 156 | 72.0% |
| EEG not attempted due to time constraint or parent declining | 6 | 2.8% |
| Child refused cap, gelling, or could not sit in chair | 8 | 3.7% |
| Parent could not be capped due to wig or hair extensions | 7 | 3.2% |
| Could not get usable connection with child scalp | 0 | 0% |
| Could not get usable connection with parent scalp | 7 | 3.2% |
| Extremely poor data quality and/or discontinued contexts due to child factors (movement, fatigue, etc.) | 15 | 6.9% |
| Extremely poor data quality and/or discontinued contexts due to child factors (movement, fatigue, etc.) | 3 | 1.4% |
| Child removed cap during session | 19 | 8.8% |
Notes: Some dyads had more than one reason that data were unusable, so percentages sum to more than 100%. Data is considered per dyad across all four contexts.
Table 4.
Number and Percentage of Usable 1-second Epochs for Child Data and Combined Child and Parent Data by Context and Timepoint
|
|
|||||
|---|---|---|---|---|---|
| Child-Only Data | Child and Parent Data | ||||
|
| |||||
| n | Mean (SD) # of Usable Epochs | Mean % of Usable Epochs | Mean (SD) # of Usable Epochs | Mean % of Usable Epochs | |
| Timepoint | |||||
| Age 2 | 114 | 220 (90) | 56% | 160 (83) | 41% |
| Age 3 | 31 | 268 (80) | 68% | 218 (67) | 57% |
| Context | |||||
| Movie | 48 | 197 (82) | 60% | 170 (77) | 52% |
| Puzzles | 25 | 252 (103) | 53% | 175 (104) | 41% |
| Books | 45 | 261 (90) | 57% | 172 (86) | 36% |
| Movie and Forms | 27 | 216 (66) | 64% | 174 (66) | 52% |
Note: This table represents 145 successful contexts which have been artifact-rejected from 49 dyads. Timepoint data are collapsed across contexts for each age; context data are collapsed across ages.
Table 5.
Mean Usable Combined Parent and Child 1-second Epochs by Engagement Code
|
|
|||
|---|---|---|---|
| Context | Person/Joint Engagement | Parallel Object Engagement | Separate Object Engagement |
| Movie | 4 (9) | 149 (70) | 1 (4) |
| Puzzles | 155 (92) | 0 (0) | 3 (14) |
| Books | 154 (89) | 0 (2) | 2 (9) |
| Movie and Forms | 0 (2) | 19 (29) | 123 (79) |
Notes: Data are presented as Mean (SD). This table represents 145 successful contexts which have been artifact-rejected from 49 dyads.
Methods
Participants
Participants were toddlers and their parents who were part of a larger longitudinal study, The When to Worry Study, focused on earlier identification of mental health and language disorder risk (e.g., Manning et al., 2019). Eligibility criteria included that the biological mother was available for surveys and study participation, therefore almost all parent participants in the sample were mothers (see Table 2). Exclusion criteria included a diagnosed developmental disability, major developmental delay or medical diagnosis (e.g., epilepsy) or parent without language proficiency to complete study measures in English. All children also met criteria of hearing English at least 80% of the time at home. The sample was enriched for children with higher levels of irritability and who were late talkers (defined as age 19–26 months with vocabulary size below 15th percentile for age and sex on the MCDI, or not yet combining words).
Table 2.
Demographic Characteristics of Toddlers and Parent Participants for Social EEG Sessions Collected to Date, by Timepoint
|
|
||||
|---|---|---|---|---|
| Year 2 | Year 3 | |||
|
|
||||
| Toddlers | Parents | Toddlers | Parents | |
| Number of participants | 180 | 180 | 31 | 31 |
| Age (mean ± SD) | 26.9 ± 1.7 months | 34.9 ± 4.7 years | 40.3 ± 2.1 months | 34.4 ± 7.2 years |
| Age range | 24–32 months | 22–46 years | 35–45 months | 20–43 years |
| Sex (% female) | 32.4% | 99.4% | 35.5% | 96.8% |
| Income-to-needs ratio | 5.6 ± 6.0 | 6.2 ± 10.1 | ||
| Race | ||||
| Asian | 1.1% | 3.3% | 0.0% | 3.2% |
| Black/African American | 16.7% | 16.7% | 22.6% | 22.6% |
| Native Hawaiian/ Pacific Islander | 0.0% | 1.1% | 0.0% | 0.0% |
| White/Caucasian | 67.8% | 71.7% | 61.3% | 67.7% |
| More than one race | 10.0% | 0.6% | 9.7% | 3.2% |
| Unknown/not reported | 4.4% | 6.7% | 6.5% | 3.2% |
| Ethnicity | ||||
| Hispanic/Latino | 11.1% | 8.3% | 6.5% | 3.2% |
| Not Hispanic/Latino | 88.9% | 90.0% | 90.3% | 93.5% |
| Unknown/not reported | 0.0% | 1.7% | 3.2% | 3.2% |
Notes: Income-to-needs ratio is the ratio of the family’s household income to the US census income cutoff for poverty, based on household size. Race and ethnicity are parent reported. Not all percentages for race sum exactly to 100% due to rounding.
Participants were recruited from pediatric clinics in the greater Chicago area, from community locations and events, and through social media. Participants are followed longitudinally and complete surveys at home via online questionnaire, assessments via videochat, and yearly lab visits for behavioral/observation measures and EEG. Parents provided informed consent and families were compensated for their time. All procedures were approved by Northwestern University’s Institutional Review Board.
Timepoints and Data Selection
Study data collection is ongoing. EEG data analyzed here were collected from 186 toddlers aged 24–47 months and their parent at two timepoints, at age 2 and age 3 years; five participants at the age 2 timepoint had two EEG attempts in order to get complete EEG data. To date, 180 sessions for the age 2 timepoint and 31 sessions for the age 3 timepoint have been collected (n = 216 unique sessions; 25 participants have completed both age 2 and age 3 timepoints). Demographic characteristics of the participants at each timepoint are given in Table 2. To assess how much clean data remained after artifact correction, we examined data from 145 successful contexts (collected from 49 dyads: 32 at age 2, and 17 at age 3) which have been processed to date. It is important to note that these contexts were from successful sessions (participants that were successfully capped and completed all contexts).
Data Collection
Toddlers and their parents completed the Social EEG paradigm as described above. EEG was recorded using two, linked BioSemi ActiveTwo Systems (BioSemi B.V., Amsterdam). Active Ag-AgCl electrodes were affixed to elastic caps appropriate for the child and parent’s head sizes (Electro-Cap Inc., Eaton, OH) secured with a fabric strap under the chin. EEG was recorded from 32 scalp sites from each participant. The parent was additionally fitted with external vertical and horizontal eye electrodes and right and left mastoid electrodes; very few children in our pilot data tolerated placement of these electrodes, so they were not used for toddlers in this study. BioSemi recordings are made in single-ended mode that amplifies the difference between each electrode site and a common mode sensor (CMS) electrode with referencing off-line. The impedances do not need to be lowered with this system due to the combination of pre-amplifiers at each electrode site, a driven right leg (DRL) circuit, and high electrical isolation (Kappenman & Luck, 2010). Offset values were kept below 40 mV during recording. EEG was recorded with a low-pass hardware filter with a half-power cutoff at 104 Hz and digitized at 512 Hz with 24 bits of resolution. Data collection occurred at the end of the lab visit, after approximately 2–3 hours of other developmental assessments. (This visit order was chosen so that with the gel-based system used for EEG, children and parents did not need to complete the rest of the visit with gel in their hair or wet hair.)
EEG Preprocessing, Event Merging, Artifact Correction, and Artifact Rejection
Data were processed using EEGLab 14.1.1 (Delorme & Makeig, 2004) and ERPLab 7.0.0 (Lopez-Calderon & Luck, 2014) software packages run in MATLAB R2019b. Data were imported, referenced to electrode Cz, and high-pass filtered at 0.1 Hz (half-power cutoff). Channels with consistently poor connection or excessive artifacts were interpolated with the average of surrounding channels using the spherical interpolation function in EEGLab; this approach was used sparingly to preserve the original data as much as possible. The list of event codes corresponding to the behavioral microcoding was merged with the list of event codes corresponding to the EEG data (based on the event codes existing in the file at the start of recording and for every elapsed minute). This merged eventlist was then imported into the continuous EEG data file.
Data were then separated into 1-second epochs; the first 500 ms of each code interval was discarded to account for the transition between engagement states (see Figure 2). Remaining time that did not fit into the 1-second epoch interval was also discarded. For example, an instance of joint engagement behavior that lasted 25.8 seconds may result in a series of epochs as follows: 6 clean epochs, 4 artifact epochs, 10 clean epochs, and 5 artifact epochs. Thus, the longest segment of continuous EEG from this one behavior code would be 10 seconds. In theory, the shortest continuous segment of EEG analyzed in a given instance of a state could be just 1 second (though behavioral codes lasted at least 2 seconds).
Eye blinks and horizontal eye movement artifacts were identified and corrected using independent component analysis in EEGLab. The moving window peak-to-peak function in ERPLab and the linear trend/variance function in EEGLab were used to reject remaining trials with artifact (including muscle activity and head/body motion). The moving window peak-to-peak function was most effective for detecting sharp artifacts, and the linear trend/variance function was most effective for detecting drift when needed. Thresholds were set individually and accuracy of artifact rejection was visually confirmed for each subject (Luck, 2014). The main moving window peak-to-peak amplitude threshold used ranged from 130–180 μV for the child data and 100–160 μV for the parent data.
Results
Child and Parent EEG Success Rates from Social EEG
We first assessed success rates across the Social EEG contexts, with “success” being defined as the dyad being able to be successfully capped and then completing all 4 contexts without excessive movement or fatigue affecting the data (per visual inspection). Based on our pilot work, these successful sessions were anticipated to result in at least 30 artifact-free, 1-second epochs per condition, which is a common minimum amount of data in previous studies to ensure reliability (e.g., McEvoy et al., 2015; Salinsky et al., 1991). EEG files not considered usable at the time of data collection (due to capping trouble, excessive movement, etc.) were not further processed.
A high percentage of individuals, 84% of children and 82% of parents, had a successful Movie context, which is most similar to typical resting state or baseline EEG paradigms. Furthermore, 83% of children and 81% of parents had a successful Movie context and at least one social interaction context (i.e., Puzzles or Books), which allows for comparison of naturalistic social interaction to baseline movie-watching akin to previous studies of manipulated social contexts. Finally, data quality was typically good across all 4 contexts; 72% of dyads successfully completed all 4 contexts with usable data for both the parent and child (see Table 3). Overall, these feasibility data indicate potential for comparing conditions of interest. To obtain these data, some contexts were repeated. At age 2, 9.7% of contexts overall were repeated (17.4% of Movie, 8.6% of Puzzle, 4.3% of Book, and 7.2% of Form). At age 3 (with fewer sessions complete overall to date), 13.9% of contexts were repeated (26.2% of Movie, 18.4% of Puzzles, 3.1% of Books, and 3.1% of Movie and Forms).
We also examined reasons for unsuccessful sessions. The most common reasons a child did not provide data for all contexts were removal of the cap (8.8%) or excessive movement during EEG (6.9%). In 2.7% of cases, the parent declined to complete the entire EEG portion of the study, often due to time constraints. The most common reason a parent did not have a successful session was due to having a wig or hair extensions incompatible with the cap (3.2%) or trouble getting a connection, often because of amount or thickness of hair (3.2%); thus, the initial n for children’s data is higher than the initial n for parents’ data. If the child could be capped but the parent could not (e.g., due to hairstyle), the EEG data collection session continued and only child data were collected. If child could not be capped but the parent could (e.g., due to child behavior), we did not continue the EEG session. Of the dyads considered for these initial feasibility analyses, 26 participated in the Social EEG paradigm at both the age 2 and age 3 to date as part of the longitudinal aims of the larger study. Of these 26 dyads, 18 children (69.2%) had usable data for all contexts at age 2 and 3 years; 16 parent-child dyads (61.5%) had usable data when children were 2 and 3 years old.
Preliminary Rates of Usable Epochs after Artifact Rejection per Context and Timepoint
The number and percent of usable epochs after event-merging and artifact correction and rejection is reported in Table 4 for a subset of 145 contexts (from 49 participants) in which data have been fully processed. These data do not fully represent the data collected to date, as they are a small subset drawn from dyads with successful sessions (as defined above) for preliminary analyses; data from dyads who could not successfully complete the EEG paradigm were not further processed. Each context yielded a large number of usable epochs for both the child and parent; on average more than 50% of data collected, and even more usable epochs when only data from the child were considered (as this can be analyzed on its own, as well). The paradigm was the same at both timepoints, but the mean number of usable epochs per context was higher for 3-year-olds (268 usable child epochs, 218 usable child and parent epochs) than 2-year-olds (220 usable child epochs, 160 usable child and parent epochs).
Preliminary Rates of Usable Epochs per Engagement Code
Lastly, we also examined the number of usable parent and child epochs after artifact rejection in the engagement codes of interest for each context (Table 5). This is important because each context was designed to elicit varied codes, and we required at least 30 usable epochs per condition for analysis based on previous literature. Overall, there were sufficient usable epochs needed to examine engagement codes either within a single context or by combining across multiple contexts depending on research questions of interest. On average, the Puzzles and Books contexts each yielded approximately 150 usable parent and child epochs coded as person engagement/joint engagement. There were, on average, 149 usable parallel object engagement epochs in the Movie context and 123 separate object engagement epochs in the Movie and Forms context.
Discussion
Here, we describe the rationale for studying the developing brain using methods that are naturalistic and reflect the real-world situations that children experience. We describe a novel naturalistic Social EEG paradigm that allows examination of naturally occurring parent-child interactions formative for early cognitive, social, and language development. Finally, we provide initial feasibility data from an ongoing study of toddlers and their parents relative to the Social EEG paradigm.
A large proportion of participating toddlers and parents successfully completed the Social EEG paradigm, and preliminary analyses indicate that this paradigm yields high numbers of usable epochs, even with relatively strict artifact detection procedures employed. Previous work has demonstrated attrition rates for EEG studies as high as 30% - 45% in toddlers aged 2–3 years (Bell & Cuevas, 2012). Successful EEG data collection in toddlers can be especially difficult as children must be tolerant of cap fitting, electrode application, and become relatively habituated to the cap in order to sit still during data collection. Our rate of successful child EEG recordings (84% of children) for the Movie context, which is most similar to typical resting state or baseline EEG paradigms, is highly comparable with a previous study showing that approximately 85% of 3-year-olds’ EEG sessions are successful (i.e., have more than 25% of data included from a 6- to 7-minute recording) while the child watches a movie (van der Velde & Junge, 2020).
Notably, the vast majority of the children in our sample completed the EEG at the end of behavioral assessment visit lasting over 2 hours. Our study’s use of engaging, naturalistic tasks, as well as relatively long intervals of data collection per context, are helpful in avoiding the challenges that some other studies face, such as children being able to understand and willing to follow task instructions and looking at a certain person or screen. We also suggest that the toddler is able to see their parent as a model wearing the EEG cap; this may have encouraged some children who would be hesitant or unhappy about wearing the cap to complete the session. There are some additional factors that may account for the fact that about 15% of children did not successfully complete the paradigm due to removing the cap or becoming too frustrated to continue. We had a higher rate of irritable children in this sample by design, and previous work indicates that children with negative temperament are less likely to complete an EEG paradigm (Marshall et al., 2009).
Considerations and Limitations of Social EEG
Despite the advantages of the Social EEG approach, there are some notable limitations. Because our goal was to capture neural correlates of naturalistic parent-toddler interaction via behaviorally defined states such as joint engagement, we used a gold-standard behavioral coding scheme, adapted for engagement state between the parent and child. This method can be more time-consuming in terms of coding and processing than traditional baseline or passive-viewing paradigms. Indeed, any type of detailed coding can be time-consuming. After this initial validation and for those interested in other research questions, future work could consider if similar information could be obtained by more streamlined coding or global measures such as a rating scale. We felt that starting with an established and detailed coding scheme was the best way to start on applying coding to interactions during EEG.
In order to maximize our ability to capture behaviors of interest during naturalistic interactions, we did not present standardized stimuli and instead used the state-based coding to identify moments of interest from natural interaction. This has many advantages including capturing more true-to-life behavior, but inevitably also reflects some variability (differences among movie choice, puzzle choice, book choice, etc.). However, the behaviors or states of interest (e.g., joint engagement, object engagement) are precisely defined and the focus of analytic comparisons. This is a markedly different approach than many studies which closely control stimuli but do not code for behavioral engagement. Beyond the rationale of aligning with our study aims, this approach also has the methodological advantage of being more feasible for young children and children with disabilities to complete. This “baseline” in some ways aligns with previous studies that aimed to have a relatively neutral context for comparison to other states or stimuli, but because we are not focused on comparing baseline across children, it also differs. Overall, it is important that researchers consider that there may be more differences than similarities across resting paradigms (Camacho et al., 2020).
Processing dyadic EEG data requires artifact correction and rejection from two participants, which requires adaptation of EEG processing pipelines and consideration of new factors. Though our design employing the various contexts seems to provide sufficient numbers of clean epochs within each code for analysis (Table 5), there are some new analytic considerations for this paradigm in which events of interest (given instances of a behavior code) vary in length. We selected a minimum state duration of 2 seconds for the behavior coding, consistent with existing behavioral coding schemes; however, within an instance of a given code, the duration of the code can be interrupted by artifacts, creating varying numbers of consecutive usable 1-second epochs within a state. Our current EEG processing pipeline utilizes ICA to correct common artifacts (e.g., eye blinks) in order to retain as much usable data as possible. We are currently also examining processing pipelines developed for child data (e.g., Debnath et al., 2020; Gabard-Durnham et al., 2018) that use more extensive artifact correction in order to retain larger segments of continuous EEG data which may be optimal for more complex parent-child synchrony analyses. The optimal number of consecutive events for EEG analysis is not yet clear; future studies may examine stability or reliability of EEG measures in relation to these indices.
Fitting toddlers and parents with caps simultaneously is challenging and requires trained research assistants familiar with working with children. Furthermore, one broad consideration with EEG is the requirement to wear a cap with gel, which is more difficult when participants have very thick or curly hair, and sometimes not possible when wearing wigs or hair extensions; these difficulties are noted in our usability data. These hair types tend to be more common in Black/African American individuals; ensuring that individuals are treated equitably and represented in research is a important consideration across EEG research (Choy et al., 2021). We work with families as much as possible to schedule visits around times when getting gel in their hair is less inconvenient (such as right before their hair is scheduled to be washed, styled, braided or rebraided, etc.) or by only collecting child EEG or only other non-EEG study measures.
Clinical Relevance
More interactive and naturalistic EEG paradigms with fewer instructions and restrictions (i.e., not requiring that the child sit still and watch stimuli on a screen) and a more engaging task, such as free play with a parent, could alleviate some of the challenges that arise when collecting brain data from toddlers. This is especially important for children that may need additional support during EEG data collection, such as children with sensory sensitivities (e.g., many children on the autism spectrum), or those who have difficulty following task instructions, including children with limited verbal and/or cognitive abilities. In the future, if naturalistic EEG markers that correspond to complex social behaviors can be identified, these markers may help to identify which children may go on to develop disorders and which may most benefit from early intervention (Dawson et al., 2012; Jeste et al., 2015). Interpersonal synchrony is also a major area of interest for researchers studying autism, which is characterized by difficulties with social communication (McNaughton & Redcay, 2020; Rolison et al., 2015) in diagnosed individuals as well as family members, who may share broader autism phenotype characteristics (Nayar et al., 2018). The present paradigm has the advantage of simultaneously capturing parent and toddler behavior, which can provide crucial information for tailoring interventions, such as whether some social skills or perceptions may be present in the absence of overt behaviors. Because children are assessed and receive treatment for various developmental disorders including autism, language disorders, and mental health conditions in naturalistic, interactive contexts with an adult, understanding how the brain functions in these dyadic interactions may inform clinical assessment and practice. Adaptations for clinical feasibility will be an important aspect of pragmatic refinement of the paradigm.
Future Directions
Our next steps are to assess the degree to which our behavioral codes reflecting engagement state align with well-validated patterns from more structured studies, for example, the finding that alpha power suppression is associated with increased attention (Perry et al., 2011), and to determine if examining neural markers of joint engagement as defined by the behavioral literature gives us a more detailed look at these constructs. Subsequent planned work will examine EEG metrics of child engagement with their parent (e.g., alpha, mu, and theta power during joint engagement versus separate object engagement), as well as parent-child neural synchrony, and how these indices may relate to child growth in language, cognition, and socio-emotional skills. We hypothesize that strong parent-child neural synchrony may support development, and perhaps serve as a protective factor for children who have high irritability or who have delayed language.
Another dimension to consider is affect and irritability in relation to socio-emotional development. For each instance of a state, we also have sub-codes for frustration and affect (negative, positive, neutral). Analyzing these data may allow additional comparisons with existing paradigms. We also have collected an additional, experimental context of frustration induction; in this context, the experimenter shows the dyad an enticing toy, a child-friendly tablet. The tablet has a small hidden switch that allows it to be turned off, before it is handed to the child. The non-functioning tablet is given to the child, and then multiple rounds of handing it back to the experimenter (who flips the switch and shows it to be working), and back to the child, are completed. We plan to analyze this task in the future.
One of our fundamental questions is how joint engagement is related to parent-child neural synchrony; certainly, a dyad who appears more “in sync” may demonstrate similarities on a neural level. However, we must also take into consideration that shared sensory input alone may be reflected in the brain. We have designed the Movie and the Movie and Forms contexts to tease apart moments when a dyad is not interacting but sharing a stimulus (audio and video from the movie) and when they are neither interacting nor sharing a primary visual stimulus. These data could help answer how much inter-brain synchrony is really explained by shared joint engagement, as opposed to the overlap in processing the same sensory stimuli.
There are also multiple measures for assessing neural synchrony which can be explored; power similarity is one way (Bowyer, 2016). Phase synchrony within a given power band is another measure of inter-brain synchrony. Because phase synchrony is meaningfully compared only within the same frequency band, this can be challenging for parent-child studies in which the frequency band shifts over development. For example, with the alpha band defined as 6–9 Hz in children and 8–13 Hz in adults, phase synchrony could be compared at 8–9 Hz. There are yet other measures related to coherence, and even Granger causality, which can be used to infer causal relations between one person and the other (e.g., Wass et al., 2018).
Future studies should consider assessing various other dimensions of caregiver-child interactions. For example, including more fathers in studies of parent-child interactions would be informative, especially when interested in inter-brain synchrony and potential genetic influences between parent and child neurobiology, as paternal and maternal effects may differentially affect child outcomes (Riva et al., 2019). The flexibility and naturalness of the Social EEG paradigm mean that the myriad factors that may affect the behavioral manifestation of caregiver-child interaction, such as culture, child age, and family risk and protective factors (stress, depression, warmth, responsiveness), and many more (Morris et al., 2020), could be examined.
Acknowledgments
We gratefully acknowledge funding for this work from NIH grants R01DC016273 (MPIs Norton & Wakschlag), R01MH107652 (PI Wakschlag), and R21DC017210 (PI Norton). Research reported in this publication was supported, in part, by grant T32NS047987 and the NIH’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422. We also acknowledge support from the Undergraduate Research Grant Program, which is administered by Northwestern University’s Office of Undergraduate Research. The conclusions, opinions, and other statements in this publication are the authors’ and not necessarily those of the sponsoring institutions. We thank our collaborators, consultants, and research assistants on this program of research: Susan Perlman, Yuri Jo, Winnie Liang, Lauren Hampton, Maranda Jones, Kamila Postolowicz, Emma Baime, Skylar Ozoh, Soujin (Jinnie) Choi, Silvia Clement-Lam, Kiera Cook, Amy Biel, Ewa Gut, Hannah Stroup, Debby Zemlock, Margaret Briggs-Gowan, Amelie Petitclerc, Michael Murias, and Lloyd Smith. We thank the participating families for their time.
Footnotes
Declarations of Interest: None
Data availability:
Upon publication, our detailed standard operating procedure (SOP) for data collection will be made freely available, and our SOP for detailed behavioral coding will be available upon request.
References
- Adamson LB, Bakeman R, & Deckner DF (2004). The development of symbol-infused joint engagement. Child Development, 75(4), 1171–1187. 10.1111/j.1467-8624.2004.00732.x [DOI] [PubMed] [Google Scholar]
- Adamson LB, Bakeman R, Suma K, & Robins DL (2019). An expanded view of joint attention: Skill, engagement, and language in typical development and autism. Child Development, 90(1), e1–e18. 10.1111/cdev.12973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AJ, & Perone S. (2018). Developmental change in the resting state electroencephalogram: Insights into cognition and the brain. Brain and Cognition, 126, 40–52. 10.1016/j.bandc.2018.08.001 [DOI] [PubMed] [Google Scholar]
- Bakeman R, & Adamson LB (1984). Coordinating attention to people and objects in mother-infant and peer-infant interaction. Child Development, 55, 1278–1289. 10.2307/1129997 [DOI] [PubMed] [Google Scholar]
- Bell MA (2020). Mother-child behavioral and physiological synchrony. Advances in Child Development and Behavior, 58, 163–188. 10.1016/bs.acdb.2020.01.006 [DOI] [PubMed] [Google Scholar]
- Bell MA, & Cuevas K. (2012). Using EEG to study cognitive development: Issues and practices. Journal of Cognition and Development, 13(3), 281–294. 10.1080/15248372.2012.691143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA, & Fox NA (1992). The relations between frontal brain electrical activity and cognitive development during infancy. Child Development, 63(5), 1142–1163. 10.1111/j.1467-8624.1992.tb01685.x [DOI] [PubMed] [Google Scholar]
- Benasich AA, Gou Z, Choudhury N, & Harris KD (2008). Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behavioural Brain Research, 195(2), 215–222. 10.1016/j.bbr.2008.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier A, Calkins SD, & Bell MA (2016). Longitudinal associations between the quality of mother–infant interactions and brain development across infancy. Child Development, 87(4), 1159–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier R, Dawson G, Webb S, & Murias M. (2007). EEG mu rhythm and imitation impairments in individuals with autism spectrum disorder. Brain and Cognition, 64(3), 228–237. 10.1016/j.bandc.2007.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom L. (1993). The Transition from Infancy to Language: Acquiring the Power of Expression. Cambridge University Press. [Google Scholar]
- Bowyer SM (2016). Coherence a measure of the brain networks: past and present. Neuropsychiatric Electrophysiology, 2(1), 1–12. [Google Scholar]
- Camacho MC, Quiñones-Camacho LE, & Perlman SB (2020). Does the child brain rest?: An examination and interpretation of resting cognition in developmental cognitive neuroscience. NeuroImage, 212, 116688. 10.1016/j.neuroimage.2020.116688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy T, Baker E. & Stavropoulos K. (2021). Systemic racism in EEG research: Considerations and potential solutions. Affective Science. 10.1007/s42761-021-00050-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway LJ, Levickis PA, Mensah F, Smith JA, Wake M, & Reilly S. (2018). The role of joint engagement in the development of language in a community-derived sample of slow-to-talk children. Journal of Child Language, 45(6), 1275–1293. 10.1017/S030500091800017X [DOI] [PubMed] [Google Scholar]
- De Jaegher H, Di Paolo E, & Gallagher S. (2010). Can social interaction constitute social cognition? Trends in Cognitive Sciences, 14(10), 441–447. 10.1016/j.tics.2010.06.009 [DOI] [PubMed] [Google Scholar]
- Debnath R, Buzzell GA, Morales S, Bowers ME, Leach SC, & Fox NA (2020). The Maryland analysis of developmental EEG (MADE) pipeline. Psychophysiology, 57(6), e13580. 10.1111/psyp.13580 [DOI] [PubMed] [Google Scholar]
- Delorme A, & Makeig S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- DePasquale CE (2020). A systematic review of caregiver–child physiological synchrony a cross systems: Associations with behavior and child functioning. Development and Psychopathology, 32(5), 1754–1777. 10.1017/S0954579420001236 [DOI] [PubMed] [Google Scholar]
- Debnath R, Buzzell GA, Morales S, Bowers ME, Leach SC, & Fox NA (2020). The Maryland analysis of developmental EEG (MADE) pipeline. Psychophysiology, 57(6), e13580. 10.1111/psyp.13580 [DOI] [PubMed] [Google Scholar]
- Dikker S, Wan L, Davidesco I, Kaggen L, Oostrik M, McClintock J, ... & Poeppel D. (2017). Brain-to-brain synchrony tracks real-world dynamic group interactions in the classroom. Current Biology, 27(9), 1375–1380. [DOI] [PubMed] [Google Scholar]
- Dumas G, Nadel J, Soussignan R, Martinerie J, & Garnero L. (2010). Inter-brain synchronization during social interaction. PloS One, 5(8), e12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. (2007). Parent–infant synchrony and the construction of shared timing: Physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry, 48(3‐4), 329–354. 10.1111/j.1469-7610.2006.01701.x [DOI] [PubMed] [Google Scholar]
- Feldman R. (2012). Parent-infant synchrony: A biobehavioral model of mutual influences in the formation of affiliative bonds. Monographs of the Society for Research in Child Development, 77(2), 42–51. 10.1111/j.1540-5834.2011.00660.x [DOI] [Google Scholar]
- Gabard-Durnam LJ, Mendez Leal AS, Wilkinson CL, & Levin AR (2018). The Harvard automated processing pipeline for electroencephalography (HAPPE): Standardized processing software for developmental and high-artifact data. Frontiers in Neuroscience, 12, 97. 10.3389/fnins.2018.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Wilkinson C, Kapur K, Tager-Flusberg H, Levin AR, & Nelson CA (2019). Longitudinal EEG power in the first postnatal year differentiates autism outcomes. Nature Communications, 10(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervain J, Mehler J, Werker JF, Nelson CA, Csibra G, Lloyd-Fox S, Shukla M, & Aslin RN (2011). Near-infrared spectroscopy: A report from the McDonnell infant methodology consortium. Developmental Cognitive Neuroscience, 1(1), 22–46. 10.1016/j.dcn.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J, Knickmeyer R. & Gao W. (2018). Imaging structural and functional brain development in early childhood. Nature Reviews Neuroscience, 19, 123–137. 10.1038/nrn.2018.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, Liu R, Lusby CM, Park JS, Bell MA, Newport DJ, & Stowe ZN (2021). Consistency of EEG asymmetry patterns in infants of depressed mothers. Developmental Psychobiology. 63(4), 768–781. [DOI] [PubMed] [Google Scholar]
- Gou Z, Choudhury N, & Benasich AA (2011). Resting frontal gamma power at 16, 24 and 36 months predicts individual differences in language and cognition at 4 and 5 years. Behavioural Brain Research, 220(2), 263–270. 10.1016/j.bbr.2011.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, & Kujala MV (2009). Brain basis of human social interaction: From concepts to brain imaging. Physiological Reviews, 89(2), 453–479. 10.1152/physrev.00041.2007 [DOI] [PubMed] [Google Scholar]
- Harrist AW, & Waugh RM (2002). Dyadic synchrony: Its structure and function in children’s development. Developmental Review, 22(4), 555–592. 10.1016/S0273-2297(02)00500-2 [DOI] [Google Scholar]
- Heggli OA, Konvalinka I, Cabral J, Brattico E, Kringelbach ML, & Vuust P. (2021). Transient brain networks underlying interpersonal strategies during synchronized action. Social Cognitive and Affective Neuroscience, 16(1–2), 19–30. 10.1093/scan/nsaa056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove MJ, & Risen JL (2009). It’s all in the timing: Interpersonal synchrony increases affiliation. Social Cognition, 27(6), 949–960. [Google Scholar]
- Jeste SS, Frohlich J, & Loo SK (2015). Electrophysiological biomarkers of diagnosis and outcome in neurodevelopmental disorders. Current Opinion in Neurology, 28(2), 110–116. 10.1097/WCO.0000000000000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EJH, Venema K, Lowy R, Earl RK, & Webb SJ (2015). Developmental changes in infant brain activity during naturalistic social experiences. Developmental Psychobiology, 57(7), 842–853. 10.1002/dev.21336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappenman E, & Luck S. (2010). The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology, 47, 888–904. 10.1111/j.1469-8986.2010.01009.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G, & Aksan N. (2004). Development of mutual responsiveness between parents and their young children. Child Development, 75(6), 1657–1676. 10.1111/j.1467-8624.2004.00808.x [DOI] [PubMed] [Google Scholar]
- Koenig T, Prichep L, Lehmann D, Sosa PV, Braeker E, Kleinlogel H, Isenhart R, & John ER (2002). Millisecond by millisecond, year by year: Normative EEG microstates and developmental stages. NeuroImage, 16(1), 41–48. 10.1006/nimg.2002.1070 [DOI] [PubMed] [Google Scholar]
- Konvalinka I, Bauer M, Stahlhut C, Hansen LK, Roepstorff A, & Frith CD (2014). Frontal alpha oscillations distinguish leaders from followers: Multivariate decoding of mutually interacting brains. NeuroImage, 94, 79–88. 10.1016/j.neuroimage.2014.03.003 [DOI] [PubMed] [Google Scholar]
- Konvalinka I, & Roepstorff A. (2012). The two-brain approach: How can mutually interacting brains teach us something about social interaction? Frontiers in Human Neuroscience, 6, 215. 10.3389/fnhum.2012.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraybill JH, & Bell MA (2013). Infancy predictors of preschool and post-kindergarten executive function. Developmental Psychobiology, 55(5), 530–538. 10.1002/dev.21057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzeczkowski JE, Van Lieshout RJ, & Schmidt LA (2020). Transacting brains: Testing an actor–partner model of frontal EEG activity in mother–infant dyads. Development and Psychopathology, 1–12. 10.1017/S0954579420001558 [DOI] [PubMed] [Google Scholar]
- Laurent HK, Duncan LG, Lightcap A, & Khan F. (2017). Mindful parenting predicts mothers’ and infants’ hypothalamic-pituitary-adrenal activity during a dyadic stressor. Developmental Psychology, 53(3), 417. [DOI] [PubMed] [Google Scholar]
- Leong V, Byrne E, Clackson K, Georgieva S, Lam S, & Wass S. (2017). Speaker gaze increases information coupling between infant and adult brains. Proceedings of the National Academy of Sciences, 114(50), 13290–13295. 10.1073/pnas.1702493114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J, & Feldman R. (2019). Synchronous interactions foster empathy. Journal of Experimental Neuroscience, 13. 10.1177/1179069519865799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J, Goldstein A, & Feldman R. (2017). Perception of social synchrony induces mother–child gamma coupling in the social brain. Social Cognitive and Affective Neuroscience, 12(7), 1036–1046. 10.1093/scan/nsx032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Acar ZA, Makeig S, & Deak G. (2015). EEG imaging of toddlers during dyadic turn-taking: Mu-rhythm modulation while producing or observing social actions. NeuroImage, 112, 52–60. 10.1016/j.neuroimage.2015.02.055 [DOI] [PubMed] [Google Scholar]
- Lindsey EW, Cremeens PR, Colwell MJ, & Caldera YM (2009). The structure of parent-child dyadic synchrony in toddlerhood and children’s communication competence and self-control. Social Development, 18(2), 375–396. 10.1111/j.1467-9507.2008.00489.x [DOI] [Google Scholar]
- Liu R, Calkins SD, & Bell MA (2021). Frontal EEG asymmetry moderates the associations between negative temperament and behavioral problems during childhood. Development and Psychopathology, 33(3), 1016–1025. 10.1017/S0954579420000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Calderon J, & Luck SJ (2014). ERPLAB: An open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience, 8, 213–213. 10.3389/fnhum.2014.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ (2014). An Introduction to the Event-Related Potential Technique, second edition. MIT Press. [Google Scholar]
- Lunkenheimer E, Hamby CM, Lobo FM, Cole PM, & Olson SL (2020). The role of dynamic, dyadic parent–child processes in parental socialization of emotion. Developmental Psychology, 56(3), 566–577. 10.1037/dev0000808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeill LA, Shewark EA, Pérez-Edgar K, & Blandon AY (2021). Sharing in the family system: Contributions of parental emotional expressiveness and children’s physiological regulation. Parenting. 10.1080/15295192.2020.1843358 [DOI] [Google Scholar]
- Mangold. (2020). INTERACT User Guide. Mangold International GmbH (Ed.). http://www.mangold-international.com [Google Scholar]
- Manning BL, Roberts MY, Estabrook R, Petitclerc A, Burns J, Briggs-Gowan M, Wakschlag LS, & Norton ES (2019). Relations between toddler expressive language and temper tantrums in a community sample. Journal of Applied Developmental Psychology, 65, 101070. 10.1016/j.appdev.2019.101070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markova G, Nguyen T, & Hoehl S. (2019). Neurobehavioral interpersonal synchrony in early development: The role of interactional rhythms. Frontiers in Psychology, 10, 2078. 10.3389/fpsyg.2019.02078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, & Fox NA (2002). Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology, 113(8), 1199–1208. 10.1016/s1388-2457(02)00163-3 [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Fox NA, & Group BC (2004). A comparison of the electroencephalogram between institutionalized and community children in Romania. Journal of Cognitive Neuroscience, 16(8), 1327–1338. 10.1162/0898929042304723 [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Reeb BC, & Fox NA (2009). Electrophysiological responses to auditory novelty in temperamentally different 9-month-old infants. Developmental Science, 12(4), 568–582. 10.1111/j.1467-7687.2008.00808.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy K, Hasenstab K, Senturk D, Sanders A, & Jeste SS (2015). Physiologic artifacts in resting state oscillations in young children: Methodological considerations for noisy data. Brain Imaging and Behavior, 9(1), 104–114. 10.1007/s11682-014-9343-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton KA, & Redcay E. (2020). Interpersonal synchrony in autism. Current Psychiatry Reports, 22(3), 12. 10.1007/s11920-020-1135-8 [DOI] [PubMed] [Google Scholar]
- McWeeny S, & Norton ES (2020). Understanding event related potentials (ERPs) in clinical and basic and language and communication disorders research: a tutorial. International Journal of Language and Communication Disorders, 55(4), 445–457. 10.1111/1460-6984.12535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AS, Wakschlag L, Krogh-Jespersen S, Fox N, Planalp B, Perlman SB, Shuffrey LC, Smith B, Lorenzo NE, Amso D, Coles CD, & Johnson SP (2020). Principles for guiding the selection of early childhood neurodevelopmental risk and resilience measures: HEALthy Brain and Child Development Study as an exemplar. Adversity and Resilience Science, 1(4), 247–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murias M, Webb SJ, Greenson J, & Dawson G. (2007). Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biological Psychiatry, 62(3), 270–273. 10.1016/j.biopsych.2006.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayar K, Gordon P, Martin G, Hogan-Brown A, La Valle C, McKinney W, Lee M, Norton ES, & Losh M. (2018). Links between looking and speaking in autism and first-degree relatives: Insights into the expression of genetic liability to autism. Molecular Autism, 9, 51. 10.1186/s13229-018-0233-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Banki A, Markova G, & Hoehl S. (2020). Studying parent-child interaction with hyperscanning. Progress in Brain Research, 254, 1–24. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Schleihauf H, Kayhan E, Matthes D, Vrtička P, & Hoehl S. (2021). Neural synchrony in mother–child conversation: Exploring the role of conversation patterns. Social Cognitive and Affective Neuroscience, 16(1–2), 93–102. 10.1093/scan/nsaa079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, & Posikera IN (1999). Theta synchronization during sustained anticipatory attention in infants over the second half of the first year of life. International Journal of Psychophysiology, 32(2), 151–172. 10.1016/s0167-8760(99)00011-2 [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Posikera IN, & Elam M. (2006). EEG theta rhythm in infants and preschool children. Clinical Neurophysiology, 117(5), 1047–1062. 10.1016/j.clinph.2005.12.027 [DOI] [PubMed] [Google Scholar]
- Perry A, Stein L, & Bentin S. (2011). Motor and attentional mechanisms involved in social interaction—Evidence from mu and alpha EEG suppression. NeuroImage, 58(3), 895–904. 10.1016/j.neuroimage.2011.06.060 [DOI] [PubMed] [Google Scholar]
- Piazza EA, Hasenfratz L, Hasson U, & Lew-Williams C. (2020). Infant and adult brains are coupled to the dynamics of natural communication. Psychological Science, 31(1), 6–17. 10.1177/0956797619878698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt M, Apter-Levi Y, Vakart A, Kanat-Maymon Y, Zagoory-Sharon O, & Feldman R. (2017). Mother-child adrenocortical synchrony; Moderation by dyadic relational behavior. Hormones and Behavior, 89, 167–175. 10.1016/j.yhbeh.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Quiñones‐Camacho LE, Fishburn FA, Camacho MC, Hlutkowsky CO, Huppert TJ, Wakschlag LS, & Perlman SB (2020). Parent–child neural synchrony: A novel approach to elucidating dyadic correlates of preschool irritability. Journal of Child Psychology and Psychiatry, 61(11), 1213–1223. 10.1111/jcpp.13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, & Schilbach L. (2019). Using second-person neuroscience to elucidate the mechanisms of social interaction. Nature Reviews Neuroscience, 20(8), 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, & Warnell KR (2018). A social-interactive neuroscience approach to understanding the developing brain. Advances in Child Development and Behavior, 54, 1–44. [DOI] [PubMed] [Google Scholar]
- Riva V, Marino C, Piazza C, Riboldi EM, Mornati G, Molteni M, & Cantiani C. (2019). Paternal—but not maternal—autistic traits predict frontal EEG alpha asymmetry in infants with later symptoms of autism. Brain Sciences, 9(12), 342. 10.3390/brainsci9120342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolison MJ, Naples AJ, & McPartland JC (2015). Interactive social neuroscience to study autism spectrum disorder. The Yale Journal of Biology and Medicine, 88(1), 17–24. [PMC free article] [PubMed] [Google Scholar]
- Ruysschaert L, Warreyn P, Wiersema JR, Metin B, & Roeyers H. (2013). Neural mirroring during the observation of live and video actions in infants. Clinical Neurophysiology, 124(9), 1765–1770. 10.1016/j.clinph.2013.04.007 [DOI] [PubMed] [Google Scholar]
- Salinsky MC, Oken BS, & Morehead L. (1991). Test-retest reliability in EEG frequency analysis. Electroencephalography and Clinical Neurophysiology, 79(5), 382–392. 10.1016/0013-4694(91)90203-G [DOI] [PubMed] [Google Scholar]
- Samadani A, Kim S, Moon J, Kang K, & Chau T. (2021). Neurophysiological synchrony between children with severe physical disabilities and their parents during music therapy. Frontiers in Neuroscience, 15, 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone G, Linkenkaer-Hansen K, Maurits NM, Plakas A, Maassen BAM, Mansvelder HD, van der Leij A, & van Zuijen TL (2014). Preliteracy signatures of poor-reading abilities in resting-state EEG. Frontiers in Human Neuroscience, 8(735). 10.3389/fnhum.2014.00735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L. (2010). A second-person approach to other minds. Nature Reviews Neuroscience, 11(6), 449–449. 10.1038/nrn2805-c1 [DOI] [PubMed] [Google Scholar]
- Schilbach L, Timmermans B, Reddy V, Costall A, Bente G, Schlicht T, & Vogeley K. (2013). Toward a second-person neuroscience. Behavioral and Brain Sciences, 36(4), 393–414. 10.1017/S0140525X12000660 [DOI] [PubMed] [Google Scholar]
- Shimada S, & Hiraki K. (2006). Infant’s brain responses to live and televised action. NeuroImage, 32(2), 930–939. 10.1016/j.neuroimage.2006.03.044 [DOI] [PubMed] [Google Scholar]
- Simon DM, Damiano CR, Woynaroski TG, Ibañez LV, Murias M, Stone WL, Wallace MT, & Cascio CJ (2017). Neural correlates of sensory hyporesponsiveness in toddlers at high risk for autism spectrum disorder. Journal of Autism and Developmental Disorders, 47(9), 2710–2722. 10.1007/s10803-017-3191-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John AM, Kao K, Choksi M, Liederman J, Grieve PG, & Tarullo AR (2016). Variation in infant EEG power across social and nonsocial contexts. Journal of Experimental Child Psychology, 152, 106–122. 10.1016/j.jecp.2016.04.007 [DOI] [PubMed] [Google Scholar]
- Stroganova TA, Orekhova EV, & Posikera IN (1997). EEG alpha rhythm in infants. Clinical Neurophysiology, 110(6), 997–1012. 10.1016/s1388-2457(98)00009-1 [DOI] [PubMed] [Google Scholar]
- Suveg C, Shaffer A, & Davis M. (2016). Family stress moderates relations between physiological and behavioral synchrony and child self-regulation in mother–preschooler dyads. Developmental Psychobiology, 58(1), 83–97. 10.1002/dev.21358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo AR, Obradović J, Keehn B, Rasheed MA, Siyal S, Nelson CA, & Yousafzai AK (2017). Gamma power in rural Pakistani children: Links to executive function and verbal ability. Developmental Cognitive Neuroscience, 26, 1–8. 10.1016/j.dcn.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney AL, Gabard-Durnam L, Vogel-Farley V, Tager-Flusberg H, & Nelson CA (2012). Developmental trajectories of resting EEG power: An endophenotype of autism spectrum disorder. PLoS ONE, 7(6), e39127. 10.1371/journal.pone.0039127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautman CH, & Rollins PR (2006). Child-centered behaviors of caregivers with 12-month-old infants: Associations with passive joint engagement and later language. Applied Psycholinguistics, 27(3), 447–463. 10.1017/S0142716406060358 [DOI] [Google Scholar]
- van der Velde B, & Junge C. (2020). Limiting data loss in infant EEG: putting hunches to the test. Developmental Cognitive Neuroscience, 45, 100809. 10.1016/j.dcn.2020.100809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elk M, van Schie HT, Hunnius S, Vesper C, & Bekkering H. (2008). You’ll never crawl alone: Neurophysiological evidence for experience-dependent motor resonance in infancy. NeuroImage, 43(4), 808–814. 10.1016/j.neuroimage.2008.07.057 [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, & Hans SL (1999). Relation of maternal responsiveness during infancy to the development of behavior problems in high-risk youths. Developmental Psychology, 35(2), 569–579. 10.1037/0012-1649.35.2.569 [DOI] [PubMed] [Google Scholar]
- Wang J, Barstein J, Ethridge LE, Mosconi MW, Takarae Y, & Sweeney JA (2013). Resting state EEG abnormalities in autism spectrum disorders. Journal of Neurodevelopmental Disorders, 5(1), 24. 10.1186/1866-1955-5-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass SV, Noreika V, Georgieva S, Clackson K, Brightman L, Nutbrown R, Covarrubias LS, & Leong V. (2018). Parental neural responsivity to infants’ visual attention: How mature brains influence immature brains during social interaction. PLoS Biology, 16(12), e2006328. 10.1371/journal.pbio.2006328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass SV, Whitehorn M, Marriott Haresign I, Phillips E, & Leong V. (2020). Interpersonal neural entrainment during early social interaction. Trends in Cognitive Sciences, 24(4), 329–342. 10.1016/j.tics.2020.01.006 [DOI] [PubMed] [Google Scholar]
- Zhang H, Shen D, & Lin W. (2019). Resting-state functional MRI studies on infant brains: a decade of gap-filling efforts. Neuroimage, 185, 664–684. 10.1016/j.neuroimage.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon publication, our detailed standard operating procedure (SOP) for data collection will be made freely available, and our SOP for detailed behavioral coding will be available upon request.