Abstract
HLA DRB1*02 and its subtypes predispose individuals for a far-advanced sputum-positive pulmonary tuberculosis transcending ethnic boundaries. Mycobacterium bovis BCG does not afford the desired protection against adult pulmonary tuberculosis, and a spectrum of immune reactivity exists in controls and hospital contacts. All of these findings have been identified and demonstrated in areas of endemicity. Skewing of immunity from protective to pathogenic may involve a shift in the Th1-Th2 paradigm. To elaborate these ideas, we studied gamma interferon (IFN-γ), interleukin-4 (IL-4), and IL-10 cytokine expression in 71 adult pulmonary tuberculosis patients and 74 controls from areas of endemicity in south India by 48-h microculture and reverse transcription-PCR. Most of the patients and controls expressed IFN-γ de novo, and in the presence of purified protein derivative (PPD), all of them expressed significantly higher levels of IFN-γ, suggesting a PPD-specific recall memory. HLA DRB1* allele-dependent IFN-γ expression was identified only in controls, suggesting a skewing of the immune response in patients. In contrast to the case for IFN-γ, only some patients and controls expressed IL-4 or IL-10 (Th2 profile); thus, the Th1 profile was identifiable only by a nonexpression of IL-4 or IL-10 in this area of endemicity. The Th2 profile was associated with HLA non-DRB1*02 and BCG scar-negative status in patients, attributing a significant risk (odds ratio = 2.074; 95% confidence interval = 0.612 to 7.07). It is possible that Mycobacterium tuberculosis (PPD)-specific IL-10 is expressed preemptively in unvaccinated (BCG scar-negative) individuals with a non-DR2 genetic background by chronic exposure in this area of endemicity and leads to pulmonary tuberculosis of adults.
Pulmonary tuberculosis has recently become a major problem in developed countries with the increase in the incidence of tuberculosis in immunocompromised human immunodeficiency virus-infected individuals and the increase in the emergence of multidrug-resistant bacilli (3, 22); it has already been recognized as the greatest killer and as a terminal disease in developing countries. Mycobacterium bovis BCG is the only prophylactic vaccine available as yet; BCG has been used throughout the world, although with varying efficacies (17). With the discovery of cytokines and the description of the Th1-Th2 paradigm, the role of cytokines in tuberculosis pathogenesis has been investigated. A standard dose of BCG induces mycobacterium-specific gamma interferon (IFN-γ), a CD4 response, and antigen-specific cytotoxicity (27). However, the development of the T-cell response itself is markedly influenced by purified protein derivative (PPD) sensitivity before vaccination (39). Studies on mouse models have equated Th1 to protective, cell-mediated immunity and Th2 to disease status (2, 32). The role of interleukin-4 (IL-4) and IL-10 in modulating IFN-γ expression has recently been described; transgenic and gene knockout mouse models have shown a suppression of Th1-like T-cell responses by IL-10 (21, 49). For humans, no concrete evidence relating Th2 to disease status has yet been described.
Our understanding of the role of host immunogenetics in infectious diseases is also limited; both major histocompatibility complex (MHC) and non-MHC genes have been implicated (6, 20a, 23). Earlier studies on HLA association with pulmonary tuberculosis did not lead to any consensus (35). With the discovery of MHC class II, HLA DR2 associations in pulmonary tuberculosis have been amply confirmed in countries where the disease is endemic, such as India, Indonesia, and Russia (7, 9, 25, 37, 38, 40, 41). Non-MHC genes such as those for the mannose receptor, vitamin D receptors, and NRAMP1 have also been implicated in the disease process (6, 20a, 23). Genome scan studies have suggested that the susceptibility is a polygenic, multifactorial phenomenon (23). Nonetheless, these genetic predispositions alone do not account for all of the cases investigated in any study.
Many variables surround key genetic epidemiological processes for Mycobacterium tuberculosis infection in regions of endemicity. These include the age of the host, postinfection disease progression, antigenic variation of the bacteria, microbial cross-reactivity, and environmental persistence and virulence of the bacilli. Whether all three categories, i.e., host genetic, immunological, and epidemiological factors, could be a reason for the reduced efficacy of BCG in protection from adult pulmonary tuberculosis in south India is a question of interest (4, 17, 39). In the study reported here, we investigated DRB1* and DQB1* alleles and PPD-RT23-recalled cytokine expression in pulmonary tuberculosis patients and controls from areas of endemicity and correlated them to the BCG vaccination status.
MATERIALS AND METHODS
Study population. (i) Pulmonary tuberculosis patients.
A total of 71 adult pulmonary tuberculosis patients, born and brought up in the Madurai district, south India, were studied in two samplings drawn from two different hospitals. The first group, of 25 patients (mean age ± standard error of the mean, 42.3 ± 1.83 years; all males), were from the state-run TB Sanatorium, Thoppur, Madurai, India, 13 km southwest of Madurai. All of the patients had advanced stages of the disease, with sputum smears positive for acid-fast bacilli and X-ray positive with bisubapical and bisubapical-apical infiltrates. These patients had been treated for 6 to 61 days at the time of sampling. The second group, of 46 patients (35.1 ± 2.04 years; male/female ratio, 26:20), were from a state-run public government hospital at Singampunari, 65 km northeast of Madurai. Patients with presenting symptoms of pulmonary tuberculosis with radiological lesions and a compatible clinical picture, with or without acid-fast bacilli in sputum smears, were included in the study. These patients had been treated for 3 to 180 days at the time of sampling. Both deltoids were examined for BCG scar status. Appropriate ethical clearance for the study from the institutional ethical committee and informed consent from patients and controls were obtained.
(ii) Controls from areas of endemicity.
Seventy-four controls from areas of endemicity, consisting of members of the staff and students of the university who were born and brought up in and around Madurai, were enrolled and studied in two groups (n = 25 and 49). The ages were 27.3 ± 1.65 and 26.4 ± 0.71 years, respectively, and the male/female ratios were 18:7 and 29:20, respectively. Both deltoids were examined for BCG scar status.
Antigen.
PPD-RT23 without preservative, at a concentration of 50,000 U per ml, was a gift from the BCG Vaccine Laboratory, Guindy, Chennai, India. A final concentration of 100 U/ml of culture medium was used.
MHC class II genotyping.
HLA-DRB1* and HLA-DQB1* genotyping of the patients and controls were performed by the PCR–sequence-specific oligo probe (SSOP) method, employing XI IHWC primers and probes as described elsewhere (38).
Short-term cultures.
Three to 5 milliliters of peripheral blood was obtained from each donor in heparin vacutainers (455 051; Greiner, Frickenhausen, Germany) and moved to the laboratory, and cultures were set up the same day.
The whole-blood culture technique described by Petrovsky and Harrison (33) was adapted; it required a smaller volume of blood (46). In short, 50 μl of heparinized whole blood, diluted to 200 μl with RPMI 1640 medium and antibiotics, was cultured for 48 h in 96-well U-bottom tissue culture plate (655 180; Greiner). RPMI 1640 medium (31870-025; Life Technologies, Gibco-BRL, Gaithersburg, Md.), supplemented with 6% pooled human AB serum (88-17-20-013; Baxter Healthcare Ltd., Norfolk, United Kingdom), 2 mM glutamine (G-1517; Sigma, St. Louis, Mo.), and antibiotics (S-9137 and P-3032; Sigma) was used. Cultures were set up in quadruplicate, with or without PPD-RT23, and incubated for 48 h in a CO2 incubator (3164; Forma Scientific, Marietta, Ohio) set at 37°C with 5% CO2 and 95% humidity. Cultures were harvested; two of the quadruplicates were pooled, one aliquot was processed further, and the other aliquot was stored. In initial experiments, a dilution of 1:4 (whole blood in medium) was found to be optimum, giving good β-actin and cytokine expression. Optimum production of IFN-γ, IL-4, and IL-10 was identified by 48 h after stimulation, as already reported by many others (13, 34, 44).
Peripheral blood mononuclear cell (PBMC) cultures with 0.6 million cells per well were set up in quadruplicate in 96-well U-bottom microtiter plates (655 180; Greiner) according to standard procedures and as described above. The first group of samples was studied by this PBMC culture method. Experiments on two patients and four controls comparing whole-blood culture with PBMC culture gave concordant results, validating the use of whole-blood culture: whenever an individual expressed IFN-γ and/or other cytokines in whole-blood culture, the same cytokines were expressed in PBMC culture as well (14). Hence, the results of the two experiments were pooled and analyzed.
RNA extraction and cDNA synthesis.
Total RNA was extracted by a single-step acid-phenol-chloroform extraction method (10), taking all of the necessary precautions to wash the glass- and plasticware with 0.1% diethyl pyrocarbonate (DEPC) (44170 3D; BDH Laboratory Supplies, Poole, United Kingdom). The cultures were harvested, washed in cold phosphate-buffered saline, and immediately suspended in 500 μl of lysis buffer and stored frozen at −70°C. To extract total RNA, the lysates were thawed and processed further as described elsewhere (10). RNA pellets were finally washed with 80% ethanol, dried, and suspended in 8 μl of DEPC water. The samples were primed with 1 μg of oligo(dT) primer (27-7858-02; Pharmacia Biotech) at 65°C for 10 min and cooled on ice, and cDNA was synthesized in a block heater set at 37°C (QBT1; Grant Instruments, Cambridge, United Kingdom) (16). The samples were finally denatured at 95°C for 5 min, cooled on crushed ice, and diluted to 100 μl using DEPC water.
PCR to identify cytokine genes.
Five microliters of the cDNA templates in a 25–μl PCR mixture were used to identify each cytokine gene. The primer sequences for β-actin, IFN-γ, and IL-4 and the procedures of Ehlers and Smith (16) were used. Primers for IL-10 were from Yamamura et al. (48). Primers were made to order by Genosys, Cambridgeshire, United Kingdom, and the sequences of primers were as follows: β-actin, 5′TGACGGGGTCACCCACACTGTGCCCATCTA3′ (forward) and 5′CTAGAAGCATTGCGGTGGACGATGGAGGG3′ (reverse) (product size, 661 bp); IFN-γ, 5′ATGAAATATACAAGTTATATCTTGGCTTT3′ (forward) and 5′GATGCTCTTCGACCTCGAAACAGCAT3′ (reverse) (product size, 501 bp); IL-4, 5′ATGGGTCTCACCTCCCAACTGCT3′ (forward) and 5′CGAACACTTTGAATATTTCTCTCTCAT3′ (reverse) (product size, 456 bp); and IL-10, 5′ATGCCCCAAGCTGAGAACCAAGACCCA3′ (forward) and 5′TCTCAAGGGGCTGGGTCAGCTATCCCA3′ (reverse) (product size, 351 bp).
PCRs were performed in 0.2-ml PCR tubes or in PCR strips, using HYBAID thermal cyclers (HBTR3 3CM 220). PCR cycles consisted of denaturation at 95°C for 45 s, annealing at 57.5°C for 45 s, and extension at 71.5°C for 90 s for 35 cycles, with a final extension of 72°C for 10 min. Twenty microliters of the amplified products was electrophoresed at 100 V for 15 min in an agarose gel with ethidium bromide and documented.
Quality control measures.
To control the relative amount of products reverse transcribed and to assess the amount of a given messenger in each sample, concurrent PCR, electrophoresis, and measurements of the housekeeping gene for β-actin and the specific cytokines were performed. The gels were visualized under UV illumination and documented in an EDAS 120 system (154 9393; Eastman Kodak Company, Rochester, N.Y.) (Fig. 1). The net band intensities were measured as pixels, using Kodak Digital Science one-dimensional image analysis software. To avoid inter- and intraexperimental variations, PCR on each sample was repeated twice and assessed for concordance, and the average was used for analysis. The net intensity of cytokine bands was normalized to the intensity of β-actin bands of the given sample and expressed as a percentage of β-actin intensity (cytokine band intensity × 100/β -actin band intensity). PPD-specific cytokine expression was obtained by deducting the percent β-actin values of no-antigen controls from the percent β-actin values of the respective PPD-induced experiments. An individual was considered negative for a given cytokine when he or she did not produce the given cytokine in the presence and absence of the antigen; the results were always confirmed by repeating the PCR for the cytokine.
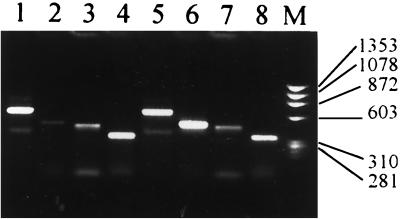
FIG. 1.
Photograph of a 1.5% agarose gel showing β-actin, IFN-γ, IL-4, and IL-10 bands identified by RT-PCR. Fifty microliters of whole blood from each patient was diluted to 200 μl using RPMI 1640 and cultured in duplicate in the absence (lanes 1 to 4) or in the presence (lanes 5 to 8) of PPD-RT23 (100 U/ml) at 37°C in a CO2 incubator. After 48 h, cultures were harvested, duplicates were pooled, total RNA was extracted, and cDNA was synthesized and diluted to 100 μl. Five microliters each of this cDNA was used as a template to amplify the β-actin, IFN-γ, IL-4, or IL-10 gene using sequence-specific primers. The results were documented in a Kodak EDAS 120 system, and band intensity was measured as pixels. The cytokine band intensity was normalized to the β-actin band intensity using the formula net intensity of cytokine/net intensity of β-actin × 100. Lane M, standard marker (φ174 digested with HaeIII).
Validation of RT-PCR.
In order to validate the reverse transcription-PCR (RT-PCR) results obtained, RT-PCR and IFN-γ ELISpot (enzyme-linked immunospot) cell titration experiments were performed simultaneously on the same samples. ELISpots were obtained as per the instructions of the kit manufacturer (3420-2; MABTECH, Stockholm, Sweden). PBMCs from two healthy volunteers were titrated from 0.1 to 2.5 million and cultured for 48 h for RT-PCR and for 18 h for ELISpot analysis. The number of PBMCs in microcultures and the net intensity of the RT-PCR bands (measured as pixels) compared well (r2 [P value]: β-actin, 0.9789 [0.003]; IFN-γ, 0.9979 [0.015]). In essence, over a wide range of 10 dilutions between 0.1 to 2.5 million PBMCs, there was a linear correlation between the RT-PCR band intensity and the number of PBMCs used. Comparison of the RT-PCR band intensity and the number of IFN-γ ELISpots also correlated well in measuring the PPD-specific cytokine expression and secretion in both donors (r2 = 0.8211 and 0.9292) (three dilutions tested). Although the recent, real-time PCR is the best method to quantify expression, it does not eliminate the inherent problems of measuring microvolumes and PCR failures; with enough precautions and stringency, the average net intensity of RT-PCR bands in the gel from repeat testing of a sample gives comparable results and can best be employed for high throughput, as suggested elsewhere (45).
Representative PCR products of IFN-γ, IL-4, IL-10, and β-actin were further sequenced using Big Dye terminator (catalog no. 4303149; Perkin-Elmer Applied Biosystems) in an ABI Prism 310 Genetic Analyzer (Perkin-Elmer Applied Biosystems). The obtained sequences were compared with human IFN-γ, IL-4, and IL-10 sequences by Blast search. There were 97% identities for the forward and reverse sequences of human IFN-γ (accession no. emb/X01992.1), 91 and 89% for human IL-4 (NM 000589.1), 97 and 98% for human IL-10 (gb/M57627.1), and 94% for human β-actin (emb/X00351.1).
Statistics.
Comparison of various groups and subgroups and their cytokine expression were analyzed by the paired t test, Wilcoxon signed rank test (WRT), Mann-Whitney (MW) test, chi-square test, Mantel-Haenszel test, and odds ratio, as applicable (42). Log-linear model analysis was employed to evaluate the contributions of the HLA-DRB1*02 allele, BCG vaccination status, and IL-10 expression in disease development (20).
RESULTS
All of the controls and patients expressed PPD-specific IFN-γ but not IL-4 or IL-10.
Peripheral blood samples from 44 controls from areas of endemicity and 43 pulmonary tuberculosis patients were cultured by the whole-blood culture method in either the presence or absence of PPD-RT23 and assessed for β-actin, IFN-γ, IL-4, and IL-10 cytokine gene expression by RT-PCR (Fig. 2). There was no difference in β-actin expression between the no-antigen control and PPD-RT23 cultures of both controls from areas of endemicity and patients (data not shown). However, 91% of the controls from areas of endemicity and 71% of the patients expressed a basal level of IFN-γ in the no-antigen control cultures, while all of them produced enhanced level of IFN-γ following PPD stimulation (paired t test, P < 0.0001; WRT, P < 0.0001). The data on the duration of treatment in patients did show a nonsignificant decline in IFN-γ level (r2/P: IFN-γ, 0.05/0.22; IL-4, 0.04/0.25; IL-10, 0.006/0.66) and reduced variance; however, this did not affect the positive status on a qualitative scale (see below).
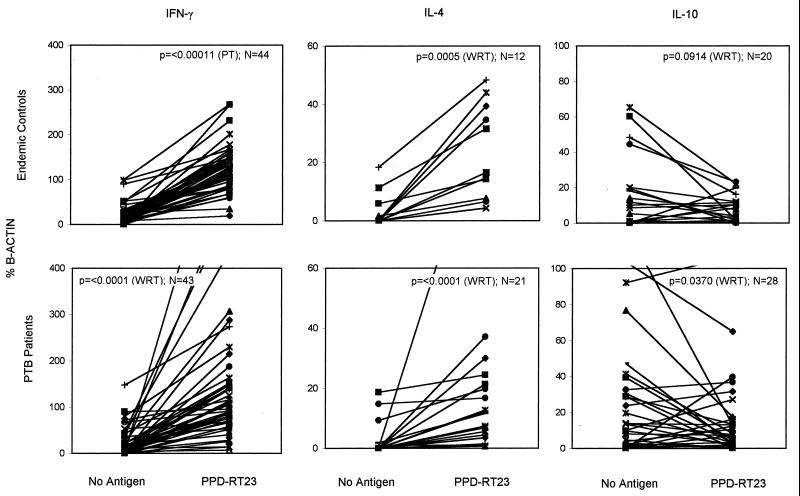
FIG. 2.
Comparison of basal (no-antigen control) and PPD-RT23-induced IFN-γ, IL-4, and IL-10 expression in pulmonary tuberculosis patients (n = 43) and controls from areas of endemicity (n = 44) from south India, studied by the whole-blood culture method. Fifty microliters of whole blood diluted to 200 μl with RPMI medium was incubated for 48 h in the presence or absence of antigen in a CO2 incubator. Ninety-one percent of the controls and 71% of the patients expressed IFN-γ in the no-antigen control mixture, while all of the patients and controls expressed IFN-γ in the presence of PPD. In contrast, only certain individuals (responders, positives), produced IL-4 and/or IL-10 in either the presence or absence of PPD. The values of cytokine expression (percentage of β-actin expression) of a sample in the absence and presence of PPD are connected by lines, and the paired t test (PT) or WRT was applied to determine the significance.
In contrast to IFN-γ, IL-4 and IL-10 were not expressed by all of the controls from areas of endemicity and pulmonary tuberculosis patients. Only 12 of 44 controls from areas of endemicity and 21 of 43 patients responded to PPD; these responders expressed an elevated level of IL-4 in the presence of PPD (by WRT, P = 0.0005 and P < 0.0001, respectively), indicating an upregulation. IL-10 expression was observed in 21 of 44 controls from areas of endemicity and in 28 of 43 pulmonary tuberculosis patients. Paradoxically, the IL-10 expression decreased following PPD stimulation in many of them (by WRT, P = 0.0914 and P = 0.037, respectively) (Fig. 2). This suggested an active suppression by PPD exposure in vitro. Thus, immunological memory specific to PPD was recalled in 48 h, and this was identifiable either by upregulation or by active suppression of the cytokines in question.
HLA-DRB1* alleles influence IFN-γ expression.
In order to determine whether the DRB1* allele correlates with the level of IFN-γ expression, the IFN-γ levels in PPD cultures were stratified and the data were analyzed. The results were significant in controls from areas of endemicity but not in patients. The highest level of IFN-γ was observed in controls with DRB1*03 alleles (mean ± standard error of the mean as percent β-actin = 189 ± 32), and the lowest was observed in controls with DRB1*08 alleles (93 ± 11) (by the MW test, P = 0.0023) (Table 1). DRB1*03, -07, -09, -10, and -1502 produced above-median values of IFN-γ, and all of their values were significantly higher than those for controls with DRB1*08 (P = 0.0023 [MW test], P = 0.0187 [t test], P = 0.0469 [t test], P = 0.0527 [t test], and P = 0.0382 [MW test], respectively). DRB1*1501, -04, and -08 produced significantly lower levels of IFN-γ than the highest producer, DRB1*03 (P = 0.0235 [t test], P = 0.038 [t test], and P = 0.0023 [MW test], respectively). PPD-specific IFN-γ expression was different only between DRB1*07 and DRB1*08 (136 ± 21 and 75 ± 13; P = 0.0356 [t test]). However, the allele dependence of IFN-γ in the PPD-stimulated culture and PPD-specific IFN-γ expression correlated well (r2 = 0.7152; P = 0.0076), suggesting an inherent nature of this response. Analysis of the data based on DQB1* alleles did not yield significant differences between various alleles. Due to a smaller number of positives with IL-4 and IL-10, it was not worthwhile to stratify and analyze these data.
TABLE 1.
PPD-RT23-induced IFN-γ expression and its HLA DRB1∗ allele dependence in controls from areas of endemicity
| DRB1* allele (na) | PPD-specific IFN-γ expression (mean ± SEM)b |
|---|---|
| DRB1*1501 (19) | 128 ± 9.8 |
| DRB1*1502 (6) | 143 ± 20 |
| DRB1*03 (7) | 189 ± 32 |
| DRB1*04 (12) | 123 ± 12 |
| DRB1*06 (8) | 134 ± 24 |
| DRB1*07 (8) | 167 ± 24 |
| DRB1*08 (7) | 93 ± 11 |
| DRB1*09 (3) | 163 ± 41 |
| DRB1*10 (4) | 150 ± 29 |
Number of samples with the indicated DRB1∗ allele.
P values for significant differences between alleles are given in the text. No significant differences were observed among patients.
HLA non-DRB1*02 status and BCG scar-negative status are associated with IL-4 and IL-10 expression status in pulmonary tuberculosis patients.
The observations that the responding controls from areas of endemicity and patients expressed significant amounts of IL-4 and IL-10 and that IFN-γ was expressed by all of the individuals in this area of endemicity led us to look for other parameters of susceptibility. In this regard, HLA DRB1*02, an allele repeatedly confirmed to have association with pulmonary tuberculosis (7, 9, 25, 37, 38), was one of the candidates. The second one was BCG vaccination status. It is known that BCG vaccination in India protects infants but not adults from pulmonary tuberculosis (4, 44a); there are many suggestions on how a BCG vaccine protecting infants may be skewed in adults (17). The cytokine expression status in 71 patients and 74 controls was thus studied qualitatively, and data were analyzed based on the above parameters (Table 2).
TABLE 2.
Correlation between BCG scar status and IL-10 and/or IL-4 expression status in pulmonary tuberculosis patients and controls
| Cytokine status | No. with the indicated BCG status
|
|||
|---|---|---|---|---|
| Patients (n = 71)
|
Controls (n = 74)
|
|||
| Negative | Positive | Negative | Positive | |
| IL-10 | ||||
| Negative | 18 | 11 | 15 | 21 |
| Positive | 37 | 5 | 18 | 20 |
| P | 0.0099 | 0.6219 | ||
| IL-4 | ||||
| Negative | 19 | 12 | 18 | 29 |
| Positive | 36 | 4 | 15 | 12 |
| P | 0.0041 | 0.1505 | ||
| IL-4 + IL-10a | ||||
| Negative | 12 | 10 | 14 | 9 |
| Positive | 30 | 3 | 14 | 10 |
| P | 0.0053 | 0.2355 | ||
Results where IL-4 and IL-10 were not both positive or both negative were not counted, and hence n was 55 and 47 for patients and controls, respectively.
Thirty-seven of the 71 patients were BCG scar negative and IL-10 positive; this was significant in a two-by-two cross tabulation (P = 0.0099). Similarly, 36 patients were BCG scar negative and IL-4 positive (P = 0.0041), and 30 of them were BCG scar negative and both IL-4 and IL-10 positive (P = 0.0053). No such skewed distribution was found in controls (Table 2). Many other parameters, such as age, sex, duration of treatment, and caste of the subjects, did not yield meaningful results.
Nested classification analysis of the three parameters, viz., DRB1*02 status, BCG status, and IL-10 status was applied to study their interactions (20). All possible log-linear models, starting from the model of DRB1*02 effect only to the model involving interaction of (DRB1*02)(IL-10), (BCG)(IL-10), and (DRB1*02)(BCG), were considered and tested for their significance. A model involving (DRB1*02)(IL-10) and (BCG)(IL-10) had the highest likelihood ratio probability, of 0.3711. The expected frequency of this log-linear model was given by log eijk = μ + λDi + λBj + λIk + λDIik + λBIjk, where eijk is the expected frequency of the cell (i, j, k) and D represents the factor DRB1*02, I represents the factor IL-10, and B represents the factor BCG. The estimates of these parameters were obtained, and the expected frequencies were calculated according to the fitted model (Table 3). The differences between observed and expected frequencies were expressed as standardized deviates (z values). All of the standardized deviates were less than 1.96 (i.e., a probability of >0.05), and hence the proposed model, (DRB1*02)(IL-10) and (BCG)(IL-10) interactions, was validated. Similar characteristics were observed with IL-4 as well; the standardized deviates were less than 1.96 for the model (DRB1*02)(IL-4) and (BCG)(IL-4) interactions. No such interactions were identified in controls.
TABLE 3.
Nested classification analysis of three factors (DRB1∗02, BCG, and IL-10 status) and their interactions in pulmonary tuberculosis patients (n = 71)
| DRB1∗02 status | BCG scar status | IL-10-negative patients
|
IL-10-positive patients
|
||||
|---|---|---|---|---|---|---|---|
| No. observed | No. expected | z valuea | No. observed | No. expected | z value | ||
| Negative | Negative | 9 | 7.4 | 0.6 | 24 | 24.7 | −0.1 |
| Positive | 3 | 4.6 | −0.7 | 4 | 3.3 | 0.4 | |
| Positive | Negative | 9 | 10.6 | −0.5 | 13 | 12.3 | 0.2 |
| Positive | 8 | 6.4 | 0.6 | 1 | 1.7 | −0.5 | |
Standardized deviate (z value) = (observed − expected)/√expected.
Further analyses taking IL-10 as the explanatory variable and disease status (patients and controls) as the outcome variable with all cross combinations of DRB1*02 and BCG revealed a significant odds ratio for non-DRB1*02, BCG scar-negative and IL-10-positive patients and controls (odds ratio = 2.07; 95% confidence interval = 0.612 to 7.072) (Table 4). Considering IL-4 as the explanatory variable, the odds ratio was only 1.53 (confidence interval, 0.51 to 4.6).
TABLE 4.
Comparison of the observed frequencies of IL-10 expression status in pulmonary tuberculosis patients and controls and the probability of occurrence (odds ratio)
| DRB1∗02 status | BCG scar status | Patients (n = 71)
|
Controls (n = 74)
|
Odds ratioa | 95% confidence interval | ||
|---|---|---|---|---|---|---|---|
| No. IL-10 negative | No. IL-10 positive | No. IL-10 negative | No. IL-10 positive | ||||
| Negative | Negative | 9 | 24 | 7 | 9 | 2.074 | 0.612–7.07 |
| Positive | 3 | 4 | 8 | 13 | 0.821 | 0.162–4.19 | |
| Positive | Negative | 9 | 13 | 8 | 9 | 1.284 | 1.282–0.37 |
| Positive | 8 | 1 | 13 | 7 | 0.054 | 0–0.53 | |
An odds ratio of 2.074 indicates that the IL-10 expression status was associated with DRB1∗02-negative status and BCG scar status in patients.
DISCUSSION
The present study has brought out some important observations on the interplay of MHC, cytokines, and BCG vaccination (immune) status in pulmonary tuberculosis susceptibility in this area of endemicity. First, a high background level and PPD-recalled IFN-γ expression observed in this area of endemicity could be attributed to the endemicity of typical and atypical mycobacterial infections and the resultant subclinical exposure. Second, a significant increase in PPD-recalled IFN-γ expression in both patients and controls irrespective of their BCG status has indicated immunological memory resulting from chronic exposure or disease, overriding the BCG vaccination-induced immunity. The DRB1* allele dependence of IFN-γ expression identified only in controls from areas of endemicity but not in patients has suggested that the IFN-γ expression is allele dependent and inherent and may be obliterated in patients by the infection per se. Our preliminary studies on ELISpot Pepscan with Esat-6 peptides show similar MHC-restricted IFN-γ ELISpots in controls from areas of endemicity but not in pulmonary tuberculosis patients (S. Vani, unpublished data). Thus, understanding the difference between subclinical exposure and the infection per se in skewing the course of the immune response and the epitope specificity of the immunological memory and immunopathogenesis in this area of endemicity has become urgent.
Third, the nested classification analysis of the data has suggested that many of the BCG scar-positive patients and controls did not express IL-10 (11 of 16 patients and 21 of 41 controls) or IL-4 (12 of 16 patients and 29 of 41 controls); this suggests a long-lasting memory of BCG vaccination identified by Th2 cytokine nonexpression. This implies that BCG vaccination induces a Th1 type of immunity in this area of endemicity but that this is of no use in protecting adults from infection, reinfection, or reactivation. Further the expression of Th2 cytokines IL-10 and IL-4 is dependent on non-DRB1*02 and BCG scar-negative status in patients. It is possible that the Th2 profile of an individual is inherent: if an individual had not been vaccinated during childhood and has a non-DR2 phenotype, the chances of infection leading to clinical disease as an adult are greater. It is interesting that (i) DRB1*02 and (ii) non-DRB1*02 but IL-10 account for 83% of the cases.
It is essential to mention here that the BCG scar-negative subjects reported in this study may include at least three groups: those who were not BCG vaccinated, those who were BCG vaccinated but did not develop the scar, and those who were BCG vaccinated and developed the scar but lost the scar over time (16a). Although BCG vaccination is mandatory in India, the BCG scar prevalence is quite varied in our study areas (unpublished observation). As identified elsewhere (16a), it is possible that a proportion of the reported BCG scar-negative patients and controls were really vaccinated. However, in the present study, despite the possible heterogeneity of the BCG scar-negative group, we find a good correlation of this group with non-DRB1*02 and IL-10 status.
It has become urgent to investigate how the endemicity of infection may nullify the childhood protective immunity conferred by BCG vaccination. Studies on animal models of infectious diseases have shown that a Th1 profile is protective and a Th2 profile is pathogenic. Although there is compelling evidences that IFN-γ is a protective cytokine in mouse tuberculosis (12, 18) and although IFN-γ is maximally expressed at the initial stages of infection and IL-4 is maximally expressed after the infection has been contained (32), their value in humans in an area of endemicity like India is not known. The protective efficacy of DNA vaccines encoding M. tuberculosis sequences is correlated to the emergence of IFN-γ-secreting T cells in mice (24). A standard dose of BCG given to unvaccinated individuals from the United States (which is not an area of endemicity) induces both delayed-type hypersensitivity and IFN-γ (27). Further, the IFN-γ levels in BCG-sensitized controls from a region that is not an area of endemicity are also high (47). Although the expression of IFN-γ is believed to be an indicator of protective immunity, active disease is also associated with IFN-γ expression and increased CD8+ cells in the bronchoalveolar lavage (43) and with high levels of IL-10, but not IFN-γ, to M. tuberculosis antigens in pulmonary tuberculosis patients (44). In a recent study from Madras, south India, BCG vaccination of PPD-negative adults did not change the level of IFN-γ or other cytokines studied (13). This supports our contention that the Mantoux status might be a host genetics-determined immune response (8, 19, 36).
The present observation that all of the samples produced PPD-recalled IFN-γ irrespective of the vaccination status of the subject, while only selected responders, mostly among the BCG scar-negative group and the non-DR2 group, produced IL-10 and IL-4 indicates an important role for IL-10 and IL-4, i.e., a role for Th2 polarization in adult pulmonary tuberculosis pathogenesis. The expression of PPD-recalled IFN-γ by all of the patients and controls may be attributed to environmental sensitization by specific and nonspecific cross-reacting pathogens and microorganisms (4), which may not be of any use in the presence of IL-4 or IL-10 expression. The present study has shown that IL-4 or IL-10 expression needs to be relied on to identify the Th2 or Th1 profile in areas of endemicity. It has been suggested that IL-10 and IL-4 expression may be one of the mechanisms to suppress IFN-γ-mediated, protective immunity (30). IL-10 is a potent inhibitor of T-cell functions, MHC class II expression, antigen-specific proliferation, and IFN-γ synthesis, and it is inversely correlated to IFN-γ in human tuberculosis (22, 44). In a mouse model, IL-4 influences the differentiation of Th0 cells into Th2 cells (1), and the M. tuberculosis antigen lipoarabinomannan is known to specifically induce IL-4 (11). Studies on gene knockout mice have shown that IL-10 is an inhibitor of early mycobacterial clearance and that it negatively regulates numerous macrophage functions and inflammatory responses (30). In humans, IL-4 and IL-10 have been identified during the early course of the immune response (5, 44), and further, the highest levels of IL-4 and transforming growth factor β, with a concomitant decrease in IFN-γ, have also been identified in advanced tuberculosis (15).
The increased expression of IL-10 in both the patients and controls implies that this may be a de novo process, reinforced by the endemicity of infections. This assumption is supported by an active depression of IL-10 expression following in vitro exposure to PPD. It is possible that following the exposure to a higher concentration of PPD in vitro, the cytokines of the Th1 arm might be produced, preemptively suppressing the in vivo sensitized IL-10 expression. A similar observation of decreased expression of IL-10 following in vitro exposure to M. tuberculosis 38-kDa antigen has been made in our laboratory (S. Shanmugalakshmi, unpublished data). In another study from south India, IL-10 expression was detectable in the absence of any in vitro stimulation and was depressed 8 weeks after BCG vaccination of healthy adults (13). The relative densities of various M. tuberculosis epitopes may be responsible for this kind of shift; further in-depth investigation may throw light on this issue.
Thus, the overall Th1-Th2 paradigm is subject to the dynamics of microbial antigens and their relative densities: once a Th2 polarization has occurred preemptively, in the absence of BCG vaccination and with a non-DRB1*02 status, a clinical disease may follow. It is possible that once the T-helper phenotype is determined it might not be further influenced by related and unrelated antigens, as suggested in the case of leprosy (29). It is possible that the endemicity of infection, chronic exposure, and heterologous immunity skew the immune response, facilitating the ability of the infection to overwhelm the host defenses (17). The underlying mechanism may be an epitope shift in antigen recognition based on the abundance and persistence of various M. tuberculosis epitopes and the immunosequestration during various stages of the disease and exposure. The observation that BCG vaccination was not able to protect in the lepromatous form of leprosy but was able to do so in a more unstable borderline form is an example of this school of thought (43a).
The present observations on the MHC class II (DRB1*) allele dependence of IFN-γ expression in controls but not in patients suggests that an inherent cytokine expression can be skewed by the infectious load. Another study employing a similar whole-blood culture system has also associated many HLA alleles (HLA DR1, -2, and -6) with high IFN-γ production and a few others (DR3, -4, -5, and -7) with low IFN-γ production in healthy individuals (34). It is known that in a mouse model C57BL/6 mice are preferentially IFN-γ producers, while BALB/c mice are preferentially IL-4 producers (31). It is possible that the MHC-linked cytokine production status and MHC-presented and antigen- or epitope-specific induction of cytokines are two different facets of cytokine expression, the first one being genetic and a priori and the second one being immunological.
In conclusion, the present study has indicated an important role for Th2 cytokines, BCG vaccination status, and MHC class II-DRB1 allelic polymorphisms in adult pulmonary tuberculosis susceptibility in this environment of endemicity. Deciphering the M. tuberculosis antigens and epitopes skewing the M. tuberculosis-specific immune responses has become urgent. Any new-generation vaccine and immunotherapeutics should induce a sterilizing immunity in patients and high-risk adults from this region of endemicity (3, 28).
ACKNOWLEDGMENTS
This project was supported by Commission of European Communities, Brussels, Belgium, jointly with Juraj Ivanyi (fixed contribution contract C/1-CT93-0079) and the Department of Biotechnology, Government of India, New Delhi (BT/PRO281/Med/09/057/96).
Permission from the Director of Rural and Public Health, Government of Tamil Nadu (H.Dis.104968/TB/1/97), to carry out the study is acknowledged.
REFERENCES
- 1.Abehsira-Amar O, Gibert M, Joliy M, Theze J, Jankovic D L. IL-4 plays a dominant role in the differential development of Th0 into Th1 and Th2 cells. J Immunol. 1992;148:3820–3829. [PubMed] [Google Scholar]
- 2.Andersen P, Herron L. Specificity of a protective memory immune response against Mycobacterium tuberculosis. Infect Immun. 1993;61:844–851. doi: 10.1128/iai.61.3.844-851.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson R M. Tuberculosis: old problems and new approaches. Proc Natl Acad Sci USA. 1998;95:13352–13354. doi: 10.1073/pnas.95.23.13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baily G V J, Narain R, Mayurnath S, Vallishayee R S, Guld J. Tuberculosis prevention trial, Madras. Indian J Med Res. 1980;72:1–74. [PubMed] [Google Scholar]
- 5.Baliko Z, Szereday L, Szekeres-Bartho J. Th2 biased immune response in cases with active Mycobacterium tuberculosis infection and tuberculin anergy. FEMS Immunol Med Microbiol. 1998;22:199–204. doi: 10.1111/j.1574-695X.1998.tb01207.x. [DOI] [PubMed] [Google Scholar]
- 6.Bellamy R, Ruwende C, Corrah T, McAdam K P, Whittle H C, Hill A V. Variations in the NRAMP1 gene and susceptibility to tuberculosis in West Africans. N Engl J Med. 1998;338:640–644. doi: 10.1056/NEJM199803053381002. [DOI] [PubMed] [Google Scholar]
- 7.Bothamley G H, Swanson-Beck J, Schreuder G M T, D'Amaro J, de Vries R R, Kardjito T, Ivanyi J. Association of tuberculosis and Mycobacterium tuberculosis specific-antibody levels with HLA. J Infect Dis. 1989;159:549–555. doi: 10.1093/infdis/159.3.549. [DOI] [PubMed] [Google Scholar]
- 8.Bothamley G H, Beck J S, Potts R C, Grange J M, Kardjito T, Ivanyi J. Specificities of antibodies and tuberculin response after occupational exposure to tuberculosis. J Infect Dis. 1992;166:182–186. doi: 10.1093/infdis/166.1.182. [DOI] [PubMed] [Google Scholar]
- 9.Brahmajothi V, Pitchappan R M, Kakkanaiah V N, Sashidhar M, Rajaram K, Ramu S, Palanimurugan K, Paramasivan C N, Prabhakar R. Association of pulmonary tuberculosis and HLA in south India. Tubercle. 1991;72:123–132. doi: 10.1016/0041-3879(91)90039-u. [DOI] [PubMed] [Google Scholar]
- 10.Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 11.Collins H L, Schaible U E, Kaufmann S H E. Early IL-4 induction in bone marrow lymphoid precursor cells by mycobacterial lipoarabinomannan. J Immunol. 1998;161:5546–5554. [PubMed] [Google Scholar]
- 12.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russel D G, Orme I M. Disseminated tuberculosis in interferon-γ-gene disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das S D, Narayanan P R, Kolappan C, Colston M J. The cytokine response to Bacille-Calmette Guerin vaccination in south India. Int J Tuberc Lung Dis. 1998;2:836–843. [PubMed] [Google Scholar]
- 14.Dheenadhayalan V. Studies on MHC restriction and immune responses in human pulmonary tuberculosis. Ph.D. thesis. Madurai, India: Madurai Kamaraj University; 2000. [Google Scholar]
- 15.Dlugovitsky D, Bay M L, Rateni L, Urizar L, Rondelli C F, Largacha C, Farroni M A, Molteni O, Bottasso O A. In vitro synthesis of interferon-gamma, interleukin-4, transforming growth factor-beta and interleukin-1 beta by peripheral blood mononuclear cells from tuberculosis patients: relationship with the severity of pulmonary involvement. Scand J Immunol. 1999;49:210–217. doi: 10.1046/j.1365-3083.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- 16.Ehlers S, Smith K A. Differentiation of T cell like lymphokine gene expression; the in vitro acquisition of T cell memory. J Exp Med. 1991;173:25–36. doi: 10.1084/jem.173.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Fine P E, Ponnighaus J M, Maine N. The distribution and implications of BCG scars in northern Malawi. Bull W H O. 1989;67:35–42. [PMC free article] [PubMed] [Google Scholar]
- 17.Fine P E M. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 18.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Ellner J. Human immune response to Mycobacterium tuberculosis antigens. J Exp Med. 1991;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fonseca L S, Biscaya D R, Saad M H F, Martins F M. The spectrum of immune response to M. tuberculosis in healthy individuals. Tuber Lung Dis. 1992;73:242. doi: 10.1016/0962-8479(92)90094-z. [DOI] [PubMed] [Google Scholar]
- 20.Fox J. Linear statistical model and related methods. New York, N.Y: John Wily & Sons; 1985. pp. 341–347. [Google Scholar]
- 20a.Greenwood C M T, Fujiwara T M, Boothroyd L J, Miller M A, Frappier D, Fanning E A, Scurr E, Morgan K. Linkage of tuberculosis to chromosome2.q35 loci, including NRAMP1, in a large aboriginal Canadian family. Am J Hum Genet. 2000;67:405–416. doi: 10.1086/303012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groux H, Cottrez F, Rouleau M, et al. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J Immunol. 1999;162:1723–1729. [PubMed] [Google Scholar]
- 22.Harries A, Maher D, Uplekar M. TB: a clinical manual for south-east Asia. WHO/TB/96.200 (SEA). Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]
- 23.Hill A V S. The immunogenetics of human infectious diseases. Annu Rev Immunol. 1998;16:593–617. doi: 10.1146/annurev.immunol.16.1.593. [DOI] [PubMed] [Google Scholar]
- 24.Kamath A T, Feng C G, Macdonald M, Briscoe H, Britton W J. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun. 1999;67:1702–1707. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khomenko A G, Litvino V I, Chukanova V P, Pospelov L E. Tuberculosis in patients with various HLA phenotypes. Tubercle. 1990;71:187–192. doi: 10.1016/0041-3879(90)90074-i. [DOI] [PubMed] [Google Scholar]
- 26.Lai C K W, Ho S, Chan C H S, Chan J, Choy D, Leung R, Lai K. Cytokine gene expression profile of circulating CD4+ T cells in active pulmonary tuberculosis. Chest. 1997;111:606–611. doi: 10.1378/chest.111.3.606. [DOI] [PubMed] [Google Scholar]
- 27.Lowry P W, Ludwig T S, Adams J A, Fitzpatrick M L, Grant S M, Andrle G A, Offerdahl M R, Cho S N, Jacobs D R., Jr Cellular immune responses to four doses of percutaneous Bacille Calmette-Guerin in healthy adults. J Infect Dis. 1998;178:138–146. doi: 10.1086/515614. [DOI] [PubMed] [Google Scholar]
- 28.Malin A S, Young D B. Designing a vaccine for tuberculosis: unraveling the tuberculosis genome—can we build a better BCG? Br Med J. 1996;312:1495. doi: 10.1136/bmj.312.7045.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Misra N, Murtaza A, Walker B, Narayan N P S, Misra R S, Ramesh V, Singh S, Colston M J, Nath I. Cytokine profile of circulating T cells of leprosy patients reflects both indiscriminate and polarized T-helper subsets: T-helper phenotype is stable and uninfluenced by related antigens of Mycobacterium leprae. Immunology. 1995;86:97–103. [PMC free article] [PubMed] [Google Scholar]
- 30.Murray P J, Young R A. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect Immun. 1999;67:3087–3095. doi: 10.1128/iai.67.6.3087-3095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishioka Y, Nakanishi K, Sugita M. BCG-induced T cell anergy and its activation by IL-4. Arerugi. 1998;47:533–542. [PubMed] [Google Scholar]
- 32.Orme I M, Roberts A D, Griffin J P, Abrams J S. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993;151:518–525. [PubMed] [Google Scholar]
- 33.Petrovsky N, Harrison L C. Cytokine-based human whole blood assay for the detection of antigen-reactive T cells. J Immunol Methods. 1995;186:37. doi: 10.1016/0022-1759(95)00127-v. [DOI] [PubMed] [Google Scholar]
- 34.Petrovsky N, Harrison L C. HLA class II-associated polymorphism of interferon-γ production. Implication for HLA-disease association. Hum Immunol. 1997;53:12–16. doi: 10.1016/S0198-8859(96)00271-6. [DOI] [PubMed] [Google Scholar]
- 35.Pitchappan R M. Genetics of tuberculosis susceptibility. Trop Med Parasitol. 1990;41:355–356. [PubMed] [Google Scholar]
- 36.Pitchappan R M, Brahmajothi V, Rajaram K, Subramaniyam P T, Balakrishnan K, Muthuveeralakshmi R. Spectrum of immune reactivity to mycobacterial (BCG) antigens in healthy hospital contacts in south India. Tubercle. 1991;72:133–139. doi: 10.1016/0041-3879(91)90040-y. [DOI] [PubMed] [Google Scholar]
- 37.Rajalingam R, Mehra N K, Jain R C, Myneedu V P, Pande J N. Polymerase chain reaction-based sequence specific oligonucleotide hybridization analysis of HLA class II antigens in pulmonary tuberculosis: relevance to chemotherapy and disease severity. J Infect Dis. 1996;173:669–676. doi: 10.1093/infdis/173.3.669. [DOI] [PubMed] [Google Scholar]
- 38.Ravikumar M, Dheenadhayalan V, Rajaram K, Shanmugalakshmi S, PaulKumaran P, Paramasivan C N, Balakrishnan K, Pitchappan R M. Associations of HLA-DRB1, DQB1 and DPB1 alleles with pulmonary tuberculosis in south India. Tuber Lung Dis. 1999;79:309–317. doi: 10.1054/tuld.1999.0213. [DOI] [PubMed] [Google Scholar]
- 39.Ravn P, Boesen H, Pedersen B K, Andersen P. Human T cell responses induced by vaccination with Mycobacterium bovis Bacillus Calmette-Guerin. J Immunol. 1997;158:1949–1955. [PubMed] [Google Scholar]
- 40.Selvaraj P, Reetha A M, Uma H, Xavier T, Janardhanam B, Prabhakar R, Narayanan P R. Influence of HLA-DR and -DQ phenotypes on tuberculin reactive status in pulmonary tuberculosis patients. Tuber Lung Dis. 1996;77:369–373. doi: 10.1016/s0962-8479(96)90104-5. [DOI] [PubMed] [Google Scholar]
- 41.Singh S P N, Mehra N K, Dingley H B, Pande J N, Vaidya M C. Human leukocyte antigen (HLA) linked control of susceptibility to pulmonary tuberculosis and association with HLA-DR types. J Infect Dis. 1983;148:676–681. doi: 10.1093/infdis/148.4.676. [DOI] [PubMed] [Google Scholar]
- 42.Snedecor G W, Cochran W G. Statistical methods. 6th ed. New Delhi, India: Oxford & IBH Publishing Co. Pvt. Ltd.; 1968. [Google Scholar]
- 43.Taha R A, Kotsimbos T C, Song Y L, Menzies D, Hamid Q. IFN-gamma and IL-12 are increased in active compared with inactive tuberculosis. Am J Respir Crit Care Med. 1997;155:1135–1139. doi: 10.1164/ajrccm.155.3.9116999. [DOI] [PubMed] [Google Scholar]
- 43a.Thuc N V, Abel L, Lap V D, Oberti J, Lagrange P H. Protective effect of BCG against leprosy and its subtypes: a case-control study in southern Vietnam. Int J Lepr Other Mycobact Dis. 1994;62:532–538. [PubMed] [Google Scholar]
- 44.Torres M, Herrera T, Villareal H, Rich E A, Sada E. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect Immun. 1998;66:176–180. doi: 10.1128/iai.66.1.176-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44a.Tuberculosis Research Centre (ICMR), Chennai, India. Fifteen year follow up of trial of BCG vaccines in south India for tuberculosis prevention. Indian J Med Res. 1999;110:56–69. [PubMed] [Google Scholar]
- 45.Vemuri N, Reddi A L, Jain S, Colston M J, Misra R S, Ramesh V, Nath I. Indian Immunological Society XXVI Annual Conference and Symposium on Cancer Immunology in the New Millennium. New Delhi, India: Indian Immunology Society; 2000. Real time PCR using flurogenic probes shows dominance of interferon-g over IL-4 in lepromatous leprosy patients; p. 28. [Google Scholar]
- 46.Weir R E, Butlin C R, Neupane K D, Failbus S S, Dockrell H M. Use of a whole blood assay to monitor the immune response to mycobacterial antigens in leprosy patients: a predictor for type 1 reaction onset. Lepr Rev. 1998;69:279–293. doi: 10.5935/0305-7518.19980029. [DOI] [PubMed] [Google Scholar]
- 47.Wilkinson R J, Wilkinson K A, De Smet K A, Haslov K, Pasvol G, Singh M, Svarcova I, Ivanyi J. Human T-and B-cell reactivity to the 16kDa alpha-crystallin protein of Mycobacterium tuberculosis. Scand J Immunol. 1998;48:403–409. doi: 10.1046/j.1365-3083.1998.00420.x. [DOI] [PubMed] [Google Scholar]
- 48.Yamamura M, Uyfumara K, Deans R J, Weinberg K, Rea T, Bloom B, Modlin R. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 49.Yang X, Gartner J, Zhu L, Wang S, Brunham R C. IL-10 gene knockout mice show enhanced Th1-like protective immunity and absent granuloma formation following Chlamydia trachomatis lung infection. J Immunol. 1999;162:1010–1017. [PubMed] [Google Scholar]