Abstract
Uncovering an informative feature of 3D genome structure, Guo et al (2022) describe chromatin jets in quiescent murine thymocytes: 1–2Mb structures formed by targeted cohesin loading at narrow accessible chromatin regions and visible as prominent off-diagonal stripes on contact maps.
Eukaryotic genomes are organized into complex 3D structures across a range of length scales. Understanding the formation and regulation of these structures is crucial, as they regulate diverse processes including gene expression, DNA repair, recombination, and replication. Chromatin conformation capture methods such as Hi-C have been instrumental in uncovering patterns of 3D genome organization including A/B compartments, TADs, and loops. A/B-compartments arise from active and inactive chromatin regions associating primarily with other regions of the same type both intra- and inter-chromosomally, and are visible on contact maps as a checkerboard pattern (Figure 1). TADs (Topologically-Associating Domains) are local chromatin domains characterized by increased contact within the region compared to outside the region; they are visible on contact maps as squares (Figure 1) (Fudenberg et al. 2017). TADs often exhibit strong stripes/flames and corner peaks on contact maps, which are indicative of cohesin-mediated loop extrusion, wherein the SMC complex cohesin loads onto DNA and extrudes bidirectionally until encountering a block or being unloaded (Davidson et al 2019, Kim et al 2019; Fudenberg et al. 2017; Gabriele et al. 2022). However, many aspects of the loop extrusion process remain poorly understood, including the factors that regulate cohesin loading onto chromatin and whether specific targeted loading sites exist in eukaryotes. A key signature of targeted loading in Hi-C maps is a stripe perpendicular to the diagonal from the site of targeted loading. Such targeted loading of loop-extruding SMC complexes resulting in an off-diagonal stripe has previously been observed in bacteria (Marbouty et al. 2015; Wang et al. 2017) and very recently in perturbed mammalian cells (Liu et al. 2021), but had not yet been seen in unperturbed mammalian cells.
Figure 1. Hi-C contact maps provide insights into the mechanisms of 3D genome structure regulation.
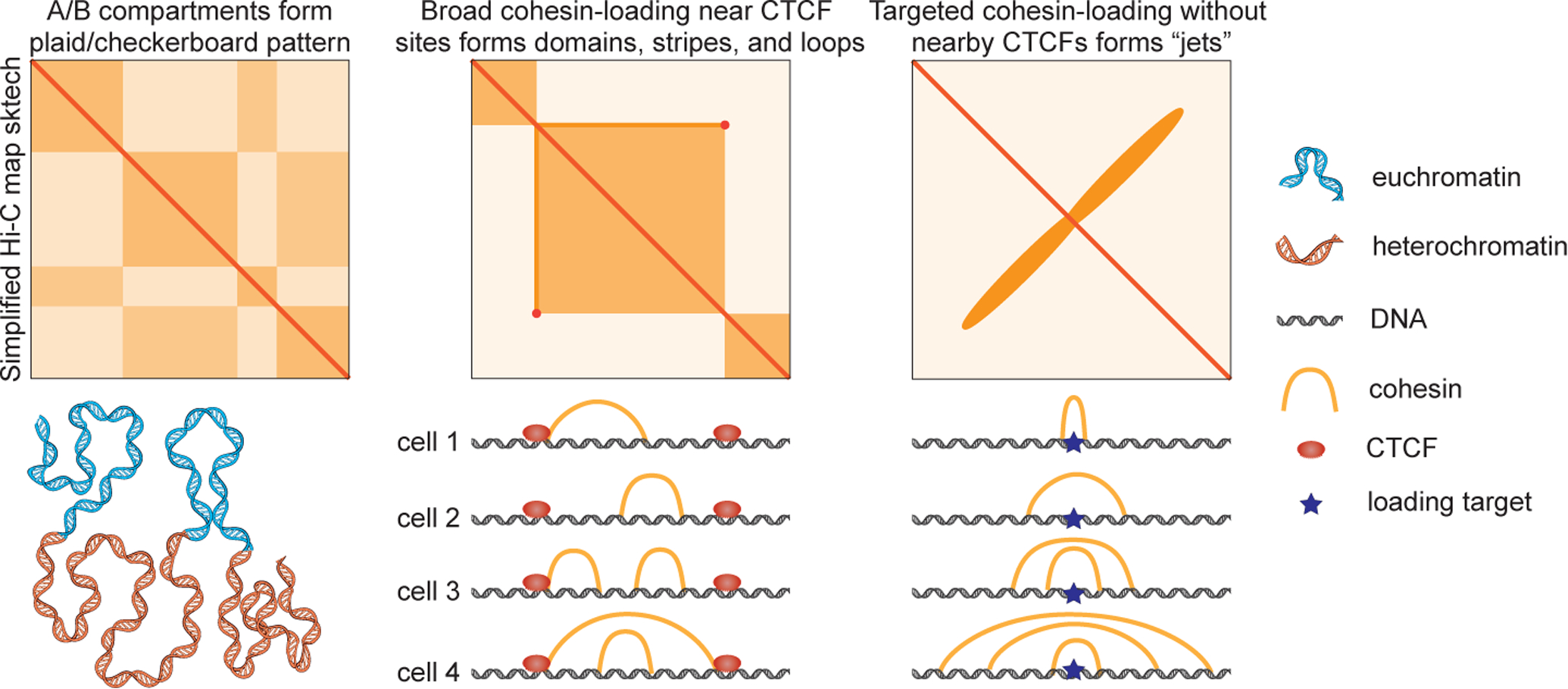
Top: Simplified sketches of Hi-C contact maps showing A/B-compartments (left), TADs, loops, and stripes (middle), and “jets” (right), with likely mechanisms shown below. A/B compartments form due to self-segregation of euchromatin and heterochromatin. TADs, stripes, and loops arise from cohesin looping in broad regions bounded by extrusion barriers such as CTCF boundaries. Jets result from focal cohesin loading in a narrow region and subsequent bidirectional extrusion without nearby extrusion barriers.
Now, Guo et al (2022) have used in situ Hi-C in quiescent murine thymocytes to reveal off-diagonal stripes, which they term jets. These structures, which are also visible in resting B cells, manifest on contact maps as strong stripes perpendicular to the main diagonal (Figure 1). Jets are observed at a subset of small open chromatin regions flanked by large heterochromatin domains. Merging Hi-C data with ChIP-seq and ATAC-seq, the authors find that the regions producing jets are especially enriched in ATAC-seq signal (indicative of accessible chromatin), occupancy by RAD21 (a cohesin subunit) and NIPBL (commonly held to be a cohesin loader), and H3K27ac marks (indicative of active regulatory regions). Partial depletion of cohesin strongly reduces jet strength across all sites, indicating that jets are cohesin-dependent structures; depletion of CTCF was not required to observe jets but did alter the strength and shape of a subset of jets. Polymer simulations reproduce jet-like structures when cohesin complexes are restricted to loading in narrow regions; by contrast, uniform cohesin loading across a broad region reproduced TAD-like structures consistent with previous findings (Fudenberg et al. 2017).
The properties of jets shed new light on several aspects of loop extrusion. Jets most closely resemble the plumes previously described by Liu et al. (2021) in that they extend perpendicular to the diagonal of a Hi-C map and appear to originate at isolated areas of accessible chromatin flanked by large heterochromatin regions (Liu et al. 2021). Unlike plumes, which require depletion of both CTCF and WAPL to form and can only be observed for 24 hours post-WAPL/CTCF depletion, jets can be observed in unperturbed wild-type cells without depleting CTCF or WAPL. Jets are also much longer than plumes, extending up to 1–2Mb from their origin. Since jets do not require perturbation to be observed, this distance provides a reasonable estimate of the physiological extrusion range of cohesin in non-cycling cells - an important insight that can help constrain loop extrusion models. Additionally, jets provide further support to the hypothesis that cohesin can continue to extrude unidirectionally even when blocked in one direction, previously put forth in modeling studies and substantiated by experimental observations of chromatin stripes/flares (Fudenberg et al. 2017). A subset of jets located near CTCF sites are deflected away from the perpendicular angle of other jets in unperturbed cells. When CTCF is depleted, these jets return to a perpendicular angle. Guo et al (2022) identify that this indicates unidirectional blockage of cohesin at the CTCF site in wild-type cells.
Guo et al (2022) also provide an answer to the long-standing question of whether cohesin loading is uniform or preferentially occurs at specific sites. They clearly show that cohesin does show preference for specific sites rather than binding uniformly across the genome. The key next step is to uncover the factors driving this site preference. Guo et al. identify high NIPBL binding and H3K27ac marks as distinguishing features of jet sites, but it is unclear if those features are sufficient for targeted cohesin loading or even causative of it. In particular, though NIPBL is commonly believed to act as a cohesin loader, it is also part of the translocating cohesin complex and may thus be present as a result of increased cohesin occupancy in the region rather than a cause (Rhodes et al. 2017, Davidson et al. 2019, Kim et al. 2019). It also remains to be determined why jets can be observed in quiescent immune cells but have not been seen in Hi-C and Micro-C data from dozens of other mouse and human cell types. It is possible that there is something unique about the chromatin environment of these non-cycling cells that gives rise to jets, akin to the flares observed in zebrafish sperm (Wike et al. 2021). As jets/plumes manifest in other cycling cell types only once CTCF and WAPL are depleted (Liu et al. 2021), another possibility is that quiescent thymocytes and B cells have unusually long-lived cohesin and less abundant CTCF, allowing cohesin to extrude long jets without dissociating or being blocked by CTCF. Interestingly, this suggests that cells may achieve cell-type specific regulation of 3D genome structure by regulating the dynamics of CTCF and cohesin, which may help to facilitate cell-type specific regulation of gene expression.
Acknowledgements
We gratefully acknowledge support from NIH grants R00GM130896, DP2GM140938, UM1HG011536, and R33CA257878, NSF 2036037, the Mather’s Foundation, a Pew-Stewart Cancer Research Scholar grant, the Broad Institute of MIT and Harvard, and MIT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- Davidson IF, Bauer B, Goetz D, et al. (2019). DNA loop extrusion by human cohesin. Science 366(6471), 1338–1345. doi: 10.1126/science.aaz3418 [DOI] [PubMed] [Google Scholar]
- Fudenberg G, Abdennur N, Imakaev M, Goloborodko A and Mirny LA (2017, January). Emerging evidence of chromosome folding by loop extrusion. In Cold Spring Harbor symposia on quantitative biology (Vol. 82, pp. 45–55). Cold Spring Harbor Laboratory Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriele M, Brandão HB, Grosse-Holz S, Jha A, Dailey GM, Cattoglio C, Hsieh THS, Mirny L, Zechner C, and Hansen AS (2022). Dynamics of CTCF-and cohesin-mediated chromatin looping revealed by live-cell imaging. Science 376(6592), 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Ediem A-J, Garcia-Millan R, et al. (2022). Chromatin jets define properties of cohesin-driven in vivo loop extrusion. Mol. Cell, This issue. [DOI] [PubMed] [Google Scholar]
- Kim Y, Shi Z, Shang H, et al. (2019). Human cohesin compacts DNA by loop extrusion. Science 366(6471), 1345–1349. doi: 10.1126/science.aaz4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NQ, Magnitov M, Schijns M, et al. (2021). Rapid depletion of CTCF and cohesin proteins reveals dynamic features of chromosome architecture. biorXiv, 2021.08.27.457977. doi: 10.1101/2021.08.27.457977 [DOI] [Google Scholar]
- Marbouty M, Le Gall A, Cattoni DI, Cournac A, Koh A, Fiche JB, Mozziconacci J, Murray H, Koszul R, and Nollmann M (2015). Condensin-and replication-mediated bacterial chromosome folding and origin condensation revealed by Hi-C and super-resolution imaging. Mol. Cell 59(4), 588–602. [DOI] [PubMed] [Google Scholar]
- Rhodes J, Mazza D, Nasmyth K, and Uphoff S (2017). Scc2/Nipbl hops between chromosomal cohesin rings after loading. eLife 6, e30000. doi: 10.7554/eLife.30000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Brandão HB, Le TB, Laub MT, and Rudner DZ (2017). Bacillus subtilis SMC complexes juxtapose chromosome arms as they travel from origin to terminus. Science 355(6324), 524–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike CL, Guo Y, Tan M, et al. (2021). Chromatin architecture transitions from zebrafish sperm through early embryogenesis. Genome Res 31(6), 981–994. doi: 10.1101/gr.269860.120 [DOI] [PMC free article] [PubMed] [Google Scholar]


