Abstract
Infection with Trypanosoma cruzi, the agent of Chagas' disease, may induce antibodies and T cells reactive with self antigens (autoimmunity). Because autoimmunity is generally thought to develop during the chronic phase of infection, one hypothesis is that autoimmunity develops only after long-term, low-level stimulation of self-reactive cells. However, preliminary reports suggest that autoimmunity may begin during acute T. cruzi infection. The goal of the present study was to investigate whether cardiac autoimmunity could be observed during acute T. cruzi infection. A/J mice infected with the Brazil strain of T. cruzi for 21 days developed severe myocarditis, accompanied by humoral and cellular autoimmunity. Specifically, T. cruzi infection induced immunoglobulin G (IgG) autoantibodies and delayed type hypersensitivity (DTH) to cardiac myosin. This autoimmunity resembles that which develops in A/J mice immunized with myosin in complete Freund's adjuvant in that myosin-specific antibodies and DTH responses both develop by 21 days postinfection or postimmunization. While the levels of myosin IgG in T. cruzi-infected mice were slightly lower than those in myosin-immunized mice, the magnitude of myosin DTH in the two groups was statistically equivalent. In contrast, C57BL/6 mice, which are resistant to myosin-induced myocarditis and its associated autoimmunity, developed undetectable or low levels of myosin IgG and did not exhibit myosin DTH or myocarditis upon T. cruzi infection. Therefore, humoral and cellular cardiac autoimmunity can develop during acute T. cruzi infection in the genetically susceptible host.
Chagas' heart disease (CHD), caused by the protozoan Trypanosoma cruzi, is a significant cause of morbidity and mortality in the Americas. Sixteen to 18 million people are infected and 120 million people are at risk of infection (20). CHD develops in 10 to 30% of infected individuals and is a common cause of fatal dilated cardiomyopathy. Infection with T. cruzi has also been shown to induce antibodies and T cells reactive with self antigens (autoimmunity) in both humans and mice (reviewed in reference 13). Specifically, T. cruzi infection may induce the production of antibodies specific for various cardiac proteins, including cardiac myosin (4, 32), β-adrenergic receptor (18, 29), and laminin (19, 30). T. cruzi infection may also induce cellular immune responses to myosin in humans and mice, though this evidence is sparse (3, 26).
Because autoimmunity has been observed most frequently in the chronic stage of disease in humans and experimental animals, it has been proposed that autoimmunity develops as a result of long-term, low-level stimulation of self-reactive cells. However, preliminary reports suggest that autoimmunity may begin to develop during acute infection. Autoantibodies reactive with tubulin and actin have been detected in acute infection in mice, and antibodies specific for laminin are found in acutely infected humans (9, 31). To our knowledge, there has been no investigation of cellular autoimmunity during acute T. cruzi infection. We set out to determine whether humoral and cellular cardiac autoimmunity might develop during acute T. cruzi infection in a strain of mouse that develops cardiac autoimmunity in response to coxsackievirus infection (21). This strain, A/J, also is susceptible to development of autoimmunity when mice are immunized with cardiac myosin (reviewed in reference 27). We also tested the C57BL/6 strain because it is resistant to cardiac autoimmunity induced by coxsackievirus infection (34) or myosin immunization (23). The results presented here provide compelling evidence that both humoral and cellular cardiac autoimmunity can develop during acute T. cruzi infection.
MATERIALS AND METHODS
Mice and T. cruzi.
Four- to six-week-old male A/J and C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and housed under specific pathogen-free conditions. Mice were infected by intraperitoneal injection of 1 × 104 T. cruzi Brazil strain trypomastigotes derived from infection of tissue culture H9C2 rat myoblasts (American Type Culture Collection, Manassas, Va.). Uninfected controls received an intraperitoneal injection of Dulbecco's phosphate-buffered saline (PBS) (GibcoBRL, Grand Island, N.Y.) of equal volume.
Antigens.
Cardiac myosin heavy chains were purified according to the method of Shiverick et al. (28). Briefly, mouse hearts were minced and homogenized in 10 volumes of ice-cold KCl buffer (0.3 M KCl, 0.15 M K2HPO4, 10 mM Na4P2O7, 1 mM MgCl2 [pH 6.80]). Myosin was extracted from the muscle homogenate by stirring at 4°C for 90 min. The suspension was centrifuged at 140,000 × g for 1 h at 4°C, and the decanted supernatant was diluted with 20 vol of water and incubated at 4°C overnight to precipitate the myosin. The precipitate was collected by centrifugation at 12,000 × g for 30 min at 4°C and suspended in ice-cold imidazole buffer (0.5 M KCl, 10 mM imidazole, 5 mM MgCl2, 5 mM Na2ATP, 2 mM dithiothreitol [pH 6.80]). The solution was then centrifuged at 43,000 × g for 30 min at 4°C to remove actin. The myosin was precipitated in 8 volumes of ice-cold water at 4°C overnight. The following day the precipitate was collected by centrifugation at 12,000 × g for 30 min at 4°C, and the pellet was suspended in the imidazole buffer and centrifuged at 43,000 × g for 30 min at 4°C to remove residual actin. The supernatant was again precipitated overnight at 4°C in 6.5 volumes of ice-cold water. The precipitate was then collected by centrifugation at 12,000 × g for 30 min at 4°C and suspended in 50 mM Na4P2O7 (pH 6.8). Protein concentration was determined by comparing dilutions of the purified myosin solution with known concentrations of purified rabbit myosin heavy chain standards (Sigma, St. Louis, Mo.) by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (15). Total heart homogenate was prepared by washing A/J hearts with PBS and mincing them with a razor blade, followed by homogenization and lyophilization were performed on hearts. T. cruzi antigen was prepared by washing T. cruzi epimastigotes three times in PBS and resuspending them in PBS before adding an equal volume of acetone. These fixed parasites were then sonicated and lyophilized prior to quantitation of protein concentration by the method of Bradford (2). T. cruzi epimastigotes were grown at 26°C in supplemented liver digest-neutralized tryptose medium as described previously (14). Myelin basic protein was purchased from Sigma, and recombinant purified myosin light chain kinase was the generous gift of R. L. Rex Chisholm.
Myosin immunization.
Mice were immunized with complete Freund's adjuvant-myosin emulsion (300 μg of myosin) in a total volume of 0.1 ml. Three sites in the dorsal flank received subcutaneous injections. Mice were boosted 7 days later in an identical manner.
Histopathology.
Hearts were removed, rinsed with PBS, and fixed for 24 h in 10% buffered formalin. Fixed hearts were embedded in paraffin, sectioned in the atrial-apical axis, stained with hematoxylin-eosin, and examined by light microscopy. Myocarditis was defined as any form of inflammation: clusters of round mononuclear cells (>5 per high-power field) confined to the interstitial spaces between nonnecrotic myocytes (10).
Serologic analysis.
Levels of cardiac myosin-specific immunoglobulin G (IgG) were analyzed by enzyme-linked immunosorbent assay (ELISA). Briefly, Maxisorp plates (Nunc, Roskilde, Denmark) were coated overnight at 4°C with 100 μl of cardiac myosin (2.5 μg/ml) in PBS. The plates were washed with PBS containing 0.05% (vol/vol) Tween 20 and then blocked with 2% bovine serum albumin (BSA) and 5% normal goat serum. The plates were then incubated with twofold serial serum dilutions (1:10 initial dilution for 16 dilutions) for 2 h at 37°C. After being washed, peroxidase-conjugated anti-mouse IgG (heavy and light chain) (0.25 μg/ml; KPL, Gaithersburg, Md.), was added for 1 h at 37°C. The bound enzyme was detected with the 3,3′,5,5′-tetramethylbenzidine substrate (KPL) and quantitated by measurement of the optical density at 450 nm (OD450) in a Kinetic MicroPlate Reader (Molecular Devices, Sunnyvale, Calif.). One-dimensional SDS-PAGE and immunoblot analysis were performed by standard methods (15, 33). In all immunoblots, the secondary antibody used was goat-anti mouse (IgG and IgM) (1 μg/ml, Caltag, Burlingame, Calif.).
DTH.
Myosin delayed-type hypersensitivity (DTH) was quantitated by a standard ear swelling assay. Prechallenge ear thickness was measured with a Mitutoyo model 7326 engineer's micrometer (Mitutoyo MTI Corporation, Aurora, Ill.). Myosin or T. cruzi antigen (10 μg in 0.15 M K2PO4–0.01 Na4P2O7–0.3 M KCl [pH 6.8]) was injected intradermally into the dorsal surface of the ear with a 100-μl Hamilton syringe fitted with a 30-gauge needle. Bovine serum albumin was injected in the opposite ear as an injection control. After 24 h, the net swelling of the injection control ear was subtracted from the net swelling of the myosin ear and expressed in units of 10−4 inch. Antigen-induced ear swelling was the result of mononuclear cell infiltration and exhibited typical DTH kinetics (i.e., minimal swelling at 4 h and maximal swelling at 24 to 48 h).
Statistical analyses.
Between any two groups of mice, comparison of the DTH responses was analyzed by Student's t test while comparison of ELISA dilution curves was analyzed by two-way analysis of variance. Values of P of <0.05 were considered significant.
RESULTS
Severe myocarditis develops in A/J mice acutely infected with the Brazil strain of T. cruzi.
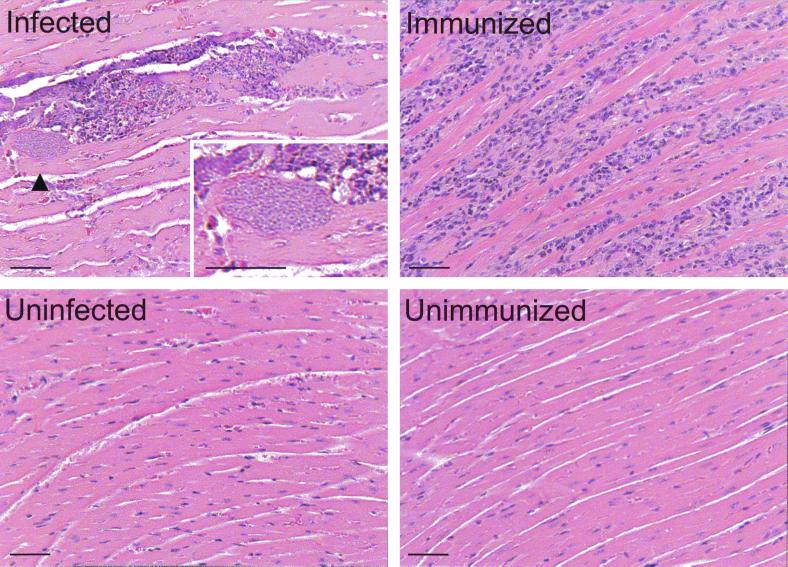
Initially we compared myocarditis induced by acute T. cruzi infection to that induced by myosin immunization. Four groups of five A/J mice were (i) injected with PBS, (ii) immunized with PBS in complete Freund's adjuvant (CFA), (iii) infected with 104 trypomastigotes of the Brazil strain of T. cruzi, or (iv) immunized with purified cardiac myosin in CFA. By 21 days postinfection or postimmunization (d.p.i.) the hearts of infected mice (five of five) exhibited multiple T. cruzi pseudocysts and severe, focal mononuclear cell infiltration (Fig. 1). Inflammatory foci were present near the apex and epicardium of the heart and were typically accompanied by fibrosis (data not shown). In contrast, myosin-immunized mice (five of five) exhibited a diffuse cellular infiltrate that was also found at the apex and epicardium and occasionally throughout the myocardium. Fibrosis was always present (data not shown). None of the control mice (n = 5) developed myocarditis.
FIG. 1.
A/J mice infected with T. cruzi or immunized with cardiac myosin develop severe myocarditis. A/J mice were infected with 104 T. cruzi trypomastigotes (infected) or immunized with 300 μg of myosin in CFA (immunized). Control mice were injected with PBS (uninfected) or immunized with CFA-PBS (unimmunized). At 21 d.p.i., cardiac sections from these mice were stained with hematoxylin and eosin. All infected (n = 5) and immunized (n = 5) mice developed severe myocarditis, with myocyte necrosis and mononuclear cellular infiltration. No myocarditis was observed in uninfected (n = 5) or unimmunized mice (n = 5). Arrowhead indicates the pseudocyst magnified in the inset. Bars, 50 μm.
Cardiac autoantibodies are induced upon acute T. cruzi infection.
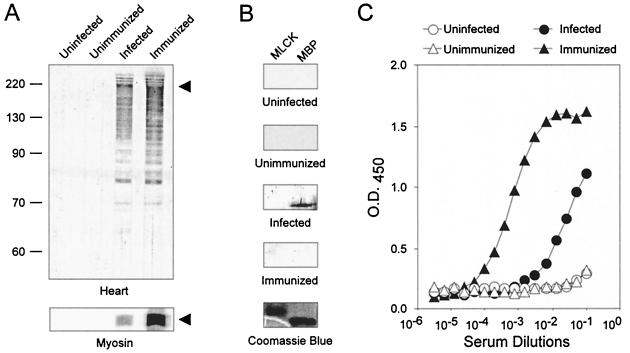
To evaluate whether humoral autoimmunity accompanies the myocarditis of acute T. cruzi infection, sera from infected mice were assayed for autoantibodies to cardiac antigens (Fig. 2A). Western blot analysis of an A/J heart homogenate revealed similar patterns of autoantibodies produced in T. cruzi-infected and myosin-immunized mice, indicating significant overlap in the repertoire of autoantibodies produced. No autoantibodies were detected in control mice. We hypothesized that the 200-kDa protein detected by sera from infected and immunized mice was the heavy chain of cardiac myosin based on its size. Indeed, sera from both infected and immunized A/J mice reacted strongly with purified syngeneic cardiac myosin by Western blot analysis (Fig. 2A). The production of antibodies reactive with cardiac myosin is specific since sera from infected or myosin-immunized mice did not react with regulatory myosin light-chain kinase (Fig. 2B). Interestingly, sera from infected mice did react with myelin basic protein, consistent with the findings of a previous report (1). Sera were also assayed by a myosin-specific IgG ELISA (Fig. 2C). High-titer myosin IgG autoantibodies were found in sera from T. cruzi-infected and myosin-immunized mice but not in sera from control mice. Myosin IgG autoantibodies could be detected as early as 7 d.p.i. (data not shown). Autoantibodies reactive with the skeletal isoform of myosin heavy chain were also found in sera of infected mice (data not shown). In conclusion, acute infection with T. cruzi induced the production of autoantibodies with high-titer cardiac myosin-specific IgG.
FIG. 2.
A/J mice produce antibodies specific for cardiac proteins after infection with T. cruzi or immunization with myosin. Serum samples from four groups of A/J mice (five mice per group) obtained 21 d.p.i. with PBS (uninfected group), with PBS-CFA (unimmunized group), with 104 T. cruzi trypomastigotes (infected group), or with myosin-CFA (immunized) were pooled for use in Western blot and ELISA analyses. (A) A/J mouse heart homogenate (top) or purified cardiac myosin (bottom) was resolved by SDS-PAGE, transferred to nitrocellulose, and blotted with 1:100 dilutions of sera from groups 1 to 4. The arrowhead indicates the position of cardiac myosin heavy chain. (B) Purified regulatory myosin light-chain kinase (MLCK) and myelin basic protein (MBP) were resolved by SDS-PAGE, transferred to nitrocellulose, and blotted with 1:100 dilutions of sera from the groups. A Coomassie blue-stained gel is also shown. (C) Sera from the four groups were tested for myosin-specific IgG by ELISA. Each datum point represents the average for three wells (standard error of the mean, <0.09).
Strong myosin-specific DTH develops during acute T. cruzi infection.
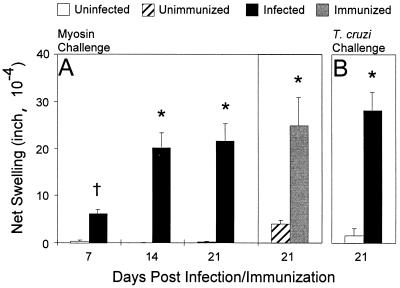
We next investigated whether acute T. cruzi infection also induces cellular autoimmunity by measuring DTH, a widely used and sensitive measure of cellular autoimmunity (11, 12, 35). Infected mice developed significant myosin-specific DTH as early as 7 d.p.i. Interestingly, at 14 and 21 d.p.i., myosin DTH from infected mice was statistically equivalent to myosin DTH from myosin-immunized mice (Fig. 3). Acutely infected A/J mice also developed vigorous antibody responses and T-cell immunity specific for T. cruzi, as measured by Western blot analysis (data not shown) and T. cruzi DTH (Fig. 3), respectively.
FIG. 3.
T. cruz-infected mice and myosin-immunized mice display myosin-specific cellular autoimmunity. Myosin-specific DTH was determined by a 24-h ear swelling assay. Error bars indicate standard errors of the means. (A) Time-course of myosin-specific DTH in T. cruzi-infected mice. Myosin-specific DTH was measured in eight mice infected with 104 T. cruzi trypomastigotes (infected) and in five mice injected with PBS (uninfected) at each d.p.i. For comparison, at 21 d.p.i., myosin-specific DTH was measured in eight myosin-immunized (immunized) mice and five PBS-CFA-immunized (unimmunized) control mice. T. cruzi-infected mice developed significant myosin-specific DTH as early as 7 d.p.i. (B) T. cruzi-specific DTH was also measured in eight infected mice and five uninfected mice at 21 d.p.i. ∗, P of <0.001; †, P = 0.002 compared to the respective controls.
Susceptibility to T. cruzi-induced myosin autoimmunity is dependent on host strain.
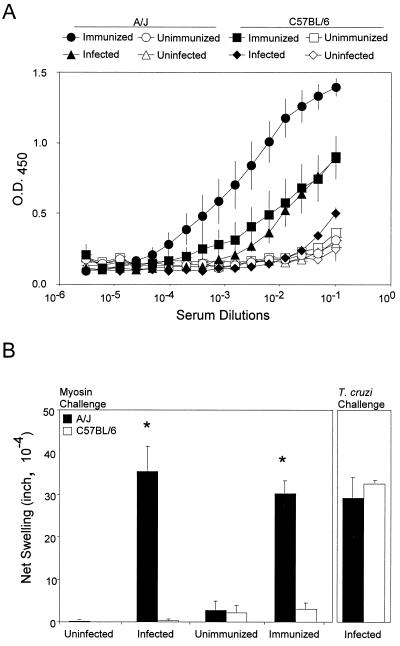
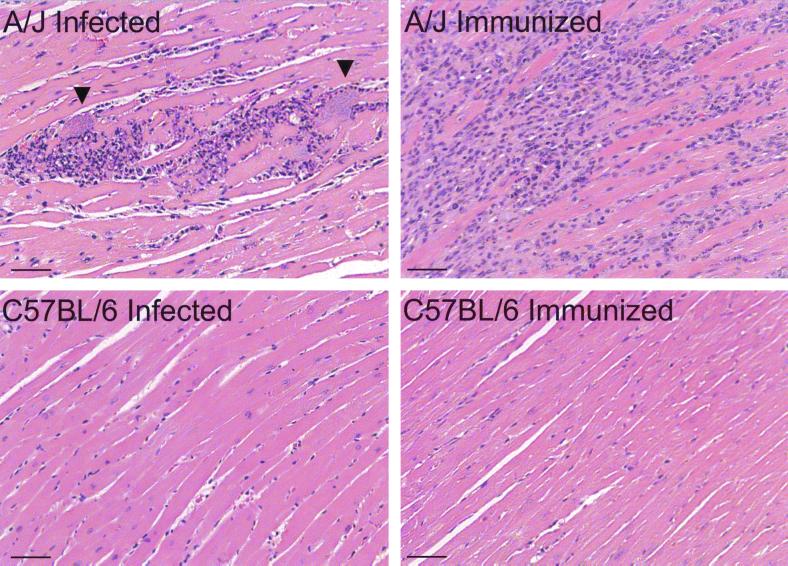
We hypothesized that susceptibility to myosin autoimmunity during acute T. cruzi infection is dependent on host immunogenetics. To test this, we compared T. cruzi-induced myosin autoimmune responses in the susceptible A/J strain to those in the C57BL/6 strain, which does not develop myocarditis or cardiac autoimmunity upon myosin immunization (23). T. cruzi infection induced the production of significantly higher levels of myosin-specific IgG in A/J mice than in C57BL/6 mice (P < 0.001) (Fig. 4A). Myosin immunization also induced significantly higher levels of myosin-specific IgG in A/J mice than in C57BL/6 mice (P = 0.002). Not surprisingly, myosin immunization induced higher levels of myosin IgG than T. cruzi infection in both A/J (P < 0.001) and C57BL/6 mice (P = 0.031). The differences in cellular autoimmunity between A/J mice and C57BL/6 mice were even more dramatic. Upon either T. cruzi infection or myosin immunization, A/J mice generated significant myosin DTH while C57BL/6 mice did not (Fig. 4B). However, T. cruzi infection did induce strong T. cruzi DTH in C57BL/6 mice, suggesting that C57BL/6 mice were still capable of mounting T cell immunity to foreign antigens. Histologic analysis of the heart revealed that only A/J mice developed myocarditis, upon either T. cruzi infection or myosin immunization (Fig. 5), while C57BL/6 mice did not.
FIG. 4.
A/J mice develop stronger myosin autoimmunity than do C57BL/6 mice both upon T. cruzi infection and upon myosin immunization. Groups of A/J or C57BL/6 mice were infected with 104 T. cruzi trypomastigotes (infected), injected with PBS (uninfected), immunized with myosin (immunized), or immunized with PBS-CFA (unimmunized). (A) Myosin autoantibody production. The infected and immunized groups contained 10 mice each while uninfected and unimmunized groups contained four mice each. At 21 d.p.i., serum from each mouse was individually tested for myosin-specific IgG in a myosin ELISA. Each datum point represents the mean OD450 value of the mice in each group, and the error bars indicate the standard errors of the means. All experimental groups were significantly different (P < 0.001) from their respective controls. Higher levels of cardiac myosin-specific IgG were detected in infected A/J than in infected C57BL/6 mice (P < 0.001) and in myosin-immunized A/J than in myosin-immunized C57BL/6 mice (P = 0.002). (B) Myosin and T. cruzi-specific DTH was determined by a 24-h ear swelling assay at 21 d.p.i. Myosin DTH was measured in mice as described for panel A. T. cruzi DTH was measured in five infected A/J and five infected C57BL/6 mice. Error bars indicate standard errors of the means. ∗, P of <0.001 compared to the C57BL/6 group.
FIG. 5.
A/J mice, but not C57BL/6 mice, develop myocarditis upon both T. cruzi infection and myosin immunization. A/J and C57BL/6 mice were infected with 104 T. cruzi trypomastigotes (infected) or immunized with 300 μg of myosin in CFA (immunized). At 21 d.p.i., cardiac sections from these mice were stained with hematoxylin and eosin. Representative sections are shown. Infected (five of five) and immunized (four of five) A/J mice developed myocarditis while no disease was observed in infected mice (n = 5), immunized C57BL/6 mice (n = 5), or any of the controls, A/J and C57BL/6 mice injected with PBS or immunized with PBS-CFA (four mice per control group). Arrowheads indicate pseudocysts. Bars, 50 μm.
DISCUSSION
The present study provides the first evidence that acute T. cruzi infection induces humoral and cellular cardiac autoimmunity together with antiparasite immunity in mice. This autoimmunity resembles that which develops in mice with myosin-induced myocarditis in that high levels of both myosin IgG antibodies and myosin DTH develop by 21 d.p.i. In contrast, C57BL/6 mice have low to undetectable myosin IgG antibodies and no myosin DTH by 21 d.p.i. Severe myocarditis develops in both T. cruzi-infected and myosin-immunized A/J mice but not in C57BL/6 mice.
The results of this study are unique in several aspects. Cellular autoimmunity has not previously been shown to develop during acute T. cruzi infection, despite its documented presence in some instances of chronic human and murine infections (3, 25). It was previously hypothesized that autoimmunity would develop in the susceptible host only after long-term, low-level stimulation of the autoreactive cells. In addition, the different susceptibilities of murine strains to myosin autoimmunity suggest that T. cruzi induced autoimmunity may have an immunogenetic component.
How can an infectious agent like T. cruzi induce humoral and cellular autoimmunity to myosin as early as 7 d.p.i.? Bystander activation and molecular mimicry are two potential mechanisms (16, 17). In bystander activation, cardiac specific T cells are stimulated to overcome their tolerant state in response to autoantigen release resulting from parasite-mediated myocytolysis. The hypothesis of molecular mimicry holds that an immune response is generated to parasite antigens that crossreact with self proteins, cardiac proteins in this case. Although there is circumstantial evidence supporting molecular mimicry between T. cruzi antigens and cardiac myosin (3, 4), bystander activation seems more likely because myosin autoimmunity develops in response to many different cardiac inflammatory insults, including viral infection (22), allograft rejection (7), and cardiac surgery (5, 6, 24).
Both T. cruzi infection and myosin immunization of A/J mice induce cardiac damage: in one case, infection and lysis of cardiac myocytes by the parasite causes the damage; in the other, peripherally sensitized myosin-specific T cells home to the target organ and initiate the inflammatory process. The cardiac damage, which occurs in both cases, may be a central autoimmunity-inducing stimulus. As a result, the tissue destruction promotes the elaboration of a polyclonal, polyspecific autoantibody response as evidenced by the observation that autoantibodies from both groups of mice reacted strongly with overlapping sets of cardiac proteins (Fig. 2A). Myosin-immunized mice developed higher levels of anti-myosin IgG than did T. cruzi-infected mice, perhaps because the amount of tissue destruction and “secondary” myosin priming were greater or the dose and route of myosin immunization were more potent (Fig. 2B). With respect to cellular autoimmunity, T. cruzi-infected mice demonstrated an increase in myosin DTH from 7 to 14 d.p.i., presumably due to the expansion of myosin-specific T cells stimulated by ongoing tissue destruction.
The development of myosin autoimmunity upon T. cruzi infection is probably dependent upon host immunogenetic susceptibility. There are several possible reasons why infected C57BL/6 mice failed to develop myosin DTH. C57BL/6 mice do not develop myosin autoimmunity upon myosin immunization (23) (Fig. 4) or coxsackievirus infection (34), while A/J mice do, suggesting that C57BL/6 mice are naturally resistant to myosin autoimmunity. Conversely, A/J mice are naturally susceptible to develop myosin autoimmunity and do so readily in response to any number of stimuli, including infection with a cardiopathogenic protozoan. However, if cardiac damage is required for the development of myosin autoimmunity during T. cruzi infection, it is possible that the C57BL/6 mice did not develop autoimmunity for this reason and not because the strain is autoimmunity resistant.
What is the role of myosin autoimmunity in acute T. cruzi myocarditis? Myosin autoimmunity may be directly responsible for tissue inflammation, may predict autoreactive inflammation developing during the course of infection, or may have no role in tissue inflammation (i.e., it is an epiphenomenon). Myosin autoimmunity is sufficient to cause myocarditis in myosin-immunized mice, and so it is possible that it is also inflammatory in acute T. cruzi infection. In this study, we provide no evidence addressing this question. However, the possibility that myosin autoimmunity is pathogenic in T. cruzi-infected A/J mice can be directly tested using myosin-specific peripheral tolerance, which effectively prevents myosin-induced myocarditis in the same animals (8). In conclusion, infection of A/J mice with the Brazil strain of T. cruzi induces vigorous humoral and cellular myosin-specific autoimmunity during acute disease in a genetically restricted manner. Whether some humans develop autoimmunity during acute T. cruzi infection as do strains of mice is worthy of further investigation.
ACKNOWLEDGMENTS
We thank A. W. Rademaker for his advice on statistical analysis, R. L. Chisholm for the gift of recombinant myosin light chain kinase, and S. D. Miller and W. J. Karpus for helpful suggestions.
This work was supported by grants from the American Heart Association, including an American Heart Association Predoctoral Fellowship to J.S.L. and Established Investigator Award to D.M.E., and the National Institutes of Health.
REFERENCES
- 1.Al-Sabbagh A, Garcia C A, Diaz-Bardales B M, Zaccarias C, Sakurada J K, Santos L M. Evidence for cross-reactivity between antigen derived from Trypanosoma cruzi and myelin basic protein in experimental Chagas disease. Exp Parasitol. 1998;89:304–311. doi: 10.1006/expr.1998.4279. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Cunha-Neto E, Coelho V, Guilherme L, Fiorelli A, Stolf N, Kalil J. Autoimmunity in Chagas' disease: Identification of cardiac myosin-B13 Trypanosoma cruzi protein crossreactive T cell clones in heart lesions of a chronic Chagas' cardiomyopathy patient. J Clin Investig. 1996;98:1709–12. doi: 10.1172/JCI118969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunha-Neto E, Duranti M, Gruber A, Zingales B, de Messias I, Stolf N, Bellotti G, Patarroyo M E, Pilleggi F, Kalil J. Autoimmunity in Chagas' disease cardiomyopathy: biological relevance of a cardiac myosin-specific epitope crossreactive to an immunodominant Trypanosoma cruzi antigen. Proc Natl Acad Sci USA. 1995;92:3541–3545. doi: 10.1073/pnas.92.8.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Scheerder I K, de Buyzere M, Delanghe J, Maas A, Clement D L, Wieme R. Humoral immune response against contractile proteins (actin and myosin) during cardiovascular disease. Eur Heart J. 1991;12:88–94. doi: 10.1093/eurheartj/12.suppl_d.88. [DOI] [PubMed] [Google Scholar]
- 6.de Scheerder I K, de Buyzere M L, Delanghe J R, Clement D L, Wieme R J. Anti-myosin humoral immune response following cardiac injury. Autoimmunity. 1989;4:51–58. doi: 10.3109/08916938909034359. [DOI] [PubMed] [Google Scholar]
- 7.Fedoseyeva E V, Zhang F, Orr P L, Levin D, Buncke H J, Benichou G. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J Immunol. 1999;162:6836–6842. [PubMed] [Google Scholar]
- 8.Godsel L M, Wang K, Schodin B A, Leon J S, Miller S D, Engman D M. Prevention of autoimmune myocarditis through the induction of antigen-specific peripheral immune tolerance. Circulation. 2001;103:1709–1714. doi: 10.1161/01.cir.103.12.1709. [DOI] [PubMed] [Google Scholar]
- 9.Grauert M R, Houdayer M, Hontebeyrie-Joskowciz M. Trypanosoma cruzi infection enhances polyreactive antibody response in an acute case of human Chagas' disease. Clin Exp Immunol. 1993;93:85–92. doi: 10.1111/j.1365-2249.1993.tb06501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herskowitz A, Wolfgram L J, Rose N R, Beisel K W. Coxsackievirus B3 murine myocarditis: a pathologic spectrum of myocarditis in genetically defined inbred strains. J Am Coll Cardiol. 1987;9:1311–1319. doi: 10.1016/s0735-1097(87)80471-0. [DOI] [PubMed] [Google Scholar]
- 11.Itoh M, Hiramine C, Mukasa A, Tokunaga Y, Fukui Y, Takouchi Y, Hojo K. Establishment of an experimental model of autoimmune epididymoorchitis induced by the transfer of a T-cell line in mice. Int J Androl. 1992;15:170–181. [PubMed] [Google Scholar]
- 12.Kennedy M K, Tan L J, Dal Canto M C, Tuohy V K, Lu Z J, Trotter J L, Miller S D. Inhibition of murine relapsing experimental autoimmune encephalomyelitis by immune tolerance to proteolipid protein and its encephalitogenic peptides. J Immunol. 1990;144:909–915. [PubMed] [Google Scholar]
- 13.Kierszenbaum F. Chagas' disease and the autoimmunity hypothesis. Clin Microbiol Rev. 1999;12:210–223. doi: 10.1128/cmr.12.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirchhoff L V, Neva F A. Chagas' disease in Latin American immigrants. JAMA. 1985;254:3058–3060. [PubMed] [Google Scholar]
- 15.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Leon J S, Engman D M. Autoimmunity in Chagas heart disease. Int J Parasitol. 2001;31:554–560. doi: 10.1016/s0020-7519(01)00163-1. [DOI] [PubMed] [Google Scholar]
- 17.Malkiel S, Kuan A P, Diamond B. Autoimmunity in heart disease: mechanisms and genetic susceptibility. Mol Med Today. 1996;2:336–342. doi: 10.1016/1357-4310(96)81799-0. [DOI] [PubMed] [Google Scholar]
- 18.Mijares A, Verdot L, Peineau N, Vray B, Hoebeke J, Argibay J. Antibodies from Trypanosoma cruzi infected mice recognize the second extracellular loop of the beta 1-adrenergic and M2-muscarinic receptors and regulate calcium channels in isolated cardiomyocytes. Mol Cell Biochem. 1996;163–164:107–112. doi: 10.1007/BF00408646. [DOI] [PubMed] [Google Scholar]
- 19.Milei J, Sanchez J, Storino R, Yu Z X, Denduchis B, Ferrans V J. Antibodies to laminin and immunohistochemical localization of laminin in chronic chagasic cardiomyopathy: a review. Mol Cell Biochem. 1993;129:161–170. doi: 10.1007/BF00926364. [DOI] [PubMed] [Google Scholar]
- 20.Moncayo A. Progress towards interruption of transmission of Chagas disease. Mem Inst Oswaldo Cruz. 1999;94(Suppl 1):401–404. doi: 10.1590/s0074-02761999000700079. [DOI] [PubMed] [Google Scholar]
- 21.Neu N, Beisel K W, Traystman M D, Rose N R, Craig S W. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to coxsackievirus B3-induced myocarditis. J Immunol. 1987;138:2488–2492. [PubMed] [Google Scholar]
- 22.Neu N, Craig S W, Rose N R, Alvarez F, Beisel K W. Coxsackievirus induced myocarditis in mice: cardiac myosin autoantibodies do not cross-react with the virus. Clin Exp Immunol. 1987;69:566–574. [PMC free article] [PubMed] [Google Scholar]
- 23.Neu N, Rose N R, Beisel K W, Herskowitz A, Gurri-Glass G, Craig S W. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139:3630–3636. [PubMed] [Google Scholar]
- 24.Nomura Y, Yoshinaga M, Haraguchi T, Oku S, Noda T, Miyata K, Umebayashi Y, Taira A. Relationship between the degree of injury at operation and the change in antimyosin antibody titer in the postpericardiotomy syndrome. Pediatr Cardiol. 1994;15:116–120. doi: 10.1007/BF00796322. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro dos Santos R, Rossi M A, Laus J L, Silva J S, Silvino W, Mengels J. Anti-CD4 abrogates rejection and reestablishes long-term tolerance to syngeneic newborn hearts grafted in mice chronically infected with Trypanosoma cruzi. J Exp Med. 1992;175:29–39. doi: 10.1084/jem.175.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizzo L V, Cunha-Neto E, Teixeira A R. Autoimmunity in Chagas' disease: specific inhibition of reactivity of CD4+ T cells against myosin in mice chronically infected with Trypanosoma cruzi. Infect Immun. 1989;57:2640–2644. doi: 10.1128/iai.57.9.2640-2644.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose N R, Hill S L. Autoimmune myocarditis. Int J Cardiol. 1996;54:171–175. doi: 10.1016/0167-5273(96)02595-8. [DOI] [PubMed] [Google Scholar]
- 28.Shiverick K T, Thomas L L, Alpert N R. Purification of cardiac myosin: application to hypertrophied myocardium. Biochim Biophys Acta. 1975;393:124–133. doi: 10.1016/0005-2795(75)90222-6. [DOI] [PubMed] [Google Scholar]
- 29.Sterin-Borda L, Perez Leiros C, Wald M, Cremaschi G, Borda E. Antibodies to beta 1 and beta 2 adrenoreceptors in Chagas' disease. Clin Exp Immunol. 1988;74:349–354. [PMC free article] [PubMed] [Google Scholar]
- 30.Szarfman A, Terranova V P, Rennard S I, Foidart J M, de Fatima Lima M, Scheinman J I, Martin G R. Antibodies to laminin in Chagas' disease. J Exp Med. 1982;155:1161–1171. doi: 10.1084/jem.155.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ternynck T, Bleux C, Gregoire J, Avrameas S, Kanellopoulos-Langevin C. Comparison between autoantibodies arising during Trypanosoma cruzi infection in mice and natural autoantibodies. J Immunol. 1990;144:1504–1511. [PubMed] [Google Scholar]
- 32.Tibbetts R S, McCormick T S, Rowland E C, Miller S D, Engman D M. Cardiac antigen-specific autoantibody production is associated with cardiomyopathy in Trypanosoma cruzi-infected mice. J Immunol. 1994;152:1493–1499. [PubMed] [Google Scholar]
- 33.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Traystman M D, Beisel K W. Genetic control of coxsackievirus B3-induced heart-specific autoantibodies associated with chronic myocarditis. Clin Exp Immunol. 1991;86:291–298. doi: 10.1111/j.1365-2249.1991.tb05812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z Y, Lee C S, Lider O, Weiner H L. Suppression of adjuvant arthritis in Lewis rats by oral administration of type II collagen. J Immunol. 1990;145:2489–2493. [PubMed] [Google Scholar]