Abstract
Importance
The effect of coronavirus disease 2019 (COVID-19) vaccination on fertility warrants clarification in women undergoing assisted reproductive treatment.
Objective
To study the association between female COVID-19 vaccination and outcomes of assisted reproductive treatment.
Data sources
PubMed, Embase, the Web of Science, Cochrane Library, and medRxiv and bioRxiv were searched for eligible studies from December 1, 2019, to November 30, 2022, with no language restrictions.
Study selection and synthesis
Observational studies comparing assisted reproductive outcomes between women with and without COVID-19 vaccination were included. The pooled estimates were calculated using the random-effects models as mean differences (MDs), standardized MDs, or odds ratios with 95% confidence intervals (CIs). Heterogeneity was evaluated using the I2 statistic.
Main Outcomes
The number of oocytes retrieved and clinical pregnancy rate.
Results
Twenty-one cohort studies involving a total of 19,687 treatment cycles were included. In a comparison of the vaccinated vs. unvaccinated groups, the pooled MD for oocyte number was −0.06 (95% CI, −0.51 to 0.39; I2 = 0), and the pooled odds ratio for clinical pregnancy was 0.95 (95% CI, 0.85–1.05; I2 = 0). Similarly, there were no statistically significant adverse effects identified in other outcomes determined a priori, including 4 cycle characteristics, 6 laboratory parameters, and 3 pregnancy indicators. Most results were consistently unchanged in subgroup and sensitivity analyses, with no evidence of publication bias according to Egger’s test.
Conclusion and relevance
Our work did not find significant differences in assisted reproductive outcomes between vaccinated and unvaccinated women. However, more data are warranted to confirm the safety of COVID-19 vaccination for assisted reproductive treatment and in female fertility in general.
Key Words: COVID-19, SARS-CoV-2, vaccine, assisted reproductive technology, fertility
Abstract
Efectos de la vacunación femenina para la enfermedad del coronavirus 2019 en los resultados de reproducción asistida: revisión sistemática y meta-análisis.
Importancia
el efecto de la vacunación para la enfermedad del coronavirus 2019 (COVID-19) en la fertilidad merece una aclaración en mujeres que se someten a un tratamiento de reproducción asistida.
Objetivo
Estudiar la asociación entre la vacunación contra el COVID-19 y los resultados de los tratamientos de reproducción asistida.
Fuentes de la data
Se buscaron estudios elegibles en PubMed, Embase, la web de la ciencia, Librería Cochrane, y medRxiv y bioRxiv desde Diciembre 1, 2019 a Noviembre 30, 2022, sin restricciones en el idioma.
Selección del estudio y síntesis
Se incluyeron estudios observacionales comparando los resultados de reproducción asistida entre mujeres con o sin vacunación contra COVID-19. La estimación agrupada fue calculada usando los modelos aleatorios-efectos como diferencias media (MDs), MDs estandarizada, o con razón de momios con 95% de intervalo de confianza (CIs). La heterogeneidad fue valorada usando estadista l2.
Resultados principales
número de óvulos recuperados y tasa de embarazo clínico.
Resultados
Se incluyeron veinte y un estudios de cohorte incluyendo un total de 19.687 ciclos de tratamiento. En una comparación de los grupos vacunados vs no vacunados, la MD agrupadas para óvulos maduros fue 0.06 (95% CI_0.51 a 0.39;I2 ¼ 0), y la razón de momios agrupadas para embarazo clínico fue 0.95 (95% CI, 0.85-1.05; I2 ¼ 0). De forma similar no hubo diferencias estadísticamente significativas en la identificación de efectos adversos en otros resultados determinados a priori, incluyendo 4 características de ciclos, 6 parámetros de laboratorio y 3 indicadores de embarazo. Muchos resultados fueron consistentemente sin cambios en los análisis sensitivos y subgrupales, sin evidencia de bias en la publicación acorde al test de Egger’s.
Conclusiones y relevancia
Nuestro trabajo no encontró diferencias significativas entre los resultados de reproducción asistida de mujeres vacunadas y no vacunadas. Sin embrago, se necesitan más datos para confirmar la seguridad de la vacunación contra COVID -19 para los tratamientos de reproducción asistida y para las fertilidad femenina en general.
Coronavirus disease 2019 (COVID-19) is induced by a novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). According to data from the World Health Organization, approximately 6.5 million mortalities have been attributed to COVID-19 as of September 6, 2022, accounting for 1.1% of all confirmed patients (>603 million) (2). Although the pandemic has lasted for nearly 3 years since its first report in December 2019, newly reported cases and deaths have been increasing worldwide with few countries spared, representing a severe and threatening challenge for public health.
Mass vaccination campaign is being conducted as an effort to create herd immunity. By the end of August 2022, a total of 124.8 billion COVID-19 vaccine doses have been administered globally, with 62.74% of the population in full vaccination (i.e., complete receipt of required vaccine doses) and 27.38% in booster vaccination (i.e., receipt of additional dose(s) after full vaccination) (2). To date, several different types of COVID-19 vaccines have been authorized for emergency use, including messenger ribonucleic acid (mRNA) vaccines, adenoviral vector vaccines, protein subunit vaccines, and inactivated vaccines (i.e., inactivation of cell culture–derived whole virus particles by chemical or physical methods) (3). These vaccines, in either randomized clinical trials or real-world studies, have shown high efficacy in preventing infection, alleviating severity, and reducing death (4, 5). In addition, most local (e.g., injection-site pain) and systematic (e.g., fatigue) symptoms are mild with limited allergic reactions and rare serious side effects (5, 6), indicating the reassuring safety of COVID-19 vaccines.
However, the negative impact of vaccines on reproductive health has been commonly raised by recipients because of the lack of follow-up data (7). In the initial rollout phase after vaccine authorization, there was also a significant upsurge in online queries regarding fertility side effects (8). These worries, as a consequence, could lead to unnecessary vaccination resistance and postponement of pregnancy plan. To address these concerns, emerging studies have investigated the association between different COVID-19 vaccines and the outcomes of assisted reproduction (9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29). However, most studies are based on small sample sizes, and the conclusions of individual studies are inconsistent.
We conducted this systematic review and meta-analysis to study the effect of COVID-19 vaccination on assisted reproductive treatment, including cycle characteristics, laboratory parameters, and fertility outcomes.
Materials and methods
This study was performed on the basis of the Meta-Analysis of Observational Studies in Epidemiology guideline (30) and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (31).
Search Strategy
A systematic literature search in PubMed, Embase, the Web of Science, Cochrane Library, and medRxiv and bioRxiv databases was conducted, covering published studies from December 1, 2019, to July 30, 2022, with no language restrictions. The search was updated on November 30, 2022. The search terms were constructed in a combination of (“COVID-19” OR “SARS-CoV-2”) AND (“vaccines”) AND (“reproductive techniques, assisted” OR “fertilization in vitro” OR “sperm injections, intracytoplasmic” OR “embryo transfer” OR “embryo implantation”). We also manually checked the reference lists of identified studies as conference proceedings of the American Society for Reproductive Medicine and European Society of Human Reproduction and Embryology to scrutinize for additional potentially relevant publications.
Study Selection
Studies were eligible if they met the following criteria: the study was an observational design, such as a cohort, cross-sectional, or case-control study; women in the experimental group underwent assisted reproductive treatment after partial, full, or booster COVID-19 vaccination, whereas the control group was unvaccinated; and the outcomes included any of the cycle characteristics, laboratory parameters, or pregnancy rates determined a priori. The cycle characteristics of interest were stimulation duration, gonadotropin dose, peak estradiol level, and endometrial thickness. The laboratory outcomes were the number of oocytes retrieved, mature oocyte rate, fertilization rate, cleavage rate, good-quality embryo rate, blastocyst formation rate, and euploidy rate. The fertility outcomes consisted of biochemical pregnancy, clinical pregnancy, embryo implantation, and ongoing pregnancy.
The exclusion criteria were as follows: case reports, cell experiments, or animal studies; nonoriginal research, such as comments, editorial letters, society statements, expert opinions, and reviews; duplicate publications in conferences; studies that evaluated the effect of male vaccination on in vitro fertilization (IVF) outcome; and studies that provided no outcomes of interest or insufficient data for statistical analysis. In the case of an ongoing cohort with overlapping populations, we retained the study containing a larger sample size.
Studies were selected by 2 independent reviewers (J.H., Z.F.) in a 2-step procedure. First, the title and/or abstract of each retrieved record was scanned for eligibility. Second, we read the full texts of all potentially relevant studies for further evaluation of inclusion. Any disputes were resolved by group discussion with the corresponding investigator (Q.W.).
Data Extraction and Quality Assessment
For eligible studies, information was collected independently by the same 2 investigators (J.H., Z.F.) and cross-checked to eliminate possible errors. A standardized form was designed, pilot-tested, and applied to extract the following data: the first investigator’s name; publication year; country of study; study design and period; number of treatment cycles; patient age; vaccine type and dose; embryo transfer (ET) strategy and number; vaccination interval to treatment; and data on the targeted outcome measures. In cases of studies with subgroup analyses, data were also collected for possible synthesis.
The methodological quality of the studies was assessed using the Newcastle-Ottawa scale (NOS) (32). With a score of 9 points in total, the NOS consists of 3 domains on selection, comparability, and outcome. Studies that scored 0–3, 4–6, and 7–9 points indicated low, moderate, and high quality, respectively. Any disagreements on scoring were resolved by consulting a third investigator (Q.W.).
Statistical Analysis
Data synthesis was performed in RevMan Manager (version 5.4; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020). For continuous outcomes, most results were pooled as mean differences (MDs) with 95% confidence intervals (CIs). In view of different measurement assays and/or laboratory standards of the included studies, the peak estradiol level was synthesized as the standardized MD. For dichotomous outcomes, the pooled effect sizes were expressed as odds ratios (ORs) with 95% CIs. Postmatching data were used for meta-analysis wherever available. When necessary, the sample means and standard deviations were estimated from medians, interquartile ranges, or 95% CIs by considering sample sizes (https://www.math.hkbu.edu.hk/∼tongt/papers/median2mean.html) (33, 34). The random-effects method was employed given the variations within and across studies (35).
Heterogeneity was assessed using Cochran’s Q test and quantified by the I 2 index, with I 2 ≥ 50% indicating high heterogeneity (36). For the primary outcomes of oocyte number and clinical pregnancy rate, subgroup analyses were further conducted according to vaccine type (mRNA, inactivated, protein-based, or mixed) and ET strategy (fresh ET, frozen-thawed ET [FET], euploid FET, or mixed). To examine the robustness of pooled estimates and leverage of individual studies, sensitivity analysis was performed by excluding 1 trial at a time. Egger’s test was applied to detect publication bias by Stata software (version 16.0; StataCorp LLC, College Station, TX). Statistical significance was set at P values of .10 for Egger’s test (37) and .05 for all other analyses.
Results
Literature Search and Study Characteristics
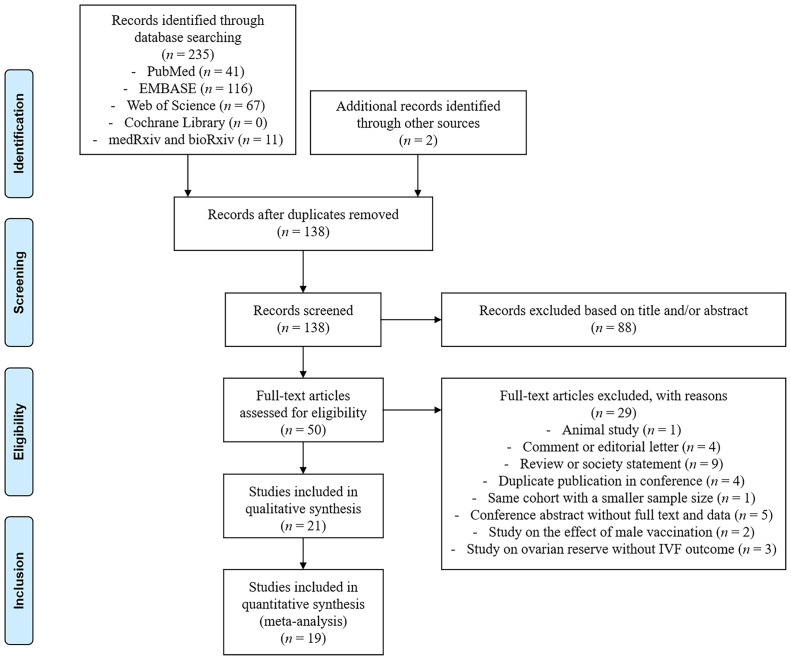
In total, 237 records were identified after a literature search, including 235 through databases and 2 through manual checks. After removing 99 duplicates, we further excluded 88 records after title and/or abstract screening and 29 records on the basis of full-text evaluation. The remaining 21 studies were finally included for our systematic review and meta-analysis (Fig. 1 ) (9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29).
Figure 1.
The flow diagram of study selection. IVF = in vitro fertilization.
The main characteristics of the included studies are summarized in Table 1 . These studies, published in 2021 and 2022, were conducted in 5 different countries, including Israel, China, the United States, Italy, and Spain. All studies were based on prospective or retrospective cohorts, with treatment cycle numbers ranging between 15 and 4,162. Participants were enrolled from a single center in most cohorts, whereas Avraham et al. (18) included women from 2 centers and Brandão et al. (20) from multiple centers. Nineteen studies compared vaccinated women with those unvaccinated, whereas 2 studies made self-controlled comparisons before and after vaccination (9, 11). Most studies involved patients undergoing IVF cycles for assisted reproductive treatment, whereas Karavani et al. (25) focused on elective oocyte cryopreservation cycles with no subsequent fertilization. The mRNA COVID-19 vaccines were used in 14 studies (9, 10, 11, 12, 13, 14, 16, 17, 18, 19, 20, 25, 26, 29), inactivated vaccines were used in 7 studies (15, 21, 22, 23, 24, 27, 28), viral-vector vaccines were used in 2 studies (17, 19), and protein-based vaccines were used in only 1 study (22). Regarding the vaccine doses, only patients vaccinated with 2 doses were included in 12 studies (9, 11, 13, 14, 15, 16, 17, 18, 21, 22, 24, 26), whereas 1 study did not provide detailed information (10) and 8 studies enrolled patients with partial, full, and/or booster vaccination for combined analyses (12, 19, 20, 23, 25, 27, 28, 29). The ET outcomes were reported in 17 of all cohorts (10, 11, 13, 14, 15, 16, 18, 19, 20, 21, 22, 23, 24, 26, 27, 28, 29). Most studies (n = 11) reported the percentage of single and double (or more) ETs (10, 14, 15, 18, 19, 20, 23, 24, 27, 28, 29), whereas some studies (n = 4) described the mean/median embryo number (13, 21, 22, 26) and 2 studies did not provide relevant data (11, 16). The time interval between vaccination and fertility treatment varied across studies, with different timepoints of the first, second, and last vaccine doses. Six of them have investigated the impact of vaccination intervals after categorization by 1, 1.8, 2, 3, 3.2, 4.5, 6, or 9 months, among which 5 reported no significant differences (15, 20, 22, 25, 27) and 1 suggested a reduced pregnancy rate after COVID-19 vaccination within 2 months (28). Overall, the analyzed studies were of low bias risk and high methodological quality with a mean NOS score of 8.05.
Table 1.
Main characteristics of THE included studies in the meta-analysis.
| Study | Country | Cohort design | Period | No. of cycles | Age (y) | Vaccine type | Vaccine dose | Embryo transfer type | Embryo transfer number | Vaccination interval to treatment | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Orvieto et al., 2021 (9) | Israel | Retrospective, single-center, self-controlled | NA | 36 | 37.3 ± 4.6 | mRNA: BNT162b2 | 2 | – | – | 32.6 ± 17.5 d (range, 7–85) (after the second dose) | 8 |
| Morris et al., 2021 (10) | United States | Retrospective, single-center | January 2021 to May 2021 | 123a | V: mean, 36.4 U: mean, 34.6 |
mRNA: BNT162b2 and mRNA-1273 | NA | FET and Euploid FET | Single | NA | 7 |
| Safrai et al., 2021 (11) | Israel | Retrospective, single-center, self-controlled | February 2021 to May 2021 | 47 | 37.4 ± 7.5 | mRNA: BNT162b2 | 2 | NA | NA | 57.3 ± 24.7 d (after the first dose) | 7 |
| Bentov et al., 2021 (12) | Israel | Prospective, single-center | February 2021 to March 2021 | 23a | V: 35.3 ± 4.0 U: 32.5 ± 5.3 |
mRNA: BNT162b2 | 1/2 | – | – | 32.2 ± 22.1 d (after the first dose) | 7 |
| Aizer et al., 2022 (13) | Israel | Retrospective, single-center | January 2021 to August 2021 | 628a | V: 30.4 ± 4.4 U: 30.9 ± 4.0 |
mRNA: BNT162b2 | 2 | FET | Median: 1 (IQR, 1–1) | 79 ± 46 d (after the second dose) | 7 |
| Aharon et al., 2022 (14) | United States | Retrospective, single-center | February 2021 to September 2021 | Cohort 1: 1,205 Cohort 2: 947 | Cohort 1: V: 36.7 ± 4.4 U: 37.1 ± 4.5 Cohort 2: V: 36.5 ± 3.7 U: 36.5 ± 4.1 |
mRNA: BNT162b2 and mRNA-1273 | 2 | Euploid FET | Single | ≥14 d (after the second dose) | 8 |
| Huang et al., 2022 (15) | China | Retrospective, single-center | June 2021 to September 2021 | 2,185 (matched: 730) | V: 33.6 ± 5.5 U: 33.4 ± 5.5 |
Inactivated: CoronaVac and BBIBP-CorV | 2 | Fresh | Single: 28.1% Double: 71.9% |
72.4 ± 57.0 d (after the second dose) | 9 |
| Odeh-Natour et al., 2022 (16) | Israel | Prospective, single-center | March 2021 to May 2021 | 55a | V: 33.3 ± 6.1 U: 35.7 ± 7.0 |
mRNA: BNT162b2 | 2 | Fresh | NA | 14–60 d (after the second dose) | 7 |
| Castiglione Morelli et al., 2022 (17) | Italy | Retrospective, single-center | March 2021 to September 2021 | 15a | V: 36.2 ± 4.3 U: 36.2 ± 4.2 |
mRNA (66.7%): BNT162b2 and mRNA-1273 Viral-vector (33.3%): ChAdOx1 nCoV-19 |
2 | – | – | Median: 29 d (range, 18–55) (after the second dose) | 7 |
| Avraham et al., 2022 (18) | Israel | Retrospective, 2-center | January 2021 to April 2021 | 400 | V: 36.1 ± 4.5 U: 36.0 ± 4.5 |
mRNA: BNT162b2 | 2 | Fresh | Single: 54.8% Double or more: 45.2% |
Mean: 30.63 d (range, 14–68) (after the second dose) | 8 |
| Jacobs et al., 2022 (19) | United States | Retrospective, single-center | December 2020 to September 2021 | 280 | V: 34 ± 4 U: 33 ± 4 |
mRNA (95.1%): BNT162b2 and mRNA-1273 Viral-vector (4.9%): Ad26.COV2.S |
1/2 | Fresh | Single: 80.0% Double: 20.0% |
93 ± 65 d (after the last dose) | 9 |
| Brandão et al., 2022 (20) | Spain | Retrospective, multicenter | NA | 4,162 | V: 38.7 ± 3.0 U: 38.2 ± 2.9b |
mRNA: BNT162b2 and mRNA-1273 | 1/2 | Euploid FET | Single | <1.8 mo: quartile 1 1.8–3.1 mo: quartile 2 3.2–4.5 mo: quartile 3 ≥4.5 mo: quartile 4 (after the last dose) |
8 |
| Wang et al., 2022 (21) | China | Retrospective, single-center | NA | 1,496 | V: 33.6 ± 4.6 U: 33.2 ± 4.1 |
Inactivated: NA | 2 | FET | Mean: 1.41 ± 0.75 | NA | 7 |
| Dong et al., 2022 (22) | China | Prospective, single-center | December 2021 to March 2022 | 735 (matched: 554) | V: 32.9 ± 3.4 U: 32.8 ± 4.1 |
Inactivated (93.7%): CoronaVac and BBIBP-CorV Protein-based (6.3%): ZF2001 |
2 | Fresh and FET | Mean: 1.43 ± 0.49 | <3 mo: 19.0% 3–6 mo: 59.2% >6 mo: 21.8% (after the last dose) |
9 |
| Wu et al., 2022 (23) | China | Retrospective, single-center | March 2021 to September 2021 | 1,583 (matched: 1,167) | V: 33.8 ± 4.7 U: 33.8 ± 4.9 |
Inactivated: CoronaVac and BBIBP-CorV | 1/2/3 | Fresh | Single: 57.4% Double: 42.6% |
≤30 d: 27.5% 31–60 d: 38.4% ≥61 d: 34.1% (after the first dose) |
9 |
| Huang et al., 2022 (24) | China | Retrospective, single-center | June 2021 to March 2022 | 133 | V: 37.7 ± 5.2 U: 37.7 ± 4.5 |
Inactivated: CoronaVac and BBIBP-CorV | 2 | Euploid FET | Single | 126.5 ± 64.0 d (range, 7–317) (after the second dose) | 9 |
| Karavani et al., 2022 (25) | Israel | Retrospective, single-center | December 2020 to January 2022 | 271 | V: 35.6 ± 2.0 U: 35.4 ± 2.3 |
mRNA: BNT162b2 | 2/3 | – | – | Range: 1–13 mo (after the first dose) | 9 |
| Safrai et al., 2022 (26) | Israel | Retrospective, single-center | February 2021 to September 2021 | 84 (matched: 58) | V: 35.1 ± 6.5 U: 32.1 ± 7.2 |
mRNA: BNT162b2 | 2 | Fresh | Mean: 1.12 ± 0.70 | 131.8 ± 43.1 d (after the first dose) | 8 |
| Cao et al., 2022 (27) | China | Retrospective, single-center | March 2021 to September 2021 |
2091 (matched: 1,755) | V: 32.9 ± 4.0 U: 32.8 ± 4.0 |
Inactivated: CoronaVac and BBIBP-CorV | 1/2 | FET | Single: 66.8% Double: 33.2% |
Median: 117.5 d (range, 7–311) (after the first dose) | 9 |
| Shi et al., 2022 (28) | China | Retrospective, single-center | May 2021 to March 2022 | 3,052 | V: 31.7 ± 4.3 U: 31.0 ± 3.0b |
Inactivated: NA | 1/2 | Fresh | Single: 82.4% Double or more: 17.6% |
≤30 d: 5.2% 31–60 d: 8.7% 61–90 d: 15.7% ≥91 d: 70.3% (after the first dose) |
9 |
| Adler Lazarovits et al., 2022 (29) | Israel | Prospective, single-center | October 2021 to November 2021 | 136a | V: 33.9 ± 6.3 U: 34.8 ± 5.6 |
mRNA: BNT162b2 | 1/2/3 | Fresh and FET | Single: 67.9% Double: 32.1% |
67.8 ± 61.5 d (after the last dose) | 8 |
FET = frozen-thawed embryo transfer; IQR = interquartile range; mRNA = messenger ribonucleic acid; NA = not available; NOS = Newcastle-Ottawa scale; U = unvaccinated; V = vaccinated.
Only vaccinated and unvaccinated groups were included for analysis, with the exclusion of infected patients.
Statistical significance was indicated by a P value of <.05.
Most studies were comparable in female age between groups, with 2 studies reporting significantly higher age in vaccinated patients (Table 1) (20, 28). Of them, Brandão et al. (20) detected no difference in fertility outcomes after euploid FET cycles, and Shi et al. (28) observed a lower oocyte number and pregnancy rate among vaccinated patients with fresh ET. Given the major confounding effect of age on IVF outcome, these 2 studies were excluded from further quantitative synthesis (meta-analysis).
Meta-Analysis of Cycle Characteristics
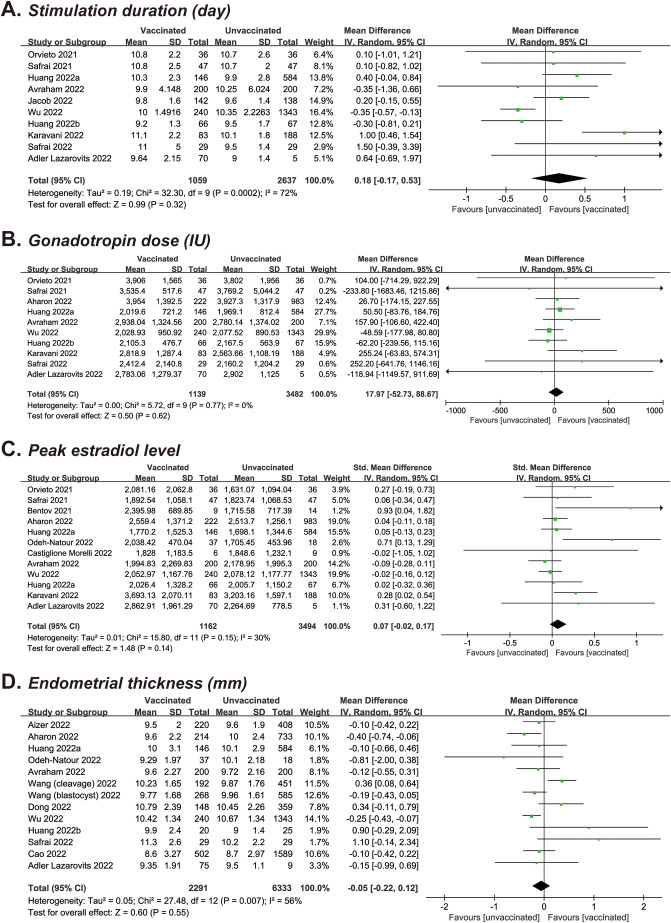
In each meta-analysis, the number of studies and cycles varied according to the characteristics reported: 10 (n = 3,696) provided data on the stimulation duration (9, 11, 15, 18, 19, 23, 24, 25, 26, 29), 10 (n = 4,621) on the gonadotropin dose (9, 11, 14, 15, 18, 23, 24, 25, 26, 29), 12 (n = 4,656) on the peak estradiol level (9, 11, 12, 14, 15, 16, 17, 18, 23, 24, 25, 29), and 13 (n = 8,624) on the endometrial thickness in fresh IVF or FET cycles (13, 14, 15, 16, 18, 21, 22, 23, 24, 26, 27, 29). As shown in Table 2 and Supplemental Figure 1 (available online), the pooled MDs/standardized MDs were 0.18 days (95% CI, −0.17 to 0.53; P=.32; I 2 = 72%) for the stimulation duration, 17.97 IU (95% CI, −52.73 to 88.67; P=.62; I 2 = 0) for the gonadotropin dose, 0.07 (95% CI, −0.02 to 0.17; P=.14; I 2 = 30%) for the peak estradiol level, and −0.05 mm (95% CI, −0.22 to 0.12; P=.55; I 2 = 56%) for the endometrial thickness in a comparison of vaccinated vs. unvaccinated cycles.
Table 2.
The pooled results of meta-analysis for the outcomes of vaccinated vs. unvaccinated cycles.
| Parameters | No. of studies | No. of cycles (vaccinated/unvaccinated) | Effect size (95% CI) | P value | I2 (%) |
|---|---|---|---|---|---|
| Cycle characteristics | |||||
| Stimulation duration | 10 | 3,696 (1,059/2,637) | MD, 0.18 d (−0.17 to 0.53) | .32 | 72 |
| Gonadotropin dose | 10 | 4,621 (1,139/3,482) | MD, 17.97 IU (−52.73 to 88.67) | .62 | 0 |
| Peak estradiol levels | 12 | 4,656 (1,162/3,494) | SMD, 0.07 (−0.02 to 0.17) | .14 | 30 |
| Endometrial thicknessa | 13 | 8,624 (2,291/6,333) | MD, −0.05 mm (−0.22 to 0.12) | .55 | 56 |
| Laboratory outcomes | |||||
| No. of oocytes retrieved | 15 | 5,132 (1,506/3,626) | MD, −0.06 (−0.51 to 0.39) | .80 | 0 |
| Mature oocyte rate | 7 | 2,760 (730/2,030) | MD, 2.01% (0.43–3.59) | .01 | 0 |
| Fertilization rateb | 10 | 3,500 (1,056/2,444) | MD, 0.64% (−1.61 to 2.89) | .58 | 35 |
| Cleavage rate | 3 | 1,417 (386/1,031) | MD, 0.42% (−0.42 to 1.26) | .33 | 31 |
| Good-quality embryo rate | 4 | 1,379 (365/1,014) | MD, −4.82% (−14.32 to 4.68) | .32 | 77 |
| Blastocyst formation rate | 5 | 2,902 (750/2,152) | MD, 1.43% (−0.90 to 3.76) | .23 | 0 |
| Euploidy rate | 2 | 1,083 (243/840) | MD, 4.49% (−0.71 to 9.69) | .09 | 18 |
| Fertility outcomes | |||||
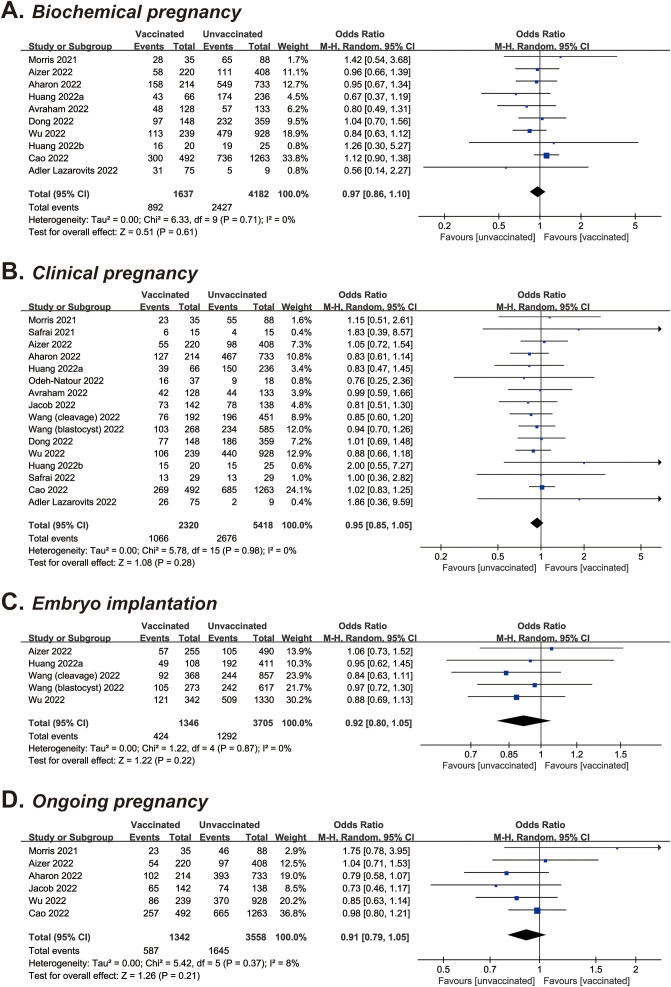
| Biochemical pregnancy | 10 | 5,819 (1,637/4,182) | OR, 0.97 (0.86–1.10) | .61 | 0 |
| Clinical pregnancya | 16 | 7,738 (2,320/5,418) | OR, 0.95 (0.85–1.05) | .28 | 0 |
| Embryo implantationa | 5 | 5,051 (1,346/3,705) | OR, 0.92 (0.80–1.05) | .22 | 0 |
| Ongoing pregnancy | 6 | 4,900 (1,342/3,558) | OR, 0.91 (0.79–1.05) | .21 | 8 |
CI = confidence interval; MD = mean difference; SMD = standardized mean difference; OR = odds ratio.
The study by Wang et al. (21) analyzed cleavage-stage embryo transfer and blastocyst transfer separately, which were included as 2 individual studies for pooled analysis.
The study by Avraham et al. (18) analyzed freeze-all cycle and fresh embryo transfer cycle separately, which were included as 2 individual studies for pooled analysis.
Meta-Analysis of Laboratory Parameters
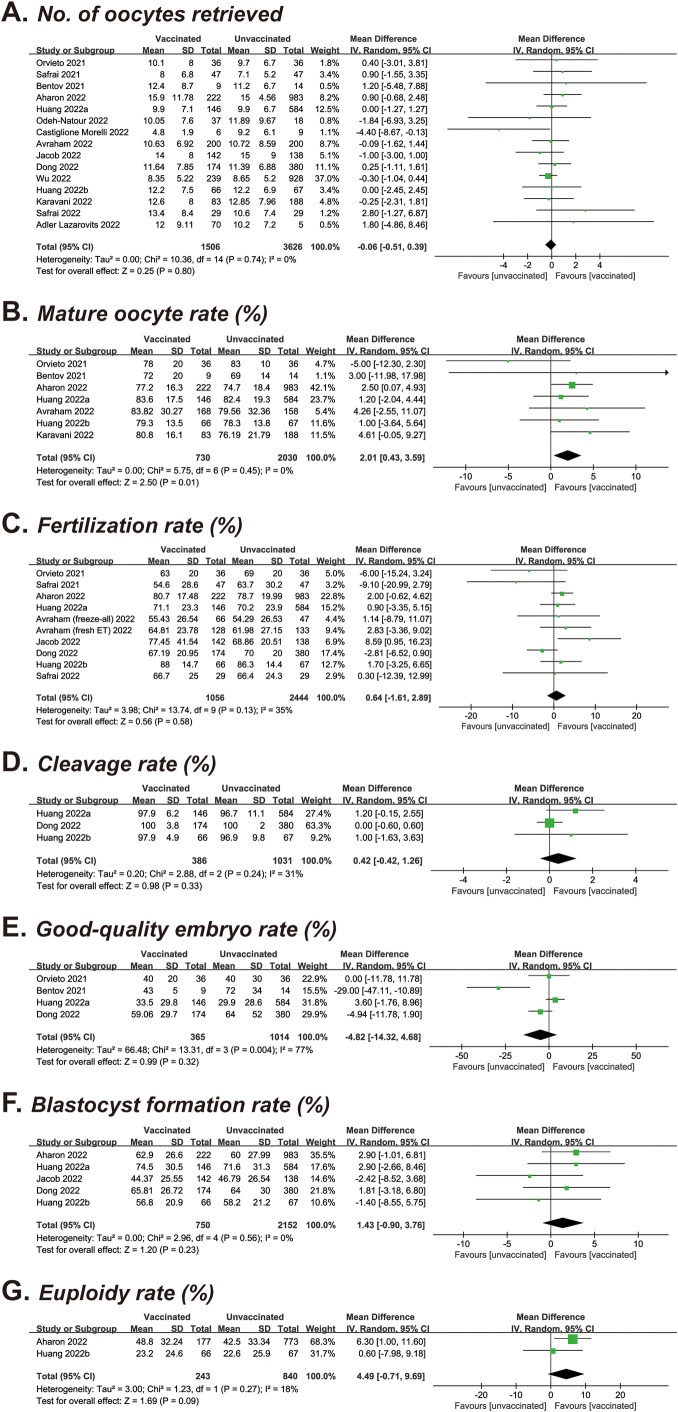
The number of oocytes retrieved was identified in 15 studies covering 1,506 vaccinated and 3,626 unvaccinated cycles (9, 11, 12, 14, 15, 16, 18, 19, 21, 22, 23, 24, 25, 26, 29). After pooled analysis, the mean oocyte number was 11.58 vs. 11.53, resulting in an MD of −0.06 (95% CI, −0.51 to 0.39; P=.80) (Table 2 and Supplemental Fig. 2A). Seven studies with a total of 2,760 cycles investigated the impact of COVID-19 vaccination on mature oocyte rate (9, 12, 14, 15, 18, 24, 25), and a significant increase was found in those vaccinated (MD, 2.01%; 95% CI, 0.43–3.59; P=.01) (Table 2 and Supplemental Fig. 2B). Regarding other laboratory parameters, the pooled MDs were 0.64% for the fertilization rate (10 studies of 3,550 cycles; 95% CI, −1.61 to 2.89; P=.58) (9, 11, 14, 15, 18, 19, 22, 24, 26), 0.42% for the cleavage rate (3 studies of 1,417 cycles; 95% CI, −0.42 t 1.26; P=.33) (15, 22, 24), −4.82% for the good-quality embryo rate (4 studies of 1,379 cycles; 95% CI, −14.32 to 4.68; P=.32) (9, 12, 15, 22), 1.43% for the blastocyst formation rate (5 studies of 2,902 cycles; 95% CI, −0.90 to 3.76; P=.23) (14, 15, 19, 22, 24), and 4.49% for the euploidy rate (2 studies of 1,083 cycles; 95% CI, −0.71 to 9.69; P=.09) (14, 24) (Table 2 and Supplemental Fig. 2C to G). Of the 7 comparisons, high heterogeneity was noted in the good-quality embryo rate (I 2 = 77%), whereas others showed no significant heterogeneity (I 2 = 0–35%).
Meta-Analysis of Fertility Outcomes
Sixteen studies, including 7,738 total cycles, provided information on the clinical pregnancy rate (10, 11, 13, 14, 15, 16, 18, 19, 21, 22, 23, 24, 26, 27, 29). According to the pooled data, the rate of clinical pregnancy was 45.9% vs. 49.4% in vaccinated vs. unvaccinated cycles, with an OR of 0.95 (95% CI, 0.85–1.05; P=.28) (Table 2 and Supplemental Fig. 3A). With regard to the effect of COVID-19 vaccination on other fertility outcomes, the ORs were 0.97 for biochemical pregnancy (10 studies of 5,819 cycles; 95% CI, 0.86–1.10; P=.61) (10, 13, 14, 15, 18, 22, 23, 24, 27, 29), 0.92 for embryo implantation (5 studies of 5,051 cycles; 95% CI, 0.80–1.05; P=.22) (13, 15, 21, 23), and 0.91 for ongoing pregnancy (6 studies of 4,900 cycles; 95% CI, 0.79–1.05; P=.21) (10, 13, 14, 19, 23, 27) (Table 2 and Supplemental Fig. 3B to D). All meta-analyses were of no or low heterogeneity (I 2 = 0–8%).
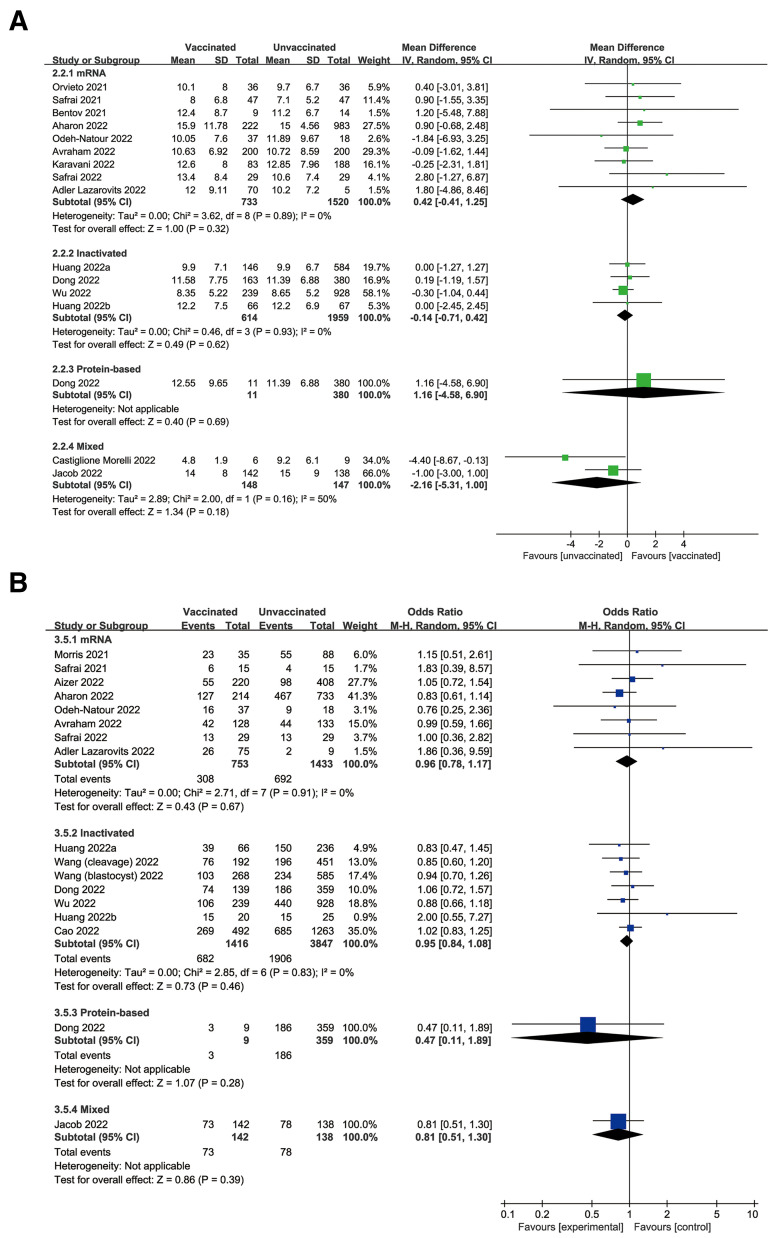
Subgroup Analysis
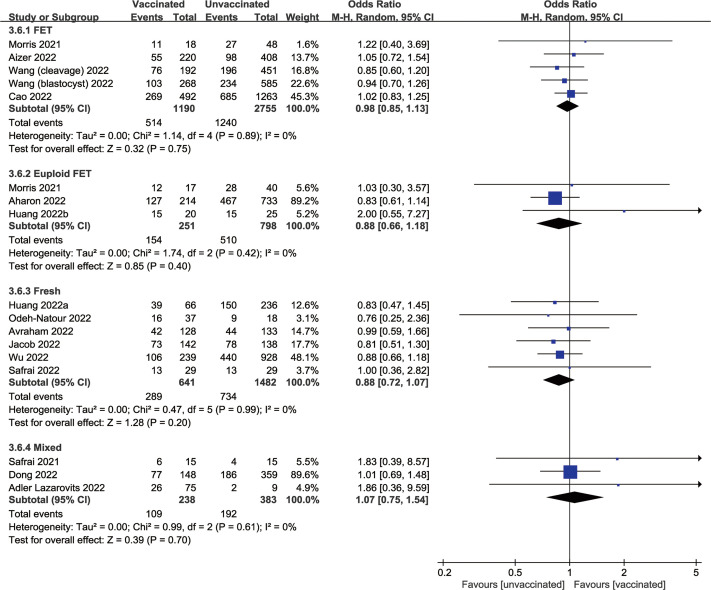
In subgroup analysis, the pooled estimate of the number of oocytes retrieved remained consistent across mRNA (MD, 0.42; 95% CI, −0.41 to 1.25; P=.32), inactivated (MD, −0.14; 95% CI, −0.71 to 0.42; P=.62), and protein-based (MD, 1.16; 95% CI, −4.58 to 6.90; P=.69) COVID-19 vaccines (Fig. 2 A). Similarly, the other primary outcome of clinical pregnancy was synthesized for mRNA (OR, 0.96; 95% CI, 0.78–1.17; P=.67), inactivated (OR, 0.95; 95% CI, 0.84–1.08; P=.46), and protein-based (OR, 0.47; 95% CI, 0.11–1.89; P=.28) vaccines (Fig. 2B). Considering the confounding effect of the ET strategy, the clinical pregnancy of vaccinated vs. unvaccinated cycles was also analyzed in subgroups of FET, euploid FET, and fresh ET, with pool ORs of 0.98 (95% CI, 0.85–1.13; P=.75), 0.88 (95% CI, 0.66–1.18; P=.40), and 0.88 (95% CI, 0.72–1.07; P=.20), respectively (Supplemental Fig. 4).
Figure 2.
Forest plot of (A) oocyte number and (B) clinical pregnancy for vaccinated vs. unvaccinated cycles according to different vaccine types. CI = confidence interval; mRNA = messenger ribonucleic acid.
Sensitivity Analysis
On excluding the study by Aharon et al. (14), the positive association between COVID-19 vaccination and mature oocyte rate became nonsignificant (MD, 1.65; 95% CI, −0.58 to 3.89). All other parameters remained unaltered after sensitivity analyses by removing individual studies, including the primary outcomes of oocyte number and clinical pregnancy.
Publication Bias
As presented in Supplemental Table 1, Egger’s test did not reveal statistical significance in 14 outcomes (P=.123 to .686), except for marginal significance in the peak estradiol level (P=.094). This suggested a limited possibility of publication bias among the included studies.
Discussion
As a systematic review and meta-analysis, the present study included 21 cohorts to investigate the association between COVID-19 vaccine uptake and assisted reproductive outcomes. On the basis of a total of 19,687 cycles, it was determined that COVID-19 vaccination had no statistically significant effects on treatment characteristics, laboratory parameters, and fertility outcomes.
In the initial analysis, patients with COVID-19 vaccination were found to have a 2% higher mature oocyte rate than those unvaccinated. Despite the lack of clinical implication, this slight change reached statistical significance. Removing the study by Aharon et al. (14) resulted in a nonsignificant change in the pooled MD, suggesting its high leverage on the overall finding. This retrospective cohort study enrolled 1,205 controlled ovarian hyperstimulation cycles during the same time frame. Compared with the unvaccinated group, the fully vaccinated group had lower parity (P= .01), an increased percentage of antagonist protocol (P=.01), and a decreased proportion of flare regimens (P=.005). Moreover, the etiologic factors of infertility (e.g., polycystic ovary syndrome) were not provided for comparison or adjustment. These baseline characteristics may, thus, lead to the variations in the mature oocyte rate (77.2% vs. 74.7%, P=.18). In this regard, the pooled results should be interpreted with caution, and further prospective studies are warranted to more valid and reliable conclusions.
Despite the lack of scientific evidences, several mechanisms have been indirectly linked to COVID-19 vaccines and female factor infertility. First, the vaccine administration could elicit the activation of CD8+ and T-helper 1 type CD4+ T-cell responses (3). Nonetheless, increased peripheral blood T cell activation has been associated with a reduced implantation rate after ET (38, 39, 40). Compared with normal fertile women, patients with recurrent pregnancy losses were also reported to have higher amounts of circulating activated T cells (40). Second, the presence of anti-SARS-CoV-2 antibody has been detected in the follicular fluid of vaccinated women (12); however, its impact on oocyte growth, development, and maturation remains unclear. In addition to antigen-specific response, vaccination may induce autoimmunity, such as the production of antiphospholipid antibodies and the development of thrombosis and thrombocytopenia in some cases (41, 42, 43). These immunologic changes, as a consequence, may lead to female factor infertility and pregnancy failure (44). Third, the use of vaccine excipients and/or adjuvants may impair oocyte quality and embryo competence by increasing oxidative stress, promoting inflammation, disrupting deoxyribonucleic acid structure, and inducing cell apoptosis and necrosis (45, 46, 47). For instance, both mRNA-1273 and BNT162b2 used lipid nanoparticles to deliver therapeutic contents, which may cross the biologic barriers to deposit in reproductive organs, such as the ovary (46). Contrarily, our pooled estimates of real-world data did not demonstrate fertility side effects after female vaccination, implying that the hypothesized biologic mechanisms may be clinically invalid and nonsignificant.
Although no consensus has been reached, the optimal time interval between vaccination completion and initiation of assisted reproductive treatment has been proposed by several academic societies. The American Society for Reproductive Medicine recommended that patients should receive COVID-19 vaccines at the soonest possible time (48). However, considering the recovery time of vaccine-associated side effects (e.g., fever), the vaccination should be avoided for at least 3 days before and after any surgical procedures (e.g., oocyte retrieval and ET). Issued on January 12, 2021, the European Society of Human Reproduction and Embryology statement advised the postponement of at least a few days for the immune response to settle (49). Given the lack of safety data on gametes, embryo implantation, and early pregnancy, it also suggested patients to wait for up to 2 months in a more prudent way. The Expert Group for Beijing Human Assisted Reproductive Technology Center for Quality Control and Improvement recommended a 1-month postponement for antibody development (50). To date, several studies have investigated the impact of vaccination intervals on reproductive outcomes (15, 20, 22, 25, 27, 28). Because different intervals with inconstant timepoints were used for categorization, we were unable to perform further stratified analysis. However, no significant differences were observed in most of these studies, which should be relatively reassuring for vaccinated patients who plan to start treatment at their earliest convenience.
The robustness of our finding is strengthened by the relatively large sample size in meta-analysis, low heterogeneity in most reported outcomes, and consistency in subgroup and sensitivity analyses. There are also several limitations that have to be acknowledged in the present study. First, although all included studies had a high methodological quality, their observational designs were associated with inherent bias and residual confounding. For instance, few studies have considered the status of male vaccination (9, 22, 23, 24, 27), which may affect treatment cycle outcomes via altered semen quality, according to some reports (51, 52). Although this effect was denied by a recent systematic review and meta-analysis (53), this confounder should be taken into account in future prospective cohorts to verify its reproductive safety in fertility treatment. In addition, some studies did not clearly differentiate or exclude infected patients in the cohorts (9, 14, 19, 20, 21), thus possibly biasing the individual and pooled results. Second, most studies focused on the fertility effects of mRNA and inactivated vaccines, with only 1 study on protein subunit vaccines and no individual study on adenoviral vector vaccines. This deserves further investigation because adenoviral vector vaccines are linked to a higher risk of thrombosis that may negatively affect pregnancy establishment and maintenance (43). Third, although this systematic review encompassed 21 cohorts, some parameters were assessed only in a small number of studies (e.g., 2 for the euploidy rate). Moreover, several outcomes tended to be worse in the vaccinated group, including the clinical pregnant rate with an OR of 0.95. Despite the lack of significance, the P value of .28 indicates a possible statistical difference when the sample size is further expanded. In this regard, larger multicenter cohort studies are still warranted, and the current meta-analysis should be updated when appropriate. Finally, owing to the short follow-up period, the primary outcome of the live birth rate in assisted reproductive treatment is still lacking (27). For safety consideration, a continuous monitoring is needed for obstetric and neonatal complications after female COVID-19 vaccination.
In conclusion, our work did not find significant differences in cycle characteristics, laboratory parameters, and fertility outcomes between vaccinated and unvaccinated women during assisted reproductive treatment. More data are needed to confirm the safety of COVID-19 vaccination in female fertility.
Footnotes
J.H. has nothing to disclose. Z.F. has nothing to disclose. Y.L. has nothing to disclose. C.X. has nothing to disclose. L.H. has nothing to disclose. J.M. has nothing to disclose. H.C. has nothing to disclose. Z.H. has nothing to disclose. L.X. has nothing to disclose. L.Ta. has nothing to disclose. Z.Z. has nothing to disclose. B.L. has nothing to disclose. H.H. has nothing to disclose. L.Ti. has nothing to disclose. X.A. has nothing to disclose. Q.W. has nothing to disclose.
J.H., Z.F., Y.L., C.X., and L.H. should be considered similar in author order.
Supported by the National Natural Science Foundation of China (Grant No. 82260315) and Central Funds Guiding the Local Science and Technology Development (Grant No. 20221ZDG020071).
Supplementary data
Supplementary Figure S1.
Forest plot of cycle characteristics for vaccinated versus unvaccinated cycles. (A) Stimulation duration. (B) Gonadotropin dose. (C) Peak estradiol level. (D) Endometrial thickness.
Supplementary Figure S2.
Forest plot of laboratory parameters for vaccinated versus unvaccinated cycles. (A) Number of oocytes retrieved. (B) Mature oocyte rate. (C) Fertilization rate. (D) Cleavage rate. (E) Good-quality embryo rate. (F) Blastocyst formation rate. (G) Euploidy rate. ET, embryo transfer.
Supplementary Figure S3.
Forest plot of fertility outcomes for vaccinated versus unvaccinated cycles. (A) Biochemical pregnancy. (B) Clinical pregnancy. (C) Embryo implantation. (D) Ongoing pregnancy.
Supplementary Figure S4.
Forest plot of clinical pregnancy for vaccinated versus unvaccinated cycles according to different embryo transfer strategies. FET, frozen-thawed embryo transfer.
References
- 1.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/ Available at:
- 3.Soleimanpour S., Yaghoubi A. COVID-19 vaccine: where are we now and where should we go? Expert Rev Vaccines. 2021;20:23–44. doi: 10.1080/14760584.2021.1875824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Q., Qin C., Liu M., Liu J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty. 2021;10:132. doi: 10.1186/s40249-021-00915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharif N., Alzahrani K.J., Ahmed S.N., Dey S.K. Efficacy, immunogenicity and safety of COVID-19 vaccines: a systematic review and meta-analysis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.714170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz P., Zizzo J., Balaji N.C., Reddy R., Khodamoradi K., Ory J., et al. Fear about adverse effect on fertility is a major cause of COVID-19 vaccine hesitancy in the United States. Andrologia. 2022;54 doi: 10.1111/and.14361. [DOI] [PubMed] [Google Scholar]
- 8.Diaz P., Reddy P., Ramasahayam R., Kuchakulla M., Ramasamy R. COVID-19 vaccine hesitancy linked to increased internet search queries for side effects on fertility potential in the initial rollout phase following Emergency Use Authorization. Andrologia. 2021;53 doi: 10.1111/and.14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orvieto R., Noach-Hirsh M., Segev-Zahav A., Haas J., Nahum R., Aizer A. Does mRNA SARS-CoV-2 vaccine influence patients' performance during IVF-ET cycle? Reprod Biol Endocrinol. 2021;19:69. doi: 10.1186/s12958-021-00757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris R.S. SARS-CoV-2 spike protein seropositivity from vaccination or infection does not cause sterility. F S Rep. 2021;2:253–255. doi: 10.1016/j.xfre.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safrai M., Rottenstreich A., Herzberg S., Imbar T., Reubinoff B., Ben-Meir A. Stopping the misinformation: BNT162b2 COVID-19 vaccine has no negative effect on women’s fertility. medRxiv. 2021:2021. 05.30.21258079. [Google Scholar]
- 12.Bentov Y., Beharier O., Moav-Zafrir A., Kabessa M., Godin M., Greenfield C.S., et al. Ovarian follicular function is not altered by SARS-CoV-2 infection or BNT162b2 mRNA COVID-19 vaccination. Hum Reprod. 2021;36:2506–2513. doi: 10.1093/humrep/deab182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aizer A., Noach-Hirsh M., Dratviman-Storobinsky O., Nahum R., Machtinger R., Yung Y., et al. The effect of coronavirus disease 2019 immunity on frozen-thawed embryo transfer cycles outcome. Fertil Steril. 2022;117:974–979. doi: 10.1016/j.fertnstert.2022.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aharon D., Lederman M., Ghofranian A., Hernandez-Nieto C., Canon C., Hanley W., et al. In vitro fertilization and early pregnancy outcomes after coronavirus disease 2019 (COVID-19) vaccination. Obstet Gynecol. 2022;139:490–497. doi: 10.1097/AOG.0000000000004713. [DOI] [PubMed] [Google Scholar]
- 15.Huang J., Xia L., Lin J., Liu B., Zhao Y., Xin C., et al. No effect of inactivated SARS-CoV-2 vaccination on in vitro fertilization outcomes: a propensity score-matched study. J Inflamm Res. 2022;15:839–849. doi: 10.2147/JIR.S347729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odeh-Natour R., Shapira M., Estrada D., Freimann S., Tal Y., Atzmon Y., et al. Does mRNA SARS-CoV-2 vaccine in the follicular fluid impact follicle and oocyte performance in IVF treatments? Am J Reprod Immunol. 2022;87 doi: 10.1111/aji.13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castiglione Morelli M.A., Iuliano A., Schettini S.C.A., Ferri A., Colucci P., Viggiani L., et al. Are the follicular fluid characteristics of recovered coronavirus disease 2019 patients different from those of vaccinated women approaching in vitro fertilization? Front Physiol. 2022;13 doi: 10.3389/fphys.2022.840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avraham S., Kedem A., Zur H., Youngster M., Yaakov O., Yerushalmi G.M., et al. Coronavirus disease 2019 vaccination and infertility treatment outcomes. Fertil Steril. 2022;117:1291–1299. doi: 10.1016/j.fertnstert.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs E., Summers K., Sparks A., Mejia R. Fresh embryo transfer cycle characteristics and outcomes following in vitro fertilization via intracytoplasmic sperm injection among patients with and without COVID-19 vaccination. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandão P., Pellicer A., Meseguer M., Remohí J., Garrido N., García-Velasco J.A. COVID-19 mRNA vaccines have no effect on endometrial receptivity after euploid embryo transfer. Reprod Biomed Online. 2022;45:688–695. doi: 10.1016/j.rbmo.2022.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Ren X., Wang Z., Feng X., Li M., Liu P. Receipt of inactivated COVID-19 vaccine had no adverse influence on embryo implantation, clinical pregnancy and miscarriage in early pregnancy. Sci China Life Sci. 2022;65:2332–2334. doi: 10.1007/s11427-022-2133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong M., Wu S., Zhang X., Zhao N., Qi J., Zhao D., et al. Effects of COVID-19 vaccination status, vaccine type, and vaccination interval on IVF pregnancy outcomes in infertile couples. J Assist Reprod Genet. 2022;39:1849–1859. doi: 10.1007/s10815-022-02543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y., Cao M., Lin Y., Xu Z., Liang Z., Huang Q., et al. Inactivated COVID-19 vaccination does not affect in vitro fertilization outcomes in women. Hum Reprod. 2022;37:2054–2062. doi: 10.1093/humrep/deac160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J., Xia L., Tian L., Fan H., Xu D., Ai X., et al. Impact of inactivated SARS-CoV-2 vaccination on embryo ploidy: a retrospective cohort study of 133 PGT-A cycles in China. Biol Res. 2022;55:26. doi: 10.1186/s40659-022-00395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karavani G., Chill H.H., Dick A., Meirman C., Gutman-Ido E., Herzberg S., et al. Pfizer SARS-CoV-2 BNT162b2 mRNA vaccination (BNT162b2) has no adverse effect on elective oocyte cryopreservation outcomes. Reprod Biomed Online. 2022;45:987–994. doi: 10.1016/j.rbmo.2022.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safrai M., Kremer E., Atias E., Ben-Meir A. BNT162b2 Covid-19 vaccine does not affect fertility as explored in a pilot study of women undergoing IVF treatment. Minerva Obstet Gynecol. 2022 doi: 10.23736/S2724-606X.22.05148-X. [DOI] [PubMed] [Google Scholar]
- 27.Cao M., Wu Y., Lin Y., Xu Z., Liang Z., Huang Q., et al. Inactivated Covid-19 vaccine did not undermine live birth and neonatal outcomes of women with frozen-thawed embryo transfer. Hum Reprod. 2022;37:2942–2951. doi: 10.1093/humrep/deac220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi W., Wang M., Xue X., Li N., Chen L., Shi J. Association between time interval from COVID-19 vaccination to in vitro fertilization and pregnancy rate after fresh embryo transfer. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.36609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adler Lazarovits C., Smadja A., Kabessa M., Allouche Kam H., Nevo L., Godin M., et al. Boosting dose of Pfizer-BioNtech mRNA vaccine against SARS-CoV-2 does not affect reproductive outcomes in in-vitro fertilization patients: a cohort study. J Womens Health (Larchmt) 2023;32:24–28. doi: 10.1089/jwh.2022.0163. [DOI] [PubMed] [Google Scholar]
- 30.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. J Am Med Assoc. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 31.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://ohri.ca/programs/clinical_epidemiology/oxford.htm Available at:
- 33.Luo D., Wan X., Liu J., Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 34.Shi J., Luo D., Weng H., Zeng X.T., Lin L., Chu H., et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020;11:641–654. doi: 10.1002/jrsm.1429. [DOI] [PubMed] [Google Scholar]
- 35.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. John Wiley & Sons, Ltd.; Chichester, UK: 2009. Introduction to meta-analysis. [Google Scholar]
- 36.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 37.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallinelli A., Roncaglia R., Matteo M.L., Ciaccio I., Volpe A., Facchinetti F. Immunological changes and stress are associated with different implantation rates in patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2001;76:85–91. doi: 10.1016/s0015-0282(01)01826-x. [DOI] [PubMed] [Google Scholar]
- 39.Coulam C.B., Roussev R.G. Increasing circulating T-cell activation markers are linked to subsequent implantation failure after transfer of in vitro fertilized embryos. Am J Reprod Immunol. 2003;50:340–345. doi: 10.1034/j.1600-0897.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 40.Yang K.M., Ntrivalas E., Cho H.J., Kim N.Y., Beaman K., Gilman-Sachs A., et al. Women with multiple implantation failures and recurrent pregnancy losses have increased peripheral blood T cell activation. Am J Reprod Immunol. 2010;63:370–378. doi: 10.1111/j.1600-0897.2010.00811.x. [DOI] [PubMed] [Google Scholar]
- 41.Cruz-Tapias P., Blank M., Anaya J.M., Shoenfeld Y. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389–393. doi: 10.1097/BOR.0b013e32835448b8. [DOI] [PubMed] [Google Scholar]
- 42.Liu T., Dai J., Yang Z., Yu X., Xu Y., Shi X., et al. Inactivated SARS-CoV-2 vaccine does not influence the profile of prothrombotic antibody nor increase the risk of thrombosis in a prospective Chinese cohort. Sci Bull (Beijing) 2021;66:2312–2319. doi: 10.1016/j.scib.2021.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharifian-Dorche M., Bahmanyar M., Sharifian-Dorche A., Mohammadi P., Nomovi M., Mowla A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J Neurol Sci. 2021;428 doi: 10.1016/j.jns.2021.117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Backos M., Rai R., Regan L. Antiphospholipid antibodies and infertility. Hum Fertil (Camb) 2002;5:30–34. doi: 10.1080/1464727992000199731. [DOI] [PubMed] [Google Scholar]
- 45.Xu L., Wang Y.Y., Huang J., Chen C.Y., Wang Z.X., Xie H. Silver nanoparticles: synthesis, medical applications and biosafety. Theranostics. 2020;10:8996–9031. doi: 10.7150/thno.45413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou C.C., Zhu J.Q. Nanoparticles and female reproductive system: how do nanoparticles affect oogenesis and embryonic development. Oncotarget. 2017;8:109799–109817. doi: 10.18632/oncotarget.19087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J., Hu S., Rao M., Hu L., Lei H., Wu Y., et al. Copper nanoparticle-induced ovarian injury, follicular atresia, apoptosis, and gene expression alterations in female rats. Int J Nanomedicine. 2017;12:5959–5971. doi: 10.2147/IJN.S139215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coronavirus/COVID-19 Task Force of the American Society for Reproductive Medicine American Society for Reproductive Medicine (ASRM) patient management and clinical recommendations during the coronavirus (COVID-19) pandemic: update no. 13 – February 22, 2021. Variants, vaccines, and vaccination. http://asrm.org/globalassets/asrm/asrm-content/news-and-publications/covid-19/covidtaskforceupdate13.pdf Available at:
- 49.ESHRE COVID-19 Working Group ESHRE statement on COVID-19 vaccination and medically assisted reproduction. http://eshre.eu/Europe/Position-statements/COVID19/vaccination Available at:
- 50.National Health Commission of the People’s Republic of China COVID-19 vaccination status. http://www.nhc.gov.cn/ Available at: [DOI] [PMC free article] [PubMed]
- 51.Gat I., Kedem A., Dviri M., Umanski A., Levi M., Hourvitz A., et al. Covid-19 vaccination BNT162b2 temporarily impairs semen concentration and total motile count among semen donors. Andrology. 2022;10:1016–1022. doi: 10.1111/andr.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzalez D.C., Nassau D.E., Khodamoradi K., Ibrahim E., Blachman-Braun R., Ory J., et al. Sperm parameters before and after COVID-19 mRNA vaccination. J Am Med Assoc. 2021;326:273–274. doi: 10.1001/jama.2021.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang J., Fang Z., Huang L., Fan L., Liu Y., Xia L., et al. Effect of COVID-19 vaccination on semen parameters: a systematic review and meta-analysis. J Med Virol. 2023;95 doi: 10.1002/jmv.28263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.