Abstract
Objective:
Tobacco dependence treatment initiated in the hospital emergency department (ED) is effective. However, trials typically use multicomponent interventions, making it difficult to distinguish specific components that are effective. In addition, interactions between components cannot be assessed. The Multiphase Optimization Strategy (MOST) allows investigators to identify these effects.
Methods:
We conducted a full-factorial, 24 or 16-condition optimization trial in a busy hospital ED to examine the performance of 4 tobacco dependence interventions: a brief negotiation interview (BNI); 6 weeks of nicotine replacement therapy (NRT) with the first dose delivered in the ED; active referral to a telephone quitline; and enrollment in SmokefreeTXT, a free short-messaging service program. Study data were analyzed with a novel mixed methods approach to assess clinical efficacy, cost effectiveness, and qualitative participant feedback. The primary endpoint was tobacco abstinence at 3 months, verified by exhaled carbon monoxide.
Results:
Between February, 2017 and May, 2019, we enrolled 1056 adult smokers visiting the ED. Biochemically confirmed abstinence rates at 3 months for each intervention, vs. control, were: BNI, 13.5% vs. 8.9% (P=0.02); NRT, 14.4% vs. 8.0% P=0.001); quitline, 12.4% vs. 10.1% (P=0.24); SmokefreeTXT, 11.6% vs. 10.8% (P=0.70). There were no statistically significant interactions among components. Economic and qualitative analyses are in progress.
Conclusions:
The BNI and NRT were efficacious. This study is the first to identify components of ED-initiated tobacco dependence treatment that are individually effective. Future work will address scalability of the BNI and NRT, by offering provider-delivered BNIs and NRT prescriptions.
Trial registration:
Trial (NCT02896400) registered in Clinicaltrials.gov on September 6, 2016.
Keywords: smoking cessation, tobacco dependence treatment, emergency department
Introduction
Background.
Nearly 60 years after the landmark 1964 Surgeon General’s report, smoking remains the leading cause of preventable death in the United States, with about 480,000 deaths per year.1 In 2019, 14% of Americans smoked cigarettes,2 still above the Healthy People 2020 goal of 12% prevalence.3 Although tobacco dependence has declined across all demographic groups, the rate of decline has been slower among individuals of lower socioeconomic status, leading to higher smoking prevalence among this group4. Individuals at particular risk include those with low income or low education, those with serious mental illness, and individuals with other substance use disorders.
These groups of smokers are frequently treated in emergency departments (EDs). ED patients are disproportionately of low socioeconomic status, more likely to smoke compared with the general population.5, 6 ED smokers often present with illnesses caused or exacerbated by tobacco use or have injuries for which smoking impedes healing.7–9 Hence the ED visit represents an opportune time to discuss patients’ tobacco use and its relevance to their current visit, and to initiate tobacco treatment and aftercare.10 This represents an evolving standard of treatment in the treatment of many ED patients with substance use disorders, including tobacco.11
It is common to treat behavioral disorders and addictions with multicomponent approaches, given the complex behavioral, genetic, physiologic, and environmental factors that mediate disorders such as addiction. Our group has shown that a multicomponent intervention delivered in the ED that includes behavioral and pharmacologic therapies can promote tobacco abstinence.9 We adapt the paradigm known as Screening, Brief Intervention, and Referral to Treatment (SBIRT).12 Because we initiate pharmacotherapy during the ED visit, we refer to our model as Screening, Treatment Initiation, and Referral (STIR).13
Importance.
It is imperative to identify the most clinically effective intervention components that are also cost-effective to create an efficacious intervention that can be delivered for the lowest cost possible in real-world ED settings. This requires assessing the performance of individual intervention components. However, an important limitation of the traditional approach is that it does not enable the investigator to disaggregate the effects of individual components or examine whether interactions exist between components.
In our prior work, the components of the intervention were: a brief adaptation of motivational interviewing called the Brief Negotiation Interview (BNI);14 initiation of nicotine replacement therapy (NRT) in the ED with provision of a 6-week supply of patches and gum; referral to the Connecticut State smokers’ telephone quitline (QL); provision of a smoking cessation brochure; and the provision of a booster phone call 3 days after enrollment.9 An additional modality added for this trial is ED-initiated short-message-service (SMS) for tobacco dependence treatment, which, in recent studies by our group, showed feasibility and potential efficacy.15, 16
In this prior trial, we assumed that each intervention component contributed to overall efficacy, but the packaged nature of the intervention did not permit quantitative analysis of their individual effects.
Goals of This Investigation.
The goal of this study is to identify components of an ED-initiated intervention which are optimally effective for treating adult smokers.
Methods
Overview and study design.
This investigation used the Multiphase Optimization Strategy (MOST) design, developed by Collins17 and outlined in Figure 1. MOST is an iterative process that employs development of a conceptual model and pilot testing of candidate intervention components (Preparation), followed by a trial (Optimization) to identify the optimal intervention package, with testing of the package in a subsequent randomized clinical trial (Evaluation). The results of the optimization trial allow investigators to efficiently identify efficacious components of an intervention, subject to a cost constraint.17–20 MOST has been used to study interventions to treat tobacco dependence,21–29 but, to our knowledge, had not been previously used in the ED setting.
Figure 1.
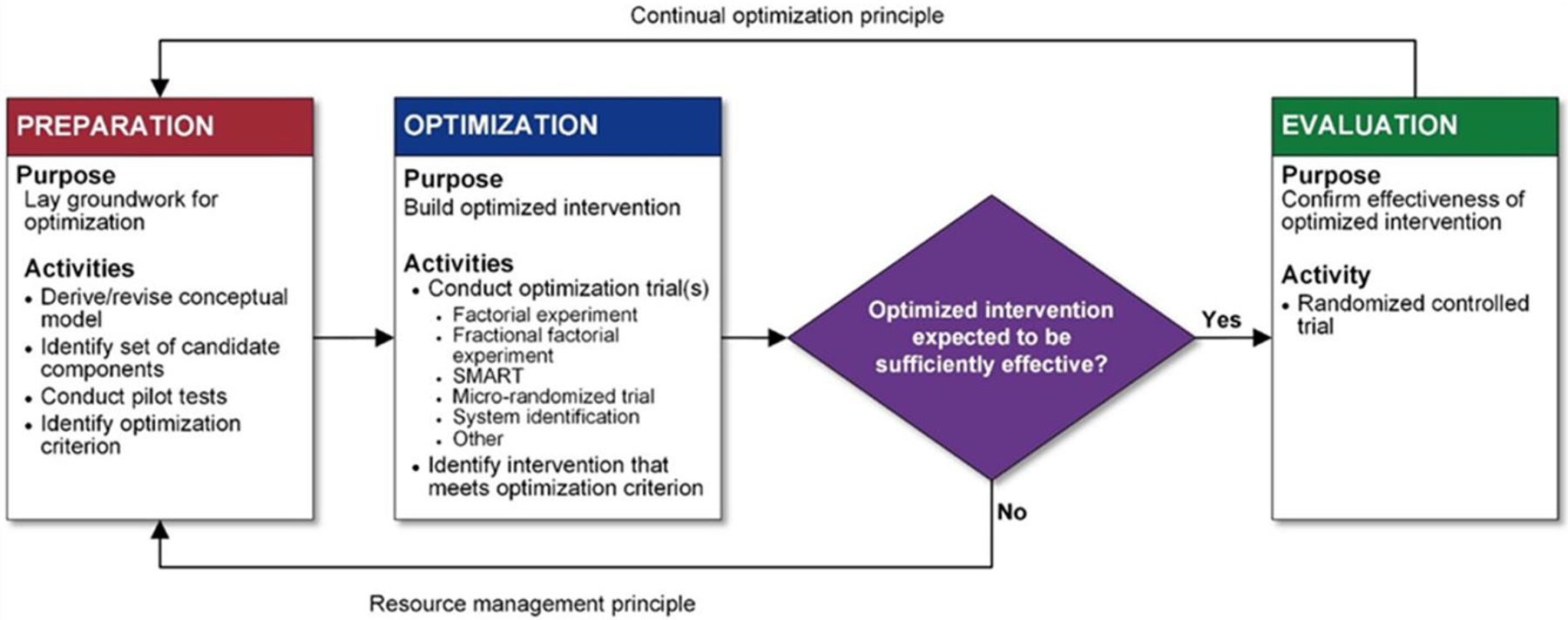
Multiphase Optimization Strategy. Reproduced from Collins.47
Details of this study protocol have been reported elsewhere.30 In brief, we examined the following intervention components: (1) a Brief Negotiation Interview (BNI, our brief adaptation of motivational interviewing31), delivered by a trained staff member; (2) provision of 6 weeks of nicotine patches and gum to the research participant, with application of the first patch in the ED; (3) active referral to the Connecticut Smokers’ Quitline; and (4) enrollment in the SmokefreeTXT short-messaging service (SMS) texting program for mobile phones. All patients received a smoking cessation brochure. Our study used a full 24 (i.e. 16-condition) factorial design to evaluate the effects of these 4 intervention components, at a fraction of the cost because this design requires only one-fourth of the participants it would take to conduct 4 individual experiments to evaluate each component separately. Although as Table 1 shows the factorial design requires the allocation of participants to 16 different combinations of the 4 components, evaluation of each individual component is performed comparing all participants receiving a component to all participants not receiving a component, making this an efficient design. For instance, evaluation of the BNI component will compare those randomized to conditions 1 through 8 to those in conditions 9 to 16. Based on the results of the screening phase in conjunction with findings from the qualitative analysis, we will design and propose a 2-arm randomized clinical trial comparing the efficacy of the multicomponent intervention package to usual care for the evaluation phase.
Table 1.
Conditions of the trial.
| Experimental Condition | Brief Negotiation Interview | Nicotine Replacement Therapy | Quitline | SmokefreeTXT |
|---|---|---|---|---|
| 1 | X | X | X | X |
| 2 | X | X | X | |
| 3 | X | X | X | |
| 4 | X | X | ||
| 5 | X | X | X | |
| 6 | X | X | ||
| 7 | X | X | ||
| 8 | X | |||
| 9 | X | X | X | |
| 10 | X | X | ||
| 11 | X | X | ||
| 12 | X | |||
| 13 | X | X | ||
| 14 | X | |||
| 15 | X | |||
| 16 |
Interventions
Our previous work employed a multicomponent approach to ED-initiated tobacco treatment, which used both pharmacologic and behavioral approaches. All components were evidence-based; most are cited in the 2008 Public Health Service clinical practice guideline.32 The newest approach, text messaging on mobile phones, is supported by a Cochrane review.33
Brief Negotiation Interview (BNI).
Brief Negotiation Intervention (BNI) is a manual-guided brief intervention that is an adaptation of motivational interviewing and has been shown to be feasible and efficacious in the ED setting. The BNI manual for this study is based on one that we used in our previous trial. The purpose of the BNI is to assist patients in recognizing the problematic nature of and changing their tobacco use. The main goals of the interview are to elicit disincentives for smoking and compelling personal reasons/motives for quitting from the patient, thereby motivating them to change their smoking behavior by reducing the number of cigarettes smoked daily or abstaining completely.
The BNI was delivered and audiotaped by trained RAs who have a bachelor’s-level education. Tapes were reviewed biweekly by a clinical psychologist with the RAs to assess fidelity to the scripted BNI protocol.
Nicotine gum and patch begun in the ED.
Participants randomized to NRT received from study personnel 6 weeks of patches (42 count) and gum (300 pieces of 2mg). As in our previous studies, NRT dosing was tailored to the participant’s cigarette consumption (5–10 cigarettes/day: 14 mg patch and 2mg gum; >10 cigarettes/day: 21mg patch and 2mg gum). Patches come in 14 and 21 mg doses. The first patch is applied by an ED nurse at the index visit. The RA then conducted a brief educational session with the participant on the use of both the patch and the gum. We use combination NRT because it is generally more efficacious than NRT monotherapy in treating tobacco dependence.32
Text Messaging.
The SmokefreeTXT program, developed by the National Cancer Institute, is an SMS text messaging service designed for adults. The program provides 24/7 encouragement, advice, and tips to quit and stay quit.
In a previous study of ED-initiated texting,16 our group modified several of SmokefreeTXT’s 130 messages. Based on the qualitative data we collected during our pilot study15, we offered participants in this trial a choice of how many messages they receive per day (2–4 messages per day) and the time of day they receive the first message. We also added 18 messages for participants presenting with ED-relevant chief complaints (cardiac, respiratory, or injury).
Active Quitline Referral.
Participant information was faxed to the state quitline’s service provider, Optum (formerly Alere Wellbeing). QL staff then telephoned participants, provided information about services standardly offered by the Connecticut QL, and enrolled interested participants in QL services. Participants who enrolled in a multiple call or web-based program were also eligible for two weeks of nicotine replacement therapy from the QL. Calls are scheduled at times convenient to participants. In prior work by our group, 32% of participants in the intervention arm engaged in at least one call with the quitline.34
Brochure.
Lastly, all participants, irrespective of assigned experimental condition, were provided with a state-produced brochure that reviewed the health hazards of smoking and provided the phone number for the quitline.
Participants.
Inclusion criteria.
Patients who presented to the adult ED at Yale-New Haven Hospital (YNHH) were eligible for the study if they: (1) were 18 years or older; (2) had smoked at least 100 cigarettes in their lifetime; (3) described themselves as every-day or some-day smokers; (4) smoked at least 5 cigarettes/day, on average; (5) owned a cellphone with texting capability; and (6) were able to give written informed consent.
Exclusion criteria.
Patients were excluded for: (1) not being able to read or understand English; (2) currently receiving formal tobacco dependence treatment; (3) having a life-threatening or unstable medical, surgical, or psychiatric condition; (4) being unable to provide at least one collateral contact; (5) living out-of-state; (6) planning to leave the ED against medical advice; (7) being pregnant (self-report or urine testing) or currently nursing or trying to conceive.
Setting.
The hospital is part of an academic medical center that serves a moderately poor city in the northeastern United States; in 2019, 26.5% of New Haven’s 130,000 residents lived in poverty.35 The racial mix of patients reflects that of the surrounding community: 65% White, not Hispanic; 23% African-American, not Hispanic, 10% Hispanic; 2% other. Payor status for ED smokers is approximately 55% Medicaid, 5% Medicare, 30% private insurance, and 10% self-pay. The Adult ED is a level one trauma center that treats 100,000 adult visits per year. The prevalence of smoking in the city is 18%, slightly above the national average.36
Selection of participants.
Participants were recruited during all days of the week from 8am-10pm, by trained research assistants (RAs) who screened all ED patients. Patients were asked for verbal consent to complete the 2-item tobacco screener used by the Behavioral Risk Factor Surveillance System (Do you smoke cigarettes every day, some days, or not at all? Have you smoked at least 100 cigarettes in your entire life?). Patients who reported smoking but were not eligible were given a handout recommending that they abstain from smoking, contact their primary care provider, and consider calling the quitline. Individuals who met inclusion and exclusion criteria and consented to participate had baseline assessments performed and were then randomized to one of 16 combinations of components (Table 1).
Randomization sequence generation, allocation, and concealment.
To assure equal intervention allocation and concealment of intervention allocation a random permuted block sequence was generated (via www.randomization.com) and intervention assignments distributed through the clinical trial data management system.
Measurements and Outcomes
Baseline measures.
A brief battery of measures was obtained at baseline, included standard demographic and clinical variables. These included self-reported brief screening instruments for depression (PHQ2), the Heaviness of Smoking Index (HIS), Rapid Alcohol Problem Screen, and Rapid Drug Screen. All are validated, commonly used instruments which we have used in our prior work. For ease of reporting, we dichotomized two instruments: the two-question PHQ2 score into categories of “likely depressed” and “likely not depressed,” and the two-question HSI into categories of “moderate-severe dependence” and “mild dependence.”
Primary outcome measure.
The primary efficacy endpoint was biochemically verified 7-day cessation at 3 months.37 Tobacco cessation was assessed by self-report and confirmatory biochemical testing with exhaled carbon monoxide. Participants who asserted abstinence by phone interview were asked to return to the hospital for assessment of exhaled carbon monoxide. All phone assessments were made by study RAs. Complete blinding was not possible because RAs were aware of which treatment components participants had received.
Secondary outcome measures.
Secondary outcomes were use of cessation medications and services, including the quitline, NRT, and other pharmacotherapies such as bupropion and varenicline, as well as changes in daily cigarette consumption and self-reported abstinence at 1 and 3 months. These were assessed by self-report and fax reports from the state quitline. A brief, structured interview called the Treatment Service Review (TSR)38 was administered by study RAs to collect information on the type and amount of services received by participants, including ED visits, hospitalizations, primary medical care visits, quitline utilization, and self-help sources.
Analysis
Assessing clinical efficacy.
Participants were contacted by telephone by study RAs at 1 and 3 months after enrollment. At 3 months, participants self-reporting tobacco abstinence were asked to return to the hospital to measure exhaled carbon monoxide (CO). Participants whose breath CO levels were 9 ppm or less were considered abstinent.37 Differences in proportions for the presence or absence of each component were estimated along with 95% confidence intervals (CIs) using the bootstrap method.39 As the goal of this study is to select promising components that may be used in a multifactorial intervention, no adjustment for multiplicity was performed.39
For the primary analysis, multivariable logistic regression was used to model the primary outcome, abstinence at 3 months. Per convention, in the primary analysis missing abstinence data, including absence of biochemical verification, was coded as smoking. For this full-factorial design, the model included main effects for each of the 4 components as well as all 2, 3 and 4-way interactions. The regression also included baseline covariates: age, sex, race/ethnicity, and smoking characteristics, such as daily cigarette consumption. Main effects were evaluated at the 0.05 significance level and 95% CIs estimated. Secondary outcomes at 1 and 3 months were evaluated by a generalized linear model. Analyses were performed with using SAS 9.4 (Cary, NC).
Sample size.
In previous work, we found a difference of 7.3% in biochemically confirmed abstinence between the control and intervention arms at 3 months. In this factorial trial, the effect of individual components would likely be less than that seen in the evaluation of our prior multicomponent intervention.18, 40 Therefore, we powered the experiment based on an absolute difference of 5% for the main effects of each component. We determined that with a two-sided 0.05 significance level, and an average abstinence proportion of 4.9% in the absence of a component, a total sample size of 860 participants would provide 80% power to detect a 5% increase in the abstinence rate. We chose to enroll 1056 participants to account for a 15% dropout rate by 3 months. We investigated whether the effect of a component was dependent on the levels of other components (i.e. interactions), although the trial was not powered to detect these. All analyses were performed under the intention-to-treat principle.
Results
Characteristics of study subjects.
Between February 2017 and May 2019, 1056 participants enrolled in the trial. Figure 2 shows the flow of patients throughout the trial. Table 2 shows their demographic and clinical profile for participants classified by intervention received. For the entire cohort of 1056 participants, the median age is 43.3 years; 520 (49.2%) are male; their racial and ethnic composition is 372 (35.2%) White non-Hispanic, 381 (36.1%) Black non-Hispanic, 231 (21.9%) Hispanic, and 72 (6.8%) Asian or other. Their insurance coverage is 733 (69.4%) Medicaid, 30 (2.8%) Medicare, 57 (5.4%) Medicaid and Medicare, 189 (17.9%) private insurance, and 40 (3.8%) uninsured. Participant demographics are comparable across all conditions.
Figure 2.
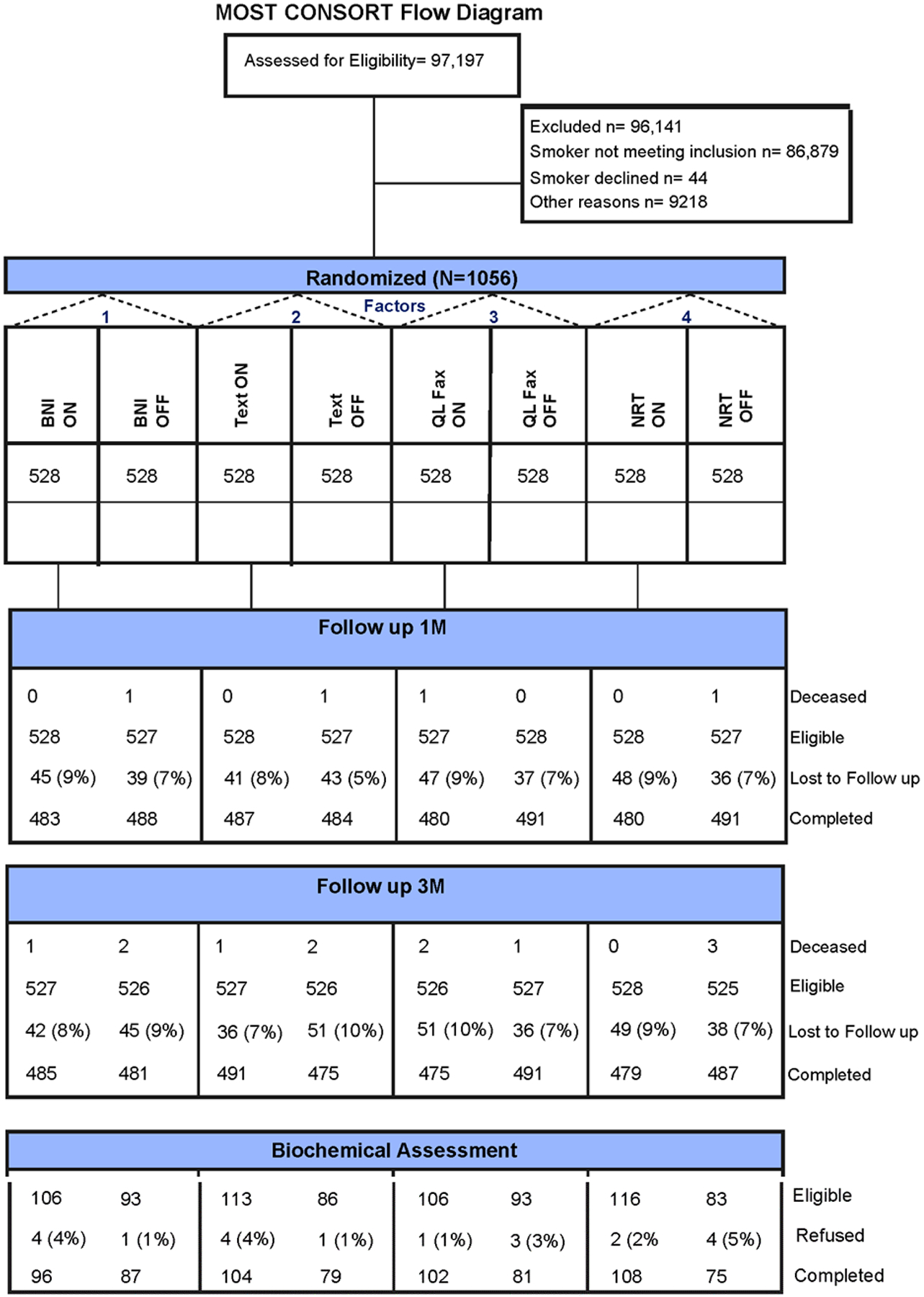
CONSORT diagram.
Table 2.
Baseline patient characteristics.
| BNI | NRT | Quitline | Texting | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Yes N = 528 |
No N=528 |
Yes N = 528 |
No N=528 |
Yes N = 528 |
No N=528 |
Yes N = 528 |
No N=528 |
| Age, mean, years (SD) | 43.8 (12.3) | 42.8 (11.9) | 44.0 (12.0) | 42.5 (12.2) * | 43.2 (11.9) | 43.3 (12.4) | 43.7 (12.1) | 42.8 (12.1) |
| Sex, no. male (%) | 269 (50.9) | 251 (47.5) | 269 (50.9) | 251 (47.5) | 260 (49.2) | 260 (49.2) | 245 (46.4) | 275 (52.1) |
| Insurance, N (%) | ||||||||
| Medicaid and Medicare | 24 (4.6) | 33 (6.3) | 24 (4.5) | 33 (6.3) | 23 (4.4) | 34 (6.4) | 30 (5.7) | 27 (5.1) |
| PHQ2 Score, N, (%) | ||||||||
| Rapid Alcohol Screen (+), N (%) | 142 (26.9) | 127 (24.1) | 138 (26.1) | 131 (24.8) | 126 (23.9) | 143 (27.1) | 128 (24.2) | 141 (26.7) |
| Rapid Drug Screen (+), N (%) | 72 (13.6) | 58 (11.0) | 67 (12.7) | 63 (11.9) | 55 (10.4) | 75 (14.2) | 66 (12.5) | 64 (12.1) |
| Cigarettes/day, median, IQR | 10.0 (6 – 15) | 10.0 (6 – 15) | 10.0 (6 – 15) | 10.0 (6 – 15) | 10.0 (6 – 15) | 10.0 (6 – 15) | 10.0 (6 – 15) | 10.0 (6 – 15) |
| Heavy Smoking Index, N (%) | ||||||||
| Subject believes ED visit related to tobacco, N (%) | 66 (12.5) | 73 (13.8) | 70 (13.3) | 69 (13.1) | 67 (12.7) | 72 (13.6) | 67 (12.7) | 72 (13.6) |
| Subject believes medical illness related to tobacco, N (%) | 224 (42.4) | 237 (44.9) | 236 (44.7) | 225 (42.6) | 221 (41.9%) | 240 (45.5%) | 230 (43.6%) | 231 (43.8%) |
About one-fourth of all participants screened positive for self-reported alcohol misuse using the Rapid Alcohol Problems Screen, and 12.3% self-reported drug misuse. Participants smoked a median of 10 cigarettes daily, and about one-fourth reported heavy smoking. About 20% of all participants screened positive for depression. Of all participants, 13.2% believed their ED visit was related to their tobacco use; 43.7% believed they have a medical illness related to tobacco use.
Main results.
Table 3 shows the proportions and differences in abstinence for each component. Both the Brief Negotiated Interview (BNI) and nicotine replacement therapies (NRT) were associated with increased smoking abstinence at 3 months: 13.5% vs. 8.9% and 14.4% vs. 8.0%, respectively. Neither the state quitline nor the SmokefreeTXT program were significantly associated with increased abstinence. The primary analysis using a factorial regression adjusted for baseline covariates is presented in table 4. BNI and NRT were both significantly associated with a greater likelihood of abstinence (OR [95% CI] = 1.8 [1.1, 2.8] for BNI and 2.1 [1.3, 3.2] for NRT. No statistically significant interactions were identified among the combinations of the individual interventions. Higher daily cigarette consumption was associated with reduced odds of abstinence. Neither race, ethnicity, gender nor age were associated with abstinence.
Table 3.
Primary efficacy endpoint: seven-day abstinence at 3 months, main effects.*
| Component | Received | Control | Differences in Proportion Abstinent (95% CI) |
|---|---|---|---|
| Brief Negotiated Interview (BNI) | 71/528 (13.5%) | 47/528 (8.9%) | 4.5% (0.74%, 8.4%) |
| Nicotine patch, gum (NRT) | 76/528 (14.4%) | 42/528 (8.0%) | 6.4% (2.7%, 10%) |
| Quitline (QL) | 65/528 (12.4%) | 53/528 (10.1%) | 2.2% (−1.6%, 6.1%) |
| SmokefreeTXT | 61/528 (11.6%) | 57/528 (10.8%) | 0.8% (−3.1%, 4.6%) |
losses to follow-up imputed to be smoking
Table 4.
Multivariable factorial logistic regression model for 7-day biochemically confirmed abstinence at 3 months.
| Odds Ratio (95% CI) | |
|---|---|
| Variable | |
| Intervention (Control is referent group) | |
| BNI | 1.8 (1.1, 2.8) |
| NRT | 2.1 (1.3, 3.2) |
| Quitline | 1.4 (0.9, 2.2) |
| Texting | 1.1 (0.7, 1.7) |
| Male gender (female is referent) | 0.8 (0.6, 1.3) |
| Age, 5 years | 1.0 (0.98, 1.02) |
| Race/ethnicity | |
| Baseline daily cigarette consumption | 0.97 (0.94, 0.99) |
missing outcomes imputed as continued smoking
Table 5 shows the comparison of secondary endpoints for each intervention component. No adjustments for multiple comparisons were made. Among the secondary endpoints, NRT was associated with an increased odds of self-reported smoking abstinence at one and three months, and a decline in daily cigarette consumption. Interestingly, the texting program was associated with an increased odds of a quit attempt at 24 hours, self-reported abstinence at one and three months, and decreased daily cigarette consumption. Neither the BNI nor the quitline were significantly associated with improvement in secondary endpoints.
Table 5.
Comparison of secondary endpoints by each component at 1 and 3 months (unadjusted).
| Variable | BNI (N = 528) | Control (N = 528) | Odds Ratio or Difference* between groups (95% CI) | NRT (N = 528) | Control (N = 528) | OR | Quitline (N = 528) | Control (N = 528) | OR | Texting (N = 528) | Control (N = 528) | OR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Secondary endpoints | ||||||||||||
| 24 hour quit attempt since ED visit, N (%) | 404 (76.5) | 394 (74.8) | 1.1 (0.8, 1.4) | 410 (77.7) | 388 (73.6) | 1.2 (0.9, 1.6) | 399 (75.6) | 399 (75.6) | 1.0 (0.7, 1.3) | 420 (79.6) | 378 (71.7) | 1.5 (1.1, 2.0) |
| 7-day abstinence at 1 m, self-report, N (%)** | 65 (12.3) | 78 (14.8) | 0.8 (0.6, 1.2) | 94 (17.8) | 49 (9.3) | 2.1 (1.5, 3.1) | 74 (14.0) | 69 (13.1) | 1.1 (0.8, 1.5) | 97 (18.4) | 46 (8.7) | 2.4 (1.6, 3.4) |
| 7-day abstinence at 3 m, self-report, N (%)** | 106 (20.1) | 93 (17.7) | 1.2 (0.9, 1.7) | 116 (22.0) | 83 (15.8) | 1.5 (1.1, 2.1) | 106 (20.2) | 93 (17.7) | 1.2 (0.9, 1.7) | 113 (21.4) | 86 (16.4) | 1.5 (1.1,2.1) |
| Change in daily cigarette consumption, mean (95% CI) | −7.4 (−7.9, – 7.0) | −6.9 (−7.3, – 6.4) | −0.6 (−1.2, 0.1) | −7.8 (−8.3, – 7.3) | −6.5 (−6.9, – 6.0) | −1.3 (−2.0, – 0.7) | −7.3 (−7.8, – 6.9) | −6.9 (−7.4, – 6.5) | −0.4 (−1.1, 0.2) | −7.7 (−8.1, – 7.2) | −6.6 (−7.0, – 6.1) | −1.1 (−1.8, – 0.5) |
compared using a factorial generalized linear model
missing outcomes imputed as continued smoking
Limitations
As with all single-site studies, our results may not be generalizable to the broader population of adult smokers who visit EDs. However, the racial, ethnic, gender, and economic diversity of the participants in this study is considerable. We have no a priori reason to believe these individuals differ in clinically pertinent ways from smokers visiting other EDs.
The duration of follow-up, three months, is relatively brief, shorter than the 6–12 months in trials of tobacco dependence treatment conducted outside EDs. This may raise questions about the durability of our component interventions. However, we have used this endpoint in prior studies, which given the limited intensity of the ED-initiated intervention, seems clinically appropriate. Furthermore, a modest (10%) proportion of our study sample were not assessed for our primary outcome. Per convention, in the primary analysis missing abstinence data, including absence of biochemical verification, was coded as smoking. Of note, sensitivity analyses (data not shown) using only completers or multiple imputation did not alter the conclusions of the study.
Discussion
This study is the first to identify components of ED-initiated tobacco dependence treatment that are individually effective. It extends and clarifies our prior work which demonstrated the efficacy of a multicomponent package of interventions for adult smokers seen in the ED. In addition, we found no interactions among components, indicating that the BNI and provision of NRT were individually efficacious. Of note, three-fourths of our participants were insured by Medicaid, suggesting the limited socioeconomic position of these individuals. That two interventions were identified as effective reaffirms the ability of ED-initiated tobacco dependence treatment to help low-income smokers achieve abstinence.
As in our prior work, our treatment model is that of STIR, with initiation of pharmacotherapy at the index visit, rather than the more traditional SBIRT. We do not set a quit date, as is typical in tobacco dependence treatment trials, rather preferring to capitalize on the “teachable moment” when the patient may be primed to initiate a behavior change. This is our second large-scale clinical trial of ED-initiated tobacco control that employed the STIR model. Both showed evidence of STIR’s efficacy and feasibility in the ED.
Both initiation of NRT and brief negotiated interviews are feasible in ED settings. NRT can be prescribed both for use during clinical care in the ED and discharge, even if several weeks of NRT cannot be handed to the patient, as was done in this study. BNIs, an abbreviated variant of motivational interviewing, have been specifically adapted by our group for use in ED settings for various substance use disorders.13 In this study, BNIs were delivered by research associates and not by frontline providers, although the latter can be trained in the technique.41 Future work might focus on integrating the delivery of BNIs and NRT by frontline ED providers and ancillary healthcare workers so as not to adversely affect patient throughput. We are planning such a study.
This trial offered some innovations to the MOST methodology. We believe it is the first ED trial to use the MOST framework with a factorial optimization trial; an ongoing study in Michigan (R01AA024755) is conducting an optimization trial using a Sequential Multiple Assignment Randomized Trial (SMART). Ours is the first study to assess the efficacy of the individual components of ED-initiated tobacco dependence treatment. And it will be the first MOST trial to incorporate a qualitative component to the analytic strategy, in addition to the quantitative assessments of clinical efficacy and cost effectiveness, using a concurrent triangulation mixed methods approach.42
Disappointingly, neither the texting program nor the quitline were found to be efficacious. These results are at variance with other studies of these modalities.43–45 Several reasons for the lack of efficacy are possible. First, smokers in the ED may differ in some way from other smokers who do respond to these modalities. Second, the “dose” of treatment delivered by texting programs and quitlines may be insufficient to motivate a behavior change in ED smokers, beyond that of the motivation conferred by the ED visit itself. Boudreaux’s Sentinel Events model of behavior change46 posits that event-related fear is a critical component of affect that mediates changes in risky behaviors. ED visits and hospitalizations for tobacco-related conditions are common events that may precipitate behavior change. Third, the texting program we used, SmokefreeTXT, may not be as efficacious as other texting programs available. We chose it because it is easily available, developed and managed by the National Cancer Institute. Other texting programs are available that have been tested in prior clinical trials, but their use may require licensing fees. Lastly, the study may have been underpowered to detect a more modest effect of texting and the quitline.
It is intriguing that although the texting program did not improve abstinence relative to the control condition, it did increase quit attempts and self-reported abstinence, and reduce daily cigarette consumption. Perhaps there is a modest benefit of texting that could not be detected by the choice of primary endpoint. However, insofar as we did not correct for multiple comparisons, these results should be interpreted with caution.
Lastly, it is notable that although the BNI was efficacious regarding the primary endpoint of abstinence, it did not show efficacy regarding the secondary endpoints. Insofar as the primary goal of the components was abstinence, we favor retaining BNI in future work. Further, the self-reported nature of the secondary endpoints, with the potential for social desirability bias, in contrast to the biochemical verification of abstinence, may explain some of these discrepancies.
We are exploring these possibilities in the study’s qualitative and economic analyses, currently in process. In light of these results, we will likely not retain SmokefreeTXT or the quitline in future ED-initiated interventions.
Conclusions
This study is the first to identify the components of ED-initiated tobacco dependence treatment that are independently efficacious. Both administration of nicotine replacement therapies and an abbreviated version of motivational interviewing were efficacious. Neither a standard texting program nor a referral to the state smokers’ quitline were efficacious. There were no interactions among the components. Both motivational interviewing and NRT can be adapted and scaled for use in other EDs. Follow-up work will focus on identifying optimal methods to scale these interventions, and testing their combined effectiveness in a new clinical trial.
Supplementary Material
Acknowledgements
The authors thank Teresa O’Leary, Kimberly Beauchemin, and Elizabeth Jurczak for their excellent research assistance.
The study is supported by Grant R01CA201873 from the National Cancer Institute of the National Institutes of Health. The funder played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
Dr. Toll consults for a Pfizer advisory board on e-cigarettes, and he testifies as an expert witness for plaintiffs who have filed litigation against the tobacco companies. All other authors declare that they have no competing interests
Ethics approval and consent to participate
This study was approved by the Human Investigation Committee of Yale University. All participants provided written informed consent before participating.
Presented at the Annual Meeting of the Society for Academic Emergency Medicine, May 2020, Denver CO, and the Annual Meeting of the Society for Research on Nicotine and Tobacco, New Orleans, 2020
References
- 1.U.S. Department of Health and Human Services. The Health Consequences of Smoking--50 Years of Progress. A Report of the Surgeon General. In: U.S. Department of Health and Human Services Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, ed. Atlanta, GA. 2014. [Google Scholar]
- 2.Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco Product Use Among Adults — United States, 2019. Accessed 8 February 2021 at DOI: 10.15585/mmwr.mm6946a4external. MMWR Morb Mortal Wkly Rep. 2020;69:1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Office of Disease Prevention and Health Promotion. Tobacco Use: Objectives. Accessed 15 December 2014 at https://www.healthypeople.gov/2020/topics-objectives/topic/tobacco-use/objectives. : U.S. Department of Health and Human Services,. [Google Scholar]
- 4.Hiscock R, Bauld L, Amos A, Fidler JA, Munafò M. Socioeconomic status and smoking: a review. Annals of the New York Academy of Sciences. 2012;1248:107–123. [DOI] [PubMed] [Google Scholar]
- 5.Lowenstein S, Tomlinson D, Koziol-McLain J, Prochazka A. Smoking habits of emergency department patients: an opportunity for disease prevention. Acad Emerg Med. 1995;2:165–171. [DOI] [PubMed] [Google Scholar]
- 6.Lowenstein SR, Koziol-McLain J, Thompson M, et al. Behavioral risk factors in emergency department patients: a multisite study. Acad Emerg Med. 1998;5:781–787. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein SL, Bijur P, Cooperman N, et al. A randomized trial of a multicomponent cessation strategy for emergency department smokers. Acad Emerg Med. 2011;18:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernstein SL, Arnsten JH, Bijur PE, et al. Concurrent use of alcohol or illicit substances improves response to ED SBIRT for adult smokers. J Substance Abuse Treatment. 2013;44:139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein SL, D’Onofrio G, Rosner J, et al. Successful Tobacco Dependence Treatment Achieved via Pharmacotherapy and Motivational Interviewing in Low-Income Emergency Department Patients. doi: 10.1016/j.annemergmed.2015.03.030. Ann Emerg Med. 2015;66:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boudreaux ED, Baumann BM, Camargo CA Jr., O’Hea E, Ziedonis DM. Changes in smoking associated with an acute health event: theoretical and practical implications. Annals of Behavioral Medicine. 2007;33:189–199. [DOI] [PubMed] [Google Scholar]
- 11.SL B Tobacco-related illnesses and management. In: Todd KH, Jr CRT, eds. Oncologic Emergency Medicine: Principles and Practice. Switzerland: Springer; 2016. [Google Scholar]
- 12.Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, Brief Intervention, and Referral to Treatment (SBIRT). Substance Abuse. 2007;28:7–30. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein SL, D’Onofrio G. Screening, treatment initiation, and referral for substance use disorders. Addiction Science & Clinical Practice. 2017;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Onofrio G, Fiellin DA, Pantalon MV, et al. A Brief Intervention Reduces Hazardous and Harmful Drinking in Emergency Department Patients. Ann Emerg Med. 2012;60:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grau LE, Pham T, O’Leary T, Rosner J, Toll B, Bernstein SL. Smokers’ Perspectives on Texting for Tobacco Dependence Treatment: A Qualitative Analysis. Nicotine & Tobacco Research. 2017;19:307–313. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein SL, Rosner J, Toll BA. A multicomponent intervention including texting to promote tobacco abstinence in emergency department smokers: a pilot study. doi: 10.1111/acem.12990. Acad Emerg Med. 2016;23:803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins LM. Optimization of Behavioral, Biobehavioral, and Biomedical Interventions: The Multiphase Optimization Strategy (MOST): Springer; 2018. [Google Scholar]
- 18.Collins LM, Dziak JJ, Kugler KC, Trail JB. Factorial Experiments: Efficient Tools for Evaluation of Intervention Components. Am J Prev Med. 2014;47:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins LM, Dziak JJ, Li R. Design of experiments with multiple independent variables: a resource management perspective on complete and reduced factorial designs. Psychological Methods. 2009;14:202–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins LM, Kugler KC, Gwadz MV. Optimization of multicomponent behavioral and biobehavioral interventions for the prevention and treatment of HIV/AIDS. AIDS and Behavior. 2016;20:197–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. American Journal of Preventive Medicine. 2007;32:S112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClure JB, Derry H, Riggs KR, et al. Questions about quitting (Q2): Design and methods of a Multiphase Optimization Strategy (MOST) randomized screening experiment for an online, motivational smoking cessation intervention. Contemporary Clinical Trials. 2012;33:1094–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker TB, Collins LM, Mermelstein R, et al. Enhancing the effectiveness of smoking treatment research: conceptual bases and progress. Addiction. 2016;111:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook JW, Collins LM, Fiore MC, et al. Comparative effectiveness of motivation phase intervention components for use with smokers unwilling to quit: a factorial screening experiment. Addiction. 2016;111:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piper ME, Fiore MC, Smith SS, et al. Identifying effective intervention components for smoking cessation: a factorial screening experiment. Addiction. 2016;111:129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlam TR, Fiore MC, Smith SS, et al. Comparative effectiveness of intervention components for producing long-term abstinence from smoking: a factorial screening experiment. Addiction. 2016;111:142–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraser D, Kobinsky K, Smith SS, Kramer J, Theobald WE, Baker TB. Five population-based interventions for smoking cessation: a MOST trial. Transl Behav Med. 2014;4:382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker TB, Mermelstein R, Collins LM, et al. New methods for tobacco dependence treatment research. Annals of Behavioral Medicine. 2011;41:192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins LM, Baker TB, Mermelstein RJ, et al. The Multiphase Optimization Strategy for Engineering Effective Tobacco Use Interventions. Annals of Behavioral Medicine. 2011;41:208–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernstein SL, Dziura J, Weiss J, et al. Tobacco Dependence Treatment in the Emergency Department: A Randomized Trial Using the Multiphase Optimization Strategy. 10.1016/j.cct.2017.12.016. Contemp Clin Trials. 2018;66:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change, 2d ed. New York: Guilford Press; 2002. [Google Scholar]
- 32.Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Department of Health and Human Services; 2008. [Google Scholar]
- 33.Whittaker R, McRobbie H, Bullen C, Borland R, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. DOI: 10.1002/14651858.CD006611.pub3. Cochrane Database of Systematic Reviews. 2012. [DOI] [PubMed] [Google Scholar]
- 34.Bernstein SL, Weiss JR, Toll BA, Zbikowski SM. Association Between Utilization of Quitline Services and Probability of Tobacco Abstinence in Low-Income Smokers. doi: 10.1016/j.jsat.2016.08.014. J Substance Abuse Treatment. 2016;71:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.United States Census Bureau. New Haven Town, New Haven County, Connecticut. Accessed 8 February 2021 at https://data.census.gov/cedsci/profile?g=0600000US0900952070: US Department of Commerce,; 2020. [Google Scholar]
- 36.Abraham M, Buchanan M. Greater New Haven Community Index 2016. Accessed 2 January 2017 at http://www.ctdatahaven.org/sites/ctdatahaven/files/DataHaven_GNH_Community_Index.pdf. New Haven, CT: DataHaven; 2016. [Google Scholar]
- 37.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 38.McLellan AT, Alterman AI, Cacciloa J, Metzger D, O’Brien CP. A new measure of substance abuse treatment: initial studies of the Treatment Service Review. J Nervous Mental Disease. 1992;180:101–110. [DOI] [PubMed] [Google Scholar]
- 39.Efron B, Tibshirani R. An Introduction to the Bootstrap. Boca Raton, FL: Chapman & Hall/CRC; 1993. [Google Scholar]
- 40.Wolbers M, Heemskerk D, Chau T, et al. Sample size requirements for separating out the effects of combination treatments: Randomised controlled trials of combination therapy vs. standard treatment compared to factorial designs for patients with tuberculous meningitis. Trials. 2011;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tetrault JM, Green ML, Martino S, et al. Developing and implementing a multispecialty graduate medical education curriculum on screening, brief intervention, and referral to treatment (SBIRT). Substance Abuse. 2012;33:168–181. [DOI] [PubMed] [Google Scholar]
- 42.Cresswell JW, Clark VLP, Gutmann ML, Hanson WE. Advanced Mixed Methods Research Designs. In: Tashakkori A, Teddlie C, eds. Handbook of Mixed Methods in Social & Behavioral Research. Thousand Oaks, CA: SAGE Publications, Inc.; 2003. [Google Scholar]
- 43.Villanti AC, West JC, Klemperer EM, et al. Smoking-Cessation Interventions for U.S. Young Adults: Updated Systematic Review. American Journal of Preventive Medicine. 2020;59:123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews. 2013. [DOI] [PubMed] [Google Scholar]
- 46.Boudreaux ED, Baumann BM, Camargo CA, O’Hea E, Ziedonis DM. Changes in smoking associated with an acute health event: theoretical and practical implications. Ann Behav Med. 2007;33. [DOI] [PubMed] [Google Scholar]
- 47.Collins LM. Optimization of behavioral, biobehavioral, and biomedical interventions: Springer; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


