Abstract
Visual phototransduction is the most extensively studied G protein-coupled receptor (GPCR) signaling pathway because of its quantifiable stimulus, non-redundancy of genes, and immense importance in vision. Here we summarize recent discoveries that have advanced our understanding of rod outer segment (ROS) morphology and the pathological basis of retinal diseases. We have combined recently published cryo-electron tomography (cryo-ET) data on the ROS and the structural knowledge on individual proteins to define the precise spatial limitations under which phototransduction occurs. Although hypothetical, the reconstruction of the rod phototransduction system highlights the potential roles of phosphodiesterase 6 (PDE6) and guanylate cyclases (GCs) in maintaining the spacing between ROS discs, suggesting a plausible mechanism by which intrinsic optical signals are generated in the retina.
Keywords: G protein-coupled receptor (GPCR), rhodopsin, phototransduction, GPCR signaling, ROS, rod cell
Rod and cone photoreceptors structure and function
Vision is so deeply embedded in our daily lives that it is easy to overlook its underlying complexity. We perceive light when photons stimulate photoreceptor cells in the retina. Vertebrate photoreceptors are highly elongated neurons that are exquisitely equipped for capturing photons and then amplifying and translating the event into an electrical response [1–4] (Figure 1A). Rod and cone photoreceptors (see Glossary) have significant biological similarities, but they also have distinct features that set them apart [5, 6]. Although rods and cones hyperpolarize in response to light via a similar phototransduction pathway, the single-photon response of cones is typically 100-fold smaller and several-fold faster in kinetics than that of rods. Cones also adapt to light much more effectively as compared to rods [7, 8]. These differences have been investigated on the molecular level in the last two decades [2, 5, 9].
Figure 1. The structure of the rod photoreceptor cell and visual phototransduction.

(A) Schematic diagram of a murine rod photoreceptor showing the major components. In mice, the rod outer segment (ROS) is comprised of ~800 bilayered disc membrane stacks [11] enclosed by a plasma membrane, and contains the components necessary for phototransduction. In WT mice, the average density of discs is 34 discs/μm for the ROS, the average ROS length is 23.8 ± 1.0 μm, and the ROS contains 810 ± 33 discs. The average diameter of the ROS in retinal sections of WT mice is 1.32 ± 0.12 μm [11]. Thus, the total calculated volumes of disc membranes and the ROS cytoplasm in the retina is ~114 × 10−6 ml and 64 × 10−6 ml, respectively [32]. (B) Distances between murine ROS membranes obtained by cryo-ET tomograms [32]. The cryo-ET structure enabled us to precisely measure the distances between ROS components, leading to an increased understanding of the spatial limits within which ROS proteins function. (C) Illustration showing the major steps involved in visual phototransduction. The light-induced conversion of rhodopsin from ground state to signaling state metarhodopsin II (Meta II) results in the activation of Gt. Upon activation, the GTP-bound α-subunit of Gt (Gtα-GTP) binds and activates PDE6, which lowers cGMP levels through hydrolysis, resulting in ion channel closure. Thus, reduction in the cation flux leads to rod cell hyperpolarization in response to a light stimulus, and decreased release of the neurotransmitter glutamate at the synapse.
Further, while rod photoreceptors contribute to vision in low light intensities, cones operate in bright light conditions and are responsible for color perception [9, 10]. Additionally for rods, a significant portion (up to half of their length) is comprised of a primary cilium referred to as the rod outer segment (ROS), containing stacks of bilayered disc membranes (about 900 in mice to 2000 in frog rods) enclosed by a plasma membrane [11]. This arrangement contrasts with cones, which typically have shorter and more conical outer segments that are much wider at the base as compared to rods. Unlike rods, cones can also survive without outer segments [12]. Also unlike rods, cone discs are connected to the plasma membrane throughout the length of the outer segment, opening them to the extracellular space [12].
ROS discs are flattened membranes comprised of two lamellar membranes restricted by a hairpin rim region and they contain the essential components for visual phototransduction [13–15] (Figure 1). Rhodopsin is the primary component of ROS disk membranes, accounting for >90 % of the total protein component and >50 % of the total disk surface area (reviewed in [16–19]). The remaining space is occupied by phospholipids, cholesterol, and other auxiliary proteins that are necessary for the visual phototransduction pathway [3, 20, 21]. Extensive characterization of rhodopsin has led to its designation as the prototype Class A GPCR [22].
Phototransduction occurs in the outer segment of retinal photoreceptors that culminates in the hydrolysis of cGMP to GMP in response to light [2–4]. The reduction in cGMP concentration results in the closure of an ion channel that controls movement of both Na+ and Ca2+ across the photoreceptor plasma membrane [23, 24] (Figure 1C), ultimately leading to hyperpolarization of the photoreceptor cell. All of the key signaling molecules have been structurally resolved, providing valuable insights into phototransduction. Recently, different forms of the cyclic nucleotide-gated channel have been structurally resolved [25–27] and one of these structures has been interpreted to include bound calmodulin [28], as discovered in previous studies [29]. Additionally, cryo-electron tomography (cryo-ET) has played a critical role in establishing the overall architecture of the ROS [30, 31]. Taken together, the cryo-ET data and the individually determined structures of phototransduction proteins serve as important resources for enhancing our understanding of ROS morphology and the pathological basis of retinal diseases. Here we have combined the cryo-ET data and the structural knowledge on individual proteins to define the precise spatial limitations under which phototransduction occurs in the ROS, highlighting the potential roles of phosphodiesterase 6 (PDE6) and guanylate cyclases (GCs) in maintaining the spacing between ROS discs.
Cryo-ET defines structural boundaries of the ROS
In low light conditions, photons are few and sporadic, and phototransduction machinery faces a considerable challenge in detecting individual photons and reliably transducing the signal downstream. This signaling cascade requires an extremely low background noise and high amplification of the underlying stimulus. Rod cells achieve low background noise by minimizing spontaneous activation of rhodopsin and varying the concentration of effector molecules. Additionally, amplification is achieved by the close spatial organization of the signaling molecules in the ROS disc membranes.
The near-native structure of the entire murine ROS has been characterized by cryo-ET at subnanometer resolution [30–32]. A combination of cryo-focused ion beam (cryo-FIB) milling and cryo-ET resulted in high quality tomograms that enabled us to precisely measure the distances between ROS components and provide intricate molecular details, facilitating an increased understanding of the spatial limits in which ROS proteins can function to carry out phototransduction [30]. The structural study of the ROS also provided evidence for the extreme radius of curvature at the disk rims and identified molecular scaffolds both between the disc membrane stacks and between the discs and plasma membrane [32]. As we describe herein, the 3D architecture of murine ROS gleaned from the cryo-ET data, together with extensive structural information from X-ray crystallography, single particle cryo-electron microscopy (cryo-EM), and high-quality computational analysis of individual components, has provided valuable insights about the overall architecture of the murine ROS. These data enabled us to produce a model that is structurally consistent with established protein dimensions and stoichiometry/density (Figure 2 and Table 1).
Figure 2. Spatial organization of the key components of visual phototransduction in the ROS.
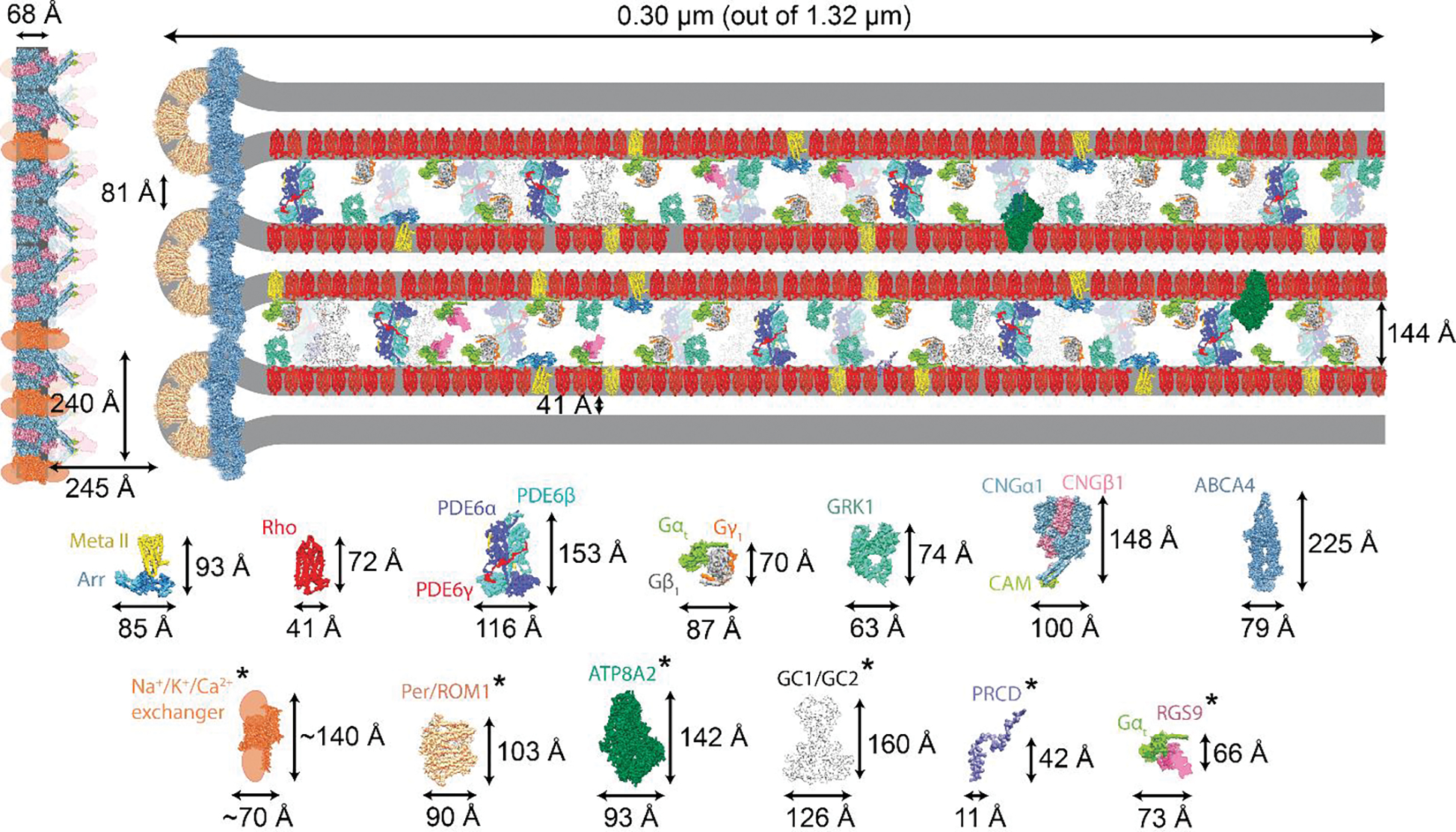
(A) Sub-section of the ROS depicting the size and abundance of visual phototransduction proteins relative to rhodopsin [67] (Table 1). Distances between the ROS membrane components were obtained from cryo-EM tomograms [30] and are drawn to scale. Individual protein components and their dimensions are displayed below the figure and in Table 1. The asterisks represent modelled protein structures or structures with modeled domains. The longest protein is ABCA4, and its localization is restricted to the rim of the discs. The central part of the disc is occupied primarily by rhodopsin. GCs and PDE6 are the only two proteins that can span the ROS interdiscal space. The separation of plasma membrane from the disc membranes is maintained by a yet unknown mechanism(s). Meta II: metarhodopsin II; Arr: arrestin; Rho: rhodopsin; PDE6: phosphodiesterase 6; GRK1: GPCR kinase 1; CNG1: cGMP-gated channel; ABCA4: Retinal-specific ATP-binding cassette transporter; PRPH2/ROM1: peripherin-ROM1; ATP8A2: ATPase phospholipid transporting 8A2; GC1 and GC2: guanylate cyclases 1 and 2; PRCD: Photoreceptor Disc Component; RGS9: Regulator of G Protein Signaling 9.
Table 1.
Proteins of the rod outer segment and their concentrations relative to rhodopsin.a
| Protein | M.W. (kDa)b Dimensions (Å) |
PDB Structure References | Estimated ratio to rhodopsinc | Interactions (protein) | Major disease associationd |
|---|---|---|---|---|---|
| Rhodopsin (dimer) | 85.39 82X75X35 |
1F88, 1U19, 6OFJ, 2I37 | 1 | Gt [37], GRK1 [41], Arrestin [84] | RP4 [85], autosomal dominant CSNB1 [86] |
| Gtα | 82 87X70X55 |
1TND | 0.11 | Rho [87], Gtβγ [88], PDE6 [89], RGS9 [90], UNC119 [91] | Autosomal dominant CSNB 3 [92], autosomal recessive CSNB 1G [93] |
| Gtβ | 218.15 116X153X108 |
1TBG, 6B20 | 0.11 | Gtα [88], Gtγ [94] | Mental retardation, autosomal dominant 42 (MRD42)[95] |
| Gtγ | 284.99 148X100X98 |
1TBG, 6B20 | 0.11 | Gtβ [94] | |
| PDE6 | 260.06 79X225X63 |
6MZB | 0.07 | Gtα [89], PrBP/delta [96] | RP43 [97], RP40 [98], autosomal CSNB 2 [99], RP57 [100] |
| CNG1 (CNGA1B1) | 153 90X103X100 |
7RHL, 7O4H, 7RHJ, 7LFY 6WEJ, 6WEK, 6WEL, | 0.060 | Calmodulin [29] (ABCA4) | RP49 [101], RP45 [102] |
| ABCA4 | 62.10 63X72X50 |
7M1Q, 7LKP, 7E7I | 0.047 | None (CNG1, rhodopsin) | Stargardt disease 1 (STGD1)[103], age-related macular degeneration 2 (AMD2)[104] |
| PRPH2/ROM1 | 155 126X160X95 |
Modelede | 0.04 | None (ABCA4, rhodopsin) | RP7 [105], Retinitis punctata albescens (RPA)[106], patterned macular dystrophy 1 (MDPT1)[107], RP7 [108] |
| GRK1 | 129 93X142X77 |
3T8O, 3C51 | 0.047 | Rhodopsin [41], Recoverin [109], PrBP/delta [110] | CSNB Oguchi type 2 (O2)[111] |
| GC1/2 | 5.99 11X42X11 |
Modeled | 0.047 | Retinal degeneration 3 (RD3) protein [112] | LCA1 [113], cone-rod dystrophy 6 (CORD6)[114], |
| ATP8A2 | 85.39 82X75X35 |
Modeled | 0.008 | Cell cycle control protein 50A [115] | Cerebellar ataxia, mental retardation, and dysequilibrium syndrome 4 (CAMRQ4)[116] |
| PRCD | 82 87X70X55 |
Modeled | 0.0035 | None (Rho) | RP36 [117] |
| Na+/K+/Ca2 exchanger 1 | 132 70X140X50 |
modeled | 0.03 | None (CNG1) | CSNB1D [118] |
ABCA4, Retinal-specific ATP-binding cassette transporter; ATP8A2, ATPase phospholipid transporting 8A2; GC, guanylate cyclase; CNG1, Cyclic nucleotide-gated channel 1; GRK1, G Protein-Coupled Receptor Kinase 1; CSNB, Congenital Stationary Night Blindness; LCA, Leber congenital amaurosis; PDE6, phosphodiesterase 6; PRPH2/ROM1, peripherin-ROM1; PRCD, Photoreceptor Disc Component; RP, retinitis pigmentosa; RGS9, Regulator of G Protein Signaling 9. Potential interactions are shown in parentheses.
Molecular masses of proteins with known structures were extracted from the protein data bank. For modeled proteins, molecular masses were calculated using the Compute pI/MW server (https://web.expasy.org/compute_pi/)
The relative abundance of visual phototransduction proteins relative to rhodopsin are obtained from [67]. The abundance of PRPH2/ROM1 relative to rhodopsin was calculated from cryo-EM tomograms [30]. The molar concentrations of Gtα and Gtβ were assumed to be equal. Note that the amount of PDE6 to rhodopsin was reported within the rage of 0.85–7% [67–69].
One defective gene can cause phenotypically multiple different diseases; more than one defective gene can cause phenotypically the same disease [119].
The structures of PRPH2 and ROM1 were individually modeled using ITASSER [120] and a 3:1 complex was generated using the M-ZDOCK program (https://zdock.umassmed.edu/m-zdock/). The structures of GC1/2, ATP8A2, Na+/K+/Ca2+ exchanger (transmembrane regions), and PRCD were obtained from the DeepMind’s program, called AlphaFold [121].
Rhodopsin - a model G protein-coupled receptor
Rhodopsin has been studied extensively over the past 150 years and many reviews have been written addressing different aspects of its structure and function [16, 22, 33]. Further, several novel advances in understanding rhodopsin structure-function relationships have been reported in recent studies. For example, Sakai et al. reported a short circuit in the visual cycle, demonstrating that a single mutation of Gly188 to Cys enabled vertebrate rhodopsin to acquire photocyclic and photoreversible properties [17]. Getter et al. identified nine small molecules that either disrupted or enhanced rhodopsin dimer interaction in vitro, including a dimer-disruptive compound that significantly slowed down the light response kinetics of intact rods, thereby reinforcing the functional importance of the native rhodopsin dimer [34]. Recently, Chen et al. used mass spectrometry to analyze native ROS membranes to demonstrate potential regulatory roles of specific lipids and ligands in rhodopsin signaling [35]. Using mass spectrometry to analyze native ROS vesicles injected directly into the instrument, Chen et al. were able to capture stages of the signaling cascade upon light stimulation, and demonstrate potential regulatory roles of specific lipids and ligands in rhodopsin signaling [35]. This native mass spectrometry approach provides a new way to investigate membrane-bound receptor pharmacology and identify novel GPCR drug targets [36].
Rhodopsin forms critical complexes with membrane associated proteins
The key elements of phototransduction are initiation and amplification of the signaling cascade followed by its quenching, and these sequential events are now structurally resolved. The initial step involves the light-activation of rhodopsin to metarhodopsin II (Meta II) and its interaction with the heterotrimeric G protein transducin (Gt) [37–39] (Figure 3A). Cryo-EM structures of a fully functional and signaling-active rhodopsin-Gt complex revealed a comprehensive picture of the structural basis of rhodopsin-Gt coupling, including stabilization of the helical domain of Gtα by Gtβγ. This provides direct evidence for the active involvement of the Gtβγ subunits in nucleotide exchange [40]. It was also noted that there is a deeper insertion of Gt into rhodopsin than for other G protein-GPCR complexes [40].
Figure 3. Rhodopsin complexes that form during the phototransduction sequence.

Structures of metarhodopsin II (Meta II) bound with (from left to right) Gt, GRK1, and arrestin. (A) The complex between photoactivated rhodopsin and the G protein of the visual system, called transducin (Gt). The interaction extends to a 1042 Å2 interface, which involves helix 3 (TM3), intracellular loop 2 (ICL2), TM5, TM6, and helix 8 (H8) of rhodopsin, and helix 5, loops a4-b6, b2-b3 and aN-b1 of Gtα (for structural details see [40]). (B) The complex between photoactivated rhodopsin and GRK1. Dynamic interaction with receptor-bound GRK1 allows formation of a unique intermediate that produces multiple phosphorylation sites in the receptor tail. Changes in rhodopsin involve TM helices 5 and 6, which adopt outward conformations relative to the transmembrane core; TM6 is rotated by about 20°, and H8 is shifted in the complex (for structural details see [45]). (C) The structure of a fusion protein between photoactivated rhodopsin and arrestin. Whether the fusion of the proteins exerts any structural constraints remains to be determined.
Photoactivated rhodopsin is a substrate for rhodopsin kinase, now known as GPCR kinase 1 (GRK1) [41–44]. Recently, this transient GRK1-rhodopsin complex was cleverly captured and its structure determined [45] (Figure 3B). GRK1 selectively phosphorylates activated rhodopsin at the C-terminal tail, on as many as 5–7 Ser/Thr residues [43]. Based on biochemical data [46], the N-terminal domain is of critical importance for the kinase function, and molecular insights into this process have been elucidated recently [45]. Cryo-EM reconstructions revealed that the N-terminus of GRK1 forms a helix that docks into the open cytoplasmic cleft of photoactivated rhodopsin, with additional stabilizing ionic interactions. A specific point of interest is the observation that the autophosphorylated residues phospho-Ser488 and phospho-Thr489 of GRK1 [47] interact with Lys66 and Arg69 of rhodopsin, respectively. These interactions enhance the activity of the kinase [47].
Finally, the phosphorylated rhodopsin binds a capping protein named arrestin [48, 49] (Figure 3C). Based on biochemical data, it is known that phosphorylation of rhodopsin leads to recruitment of arrestin. Upon phosphorylation of photoactivated rhodopsin, the acidic C-terminal region of arrestin is displaced by the phosphorylated C-terminal region of rhodopsin, initiating the binding of the two proteins [50]. This mechanism has been confirmed by an X-ray free electron laser (XFEL) crystal structure of the rhodopsin-arrestin complex [51]. To accomplish this challenging project, the authors opted for a T4 lysozyme (T4L)-rhodopsin-arrestin tri-part fusion complex. Two phospho-residues, phospho-Thr336 and phospho-Ser338, along with Glu341, form an extended network of electrostatic interactions with three positively charged pockets in arrestin. Some details of this complex will need to be verified by further studies in its native state, but overall, the current structural data provides an informative view of how this important complex is assembled. Our recently solved structure of arrestin in a ligand-free state features a near-complete model of the previously unresolved C-tail, which plays a crucial role in regulating arrestin activity [52]. We also found that inositol phosphates bind to the N-terminal domain of arrestin displacing the C-tail [52], analogous to the action of the phosphorylated C-terminal region of rhodopsin. The three consecutive complexes of rhodopsin with Gt, GRK1, and arrestin are critical for the initiation and termination of light signals, as represented in the ROS model [53–56] (Figures 2 and 3).
In all three complexes with the regulatory proteins, rhodopsin engages the space that opens upon illumination to receive the complementary moiety of each of the associated proteins. Thus, each protein inserts a critical intradiscal region into the rhodopsin pocket of the 7-transmembrane bundle: Gt (C-terminal), GRK1 (N-terminal), and arrestin (central core).
Downstream in the signaling cascade, the complex between activated G protein and PDE6 is highly relevant [38] (Figure 1C). Recently, Gao et al. determined the cryo-EM structure of the PDE6αγβγ-(Gtα-GTP)2 complex, shedding new light on Gtα-GTP-mediated activation of the PDE6αγβγ complex [57]. The interaction of Gtα-GTP with tetrameric PDE6 almost exclusively involves the PDE6γ subunits, suggesting that the supercomplex is highly dynamic in nature. This model, however, will require further validation due to the absence of a lipidic environment and the use of a bivalent antibody to simultaneously present two Gtα-GTP subunits to PDE6. The bivalent antibody resulted in a 2-fold enhancement in the maximal achievable catalytic activity of PDE6, and therefore would have posed some restrictions on the dynamics of Gtα·GTP binding with PDE6.
ROS composition and lipids
In addition to proteins, lipids are integral components of the ROS (extensively reviewed in [20]). For vertebrate ROS membranes, phospholipids represent 80–85% (wt/wt) of the total lipid, while neutral lipids represent about 10–15 mol %; cholesterol represents about 2% of the membrane dry weight, or about 4–6% (wt/wt) of the total lipid, and there are smaller amounts of gangliosides [20] (Figure 4A). The major phospholipids are phosphatidylethanolamine (PE, ~38%) and phosphatidylcholine (PC, ~32%), along with relatively large amounts of phosphatidylserine (PS, ~12%). The most abundant neutral lipid is cholesterol. The cholesterol/phospholipid mole ratio for these ROS membranes is in the range of 0.09–0.11 [20].
Figure 4. Major lipid composition of the ROS.

(A) Fatty acid composition of phospholipids from human ROS membranes. Values are expressed as mol % of total fatty acids. At least 70% of the amino phospholipids (i.e., phosphatidylethanolamine and phosphatidylserine) are localized to the outer (cytoplasmic) leaflet of the disc membrane bilayer, while most of the choline phospholipids (i.e., phosphatidylcholine and sphingomyelin) were presumed (by difference) to be localized to the inner (lumenal) membrane leaflet. About 73–87% of the phosphatidylethanolamine and 77–88% of the phosphatidylserine were distributed in the outer monolayer of the disc membrane, while calculations indicated that 65–100% of the phosphatidylcholine resides in the inner monolayer [20]. The most striking feature of ROS lipid composition is the unusually high content of long-chain polyunsaturated acids, which generally comprise 50–60 mol % of the membrane fatty acids. Saturated fatty acids are esterified primarily at the sn1-position, while polyunsaturated fatty acids (PUFAs) are found primarily at the sn2-position of the glycerol moiety. (B) Intradiscal view of ROS showing the localization of major proteins on the surface (cross-section is represented in Figure 2). Tight packing between rhodopsin dimers (pink color) leaves a limited space for lipid binding. Gtα (green); Gtβ (gray); Gtγ (orange); PDE6α (blue); PDE6β (purple); PDE6γ (red). (C) Schematic diagram depicting the lipid composition surrounding a rhodopsin molecule. The relative concentrations of individual lipids are obtained from [20]. The difference between the extracellular and the cytoplasmic total flat surface areas is a result of the number of phospholipids residing at each side and the different packing forces. PC, phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine; CHOL, cholesterol.
Remarkable regional differences in lipid composition of the ROS were documented recently by employing styrene-maleic acid lipid particles (SMALPs) and specific antibodies to selectively copurify membrane proteins with associated lipids from each region. Significant differences between the ROS center (rhodopsin) and rim (ABCA4 and PRPH2/ROM1) regions were observed. A lower PC to PE ratio and increased long-chain (C20–24) (LC-PUFAs) and very-long-chain (>C24) polyunsaturated fatty acids (VLC-PUFAs) were found in the center relative to the rim, which was more enriched in shorter saturated FAs [58]. To illustrate how much space is occupied by rhodopsin and lipids, a top-to-bottom intradiscal image of ROS is presented, showing tight packing between rhodopsin dimers, leaving the remaining space for lipid binding (Figure 4B). A general description of rhodopsin and lipids in the ROS was published previously [59] and is illustrated in cartoon form in Figure 4C. Each rhodopsin molecule is surrounded by a little more than a single layer of phospholipids, as viewed from the extracellular side and from the cytoplasmic side (Figure 4C). Chemical analysis of the phospholipid and rhodopsin contents of the ROS from mouse, rat, and cow revealed a phospholipid:rhodopsin molar ratio of 63:1 [60].
Maintaining spacing between discs and the plasma and discs membranes
The distance between the ROS discs is strictly maintained, and it has been proposed that a structural protein(s), the so-called “pillar protein” is responsible [30, 32]. However, insufficient structural resolution has hindered the identification of the pillar protein. With improvements in transmission electron microscopes, sample vitrification, detector sensitivity, and image processing software, one can now use a combination of cryo-ET, where the sample is processed in a near-native state, and cryo-FIB micromachining to investigate the near-native organization of pillar protein molecules in the ROS (Figure 1B). These pillar proteins were found to be distributed throughout the ROS outer leaflet and are ~144 Å in length [30]. In our opinion, there are three proteins in the ROS that could fulfill the role of pillar molecules based on their size, PDE6 and GC1 and GC2 (see below). Notably, the PDE6αβ heterodimer extends to a length of ~154 Å (Figure 2), and the dimeric GCs are predicted to be about 160 Å. These findings suggest a potential role of PDE6 and/or GCs as the pillar proteins that maintain ROS interdiscal distances.
The distance between the plasma membrane and the discs is maintained throughout the ROS. Based on their localization, we believe the key players in this spacing are the cGMP-gated channel (CNG1) and its splice variants containing glutamic-acid rich protein (GARP), and disc membrane proteins ABCA4 and PRPH2/ROM1 [61, 62], though neither of the latter two proteins can span the distance of 245 Å (Figure 2). A hypothetical model was proposed that involves the interaction between GARPs and CNG1 [62]. However, knockout mice devoid of CNGA1, displayed only a progressive retinal thinning (a typical phenotype for retinitis pigmentosa), rather than inhibition of ROS formation or collapse of the ultrastructure [63]. Therefore, determining how the distance between the plasma membrane and the ROS discs is maintained is still an open topic.
Hypothetical role of PDE6 and GC1/2 in maintaining spacing between discs
As mentioned above, the limited number of proteins, their relative density, and their physical dimensions restrict how the ROS can maintain physical distances. Possibly, some inter-protein complexes could form, but experimental documentation to support this is lacking. The mathematical constraints leave very few options for identifying the pillar proteins, and one of them is discussed below.
The N- and C-termini of PDE6, located at opposite ends in space, could link the neighboring discs. Analogous to the C-termini of the PDE6 subunits, which contain farnesyl (PDE6α) or geranylgeranyl (PDE6β) moieties, the N-terminal pony-tail helical structure (Pt-motif) that forms between the PDE6α and PDE6β subunits could insert into the disc membranes, and thereby physically anchor adjacent ROS disc leaflets (Figure 2). This hypothesis is supported by studies of PDEα and PDEβ (rd1) knockout mouse models, where mutations in the PDE6α or PDE6β gene result in rapid degeneration of rod photoreceptors [64, 65]. Although the rapid onset of the phenotype could be explained by the loss of cGMP homeostasis [64], transfection of rd1 mouse photoreceptors to overexpress non-photoreceptor-specific PDE5 preserves the photoreceptors to some degree. The lack of a high-resolution structural study of the retina from these mice hinders the understanding of the structural component responsible for the degeneration [66]. However, these studies do not exclude an alternate and complementary mechanism in which the structural deformation of the PDE6αβ heterodimer, caused by the PDE6β mutation, results in the collapse of interdiscal spaces within the ROS.
Previous studies have determined the ratio between PDE6 and rhodopsin concentrations as 1:14 [67]. Notably, the cryo-ET structure of murine ROS reported the pillar molecule density as 920 connectors per μm2, or ~2% relative to rhodopsin concentration, which is within the concentration range of 0.85–7% that has been reported for PDE6 in the literature [30, 67–70], thereby making it quantitatively feasible for PDE6 to serve as a ROS disc pillar protein. However, to verify this interpretation, one needs to perform large-scale cryo-ET data acquisition on murine ROS to generate ultra-high-resolution tomograms. Although not addressing the issue presented here directly, Morshedian et al. reported an interesting mouse model that provided data pertinent to deducing the functions of PDE6 in generation of reproducible rod photoreceptor response [71]. Thus, a D167A point mutation in the α subunit of PDE6 likely removed a noncatalytic binding site for cGMP and not only caused dramatic reduction in PDE6α expression, but also diminished PDEβ expression to some degree, indicating an interdependence between the α and β subunits of PDE6 [64]. However, the resulting degeneration was slow, with 50%-60% of rods remaining after 6 months, which suggests that PDE6 might not be the only protein responsible for maintaining the interdiscal spaces.
The second candidate for the ROS pillar protein is GC1/2. There are seven mammalian membrane-bound guanylate cyclase enzymes that synthesize the second-messenger cGMP, and they all have a similar structural topology [72]. They each consist of an extracellular (or intradiscal) domain, a short transmembrane region, and a large intracellular domain, which contains a kinase-homology domain, a hinge region, and the catalytic region [73]. GC1 and GC2 are colocalized within the rod and cone photoreceptors of the retina, and they are key enzymes for restoring dark conditions through adjusting cGMP concentration post-light activation, and as a result Ca2+ concentration is reset. Recent quantitative characterization of ROS ultrastructure suggests a potential role of GCs as additional or alternative pillar proteins that maintain ROS interdiscal distances. As with PDE6, mutation in GC1 causes a severe retinal degenerative disorder called Leber congenital amaurosis [74, 75]. Analogous to PDE6 deficiency, it remains to be determined whether lack of GC proteins and/or the dysregulation of Ca2+ homeostasis by GCAP1/GCAP2, or both, could be the cause of this severe blinding disease. In mice, double GC1/2 knockout results in unconventional ROS membrane assembly [76], indicative of their structural role.
Optoretinography
Intrinsic optical signals, now known as optoretinogram (ORG), result from changes in the optical path length of individual photoreceptors in response to light stimulation. ORG is measured by optical coherence tomography (OCT) and the signals can be connected directly to photoreceptor activity and phototransduction. As discussed above, PDE6, GC1, and GC2 are candidates for ROS pillar proteins. Among these proteins, the PDE6αβ heterodimer assumes a pseudo-two-fold symmetry which can be attributed to its N-terminal Pt-motif [77] (Figure 5). PDE6 displays a conserved catalytic phosphohydrolase domain, the activity of which is controlled by regulatory GAF domains (purple and red in Figure 5B) and PDE6γ-mediated regulatory mechanisms [78]. PDE6 undergoes conformational changes during phototransduction that accompany its allosteric activation [77]. PDE6 is expected to elongate by about 30 Å upon Gtα-GTP-mediated activation [78] (Figures 5A and 5B). The elongation of PDE6 would be achieved by the rotation of the catalytic domains around an axis parallel to the lamellar membranes and would increase the ROS intradiscal spacing to ~17 nm. A prolonged light stimulus with full saturation is expected to result in an increase of the overall ROS length from 23.8 ± 1.0 μm (dark) to 26.23 ± 1.1 μm (light) (a cumulative change for 11 intradiscal spaces is shown in Figure 5C). The time frame for activation would be within the millisecond range [2]. The potential role of GC1/2 in the ROS elongation process is currently unknown due to the lack of structural knowledge of GC1/2 and their complexes with corresponding GCAP proteins. It is conceivable that the GC/GCAP complexes could elongate upon release of Ca2+ from myristoylated GCAP after illumination because they are less ordered in the Ca2+-free form, so release of Ca2+ could induce conformational changes in GCs, extending their length. However, this interpretation still needs to be evaluated with further studies.
Figure 5. Hypothetical structural model of PDE6-guided change in ROS morphology.

(A) The cryo-EM structure of the PDE6αβ2γ tetramer, displaying PDE6α (teal), PDE6β (pink) and two molecules of PDE6γ (light gray) [77]. The PDE6αβ heterodimer attains a pseudo-twofold symmetry, where the three domains of PDE6α and PDE6β are organized in a head-to-head arrangement with dimensions of 154 Å X 115 Å X 74 Å. Each of the PDE6α and PDE6β subunits contain two regulatory N-terminal GAF domains (GAF-A and GAF-B) and a C-terminal catalytic domain. The PDE6αβ2γ complex has a 34Å-long N-terminal pony-tail domain (Pt motif). The Pt motif is among the most flexible regions within the PDE6αβ2γ complex and could provide structural stability to the PDE6αβ heterodimer by providing an additional interaction interface of ~895 Å2 between the PDE6α and PDE6β subunits. Due to its hydrophobic properties, this region could provide tethering into the ROS membranes. (B) Light-activation of the dark-adapted ROS leads to conformational rearrangements in the N-terminal GAF (red and purple domains) and C-terminal catalytic domains (green) of the PDE6 heterodimer, resulting in elongation of the PDE6 heterodimer. One side of the PDE6 is tethered to membranes by the isoprenylated moieties on each of the catalytic subunits. The N-terminal side contains the Pt motif, which could intercalate into the adjacent disc membrane, spanning the entire space between discs. (C) The extent of potential conformational changes and ROS extension that occurs during PDE6 activation is illustrated. These structural changes result in the elongation of the PDE6 heterodimer, culminating in the elongation of the ROS.
Hillmann and colleagues observed both spatial and temporal changes in the optical path length of the photoreceptor outer segment in response to an optical stimulus in the living human eye [79]. This noninvasive detection of changes in the optical path length provides information about the activity of single cones in the living human retina, and thereby creates diagnostic opportunities related to phototransduction. In another study, visible light stimulation over a 200-fold intensity range caused ROS elongation and increased light scattering in the eyes of wild-type mice, but not in mice lacking the rod Gtα, indicating that these responses were triggered by phototransduction [80]. The increased backscattering from the base of the ROS can be explained by a model that combines cytoplasmic swelling, translocation of dissociated G-protein subunits from the disc membranes into the cytoplasm, and a relatively higher H2O-permeability of nascent discs in the basal region of the ROS. However, the slow rise in the signal is contradictory with the rapid time course of phototransduction. In another elegant study, Miller and colleagues showed that optical phase changes induced by light occur in cone cells [80], and this signal could be used to assess dysfunction of these cells [81]. The authors suggested that these phase dynamics occur on dramatically different timescales (from milliseconds to seconds) inside the cone outer segment, thus encompassing the phototransduction cascade and subsequent downstream effects. The results from this study could offer methods for assessing color vision and for investigating retinal diseases that affect cone function. Ma et al. have claimed that in vivo optoretinography can detect both phototransduction activation and energy metabolism in retinal photoreceptors [82]. Overall, optoretinography measurements could be used as an objective assessment of the health of photoreceptor cells.
In short, the observed ORG requires a molecular explanation. Measurement of an ORG in dark- and light-adapted conditions would be related directly to the responsiveness of individual photoreceptors. Based on structural insights, here we propose plausible mechanisms by which these signals are generated. Upon light activation, PDE6 activation triggers elongation of 18,300 ± 200 PDE molecules per rod (Figure 5). In the dark, PDE6 resembles a coiled spring, and upon light activation a 1–3 μm extension takes place which increases the ROS volume. This is followed by the termination of the phototransduction, and the dark state is then reestablished. Again, GCs could also play a role in the observed ORG.
Concluding remarks
This review has primarily focused on placing the major components of the phototransduction pathway in the ROS disc membranes, illustrating the spatial limitations imposed on the phototransduction machinery. The confined space could increase the overall probability of productive collisions between key proteins to propagate the visual signal. Thus, the intricate architecture of the ROS and the flexibility of its protein components represent an efficient interfacial reactor as shown in Figure 2. Distances between the ROS membrane components were obtained from cryo-EM tomograms and are drawn to scale. The relative abundance of visual phototransduction proteins is in accordance with a previously conducted tandem mass spectrometry-based proteomics study [67, 68]. The displayed proteins are structurally accurate with correct dimensions and established protein stoichiometry/density relative to rhodopsin. To appreciate the complexity and fluidity of the ROS disc membranes, one must consider the juxtaposition of phototransduction proteins within the ROS membrane, as has been described herein. However, many questions still remain unanswered (see Outstanding Questions). Particularly, the low primary sequence similarity of the Pt-motif and different dimeric configurations of rod and cone PDE6 could potentially reform our basic understanding of vision. Further advances in cryo-ET and data processing will be required to achieve a resolution at which individual proteins can be accurately identified. Conclusive identification of the ROS pillar molecules will greatly enhance our understanding of the development of photoreceptors and their degeneration in progressive retinal diseases. The relative distribution of these pillar molecules between photoreceptor types could explain why rod and cone photoreceptors have acquired different morphologies to carry out their analogous functions. Insights obtained from the detailed structural characterization of this unique GPCR signaling system are likely to be applicable to advancing our understanding of other GPCRs.
OUTSTANDING QUESTIONS.
What precise structural changes occur in rhodopsin post-photoactivation to initiate the signaling cascade? How does photoactivated rhodopsin so perfectly, like a molecular clock, undergo these conformational changes in a unidirectional manner? How do chromophores participate in the process?
How is the specific distance maintained between the plasma membrane and the disc membranes? What are the roles of the cGMP-gated cation channel and its splice forms, ABCA4, or PRPH2/ROM1? Are there other protein(s) involved in creating the scaffold?
Are PDEs and/or GCs actually serving as interdiscal spacers (pillar proteins) of the ROS? How would they anchor to both leaflets between the two disc membranes? How would mutations in these proteins lead to rapid rod degeneration or malformation of the ROS? Can the degenerative disorders be explained exclusively by dysregulation of cGMP and/or Ca2+ levels and subsequent toxicity, or are they also due to disruption of the structural roles of the PDE6 or GC proteins?
How does the dynamic/fluid nature of the rod outer segment change between the dark state and after photoactivation?
How do the structures of GC1 and its activating Ca2+-binding proteins relate to their functions? What are the molecular differences in the activation mechanisms of GC1 and 2 and their activating proteins GCAP1 and 2?
Would improvements in hardware and software used for cryo-ET enable visualization of phototransduction at the level of individual proteins? Can we resolve individual rhodopsin molecules in the discs?
What are the forces behind lipid segregation in the ROS? How are lipids segregated during the elongation of ROS when cell differentiation occurs?
What is the full molecular explanation for IOS? Is the PDE6 pillar hypothesis correct? How quickly does PDE6 change its conformation? Can we observe responsiveness of all photoreceptors in the retina at once?
Obtaining better quantitative estimates of the relative protein abundances in ROS requires special attention. Historically, different methods have been used to calculate protein stoichiometry relative to rhodopsin. Lately, three reports were presented that took a more comprehensive approach [67, 68, 83]. However, we find there are internal inconsistencies between these reports that are likely due to the limitations of the methods used and the relative purity of ROS samples. Perhaps this issue will remain unresolved with respect to the precision required until stable isotope (13C,15N,18O)-enriched whole mice are generated. Such isotope-enriched mice would enable quantitative analysis of all proteins in the retina without any concerns regarding ROS contamination or ROS integrity. We anticipate that this model, however, will remain valuable in a global sense, with a few quantitative adjustments as more precise results become available in the future.
HIGHLIGHTS.
Cryo-EM and cryo-ET analysis of the ROS has characterized the functional components, specific molecular interactions, and physical boundaries for GPCR phototransduction.
Rhodopsin is the only GPCR with known structures of its intermolecular complexes with all three effectors in its signal transduction sequence.
When activated, rhodopsin transiently binds transducin to propagate the cascade of reactions that lead to vision; the activity is quenched by GRK1-mediated phosphorylation, and binding of arrestin.
Phototransduction constitutes interfacial catalysis; thus, lipids are important constituents and display remarkable microheterogeneity in support of signaling.
Phototransduction changes the size of the ROS, generating the basis for Intrinsic optical signals (IOS) observed by optoretinography.
These discoveries advance understanding of the structure/function relationships in GPCR signaling in vision and thus help in interpreting the cellular physiology and pathological basis of retinal diseases.
Acknowledgments:
We thank Drs. Vladimir Kefalov and Philip D. Kiser, Molly Gulati, and members of the Palczewski laboratory for their helpful comments on this manuscript. K. Palczewski is the Irving H. Leopold Chair of Ophthalmology at the Gavin Herbert Eye Institute, Department of Ophthalmology, University of California, Irvine. This research was supported in part by a grant to K.P. from the National Institutes of Health (NIH) (R01EY030873). The authors also acknowledge support from an RPB unrestricted grant to the Department of Ophthalmology, University of California, Irvine.
Glossary
- Retinal-specific ATP-binding cassette transporter (ABCA4)
Mammalian rod outer segments (ROS) contain three abundant proteins, which localize to the disc rim and harbor the large disc luminal domain protein ABCA4, involved in retinoid transport.
- cGMP-gated cation channel
The cGMP-gated channel of the rod and cone photoreceptor cells controls the flow of Na+ and Ca2+ in response to an increase in cGMP concentration. The rod channel is a tetramer of α and β subunits with 3:1 stoichiometry.
- Cone photoreceptors
Cone cells are photoreceptor cells in vertebrate retinas. In humans, they are less abundant than the rod photoreceptor cells (6 million cones versus 120 million rods). Cones are photoactivated by light like rods, but are less sensitive, and thus are responsible for high spatial-acuity photopic vision. Color discrimination is possible due to different visual pigments present in subpopulations of different cones.
- G protein-coupled receptor (GPCR)
G protein-coupled receptors (GPCRs) share a common architecture, comprising seven transmembrane-spanning alpha-helices. They are transformed by ligands to active conformations, propagating the signal to heterotrimeric G proteins. GPCRs are key regulatory elements in a broad range of normal and pathological processes, and they are the most prevalent target of modern medications.
- Guanylate cyclase 1 and 2 (GC1 and GC2)
GC1 and GC2 are enzymes responsible for catalyzing synthesis of cyclic GMP in rods and cones; they are integral membrane proteins, each with a single transmembrane domain. GC1 and GC2 are activated by respective Ca2+-binding proteins called guanylate cyclase-activating proteins 1 and 2 (GCAP1 and GCAP2).
- Optical coherence tomography (OCT)
Optical Coherence Tomography (OCT) is a non-invasive technique that measures light back-scattered from the tissue. OCT generates two- and three-dimensional images with micron scale resolution, based on the intensity of the back-scattered light as a function of location in the tissue. OCT can also measure optical path length changes, which for instance are induced by light stimuli in photoreceptors.
- Rhodopsin
The visual pigment of rod cells, rhodopsin, consists of a membrane protein opsin that covalently couples to the visual chromophore, 11-cis-retinal, through a protonated Schiff base linkage. Upon photoisomerization the chromophore undergoes geometric isomerization to all-trans-retinal. Rhodopsin is a class A GPCR that converts a light stimulus into a signaling conformation of its seven-transmembrane helical bundle, facilitating propagation of the signal to G protein transducin.
- Rod photoreceptors
Rods are specialized neurons responsible for conversion of visual stimuli in the form of photons (particles of light) into neuronal activity. Rods have been evolved to respond reliably to single photons and enable vision in dim conditions (scotopic vision). They are cylindrically shaped photoreceptors concentrated at the outer edges of the retina and are used in peripheral vision.
- Rod outer segment (ROS)
The rod cell features a modified primary cilium known as the rod outer segment (ROS), composed of bilayered disc membrane stacks enclosed by a plasma membrane, which together contain the components necessary for phototransduction. The ROS stacks are flattened discs that are made up of two lamellar membranes restricted by a hairpin rim region.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rodieck RW (1998) The first steps in seeing, Sinauer Associates. [Google Scholar]
- 2.Luo DG et al. (2008) How vision begins: an odyssey. Proc Natl Acad Sci U S A 105 (29), 9855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polans A et al. (1996) Turned on by Ca2+! The physiology and pathology of Ca(2+)-binding proteins in the retina. Trends Neurosci 19 (12), 547–54. [DOI] [PubMed] [Google Scholar]
- 4.Arshavsky VY and Wensel TG (2013) Timing is everything: GTPase regulation in phototransduction. Invest Ophthalmol Vis Sci 54 (12), 7725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawamura S and Tachibanaki S (2021) Molecular bases of rod and cone differences. Prog Retin Eye Res, 101040. [DOI] [PubMed] [Google Scholar]
- 6.Arshavsky V (2002) Like night and day: rods and cones have different pigment regeneration pathways. Neuron 36 (1), 1–3. [DOI] [PubMed] [Google Scholar]
- 7.Baylor DA (1987) Photoreceptor signals and vision. Proctor lecture. Invest Ophthalmol Vis Sci 28 (1), 34–49. [PubMed] [Google Scholar]
- 8.Yau KW and Hardie RC (2009) Phototransduction motifs and variations. Cell 139 (2), 246–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Y and Yau KW (2007) Phototransduction in mouse rods and cones. Pflugers Arch 454 (5), 805–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kefalov VJ (2012) Rod and Cone Visual Pigments and Phototransduction through Pharmacological, Genetic, and Physiological Approaches. J Biol Chem 287 (3), 1635–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang Y et al. (2004) Rhodopsin signaling and organization in heterozygote rhodopsin knockout mice. J Biol Chem 279 (46), 48189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H et al. (2022) Visual pigment-deficient cones survive and mediate visual signaling despite the lack of outer segments. Proc Natl Acad Sci U S A 119 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roof DJ and Heuser JE (1982) Surfaces of rod photoreceptor disk membranes: integral membrane components. J Cell Biol 95 (2 Pt 1), 487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsukamoto Y (1987) The number, depth and elongation of disc incisures in the retinal rod of Rana catesbeiana. Exp Eye Res 45 (1), 105–16. [DOI] [PubMed] [Google Scholar]
- 15.Molday RS et al. (1987) Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest Ophthalmol Vis Sci 28 (1), 50–61. [PubMed] [Google Scholar]
- 16.Filipek S et al. (2003) G protein-coupled receptor rhodopsin: a prospectus. Annu Rev Physiol 65, 851–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai K et al. (2022) Creation of photocyclic vertebrate rhodopsin by single amino acid substitution. Elife 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arshavsky VY et al. (2002) G proteins and phototransduction. Annu Rev Physiol 64, 153–87. [DOI] [PubMed] [Google Scholar]
- 19.Wensel TG et al. (2016) Structural and molecular bases of rod photoreceptor morphogenesis and disease. Prog Retin Eye Res 55, 32–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fliesler SJ and Anderson RE (1983) Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res 22 (2), 79–131. [DOI] [PubMed] [Google Scholar]
- 21.Pearring JN et al. (2013) Protein sorting, targeting and trafficking in photoreceptor cells. Prog Retin Eye Res 36, 24–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palczewski K (2006) G protein-coupled receptor rhodopsin. Annu Rev Biochem 75, 743–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fesenko EE et al. (1985) Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature 313 (6000), 310–3. [DOI] [PubMed] [Google Scholar]
- 24.Yau KW and Baylor DA (1989) Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci 12, 289–327. [DOI] [PubMed] [Google Scholar]
- 25.Xue J et al. (2022) Structural mechanisms of assembly, permeation, gating, and pharmacology of native human rod CNG channel. Neuron 110 (1), 86–95 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barret DCA et al. (2022) The structure of the native CNGA1/CNGB1 CNG channel from bovine retinal rods. Nat Struct Mol Biol 29 (1), 32–39. [DOI] [PubMed] [Google Scholar]
- 27.Zheng X et al. (2020) Mechanism of ligand activation of a eukaryotic cyclic nucleotide-gated channel. Nat Struct Mol Biol 27 (7), 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barret DCA et al. (2022) Structural basis of the partially open central gate in the human CNGA1/CNGB1 channel explained by additional density for calmodulin in cryo-EM map. J Struct Biol 214 (1), 107828. [DOI] [PubMed] [Google Scholar]
- 29.Hsu YT and Molday RS (1993) Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature 361 (6407), 76–9. [DOI] [PubMed] [Google Scholar]
- 30.Poge M et al. (2021) Determinants shaping the nanoscale architecture of the mouse rod outer segment. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilliam JC et al. (2012) Three-dimensional architecture of the rod sensory cilium and its disruption in retinal neurodegeneration. Cell 151 (5), 1029–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nickell S et al. (2007) Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J Cell Biol 177 (5), 917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada T et al. (2001) Activation of rhodopsin: new insights from structural and biochemical studies. Trends Biochem Sci 26 (5), 318–24. [DOI] [PubMed] [Google Scholar]
- 34.Getter T et al. (2021) Identification of small-molecule allosteric modulators that act as enhancers/disrupters of rhodopsin oligomerization. J Biol Chem 297 (6), 101401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S et al. (2022) Capturing a G-protein coupled receptor signalling cascade across a native membrane. Nature, (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salon JA et al. (2011) The significance of G protein-coupled receptor crystallography for drug discovery. Pharmacol Rev 63 (4), 901–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwok-Keung Fung B and Stryer L (1980) Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci U S A 77 (5), 2500–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurley JB (1987) Molecular properties of the cGMP cascade of vertebrate photoreceptors. Annu Rev Physiol 49, 793–812. [DOI] [PubMed] [Google Scholar]
- 39.Wensel TG (2008) Signal transducing membrane complexes of photoreceptor outer segments. Vision Res 48 (20), 2052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao Y et al. (2019) Structures of the Rhodopsin-Transducin Complex: Insights into G-Protein Activation. Mol Cell 75 (4), 781–790 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuhn H (1974) Light-dependent phosphorylation of rhodopsin in living frogs. Nature 250 (467), 588–90. [DOI] [PubMed] [Google Scholar]
- 42.Wilden U et al. (1986) Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci U S A 83 (5), 1174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maeda T et al. (2003) Rhodopsin phosphorylation: 30 years later. Prog Retin Eye Res 22 (4), 417–34. [DOI] [PubMed] [Google Scholar]
- 44.Ohguro H et al. (1995) Rhodopsin phosphorylation and dephosphorylation in vivo. J Biol Chem 270 (24), 14259–62. [DOI] [PubMed] [Google Scholar]
- 45.Chen Q et al. (2021) Structures of rhodopsin in complex with G-protein-coupled receptor kinase 1. Nature 595 (7868), 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palczewski K et al. (1993) Identification of the N-terminal region in rhodopsin kinase involved in its interaction with rhodopsin. J Biol Chem 268 (8), 6004–13. [PubMed] [Google Scholar]
- 47.Buczylko J et al. (1991) Regulation of rhodopsin kinase by autophosphorylation. Proc Natl Acad Sci U S A 88 (6), 2568–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuhn H and Wilden U (1987) Deactivation of photoactivated rhodopsin by rhodopsin-kinase and arrestin. J Recept Res 7 (1–4), 283–98. [DOI] [PubMed] [Google Scholar]
- 49.Wilden U et al. (1986) Rapid affinity purification of retinal arrestin (48 kDa protein) via its light-dependent binding to phosphorylated rhodopsin. FEBS Lett 207 (2), 292–5. [DOI] [PubMed] [Google Scholar]
- 50.Palczewski K (1994) Structure and functions of arrestins. Protein Sci 3 (9), 1355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou XE et al. (2017) Identification of Phosphorylation Codes for Arrestin Recruitment by G Protein-Coupled Receptors. Cell 170 (3), 457–469 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sander CL et al. (2022) Structural evidence for visual arrestin priming via complexation of phosphoinositols. Structure 30 (2), 263–277 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cideciyan AV et al. (1998) Null mutation in the rhodopsin kinase gene slows recovery kinetics of rod and cone phototransduction in man. Proc Natl Acad Sci U S A 95 (1), 328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palczewski K et al. (1992) The influence of arrestin (48K protein) and rhodopsin kinase on visual transduction. Neuron 8 (1), 117–26. [DOI] [PubMed] [Google Scholar]
- 55.Sitaramayya A and Liebman PA (1983) Phosphorylation of rhodopsin and quenching of cyclic GMP phosphodiesterase activation by ATP at weak bleaches. J Biol Chem 258 (20), 12106–9. [PubMed] [Google Scholar]
- 56.Miller JL et al. (1986) Amplification of phosphodiesterase activation is greatly reduced by rhodopsin phosphorylation. Biochemistry 25 (18), 4983–8. [DOI] [PubMed] [Google Scholar]
- 57.Gao Y et al. (2021) Structure of the visual signaling complex between transducin and phosphodiesterase 6. Mol Cell 81 (11), 2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sander CL et al. (2021) Nano-scale resolution of native retinal rod disk membranes reveals differences in lipid composition. J Cell Biol 220 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Filipek S et al. (2004) A concept for G protein activation by G protein-coupled receptor dimers: the transducin/rhodopsin interface. Photochem Photobiol Sci 3 (6), 628–38. [DOI] [PubMed] [Google Scholar]
- 60.Watts A et al. (1979) Rhodopsin-lipid associations in bovine rod outer segment membranes. Identification of immobilized lipid by spin-labels. Biochemistry 18 (22), 5006–13. [DOI] [PubMed] [Google Scholar]
- 61.Batra-Safferling R et al. (2006) Glutamic acid-rich proteins of rod photoreceptors are natively unfolded. J Biol Chem 281 (3), 1449–60. [DOI] [PubMed] [Google Scholar]
- 62.Korschen HG et al. (1999) Interaction of glutamic-acid-rich proteins with the cGMP signalling pathway in rod photoreceptors. Nature 400 (6746), 761–6. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y et al. (2021) Retinal degeneration in mice lacking the cyclic nucleotide-gated channel subunit CNGA1. FASEB J 35 (9), e21859. [DOI] [PubMed] [Google Scholar]
- 64.Sakamoto K et al. (2009) New mouse models for recessive retinitis pigmentosa caused by mutations in the Pde6a gene. Hum Mol Genet 18 (1), 178–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Viczian A et al. (1992) Photoreceptor-specific mRNAs in mice carrying different allelic combinations at the rd and rds loci. Exp Eye Res 54 (6), 853–60. [DOI] [PubMed] [Google Scholar]
- 66.Zou T et al. (2022) Expression of exogenous PDE5 rescues photoreceptors in rd1 mice. Curr Med Chem. [DOI] [PubMed] [Google Scholar]
- 67.Kwok MC et al. (2008) Proteomics of photoreceptor outer segments identifies a subset of SNARE and Rab proteins implicated in membrane vesicle trafficking and fusion. Mol Cell Proteomics 7 (6), 1053–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skiba NP et al. (2022) Absolute quantification of phototransduction and other rod outer segment proteins using a novel technique of MS-Western. Invest Ophthalmol Vis Sci 63, 4121–F0358. [Google Scholar]
- 69.Pugh EN Jr. and Lamb TD (1993) Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta 1141 (2–3), 111–49. [DOI] [PubMed] [Google Scholar]
- 70.Fotiadis D et al. (2003) Atomic-force microscopy: Rhodopsin dimers in native disc membranes. Nature 421 (6919), 127–8. [DOI] [PubMed] [Google Scholar]
- 71.Morshedian A et al. (2022) Reproducibility of the Rod Photoreceptor Response Depends Critically on the Concentration of the Phosphodiesterase Effector Enzyme. J Neurosci 42 (11), 2180–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuhn M (2016) Molecular Physiology of Membrane Guanylyl Cyclase Receptors. Physiol Rev 96 (2), 751–804. [DOI] [PubMed] [Google Scholar]
- 73.Dizhoor AM (2000) Regulation of cGMP synthesis in photoreceptors: role in signal transduction and congenital diseases of the retina. Cell Signal 12 (11–12), 711–9. [DOI] [PubMed] [Google Scholar]
- 74.Bouzia Z et al. (2020) GUCY2D-Associated Leber Congenital Amaurosis: A Retrospective Natural History Study in Preparation for Trials of Novel Therapies. Am J Ophthalmol 210, 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharon D et al. (2018) Genotype-functional-phenotype correlations in photoreceptor guanylate cyclase (GC-E) encoded by GUCY2D. Prog Retin Eye Res 63, 69–91. [DOI] [PubMed] [Google Scholar]
- 76.Baehr W et al. (2007) The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J Biol Chem 282 (12), 8837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gulati S et al. (2019) Cryo-EM structure of phosphodiesterase 6 reveals insights into the allosteric regulation of type I phosphodiesterases. Sci Adv 5 (2), eaav4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gulati S and Palczewski K (2021) New focus on regulation of the rod photoreceptor phosphodiesterase. Curr Opin Struct Biol 69, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hillmann D et al. (2016) In vivo optical imaging of physiological responses to photostimulation in human photoreceptors. Proc Natl Acad Sci U S A 113 (46), 13138–13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang P et al. (2017) In vivo optophysiology reveals that G-protein activation triggers osmotic swelling and increased light scattering of rod photoreceptors. Proc Natl Acad Sci U S A 114 (14), E2937–E2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lassoued A et al. (2021) Cone photoreceptor dysfunction in retinitis pigmentosa revealed by optoretinography. Proc Natl Acad Sci U S A 118 (47). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma G et al. (2021) In vivo optoretinography of phototransduction activation and energy metabolism in retinal photoreceptors. J Biophotonics 14 (5), e202000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Skiba NP et al. (2018) Identification of protein components of the rod outer segment plasma membrane by label-free protein correlation profiling. Investigative Ophthalmology & Visual Science 59 (9). [Google Scholar]
- 84.Kuhn H et al. (1984) Light-induced binding of 48-kDa protein to photoreceptor membranes is highly enhanced by phosphorylation of rhodopsin. FEBS Lett 176 (2), 473–8. [DOI] [PubMed] [Google Scholar]
- 85.Dryja TP et al. (1990) A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature 343 (6256), 364–6. [DOI] [PubMed] [Google Scholar]
- 86.Dryja TP et al. (1993) Heterozygous missense mutation in the rhodopsin gene as a cause of congenital stationary night blindness. Nat Genet 4 (3), 280–3. [DOI] [PubMed] [Google Scholar]
- 87.Ramachandran S and Cerione RA (2011) A dominant-negative Galpha mutant that traps a stable rhodopsin-Galpha-GTP-betagamma complex. J Biol Chem 286 (14), 12702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Noel JP et al. (1993) The 2.2 A crystal structure of transducin-alpha complexed with GTP gamma S. Nature 366 (6456), 654–63. [DOI] [PubMed] [Google Scholar]
- 89.Fung BK et al. (1981) Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A 78 (1), 152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He W et al. (1998) RGS9, a GTPase accelerator for phototransduction. Neuron 20 (1), 95–102. [DOI] [PubMed] [Google Scholar]
- 91.Zhang H et al. (2011) UNC119 is required for G protein trafficking in sensory neurons. Nat Neurosci 14 (7), 874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gal A et al. (1994) Gene for autosomal dominant congenital stationary night blindness maps to the same region as the gene for the beta-subunit of the rod photoreceptor cGMP phosphodiesterase (PDEB) in chromosome 4p16.3. Hum Mol Genet 3 (2), 323–5. [DOI] [PubMed] [Google Scholar]
- 93.Szabo V et al. (2007) p.Gln200Glu, a putative constitutively active mutant of rod alpha-transducin (GNAT1) in autosomal dominant congenital stationary night blindness. Hum Mutat 28 (7), 741–2. [DOI] [PubMed] [Google Scholar]
- 94.Sondek J et al. (1996) Crystal structure of a G-protein beta gamma dimer at 2.1A resolution. Nature 379 (6563), 369–74. [DOI] [PubMed] [Google Scholar]
- 95.Petrovski S et al. (2016) Germline De Novo Mutations in GNB1 Cause Severe Neurodevelopmental Disability, Hypotonia, and Seizures. Am J Hum Genet 98 (5), 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qureshi BM et al. (2018) Mechanistic insights into the role of prenyl-binding protein PrBP/delta in membrane dissociation of phosphodiesterase 6. Nat Commun 9 (1), 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang SH et al. (1995) Autosomal recessive retinitis pigmentosa caused by mutations in the alpha subunit of rod cGMP phosphodiesterase. Nat Genet 11 (4), 468–71. [DOI] [PubMed] [Google Scholar]
- 98.McLaughlin ME et al. (1993) Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat Genet 4 (2), 130–4. [DOI] [PubMed] [Google Scholar]
- 99.Gal A et al. (1994) Heterozygous missense mutation in the rod cGMP phosphodiesterase beta-subunit gene in autosomal dominant stationary night blindness. Nat Genet 7 (1), 64–8. [DOI] [PubMed] [Google Scholar]
- 100.Dvir L et al. (2010) Autosomal-recessive early-onset retinitis pigmentosa caused by a mutation in PDE6G, the gene encoding the gamma subunit of rod cGMP phosphodiesterase. Am J Hum Genet 87 (2), 258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dryja TP et al. (1995) Mutations in the gene encoding the alpha subunit of the rod cGMP-gated channel in autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci U S A 92 (22), 10177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bareil C et al. (2001) Segregation of a mutation in CNGB1 encoding the beta-subunit of the rod cGMP-gated channel in a family with autosomal recessive retinitis pigmentosa. Hum Genet 108 (4), 328–34. [DOI] [PubMed] [Google Scholar]
- 103.Allikmets R et al. (1997) A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet 15 (3), 236–46. [DOI] [PubMed] [Google Scholar]
- 104.Allikmets R et al. (1997) Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science 277 (5333), 1805–7. [DOI] [PubMed] [Google Scholar]
- 105.Farrar GJ et al. (1991) A three-base-pair deletion in the peripherin-RDS gene in one form of retinitis pigmentosa. Nature 354 (6353), 478–80. [DOI] [PubMed] [Google Scholar]
- 106.Kajiwara K et al. (1993) A null mutation in the human peripherin/RDS gene in a family with autosomal dominant retinitis punctata albescens. Nat Genet 3 (3), 208–12. [DOI] [PubMed] [Google Scholar]
- 107.Nichols BE et al. (1993) Butterfly-shaped pigment dystrophy of the fovea caused by a point mutation in codon 167 of the RDS gene. Nat Genet 3 (3), 202–7. [DOI] [PubMed] [Google Scholar]
- 108.Kajiwara K et al. (1994) Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science 264 (5165), 1604–8. [DOI] [PubMed] [Google Scholar]
- 109.Higgins MK et al. (2006) Recoverin binds exclusively to an amphipathic peptide at the N terminus of rhodopsin kinase, inhibiting rhodopsin phosphorylation without affecting catalytic activity of the kinase. J Biol Chem 281 (28), 19426–32. [DOI] [PubMed] [Google Scholar]
- 110.Zhang H et al. (2004) Photoreceptor cGMP phosphodiesterase delta subunit (PDEdelta) functions as a prenyl-binding protein. J Biol Chem 279 (1), 407–13. [DOI] [PubMed] [Google Scholar]
- 111.Yamamoto S et al. (1997) Defects in the rhodopsin kinase gene in the Oguchi form of stationary night blindness. Nat Genet 15 (2), 175–8. [DOI] [PubMed] [Google Scholar]
- 112.Azadi S et al. (2010) RD3, the protein associated with Leber congenital amaurosis type 12, is required for guanylate cyclase trafficking in photoreceptor cells. Proc Natl Acad Sci U S A 107 (49), 21158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Perrault I et al. (1996) Retinal-specific guanylate cyclase gene mutations in Leber’s congenital amaurosis. Nat Genet 14 (4), 461–4. [DOI] [PubMed] [Google Scholar]
- 114.Perrault I et al. (1998) A retGC-1 mutation in autosomal dominant cone-rod dystrophy. Am J Hum Genet 63 (2), 651–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van der Velden LM et al. (2010) Heteromeric interactions required for abundance and subcellular localization of human CDC50 proteins and class 1 P4-ATPases. J Biol Chem 285 (51), 40088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Onat OE et al. (2013) Missense mutation in the ATPase, aminophospholipid transporter protein ATP8A2 is associated with cerebellar atrophy and quadrupedal locomotion. Eur J Hum Genet 21 (3), 281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zangerl B et al. (2006) Identical mutation in a novel retinal gene causes progressive rod-cone degeneration in dogs and retinitis pigmentosa in humans. Genomics 88 (5), 551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Riazuddin SA et al. (2010) A mutation in SLC24A1 implicated in autosomal-recessive congenital stationary night blindness. Am J Hum Genet 87 (4), 523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kiser PD and Palczewski K (2021) Pathways and disease-causing alterations in visual chromophore production for vertebrate vision. J Biol Chem 296, 100072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zheng W et al. (2021) Folding non-homologous proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Rep Methods 1 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jumper J et al. (2021) Highly accurate protein structure prediction with AlphaFold. Nature 596 (7873), 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]


