Abstract
Porphyromonas gingivalis cysteine proteinases (gingipains) have been associated with virulence in destructive periodontitis, a disease process variously considered to represent an unregulated stimulation of either T helper type 1 (Th1)- or Th2-type cells. Critical in maintaining Th1 activity is the response of T lymphocytes to environmental interleukin 12 (IL-12) in the form of up-regulation of gamma interferon (IFN-γ) production. Here we demonstrate that in the presence or absence of serum, gingipains were able to hydrolyze IL-12 and reduce the IL-12-induced IFN-γ production from CD4+ T cells. However, the induction of IL-12 receptors on T cells by gingipains did not correlate with the enhancement of IFN-γ production. The gingipains cleaved IL-12 within the COOH-terminal region of the p40 and p35 subunit chains, which leads to IL-12 inactivity, whereas IL-2 in these assays was not affected. Inactivation of IL-12 by the gingipains could disrupt the cytokine balance or favor Th2 activities in the progression of periodontitis.
Porphyromonas gingivalis has been implicated as a major etiological agent in periodontitis, with the cysteine proteinases having received considerable attention due to their ability to activate and/or degrade a broad range of host proteins and cytokines (11, 17, 23, 32, 61). The mechanisms used by the pathogen and its cellular constituents to evade the immune response include modulation of the host cytokine signaling networks, for instance, by the induction of antiinflammatory mediators, such as interleukin 1 (IL-1) receptor antagonist (60), at the site of periodontal infection. Further, major proinflammatory cytokines have recently been shown to be susceptible to degradation and inactivation by P. gingivalis cysteine proteinases, including gamma interferon (IFN-γ) (61), IL-1β (18), IL-6 (1), tumor necrosis factor alpha (6), and IL-8 (37). The cysteine proteinases referred to as gingipains cleave synthetic and natural substrates after arginine (gingipain R, RgpA or RgpB) (42) or lysine residues (gingipain K, Kgp) (41). The major forms of high-molecular-mass gingipain (RgpA or Kgp) released by P. gingivalis are purified as noncovalent complexes of the catalytic domain with several polypeptide chains (GP44, GP15, GP17, and GP27) derived from the nascent hemagglutinin domain via putative autocatalytic processing (12, 23, 43).
Antigen-specific immune responses tend to be categorized as T helper type 1 (Th1)- and Th2-type activities, each governed by the set of cytokines produced by the T cells involved. Based on the dominance of immunological parameters pertaining to stages of the disease process (40, 48), Gemmell and Seymour (22) proposed that the predominant lymphocytes in the stable lesion are CD4+ Th1 cells, while the progressive lesion involves Th2 cells which secrete cytokines acting mainly on B cells. In contrast, Ebersole and Taubman (15, 52) have proposed that Th1 cells are involved in destructive lesions, while Th2 cells are rather protective. Their concept has been supported by the demonstration that adoptive transfer of Th2 cells into rats infected with Actinobacillus actinomycetemcomitans improved the outcome of experimental periodontitis (59). These studies suggested that an imbalance of selected cytokines derived from Th1 and/or Th2 cells may be responsible for periodontal destruction through cellular and/or humoral hyperimmune responses.
The single major factor known for the efficient differentiation of naive CD4+ T cells towards the Th1 phenotype is IL-12, which induces production of IFN-γ and consequent development of a cell-mediated immune response (34, 36). IFN-γ is considered to account for an important characteristic of the Th1 response (4) and has a positive feedback effect by enhancing the production of IL-12 by monocytes and macrophages (31). Evidence indicates that IFN-γ favors induction of Th1-type isotype balance (low immunoglobulin E [IgE] and IgG1 levels and high IgG2a levels) (29). The induction of IFN-γ by IL-12 is characterized by a synergistic effect with IL-2, an important autocrine growth factor for T-cell proliferation and cytokine production (57). Possible lower IFN-γ and IL-2 levels in periodontitis suggest a depression of Th1 responses (21, 22). However, there are reports of high levels of IFN-γ mRNA in inflamed gingival tissue (49, 52), suggesting that Th1 cells were prominent in the diseased sites.
IL-12 is a proinflammatory heterodimer with a mass of 70 kDa, consisting of disulfide-linked glycosylated chains of 35 kDa (p35) and 40 kDa (p40) encoded by separate and unrelated genes (14, 56, 57). The p35 chain is significantly homologous to the helical cytokines IL-6 and granulocyte colony stimulating factor (34), whereas the p40 chain subunit is homologous to the extracellular portions of the receptors for ciliary neurotrophic factor and IL-6 (20). Both units are required for biological activity of IL-12 (24). IL-12, which is produced by antigen-presenting cells such as monocytes/macrophages, dendritic cells, and B cells, has pleiotropic effects on T cells and natural killer (NK) cells, including enhancement of cell-mediated cytotoxicity and comitogenic effects on resting T cells (30, 55, 56). Decreased IL-12 levels at sites of periodontal disease have been reported (16). IL-12 exerts its pleiotropic effects through binding to specific IL-12 receptors (IL-12R) that are expressed on T and NK cells (19). IL-12R has been shown to contain at least two protein subunits, IL-12Rβ1 and IL-12Rβ2 (8, 46, 50). Coexpression of both human IL-12R β1 and β2 subunits results in both high-affinity (Kd of about 50 pM) and low-affinity (Kd of about 5 nM) IL-12 binding sites (46, 58).
Costimulation signals are essential to induce maximal T-cell cytokine secretion, proliferation, and induction of effector function (10). A role for vascular endothelial cells in the priming of CD4+ T cells for costimulatory effects of IL-12 has been reported (35). In this context, the leukocyte function-associated antigen 3 has been identified in human umbilical vein endothelial (HUVE) cells as the major ligand for CD2 expressed on all T cells (5) and has been shown to augment IFN-γ production.
Although studies aimed at determining the possible association between the Th1 and Th2 lymphocyte phenotype ratios and disease status have been performed, the results are not conclusive. In earlier studies we showed that a preparation of gingipains from P. gingivalis was able to cleave and inactivate IFN-γ at a single site at the carboxyl terminus (61). The results in the present study demonstrate (i) that RgpA induces IL-12R but the presence of the active gingipains suppresses the accumulation of IFN-γ in stimulated T-cell cultures, (ii) that the gingipains are capable of hydrolyzing IL-12, (iii) that both the p40 and p35 subunits of IL-12 are affected by the gingipains, and (iv) that hydrolysis of IL-12 by the gingipains leads to IL-12 inactivity by removal of the COOH terminus.
MATERIALS AND METHODS
Chemicals and reagents.
Ficoll-Hypaque was purchased from Pharmacia, Uppsala, Sweden. Collagenase type 1A, endothelial cell growth factor, leupeptin, l-arginine, l-lysine, l-cysteine, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), N-α-tosyl-l-lysyl chloromethyl ketone (TLCK), phytohemagglutinin (PHA), polymyxin B, propanol, sodium dodecyl sulfate (SDS), tosyl-Gly-l-Pro-l-Arg p-nitroanilide, tosyl-Gly-l-Pro-l-Lys p-nitroanilide, Trizma base, Tris-HCl, and trypsin were purchased from Sigma (St. Louis, Mo.). Trypticase soy broth was purchased from Difco (Detroit, Mich.). Tween 20, RPMI, and M199 medium were obtained from ICN Biochemicals (Irvine, Calif.). 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) was purchased from Calbiochem (La Jolla, Calif.). Phosphate-buffered saline (PBS) was purchased from Oxoid (Basingstoke, United Kingdom). All reagents for electrophoresis and Western blotting were from Bio-Rad (Richmond, Calif.).
Recombinant cytokines and antibodies.
Recombinant human interleukin-12 (rIL-12) expressed in a eukaryotic baculovirus system and IL-2 expressed in Escherichia coli were obtained from R&D Systems (Minneapolis, Minn.). Recombinant human IFN-γ was purchased from Endogen (Cambridge, Mass.). Goat polyclonal antibodies (Abs) specific for human IL-12 and IL-2 were purchased from R&D Systems. Monoclonal and polyclonal rabbit anti-human IFN-γ Abs were purchased from Endogen. Monoclonal Ab specific for human CD25 was obtained from PharMingen (San Diego, Calif.). Monoclonal Ab specific for human HLA-DR β-chain was purchased from Dako (Glostrup, Denmark). Affinity-purified goat Abs specific for the human IL-12 p35 and p40 subunits and rabbit polyclonal IgG Abs specific for IL-12R were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif).
RgpA and Kgp isolation.
P. gingivalis (ATCC 33277) cells were grown in enriched Trypticase soy broth under anaerobic conditions for 48 h as described previously (61). Briefly, the bacterial pellet was then extracted in a buffer containing 0.05 M Tris and 1 mM CaCl2, pH 7.5 (Tris buffer) with 1% CHAPS, a nondenaturing zwitterionic detergent, by gentle mixing for 2 h (9). Gingipain R (or RgpA) and gingipain K proteinase-adhesin complexes were then isolated by the sequential use of a Mono-Q fast-protein liquid chromatography column and arginine-Sepharose chromatography (61).
Cell isolation and preparation.
HUVE cells were isolated and cultured as previously described (53, 61). Briefly, the cells were obtained by treatment of fresh human umbilical cord with collagenase type 1A and then serially cultured. Cells used in these experiments were confluent and at passage levels 4 through 6. HUVE cells were identified by reaction with Ulex agglutinin (Dako). Human peripheral blood mononuclear cells (PBMC) from healthy volunteers (Blood Bank, Red Cross Transfusion Service, New South Wales, Australia) were separated using Ficoll-Hypaque gradients (3). CD4+ T cells were obtained from PBMC by positive selection using magnetic beads coated with anti-CD4 Ab (Dynal Inc., Lake Success, N.Y.). Briefly, PBMC were incubated with Ab-coated beads for 1 h at room temperature, and CD4+ T cells were selected using a magnet. The magnetic beads were finally removed from the CD4+ T cells using Detachabead (Dynal) (5). The purity of the CD4+ T cells isolated by this method was >99% with <1% monocytes as analyzed by flow-cytometric (fluorescence-activated cell sorter) analysis (data not shown); the cells were not activated as analyzed by the lack of major histocompatibility complex class II antigen and CD25 expression. CD4+ T cells were cultured in complete medium (RPMI 1640 containing 10% fetal calf serum [FCS], 1% penicillin-streptomycin, 2 mM glutamine, and 50 μM 2-mercaptoethanol) in a humidified atmosphere with 5% CO2 at 37°C. T-cell populations were used within 4 h of isolation.
Biological assay for IL-12 with gingipains.
Tissue culture flat-bottom 96-well plates were coated with gelatin and then seeded with HUVE cells at a density of 105 cells/cm2 in 200 μl of supplemented M199 media containing 20% FCS for 2 h before coculture. Upon removal of 100 μl from each well, purified T cells (2 × 104/well) in RPMI medium were added to each well for a final volume of 200 μl. PHA was further added at a final concentration of 1 μg/ml. In all experiments, the RgpA or Kgp preparations were pretreated with polymyxin B (final concentration, 10 ng/ml) for 30 min at room temperature to inhibit activity of potentially contaminating lipopolysaccharide (LPS) (38). RgpA or Kgp was then incubated for 15 min at 37°C with 5 mM l-cysteine. rIL-12 (10 pg/ml) and RgpA or Kgp at various concentrations were added simultaneously to the wells after the PHA, and the cultures were then incubated for 48 h. Cell coculture supernatants (100 μl each) were taken and assayed for IFN-γ after 48 h. Total viable cell numbers in coculture were assessed at 48 h by colorimetric MTT (tetrazolium) assay. Briefly, 50 μl of a 1-mg/ml solution of MTT in RPMI-phenol red free medium was added to the cells in each well, and plates were incubated at 37°C for 3 h. Propanol (50 μl) was added to all wells. After a few minutes at room temperature in order to ensure solubilization of the blue formazan, the reactions were analyzed using a Titertek Twinreader PLUS photometer (Flow Lab, Sydney, Australia), with a 560-nm test wavelength and a 690-nm reference wavelength (13).
Analysis of IL-12R expression.
Tissue culture flat-bottom 12-well plates (Corning, Corning, N.Y.) were seeded with HUVE cells at a density of 105 cells/cm2 for 2 h before coculture. CD4+ T cells (105/well) were cocultured without PHA in the absence or presence of RgpA preparation (13.3 nM) or TLCK-inhibited RgpA or with PHA (2 μg/ml) in the absence or presence of RgpA preparation (13.3 nM), TLCK-inhibited RgpA, IL-12 (10 pg/ml), RgpA preparation (13.3 nM), or TLCK-inhibited RgpA plus IL-12. Supernatants were isolated at 48 h and analyzed for IFN-γ by specific enzyme-linked immunosorbent assay (ELISA). Samples containing T cells cocultured with HUVE cells were further purified with immunomagnetic beads specific for CD4+ T cells. Purified T cells were washed and then incubated with primary rabbit anti-human IL-12R polyclonal antibody, followed by the addition of fluorescein isothiocyanate-conjugated mouse anti-rabbit IgG1 (Dako), and quantitated as described above.
Proteolytic digestion of IL-12.
RgpA or Kgp was preincubated for 15 min at 37°C with 5 mM l-cysteine. Activated RgpA (61) or Kgp was incubated with IL-12 at a final substrate-to-enzyme (S/E) ratio of 100:1 in the absence of serum (35 nM IL-12 with 0.35 nM RgpA or Kgp) or 1:1 (35 nM IL-12 with 35 nM RgpA or Kgp) in the presence of 20% FCS. Both reaction mixtures were then incubated at 37°C for a time course study. Reactions were stopped at various times with TLCK (2 mM final concentration). Aliquots were then resolved on 12% polyacrylamide gels (SDS–12% polyacrylamide gel electrophoresis [SDS–12% PAGE]) (33) and transferred to polyvinylidene difluoride membranes (54). IL-12 was detected with goat anti-human IL-12 polyclonal antibody. The p35 and p40 subunits were detected with affinity-purified goat anti-human IL-12 p35 and p40 accordingly specific for amino acids mapping at the COOH-terminal domain of the human p35 chain or p40 chain. Alkaline phosphatase rabbit anti-goat (Dako) was used as the secondary antibody. Color was developed in a solution containing nitroblue tetrazolium chloride (1.65 mg) and 5-bromo-4-chloro-3-indolylphosphate p-toluidine salt (0.8 mg) in 5 ml of 100 mM Tris-HCl (pH 9.5). Membranes were washed three times in Tris-buffered saline–0.1% Tween between each step (61).
Measurement of kinetic constants for Arg-gingipain and Lys-gingipain.
Experiments were conducted in which RgpA or Kgp was preincubated in Tris buffer containing 5 mM l-cysteine for 15 min at 37°C. The activated RgpA or Kgp (0.7 nM each) was then added to the stock IL-12 substrate solution (70 nM) at 37°C for 10 min, and the reaction was stopped in aliquots with TLCK (2 mM). The reaction with Lys-gingipain was carried out in the presence of 0.1 mM leupeptin to compensate for the percentage of RgpA in the Kgp preparations. Aliquots were resolved on 12% polyacrylamide gels by SDS-PAGE for immunoblot analysis with goat anti-human IL-12 polyclonal antibodies (R&D Systems). Hydrolysis of IL-12 was measured by densitometry as the fraction degraded from the native 70-kDa IL-12 molecule.
Cytokine assays.
IFN-γ was determined by specific ELISA. Briefly, monoclonal antibody was used as a capture antibody to coat 96-well flat-bottom ELISA plates (Sarstedt, Sydney, Australia) overnight at room temperature. Blocking was performed with 0.1% Tween in PBS for 2 h at room temperature. Subsequently, neat coculture supernatants or standards were added to wells overnight at room temperature, and secondary polyclonal anti-IFN-γ antibody was added to wells for 3 h at room temperature. Between each step, the plates were washed twice in PBS with 0.1% Tween. The ELISA was developed using alkaline phosphatase (Dako) and phosphatase substrate (Bio-Rad, Richmond, Calif.). Plates were read at 405 nm in a Titertek ELISA plate reader.
Statistics.
Data are presented as means ± standard errors. Statistical analysis was performed by Student t testing using SigmaStat software (Jandel Corp.), and P values less than 0.05 were considered significant.
RESULTS
Effect of the cysteine proteinases on IL-12-induced T-cell IFN-γ production.
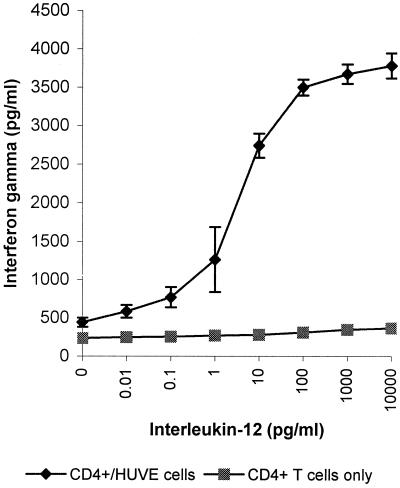
PHA-activated CD4+ T cells produced little IFN-γ (∼300 pg/ml) in the absence of accessory cells (Fig. 1). Coculturing of PHA-treated CD4+ T cells with HUVE cells slightly increased levels of IFN-γ (∼600 pg/ml) in the presence of PHA (1 μg/ml). As IL-12 is a potent inducer of IFN-γ production and Th1 differentiation, addition of IL-12 (0.01 to 10,000 pg/ml) to cocultures for 48 h greatly enhanced the production by CD4+ T cells of IFN-γ in a dose-dependent manner as expected (Fig. 1).
FIG. 1.
Effect of IL-12 on HUVE cell costimulation of IFN-γ production by human CD4+ T cells. Freshly isolated human CD4+ T cells were cultured with various concentrations of IL-12 in the absence or presence of HUVE cells in medium containing PHA (1 μg/ml). Culture supernatants were taken after 48 h, and the IFN-γ concentration was assessed by ELISA. Error bars show the means and standard errors derived by pooling data from three independent experiments.
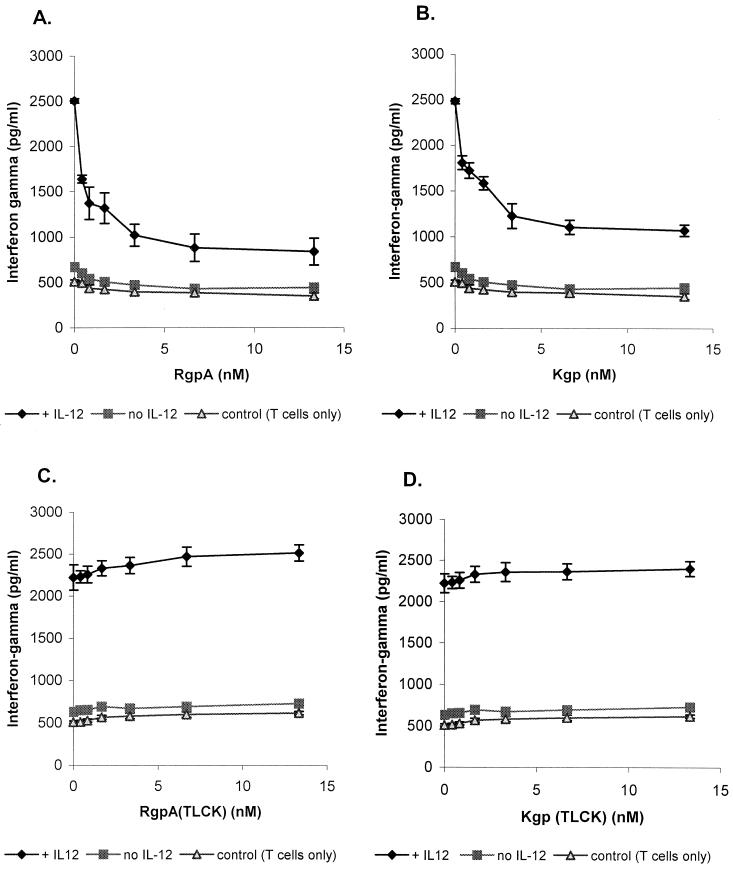
In order to evaluate the ability of gingipains to modify the biological activity of IL-12, the IL-12-enhanced T-cell IFN-γ production was measured following incubation with RgpA or Kgp. The purity and biological activities of the gingipains were characterized as previously described (61). In the absence of exogenous IL-12, activated RgpA or Kgp reduced the limited IFN-γ accumulation in the PHA-activated T-cell cultures (Fig. 2A and B). In cocultures stimulated to express IFN-γ by IL-12 (10 pg/ml), the addition of catalytically active gingipain preparations (0.4 to 13.3 nM) reduced the accumulation of IFN-γ measured in the wells up to 2.5-fold in a dose-dependent manner. TLCK inactivation of the gingipain proteolytic activity in this assay suppressed the inhibitory effect, indicating that the effect was dependent on the catalytic actions of RgpA or Kgp (Fig. 2C and D). The suppressive effects of active gingipains were not associated with significant changes in numbers of viable cells during the assay as determined by MTT assay (data not shown).
FIG. 2.
Inactivation of IL-12-inducing activities on T lymphocyte IFN-γ production by RgpA or Kgp cocultured with HUVE cells. (A and B) RgpA and Kgp preparations were preincubated for 15 min at 37°C with 5 mM l-cysteine. HUVE cells were seeded to confluence. Purified CD4+ T-cell preparations were then cultured with PHA at a concentration of 1 μg/ml and a range of concentrations of activated RgpA (A) or Kgp (B) in the absence or presence of IL-12 (10 pg/ml). (C and D) RgpA (C) and Kgp (D) were preincubated with thiol-protease inhibitor TLCK (2 mM final concentration) for 1 h at 37°C, dialyzed, and cocultured in the absence or presence of IL-12 (10 pg/ml). Controls were CD4+ T cells in medium containing PHA. The production of IFN-γ was measured by ELISA after 48 h. Error bars show the means and standard errors derived by pooling data from three independent experiments.
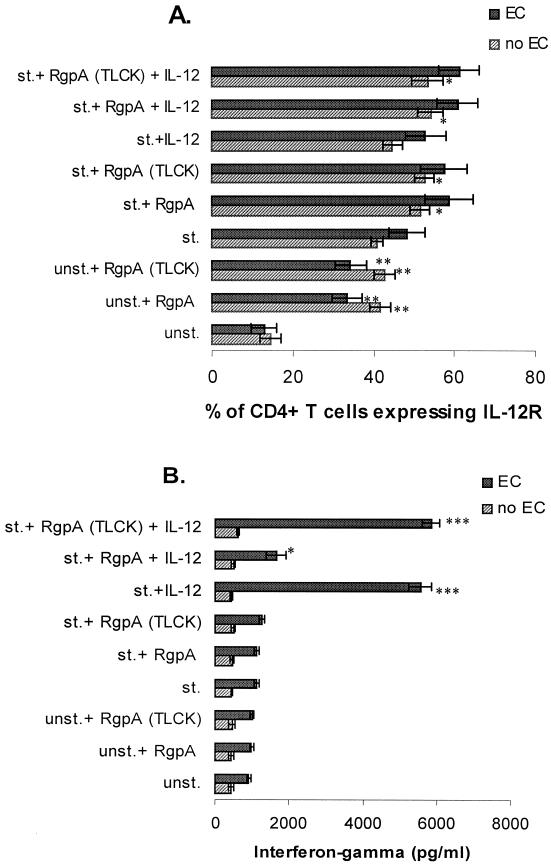
Gingipain-R affects the PHA-induced up-regulation of IL-12R on CD4+ T cells.
Determination of IL-12 as a critical monocyte-derived cofactor in IFN-γ secretion by T cells prompted investigation into the regulation of IL-12R during these experiments. The addition of catalytically active RgpA to unstimulated CD4 T-cell cocultures produced an increase in IL-12R in the presence or absence of endothelial costimulation (Fig. 3A). Treatment of the cell cultures with IL-12 in the presence of RgpA also stimulated relatively high levels of IL-12R, but little IFN-γ was detected within the wells (Fig. 3A and B). RgpA did not, however, stimulate a detectable accumulation of IFN-γ, and the addition of activated RgpA to cocultures stimulated by IL-12 reduced the levels of accumulated IFN-γ. Up-regulation of IL-12R was not dependent upon proteolytic activity of RgpA, since TLCK-inhibited RgpA was effective in IL-12R stimulation, as well.
FIG. 3.
Up-regulation of the expression of IL-12 receptors resulting from binding of RgpA to T cells. (A) CD4+ T cells (105/well) were cocultured without PHA (unst.) in the absence or presence of RgpA preparation (13.3 nM) or TLCK-inhibited RgpA, or with PHA (st.) (2 μg/ml) in the absence or presence of RgpA preparation (13.3 nM) or TLCK-inhibited RgpA, IL-12 (10 pg/ml), or RgpA preparation (13.3 nM) or TLCK-inhibited RgpA plus IL-12 for 48 h. Supernatants were assessed at 48 h for IFN-γ by specific ELISA. Samples were stained with anti-IL-12R after 48 h in culture followed by flow-cytometric analysis as described in Materials and Methods. EC, endothelial cells. Error bars show the means and standard errors derived by pooling data from three independent experiments. (B) Results are parallel to studies shown in Fig. 4A. Supernatants (100 μl each) were assessed at 48 h and analyzed for IFN-γ by specific ELISA. EC, endothelial cells. Error bars show the means and standard errors derived by pooling data from three independent experiments. The difference between the unstimulated or stimulated samples with or without RgpA or RgpA (TLCK), IL-12, and RgpA or RgpA (TLCK) plus IL-12 was significant by Student's t test (∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001).
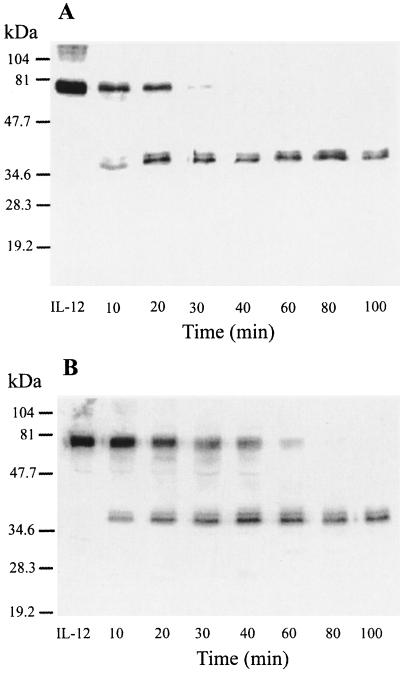
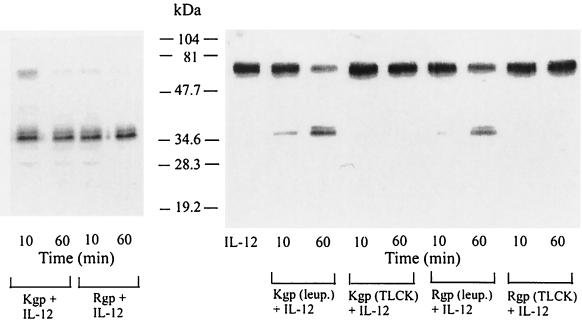
Cleavage of IL-12 by purified RgpA and Kgp in the absence or presence of serum.
To investigate whether proteolysis of the critical soluble mediator IL-12 could have a role in the suppressive effect of RgpA, purified IL-12 was incubated with the activated gingipains; IL-12 degradation in the presence or absence of serum was assessed by Western blot analysis (Fig. 4 and 5). The cleavage of IL-12 by RgpA and Kgp as a function of enzyme concentration was tested at the range of an enzyme-substrate (E/S) molar ratio of 1:100. In the absence of serum, both RgpA and Kgp were capable of degrading IL-12 efficiently (Fig. 4). As the serum proteins competed for gingipain activity, more gingipain was added in serum-inclusive conditions (Fig. 5). IL-12 was incubated with gingipains at an E/S molar ratio of 1:1 at 37°C for up to 100 min. The results indicated that both RgpA and Kgp were capable of cleaving IL-12 in serum, and the intensity of the band at 70 kDa (under nonreducing conditions) was decreased in a time-dependent manner (Fig. 5A and B). Peptides with molecular masses of less than 35 kDa were not detected. Incubation with either RgpA or Kgp gave similar results. Separation of the IL-12 p40 and p35 subunits by boiling and reduction of reaction products prior to electrophoresis demonstrated the partially degraded products of both the 40- and the 35-kDa immunoreactive bands by RgpA or Kgp (Fig. 5C).
FIG. 4.
Time course of IL-12 degradation by gingipains in the absence of serum. RgpA (A) or Kgp (B) was preincubated for 15 min at 37°C with 5 mM l-cysteine. Preactivated RgpA or Kgp was incubated with IL-12 at a final S/E ratio of 100:1 in the absence of serum (35 nM IL-12 with 0.35 nM RgpA or Kgp in each reaction). Both reaction mixtures (A and B) were then incubated at 37°C for a time course study. Reactions were stopped at the indicated time with TLCK (2 mM final concentration). Control samples incubated without RgpA or Kgp are labeled IL-12. Aliquots were resolved by SDS–12% PAGE for Western blot analysis with polyclonal antibodies against IL-12 as described in Materials and Methods. Control samples incubated without gingipains are labeled IL-12. Data are representative of three separate experiments.
FIG. 5.
Cleavage of IL-12 by RgpA or Kgp in the presence of serum. Cysteine-activated (5 mM final concentration) RgpA (A) or Kgp (B) was mixed with whole bovine serum and combined with an equimolar ratio of IL-12 (35 nM gingipains with 35 nM IL-12 in each reaction) for a final serum concentration of 20%. Digestions were incubated at 37°C for various times then stopped in aliquots with TLCK (2 mM final concentration). Unboiled aliquots (with nonreduced sample buffers added) were resolved by SDS–12% PAGE for Western blot analysis with polyclonal antibodies against IL-12 as described in Materials and Methods. (C) Cysteine-activated RgpA or Kgp was combined with whole bovine serum and combined with an equimolar ratio of IL-12 as in panel A or B, and digestion mixtures were incubated at 37°C for various times and then stopped in aliquots with TLCK (2 mM final concentration). The samples were then boiled under reducing conditions for 10 min, and aliquots were resolved by SDS–12%. PAGE for Western blot analysis with polyclonal antibodies against IL-12 as described in Materials and Methods. Control samples incubated without gingipains are labeled IL-12. Data are representative of three separate experiments.
Interactions between IL-12 and RgpA or Kgp in the presence of leupeptin: kinetic characteristics.
To evaluate the relative roles of RgpA and Kgp in IL-12 degradation, reactions with RgpA and Kgp were carried out in the presence of 5 mM cysteine and with or without 0.1 mM leupeptin, a known inhibitor of RgpA and not Kgp. Although the RgpA preparation contained a low level of Kgp (L. W. P. Yun, A. A. DeCarlo, C. Collyer, and N. Hunter, unpublished data), addition of leupeptin to the RgpA preparation significantly reduced IL-12 degradation, indicating that RgpA degrades IL-12 (Fig. 6). As for Kgp, almost complete cleavage of IL-12 occurred within 60 min in the presence of 0.1 nM leupeptin, showing that Kgp also degrades IL-12. IL-12 degradation was also almost completely blocked by the cysteine proteinase inhibitor TLCK (at 2 mM) for low levels of gingipain-R and gingipain-K (0.35 nM each) in the absence of serum, demonstrating that the cleavage of IL-12 was due to the enzymatic activity of gingipains.
FIG. 6.
Degradation of IL-12 by the gingipains is thiol mediated. RgpA or Kgp was preincubated with the thiol-protease inhibitors TLCK (2 mM final concentration) or leupeptin (0.1 mM final concentration) for 1 h at 37°C and then dialyzed against PBS. TLCK- or leupeptin (leup.)- treated RgpA or Kgp was then mixed with IL-12 at a final S/E ratio of 100:1 (35 nM IL-12 with 0.35 nM RgpA or Kgp in each reaction), and the samples were incubated for 10 min and 1 h in the absence of serum. Aliquots were resolved by SDS–12% PAGE for Western blot analysis with polyclonal antibodies against IL-12 as described in Materials and Methods. Control samples incubated without gingipains are labeled IL-12.
In the absence of serum, both RgpA and Kgp were capable of degrading IL-12 efficiently. The Km and Vmax values for Arg-gingipain and Lys-gingipain were determined by measuring the fraction degraded from the 70-kDa IL-12 substrate in Western blot analysis. The Km values for the conversion of the IL-12 70-kDa molecule were 240 nM for Arg-gingipain and 10 nM for Lys-gingipain. The Vmax values for Arg-gingipain and Lys-gingipain were 6 and 1 fM/s, respectively. (The reaction with Lys-gingipain was carried out in the presence of 0.1 mM leupeptin to compensate for the percentage of RgpA in the Kgp preparation.) Kgp exhibited a higher affinity for IL-12 as supported by a lower Km value, while RgpA degraded the IL-12 more efficiently, with a higher Vmax value.
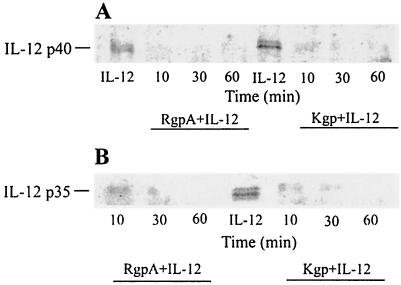
Loss of IL-12 COOH-terminal epitope by RgpA or Kgp.
We next investigated whether the gingipains might cleave near these Arg or Lys sites of the COOH-terminal regions of the p40 and p35 subunits. Western blot analysis using affinity-purified antibodies specific for the C terminus of p40 or p35 subunits was performed (Fig. 7). After 30 min of incubation in the absence of serum at 37°C, both RgpA and Kgp completely eliminated detection of the COOH-terminal regions of the p40 and p35 subunits (E/S ratio of 1:100).
FIG. 7.
Gingipain treatment induces loss of the COOH-terminal epitopes of the p40 and p35 subunits of IL-12. Cysteine-activated gingipains were incubated in serum-free medium at a final E/S ratio of 1:100 (0.35 nM gingipains with 35 nM IL-12 in each reaction) at 37°C for 1 h. Digestion mixtures were incubated at 37°C for various times and then stopped in aliquots with TLCK (2 mM final concentration). The samples were then boiled under reducing conditions for 10 min, and aliquots were resolved by SDS–12%. PAGE for Western blot analysis with polyclonal antibodies specific for the COOH terminus of the p40 (A) or p35 (B) subunit of IL-12. Data are representative of multiple experiments.
Western blot analysis of IL-2.
As IL-2 has been demonstrated to augment IL-12 in the activation of T cells, we also assessed whether the gingipains degraded IL-2. Supernatants of activated T-cell cocultures containing RgpA in the presence of IL-12 were examined by Western blotting. IL-2 produced in the cocultures appeared to be resistant to proteolytic degradation by various concentrations of RgpA in the presence of serum (data not shown).
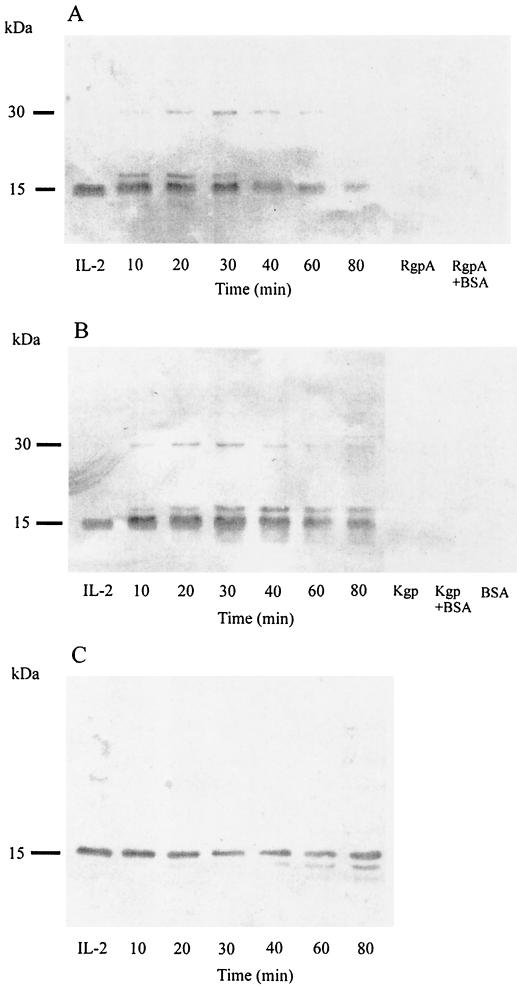
To evaluate whether the gingipains might modify the activity of IL-2 in its purified state, IL-2 was incubated with activated gingipains at an E/S ratio of 1:100 under serum-free conditions (Fig. 8). The polyclonal antibody preparation reactive with IL-2 did not recognize the carrier protein bovine serum albumin or the RgpA or Kgp preparations but detected human IL-2 as an ∼15-kDa band under nonreducing conditions (Fig. 8A and B). Under serum-free conditions, within 10 min of incubation with either RgpA or Kgp, additional bands at ∼17 and ∼30 kDa were evident (Fig. 8A and B). Following more prolonged incubation with RgpA, these bands faded coincident with reduction of the 15-kDa peptide (Fig. 8A). The higher-molecular-mass peptides were more persistent following extended incubation with Kgp with lower-molecular-mass fragments (<15 kDa) indicative of further cleavage of IL-2 (Fig. 8B). Incubation of IL-2 with Kgp for 40 to 60 min yielded a lower-mass immunoreactive fragment of ∼13 kDa in the presence of a reducing agent (Fig. 8C). The IL-2 degradation was specific to the gingipains and not to contaminant, since the cleavage was completely blocked by TLCK, an inhibitor specific for both RgpA and Kgp, after 60 min of incubation (data not shown). However, no hydrolysis of IL-2 was observed when RgpA or Kgp was mixed with an equal molar ratio of IL-2 in a final fetal bovine serum concentration of 20% and up to 80 min of incubation (data not shown).
FIG. 8.
Time course of IL-2 degradation by gingipains in the absence of serum. RgpA (A) or Kgp (B and C) was preincubated for 15 min at 37°C with 5 mM l-cysteine. The reaction with Kgp was carried out in the presence of 0.1 mM leupeptin to compensate for the percentage of RgpA in the Kgp preparation. In panels A and B, preactivated RgpA or Kgp was incubated with IL-2 at a final S/E ratio of 100:1 in the absence of serum (35 nM IL-2 with 0.35 nM RgpA or Kgp in each reaction). Both reactions were then incubated at 37°C for a time course study. Reactions were stopped at the indicated time with TLCK (2 mM final concentration). Control samples are labeled IL-2. Reactions were carried out with RgpA or Kgp alone, RgpA or Kgp with bovine serum albumin (BSA), or BSA alone (0.1% in PBS). Unboiled aliquots (with nonreduced sample buffers added) were resolved by SDS–14% PAGE for Western blot analysis with polyclonal antibodies against IL-2. (C) Cysteine-activated Kgp was combined with IL-2 at a final S/E ratio of 100:1 in the absence of serum (35 nM IL-2 with 0.35 nM Kgp in each reaction). Following timed incubation aliquots containing gingipains were inhibited with TLCK (2 mM final concentration). The samples were then boiled under reducing conditions for 10 min, and aliquots were resolved by SDS–14% PAGE for Western blot analysis with polyclonal antibodies against IL-2. Control samples incubated without gingipains are labeled IL-2. Data are representative of three separate experiments. combined with IL-2 at a final S/E ratio of 100:1 in the absence of serum (35 nM IL-2 with 0.35 nM Kgp in each reaction). Following timed incubation aliquots containing gingipains were inhibited with TLCK (2 mM final concentration). The samples were then boiled under reducing conditions for 10 min, and aliquots were resolved by SDS–14% PAGE for Western blot analysis with polyclonal antibodies against IL-2. Control samples incubated without gingipains are labeled IL-2. Data are representative of three separate experiments.
DISCUSSION
The mechanism for the preferential activation of either Th1 or Th2 pathways during progression of periodontitis is undetermined. Th0 cells express some differentiated effector functions that are characteristic of both the inflammatory and the helper T cells. Several lines of evidence have demonstrated that IL-4 stimulates differentiation into Th2 cells, whereas IFN-γ, IL-12, and TGF-β enhance Th1 development (39). The cytokines present during T-cell expansion influence the ability of Th cells to differentiate into Th1 cells, which produce IL-2, IFN-γ, and lymphotoxin and favor cell-mediated responses, or Th2 cells, which produce IL-4, IL-5, IL-6 and IL-10 and favor the induction of the humoral response. IL-12 is known as a potent inducer of IFN-γ, and interference with Th1 differentiation by hydrolysis and inactivation as demonstrated herein may have a significant role in the pathogenesis of periodontal disease. We will continue to investigate the interactions of the gingipains with other cytokines involved with T-cell differentiation.
Expression of IL-12R was up-regulated by activated or proteolytically inhibited gingipains (Fig. 4), possibly as a result of binding by the gingipains to the T-cell surface (data not shown). This effect was not due to potentially contaminating traces of LPS in these experiments, since LPS activity was sufficiently inhibited by the addition of polymyxin B. Purified LPS did not affect IL-12R levels (Yun et al., unpublished).
Further, these data suggest that the gingipains do not degrade IL-12R, although this was not measured directly. Up-regulated expression of the IL-12R in the presence of IL-12 was futile, however, since IL-12 was degraded and inactivated by gingipain proteolytic activity. Expression of high-affinity IL-12 receptors is required for IL-12-mediated IFN-γ production. Activation of this pathway has been shown to be critical in generating optimal cell-mediated immunity. Therefore, increased IL-12 receptor expression might be expected in the host response after infection by a bacterial pathogen. The results suggest that gingipains can enhance an optimal host immune response by inducing the expression and activity of high-affinity IL-12 receptors. Therefore, the net outcome with respect to Th1 activity would depend on the balance of the induction of IL-12 receptor expression versus inactivation of IL-12 by the gingipains within microenvironments. Also, the IL-12 produced by the small percentage of monocytic cells within the T-cell culture (<0.5%) in response to T-cell IFN-γ was significant enough to increase IFN-γ secretion through a feedback regulatory cycle (data not shown). In a complex environment such as the periodontium, hydrolysis and inactivation of IL-12 (this report) and of IFN-γ (61) by gingipain proteolytic activity could play a very significant role in minimizing Th1 T-cell switching and development.
Gingipain hydrolysis of IL-12 was demonstrated to occur in the presence of serum, which is significant considering that the periodontium and inflamed periodontal pocket contain serum components and protease inhibitors. Previous studies have reported decreased levels of IL-12 at sites of active periodontal disease (16). The catalytic efficiency of the gingipains has been demonstrated to be quite high (43, 45), suggesting that they are highly disruptive of many processes related to cellular homeostasis, immunity, and structural integrity (32).
Cleavage rates of IL-12 by RgpA and Kgp are similar and do not follow significantly different kinetics. Considering the high conservation of the noncatalytic regions of these enzymes, the detected differences are potentially attributable to structural heterogeneity within the catalytic domains.
Immunoblot analysis using polyclonal antibody to detect the RgpA or Kgp effect on IL-12 under reducing conditions demonstrated that the partially degraded products were derived from both the p40 and p35 subunit chains of IL-12. Antibodies specific for COOH termini of the p40 and p35 subunit chains failed to detect either of these two bands after prolonged incubation with the gingipains, indicating that the partially cleaved fragments from p40 and p35 chains lack the COOH-terminal epitopes. There are 21 Arg-X and 39 Lys-X bonds in the IL-12 amino acid sequence, which are possible sites of cleavage by RgpA and Kgp (57). Given the acidic nature of the gingipains, it is notable that human p40 possesses near its C terminus a cluster of six basic residues within a nine-amino-acid sequence (Lys258 to Arg266). This unusually dense basic sequence is a crucial candidate for a gingipain binding site.
IL-12 provides a link between natural resistance mediated by phagocytic cells or NK cells and adaptive immunity mediated by T-helper cells, cytolytic T cells, and B cells (56). The early production of IL-12 represents a key process in natural killer activation and innate resistance. NK cells can influence the pathway of Th1 or Th2 development when antigen-specific T cells commence clonal expansion and differentiation. NK cells may represent an early source of IFN-γ, which would contribute to the development of a Th1 response. Lower levels of IFN-γ and IL-2 have been shown to occur in periodontal disease lesions (21, 22). The pattern of IL-2 peptides detected after incubation with either RgpA or Kgp suggests the spontaneous formation of disulfide-linked products of hydrolysis, and in this context, there is an unpaired cysteine at position 124 of the processed peptide. This was confirmed by the treatment with the reducing agent. In the experimental system in the presence of fetal bovine serum, there was no evidence for hydrolysis of IL-2. The capacity of gingipains to cleave and inactivate IL-12 and IFN-γ (Yun et al., unpublished) may represent the disruption of the early inflammatory responses in the affected periodontal site and could contribute to the development of a Th2 response. This is consistent with the findings that the predominant infiltration of chronically inflamed tissues of adult periodontitis is generally characterized as a hyperresponsiveness of B-lineage cells (40, 48). The finding of higher proportions of T cells expressing mRNA for IL-2 and IFN-γ in a study of cells extracted from periodontitis sites is of interest in this context (51). However, in this case patients had been treated prior to surgery, with the implication that cells were extracted for analysis from healing sites rather than giving a representation of the natural history of the disease.
In addition to dysregulation of cytokine networks, P. gingivalis and its components, such as LPS, can induce the host to express a variety of cytokines. We have recently demonstrated that P. gingivalis LPS could upregulate IFN-γ and IL-12 through a synergistic feedback regulatory loop cycle that was not related to induction of IL-12 receptor expression (Yun et al., unpublished) and which may contribute to the increased inflammation at disease sites. In contrast, gingipains from P. gingivalis down-regulate these activities by hydrolysis. Apart from disruption of cytokine networks, P. gingivalis proteinases have also been reported to exhibit enzymatic activity against a broad range of host proteins, including host proteinase inhibitors (7, 44), immunoglobulin (2), matrix metalloproteinases (11), and proteins involved in the complement (28), coagulation (47), and kallikrein/kinin cascades (25–27). Hence, combined action of the gingipains and LPS which are colocated in outer membrane vesicles released from this pathogenic organism would provide a mechanism for inappropriate stimulation of CD4+ T cells related to the development of destructive periodontitis.
ACKNOWLEDGMENT
This study was supported by a grant from the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Banbula A, Bugno M, Kuster A, Heinrich P C, Travis J, Potempa J. Rapid and efficient inactivation of IL-6 gingipains, lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Biochem Biophys Res Commun. 1999;261:598–602. doi: 10.1006/bbrc.1999.1075. [DOI] [PubMed] [Google Scholar]
- 2.Bedi G S, William T. Purification and characterization of a collagen-degrading protease from Porphyromonas gingivalis. J Biol Chem. 1994;269:599–606. [PubMed] [Google Scholar]
- 3.Bernzweig E, Payne J B, Reinhardt R A, Dyer J K, Patil K D. Nicotine and smokeless tobacco effects on gingival and peripheral blood mononuclear cells. J Clin Periodontol. 1998;25:246–252. doi: 10.1111/j.1600-051x.1998.tb02435.x. [DOI] [PubMed] [Google Scholar]
- 4.Boehm U, Howard J C. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 5.Briscoe D M, Henault L E, Geehan C, Alexander S I, Lichtman A H. Human endothelial cell costimulation of T cell IFN-γ production. J Immunol. 1997;159:3247–3256. [PubMed] [Google Scholar]
- 6.Calkins C C, Platt K, Potempa J, Trávis J. Inactivation of tumor necrosis factor-α by proteinases (gingipains) from the periodontal pathogen, Porphyromonas gingivalis. J Biol Chem. 1998;273:6611–6614. doi: 10.1074/jbc.273.12.6611. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson J, Herrmann B F, Höfling J F, Sundqvist G K. Degradation of human proteinase inhibitors alpha-1-antitrypsin and alpha-2-macroglobulin by Bacteroides gingivalis. Infect Immun. 1984;43:644–648. doi: 10.1128/iai.43.2.644-648.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chua A O, Chizzonite R, Desai B B, Truitt T P, Nunes P, Minetti L J, Warrier R R, Presky D H, Levine J F, Gately M K, Gubler U. Expression cloning of a human IL-12 receptor component: a new member of cytokine receptor superfamily with strong homology to gp130. J Immunol. 1994;153:128–136. [PubMed] [Google Scholar]
- 9.Ciborowski P, Nishikata M, Allen R D, Lantz M S. Purification characterization of two forms of a high-molecular-weight cysteine proteinase (porphypain) from Porphyromonas gingivalis. J Bacteriol. 1994;176:4549–4559. doi: 10.1128/jb.176.15.4549-4557.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croft M, Dubey C. Accessory molecule and costimulation requirements for CD4 T cell response. Crit Rev Immunol. 1997;17:89–118. doi: 10.1615/critrevimmunol.v17.i1.40. [DOI] [PubMed] [Google Scholar]
- 11.DeCarlo A A, Windsor L J, Bodden M K, Harber G J, Birkedal-Hansen B, Birkedal-Hansen H. Activation and novel processing of matrix metalloproteinases by a thiol-proteinase from the oral anaerobe Porphyromonas gingivalis. J Dent Res. 1997;76:1260–1270. doi: 10.1177/00220345970760060501. [DOI] [PubMed] [Google Scholar]
- 12.DeCarlo A A, Paramaesvaran M, Yun L W P, Collyer C, Hunter N. Porphyrin-mediated binding to hemoglobin by the HA2 domain of cysteine proteinases (gingipains) and hemagglutinins from the periodontal pathogen Porphyromonas gingivalis. J Bacteriol. 1999;181:3784–3791. doi: 10.1128/jb.181.12.3784-3791.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 14.Dwyer D S. Molecular model of interleukin 12 that highlights amino acid sequence homologies with adhesion domains and gastrointestinal peptides. J Mol Graph. 1996;14:148–157. doi: 10.1016/s0263-7855(96)00044-6. [DOI] [PubMed] [Google Scholar]
- 15.Ebersole J L, Taubman M A. The protective nature of host responses in periodontal diseases. Periodontol 2000. 1994;5:112–141. doi: 10.1111/j.1600-0757.1994.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 16.Ellis E D, Tucci M A, Serio F G, Johnson R B. Factors for progression of periodontal diseases. J Oral Pathol Med. 1998;27:101–105. doi: 10.1111/j.1600-0714.1998.tb01923.x. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher H M, Schenkein H A, Macrina F L. Cloning and characterization of a new protease gene (prtH) from Porphyromonas gingivalis. Infect Immun. 1994;62:4279–4286. doi: 10.1128/iai.62.10.4279-4286.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fletcher J, Reddi K, Poole S, Nair S, Henderson B, Tabona P, Wilson M. Interactions between periodontopathogenic bacteria and cytokines. J Periodontal Res. 1997;32:200–205. doi: 10.1111/j.1600-0765.1997.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 19.Gately M K, Renzetti L M, Magram J, Stern A S, Adorini L, Gubler U, Presky D H. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 20.Gearing D P, Cosman D. Homology of the p40 subunit of natural killer cell stimulatory factor (NKSF) with the extracellular domain of the interleukin-6 receptor. Cell. 1991;66:9–10. doi: 10.1016/0092-8674(91)90131-h. [DOI] [PubMed] [Google Scholar]
- 21.Gemmell E, Seymour G J. Cytokine profiles of cells extracted from humans with periodontal diseases. J Dent Res. 1998;77:16–26. doi: 10.1177/00220345980770010101. [DOI] [PubMed] [Google Scholar]
- 22.Gemmell E, Seymour G J. Modulation of immune responses to periodontal bacteria. Curr Opin Periodontol. 1994;2:28–38. [PubMed] [Google Scholar]
- 23.Genco C A, Potempa J, Mikolajczyk-Pawlinska J, Travis J. Role of gingipains R in the pathogenesis of Porphyromonas gingivalis-mediated periodontal disease. Clin Infect Dis. 1999;28:456–656. doi: 10.1086/515156. [DOI] [PubMed] [Google Scholar]
- 24.Gubler U, Chua A O, Schoenhaut D S, Dwyer C M, McComas W, Motyka R, Nabavi N, Wolitzky A G, Quinn P M, Familetti P C, Gately M K. Co-expression of two distinct genes is required to generate secreted, bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA. 1991;88:4143–4147. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imamura T, Pike R N, Potempa J, Travis J. Pathogenesis of periodontitis: a major arginine-specific cysteine proteinase from Porphyromonas gingivalis induces vascular permeability enhancement through activation of the kallikrein/kinin pathway. J Clin Investig. 1994;94:361–367. doi: 10.1172/JCI117330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imamura T, Potempa J, Pike R N, Travis J. Dependence of vascular permeability enhancement on cysteine proteinases in vesicles of Porphyromonas gingivalis. Infect Immun. 1995;63:1999–2003. doi: 10.1128/iai.63.5.1999-2003.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imamura T, Potempa J, Tanase S, Travis J. Activation of blood coagulation factor X by arginine-specific cysteine proteinases (gingipain-Rs) from Porphyromonas gingivalis. J Biol Chem. 1997;272:16062–16067. doi: 10.1074/jbc.272.25.16062. [DOI] [PubMed] [Google Scholar]
- 28.Jagles M A, Travis J, Potempa J, Pike R, Hugli T E. Proteolytic inactivation of the leukocyte C5a receptor by proteinases derived from Porphyromonas gingivalis. Infect Immun. 1996;64:1984–1991. doi: 10.1128/iai.64.6.1984-1991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawano Y, Noma T, Yata J. Regulation of human IgG subclass production by cytokines: IFN-gamma and IL-6 act antagonistically in the induction of human IgG1 but additively in the induction of IgG2. J Immunol. 1994;153:4948–4958. [PubMed] [Google Scholar]
- 30.Kobayashi M, Fitz L, Ryan M, Hewick R M, Clark S C, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubin M, Chow J M, Trinchieri G. Differential regulation of interleukin-12 (IL-12), tumor necrosis factor-alpha, and IL-1-beta production in human myeloid leukemia cell lines and peripheral blood mononuclear cells. Blood. 1994;83:1847–1855. [PubMed] [Google Scholar]
- 32.Kuramitsu H K. Proteases of Porphyromonas gingivalis: what don't they do? Oral Microbiol Immunol. 1998;13:263–270. doi: 10.1111/j.1399-302x.1998.tb00706.x. [DOI] [PubMed] [Google Scholar]
- 33.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Lamont A G, Adorini L. IL-12: a key cytokine in immune regulation. Immunol Today. 1996;17:214–217. doi: 10.1016/0167-5699(96)30011-x. [DOI] [PubMed] [Google Scholar]
- 35.Ma W, Pober J S. Human endothelial cells effectively costimulate cytokine production by, but not differentiation of, naive CD4+ T cells. J Immunol. 1998;161:2158–2167. [PubMed] [Google Scholar]
- 36.Magram J, Connaughton S E, Warrier R R, Carvajal D M, Wu C Y, Ferrante J, Stewart C, Sarmiento U, Faherty D A, Gately M K. IL-12 deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 37.Mikolajczyk-Pawlinska J, Travis J, Potempa J. Modulation of interleukin-8 activity by gingipains from Porphyromonas gingivalis: implications for pathogenicity of periodontal disease. FEBS Lett. 1998;440:282–286. doi: 10.1016/s0014-5793(98)01461-6. [DOI] [PubMed] [Google Scholar]
- 38.Morrison D C. Bacterial endotoxins and pathogenesis. Rev Infect Dis. 1983;5:S733–S747. doi: 10.1093/clinids/5.supplement_4.s733. [DOI] [PubMed] [Google Scholar]
- 39.Mosmann T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 40.Okada H, Kida T, Yamagami H. Identification and distribution of immunocompetent cells in inflamed gingiva of human chronic periodontitis. Infect Immun. 1983;41:365–374. doi: 10.1128/iai.41.1.365-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavloff N, Pemberton P A, Potempa J, Chen W C A, Pike N R, Prochazka V, Kiefer M C, Travis J, Barr P. Molecular cloning and characterization of Porphyromonas gingivalis Lys-gingipain. A new member of an emerging family of pathogenic bacterial cysteine proteinases. J Biol Chem. 1997;272:1595–1600. doi: 10.1074/jbc.272.3.1595. [DOI] [PubMed] [Google Scholar]
- 42.Pavloff N, Potempa J, Pike R N, Prochazka V, Kiefer M C, Travis J, Barr P J. Molecular cloning and structural characterization of the Arg-gingipain proteinase of Porphyromonas gingivalis: biosynthesis as a proteinase-adhesin polyprotein. J Biol Chem. 1995;270:1007–1010. doi: 10.1074/jbc.270.3.1007. [DOI] [PubMed] [Google Scholar]
- 43.Pike R, McGraw W, Potempa J, Travis J. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis: isolation and evidence for the existence of complexes with hemagglutinins. J Biol Chem. 1994;269:406–411. [PubMed] [Google Scholar]
- 44.Pizzo S V, Grøn H, Pike R, Potempa J, Travis J, Thøgersen I B, Enghild J J. The potential role of α2-macroglobulin in the control of cysteine proteinases (gingipains) from Porphyromonas gingivalis. J Periodontal Res. 1997;32:61–68. doi: 10.1111/j.1600-0765.1997.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 45.Potempa J, Mikolajczyk-Pawlinska J, Brassell D, Nelson D, Thøgersen I B, Enghild J J, Travis J. Comparative properties of two cysteine proteinases (gingipains R), the products of two related but individual genes of Porphyromonas gingivalis. J Biol Chem. 1998;273:21648–21659. doi: 10.1074/jbc.273.34.21648. [DOI] [PubMed] [Google Scholar]
- 46.Presky D H, Yang H, Minetti L J, Chua A O, Nabavi N, Wu C Y, Gately M K, Gubler U. A functional interleukin 12 receptor complex is composed of two β type cytokine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott C F, Whitlaker E J, Hammond B F, Colman R W. Purification and characterization of a potent 70-kDa thiol lysyl-proteinase (Lys-gingivain) from Porphyromonas gingivalis that cleaves kininogens and fibrinogen. J Biol Chem. 1993;268:7935–7942. [PubMed] [Google Scholar]
- 48.Seymour G J, Crouch M S, Powell R N, Brooks D, Beckman I, Zola H. The identification of lymphoid cell subpopulations in sections of human lymphoid tissue and gingivitis in children using monoclonal antibodies. J Periodontal Res. 1982;17:247–256. doi: 10.1111/j.1600-0765.1982.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 49.Shimabukuro Y, Murakami S, Okada H. Antigen-presenting-cell function of interferon gamma-treated human gingival fibroblasts. J Periodontal Res. 1996;31:217–228. doi: 10.1111/j.1600-0765.1996.tb00486.x. [DOI] [PubMed] [Google Scholar]
- 50.Stern A S, Gubler U, Presky D H, Magram J. Structural and functional aspects of the IL-12 receptor complex. Chem Immunol. 1997;68:23–37. doi: 10.1159/000058692. [DOI] [PubMed] [Google Scholar]
- 51.Takeichi O, Haber J, Kawai T, Smith D J, Moro I, Taubman M A. Cytokine profiles of T-lymphocytes from gingival tissues with pathological pocketing. J Dent Res. 2000;79:1548–1555. doi: 10.1177/00220345000790080401. [DOI] [PubMed] [Google Scholar]
- 52.Taubman M A, Eastcott J W, Shimauchi H, Takeichi O, Smith D J. Modulatory role of T lymphocytes in periodontal inflammation. In: Genco R, et al., editors. Molecular pathogenesis of periodontal diseases. Washington, D.C.: ASM Press; 1994. pp. 147–159. [Google Scholar]
- 53.Thornton S C, Mueller S N, Levine E M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983;222:623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- 54.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci: USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-γ) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 56.Trinchieri G, Scott P. Interleukin-12: basic principles and clinical applications. Curr Top Microbiol Immunol. 1999;238:59–78. doi: 10.1007/978-3-662-09709-0_4. [DOI] [PubMed] [Google Scholar]
- 57.Wolf S F, Temple P A, Kobayashi M, Young D, Dicig M, Lowe L, Dziabo R, Fitz L, Ferenz C, Hewick R M, Kelleher K, Chan S H, Trinchieri G, Perussia B. Cloning of cDNA for natural killer cell stimulation factor, a heterodimeric cytokine with multiple biological effects on T and natural killer cells. J Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- 58.Wu C Y, Warrier R R, Carvajal D M, Chua A O, Minetti L J, Chizzonite R, Mongini P K A, Stern A S, Gubler U, Presky D H, Gately M K. Biological function and distribution of human interleukin-12 receptor β chain. Eur J Immunol. 1996;26:345–350. doi: 10.1002/eji.1830260212. [DOI] [PubMed] [Google Scholar]
- 59.Yamashita K, Eastcott J W, Taubman M A, Smith D J, Cox D S. Effect of adoptive transfer of cloned Actinobacillus actinomycetemcomitans-specific T helper cells on periodontal disease. Infect Immun. 1991;59:1529–1534. doi: 10.1128/iai.59.4.1529-1534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshimura A, Hara Y, Kaneko T, Kato I. Secretion of IL-1-beta, TNF-alpha, IL-8 and IL-1ra by human polymorphonuclear leukocytes in response to lipopolysaccharides from periodontopathic bacteria. J Periodontal Res. 1997;32:279–286. doi: 10.1111/j.1600-0765.1997.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 61.Yun L W P, DeCarlo A A, Hunter N. Modulation of major histocompatibility complex protein expression by human gamma interferon mediated by cysteine proteinase-adhesin polyproteins of Porphyromonas gingivalis. Infect Immun. 1999;67:2986–2995. doi: 10.1128/iai.67.6.2986-2995.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]