Abstract
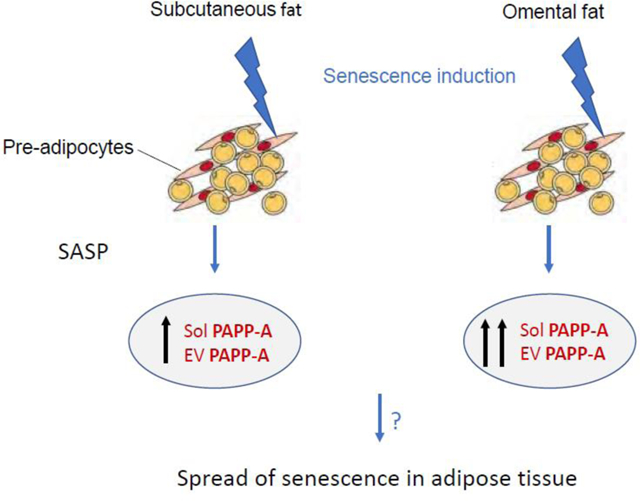
Senescence is a cellular response to various stressors characterized by irreversible cell cycle arrest, resistance to apoptosis and expression of a senescence-associated secretory phenotype (SASP). Interestingly, studies where senescent cells were deleted in mice produced beneficial effects similar to those where the zinc metalloproteinase, PAPP-A, was deleted in mice. In this study, we investigated the effect of senescence on PAPP-A secretion and activity in primary cultures of adult human pre-adipocytes. Cultured pre-adipocytes were isolated from subcutaneous (Sub) and omental (Om) fat. Senescence was induced with low dose etoposide. PAPP-A protein was measured by an ultrasensitive PAPP-A ELISA. PAPP-A proteolytic activity was measured by a specific substrate cleavage assay. Senescence significantly increased PAPP-A levels in both Sub and Om conditioned medium (CM) 8- to 15-fold over non-senescent CM. Proteolytic activity reflected PAPP-A protein with 12- to 18-fold greater activity in senescent CM versus non-senescent CM. Furthermore, PAPP-A was found at high levels on the surface of extracellular vesicles secreted by senescent pre-adipocytes and was proteolytically active. In conclusion, we identified enzymatically active PAPP-A as a component of human pre-adipocyte SASP. This recognition warrants further investigation of PAPP-A as a new biomarker for senescence and a potential therapeutic target to control the of spread of senescence in adipose tissue.
Keywords: senescence, PAPP-A, protease, human pre-adipocytes, extracellular vesicles
Graphical Abstract
INTRODUCTION
Senescence is a complex response of a cell to various stressors that is characterized by irreversible cell cycle arrest, resistance to apoptosis, and expression of a senescence-associated secretory phenotype (SASP) comprised of cytokines, chemokines, proteases, inhibitors, and growth factors1–3. Identification and understanding of the different factors present in various SASPs and their function is incomplete. SASP can vary depending on the inducer of senescence, duration in senescence, and cell type. Importantly, the effects of SASP can spread to nearby non-senescent cells4. This is a paracrine mechanism by which senescent cells can impact tissue and organ function despite initial small numbers. Interestingly, the secretion of extracellular vesicles/exosomes by senescent cells has been reported recently and may be involved in far-reaching communication5, 6. These senescent-associated extracellular vesicles (EVs) appear to be novel SASP factors7.
Cellular senescence can be beneficial in preventing proliferation of pre-neoplastic cells, regulating tissue remodeling during embryogenesis, and promoting optimal wound healing. However, accumulation of senescent cells with age appears to have adverse consequences on tissue and organ function3. Indeed, SASP has been postulated to make a significant contribution to age-related inflammation and associated pathologies8, 9. Genetic and pharmacological approaches have been used to selectively target and clear senescent cells in various mouse models of aging and age-related disease10. Removal of senescent cells resulted in decreased atherosclerotic plaque progression11, prevention of pulmonary fibrosis12, alleviation of obesity-related metabolic dysfunction13, 14, reduced liver lipid13, 14, delay in thymic involution15, 16, decreased indices of frailty17, and enhanced insulin sensitivity13. Baker et al.18 demonstrated that late-life clearance of senescent cells in wild-type mice could attenuate progress of several established age-related disorders and extend median lifespan. Several papers have identified senescent cells associated with aging and age-related pathologies in human tissues, as well19. Although senolytic drugs that target cell death-resistant pathways show great promise20–22, a better understanding of cell senescence, and especially SASP, will be critical for guiding successful clinical trials.
Increased insulin-like growth factor (IGF) signaling has been associated with aging and many age-related diseases. Indeed, reduction of IGF-I signaling has been shown to prolong lifespan in diverse species23, 24. However, very little is known about the IGF system in senescence. It has been shown that acute treatment of cells with IGF-I stimulates proliferation, while prolonged treatment of cells with IGF-I promotes senescence in a variety of cell types25–27.
It seemed more than coincidental that studies where senescent cells were deleted in mice produced a beneficial phenotype similar to those where PAPP-A was deleted in mice (Table 1). PAPP-A is a novel zinc metalloprotease that can increase pericellular IGF bioavailability through cleavage of inhibitory IGFBPs, in particular IGFBP-428. PAPP-A is a secreted protein that tethers to the surface of cells through heparan sulfate-like proteoglycan moieties in an autocrine/paracrine fashion. IGF bound to IGFBP-4 is unable to activate receptors. However, upon cleavage of IGFBP-4 by PAPP-A, IGF is liberated from the complex in the pericellular environment and IGF signaling is initiated. PAPP-A-induced enhancement of local IGF action through proteolysis of IGFBP-4 has been demonstrated in several in vitro systems and appears to serve a similar function in vivo28. Conversely, inhibition of PAPP-A expression or its proteolytic activity represents an innovative approach to decrease IGF availability with resultant restraint of IGF-I receptor signaling. Thus, inhibition of PAPP-A through gene deletion in mice had many beneficial effects, including a remarkable extension of lifespan by ~30%29. A significant increase in lifespan was also seen with PAPP-A knock-out (KO) mice on a high fat diet29, and when PAPP-A gene expression was knocked-down in adult mice30. PAPP-A KO mice, like senescent cell-targeted mice, showed resistance to atherosclerotic plaque progression31, 322, resistance to visceral obesity with high fat diet33, 34, reduced liver lipid33, 34, delay of thymic involution with persistence of a youthful T-cell phenotype in aged mice35, prevention of sarcopenia with age36, and enhanced insulin sensitivity37. We can also inhibit the ability of PAPP-A to cleave IGFBP-4 with a novel neutralizing monoclonal antibody generated against a unique exosite in PAPP-A (mAb-PA1/41)38, 39. Weekly treatment of apolipoprotein E KO mice (wild-type alleles for PAPP-A) with this monoclonal antibody reduced atherosclerotic plaque burden by 70–80%31. Visceral obesity in mice on high fat diet was also prevented with genetic or pharmacological inhibition of PAPP-A34.
Table 1.
PAPP-A and Cell Senescence
| Effects on aging and age-related disorders | Delete Senescent Cells | Delete PAPP-A |
|---|---|---|
| Increase lifespan | Yes10,18 | Yes28–30 |
| Impede atherosclerosis | Yes11 | Yes31,32 |
| Prevent visceral obesity | Yes13,14 | Yes33,34 |
| Reduced liver lipid | Yes13,14 | Yes33,34 |
| Delay Thymic involution | Yes15,16 | Yes35 |
| Prevent sarcopenia | Yes17 | Yes36 |
| Enhance insulin sensitivity | Yes13 | Yes37 |
For this study, we posed specific questions about PAPP-A and senescence using primary cultures of adult human pre-adipocytes. Pre-adipocytes are also referred to as adipocyte-derived stem cells or fat progenitor cells. Adipose tissue is the largest source of progenitor cells2, 10, and human adipose tissue exhibits a very high accumulation of senescent cells with age19.
MATERIALS and METHODS
Materials.
Cell culture components were from Gibco Life Technologies. Bovine serum albumin (BSA) was from MilliporeSigma. Recombinant human IGF-II was from R&D Systems and IGFBP-4 was a gift from our colleague, Professor Claus Oxvig (Aarhus University, Denmark). Anti-IGFBP-4 antibodies were purchased from Abcam: N-terminal ab92625 and C-terminal ab77350. Secondary antibodies were from LiCor, Inc: Donkey anti-goat (926–68074) and donkey anti-rabbit (926–32213). Etoposide was obtained from Enzo Life Sciences. picoPAPP-A ELISA kits and monoclonal antibody generated against a unique exosite in PAPP-A (mAb-PA1/41)38, 39 were obtained from Ansh Labs (Webster, TX).
Cells.
Primary human pre-adipocytes isolated from subcutaneous (Sub) and omental (Om) fat from the same subject were plated and cultured, as previously described40. These are essentially pure populations of preadipocytes41. All experiments used isolations from three different donors; age and sex are presented in Supplemental Table 1. Cells were grown in αMEM supplemented with penicillin (100 IU/ml), streptomycin (100 μg/ml), and 10% heat-inactivated fetal bovine serum. After washing in phosphate-buffered saline (PBS), experiments were performed in serum-free DMEM containing 0.1% BSA. Cells were used at passages 4–9.
Low dose etoposide was used to induce DNA damage as a genotoxic stress leading to cell senescence, as previously described42. For induction of senescence, human pre-adipocytes at 60% confluency were treated for 48 h with 25 μM etoposide in growth media. For non-senescent cells, vehicle (0.025% DMSO) was added to the growth media. Etoposide and DMSO were removed and replaced with fresh media every three days. Senescent and non-senescent cells were changed to serum-free media with experimental additions for 72 h and conditioned medium (CM) was collected, aliquoted and stored frozen at −80°C.
Senescence-associated β-galactosidase staining and cell number.
SA-β-gal staining was performed as previously described42. DAPI (Thermo Scientific 62248) was used at a 1:1000 dilution for cell number. Cytation 5 Imaging Reader (Bio Tek) was used for imaging and quantification of SA-β-gal and DAPI.
RT-qPCR.
Total RNA was isolated from cultured cells by washing twice with cold phosphate buffered saline (PBS), lysing with 1 ml of Trizol (Ambion Life Technologies, Carlsbad, CA) and further processed as per manufacturer’s instruction. RNA (1 μg) was reversed transcribed with the SuperScript III Frist-Strand Synthesis System (Life Technologies) and evaluated by quantitative real-time PCR using the CFX Connect Real-Time System with iTAQ Universal SYBR Green Supermix (Bio-Rad, Hercules, CA). Amplification plots were analyzed using CFX Maestro Software version 4.1 (Bio-Rad). [p21 primers for RT-qPCR were forward: TGTCCGTCAGAACCCATGC; reverse: AAAGTCGAAGTTCCATCGCTC]. To create standard curves, designed primers were PCR’d with cDNA from known cell culture samples. Amplified PCR products were purified through QIAquick Gel Extraction Kit (Qiagen Hilden, Germany), quantified and serial diluted from 108 to 103 molecules. Relative quantification and fold changes were based on the standard curve for each gene.
PAPP-A protein and enzymatic activity.
Ultrasensitive ELISA kits that measure human PAPP-A were used according to manufacturer’s instructions to determine levels of PAPP-A in CM. For proteolysis assays, human IGFBP-4 (4 nM) pre-complexed to IGF-II (2 nM) was incubated in cell-free CM without and with inhibitory PAPP-A monoclonal antibody (mAb-PA1/41, 10−7 M) for 2 hours at 37°C, as previously described43. IGF must be bound to IGFBP-4 for IGFBP-4 to be a substrate for PAPP-A proteolysis, and IGF-II is more effective than IGF-I in this cell-free assay44–46. mAb-PA1/41 specifically inhibits PAPP-A-mediated IGFBP-4 proteolysis38, 39. CM was then incubated for the indicated times at 37°C. Western blotting was performed as previously described43. Briefly, samples were separated by 12% SDS-PAGE under reducing conditions, blotted onto a PVDF membrane, blocked, and probed for IGFBP-4 fragments using specific N- and C-terminal antibodies. Fluorescently-labeled secondary antibodies were used for detection of intact and cleaved IGFBP-4 by LI-COR Odyssey imager and Image Studio software.
Extracellular vesicle isolation.
The isolation procedure for EVs was taken from Barile et al. using serial ultracentrifugation47. Freshly collected CM from T75 flasks was centrifuged 3,000 × g for 15 minutes at 4°C to remove any cells. The supernatant was moved to a new tube and centrifuged at 10,000 × g for 15 minutes at 4°C to remove cellular debris. This supernatant was collected into a 10 ml conical bottom ultracentrifuge tube (Beckman Coulter) and centrifuged 100,000 × g for 90 minutes in an L-70 ultracentrifuge (Beckman) using an SW 41 swinging bucket. The supernatant was discarded and the EV-enriched pellet reconstituted in PBS or solubilized in RIPA buffer (0.15 M NaCl, 0.5% NP-40, 0.1% SDS, 50 mM Tris). Total protein concentrations were determined by using Pierce BCA Protein Assay Kit (ThermoFisher) according to manufacturer’s instructions.
Adipokines.
Pre-adipocyte CM were analyzed using a Luminex Discovery Panel from R&D Systems by the Immunochemical Core Laboratory of Mayo Clinic.
Statistical analyses.
Comparisons of Sub and Om pre-adipocytes from each patient were analyzed by paired t-tests. Comparisons of non-senescent vs. senescent cells were analyzed by non-paired t-test. Significance was set at P < 0.05
RESULTS
Are subcutaneous- and visceral fat-derived pre-adipocytes differentially responsive to etoposide-induced senescence?
To address this question, we assessed etoposide-induced senescence by observing characteristic physical changes in the cells, i.e., enlarged and flatten cell morphology, and SA-β-gal staining of (Sub) and omental (Om) pre-adipocyte pairs. There was a marked 50–65% decrease in cell number in etoposide-treated compared to non-treated cells, with apparent cessation of proliferation (Fig.1A), at which times 90–100% of cells were positive for SA-β-gal staining. Representative SA-β-gal staining is shown in Figure 1B.
Figure 1.
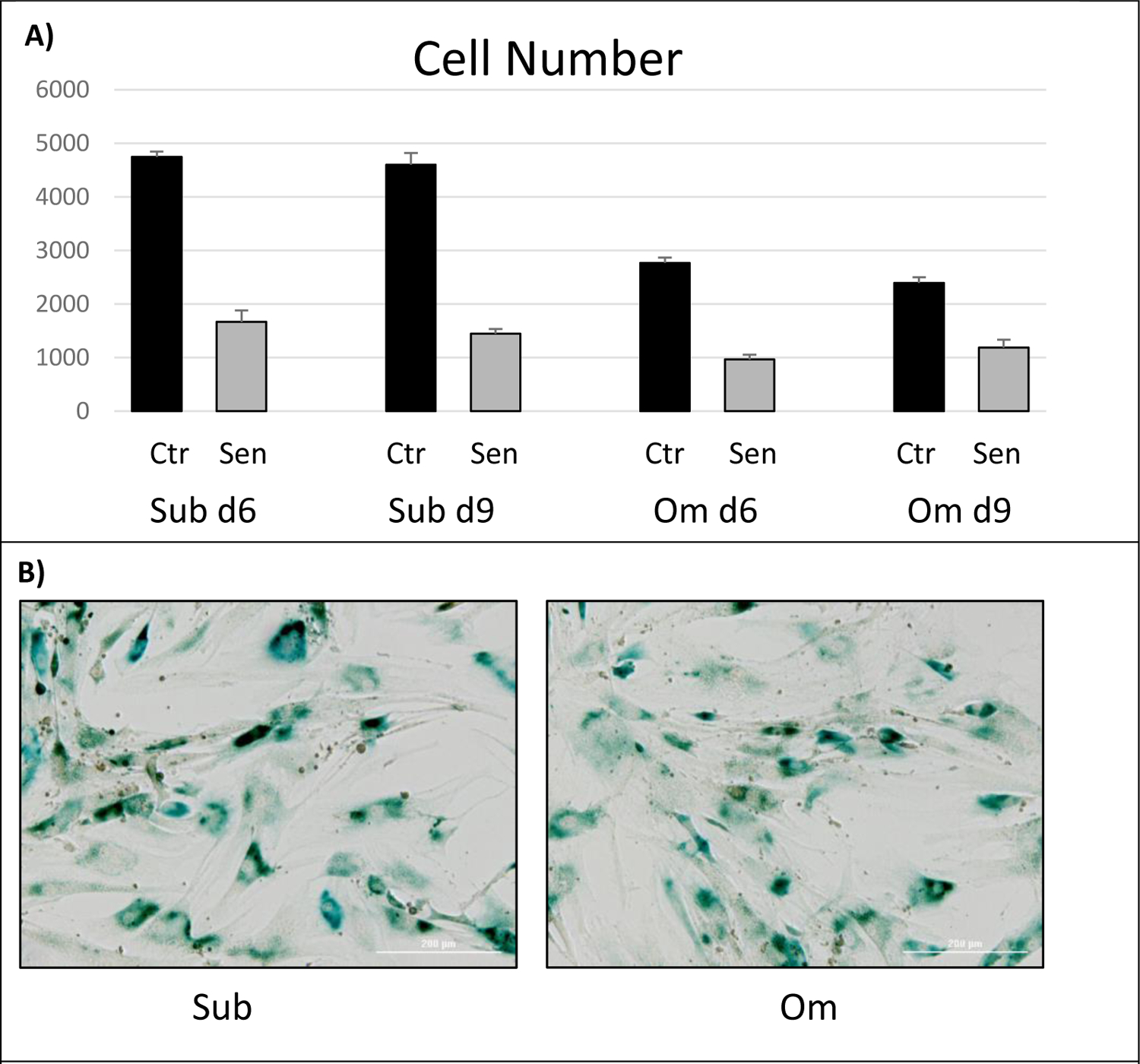
Effect of etoposide-induced cell senescence (Sen) on (A) cell number and (B) senescence-associated B-galactosidase staining in Subcutaneous (Sub) and Omental (Om) pre-adipocytes
The p21 gene is a marker of cell cycle arrest and its upregulation has been shown to maintain viability of DNA damage-induced senescent cells48. There was a significant elevation (~2-fold) of p21 expression in both Sub and Om pre-adipocytes in response to etoposide-induced senescence (Table 2).
Table 2.
p21 mRNA Expression (x104)
| Non-Senescent | Senescent | P-value | |
|---|---|---|---|
| Sub | 3.5 ± 0.14 | 5.7 ± 0.21 | 0.001 |
| Om | 4.1 ± 0.21 | 5.6 ± 0.23 | 0.008 |
Expression of p21, a marker of cell cycle inhibition, was measured by RT-qPCR.
Results are mean ± SEM, n = 3 subjects.
Levels of another gene marker of cell cycle arrest, p16, did not change in these cells with induction of senescence (data not shown). p21 and p16 mediate different pathways and at different phases of senescence and are not always up-regulated in parallel49, 50. Thus, morphological changes, increased SA-β-gal staining, decreased cell proliferation, up-regulation of p21, and induction of common SASP factors, IL-6 and MCP-1 (see Table 6), are consistent with a senescent phenotype in both Sub and Om pre-adipocytes.
Table 6.
Cytokine/SASP factor expression in CM from Non-Senescent and Senescent pre-adipocytes Seventy-two hour CM from pre-adipocyte isolates from subcutaneous and omental fat depots. Results are mean ± SEM of three subjects and expressed as pg/104 cells. All samples (in duplicate) were run together in the same assay. IL (Interleukin)-6, IL-8, MCP (Monocyte Chemoattractant Protein)-1, HGF (Hepatocyte Growth Factor), PAI-1 (Plasminogen Activator Inhibitor)-1 IL-1β and TNF (Tumor Necrosis Factor)-α were below the limits of quantitation in the Luminex Discovery Panel.
| pg/104 Cells | |||
|---|---|---|---|
| Non-Senescent | Senescent | P-value | |
| IL-6 | |||
| Sub | 235 ± 97 | 1,695 ± 136 | 0.0009 |
| Om | 589 ± 304 | 1,972 ± 152 | 0.0152 |
| IL-8 | |||
| Sub | 115 ± 103 | 117 ± 19 | 0.986 |
| Om | 109 ± 93 | 176 ± 48 | 0.557 |
| MCP-1 | |||
| Sub | 75 ± 56 | 5,647 ± 229 | <0.0001 |
| Om | 143 ± 83 | 2,507 ± 581 | 0.0158 |
| HGF | |||
| Sub | 49 ± 44 | 60 ± 35 | 0.854 |
| Om | 15 ± 6 | 104 ± 73 | 0.291 |
| PAI-1 | |||
| Sub | 17,430 ± 7154 | 163,486 ± 111,421 | 0.261 |
| Om | 33,200 ± 10,813 | 46,020 ± 23,718 | 0.649 |
Does senescence affect PAPP-A expression and proteolytic activity?
Senescent and non-senescent Sub and Om cells were washed and changed to serum-free medium for 72 hr. Results (expressed as ng PAPP-A protein/104 cells) are presented in Table 3.
Table 3.
PAPP-A Expression in CM from Non-Senescent and Senescent pre-adipocytes.
| PAPP-A (ng/104 Cells) | ||
|---|---|---|
| Sub | Om | |
| Non-Senescent | ||
| Subject A | 0.7 | 2.0 |
| Subject B | 1.1 | 6.2 |
| Subject C | 0.7 | 2.1 |
| Senescent | ||
| Subject A | 8.8 | 19.4 |
| Subject B | 16.2 | 55.1 |
| Subject C | 5.9 | 23.8 |
Human PAPP-A protein levels in CM were measured by picoPAPP-A ELISA and expressed as ng/104 cells. All samples (in duplicate) were run together in the same assay.
In non-senescent cells, PAPP-A levels in Om CM were 3- to-6 fold higher than in Sub CM. This is similar to what we have previously reported40, but did not reach statistical significance in this study. Senescence increased PAPP-A levels 8- to 15-fold over non-senescent cells in both Sub and Om CM, with absolute levels of PAPP-A being 2- to 4-fold higher in Om CM than in Sub CM. The effect of senescence was significant for Sub CM (P = 0.004) and Om CM (P = 0.011), Figure 2.
Figure 2.
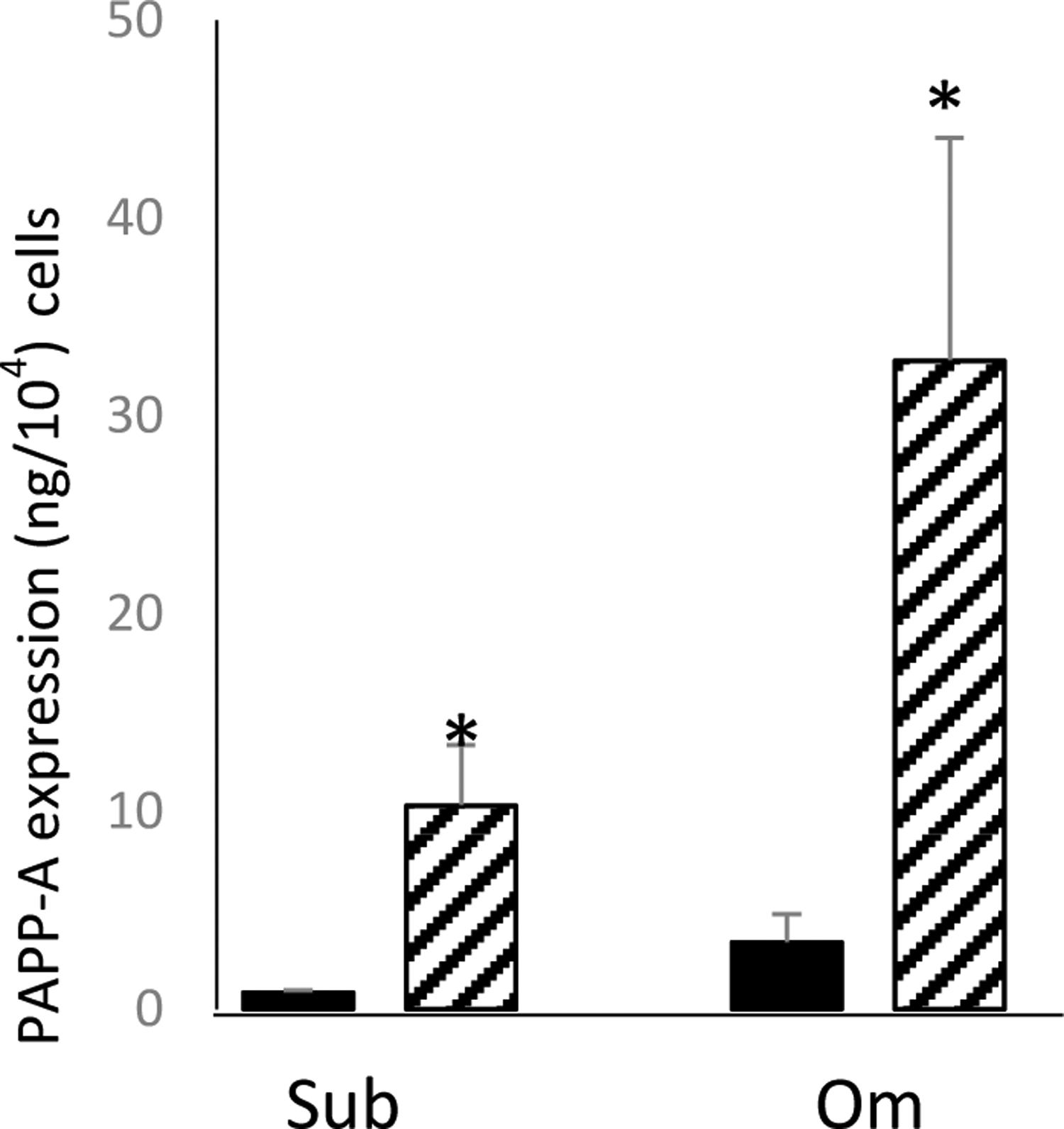
Effect of senescence on PAPP-A protein secretion in pre-adipocytes isolated from subcutaneous (Sub) and omental (Om) fat depots.
Results are mean ± SEM, n=3
*P < 0.05 Senescence (hatched bars) vs. non-senescence (solid bars)
Proteolytic activity in CM reflected PAPP-A protein levels. An example is presented in Figure 3, but the results were similar in all three subjects (Table 4 and full Westerns in supplemental Figures 1–3).
Figure 3.
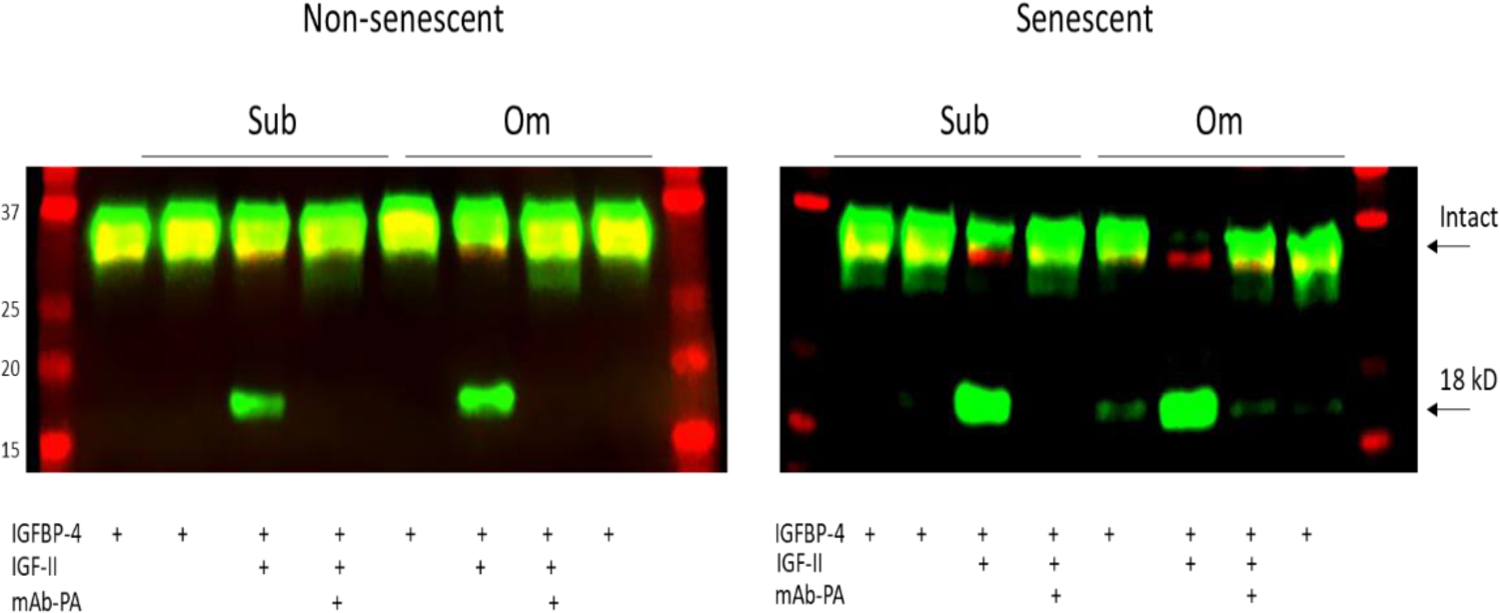
PAPP-A-mediated IGF-dependent IGFBP-4 proteolysis in CM from non-senescent and senescent subcutaneous (Sub) and omental (Om) pre-adipocytes. Arrows indicate intact IGFBP-4 and 18-kD N-terminal fragment of IGFBP-4. Molecular weight standards are on the left.
Table 4.
PAPP-A-mediated IGF-Independent IGFBP-4 proteolysis in CM from Non-Senescent and Senescent pre-adipocytes.
| 18 kD IGFBP-4 fragment (densitometry units) | ||
|---|---|---|
| Sub | Om | |
| Non-Senescent | ||
| Subject A | 0.18 | 0.25 |
| Subject B | 0.06 | 0.16 |
| Subject C | 0.10 | 0.19 |
| Senescent | ||
| Subject A | 0.80 | 1.12 |
| Subject B | 1.10 | 0.86 |
| Subject C | 0.83 | 0.96 |
PAPP-A-mediated IGFBP-4 proteolysis in CM was measured as described in Materials and Methods.
In CM from non-senescent cells, proteolytic fragments of IGFBP-4 were only seen in the presence of exogenous IGF-II, indicating the IGF-dependent nature of PAPP-A-mediated proteolysis of IGFBP-444–46. This activity was inhibited by mAb-PA1/41 indicating specificity for PAPP-A38, 39. As measured by the fluorescent intensity of the 18 kD IGFBP-4 fragment, proteolytic activity in Om CM was 2- to 3-fold greater than in Sub CM from non-senescent cells. CM from senescent cells had markedly increased proteolytic activity that was 12- to 28-fold over CM from non-senescent cells (Fig. 4). With the 6h incubation time, there was no apparent difference in proteolytic activity between Sub and Om CM from senescent cells. There were some IGFBP-4 fragments in the absence of added IGF-II (Control lanes) in the senescent cell CM of all three subjects. This could reflect endogenous IGF secretion by senescent cells51 or, more likely, the very high levels of PAPP-A in CM from senescent cells.
Figure 4.
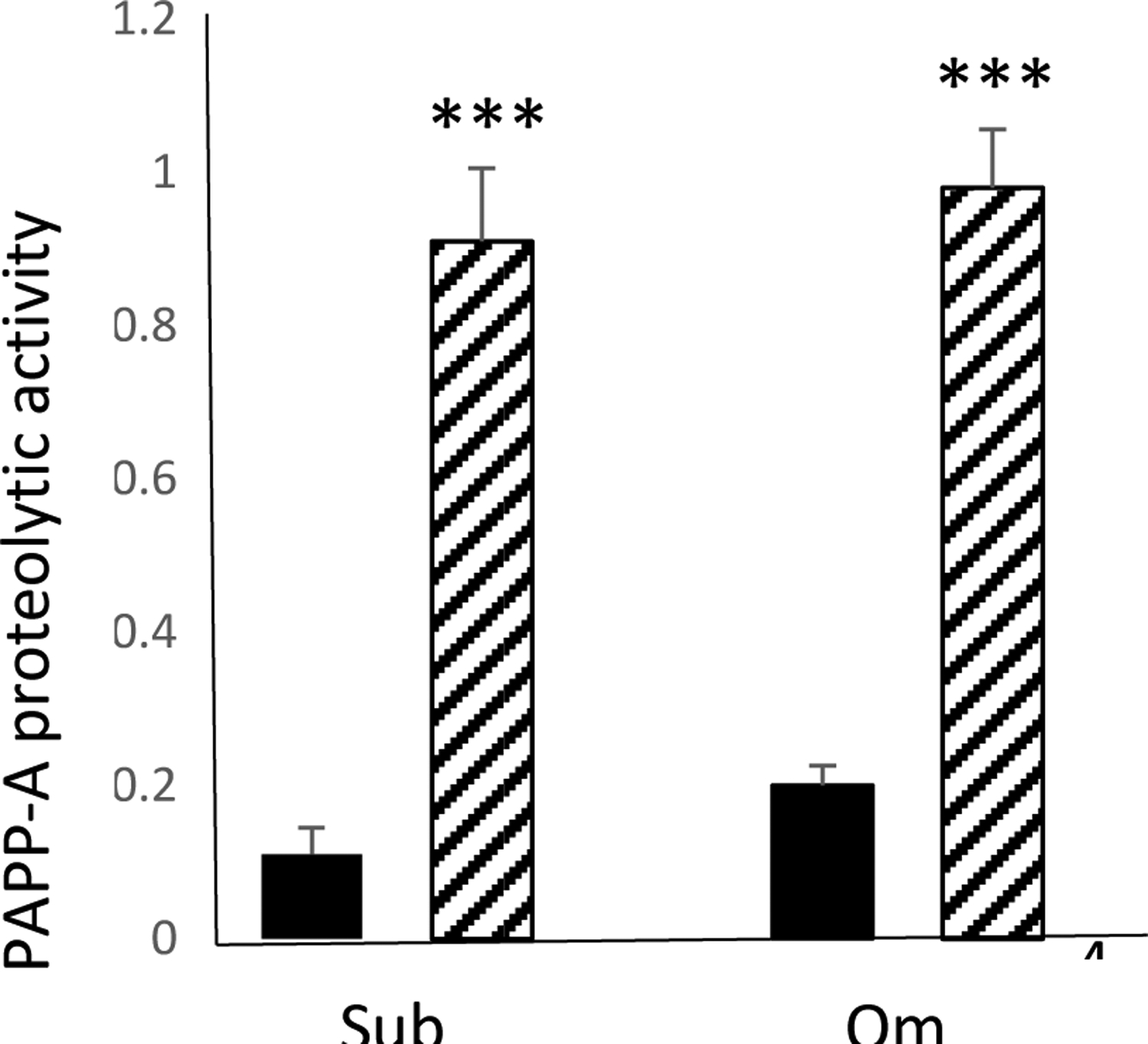
Effect of senescence on PAPP-A-mediated IGFBP-4 proteolysis in CM from pre-adipocytes isolated from subcutaneous (Sub) and omental (Om) fat depots, as measured by IGFBP-4 fragments (in densitometry units).
Results are mean ± SEM, n=3
***P < 0.001 Senescence (hatched bars) vs. non-senescence (solid bars)
Is PAPP-A found on extracellular vesicles (EVs) released by senescent pre-adipocytes?
Subcutaneous and omental pre-adipocytes were treated and cultured as above. CM from senescent and control cells were collected and processed for EVs; the isolation procedure is taken from Barile et al. using sequential ultra-centrifugation47. EVs were assayed for PAPP-A protein and proteolytic activity, as above. EV PAPP-A was expressed as ng/mg protein (Table 5). The difference in Sub vs Om in non-senescent cells was approximately 2-fold (Om > Sub), but there was considerable variability among the three subjects. Likewise, senescence increased EV-PAPP-A but with variable results (2- to 7-fold). Proteolytic activity in senescent EVs is presented in Figure 5 (full Western in supplemental Figure 4).
Table 5.
PAPP-A expression in EVs secreted by Non-Senescent and Senescent pre-adipocytes.
| PAPP-A (ng/mg protein) | ||
|---|---|---|
| Sub | Om | |
| Non-Senescent | ||
| Subject A | 23 | 98 |
| Subject B | 25 | 31 |
| Subject C | 35 | 61 |
| Senescent | ||
| Subject A | 59 | 237 |
| Subject B | 175 | 226 |
| Subject C | 44 | 477 |
Human PAPP-A protein levels in solubilized EVs were measured by ELISA and expressed as ng/mg protein. All samples (in duplicate) were run together in the same assay.
Figure 5.
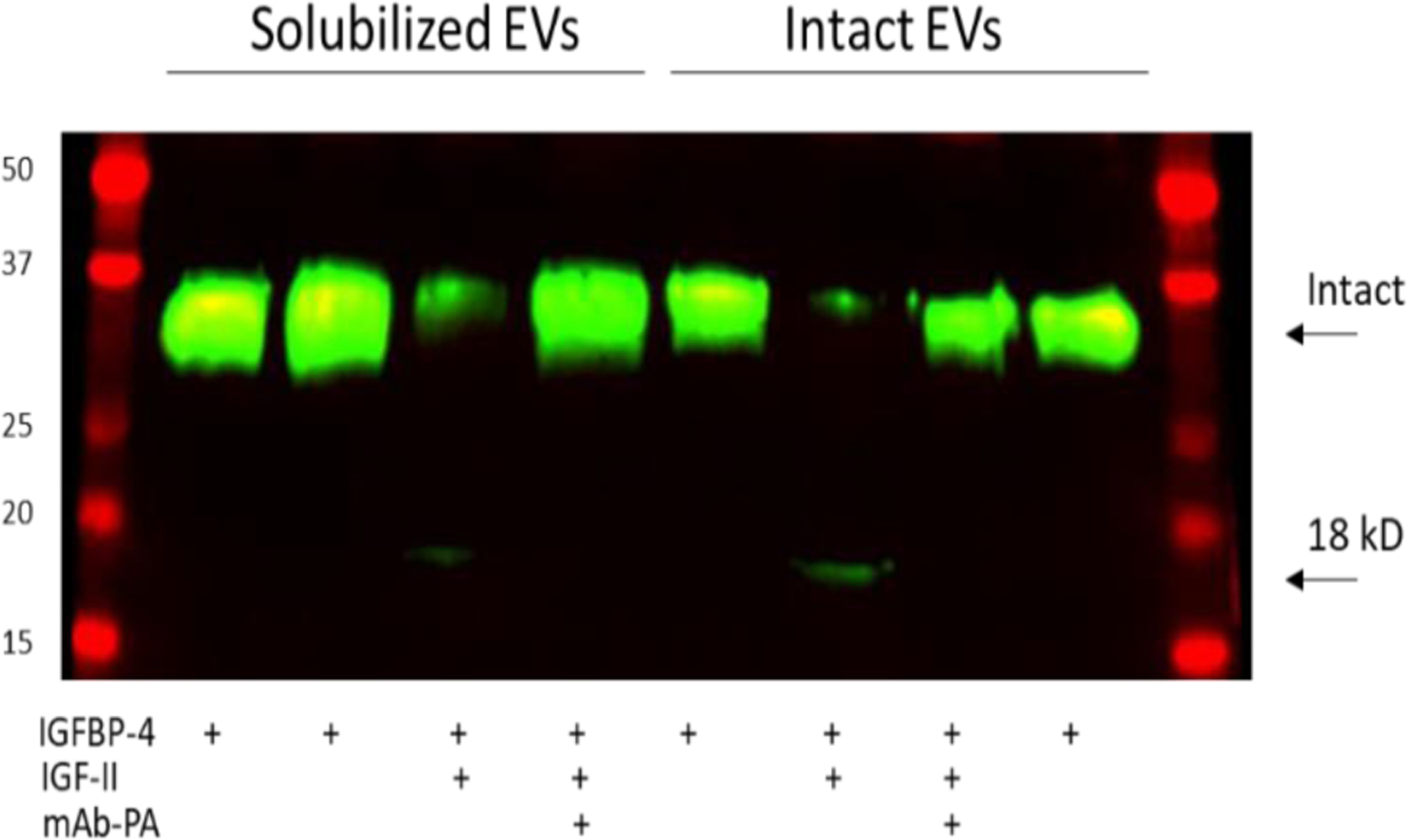
PAPP-A-mediated IGF-dependent IGFBP-4 proteolysis in solubilized and intact extracellular vesicles (EVs) from senescent pre-adipocytes. Arrows indicate intact IGFBP-4 and 18-kD N-terminal fragment of IGFBP-4. Molecular weight standards are on the left.
We compared EVs solubilized in RIPA buffer (total) to those brought up in PBS (intact). We found marked IGF-dependent IGFBP-4 proteolytic activity (measured as decrease in intact IGFBP-4 fluorescence) in both RIPA (11% of control) and PBS (8% of control) preparations which was completely inhibited by mAb-PA1/41. The difference in the protease assay for CM and EV protein likely reflects the difference in incubation times, i.e., 6 hr. for CM and 24 hr. for EVs. Nevertheless, we demonstrated that PAPP-A was found on the surface of EVs from senescent preadipocytes and was proteolytically active.
Is PAPP-A expression induced in senescence driven by increases in pro-inflammatory cytokines?
Interleukin (IL)-1β and tumor necrosis factor (TNF)-α are potent stimulators of PAPP-A expression in human pre-adipocytes, with response of Om cells > Sub cells40. However, these pro-inflammatory cytokines were below the level of detection in 72 hr. CM (Table 6). Common SASP factors, IL-6 and Monocyte Chemoattractant protein (MCP)-1 were significantly elevated in CM from senescent Sub and Om cells. Other recognized SASP factors IL-8, Hepatocyte Growth Factor (HGF) and Plasminogen Activator Inhibitor (PAI)-1 were detectable in the Sub and Om CM but not significantly changed with induced senescence.
DISCUSSION
It is known that visceral pre-adipocytes are relatively resistant to differentiation52, which affects function, but it is not known whether this also applies to senescence. Therefore, we first asked ‘Are subcutaneous- and visceral fat-derived pre-adipocytes differentially responsive to etoposide-induced senescence?’. The answer was no. We found very similar induction and extent of SA-β-gal staining, elevation in p21 expression, and induction of common SASP factors, IL-6 and MCP-1, in Sub and Om pre-adipocytes. To our knowledge, this is the first study to directly compare senescence induction and SASP in primary human subcutaneous and omental preadipocytes. There are studies looking at senescence in subcutaneous pre-adipocytes and one which showed that omental pre-adipocytes are capable of replicative senescence17, 53, but there are no paired studies in Sub and Om cells from the same subject and cultured under identical conditions. We acknowledge that there may be differences in Sub and Om with other inducers of senescence.
The answer to our next question ‘Does senescence affect PAPP-A expression and proteolytic activity?’ was yes. There were highly significant effects of senescence on PAPP-A secretion and activity in both Sub and Om CM. PAPP-A protein was increased 8- to 15-fold in Sub CM and 9- to 11-fold in Om CM from senescent cells compared to non-senescent counterparts. Overall PAPP-A levels in Om CM were ~ 3-fold higher than in Sub CM from senescent cells. Large increases in IGF-dependent proteolytic activity against IGFBP-4 in CM were also seen following induced senescence. Specificity of PAPP-A-mediated IGFBP-4 proteolysis was confirmed with mAb-PA1/41, which inhibits this activity38, 39. Thus, active PAPP-A that can enhance local IGF bioavailability and inhibit apoptosis is part of soluble SASP in pre-adipocytes. Resistance to apoptosis is a hallmark of cellular senescence and can promote accumulation of senescent cells with deleterious effects on the tissue1–3.
The answer to our third question ‘Is PAPP-A found on extracellular vesicles (EVs) released by senescent preadipocytes?’ was also a yes. We found that senescent pre-adipocytes secreted EVs with active PAPP-A on their membrane surface, which could impact non-senescent cells. Little is known about active components on the surface of EVs and their effect on signaling. EVs released by senescent human pre-adipocytes could be a novel mechanism by which PAPP-A contributes to spread of premature senescence in adipose tissue, likely through enhanced paracrine IGF signaling.
We also asked whether PAPP-A expression could be regulated by pro-inflammatory cytokines co-secreted in the SASP. IL-1β and TNF-α are known potent stimulators of PAPP-A expression in a variety of cells, including pre-adipocytes40, 54. However, neither IL-1β nor TNF-α was detected in pre-adipocyte CM even upon induction of senescence. IL-6 is generally the most abundant pro-inflammatory cytokine in various SASPs, including our Sub and Om pre-adipocyte SASP. However, we have shown that IL-6 does not stimulate PAPP-A expression in human pre-adipocytes40. The other analyte that was significantly increased with senescence in these cells was MCP-1, a regulator of migration and infiltration of immune cells. It was found to be elevated during replicative senescence of mesenchymal stromal cells55. There is no known association of MCP-1 with PAPP-A expression. IL-8 and HGF have been considered markers of senescence in different cell types56, 57, but levels were low in Sub and Om pre-adipocyte CM with no significant elevation with senescence. PAI-1 levels in CM were high and showed considerable variability. Its relationship to the senescent phenotype is unclear as it is multifunctional protein58. Thus, increased PAPP-A expression with senescence does not appear to be secondary to other factors in Sub and Om SASP and, therefore, may be an independent biomarker of senescence at least in adipose tissue.
We acknowledge that senescence is an interactive process involving several cell types, various growth factors and cytokines, crosstalk and feedback loops that cannot be fully appreciated in cell cultures studies. Nevertheless, the experiments were carefully designed and controlled to answer the specific questions that relate to a proposed new pathologic mechanism underlying cellular senescence. Answers to these questions are important for recognizing PAPP-A as a novel SASP component that could contribute to the spread of cellular senescence and for providing rationale for potential PAPP-A-targeted therapies in aging and age-related disorders where senescence is known to play a role.
Supplementary Material
HIGHLIGHTS.
Senescence was induced in primary cultures of adult human pre-adipocytes
Senescence significantly increased PAPP-A secretion and proteolytic activity
Senescence increased active PAPP-A on the surface of extracellular vesicles (EV)
Soluble and EV-PAPP-A are novel components of SASP in human pre-adipocytes
ACKNOWLEDGEMENTS
The authors would like to thank Dr. James L. Kirkland for the primary human pre-adipocytes originally collected under IRB #11-009182 and kept frozen at −80°C, Nino Giorgadze for the initial culturing of these cells, and Rebekah Pringle for aid in formatting and submitting the manuscript.
ACKNOWLEDGEMENT OF SUPPORT
This publication was made possible by support of the Immunochemical Core Lab, Mayo Clinic, Rochester, MN
The authors would like to thank Maunik Lefin Koloko Ngassie for assistance using Cytation 5 and Rebekah Pringle for her help in formatting this manuscript
FUNDING
This work was supported by NIH Relief AG0-68020
AUTHOR CONTRIBUTIONS
CAC designed the experiments with input from LKB, analyzed and interpreted data, created Tables and Figures, and wrote the draft of the manuscript. LKB performed the experiments, generated the raw data, and reviewed and approved the manuscript. The authors agree to be personally accountable for their contributions.
This work was supported in part by NIH Relief AG068020 to CAC.
ABBREVIATIONS
- PAPP-A
pregnancy-associated plasma protein-A
- Sub
subcutaneous
- Om
omental
- CM
conditioned medium
- SASP
senescence-associated secretory phenotype
- EV
extracellular vehicle
- IGF
insulin-like growth factor
- IGFBP-4
insulin-like growth factor binding protein-4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The Authors have nothing to declare
DISCLOSURE STATEMENT
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Childs BG, Durik M, Baker DJ, van Deursen JM Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21(12):1424–35. doi: 10.1038/nm.4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9(5):667–884. doi: 10.1111/j.1474-9726.2010.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He S, Sharpless NE Senescence in Health and Disease. Cell. 2017;169(6):1000–11. doi: 10.1016/j.cell.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson G, Wordsworth J, Wang C, Jurk D, Lawless C, Martin-Ruiz C, et al. A senescent cell bystander effect: senescence-induced senescence. Aging Cell. 2012;11(2):345–9. doi: 10.1111/j.1474-9726.2012.00795.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan Basisty, Abhijit Kale, Okhee Jeon, Chisaka Kuehnemann, Therese Payne, Chirag Rao, et al. A Proteomic Atlas of Senescence-Associated Secretomes for Aging Biomarker Development. bioRxiv. 2019:604306. doi: 10.1101/604306 [DOI] [Google Scholar]
- 6.Takasugi M Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell. 2018;17(2). doi: 10.1111/acel.12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadota T, Fujita Y, Yoshioka Y, Araya J, Kuwano K, Ochiya T Emerging role of extracellular vesicles as a senescence-associated secretory phenotype: Insights into the pathophysiology of lung diseases. Mol Aspects Med. 2018;60:92–103. doi: 10.1016/j.mam.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 8.Freund A, Orjalo AV, Desprez PY, Campisi J Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16(5):238–46. doi: 10.1016/j.molmed.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khosla S, Farr JN, Tchkonia T, Kirkland JL The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol. 2020;16(5):263–75. doi: 10.1038/s41574-020-0335-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkland JL, Tchkonia T Cellular Senescence: A Translational Perspective. EBioMedicine. 2017;21:21–8. doi: 10.1016/j.ebiom.2017.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354(6311):472–7. doi: 10.1126/science.aaf6659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schafer MJ, Miller JD, LeBrasseur NK Cellular senescence: Implications for metabolic disease. Mol Cell Endocrinol. 2017;455:93–102. doi: 10.1016/j.mce.2016.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM, et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019;18(3):e12950. doi: 10.1111/acel.12950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schafer MJ, White TA, Evans G, Tonne JM, Verzosa GC, Stout MB, et al. Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes. 2016;65(6):1606–15. doi: 10.2337/db15-0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbouti A, Evangelou K, Pateras IS, Papoudou-Bai A, Patereli A, Stefanaki K, et al. In situ evidence of cellular senescence in Thymic Epithelial Cells (TECs) during human thymic involution. Mech Ageing Dev. 2019;177:88–90. doi: 10.1016/j.mad.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 16.Aw D, Silva AB, Maddick M, von Zglinicki T, Palmer DB Architectural changes in the thymus of aging mice. Aging Cell. 2008;7(2):158–67. doi: 10.1111/j.1474-9726.2007.00365.x [DOI] [PubMed] [Google Scholar]
- 17.Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A. 2015;112(46):E6301–10. doi: 10.1073/pnas.1515386112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–6. doi: 10.1038/nature10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuttle CSL, Waaijer MEC, Slee-Valentijn MS, Stijnen T, Westendorp R, Maier AB Cellular senescence and chronological age in various human tissues: A systematic review and meta-analysis. Aging Cell. 2020;19(2):e13083. doi: 10.1111/acel.13083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–56. doi: 10.1016/j.ebiom.2019.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morsli S, Doherty GJ, Muñoz-Espín D Activatable senoprobes and senolytics: Novel strategies to detect and target senescent cells. Mech Ageing Dev. 2022;202:111618. doi: 10.1016/j.mad.2021.111618 [DOI] [PubMed] [Google Scholar]
- 22.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–56. doi: 10.1038/s41591-018-0092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenyon C The plasticity of aging: insights from long-lived mutants. Cell. 2005;120(4):449–60. doi: 10.1016/j.cell.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 24.Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol. 2013;9(6):366–76. doi: 10.1038/nrendo.2013.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Handayaningsih AE, Takahashi M, Fukuoka H, Iguchi G, Nishizawa H, Yamamoto M, et al. IGF-I enhances cellular senescence via the reactive oxygen species-p53 pathway. Biochem Biophys Res Commun. 2012;425(2):478–84. doi: 10.1016/j.bbrc.2012.07.140 [DOI] [PubMed] [Google Scholar]
- 26.Tran D, Bergholz J, Zhang H, He H, Wang Y, Zhang Y, et al. Insulin-like growth factor-1 regulates the SIRT1-p53 pathway in cellular senescence. Aging Cell. 2014;13(4):669–78. doi: 10.1111/acel.12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bitto A, Lerner C, Torres C, Roell M, Malaguti M, Perez V, et al. Long-term IGF-I exposure decreases autophagy and cell viability. PLoS One. 2010;5(9):e12592. doi: 10.1371/journal.pone.0012592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conover CA Key questions and answers about pregnancy-associated plasma protein-A. Trends Endocrinol Metab. 2012;23(5):242–9. doi: 10.1016/j.tem.2012.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conover CA, Bale LK, Mader JR, Mason MA, Keenan KP, Marler RJ Longevity and age-related pathology of mice deficient in pregnancy-associated plasma protein-A. J Gerontol A Biol Sci Med Sci. 2010;65(6):590–9. doi: 10.1093/gerona/glq032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bale LK, West SA, Conover CA Inducible knockdown of pregnancy-associated plasma protein-A gene expression in adult female mice extends life span. Aging Cell. 2017. doi: 10.1111/acel.12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conover CA, Bale LK, Oxvig C Targeted inhibition of pregnancy-associated plasma protein-A activity reduces atherosclerotic plaque burden in mice. J Cardiovasc Transl Res. 2016;9(1):77–9. doi: 10.1007/s12265-015-9666-9 [DOI] [PubMed] [Google Scholar]
- 32.Harrington SC, Simari RD, Conover CA Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ Res. 2007;100(12):1696–702. [DOI] [PubMed] [Google Scholar]
- 33.Conover CA, Harstad SL, Tchkonia T, Kirkland JL Preferential impact of pregnancy-associated plasma protein-A deficiency on visceral fat in mice on high-fat diet. Am J Physiol: Endo Metab. 2013;305(9):E1145–53. doi: 10.1152/ajpendo.00405.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akhila Ramakrishna, Bale Laurie K., West Sally A, Conover Cheryl A. Genetic and Pharmacological Inhibition of PAPP-A Protects Against Visceral Obesity in Mice. 2020. doi: 10.1210/endocr/bqaa160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallejo AN, Michel JJ, Bale LK, Lemster BH, Borghesi L, Conover CA Resistance to age-dependent thymic atrophy in long-lived mice that are deficient in pregnancy-associated plasma protein A. Proc Natl Acad Sci USA. 2009;106(27):11252–7. doi: 10.1073/pnas.0807025106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conover CA, Bale LK, Nair KS Comparative gene expression and phenotype analyses of skeletal muscle from aged wild-type and PAPP-A-deficient mice. Exp Gerontol. 2016;80:36–42. doi: 10.1016/j.exger.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill CM, Arum O, Boparai RK, Wang F, Fang Y, Sun LY, et al. Female PAPP-A knockout mice are resistant to metabolic dysfunction induced by high-fat/high-sucrose feeding at middle age. Age (Dordr). 2015;37(3):9765. doi: 10.1007/s11357-015-9765-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mikkelsen JH, Gyrup C, Kristensen P, Overgaard MT, Poulsen CB, Laursen LS, et al. Inhibition of the proteolytic activity of pregnancy-associated plasma protein-A by targeting substrate exosite binding. J Biol Chem. 2008;283(24):16772–80. doi: 10.1074/jbc.M802429200 [DOI] [PubMed] [Google Scholar]
- 39.Mikkelsen JH, Resch ZT, Kalra B, Savjani G, Kumar A, Conover CA, et al. Indirect targeting of IGF receptor signaling in vivo by substrate-selective inhibition of PAPP-A proteolytic activity. Oncotarget. 2014;5(4):1014–25. doi: 10.18632/oncotarget.1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davidge-Pitts C, Escande CJ, Conover CA Preferential expression of PAPPA in human preadipocytes from omental fat. J Endocrinol. 2014;222(1):87–97. doi: 10.1530/JOE-13-0610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. A J Physiol Endo Metab. 2007;292(1):E298–307. doi: 10.1152/ajpendo.00202.2006 [DOI] [PubMed] [Google Scholar]
- 42.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. doi: 10.1038/ncomms14532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conover CA, Bale LK, Frye RL, Schaff HV Cellular characterization of human epicardial adipose tissue: highly expressed PAPP-A regulates insulin-like growth factor I signaling in human cardiomyocytes. Physiol Rep. 2019;7(4):e14006. doi: 10.14814/phy2.14006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conover CA, Kiefer MC, Zapf J Posttranslational regulation of insulin-like growth factor binding protein-4 in normal and transformed human fibroblasts. Insulin-like growth factor dependence and biological studies. J Clin Invest. 1993;91(3):1129–37. doi: 10.1172/JCI116272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laursen LS, Overgaard MT, Soe R, Boldt HB, Sottrup-Jensen L, Giudice LC, et al. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett. 2001;504:36–40. doi: 10.1016/s0014-5793(01)02760-0 [DOI] [PubMed] [Google Scholar]
- 46.Qin X, Byun D, Lau K-HW, Baylink DJ, Mohan S Evidence that the interaction between insulin-like growth factor (IGF)-II and IGF binding protein (IGFBP)-4 is essential for the action of the IGF-II-dependent IGFBP-4 protease. Arch Biochem Biophys. 2000;379:209–16. [DOI] [PubMed] [Google Scholar]
- 47.Barile L, Cervio E, Lionetti V, Milano G, Ciullo A, Biemmi V, et al. Cardioprotection by cardiac progenitor cell-secreted exosomes: role of pregnancy-associated plasma protein-A. Cardiovasc Res. 2018;114(7):992–1005. doi: 10.1093/cvr/cvy055 [DOI] [PubMed] [Google Scholar]
- 48.Yosef R, Pilpel N, Papismadov N, Gal H, Ovadya Y, Vadai E, et al. p21 maintains senescent cell viability under persistent DNA damage response by restraining JNK and caspase signaling. Embo J. 2017;36(15):2280–95. doi: 10.15252/embj.201695553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saul D, Kosinsky RL, Atkinson EJ, Doolittle ML, Zhang X, LeBrasseur NK, et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat Commun. 2022;13(1):4827. doi: 10.1038/s41467-022-32552-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci U S A. 1996;93(24):13742–7. doi: 10.1073/pnas.93.24.13742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Durham SK, De León DD, Okazaki R, Riggs BL, Conover CA Regulation of insulin-like growth factor (IGF)-binding protein-4 availability in normal human osteoblast-like cells: role of endogenous IGFs. J Clin Endocrinol Metab. 1995;80(1):104–10. doi: 10.1210/jcem.80.1.7530254 [DOI] [PubMed] [Google Scholar]
- 52.Tchkonia T, Giorgadze N, Pirtskhalava T, Tchoukalova Y, Karagiannides I, Forse RA, et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol. 2002;282(5):R1286–R96. doi: 10.1152/ajpregu.00653.2001 [DOI] [PubMed] [Google Scholar]
- 53.Ksiazek K, Piwocka K, Brzezinska A, Sikora E, Zabel M, Breborowicz A, et al. Early loss of proliferative potential of human peritoneal mesothelial cells in culture: the role of p16INK4a-mediated premature senescence. J Appl Physiol (1985). 2006;100(3):988–95. doi: 10.1152/japplphysiol.01086.2005 [DOI] [PubMed] [Google Scholar]
- 54.Resch ZT, Chen BK, Bale LK, Oxvig C, Overgaard MT, Conover CA Pregnancy-associated plasma protein a gene expression as a target of inflammatory cytokines. Endocrinology. 2004;145(3):1124–9. doi: 10.1210/en.2003-1313 [DOI] [PubMed] [Google Scholar]
- 55.Jin HJ, Lee HJ, Heo J, Lim J, Kim M, Kim MK, et al. Senescence-Associated MCP-1 Secretion Is Dependent on a Decline in BMI1 in Human Mesenchymal Stromal Cells. Antioxid Redox Signal. 2016;24(9):471–85. doi: 10.1089/ars.2015.6359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci U S A. 2009;106(40):17031–6. doi: 10.1073/pnas.0905299106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boichuck M, Zorea J, Elkabets M, Wolfson M, Fraifeld VE c-Met as a new marker of cellular senescence. Aging (Albany NY). 2019;11(9):2889–97. doi: 10.18632/aging.101961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lijnen HR Pleiotropic functions of plasminogen activator inhibitor-1. J Thromb Haemost. 2005;3(1):35–45. doi: 10.1111/j.1538-7836.2004.00827.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


