Abstract
The main objective of this work was to perform a comprehensive analysis and provide a race-stratified epidemiological report accounting for differences in treatment patterns and treatment related adverse events in Non-Hispanic women with breast cancer (BC). The cohort included women ≥ 18 years diagnosed with in-situ, early-stage, and late-stage BC (2005–2022). Treatment patterns included: surgery, breast radiation, chemotherapy, endocrine therapy, or biologic therapy. Treatment related adverse events were: chemotherapy complications, cardiovascular toxicities, immune-related adverse events, psychological affectations, or cognitive decline/dementia. The influence of race on the outcomes was measured via Cox proportional-hazards models. We included 17,454 patients (82% non-Hispanic Whites [NHW]). Most of the patients had a Charlson Comorbidity Score between 1 and 2 (68%), and TNM stage I (44.5%). Surgery was performed in 51.5% of the cases, while 30.6% received radiotherapy, 26.4% received chemotherapy, 3.1% received immunotherapy, and 41.2% received endocrine therapy. Non-Hispanic Blacks (NHB) had a lower probability of undergoing breast cancer surgery (aHR = 0.92, 95% CI 0.87–0.97) and of being prescribed endocrine therapy (aHR = 0.83, 95% CI 0.79–0.89), but a higher probability of receiving adjuvant radiotherapy (aHR = 1.40, 95% CI 1.29–1.52). Moreover, NHBs had lower risk of being diagnosed with psychological issues (aHR = 0.71, 95% CI 0.63–0.80) but a higher risk for cognitive decline/dementia (aHR = 1.30, 95% CI 1.08–1.56). In conclusion, NHB women diagnosed with BC were less likely than NHW to undergo curative intent surgery or receive endocrine therapy, and had a higher risk of cognitive decline/dementia after cancer treatment. Public policy measures are urgently needed which equalize access to quality healthcare for all patients and that promote a learning healthcare system which can improve cancer outcomes.
Subject terms: Breast cancer, Oncology, Public health
Introduction
Breast cancer (BC) is the most frequently diagnosed malignancy among women globally, and the leading cause of cancer death, and in 2020 was responsible for 2,261,419 new cases (11.7% of the total), and 684,996 deaths (6.9% of the total)1. In the United States (US) alone, there will be an estimated 287,850 new cases (15% of the total) and 43,250 deaths (7.1% of the total) in 20222,3. According to the National Cancer Institute (NCI), approximately 13% of women will be diagnosed with BC at some point during their life3.
With a 5-year relative survival of approximately 91%, treatment of BC involves one or a combination of surgery (lumpectomy or mastectomy), radiotherapy, and/or neoadjuvant or adjuvant systemic therapy (endocrine therapy, chemotherapy, and/or anti-HER2 directed antibody therapy or immunotherapy), depending on staging, pathological, and molecular features4,5. Despite cure rates and efficacy, BC treatments are accompanied by acute and late effects6–9. Common effects include both physical symptoms such as fatigue, erythema, pneumonia, heart problems, neutropenic infection, nausea, vomiting, diarrhea, neuropathy, alopecia and menopausal symptoms, as well as psychological symptoms, mainly involving anxiety and depression7–9. Late toxicities of breast cancer treatments can also include permanent cardiovascular and bone marrow toxicities. The combination of adverse effects, both acute and late, is responsible for changes in quality of life—an important prognostic factor6–8.
In the published literature, the reality of racial cancer disparities is well documented10–12. In BC, Black patients, when compared to other groups, are more likely to be diagnosed at more advanced stages, have limited access to quality health care, and have a higher risk of having triple negative breast cancer (TNBC)—the BC subtype with the poorest prognosis10,13–16. Consequently, because of these myriad factors Black women with breast cancer have a higher mortality risk. It is believed that part of the BC disparities in survival outcomes are explained by disparities in treatment as Black patients are less likely to receive adequate treatment and more likely to experience significant treatment delays compared to White patients17,18. Reports also indicate that, for cancer as overall, racial minorities are at increased risk for poorer health outcomes, e.g. hospitalization, health-care associated infections, and emergency department (ED) visits19–21.
Despite racial disparities in BC outcomes and in access to treatment being well documented in the medical literature, to our knowledge there is scant information on whether racial disparities also exist with respect to treatment patterns and treatment related adverse events in BC patients. We hypothesize that there are differences in the incidence of adverse treatment effects which might contribute to racial disparities in BC outcomes. The primary objective of this study is to perform a comprehensive analysis and provide an epidemiological report stratified by race accounting for treatment patterns and treatment adverse events in Non-Hispanic women with BC.
Methods
The study setting was the University Hospitals (UH) Seidman Cancer Center (Northeast Ohio, US). All patient data were obtained from the UH data repository based on the CAISIS platform, which consists of an open-source, web-based cancer data management system composed by disparate sources of cancer patient data (i.e., Soarian, NGS Labs, Sunrise Clinical Manager, Tumor Registry, Via Oncology, OnCore, MosiaQ, PRO tools, and others)22–24. All patient records were de-identified, and all analyses were performed in accordance with relevant guidelines and regulations, respecting the Declaration of Helsinki. The study with the waiver of the informed consent was approved by the University Hospitals of Cleveland Institutional Review Board (IRB). All the information obtained from the UH database was subsequently complemented with electronic health record (EHR) information captured via EMERSE (Electronic Medical Record Search Engine) in order to obtain the most accurate and complete information per patient, avoiding high missingness25.
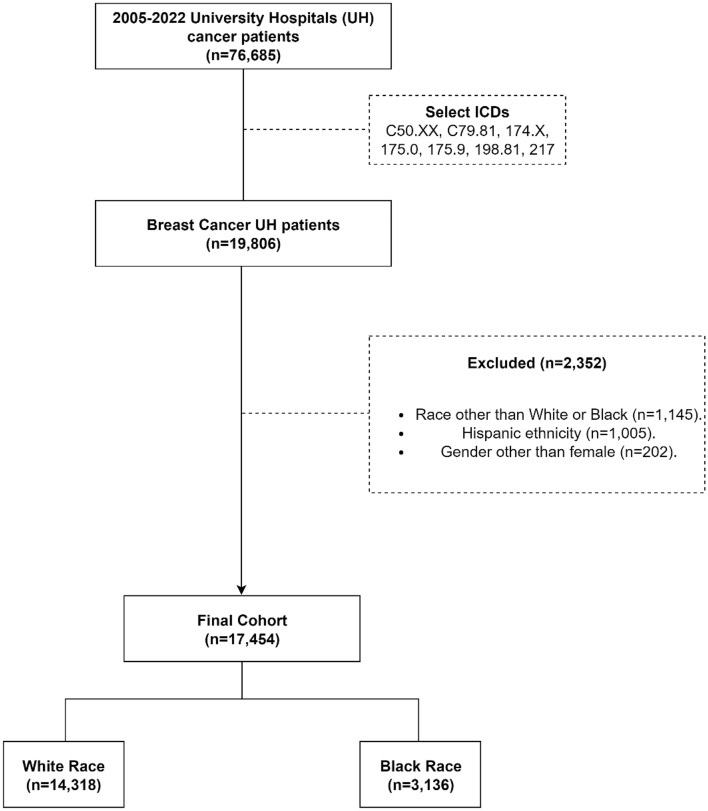
The initial cohort included women ≥ 18 years diagnosed with in-situ, early-stage, or late-stage breast cancer (henceforth, breast cancer; determined using Tumor Registry (TR) or Electronic Medical Record (EMR) International Classification of Diseases [ICD] 9/10 codes: C50.XX, C79.81, 174.X, 175.0, 175.9, 198.81, 217, with X standing for any integer) between 01/01/2005 and 03/31/202226. Patients were excluded from the analysis if they had race other than Black or White, ethnicity different than Non-Hispanic (due to the low number of Hispanic patients in the dataset), and gender different than female. Hispanics were excluded due very low patient numbers, while other races were excluded to focus the analysis exclusively on disparities on treatment related toxicities in White vs. Black BC patients. The cohort selection consort diagram is presented in Fig. 1.
Figure 1.
Study consort diagram detailing inclusion and exclusion criteria for Breast Cancer University Hospitals (UH) population (2005–2022). The final cohort included 17,454 patients, of which 3136 (18%) self-reported as Black.
Outcomes
Study outcomes included: (I) treatment and time-to-treatment following index breast cancer diagnosis; (II) the diagnosis and time-to-event of a treatment adverse event.
Covariates
Demographics, BC pathological characteristics, treatment patterns, and treatment adverse events data were obtained for all eligible patients. The demographic characteristics included: age at breast cancer diagnosis, self-reported race (White, Black), smoking status (yes, no, former, unknown), and Charlson comorbidity index27. Tumor characteristics included: date of BC diagnosis, hormone receptor status (estrogen receptor [ER], progesterone receptor [PR]) and HER2 status (positive or negative as per ASCO/CAP guidelines), histological type (ductal carcinoma, ductal or lobular carcinoma in-situ [DCIS/LCIS], and other), and TNM staging group (stage 0–IV)28–30.
Treatment patterns examined in this study included: treatment adherence (number of appointments per patient and % of appointments attended), surgery (mastectomy or lumpectomy), breast radiation (right, left, or bilateral), chemotherapy, endocrine therapy, or immunotherapy (HER-2 or PDL1 antibody therapy). The use of single or multiple treatment modalities were accounted. The medical treatments included as covariates included: anthracyclines, PIK3CA/mTOR inhibitors, HER2-targeted agents, ER antagonists, LHRH agonists, aromatase inhibitors (AI), or other novel biologic therapies. Time-to-treatment variables were extracted based on the date of BC diagnosis and the date of the first treatment (surgery, radiation, or systemic therapy). The medications included in each category are described in Supplemental Table I.
Treatment adverse events were extracted on the basis of ICD 9/10 codes, where only diagnoses occurring after the date of first treatment were considered. Complications from any treatment included: cognitive decline or dementia (yes, no), or the presence of psychological a disorder (yes, no) and characterization of the psychological disorder (depression, anxiety, or bipolar disorder). Chemotherapy complications included those most frequently reported in the literature22,23,31. Complications from immunotherapy were described as immune-related adverse events (irAEs) (yes, no) and the specific irAEs22,23,31. The ICD codes and categorizations are summarized in Supplemental Table 1.
Statistical analysis
The population was described via percentages, median and interquartile range (IQR). All descriptive and inferential statistics were race-stratified to examine racial differences. The Pearson Chi-Square test was used to compare categorical variables. Data distribution assumptions for continuous variables were confirmed using histograms and the Kolmogorov–Smirnov test, followed by Student’s T-tests for normally distributed factors and non-parametric Kruskal–Wallis tests for non-normal distributed factors. The influence of race on type of treatment received, and treatment adverse events was assessed via Hazard Ratios (HR) or adjusted Hazard Rations (aHR) with 95% confidence intervals (CIs), using univariable and multivariable Cox proportional-hazards models, after confirming the model’s assumptions. Patients were censored according to the last follow-up date and the models for treatment adverse events were performed only in patients who received the respective treatment. Sensitivity analysis was performed in patients diagnosed after 2015 to mitigate the effect of temporal changes.
The multivariable selected were those that achieved a p < 0.10 in univariable analyses for the primary outcome and those deemed to have clinical importance by study investigators. Independent variable correlations were checked by correlation plots, and those variables found to be correlated were not included simultaneously in the final multivariable models. A p-value < 0.05 was considered significant in the final models, and missing values were not included in the final analysis. All analyses were performed using RStudio software32. We used the STROBE cohort checklist when writing our report33.
Ethical approval
Patient records were deidentified, and the study was approved by the University Hospitals of Cleveland Institutional Review Board (IRB).
Results
Population
Using data from 2005 to 2022, we analyzed 17,454 BC Non-Hispanic women with a BC diagnosis. The cohort’s median age was 63 (interquartile range [IQR] 53–73) years, with a predominance of Non-Hispanic Whites (NHWs) (82%). Most of the patients had a Charlson Comorbidity Score between 1 and 2 (68%), and TNM stage I (44.5%). Surgery was performed in 51.5% of patients, while 30.6% received radiotherapy, 26.4% received chemotherapy, 3.1% received immunotherapy, and 41.2% received endocrine therapy.
Racial disparities in demographics and tumor characteristics
Among 17,454 patients analyzed, 18% were Non-Hispanic Blacks (NHBs). NHB were followed-up for a median of 4.4 years, while NHW were followed-up for a median of 8.1 years. Compared to NHWs, NHB patients had a significantly lower median age at diagnosis (62, IQR 52–72 vs. 63, IQR 53–73, p = 0.001), and a significantly higher: probability of a prior smoking history (13.5% vs. 9%, p < 0.001), proportion of women with Charlson comorbidity scores ≥ 5 (21.4% vs. 9.2%, p < 0.001), Ductal carcinoma (48.8% vs. 39.9%, p < 0.001), and stage IV (6.4% v 4.9%, p < 0.001, Table 1).
Table 1.
Breast Cancer University Hospitals (UH) population (2005–2022) description and comparison stratified by race in demographics and tumor characteristics.
| Breast cancer UH population (n = 17,454) | |||
|---|---|---|---|
| Black | White | p value | |
| 3136 (18%) | 14,318 (82%) | ||
| Age at diagnosis—median (IQR) | 62 (52–72) | 63 (53–73) | 0.001 |
| Year of diagnosis—n (%) | |||
| 2005–2010 | 669 (21.3%) | 2890 (20.2%) | < 0.001 |
| 2010–2015 | 914 (29.1%) | 3622 (25.3%) | |
| > 2015 | 1553 (49.5%) | 7806 (54.5%) | |
| Smoking status—n (%) | |||
| Smoker | 283 (13.5%) | 902 (9%) | < 0.001 |
| Never smoker | 1205 (57.7%) | 6232 (62.5%) | |
| Former smoker | 601 (28.5%) | 1840 (28.5%) | |
| Unknown | 1047 | 4,344 | |
| Charlson score—n (%) | |||
| 1–2 | 1599 (51%) | 10,278 (71.8%) | < 0.001 |
| 3–4 | 865 (27.6%) | 2716 (19%) | |
| ≥ 5 | 672 (21.4%) | 1324 (9.2%) | |
| Histology—n (%) | |||
| DCIS/LCIS | 164 (5.2%) | 532 (3.7%) | < 0.001 |
| Ductal | 1529 (48.8%) | 5706 (39.9%) | |
| Other | 1443 (46%) | 8080 (56.4%) | |
| Stage—n (%) | |||
| 0 | 348 (15.3%) | 1174 (12.8%) | < 0.001 |
| I | 843 (37.1%) | 4250 (46.4%) | |
| II | 655 (28.8%) | 2425 (26.5%) | |
| III | 282 (12.4%) | 864 (9.4%) | |
| IV | 145 (6.4%) | 451 (4.9%) | |
| Unknown | 863 | 5154 | |
| ER + − n (%) | 1364 (43.5%) | 5998 (41.9%) | 0.1 |
| PR + − n (%) | 1201 (38.3%) | 5358 (37.4%) | 0.36 |
| HER2 + − n (%) | 119 (3.8%) | 454 (3.2%) | 0.08 |
| Triple positive—n (%) | 52 (1.7%) | 229 (1.6%) | 0.87 |
A total of 17,454 patients were analyzed, with 3,136 (18%) self-reporting as Black. IQR interquartile range.
Racial disparities in treatment patterns
When comparing treatment rates in NHBs vs. NHWs (Table 2), we found that women from the first group had higher rates of surgery (58% vs. 50.1%, p < 0.001), radiotherapy (42.1% vs. 28%, p < 0.001), chemotherapy (34.6% vs. 24.6%, p < 0.001), hormone therapy (42.6% vs. 40.9%), immunotherapy (4.1% vs. 2.8%, p < 0.001), and combined therapy (including combined modality and combined systemic therapy). Stratifying the analysis by specific medications (Table 2), NHBs were prescribed both anthracycline containing (11.4% vs. 7.4%, p < 0.001), and non-anthracycline containing chemotherapy regimens (22.1% vs. 15.5%, p < 0.001) at a significantly higher rates. We also found that NHB were prescribed endocrine therapies at higher rates: aromatase inhibitors (12% vs. 7.7%, p < 0.001), LHRH agonists (2.6% vs. 1.9%, p = 0.01), ER antagonists (6.2% vs. 4.4%, p < 0.001), as well as other biologic therapies (6.7% vs. 5.3%, p < 0.001).
Table 2.
Breast Cancer University Hospitals (UH) population (2005–2022) description and comparison stratified by race in treatment patterns. IQR = interquartile range.
| Breast cancer UH population (n = 17,454) | |||
|---|---|---|---|
| Black | White | p value | |
| 3136 (18%) | 14,318 (82%) | ||
| Surgery—n (%) | 1819 (58%) | 7167 (50.1%) | < 0.001 |
| Mastectomy—n (%) | 490 (15.6%) | 1790 (12.5%) | < 0.001 |
| Lumpectomy—n (%) | 583 (18.6%) | 1771 (12.4%) | < 0.001 |
| Time to surgery (days)—median (IQR) | 42 (27–106) | 34 (21–62) | < 0.001 |
| Radiotherapy (R)—n (%) | 1320 (42.1%) | 4015 (28%) | < 0.001 |
| Right side only radiotherapy—n (%) | 371 (11.8%) | 1136 (7.9%) | < 0.001 |
| Left side only radiotherapy—n (%) | 315 (10%) | 967 (6.8%) | < 0.001 |
| Time to radiotherapy (days)—median (IQR) | 204 (99–287) | 138 (77–253) | < 0.001 |
| Chemotherapy (C)—n (%) | 1085 (34.6%) | 3528 (24.6%) | < 0.001 |
| Time to chemotherapy (days)—median (IQR) | 70 (37–112) | 62 (36–95) | < 0.001 |
| Hormone therapy (H)—n (%) | 1335 (42.6%) | 5853 (40.9%) | 0.08 |
| Time to hormone therapy (days)—median (IQR) | 138 (72–245) | 126 (72–218) | 0.003 |
| Immunotherapy (I)—n (%) | 129 (4.1%) | 406 (2.8%) | < 0.001 |
| Time to immunotherapy (days)—median (IQR) | 68 (43–127) | 59 (34–128) | 0.44 |
| Combined therapy | |||
| C + R − n (%) | 718 (22.9%) | 1,558 (10.9%) | < 0.001 |
| I + R − n (%) | 87 (2.8%) | 206 (1.4%) | < 0.001 |
| H + R − n (%) | 733 (23.4%) | 2,285 (16%) | < 0.001 |
| H + C + R − n (%) | 383 (12.2%) | 1,008 (7%) | < 0.001 |
| H + C + R + I − n (%) | 39 (1.2%) | 111 (0.8%) | 0.01 |
| Agents | |||
| Anthracyclines—n (%) | 357 (11.4%) | 1,053 (7.4%) | < 0.001 |
| Non-anthracycline cytotoxic chemotherapy—n (%) | 694 (22.1%) | 2,214 (15.5%) | < 0.001 |
| PIK3CA/mTOR inhibitors—n (%) | 4 (0.1%) | 9 (0.1%) | 0.4 |
| Aromatase inhibitors—n (%) | 375 (12%) | 1,098 (7.7%) | < 0.001 |
| LHRH agonists—n (%) | 82 (2.6%) | 270 (1.9%) | 0.01 |
| ER antagonists—n (%) | 196 (6.2%) | 631 (4.4%) | < 0.001 |
| Newer therapies—n (%) | 211 (6.7%) | 752 (5.3%) | < 0.001 |
| Appointments per patient—median (IQR) | 10 (5–23) | 8 (4–17) | < 0.001 |
| % of appointments attended—median (IQR) | 66 (44–80) | 69 (50–85) | < 0.001 |
By contrast, NHBs had longer delays for surgery (median of 42 days [IQR 27–106] vs. 34 days [IQR 21–62]), radiotherapy (median of 204 days [IQR 99–287] vs. 138 days [77–253]), chemotherapy (median of 70 days [IQR 37–112] vs. 62 days [IQR 39–95]), as well as time to initiation of endocrine therapy (median of 138 days [IQR 72–245] vs. 126 days [IQR 72–218], Table 2). Despite a higher number of appointments per patient (10 [IQR 5–23] vs. 8 [IQR 4–17]), NHBs had lower median appointment completion rates (66% appointments attended [IQR 44–80] vs. 69% [IQR 50–85]).
Racial disparities in treatment related adverse events
Analyzing treatment related adverse events (Table 3), considering only patients that received the respective treatments, NHBs had higher rates of chemotherapy related complications (20.9% vs. 12.2%, p < 0.001), including: cardiomyopathy, diarrhea/enteritis, fatigue, nausea/vomiting, neuropathy, lung disease, pain, dehydration/hypovolemia, rash, and infusion reactions, as well as higher reported rates of cognitive decline/dementia (13.6% vs. 6.7%, p < 0.001). Although no differences were seen in the incidence of overall immune related toxicities (irAEs), NHBs had higher rates cardiac toxicties acute myocardial infarction (3.1% vs. 0.2%, p = 0.01), and pneumonitis (7.8% vs. 2%, p = 0.003).
Table 3.
Breast Cancer University Hospitals (UH) population (2005–2022) description and comparison stratified by race in treatment adverse events, including chemotherapy complications, irAEs (immune-related adverse events), psychological affections, and cognitive decline/dementia. IQR interquartile range; MI myocardial infarction; AKI acute kidney injury.
| Breast cancer UH population (n = 17,454) | |||
|---|---|---|---|
| Black | White | p value | |
| Chemotherapy complications—n (%) | 227 (20.9%) | 431 (12.2%) | < 0.001 |
| Adverse reaction—n (%) | 11 (1%) | 21 (0.6%) | 0.21 |
| Cardiomyopathy—n (%) | 14 (1.3%) | 16 (0.5%) | 0.005 |
| Diarrhea/enteritis—n (%) | 41 (3.8%) | 64 (1.8%) | < 0.001 |
| Fatigue—n (%) | 54 (5%) | 80 (2.3%) | < 0.001 |
| Nausea/vomiting—n (%) | 62 (5.7%) | 94 (2.7%) | < 0.001 |
| Steatohepatitis—n (%) | 6 (0.6%) | 19 (0.5%) | 1 |
| Neuropathy—n (%) | 27 (2.5%) | 37 (1%) | < 0.001 |
| Thrombocytopenia—n (%) | 2 (0.2%) | 6 (0.2%) | 1 |
| Lung disease—n (%) | 35 (3.2%) | 27 (0.8%) | < 0.001 |
| Pain—n (%) | 53 (4.9%) | 54 (1.5%) | < 0.001 |
| Anemia—n (%) | 10 (0.9%) | 15 (0.4%) | 0.08 |
| Agranulocytosis—n (%) | 46 (4.2%) | 156 (4.4%) | 0.86 |
| Mouth sore—n (%) | 0 | 2 (0.1%) | 1 |
| Dehydration/hypovolemia—n (%) | 38 (3.5%) | 57 (1.6%) | < 0.001 |
| Renal failure—n (%) | 1 (0.1%) | 0 | 0.53 |
| Rash—n (%) | 7 (0.6%) | 1 | < 0.001 |
| Infusion reaction—n (%) | 6 (0.6%) | 5 (0.1%) | 0.03 |
| IRAES—n (%) | 47 (36.4%) | 153 (37.7%) | 0.06 |
| Anemia—n (%) | 10 (7.8%) | 36 (8.9%) | 0.83 |
| Thrombocytopenia—n (%) | 2 (1.6%) | 20 (4.9%) | 0.15 |
| Leukopenia—n (%) | 9 (7%) | 45 (11.1%) | 0.23 |
| Hypothyroidism—n (%) | 11 (8.5%) | 43 (10.6%) | 0.60 |
| Hyperthyroidism—n (%) | 2 (1.6%) | 4 (1%) | 0.95 |
| Hypophysitis/PGA—n (%) | 3 (2.3%) | 5 (1.2%) | 0.63 |
| Hyper/hypo- parathyroidism—n (%) | 0 | 4 (1%) | 0.58 |
| AKI—n (%) | 8 (6.2%) | 14 (3.4%) | 0.26 |
| Neuritis—n (%) | 4 (3.1%) | 11 (2.7%) | 1 |
| Hepatitis—n (%) | 0 | 3 (0.7%) | 0.76 |
| Colitis—n (%) | 3 (2.3%) | 16 (3.9%) | 0.55 |
| Pancreatitis—n (%) | 3 (2.3%) | 1 (0.2%) | 0.07 |
| Mucositis—n (%) | 1 (0.8%) | 3 (0.7%) | 1 |
| Arrhythmia—n (%) | 7 (5.4%) | 28 (6.9%) | 0.70 |
| Acute MI—n (%) | 4 (3.1%) | 1 (0.2%) | 0.01 |
| Myocarditis—n (%) | 7 (5.4%) | 24 (5.9%) | 1 |
| Pericarditis—n (%) | 0 | 2 (0.5%) | 1 |
| Cardiomyopathy—n (%) | 3 (2.3%) | 9 (2.2%) | 1 |
| Pneumonitis—n (%) | 10 (7.8%) | 8 (2%) | 0.003 |
| Type I diabetes—n (%) | 2 (1.6%) | 3 (0.7%) | 0.75 |
| Meningitis—n (%) | 0 | 0 | - |
| Encephalitis, myelitis, encephalomyelitis—n (%) | 0 | 0 | - |
| Vitiligo—n (%) | 1 (0.8%) | 0 | 0.54 |
| Psychological affections—n (%) | 598 (26.4%) | 2,505 (27.5%) | 0.30 |
| Depression—n (%) | 381 (16.8%) | 1,516 (16.6%) | 0.86 |
| Anxiety—n (%) | 440 (19.4%) | 1,893 (20.8%) | 0.15 |
| Bipolar Disorder—n (%) | 36 (1.6%) | 96 (1.1%) | 0.04 |
| Cognitive decline/dementia—n (%) | 308 (13.6%) | 608 (6.7%) | < 0.001 |
Bold values indicates the psychological affections category is composed of depression + anxiety + bipolar disorder.
Association between race and treatment or treatment adverse events
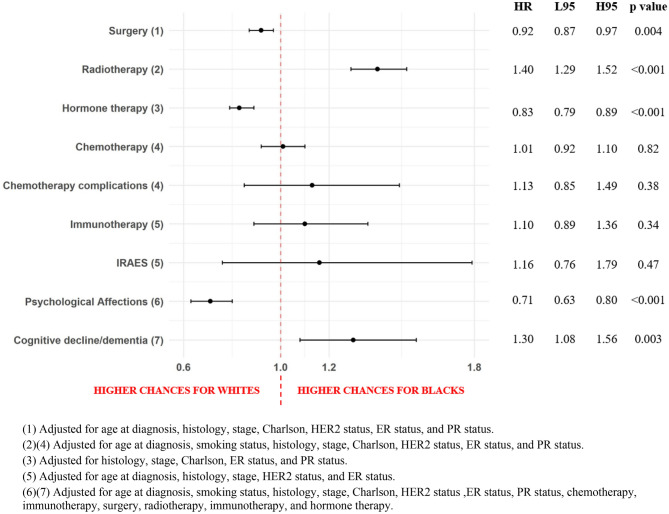
Multivariable cox proportional-hazards regressions (Fig. 2) revealed that, using NHWs as reference, NHBs had a lower probability of undergoing curative intent breast cancer surgery (aHR = 0.92, 95% CI 0.87–0.97) and of being prescribed endocrine therapy (aHR = 0.83, 95% CI 0.79–0.89), but a higher probability of receiving adjuvant radiotherapy (aHR = 1.40, 95% CI 1.29–1.52). For treatment related adverse mental health events, NHBs had lower risk of being diagnosed with psychological issues (aHR = 0.71, 95% CI 0.63–0.80) but a higher risk for cognitive decline/dementia (aHR = 1.30, 95% CI 1.08–1.56).
Figure 2.
Forest plot detailing association between race and treatment patterns or treatment adverse events for Breast Cancer University Hospitals (UH) population (2005–2022). Results are presented in hazard ratios (HR) for Blacks, lower 95% confidence intervals (L95), higher 95% confidence intervals (H95), and p-value.
Sensitivity analysis considering patients diagnosed after 2015 (n = 8,635) showed similar results (Supplemental Table II).
Discussion
The primary objective of this study was to perform a comprehensive analysis and provide an epidemiological exploration of racial disparities in treatment patterns and treatment related adverse events in Non-Hispanic women diagnosed with BC. With a retrospective design, our analyses included over 17,000 patients over a 17-year period and found that, when compared to NHW, NHB had lower probability of undergoing curative intent breast cancer surgery or of being prescribed endocrine therapy. At the same time NHB had lower rates of adherence to outpatient medical visits. Furthermore, we found that NHB compared to NHW had a 19% lower risk of being diagnosed with a psychological disorder, but a 30% higher risk of being diagnosed with cognitive decline/dementia after BC treatment. Our findings are important because it adds another dimension to BC racial disparities described in the literature, and point to the need for more personalized care and the development of public policies that equalize access to quality healthcare for minorities to mitigate poor outcomes.
In addition to our main findings, we also found that, besides breast cancer diagnosis occurring at younger age, NHBs had worse baseline health (characterized by higher levels of Charlson comorbidity score, and higher rates of smokers), and were more often diagnosed at advanced stages. Moreover, NHBs had higher rates of all treatment types alone or combined and a higher number of appointments per patient.
Racism is a central topic when analyzing racial disparities, and can manifest in a variety of ways which can impact health directly or indirectly34. Several published studies have demonstrated a direct correlation between self-reported personally-mediated racism and negative physical and mental health outcomes35,36. For example, inequities in income, education, employment and living standards, can greatly impact individual living environments and exposure to risk and protective factors35,37–40. Health consequences also occurs due to physical violence and stress pathways, which have negative psychological and physiological impacts35. In addition, racism is present in healthcare both in institutions and providers, leading to difficulties both in accessing and obtaining quality care35,41.
Historically, BC incidence is lower in Black when compared to White patients, however temporal trends show an increasingly incidence in the first group, and a stable pattern in the second group, probably as a result of improvement in health care access by Black patients42. Black women are diagnosed with BC at younger ages, and later stages, in addition to higher risks of lymph node or distant metastasis43,44. Several factors may explain these differences, which are consistent with our findings. There are biologic factors at play: Black women are diagnosed with TNBC at higher rates, are more likely to have somatic TP53 mutation, and less likely to have somatic PIK3CA mutations45–49. There are also environmental factors, such as social determinants of health (SDOH) which greatly influence cancer outcomes. Unemployment rates are higher in the Black community and are correlated with less job-based medical insurance and lower financial stability, creating barriers to health care access and leading to a delayed screening and diagnosis45,50,51.
For treatment, as stated above, Black women have higher rates of unemployment and a higher financial insecurity, which can negatively impact timely access to health care. This adds to the neighborhood context, as poor neighborhoods tends to be distant from health services45.
Prior studies have also previously reported that Black women experience greater time to BC treatment delays , and are more likely to experience very long treatment delays, and, even when they have access to treatment are less likely to receive treatments that are in accordance with national evidence based guidelines52–54. Furthermore, prior studies have also shown that Black BC patients are more prone to receive lower chemotherapy doses and greater likelihood of treatment modifications55–58. Our treatment patterns findings are consistent with the racial cancer disparities literature. The lower appointment completion rates in NHB may be linked to the barriers to accessing healthcare due to adverse SDOH and racism. We found higher rates of treatment in the NHB population, likely due to the more advanced breast cancer stage and histological type of disease at the time of initial diagnosis, factors that may also be the explanation for lower chances of surgery and hormone therapy, and higher chances of radiotherapy reported. Interestingly, previous studies have shown that NHB in the general population are less likely to be offered or receive radiotherapy59.
Prior cancer disparities work has not examined to what extent differences in the frequency or types of adverse effects of systemic treatments among NHB and NHW BC patients contributes to disparities in BC outcomes. Some studies have previously reported racial disparities in adverse drug events (ADE) in the general population being treated with anticoagulants, diabetic medications, and opioids60. We found that NHBs are at higher risk of cognitive decline/dementia, and cardiovascular (CV) subtypes of treatment adverse events. Higher risk of cognitive decline/dementia for NHB in the general population have been reported, but there are no published reports on the differential impact of breast cancer treatment on cognitive decline among NHB patients61. These poorer BC outcomes for NHB women and differences in treatment patterns suggest rather stark differences in the quality of cancer care which is a function of race19,62–66.
The racial disparities existent in demographics, treatment patterns, and treatment adverse events ultimately leads to disparities in BC mortality. Due to evolutions in treatment and screening, BC mortality reduced a 40% overall in the US since 199067. However, differences between races in BC mortality still existing, with an estimated 40% higher risk in NHBs than in NHW45,67,68.
This study has several limitations. Our institutional database is EMR-based, and some of the information in the EMR may be incomplete. As a study in a single institution, some patients may have been to lost follow-up or sought emergency care at other institutions, and the lack of available data could have impacted our analysis. Adverse event rates were based on EMR ICD codes and can be underreported. Some of the ICD codes utilized to identify treatment related adverse effects are not treatment-specific and therefore may not be treatment-related. The extended timeframe employed can encompass generational changes in treatment, which we mitigated with sensitivity analysis of patients diagnosed after 2015. Also, we used a database that integrates disparate sources and includes detailed and longitudinal information on each patient, rarely seen in other databases. Finally, as an oncology center, we maintain a close follow-up with patients who usually come to our emergency (ED).
In summary, we found not only racial disparities in curative intent BC surgery, as well as important differences in endocrine and/or chemotherapy, but we also identified differences between NHB and NHW BC patients in the frequency and types of treatment related adverse events they experience. Taken together, racial disparities in BC outcomes is related not only to socioeconomic differences and differences in access to care, but also differences in the types of curative interventions they undergo and differences in treatment related toxicities.
Supplementary Information
Author contributions
Planning of study: N.S. conceived the study concept, and the study design was by N.S., A.J.M., and J.B.S. Conduct of Study: N.S. had full access to all the data in the study and took responsibility for the data’s integrity and the accuracy of the data analysis. Reporting of Study: N.S. drafted the first version of the manuscript. All authors provided critical revisions of the manuscript for important intellectual content.
Funding
NS is supported through funding from the Sociedade Beneficente Israelita Brasileira Albert Einstein on the program “Marcos Lottenberg & Marcos Wolosker International Fellowship for Physicians Scientist—Case Western”. This work was supported by ACHIEVE GreatER (Addressing Cardiometabolic Health Inequities by Early PreVEntion in the Great LakEs Region).
Data availability
University Hospitals (UH) Seidman Cancer Center database is available at University Hospitals Cleveland Medical Center and has access restricted to researchers with IRB approval. Data are however available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests. JBS is a full-time, paid employee of the NCI/NIH. KW is a full-time, paid contractor of the NCI/NIH.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Jill S. Barnholtz-Sloan and Alberto J. Montero.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-27578-4.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Cancer of the Breast (Female)—Cancer Stat Facts [Internet]. SEER. [cited 2022 Jun 1]. https://seer.cancer.gov/statfacts/html/breast.html.
- 4.Waks AG, Winer EP. Breast cancer treatment: A review. JAMA. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 5.Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. The Lancet. 2021;397(10286):1750–1769. doi: 10.1016/S0140-6736(20)32381-3. [DOI] [PubMed] [Google Scholar]
- 6.Ewertz M, Jensen AB. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncol. 2011;50(2):187–193. doi: 10.3109/0284186X.2010.533190. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. N. Engl. J. Med. 2001;344(26):1997–2008. doi: 10.1056/NEJM200106283442607. [DOI] [PubMed] [Google Scholar]
- 8.Paraskevi T. Quality of life outcomes in patients with breast cancer. Oncol Rev. 2012;6(1):e2. doi: 10.4081/oncol.2012.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leclerc AF, Jerusalem G, Devos M, Crielaard JM, Maquet D. Multidisciplinary management of breast cancer. Arch. Public Health. 2016;74(1):50. doi: 10.1186/s13690-016-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zavala VA, Bracci PM, Carethers JM, Carvajal-Carmona L, Coggins NB, Cruz-Correa MR, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br. J. Cancer. 2021;124(2):315–332. doi: 10.1038/s41416-020-01038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esnaola NF, Ford ME. Racial differences and disparities in cancer care and outcomes: Where’s the rub? Surg Oncol Clin N Am. 2012;21(3):417-viii. doi: 10.1016/j.soc.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross CP, Smith BD, Wolf E, Andersen M. Racial disparities in cancer therapy. Cancer. 2008;112(4):900–908. doi: 10.1002/cncr.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. 2015;313(2):165–173. doi: 10.1001/jama.2014.17322. [DOI] [PubMed] [Google Scholar]
- 14.Nahleh Z, Otoukesh S, Mirshahidi HR, Nguyen AL, Nagaraj G, Botrus G, et al. Disparities in breast cancer: A multi-institutional comparative analysis focusing on American Hispanics. Cancer Med. 2018;7(6):2710–2717. doi: 10.1002/cam4.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Alcalá ME, Qin H, Jeanetta S. The role of acculturation and social capital in access to health care: A meta-study on hispanics in the US. J. Commun. Health. 2019;44(6):1224–1252. doi: 10.1007/s10900-019-00692-z. [DOI] [PubMed] [Google Scholar]
- 16.Parise CA, Bauer KR, Caggiano V. Variation in breast cancer subtypes with age and race/ethnicity. Crit. Rev. Oncol. Hematol. 2010;76(1):44–52. doi: 10.1016/j.critrevonc.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Wheeler SB, Reeder-Hayes KE, Carey LA. Disparities in breast cancer treatment and outcomes: Biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18(9):986–993. doi: 10.1634/theoncologist.2013-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lund MJ, Brawley OP, Ward KC, Young JL, Gabram SSG, Eley JW. Parity and disparity in first course treatment of invasive breast cancer. Breast Cancer Res. Treat. 2008;109(3):545–557. doi: 10.1007/s10549-007-9675-8. [DOI] [PubMed] [Google Scholar]
- 19.Pinheiro LC, Reshetnyak E, Safford MM, Kern LM. Racial disparities in preventable adverse events attributed to poor care coordination reported in a national study of older US adults. Med. Care. 2021;59(10):901–906. doi: 10.1097/MLR.0000000000001623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potentially Preventable Hospitalizations—United States, 2001–2009 [Internet]. [cited 2022 Jun 24]. https://www.cdc.gov/mmwr/preview/mmwrhtml/su6203a23.htm.
- 21.Bakullari A, Metersky ML, Wang Y, Eldridge N, Eckenrode S, Pandolfi MM, et al. Racial and ethnic disparities in healthcare-associated infections in the United States, 2009–2011. Infect. Control Hosp. Epidemiol. 2014;35(S3):S10–S16. doi: 10.1086/677827. [DOI] [PubMed] [Google Scholar]
- 22.Stabellini N, Chandar AK, Chak A, Barda AJ, Dmukauskas M, Waite K, et al. Sex differences in esophageal cancer overall and by histological subtype. Sci. Rep. 2022;12(1):5248. doi: 10.1038/s41598-022-09193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stabellini N, Bruno DS, Dmukauskas M, Barda AJ, Cao L, Shanahan J, et al. Sex differences in lung cancer treatment and outcomes at a large hybrid academic-community practice. JTO Clin. Res. Rep. 2022;3(4):100307. doi: 10.1016/j.jtocrr.2022.100307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caisis | The Free & Open Source Cancer Data Management System [Internet]. [cited 2021 Apr 15]. http://www.caisis.org/.
- 25.EMERSE: Electronic Medical Record Search Engine [Internet]. [cited 2022 May 16]. https://project-emerse.org/index.html.
- 26.International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) [Internet]. [cited 2021 Apr 15]. https://www.who.int/standards/classifications/other-classifications/international-classification-of-diseases-for-oncology.
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Giuliano AE, Edge SB, Hortobagyi GN. Eighth edition of the AJCC cancer staging manual: Breast cancer. Ann. Surg. Oncol. 2018;25(7):1783–1785. doi: 10.1245/s10434-018-6486-6. [DOI] [PubMed] [Google Scholar]
- 29.Makki J. Diversity of Breast carcinoma: Histological subtypes and clinical relevance. Clin. Med. Insights Pathol. 2015;21(8):23–31. doi: 10.4137/CPath.S31563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.HER2 Testing in Breast Cancer—2018 Focused Update [Internet]. College of American Pathologists. [cited 2022 Dec 11]. https://www.cap.org/protocols-and-guidelines/cap-guidelines/current-cap-guidelines/recommendations-for-human-epidermal-growth-factor-2-testing-in-breast-cancer.
- 31.Devlin EJ, Denson LA, Whitford HS. Cancer treatment side effects: A meta-analysis of the relationship between response expectancies and experience. J. Pain Symptom. Manag. 2017;54(2):245–258.e2. doi: 10.1016/j.jpainsymman.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 32.RStudio | Open source & professional software for data science teams [Internet]. [cited 2022 May 3]. https://www.rstudio.com/.
- 33.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vince RA, Eyrich NW, Mahal BA, Stensland K, Schaeffer EM, Spratt DE. Reporting of racial health disparities research: Are we making progress? J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022;40(1):8–11. doi: 10.1200/JCO.21.01780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanley J, Harris R, Cormack D, Waa A, Edwards R. The impact of racism on the future health of adults: Protocol for a prospective cohort study. BMC Public Health. 2019;19(1):346. doi: 10.1186/s12889-019-6664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Racism as a Determinant of Health: A Systematic Review and Meta-Analysis [Internet]. [cited 2022 Jan 21]. 10.1371/journal.pone.0138511
- 37.Williams DR, Mohammed SA. Racism and health I: Pathways and scientific evidence. Am. Behav. Sci. 2013;57(8):1152–1173. doi: 10.1177/0002764213487340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones CP. Confronting institutionalized racism. Phylon 1960. 2002;50(1/2):7–22. doi: 10.2307/4149999. [DOI] [Google Scholar]
- 39.Paradies YC. Defining, conceptualizing and characterizing racism in health research. Crit. Public Health. 2006;16(2):143–157. doi: 10.1080/09581590600828881. [DOI] [Google Scholar]
- 40.Krieger N. Methods for the scientific study of discrimination and health: An ecosocial approach. Am. J. Public Health. 2012;102(5):936–944. doi: 10.2105/AJPH.2011.300544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones CP. Invited commentary: “Race”, racism, and the practice of epidemiology. Am. J. Epidemiol. 2001;154(4):299–304. doi: 10.1093/aje/154.4.299. [DOI] [PubMed] [Google Scholar]
- 42.SEER Cancer Statistics Review, 1975–2018 [Internet]. SEER. [cited 2022 Jun 26]. https://seer.cancer.gov/csr/1975_2018/index.html.
- 43.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019;69(6):438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 44.Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J. Clin. 2015;65(3):221–238. doi: 10.3322/caac.21271. [DOI] [PubMed] [Google Scholar]
- 45.Stringer-Reasor EM, Elkhanany A, Khoury K, Simon MA, Newman LA. Disparities in breast cancer associated With African American identity. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 2021;41:e29–46. doi: 10.1200/EDBK_319929. [DOI] [PubMed] [Google Scholar]
- 46.Pitt JJ, Riester M, Zheng Y, Yoshimatsu TF, Sanni A, Oluwasola O, et al. Characterization of Nigerian breast cancer reveals prevalent homologous recombination deficiency and aggressive molecular features. Nat. Commun. 2018;9(1):4181. doi: 10.1038/s41467-018-06616-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goel N, Kim DY, Guo JA, Zhao D, Mahal BA, Alshalalfa M. Racial differences in genomic profiles of breast cancer. JAMA Netw. Open. 2022;5(3):e220573. doi: 10.1001/jamanetworkopen.2022.0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keenan T, Moy B, Mroz EA, Ross K, Niemierko A, Rocco JW, et al. Comparison of the genomic landscape between primary breast cancer in African American versus white women and the association of racial differences with tumor recurrence. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015;33(31):3621–3627. doi: 10.1200/JCO.2015.62.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dietze EC, Sistrunk C, Miranda-Carboni G, O’Regan R, Seewaldt VL. Triple-negative breast cancer in African–American women: Disparities versus biology. Nat. Rev. Cancer. 2015;15(4):248–254. doi: 10.1038/nrc3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ansell D, Grabler P, Whitman S, Ferrans C, Burgess-Bishop J, Murray LR, et al. A community effort to reduce the black/white breast cancer mortality disparity in Chicago. Cancer Causes Control CCC. 2009;20(9):1681–1688. doi: 10.1007/s10552-009-9419-7. [DOI] [PubMed] [Google Scholar]
- 51.Press R, Carrasquillo O, Sciacca RR, Giardina EGV. Racial/ethnic disparities in time to follow-up after an abnormal mammogram. J. Womens Health 2002. 2008;17(6):923–930. doi: 10.1089/jwh.2007.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silber JH, Rosenbaum PR, Clark AS, Giantonio BJ, Ross RN, Teng Y, et al. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013;310(4):389–397. doi: 10.1001/jama.2013.8272. [DOI] [PubMed] [Google Scholar]
- 53.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch. Intern. Med. 2003;163(1):49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 54.Griggs JJ, Culakova E, Sorbero MES, Poniewierski MS, Wolff DA, Crawford J, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007;25(18):2522–2527. doi: 10.1200/JCO.2006.10.2749. [DOI] [PubMed] [Google Scholar]
- 55.Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009;27(5):713–719. doi: 10.1200/JCO.2008.17.9234. [DOI] [PubMed] [Google Scholar]
- 56.Griggs JJ, Sorbero MES, Stark AT, Heininger SE, Dick AW. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res. Treat. 2003;81(1):21–31. doi: 10.1023/A:1025481505537. [DOI] [PubMed] [Google Scholar]
- 57.Bickell NA, Wang JJ, Oluwole S, Schrag D, Godfrey H, Hiotis K, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006;24(9):1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 58.Smith K, Wray L, Klein-Cabral M, Schuchter L, Fox K, Glick J, et al. Ethnic disparities in adjuvant chemotherapy for breast cancer are not caused by excess toxicity in black patients. Clin. Breast Cancer. 2005;6(3):260–266. doi: 10.3816/CBC.2005.n.029. [DOI] [PubMed] [Google Scholar]
- 59.Smith GL, Shih YCT, Xu Y, Giordano SH, Smith BD, Perkins GH, et al. Racial disparities in the use of radiotherapy after breast-conserving surgery: A national medicare study. Cancer. 2010;116(3):734–741. doi: 10.1002/cncr.24741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baehr A, Peña JC, Hu DJ. Racial and ethnic disparities in adverse drug events: A systematic review of the literature. J. Racial. Ethn. Health Dispar. 2015;2(4):527–536. doi: 10.1007/s40615-015-0101-3. [DOI] [PubMed] [Google Scholar]
- 61.Wright CB, DeRosa JT, Moon MP, Strobino K, DeCarli C, Cheung YK, et al. Race/ethnic disparities in mild cognitive impairment and dementia: The northern manhattan study. J. Alzheimers Dis. 2021;80(3):1129–1138. doi: 10.3233/JAD-201370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martino SC, Elliott MN, Hambarsoomian K, Weech-Maldonado R, Gaillot S, Haffer SC, et al. Racial/ethnic disparities in medicare beneficiaries’ care coordination experiences. Med. Care. 2016;54(8):765–771. doi: 10.1097/MLR.0000000000000556. [DOI] [PubMed] [Google Scholar]
- 63.Hicks LS, Ayanian JZ, Orav EJ, Soukup J, McWilliams JM, Choi SS, et al. Is hospital service associated with racial and ethnic disparities in experiences with hospital care? Am. J. Med. 2005;118(5):529–535. doi: 10.1016/j.amjmed.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 64.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N. Engl. J. Med. 2004;351(6):575–584. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 65.Hall WJ, Chapman MV, Lee KM, Merino YM, Thomas TW, Payne BK, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: A systematic review. Am. J. Public Health. 2015;105(12):e60–76. doi: 10.2105/AJPH.2015.302903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen MJ, Peterson EB, Costas-Muñiz R, Hernandez MH, Jewell ST, Matsoukas K, et al. The effects of race and racial concordance on patient-physician communication: A systematic review of the literature. J. Racial Ethn. Health Dispar. 2018;5(1):117–140. doi: 10.1007/s40615-017-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jatoi I, Sung H, Jemal A. The emergence of the racial disparity in U.S. breast-cancer mortality. N. Engl. J. Med. 2022;386(25):2349–2352. doi: 10.1056/NEJMp2200244. [DOI] [PubMed] [Google Scholar]
- 68.Yedjou CG, Sims JN, Miele L, Noubissi F, Lowe L, Fonseca DD, et al. Health and racial disparity in breast cancer. Adv Exp Med Biol. 2019;1152:31–49. doi: 10.1007/978-3-030-20301-6_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
University Hospitals (UH) Seidman Cancer Center database is available at University Hospitals Cleveland Medical Center and has access restricted to researchers with IRB approval. Data are however available from the corresponding author upon reasonable request.