Abstract
Matrix metalloproteinases (MMPs) constitute a large family of enzymes with specificity for the various proteins of the extracellular matrix which are implicated in tissue remodeling processes and chronic inflammatory conditions. To investigate the role of MMPs in immunity to mycobacterial infections, we incubated murine peritoneal macrophages with viable Mycobacterium bovis BCG or Mycobacterium tuberculosis H37Rv and assayed MMP activity in the supernatants by zymography. Resting macrophages secreted only small amounts of MMP-9 (gelatinase B), but secretion increased dramatically in a dose-dependent manner in response to either BCG or M. tuberculosis in vitro. Incubation with mycobacteria also induced increased MMP-2 (gelatinase A) activity. Neutralization of tumor necrosis alpha (TNF-α), and to a lesser extent interleukin 18 (IL-18), substantially reduced MMP production in response to mycobacteria. Exogenous addition of TNF-α or IL-18 induced macrophages to express MMPs, even in the absence of bacteria. The immunoregulatory cytokines gamma interferon (IFN-γ), IL-4, and IL-10 all suppressed BCG-induced MMP production, but through different mechanisms. IFN-γ treatment increased macrophage secretion of TNF-α but still reduced their MMP activity. Conversely, IL-4 and IL-10 seemed to act by reducing the amount of TNF-α available to the macrophages. Finally, infection of BALB/c or severe combined immunodeficiency (SCID) mice with either BCG or M. tuberculosis induced substantial increases in MMP-9 activity in infected tissues. In conclusion, we show that mycobacterial infection induces MMP-9 activity both in vitro and in vivo and that this is regulated by TNF-α, IL-18, and IFN-γ. These findings indicate a possible contribution of MMPs to tissue remodeling processes that occur in mycobacterial infections.
Infection with virulent mycobacterial species results in a granulomatous disease of the affected organs. Granuloma formation and activation of macrophages and T cells are crucial parts of a protective cellular immune response (28, 31, 39). T-cell immunity is mainly mediated by secretion of gamma interferon (IFN-γ), which induces effective macrophage killing of ingested bacteria. Macrophages also secrete cytokines and chemokines important for containing the infection. Among these, tumor necrosis factor alpha (TNF-α) is important for granuloma formation and host resistance (8, 15, 45), but if it is produced in excess, or late in the infection, the effects can be detrimental (3, 32, 49). Thus, the same effector mechanisms can result in both protective immune responses and pathology, depending on their strength and kinetics (11). These pathological processes are often associated with tissue remodeling and breakdown of the extracellular matrix (ECM).
Matrix metalloproteinases (MMPs) constitute a large family of Zn2+- and Ca2+-dependent endopeptidases, implicated in tissue remodeling and chronic inflammation. They possess broad and overlapping specificities and collectively have the capacity to degrade all the components of the ECM (42, 52). MMPs are produced by many cell types, including lymphocytes and granulocytes, but in particular by activated macrophages (17). MMPs are secreted as proenzymes, which are activated by proteolytic cleavage and regulated by a family of inhibitors called the tissue inhibitors of matrix metalloproteinases (TIMPs), which are constitutively produced by a variety of cells. Changes in actual MMP activity are thus dependent on the balance between production and activation of MMPs and the local levels of TIMPs. In rheumatoid arthritis, pulmonary emphysema, periodontal disease, and inflammatory bowel disease, MMPs are believed to be responsible for much of the associated tissue destruction (4, 35, 43, 50). In addition to their direct effects on ECM proteins, MMPs can exacerbate inflammation by activating the proinflammatory cytokine interleukin-1β (IL-1β) or releasing cytokines such as TNF-α and IL-6 from cell surfaces (1, 21, 23). Their generation of chemotactic fragments from ECM proteins may also contribute to the recruitment of inflammatory cells (22, 40).
Little is known about MMP production during bacterial infections and the contribution they make to immunity versus pathology. Systemic Escherichia coli infection, acute Lyme neuroborreliosis, and pneumococcal meningitis can all lead to secretion of significant amounts of MMP-9 (25, 29, 34), and in the last infection MMP-9 is suggested to contribute to destruction of the blood-brain barrier and to neuronal injury. In cell culture, MMP production is induced by bacterial products such as lipopolysaccharide (LPS), phospholipase C, and clamydial heat shock proteins (10, 14, 26, 54). Several proinflammatory cytokines produced in response to bacterial infections, including TNF-α, IL-1, and granulocyte-macrophage colony-stimulating factor (GM-CSF), have also been shown to up-regulate monocyte and macrophage MMP production in vitro (38, 55). Significantly, a recent report has demonstrated increased levels of MMP-9 in bronchoalveolar lavage fluids from tuberculosis patients and that heat-killed Mycobacterium tuberculosis as well as mycobacterial cell wall components increase MMP-9 mRNA production by a myelomonocytic cell line (7).
In order to investigate the regulation of MMP expression by live mycobacteria in more detail, we have compared the effects of Mycobacterium bovis BCG and M. tuberculosis on MMP expression by purified macrophages in culture and also in the lungs, livers, and spleens of infected mice. We now show that mycobacteria induce extensive production of the gelatinases MMP-9 and MMP-2 both in vivo and in vitro and that this process is regulated by both macrophage- and T-cell-derived cytokines.
MATERIALS AND METHODS
Animals.
CB-17/ICR severe combined immunodeficiency (SCID) mice were bred under aseptic conditions and maintained in microisolator cages at the London School of Hygiene and Tropical Medicine. BALB/c mice were purchased from Harlan (Oxon, United Kingdom). Female animals between 8 and 15 weeks of age were used.
Bacterial strains and infections.
M. tuberculosis strain H37Rv was obtained from the American Type Culture Collection (ATCC 25618), and M. bovis BCG was obtained from Statens Seruminstitut (Copenhagen, Denmark). Bacteria were grown to mid-log phase, washed twice in phosphate-buffered saline, and kept frozen at −70°C, and a fresh aliquot was thawed for each experiment. Mice were infected with 106 CFU of M. tuberculosis H37Rv or BCG in 200 μl of normal saline, via a lateral tail vein. The multiplicity of infection was calculated from mycobacterial stocks which were stored at −70°C and regularly confirmed by plating the bacterial input at the time of experimentation. In the case of M. tuberculosis, the multiplicity of infection was confirmed by direct plating of the inoculum in each experiment. Sham-infected mice received saline only. For in vitro experiments, thawed aliquots of bacteria were kept at 4°C for up to 3 weeks without loss of activity.
Stimulation of macrophage MMP expression in vitro.
Peritoneal exudate cells were collected 3 to 4 days following intraperitoneal injection of 10% Proteose Peptone into naive mice by injection and retrieval of 10 ml of ice-cold RPMI medium containing 1% fetal calf serum. Isolated cells were washed in medium and plated at 2 × 106 cells/well in 24-well tissue culture plates in RPMI containing 2.5% fetal calf serum, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, and 100 mM l-glutamine (Life Technologies, Paisley, United Kingdom). Two hours later, plates were washed extensively with room temperature RPMI to remove nonadherent cells, and the remaining adherent cells were subsequently cultured in 0.5 ml of macrophage serum-free medium (MØ-SFM; Life Technologies) supplemented with penicillin and streptomycin as above. In the experiments comparing BCG and M. tuberculosis, no antibiotics were used. Recombinant IL-12, IL-18 (both from Genzyme, Cambridge, Mass.), TNF-α (Life Technologies), or thawed and gently resuspended viable mycobacteria were added and incubated at 37°C for up to 4 days. Input bacterial concentrations ranged between 0.001 and 30 bacteria per macrophage. Culture medium was collected, centrifuged at 10,000 rpm for 10 min in an Eppendorf centrifuge, aliquoted, and stored at −20°C until analysis of MMP activity. In some experiments, neutralizing antibodies to TNF-α (clone TN3, a kind gift from R. D. Schreiber, Washington University School of Medicine, St. Louis, Mo.), IL-12 (clone C17.8, a kind gift from G. Trinchieri, Wistar Institute, Philadephia, Pa.) (53), IL-18 (MBL Co., Ltd., Nagoya, Japan), GM-CSF (clone MPI.22E9.11, a kind gift from J. Abrams, DNAX, Palo Alto, Calif.), blocking antibodies to the IL-1β receptor (Pharmingen, San Diego, Calif.), or 5 μg of indomethacin (Sigma, St. Louis, Mo.) per ml were added to the macrophage cultures immediately prior to addition of bacteria or cytokines. In other cultures, recombinant IL-4 (Life Technologies), IL-10 (R&D, Abingdon, United Kingdom), or IFN-γ (Life Technologies) either were added for 20 h and then washed off before BCG stimulation or were added at the same time as the BCG. Macrophage-conditioned medium was generated by incubating naive peritoneal macrophages prepared as above with 10 BCG bacteria per macrophage. The supernatant was removed after 18 h and sterile filtered through a 22-μm-pore-size filter. Conditioned medium was added to uninfected macrophages for 18 h and removed by washing the macrophages twice in fresh medium, and MMP activity was assessed in the supernatants after a further 24 or 72 h of incubation.
Specimen collection and extraction of MMP activity from tissues.
Mice infected intravenously (i.v.) with BCG or M. tuberculosis H37Rv were killed at various time points after infection, and lungs, spleens, and livers were collected for analysis of histology, bacterial burden, and protein extraction. For determination of pathology and granuloma formation in M. tuberculosis-infected mice, approximately 200 mg from each organ was fixed in formalin, embedded in paraffin, and subsequently used for routine hematoxylin and eosin staining for morphology and Ziehl-Neelsen staining to detect mycobacteria. The bacterial burden in each tissue was assessed in organs homogenized by passage through 100-μm nylon membranes in water containing 0.05% Tween 80. Serial 10-fold dilutions of the homogenate in 7H10 medium were plated on Middlebrook 7H11 agar plates supplemented with oleic acid, albumin, dextrose, and catalase (all from Difco, Detroit, Mich.) and incubated for 3 to 4 weeks at 37°C before the counting of CFU. The remaining homogenized tissue was incubated for 2 h at 4°C, centrifuged at 10,000 rpm in an Eppendorf centrifuge, and sterile filtered. The protein concentration was determined using the BCA kit (Pierce, Rockford, Ill.), and the protein extract was frozen in aliquots at −20°C until analysis of MMP activity. The organs of BCG-infected mice were prepared as above and used directly for CFU determination and formalin fixation, and the remaining snap-frozen tissue was kept at −70°C until used for protein extraction in water-Tween 80 as above.
Detection of MMPs using zymography.
The MMP activity in tissue extracts and supernatants was analyzed using substrate gel sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) zymography (20). Tissue extracts were adjusted to the same protein concentration (see below) and mixed with an equal volume of nonreducing SDS-PAGE sample buffer (0.2 M Tris-HCl, 20% glycerol, 6% SDS, 0.05% bromophenol blue, pH 6.7), and 40 μl of this mixture was loaded per lane. Cell culture medium was mixed with an equal volume of sample buffer, and 40 μl was loaded per lane. In some assays, the supernatants were concentrated 20 times using Centricon tubes with a 10-kDa cutoff (Millipore Corporation, Bedford, Mass.) before analysis. The samples were separated in 7.5% polyacrylamide gels containing 1 mg of gelatin (type B, 225 Bloom; Sigma) per ml or 0.5 mg of α-casein (Sigma) per ml at 120 V (gelatin gels) or 60 V (casein gels). The gels were then incubated for 30 min on a rotating platform in Tris-buffered saline (10 mM Tris-HCl, 0.15 M NaCl, pH 7.6 [TBS]) containing 2.5% Triton X-100. They were washed three times in TBS and then incubated for 20 h at 37°C in TBS containing 5 mM CaCl2, 1% Triton X-100, and 0.02% NaN3. Coomassie blue staining revealed the presence of gelatinolytic or caseinolytic activity as clear bands against the blue background. Recombinant murine MMP-9 or purified murine MMP-3 (both from Chemicon, Harrow, United Kingdom) were used as positive controls, and the sensitivity of the assays was >50 pg. Inclusion of 10 mM EDTA in assay buffers completely inhibited all MMP activity in the samples, and the identity of MMP-9 in macrophage cultures stimulated with BCG was further confirmed by Western blotting, using a polyclonal goat antibody reacting with murine MMP-9 (Santa Cruz Biotechnology, Santa Cruz, Calif.).
To estimate the amount of active MMPs in a certain sample, the intensity of the lytic bands was determined using Phoretix 1d software. Data are presented as relative enzymatic activity obtained by dividing the optical density of the experimental sample with that resulting from the activity of 2 ng of rMMP-9 separated on the same gel. The procedure was validated by analyzing serial dilutions of rMMP-9 by zymography, which yielded a straight-line relationship between MMP concentration and optical density for samples containing between 0.5 and 8 ng of MMP-9 (relative enzymatic activities between 0.25 and 3). The total amount of tissue-extracted protein that was loaded onto the gel was adjusted to fall within this range, resulting in 3, 40, and 20 μg of total spleen, liver, and lung proteins, respectively, being loaded in individual lanes.
Cytokine detection.
The concentration of TNF-α in culture supernatants was determined by enzyme-linked immunosorbent assay (ELISA). Ninety-six-well plates were coated with 5 μg of anti-murine TNF-α monoclonal antibody TN3 per ml. Captured TNF-α was detected by stepwise addition of a polyclonal rabbit serum raised against murine TNF-α, horseradish peroxidase-labeled goat anti-rabbit antibodies (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.), 1 mg of 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS), and 0.04% H2O2 substrate. Standard curves were constructed using recombinant mouse TNF-α, and the sensitivity of the assay was >1 ng/ml.
Statistical evaluation.
Differences in enzymatic activity between groups of mice were evaluated using the two-tailed t test for independent samples.
RESULTS
Stimulation with BCG induces macrophage secretion of MMPs.
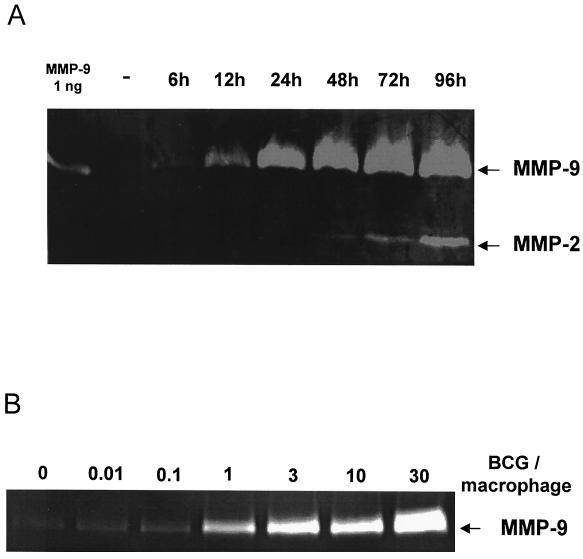
To determine if mycobacteria can induce secretion of MMPs from macrophages, peritoneal macrophages were incubated with 10 live M. bovis BCG bacteria per macrophage, and culture supernatants were collected at various time points. Zymography analyses showed a low but consistent secretion of MMP-9 (gelatinase B) in media from unstimulated cells cultured for 48 h. Stimulation with BCG increased MMP-9 expression within 6 h, and it rose progressively, reaching a maximum after 48 h (Fig. 1A). The identity of MMP-9 was further confirmed by Western blotting. In contrast, production of MMP-2 (gelatinase A) was not detected until after 48 h of incubation with BCG, and maximal MMP-2 activity was consistently less than that of MMP-9. Neither MMP-2 nor MMP-9 production was influenced by addition of the PGE2 synthase inhibitor indomethacin (data not shown).
FIG. 1.
M. bovis BCG induces gelatinase activity in macrophages. Macrophage monolayers were incubated with either medium alone or 10 BCG bacteria per macrophage for various times (A) or for 48 h with 0.01 to 30 BCG bacteria per macrophage (B). Cell culture supernatants were analyzed for gelatinase activity using zymography. Data presented are representative of four independent experiments.
Reproducible induction of MMP-9 activity was observed with 0.01 BCG bacterium per macrophage and increased in a dose-dependent manner at least up to 30 BCG bacteria per macrophage (Fig. 1B). Our results indicate that upregulation of MMP activity by mycobacteria occurs equally well in response to live and dead bacteria. Thus, the presence (Fig. 1; see also Fig. 2, 3, and 5) or absence (see Fig. 7) of streptomycin in the culture media has no effect on the magnitude of the response observed under these conditions. Production of other gelatinolytic enzymes, particularly MMP-3 or MMP-7, was not observed under these conditions or in casein zymograms, even after concentrating the culture supernatants 20 times. Together these results demonstrate that BCG is a relatively selective inducer of MMP-9, and to a lesser extent MMP-2, expression by murine macrophages in vitro.
FIG. 2.
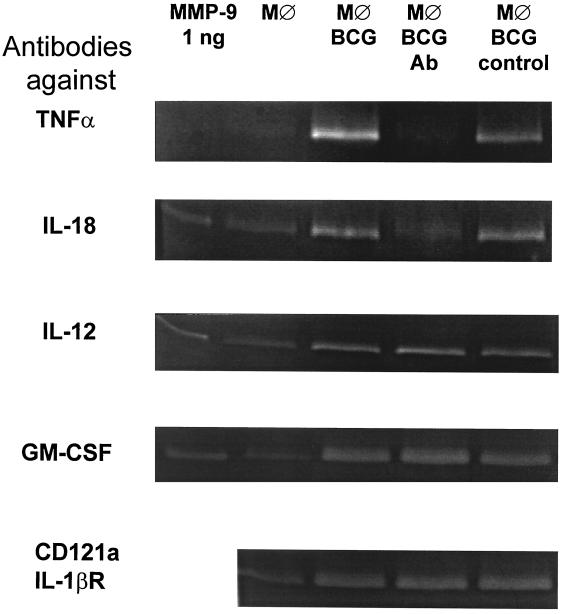
Neutralization of TNF-α or IL-18 inhibits M. bovis BCG-induced MMP secretion by macrophages. Macrophage monolayers were incubated with either medium alone (MØ) or with 0.1 BCG bacterium per macrophage (MØ BCG) in the absence or presence of neutralizing antibodies to TNF-α, IL-18, IL-12, GM-CSF, or the IL-1β receptor CD121a (MØ BCG Ab), or relevant isotype control antibodies (MØ BCG control). Cell culture supernatants were harvested after 72 h and analyzed for gelatinase activity using zymography. Data presented are representative of two to four independent experiments.
FIG. 3.
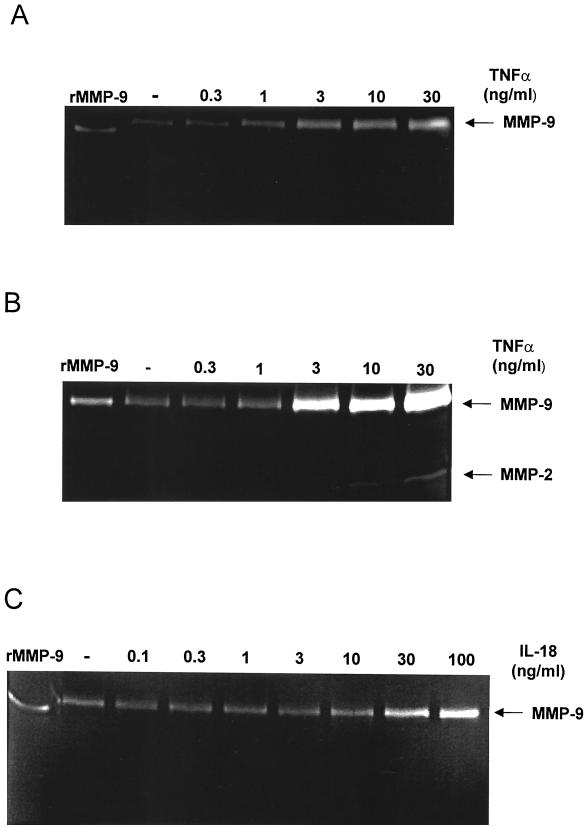
Recombinant TNF-α and IL-18 induce macrophage MMP activity. Macrophage monolayers were incubated in medium alone or with increasing concentrations of recombinant murine TNF-α for 24 h (A) or 72 h (B) or with recombinant murine IL-18 for 72 h (C). Cell culture supernatants were analyzed for gelatinase activity using zymography. No MMP activity could be detected in IL-18-stimulated cultures by 24 h. Data presented are representative of three to five independent experiments.
FIG. 5.
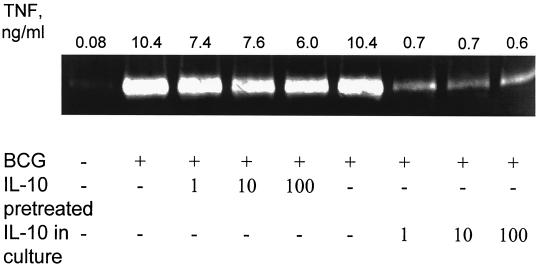
IL-10 regulates macrophage production of MMP-9. Macrophage monolayers were incubated with medium alone or with 1 to 100 IU of IL-10 per ml either for 20 h prior to (pretreated) or at the time of addition of 10 M. bovis BCG bacteria per macrophage (IL-10 in culture). Cell culture supernatants were harvested 48 h later, assayed for gelatinase activity using zymography, and analyzed for TNF-α content in ELISA. Variation between duplicates in ELISA was consistently <5%. Data presented are representative of three independent experiments, with the TNF-α concentrations from the same cultures given above each zymogram lane.
FIG. 7.
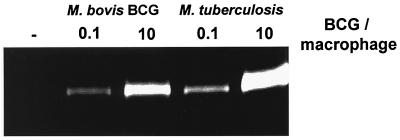
M. tuberculosis induces gelatinase activity in macrophages in vitro. Macrophage monolayers were incubated either with medium alone or with 0.1 or 10 M. tuberculosis H37Rv or M. bovis BCG bacteria per macrophage. Cell culture supernatants were collected after 48 h and analyzed for gelatinase activity using zymography. Data presented are representative of two independent experiments.
The role of macrophage-derived cytokines in MMP induction by BCG.
Under the culture conditions we used, BCG is readily phagocytosed (13), which induces the secretion of a range of macrophage-derived cytokines. To compare the role of ingestion per se versus cytokine secretion in MMP induction, macrophages were incubated with BCG and neutralizing antibodies to selected proinflammatory cytokines known to be produced after BCG ingestion (12, 13, 46, 51), and the resulting supernatants were assayed by zymography. When low numbers of BCG bacteria were incubated with the macrophages (0.1 BCG bacterium per macrophage), neutralization of TNF-α abolished MMP-9 induction (Fig. 2), while antibodies to IL-18 partially reduced MMP-9 secretion. When higher bacterial burdens were used (10 BCG bacteria per macrophage), the effect of anti-TNF-α antibodies was still evident, whereas antibodies to IL-18 had no detectable effect. In the latter setting, however, the combination of antibodies to TNF-α and IL-18 resulted in a further decrease in MMP activity, compared to the effect of anti-TNF-α alone (data not shown). In contrast, addition of antibodies to IL-12 or GM-CSF, or blocking of the IL-1βR, did not affect the MMP response (Fig. 2).
In order to determine if cytokines alone could induce MMP secretion to the same extent as BCG, macrophages were cultured with purified recombinant TNF-α or IL-18 in the absence of mycobacteria. TNF-α induced a dose-dependent production of MMP-9, with concentrations as low as 1 ng/ml resulting in increased levels of MMP-9 after 24 h of stimulation (Fig. 3A). In contrast, 10 ng of TNF-α per ml was needed to induce MMP-2, which was not detected until after 72 h of stimulation (Fig. 3B). When macrophages were cultured with IL-18, only ≥30 ng of IL-18 per ml induced increased production of MMP-9, but not until 72 h after stimulation (Fig. 3C). Addition of TNF-α plus IL-18 did not induce any further MMP production than that observed with either cytokine alone (data not shown). We have no evidence to suggest that the MMP activity induced by IL-18 results from increased TNF-α secretion, since this cytokine could not be detected in supernatants from IL-18-stimulated macrophages, and neutralization of TNF-α in these cultures did not inhibit the IL-18-induced MMP production. Furthermore, the ability of secreted products from BCG-stimulated macrophages to influence MMP production was tested using conditioned media from BCG-stimulated macrophages, which induced a slightly increased MMP-9 activity, but not to the same extent as did infection with BCG (data not shown). Together, these results indicate that TNF-α and IL-18 produced by macrophages in response to mycobacterial infection induce MMP activity in an autocrine fashion.
Effects of immunoregulatory cytokines on macrophage MMP production.
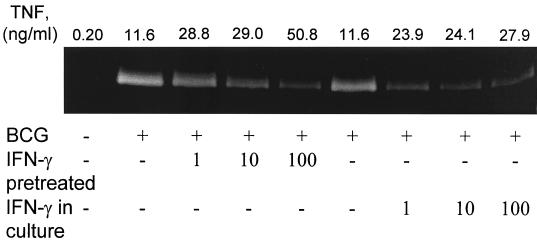
In granulomas, macrophages are subjected to the effects of several immunomodulatory cytokines, some of the more prominent being the activating cytokine IFN-γ and the inhibitory cytokines IL-10 and IL-4 (19, 33). To examine if BCG-induced MMP production could be further modulated by these cytokines, macrophages were preincubated with any one of these cytokines and then stimulated with BCG as described above. In parallel experiments, the cytokines were added at the same time as BCG, and all cell cultures were analyzed by zymography. Addition of IFN-γ inhibited MMP-9 production either when the macrophages were pretreated for 20 h before addition of BCG or when IFN-γ was added at the same time (Fig. 4). Paradoxically, IFN-γ treatment resulted in reduced MMP production despite increasing levels of TNF-α in the cultures compared to the MMP production of those with BCG alone (Fig. 4).
FIG. 4.
IFN-γ regulates macrophage production of MMP-9. Macrophage monolayers were incubated with medium alone or with 1 to 100 IU of IFN-γ per ml either for 20 h prior (pretreated) or at the time of addition of 10 M. bovis BCG bacteria per macrophage (IFN-γ in culture). Cell culture supernatants were harvested 48 h later, assayed for gelatinase activity using zymography, and analyzed for TNF-α content in ELISA. Variation between duplicates in ELISA was consistently <5%. Data presented are representative of three independent experiments, with the TNF-α concentrations from the same cultures given above each zymogram lane.
We also examined the effect of IL-10, a cytokine with inhibitory effects on macrophage effector function, on BCG-induced MMP-9 activity. Minimal effect was seen on MMP activity when macrophages were pretreated with different concentrations of IL-10 and then stimulated with BCG (Fig. 5). On the other hand, addition of IL-10 at the same time as BCG resulted in a clear reduction in the amount of MMP-9 secreted by the macrophages. In contrast to what was observed with IFN-γ, the inhibitory effect of IL-10 on MMP-9 expression correlated with reduced TNF-α levels (Fig. 5) and could be overcome by addition of exogenous TNF-α to the cultures (data not shown). Treatment with IL-4 resulted in a modest reduction of MMP-9 activity in both assay protocols. As with IL-10, the reduction in MMP-9 activity correlated with reduced levels of TNF-α in the culture supernatants (data not shown).
In vivo production of MMPs during BCG infection.
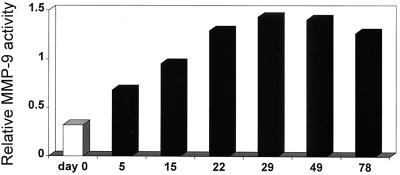
To determine if increased MMP activity was also a feature of mycobacterial infections in vivo, MMP levels were assayed in tissue homogenates prepared from spleens, livers, and lungs of BALB/c mice during the course of infection with BCG. The baseline MMP activity in uninfected or saline-treated mice differed between the organs but was consistent between animals. The spleen had the highest baseline MMP-9 activity (relative enzyme activity, 0.85 ± 0.17 [mean ± standard deviation] in 20 μg of total proteins), whereas the lung activity was lower (0.38 ± 0.11 in 20 μg) and the liver activity was almost undetectable in naive animals (0.13 ± 0.03 in 40 μg). The MMP-9 activity in extracts from spleens increased over the first 4 weeks after infection compared to that of uninfected controls, and then it remained elevated (Fig. 6). MMP-9 activity in the lungs and livers of BCG-infected mice was also increased compared to that of controls, particularly at day 78 postinfection (data not shown). A low, constitutive MMP-2 activity was observed in all uninfected tissues tested and did not increase over the time course of the experiment. No MMP-3 or MMP-7 activity could be detected in spleens, livers, or lungs collected 5, 29, and 78 days after infection.
FIG. 6.
Infection with M. bovis BCG increased spleen MMP-9 activity in vivo. Sham-infected BALB/c mice (white bars) and mice infected i.v. with 106 BCG bacteria (black bars) were killed at various time points after infection. Tissue homogenates from spleens were analyzed by gelatin zymography. Data are presented as the relative enzymatic activity in pooled samples consisting of 1 μg of total protein per animal from spleen homogenates from three mice per group. When representative samples were assayed from individual mice, the standard deviations of relative MMP activity ranged from 5 to 19% of the means.
The course of infection followed a classical pattern for BCG in immunocompetent mice. The bacterial load rose from 5.4 × 105 ± 0.03 × 105 CFU in the spleen to the highest seen in the organs (2.7 × 106 ± 0.01 × 106 CFU/spleen) 22 days after infection. After this initial replication, the bacterial growth was controlled and CFU numbers returned to the level seen at the beginning of the experiment.
Infection with M. tuberculosis induces MMP production in vivo and in vitro.
We next asked how virulent M. tuberculosis affected macrophage MMP production in comparison to BCG. In peritoneal macrophages, M. tuberculosis induced a vigorous MMP-9 response that was dose dependent and of at least the same magnitude as that induced by BCG (Fig. 7).
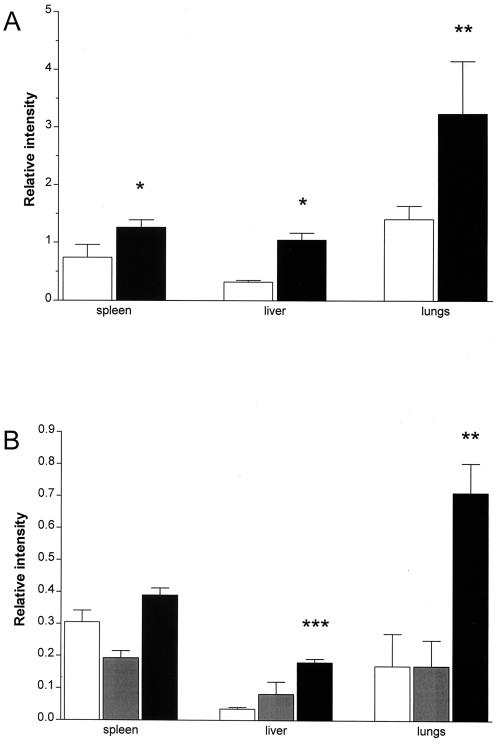
The presence of MMPs in protein extracts from spleens, livers, and lungs was then determined in BALB/c mice infected i.v. with 106 M. tuberculosis H37Rv organisms for 5 months. At this time point, there was substantial inflammation and mature organized granulomas were seen in the livers. In the lungs, granulomas corresponded to lesions of categories 3 to 4 as defined by Rhoades et al. (37), i.e., focal lesions of epithelioid macrophages with some scattered lymphocytic foci. In this experiment, spleen, liver, and lung tissues from infected animals all had levels of MMP-9 which were significantly increased compared to those of age-matched sham-infected controls (Fig. 8A).
FIG. 8.
Infection with M. tuberculosis induces increased MMP-9 activity in vivo. (A) BALB/c mice were infected i.v. with 106 M. tuberculosis H37Rv bacteria, and homogenates were prepared from spleens, livers, and lungs of sham-infected age-matched controls (white bars) and of infected mice (black bars) 5 months later and analyzed by gelatin zymography. (B) SCID mice were infected i.v. with 106 M. tuberculosis H37Rv bacteria, and homogenates were prepared from spleens, livers, and lungs of sham-infected age-matched controls (white bars) and of infected mice 8 days (grey bars) and 22 days (black bars) later and analyzed by gelatin zymography. Data are presented as means + standard deviations of the relative enzymatic activity in samples from individual mice (three per group) with the same total protein content loaded (lungs, 20 μg; spleens, 3 μg; and liver, 40 μg of total protein/lane). ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 when comparing with the uninfected animals.
In order to assess MMP activity in vivo in a situation without the T-cell-dependent control of mycobacterial growth, gelatinase activity was determined in organs from M. tuberculosis-infected SCID mice on a BALB/c background. These mice were infected with M. tuberculosis H37Rv, and tissues were collected both early (8 days) and relatively late (22 days) after infection. The lack of T cells resulted in an accelerated pathology with necrosis and a large influx of neutrophils in the late stage of the infection. Bacterial replication was not controlled in any of the organs studied. Mean bacterial numbers were as high as 1.4 × 109 in the spleens, 3.0 × 108 in the livers, and 5.0 × 108 in the lungs, and the mice died about 35 days postinfection. Eight days after infection, no effects on the MMP levels of the SCID mice were seen. In contrast, 2 weeks later a fourfold increase in MMP-9 could be seen in the livers and lungs of these animals (Fig. 8B). These experiments demonstrate that M. tuberculosis is a potent inducer of gelatinase activity both in vivo and in vitro.
DISCUSSION
In this article we show that mycobacterial infection is a powerful stimulus for production of MMP-9 (gelatinase B) and MMP-2 (gelatinase A) by murine macrophages. The mycobacterial induction of MMPs was mediated by macrophage secretion of TNF-α and IL-18 and was regulated by the T-cell-associated cytokines IFN-γ and IL-4, as well as by IL-10. Infection of both immunocompetent and SCID mice with M. tuberculosis or BCG also resulted in increased MMP-9 activity in several organs.
In order to study MMP responses from normal, nontransformed macrophages after interaction with mycobacteria, we used an in vitro model in which peritoneal macrophages were stimulated with BCG or M. tuberculosis. In this system, small numbers of bacteria from both species were able to induce prominent production of MMP-9. Preliminary experiments also showed that stimulation with Mycobacterium avium induced comparable levels of MMP-9 activity in macrophages, and therefore, MMP-9 induction seems to be a common feature of mycobacterial infection. Apart from MMP-9 and MMP-2, no other MMPs could be detected after stimulation with BCG, an MMP profile that is in agreement with the range of MMPs induced by in vitro stimulation of macrophages with LPS or in vitro infection with Borrelia spp. (25, 54). A previous study (7) also demonstrated that inactivated M. tuberculosis induced increased MMP-9 production in a myelomonocytic cell line. We have now extended these findings by demonstrating the effects of both live and inactivated mycobacteria on freshly isolated macrophages and have also characterized the cytokine network regulating mycobacterium-induced macrophage MMP-production.
Several observations indicate that the most important factor contributing to MMP production after BCG stimulation in vitro is the cytokine response which the mycobacteria induce. Thus, blocking of TNF-α inhibited BCG-induced MMP production, and addition of recombinant TNF-α to untreated macrophages induced MMP-9 activity to the same extent as live BCG bacteria. Indeed, the concentration of recombinant TNF-α needed to induce MMP-9 activity was similar to the levels of TNF-α induced in macrophage cultures by stimulation with BCG. Finally, conditioned, sterile-filtered media from BCG-infected macrophages induced increased levels of MMP activity. Previous studies have demonstrated the ability of TNF-α to induce MMP production by different cell types in vitro (24, 38, 50, 51, 55), but this study is the first to demonstrate the involvement of TNF-α in MMP production induced by a pathogenic bacterium. In addition to the effect of TNF-α neutralization, antibodies to IL-18 also had a reproducible, but less pronounced, inhibitory effect and stimulation with recombinant IL-18 also enhanced MMP-9 activity. This effect of IL-18 on MMP production has not been documented before, and the previously reported effects of IL-18 on macrophages have mainly been inhibitory, such as attenuating LPS-induced IL-12 and TNF-α secretion (6, 36). Other proinflammatory cytokines produced by macrophages in response to ingestion of mycobacteria, such as IL-1β and GM-CSF (12, 51), have been shown to promote MMP secretion in vitro, alone or in synergy with TNF-α (38, 55). However, the BCG-induced secretion of MMP-9 and MMP-2 shown here was not influenced by neutralization of IL-1, IL-12, or GM-CSF. Taken together, these results indicate the existence of an autocrine loop involving key cytokines (among them TNF-α and IL-18) which induce macrophage production of MMPs in response to mycobacteria. Indomethacin treatment did not affect macrophage MMP production, suggesting that the BCG-induced MMP production is largely independent of prostaglandin E2. This again supports the view that a major part of BCG-induced MMP production is mediated by macrophage cytokines produced in response to the infection, since cytokine- but not LPS-induced MMP-9 production is independent of prostaglandin E2 (41, 55).
Macrophage function and activation stage are dependent on the net effect of the regulatory cytokines that the macrophage encounters (48). TNF-α and IL-18 are produced during mycobacterial infection and are important for containing the infection, presumably by promoting IFN-γ secretion from T cells and NK cells (2, 15, 16, 30). TNF-α and IFN-γ often act in synergy to enhance macrophage effector functions, partly since IFN-γ induces expression of TNF-R and increases the activation of NF-κB induced by TNF-α stimulation (5, 18). Nevertheless, IFN-γ and TNF-α have opposing effects on macrophage MMP secretion induced by mycobacteria. In support of this finding, IFN-γ has previously been shown to down-regulate LPS- and cytokine-induced MMP production (38, 54), but the intracellular signaling events resulting in decreasing MMP production in IFN-γ-containing cultures are presently unknown. We cannot determine if IFN-γ binding results in a direct decrease in the amounts of MMPs produced in our system or rather in increased TIMP production. Earlier studies suggest, however, that the potential of IFN-γ to down-regulate macrophage gelatinase activity in cytokine- or LPS-stimulated macrophages does not result from increased TIMP synthesis (44, 47). NO has been suggested to decrease MMP secretion from macrophages (27), and IFN-γ-induced NO production might therefore be one of the mechanisms resulting in decreased MMP-9 activity.
In addition to IFN-γ, the immunomodulatory cytokines IL-10 and IL-4 also down-regulate BCG-induced MMP activity. However, in this case the effects seem to be indirect, accomplished by reducing the amount of TNF-α available to the macrophages. It seems, therefore, that macrophage MMP production can be modulated in two ways by regulatory cells, either directly by IFN-γ or indirectly by altering the magnitude of the TNF-α response with macrophage-deactivating cytokines such as IL-10 and IL-4.
Increased MMP activity in response to mycobacterial infection was not only an in vitro phenomenon, since infection with BCG or M. tuberculosis results in significantly increased MMP-9 activity in several tissues in immunocompetent mice. These observations are consistent with the finding of increased MMP levels in bronchoalveolar lavage specimens from patients with active tuberculosis (7) and now provide an experimental model in which to dissect their contribution to immunopathology versus resistance in vivo. Thus, we could monitor the different kinetics of induction of MMP-9 activity in several organs after infection with BCG.
The increased tissue MMP activity in SCID mice after infection with M. tuberculosis is of particular interest. Vigorous MMP-9 responses were seen during the late stage of the infection in lungs and livers of SCID mice infected with M. tuberculosis. At this time point, the infection is characterized by extensive tissue damage combined with a large neutrophil infiltration, especially in the lungs. The excessive MMP production in SCID mice may partly result from a reduced level of the T-cell-associated cytokine IFN-γ, which would down-regulate MMP activity in an immunocompetent mouse. The influence of large bacterial loads in the tissues may also be one of the reasons for the high MMP-9 activity, and the large number of infiltrating neutrophils may also contribute to the tissue content of MMP-9, since neutrophils harbor preformed MMP-9 in their granulae (43).
The implications of increased MMP activity in the various organs during mycobacterial infections are still unclear, but we speculate that the breakdown of the extracellular matrix is necessary for cell recruitment and efficient granuloma formation. Thus, MMP production may be essential for the development of protective immune responses to mycobacteria. However, MMP activity may also play an important role in the pathology of mycobacterial infections by destroying the tissue integrity. Dysregulation of MMP production at late stages of the infection could be one of the factors resulting in tissue damage. The generation of chemotactic fragments from the ECM may also contribute to cell recruitment to inflammatory foci (22, 40). In view of recent in vitro data demonstrating that M. avium-induced MMP activity promotes human immunodeficiency virus replication and spread in T cells, MMP production in vivo during mycobacterial infections is probably a contributing factor to the mutual exacerbation of both diseases in patients coinfected with M. tuberculosis and human immunodeficiency virus type 1 (9).
In conclusion, we show that mycobacterial infections induce increased MMP-9 activity both in vitro and in vivo and that this activity is regulated by TNF-α, IL-18, and IFN-γ. These findings indicate possible contributions of these enzymes to tissue remodeling processes in tuberculous lesions in vivo.
ACKNOWLEDGMENTS
M.Q.J. was supported by a grant from the Swedish Foundation for International Cooperation in Research and Higher Education.
We are grateful for the technical assistance of Helen Counihan and the staff of the Biological Services Facility at the London School of Hygiene and Tropical Medicine. We thank Paul Kaye for helpful comments on the manuscript.
REFERENCES
- 1.Arribas J, Coodly L, Vollmer P, Kishimoto T K, Rose-John S, Massague J. Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J Biol Chem. 1996;271:11276–11282. doi: 10.1074/jbc.271.19.11376. [DOI] [PubMed] [Google Scholar]
- 2.Bancroft G J. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993;5:503–510. doi: 10.1016/0952-7915(93)90030-v. [DOI] [PubMed] [Google Scholar]
- 3.Bekker L G, Maartens G, Steyn L, Kaplan G. Selective increase in plasma tumor necrosis factor-α and concomitant clinical deterioration after initiating therapy in patients with severe tuberculosis. J Infect Dis. 1998;178:580–584. doi: 10.1086/517479. [DOI] [PubMed] [Google Scholar]
- 4.Birkedal-Hansen H, Taylor R E, Zambon J J, Barwa P K, Neiders M E. Role of matrix metalloproteinases in human periodontal diseases. J Periodontal Res. 1993;64:474–483. [Google Scholar]
- 5.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 6.Bohn E, Sing A, Zumbihl R, Bielfeldt C, Okamura C, Kurimoto M, Heesemann J, Autenrieth I B. IL-18 (IFN-gamma-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J Immunol. 1998;160:299–307. [PubMed] [Google Scholar]
- 7.Chang J C, Wysocki A, Tchou-Wong K M, Moskowitz N, Zhang Y, Rom W N. Effect of Mycobacterium tuberculosis and its components on macrophages and the release of matrix metalloproteinases. Thorax. 1996;51:306–311. doi: 10.1136/thx.51.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denis M. Involvement of cytokines in determining resistance and acquired immunity in murine tuberculosis. J Leukoc Biol. 1991;50:495–501. doi: 10.1002/jlb.50.5.495. [DOI] [PubMed] [Google Scholar]
- 9.Dezutti C S, Swords W E, Guenthner P C, Sasso D R, Wahl L M, Drummond A H, Newman G W, King C H, Quinn F D, Lal R B. Involvement of matrix metalloproteinases in human immunodeficiency virus type 1-induced replication by clinical Mycobacterium avium isolates. J Infect Dis. 1999;180:1142–1152. doi: 10.1086/314992. [DOI] [PubMed] [Google Scholar]
- 10.Ding Y, Uitto V J, Firth J, Slo T, Haapasalo M, Konttinen Y T, Sorsa T. Modulation of host matrix metalloproteinases by bacterial virulence factors relevant to human periodontal diseases. Oral Dis. 1995;1:279–286. doi: 10.1111/j.1601-0825.1995.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 11.Ehlers S. Immunity to tuberculosis: a delicate balance between protection and pathology. FEMS Immunol Med Microbiol. 1999;23:149–158. doi: 10.1111/j.1574-695X.1999.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 12.Fattorini L, Xiao Y, Li B, Santoro C, Ippoliti F, Orefici G. Induction of IL-1 beta, IL-6, TNF-alpha, GM-CSF, and G-CSF in human macrophages by smooth transparent and smooth opaque colonial variants of Mycobacterium avium. J Med Microbiol. 1994;40:129–133. doi: 10.1099/00222615-40-2-129. [DOI] [PubMed] [Google Scholar]
- 13.Fenton M J, Vermeulen M W. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect Immun. 1996;64:683–690. doi: 10.1128/iai.64.3.683-690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Firth J D, Putnins E E, Larjava H, Uitto V J. Bacterial phospholipase C upregulates matrix metalloproteinase expression by cultured epithelial cells. Infect Immun. 1997;65:4931–4936. doi: 10.1128/iai.65.12.4931-4936.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Shreiber R, Mak T W, Bloom B R. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 16.Garcia V E, Uyemura K, Sieling P A, Ochoa M T, Morita C T, Okamura H, Kurimoto M, Rea T H, Modlin R L. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J Immunol. 1999;162:6114–6121. [PubMed] [Google Scholar]
- 17.Goetzl E J, Banda M J, Lepper D. Matrix metalloproteinases in immunity. J Immunol. 1996;156:1–4. [PubMed] [Google Scholar]
- 18.Held T K, Welhua X, Yuan L, Kalvakolanu D V, Crass A S. Gamma interferon augments macrophage activation by lipopolysaccharide by two distinct mechanisms, at the signal transduction level and via an autocrine mechanism involving tumor necrosis factor alpha and interleukin-1. Infect Immun. 1999;67:206–212. doi: 10.1128/iai.67.1.206-212.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez-Pando R, Orozcoe H, Sampieri A, Pavon L, Velasquillo C, Larriva-Sahd J, Alcocer J M, Madrid M V. Correlation between the kinetics of Th1/Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89:26–33. [PMC free article] [PubMed] [Google Scholar]
- 20.Heussen C, Dowdle E B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulphate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- 21.Hooper N M, Karran E H, Turner J. Membrane protein secretases. Biochem J. 1997;321:265–279. doi: 10.1042/bj3210265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunninghake G W, Davidson J M, Rennard S, Szapiel S, Gadek J E, Crystal R G. Elastin fragments attract macrophage precursors to diseased sites in pulmonary emphysema. Science. 1981;212:925–927. doi: 10.1126/science.7233186. [DOI] [PubMed] [Google Scholar]
- 23.Ito A, Mukaiyama A, Itoh Y, Nagase H, Thøgersen I B, Enghild J, Sasaguri Y, Moti Y. Degradation of interleukin 1β by matrix metalloproteinases. J Biol Chem. 1996;271:14657–14660. doi: 10.1074/jbc.271.25.14657. [DOI] [PubMed] [Google Scholar]
- 24.Johnatty R, Taub D D, Reeder S P, Turcovski-Corrales S M, Cottam D W, Stephenson T J, Rees R C. Cytokine and chemokine regulation of proMMP-9 and TIMP-1 production by human peripheral blood lymphocytes. J Immunol. 1997;158:2327–2333. [PubMed] [Google Scholar]
- 25.Kirchner A, Koedel U, Fingerle V, Paul R, Wilske B, Pfister H W. Upregulation of matrix metalloproteinase-9 in the cerebrospinal fluid of patients with acute Lyme neuroborreliosis. J Neurol Neurosurg Psychiatry. 2000;68:368–371. doi: 10.1136/jnnp.68.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kol A, Sukhova G K, Lichtman A H, Libby P. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation. 1998;98:300–307. doi: 10.1161/01.cir.98.4.300. [DOI] [PubMed] [Google Scholar]
- 27.Kumar R, Xie K, Eue I, Dong Z, Killion J J, Fiddler I J. Differential regulation of type IV collagenases and metalloelastase in murine macrophages by the synthetic bacterial lipopeptide JBT 3002. Int J Immunopharmacol. 2000;22:431–443. doi: 10.1016/s0192-0561(00)00008-4. [DOI] [PubMed] [Google Scholar]
- 28.Ladel C H, Daugelat S, Kaufmann S H E. Immune response to Mycobacterium bovis bacille Calmette Guérin infection in major histocompatibility complex class I- and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur J Immunol. 1995;25:377–384. doi: 10.1002/eji.1830250211. [DOI] [PubMed] [Google Scholar]
- 29.Leib S L, Leppert D, Clements J, Täuber M G. Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect Immun. 2000;68:615–620. doi: 10.1128/iai.68.2.615-620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-γ production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–264. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 31.Orme I M, Andersen P, Bloom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 32.Orme I M, Cooper A M. Cytokine/chemokine cascades in immunity to tuberculosis. Immunol Today. 1999;20:307–312. doi: 10.1016/s0167-5699(98)01438-8. [DOI] [PubMed] [Google Scholar]
- 33.Orme I M, Robert A D, Griffin J P, Abrams J S. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993;151:518–525. [PubMed] [Google Scholar]
- 34.Paemen L, Jansen P M, Proost P, Van Damme J, Opdenakker G, Hack E, Taylor F B. Induction of gelatinase B and MCP-2 in baboons during sublethal and lethal bacteraemia. Cytokine. 1997;9:412–415. doi: 10.1006/cyto.1996.0183. [DOI] [PubMed] [Google Scholar]
- 35.Pender S L F, Tickle S P, Docherty A J P, Howie D, Wathen N C, MacDonald T T. A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol. 1997;158:1582–1590. [PubMed] [Google Scholar]
- 36.Prinz M, Hanisch U K. Murine microglia cells produce and respond to interleukin-18. J Neurochem. 1999;72:2215–2218. doi: 10.1046/j.1471-4159.1999.0722215.x. [DOI] [PubMed] [Google Scholar]
- 37.Rhoades E R, Frank A A, Orme I M. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber Lung Dis. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 38.Saren P, Welgus H, Kovanen P. TNF-α and IL-1β selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol. 1996;157:4159–4165. [PubMed] [Google Scholar]
- 39.Saunders B M, Frank A A, Orme I M. Granuloma formation is required to contain bacillus growth and delay mortality in mice chronically infected with Mycobacterium tuberculosis. Immunology. 1999;98:324–328. doi: 10.1046/j.1365-2567.1999.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Senior R M, Griffin G L, Mecham R P. Chemotactic activity of elastin-derived peptides. J Clin Investig. 1980;66:859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shankavaram U T, DeWitt D L, Wahl L M. Lipopolysaccharide induction of monocyte matrix metalloproteinases is regulated by the tyrosine phosphorylation of cytosolic phospholipase A2. J Leukoc Biol. 1998;64:221–227. doi: 10.1002/jlb.64.2.221. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro S D. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol. 1998;10:602–608. doi: 10.1016/s0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- 43.Shapiro S D. The macrophage in chronic obstructive pulmonary disease. Am J Crit Care Med. 1999;160:S29–S32. doi: 10.1164/ajrccm.160.supplement_1.9. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro S D, Campbel E J, Kobayashi D K, Welgus H G. Immune modulation of metalloproteinase production in human macrophages. Selective pretranslational suppression of interstitial collagenase and stromelysin biosynthesis by interferon-gamma. J Clin Investig. 1990;86:1204–1210. doi: 10.1172/JCI114826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith D, Hänsch H, Bancroft G J, Ehlers S. T-cell-independent granuloma formation in response to Mycobacterium avium: role of tumour necrosis factor-α and interferon-γ. Immunology. 1997;92:413–421. doi: 10.1046/j.1365-2567.1997.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishomoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18 deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 47.Tamai K, Ishikawa H, Mauviel A, Uitto J. Interferon-gamma co-ordinately upregulates matrix metalloproteinase (MMP)-1 and MMP-3, but not tissue inhibitor of metalloproteinases (TIMP) expression in cultured keratinocytes. J Investig Dermatol. 1995;104:384–390. doi: 10.1111/1523-1747.ep12665857. [DOI] [PubMed] [Google Scholar]
- 48.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-γ) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 49.Tsenova L, Bergtold A, Freedman V H, Young R A, Kaplan G. Tumor necrosis factor α is a determinant of pathogenesis and disease progression in mycobacterial infection in the central nervous system. Proc Natl Acad Sci USA. 1999;96:5657–5662. doi: 10.1073/pnas.96.10.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Unemori E N, Hibbs M S, Amento E P. Constitutive expression of a 92-kD gelatinase (type V collagenase) by rheumatoid synovial fibroblasts and its induction in normal human fibroblasts by inflammatory cytokines. J Clin Investig. 1991;88:1656–1662. doi: 10.1172/JCI115480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Wakeham J, Harkness R, Xing Z. Macrophages are a significant source of type 1 cytokines during mycobacterial infection. J Clin Investig. 1999;103:1023–1029. doi: 10.1172/JCI6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 53.Wysocka M, Kubin M, Vieira L, Ozen L, Garotta G, Scott P, Trinchieri G. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- 54.Xie B, Dong Z, Fidler I. Regulatory mechanisms for the expression of type IV collagenases/gelatinases in murine macrophages. J Immunol. 1994;152:3637–3644. [PubMed] [Google Scholar]
- 55.Zhang Y, McCluskey K, Fuji K, Wahl L M. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-α, granulocyte-macrophage-CSF, and IL-1β through prostaglandin-dependent and independent mechanisms. J Immunol. 1998;161:3071–3076. [PubMed] [Google Scholar]