Abstract
Introduction
This study aimed to evaluate the clinical and economic outcomes of implementing a Clostridiodes difficile infection (CDI) Treatment Optimization and Access Pathway (treatment pathway) directing first-line use of fidaxomicin for CDI.
Methods
This was a retrospective, quasi-experimental study of adult patients with CDI using Electronic Health Record data from a single center. The primary intervention was implementation of a treatment pathway directing first-line use of fidaxomicin for patients with first/second CDI episode and at high risk of recurrence. The primary clinical outcome was CDI recurrence within 30 days of completing therapy in patients achieving clinical cure. Secondary clinical outcomes included clinical cure and sustained response evaluated at 90 days after completion of CDI treatment. Economic outcomes included costs associated with hospital stay at index admission and 30- and 90-day readmission. Differences between the pre- and post-implementation cohorts were assessed for baseline characteristics, CDI treatment utilization, clinical outcomes, and economic outcomes. The budget impact was calculated for the pre- vs. post-implementation cohorts, each normalized to 100 patients.
Results
Post- vs. pre-implementation, 30-day recurrence (6.4% vs. 18.0%., p = 0.001), 90-day recurrence (14.9% vs. 27.1%, p = 0.009), and 30-day (4.6% vs. 12.7%, p = 0.007) and 90-day CDI-related readmissions (8.5% vs. 18.9%, p = 0.007) were lower. The clinical cure (94.1% vs. 84.4%, p = 0.002) and 90-day sustained response rates were higher (73.3% vs. 55.9%, p < 0.001). Median total costs were also lower in the post- vs. pre-implementation cohorts at index admission ($11,934.64 vs. $14,523.27, p = 0.048), and 30-day ($7685.82 vs. $12,424.44, p = 0.102) and 90-day CDI-related readmission episodes ($8246.69 vs. $12,729.57, p = 0.042). The budget impact analyses of 100 patients post- vs. pre-implementation found saving of $222,895 overall and $9432 per CDI-readmission avoided.
Conclusions
Implementation of the CDI treatment pathway was associated with better clinical outcomes and hospital cost savings. The findings help validate real-world value of fidaxomicin for CDI disease management.
Keywords: Budget impact, Clostridioides difficile, Fidaxomicin, Oral vancomycin, Retrospective analyses, Treatment pathway
Key Summary Points
| Clostridioides difficile infection (CDI) is the leading cause of nosocomial diarrhea in developed countries, causing 30% of antibiotic-related diarrhea. |
| The 2021 Focused Infectious Diseases Society of America (IDSA) Guideline updated its recommendation of fidaxomicin to a preferred option over vancomycin in patients with initial or recurrent CDI. Some institutions consider it cost-prohibitive to implement this recommendation. |
| This study aimed to assess how implementation of a CDI Treatment Optimization and Access Pathway (treatment pathway) directing use of fidaxomicin for first and second- episodes of CDI impacted real-world clinical and economic outcomes at a US Health System. |
| We found that the implementation of the treatment pathway was associated with decreases in CDI recurrences and CDI-related readmissions within 30 and 90 days, increases in clinical cure and sustained response at 90-days post treatment, and overall cost-savings to the health system driven by reduced recurrences. |
| Implementation of the treatment pathway for fidaxomicin at this Health System was associated with real-world clinical and economic benefits. Findings suggest fidaxomicin is a cost-effective CDI treatment option even though more costly. |
Introduction
Clostridioides difficile infection (CDI) is the leading cause of nosocomial diarrhea in developed countries, causing 30% of antibiotic-related diarrhea [1, 2]. Patients are susceptible to CDI when there is disruption in the intestinal microbiome, often following long-term antibiotic use, gastric acid suppressant therapy, immunodeficiency, and increased age [2–4]. The Centers for Disease Control and Prevention 2019 Antibiotic Resistant Threats Report estimated that the incidence of CDI was 223,900 cases in hospitalized patients in 2017, with 12,800 deaths and a recurrence rate of 25%. While hospital-associated cases have decreased, community-associated cases have not [2].
The 2017 Infectious Diseases Society of America (IDSA)/Society of Healthcare Epidemiology of America (SHEA) CDI treatment guidelines recommended fidaxomicin or vancomycin as first-line treatment options for adult patients with CDI, with metronidazole demoted to an alternative agent when the first-line agents were not available [5]. The 2021 Focused IDSA guideline updated its recommendation of fidaxomicin to a preferred option over vancomycin for patients with initial or recurrent CDI [6]. This recommendation was not surprising as fidaxomicin has been reported to have many clinical benefits over vancomycin. As a bactericidal agent that is selective against C. difficile, with minimal activity against enteric gram-negative organisms, fidaxomicin provides an effective option that preserves intestinal microflora and is not associated with colonization with Candida and vancomycin-resistant Enterococcus, which has been observed with oral vancomycin [7, 8]. Despite clinical benefits of fidaxomicin over vancomycin, cost remains a primary factor limiting widespread use as fidaxomicin’s broader economic value in managing CDI in real-world settings is not well understood [9].
Carilion Roanoke Memorial Hospital (CRMH) implemented a CDI Treatment Optimization and Access Pathway (further referred to as treatment pathway) in January 2018 directing first-line use of fidaxomicin for first and second occurrences of CDI. Prior to this change, most patients presenting with uncomplicated CDI were managed with oral metronidazole or oral vancomycin. Implementation of the treatment pathway provided an opportunity to examine clinical and economic outcomes in patients with CDI given more fidaxomicin use in a real-world hospital setting. This study aimed to evaluate clinical and economic outcomes pre- vs. post-implementation of the CDI treatment pathway. Findings should provide information to help inform clinical practice and CDI disease management strategies.
Methods
Study Design and Population
This was a retrospective, quasi-experimental study of adult patients diagnosed with and treated for CDI at CRMH, a 763-bed level one trauma center in Southwest Virginia. CRMH is a part of the Carilion Clinic health system, which provides healthcare to patients across the region. The study was reviewed by the institution’s internal review board prior to commencement and was in compliance with ethics guidelines. This study was determined to be exempt by the institutional review board at Carilion Clinic. Subjects were not consented as this was waived by IRB due to the nature of the retrospective chart review design.
Two time periods, a pre-implementation period from August 1, 2016, to November 31, 2017, and a post-implementation period from May 1, 2018, to January 31, 2020, were evaluated. A washout period between the pre- and post-implementation periods was included as the CDI treatment pathway was implemented in January 2018, and there was significant provider education that occurred during the washout period. The treatment pathway consisted of an update to the institutional treatment guidelines, a fidaxomicin access algorithm developed with case management, and a medication assistance program that began immediately upon order of the medication. Additionally, to drive change in clinical practice, oral vancomycin and fidaxomicin were only orderable through an order set in the electronic health record (EHR) that directed providers to appropriate management per patient characteristics. This treatment pathway indicated a 10-day course of fidaxomicin for patients with their first or second episode of CDI who were also at high risk of recurrence, defined as meeting at least one of these criteria: age ≥ 65 years, concurrent systemic antibiotic use, current proton pump inhibitor use, history of cancer, baseline renal insufficiency, and/or a previous episode of CDI.
A patient list for screening was generated from Sentri7® (2005–2021 Wolters Kluwer), the clinical decision software system utilized by CMRH. This list captures all CDI-positive results and is validated by the infection control team at CRMH. All data were extracted from EPIC, CRMH’s EHR, using a standardized electronic case report form generated through REDCap (v. 10.1.5, 2021 Vanderbilt University) and hosted on secure internal servers.
Sample Selection
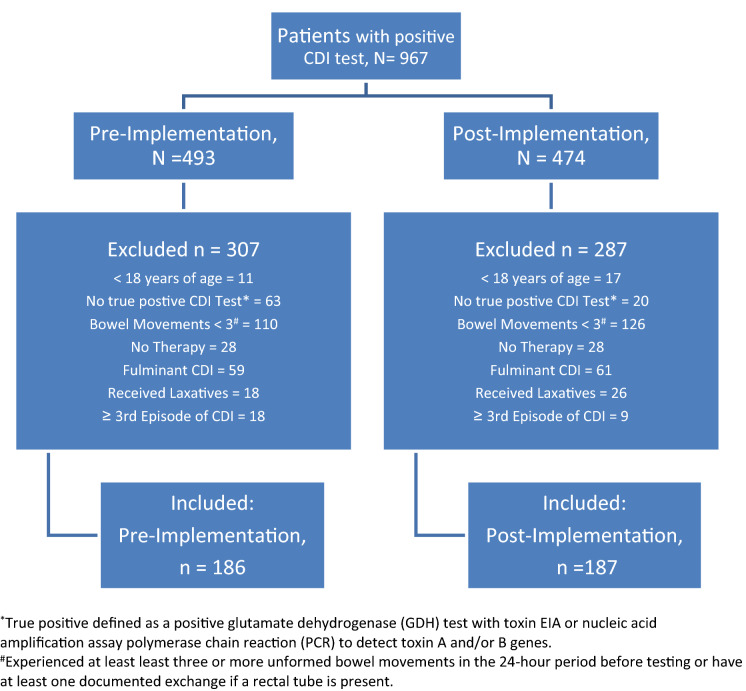
All adult patients with a positive CDI test were included in the study sample (Fig. 1). A CDI-positive test was defined as a positive glutamate dehydrogenase (GDH) test with toxin enzyme immunoassay (EIA) or nucleic acid amplification assay polymerase chain reaction (PCR) to detect toxin A and/or B genes and receipt of CDI-active treatment during the index CDI episode. Diagnosis via PCR may be reflective of colonization, so PCR-tested patients were required to have had at least three bowel movements in the 24-h period before testing. Patients were excluded if categorized as having fulminant CDI [5], received laxatives within 24 h prior to testing, or index CDI episode was third or later. Patients meeting inclusion criteria in both the pre- and post-implementation cohorts were excluded from the post-implementation cohort to avoid repeated observations.
Fig. 1.
Sample selection flowchart
Clinical Outcomes
The primary clinical outcome of the study was CDI recurrence within 30 days of completing therapy in patients who achieved clinical cure. Recurrence was defined as a positive CDI test and CDI treatment initiated by provider. Secondary clinical outcomes included clinical cure defined as resolution of diarrhea such that there was no further requirement for therapy within 2 days of completion of index therapy and sustained response defined as clinical cure with no CDI recurrence and evaluated at 90 days after the completion of CDI treatment. Additional secondary outcomes included CDI-related readmissions and all-cause readmissions at both 30 and 90 days post-discharge from index admission. CDI-related readmissions were identified as recorded in the EHR by the treating provider.
Index CDI therapy was defined as the first CDI-active treatment (oral metronidazole, oral vancomycin, or fidaxomicin) that continued for 5 days. Baseline characteristics evaluated included sociodemographic information, relevant comorbidities, CDI risk factors, and concomitant non-CDI antibiotic use at time of CDI. Non-CDI antibiotic use was evaluated within 4 weeks prior to diagnosis and defined as any systemic antibiotic therapy. The use of high-risk antibiotics was defined as use of fluoroquinolones, third- or fourth- generation cephalosporins, clindamycin, intravenous vancomycin, or carbapenem therapy.
Economic Outcomes
Total Direct Costs, Total Indirect Costs, and Total Treatment Costs were captured at the index CDI admission and 30- and 90-day CDI-related readmission episodes. Total Direct Costs included all direct costs associated with the hospital stay, i.e., Pharmacy Direct Costs (medications), Laboratory Costs, and Other Costs (procedures, room/board, salaries, and supplies). Total Indirect Costs included overhead costs of non-revenue-generating hospital operations applied per day based on unit and modeling. Total Treatment Costs included all direct and indirect costs. All cost data, except fidaxomicin cost, were derived from aggregate charge captures for the study population. To control for variable institution-specific contract costs of fidaxomicin, its 2020 Wholesale Acquisition Cost (WAC) was utilized for all administered doses [10]. All other costs were inflation adjusted to 2020 dollars using the medical care consumer price index (CPI) from the Bureau of Labor Statistics (series CUUR0000SAM) [11].
Statistical Analyses
Power Calculation
A total sample of 248 participants (n = 124 in each cohort) was needed for 80% power to detect a 30% difference in recurrence rate (α = 0.05). This estimate was obtained via an a priori power analysis using PASS 2019, v19.0.5. The 30% effect size was applied as a conservative estimate for power calculations in the current real-world study relative to a 45% recurrence reduction reported in a clinical trial comparing fidaxomicin to vancomycin [8].
Clinical
Differences between the pre- and post-implementation cohorts were assessed for baseline characteristics, CDI treatment utilization, and clinical outcomes. Continuous variables were reported as medians and interquartile range (IQR) and assessed using Mann-Whitney U tests. Frequencies were reported for categorical variables and analyzed using chi-square or Fisher’s exact tests as appropriate. A multivariable regression analysis with backward elimination was used to assess associations between treatment pathway implementation and sustained response, controlling for baseline characteristics. Baseline characteristics were selected for initial inclusion in the multivariable analysis based on having p-values < 0.2 in bivariate analysis. Other variables of clinical interest were considered. Variables with p-values > 0.2 were removed from the model. Data were analyzed using SAS Enterprise Guide 7.1 and p-values ≤ 0.05 considered statistically significant.
Economic
Economic outcomes were compared pre- and post-implementation of the treatment pathway and presented as medians. To control for significant cost outliers, a standard methodology was applied to remove all Total Direct Costs values above or below 1.5 × IQR from the statistical analysis. Cost comparisons were conducted using the Mann-Whitney U (Wilcoxon rank sum) with statistical significance defined as p ≤ 0.05.
The budget impact of the treatment pathway was calculated for the pre- vs. post-implementation cohorts, each normalized to 100 patients for ease of extrapolation as many institutions have annual CDI case rates between 100 and 200 patients [12]. Clinical and economic outcomes for the pre- and post-implementation cohorts were used as inputs. Parameter estimates were obtained by multiplying these inputs with the normalized patient count for each cohort and rounding values to the nearest whole number. Clinical and economic inputs were based on 90-day endpoints, with median Total Direct Costs used for the latter. Median length of stay for 90-day CDI-related readmissions was used for bed days calculation. Where outcomes were not significantly different between cohorts, the more conservative value was used. Budget impact metrics calculated included overall cost savings, savings per additional patient with sustained response, savings per CDI-related readmission avoided, and savings per bed day avoided (see Table 4 for definitions).
Table 4.
Budget impact analyses
| Parameter | Modeled for 100 CDI patients | Differencea | |
|---|---|---|---|
| Pre-implementation | Treatment pathway | ||
| Clinical impact at 90-day follow-up | |||
| CDI recurrence (n, patients) | 27 | 15 | − 12 |
| Sustained response (n, patients) | 56 | 73 | 17 |
| CDI-related readmissions (n, patients) | 19 | 9 | − 10 |
| Readmission length of stay (n, bed-days)b | 95 | 45 | − 50 |
| Economic impact at 90-day follow-up | |||
| Index admission direct costs, overall, $ | $836,233 | $707,660 | − $128,573 |
| CDI-related readmission direct costs, overall, $ | $135,236 | $40,914 | − $94,322 |
| Total direct costs (index + readmission), $ | $971,469 | $748,574 | − $222,895 |
| Overall impact | |||
| Overall Direct Cost Savings,a $ | $222,895 | ||
| Savings per additional patient with sustained response,c $ | $5548 | ||
| Savings per CDI-related readmission avoided,d $ | $9432 | ||
| Savings per bed-day avoided,e $ | $1886 | ||
aPost-implementation of treatment pathway minus pre-implementation
bMedian of 5 days used for both due to non-significant difference [6 (4–10) vs. 4.5 (2–4.5), p = 0.251]
cEquals savings from CDI-related readmission costs/the additional number of patients with sustained response
dEquals savings from CDI-related readmission costs/the number of CDI-related readmissions avoided
eEquals savings from CDI-related readmission costs/the number of bed-days saved
Results
A total of 186 patients in the pre-implementation cohort and 187 patients in the post-implementation cohort met sample selection criteria (Fig. 1). The most common reason for exclusion was not experiencing diarrhea (≥ 3 bowel movements) 24 h prior to testing, if diagnosed by positive GDH and PCR (24% of entire sample).
Baseline characteristics were not significantly different between the pre- and post-implementation cohorts (Table 1). The median ages for the pre- and post- cohorts were 67 and 68 years, respectively. Most patients in each cohort were female and white. In either cohort, about 90% of patients were experiencing their first episode of CDI and about 50% were experiencing severe CDI. The most common risk factor for developing CDI in either cohort was non-CDI antibiotic use within 4 weeks preceding diagnosis (68% in combined sample), with > 70% these antibiotics considered high risk.
Table 1.
Baseline characteristics
| Characteristic, no. (%) | Pre-implementation (n = 186) | Post-implementation (n = 187) | p-value |
|---|---|---|---|
| Age, median (IQR) | 67 [57–75] | 68 [59–77] | 0.304 |
| Female | 96 (51.6%) | 103 (55.1%) | 0.502 |
| Race/ethnicity | 0.353 | ||
| White | 152 (81.7%) | 158 (84.5%) | |
| Black | 32 (17.2%) | 23 (12.3%) | |
| Other | 2 (1%) | 6 (3.2%) | |
| Intensive care unit admission at diagnosis | 23 (12.4%) | 24 (12.8%) | 0.892 |
| Charlson comorbidity index score, median (IQR) | 5 [3–7] | 5 [3–7] | 0.441 |
| Renal impairment | 42 (22.6%) | 48 (25.7%) | 0.486 |
| Crohn’s disease or ulcerative colitis | 6 (3.2%) | 4 (2.1%) | 0.543 |
| Immunosuppresseda | 32 (17.2%) | 33 (17.7%) | 0.910 |
| History of cancer | 26 (14.0%) | 29 (15.5%) | 0.677 |
| Active malignancy | 25 (13.4%) | 32 (17.1%) | 0.325 |
| Solid organ transplant recipient | 6 (3.2%) | 1 (0.5%) | 0.067 |
| CDI history | 0.533 | ||
| Primary episode | 166 (89.3%) | 163 (87.2%) | |
| Second episode | 20 (10.8%) | 24 (12.8%) | |
| Concomitant antibiotic during CDI | 72 (38.7%) | 57 (30.5%) | 0.095 |
| High-risk antibiotic, 4 weeks prior | 103 (55.4%) | 89 (47.6%) | 0.133 |
| Gastric acid suppressant use | 107 (57.5%) | 105 (56.2%) | 0.788 |
| CDI risk factors | |||
| Age ≥ 65 years | 101 (54.3%) | 110 (58.8%) | 0.378 |
| Severe CDI | 95 (51.1%) | 94 (50.3%) | 0.876 |
| Prior CDI within 12 months | 21 (11.3%) | 23 (12.3%) | 0.763 |
| Non-CDI antibiotic, 4 weeks prior | 134 (72%) | 120 (64.2%) | 0.103 |
| Deep vein thrombosis prophylaxisb | 177 (95.2%) | 179 (95.7%) | 0.795 |
aDefined as receiving antineoplastics, steroids > 20 mg prednisone equivalents per day for at least 2 weeks, transplant medication or immunomodulators, absolute neutrophil cell count < 500 cells/ul or diagnosis of immune deficiency, solid organ transplant, or hematopoietic stem cell transplant (HSCT)
bDeep vein thrombosis prophylaxis was used as a non-dependent control variable
There was a reduction in CDI drug utilization to almost no use of metronidazole (47.8% vs. 1.6%), slightly less vancomycin use (50.5% vs. 41.7%), and increased use of fidaxomicin (1.6% vs. 56.7%) after the implementation of the treatment pathway (Table 2). The distribution of CDI treatment was significantly different (P < 0.001) pre vs. post-implementation. About 80% of the study population received a full treatment course, with the remaining 20% switching agents during the treatment course.
Table 2.
Treatment utilization and clinical outcomes
| Outcome, no. (%) | Pre-implementation (n = 186) | Post-implementation (n = 187) | p-value |
|---|---|---|---|
| Treatment utilization | |||
| Primary treatment agent | < 0.001 | ||
| Metronidazole | 89 (47.8%) | 3 (1.6%) | |
| Oral vancomycin | 94 (50.5%) | 78 (41.7%) | |
| Fidaxomicin | 3 (1.6%) | 106 (56.7%) | |
| Oral vancomycin dose | 0.036 | ||
| 125 mg four times per day | 94 (50.5%) | 88 (47.1%) | |
| 250 mg four times per day | 5 (2.7%) | 0 (0%) | |
| 500 mg four times per day | 4 (2.2%) | 1 (0.5%) | |
| Oral vancomycin taper | 6 (3.2) | 3 (1.6%) | |
| Adjunctive therapies | 0.248 | ||
| Fecal microbiota transplant | 2 (1.1%) | 0 (0%) | |
| Bezlotoxumab | 0 (0%) | 0 (0%) | |
| Duration of therapy, median (IQR) | 14 (11–15) | 10 (10–13) | < 0.001 |
| Received full treatment coursea | 151 (81.2%) | 147 (78.7%) | 0.535 |
| Combination intravenous metronidazole | 25 (13.4%) | 9 (4.8%) | 0.004 |
| Clinical outcomes | |||
| Clinical cure, n/N | 157/186 (84.4%) | 176/187 (94.1%) | 0.002 |
| Recurrence, n/Nb | |||
| 30-day | 27/150 (18.0%) | 11/171 (6.4%) | 0.001 |
| 90-day | 39/144 (27.1%) | 24/161 (14.9%) | 0.009 |
| Sustained response at 90 days, n/N | 104/186 (55.9%) | 137/187 (73.3%) | < 0.001 |
| CDI-related readmission, n/Nc | |||
| 30-day | 21/166 (12.7%) | 8 /176 (4.6%) | 0.007 |
| 90-day | 30/159 (18.9%) | 14/164 (8.5%) | 0.006 |
| All-cause readmission, n/Nc | |||
| 30-day | 60/168 (35.7%) | 49/177 (27.7%) | 0.109 |
| 90-day | 75/165 (45.4%) | 65/170 (38.2%) | 0.181 |
| Index admission length of stay for index, median (IQR) | 8 (5–14) | 6 (4–13) | 0.093 |
aPartial courses occurred in 18.8% pre-implementation and 21.4% post-implementation
bRecurrence was evaluable in those patients with clinical cure. Patients who died prior to 30 or 90 days were not evaluated for the outcome unless recurrence occurred prior to death
cPatients who died prior to 30 and 90 days were not evaluated for the outcome unless readmission occurred prior to death
Clinical Outcomes
Compared to the pre-implementation cohort, the post-implementation cohort had a higher proportion of patients with clinical cure (84.4% vs. 94.1%, p = 0.002) and higher sustained response (73.3% vs. 55.9%, p < 0.001) (Table 2). In the post-implementation cohort, the proportions of patients with CDI recurrences at 30 days (18.0% vs. 6.3%, p = 0.001) and 90 days (27.1% vs. 14.9%, p = 0.009), and proportions with 30-day (4.6% vs. 12.7%, p = 0.007) and 90-day CDI-related readmissions (8.5% vs. 18.9%, p = 0.007) were lower. There were no significant differences between the two cohorts in all-cause readmissions and number of emergency room visits at 30 and 90 days.
In the multivariable regression analysis, fidaxomicin use was found to be associated with a higher likelihood of sustained response [odds ratio (OR) = 1.96, 95% CI 1.03–3.7196, p < 0.037], whereas, there were no significant associations found for post- vs. pre-implementation cohort membership (OR = 1.52, 95% CI 0.89–1.52, p < 0.124). No other variable met the criterion (i.e., p < 0.2) for remaining in the model. Variables that met criteria for inclusion in the regression model from bivariate analyses were non-CDI antibiotic use within 4 weeks prior, concomitant non-CDI antibiotic use, and solid organ transplant recipient. Fidaxomicin use and age > 65 were included out of clinical interest.
Economic Outcomes
For the index admission episode, the post- vs. pre-implementation cohort had lower median Total Treatment ($11,934.64 vs. $14,523.27, post–pre difference − $2588.63, p = 0.048), Total Direct ($7076.60 vs. $8362.33, difference − $1,285.73, p = 0.073), Laboratory Direct ($507.89 vs. $808.73, difference − $300.84, p < 0.001) and Other Direct Costs ($4851.23 vs. $6561.11, difference − $1709.88, p < 0.001), but higher Pharmacy Direct Costs ($1503 vs. $810.08, difference $693.45, p = 0.001) (Table 3). Total Indirect Costs were rolled up into calculation of median Total Treatment Costs but were not reported separately because of institution confidentiality requirements. Median Total Treatment Costs and Total Direct Costs were also lower post- vs. pre-implementation for 30-day CDI readmission ($7685.82 vs. $12,424.44, difference − $4738.62, p = 0.102 and $4395.45 vs. $7069.54, difference − $2674.09, p = 0.083) and 90-day readmission episodes ($8246.69 vs. $12,729.57, difference − $4482.88, p = 0.042 and $4546.03 vs. $7117.66, difference − $2571.63, p = 0.029). Pharmacy Direct Costs were not significantly different between the post- and pre-implementation cohorts for the 30- and 90-day readmission episodes.
Table 3.
Economic outcomes
| Cost type, per patient | Pre-implementation (median) | Post-implementation (median) | Difference (post–pre) | p-value |
|---|---|---|---|---|
| Index admission | n = 154 | n = 160 | ||
| Total treatment costa | $14,523.27 | $11,934.64 | − $2588.63 | 0.048 |
| Total direct cost | $8362.33 | $7076.60 | − $1285.73 | 0.073 |
| Pharmacy cost | $810.08 | $1503.53 | $693.45 | 0.001 |
| Laboratory cost | $808.73 | $507.89 | − $300.84 | < 0.001 |
| Other costb | $6,561.11 | $4851.23 | − $1709.88 | 0.004 |
| 30-day CDI-related readmission | n = 21 | n = 8 | ||
|---|---|---|---|---|
| Total treatment cost | $12,424.44 | $7685.82 | − $4738.62 | 0.102 |
| Total direct cost | $7069.54 | $4395.45 | − $2674.09 | 0.083 |
| Pharmacy cost | $759.95 | $648.98 | -$110.97 | 14 |
| Laboratory cost | $550.70 | $233.24 | − $317.46 | 0.006 |
| Other cost | $5451.18 | $2561.17 | − $2890.01 | 0.018 |
| 90-day CDI-related readmission | n = 30 | n = 14 | ||
|---|---|---|---|---|
| Total treatment cost | $12,729.57 | $8246.69 | − $4482.88 | 0.042 |
| Total direct cost | $7117.66 | $4546.03 | − $2571.63 | 0.029 |
| Medication cost | $766.43 | $691.77 | − $74.66 | 0.539 |
| Laboratory cost | $584.70 | $330.02 | − $254.68 | 0.007 |
| Other cost | $5718.38 | $3672.75 | − $2045.63 | 0.010 |
aTreatment Cost = Total Direct Cost + Total Indirect Costs
bOther Direct Costs include procedures, room/board, salaries and supplies
In budget impact analyses of 100 patients with CDI post- vs. pre-implementation (i.e., treatment pathway vs. traditional management), there were 12 fewer patients with recurrence, 17 more patients with sustained response, 10 fewer patients readmitted for CDI recurrence, and 50 fewer readmission bed days (Table 4) within 90 days after the index CDI therapy. These clinical improvements translated into $222,895 total budget savings per 100 patients with CDI or savings of $5548 per additional patient with sustained response, $9432 per CDI-related readmission avoided, and $1886 per bed day avoided.
Discussion
This study evaluated clinical and economic outcomes pre- and post-implementation of a treatment pathway at CMRH directing first-line use of fidaxomicin for first and second occurrences of CDI. Implementation increased fidaxomicin use and was associated with better clinical outcomes and hospital cost-savings. These findings validate the real-world value of fidaxomicin for CDI disease management in health systems.
Implementation of the treatment pathway shifted drug utilization from metronidazole to fidaxomicin, with similar oral vancomycin use. The shift from metronidazole to fidaxomicin was not surprising given CDI treatments were orderable only via an order set that directed providers to the appropriate management for patients with CDI in alignment with the 2017 IDSA/SHEA CDI guideline recommendations [5]. These CDI treatment utilization changes are consistent with prior literature reporting more fidaxomicin use and less metronidazole use in institutions implementing the 2017 IDSA/SHEA CDI guideline recommendations [13, 14].
The treatment pathway was also associated with a significant increase in clinical cure and sustained response, and significant decrease in CDI recurrence and CDI-related readmissions. Previous studies have reported similar clinical cure rates between fidaxomicin and oral vancomycin [8], but superiority of both agents over metronidazole [15, 16], indicating that reduction in metronidazole use and shift to fidaxomicin (given vancomycin use was similar pre-post) were drivers of increased clinical cure in the current study. The superiority of fidaxomicin over vancomycin and metronidazole in increasing sustained response has also been reported in prior studies [8, 17]. Findings from multivariable regression analysis in this study suggest it was fidaxomicin use more so than the overall treatment pathway that increased sustained response pre-post, further highlighting the important contribution of fidaxomicin. Reductions in recurrences and CDI readmissions post treatment pathway implementation demonstrate real-world experience consistent with prior literature, with the current study also providing a larger sample-size than available retrospective studies [17–19].
Pharmacy Costs were higher at index admission post- compared to pre-implementation, which is not surprising as fidaxomicin acquisition cost is higher than metronidazole or oral vancomycin [10]. However, Total Treatment Costs and Total Direct Costs were lower post-implementation, with the latter driven by lower Laboratory Direct and Other Direct Costs. The length of stay was numerically lower in the post-implementation cohort, which is a likely major contributor to the lower Other Direct Costs found, given room and board are included in this cost bucket. Lower Total Treatment Costs and lower Total Direct Costs were also observed in the post-implementation cohorts for patients with 30- and 90-day readmission episodes.
In budget impact analyses of 100 patients with CDI managed post-implementation (i.e., using treatment pathway) compared to pre-implementation (i.e., traditional care management), we found an overall cost saving of $222,895, as well as a saving of $9432 per readmission avoided, $5548 per additional patient with sustained response, and $1886 per bed day avoided. These cost savings were driven mostly by reductions in CDI-related recurrences, readmissions, and resource use, consistent with previous literature [19, 20]. This speaks to the true benefit of fidaxomicin, including less impact on intestinal microflora, which leads to a reduction in recurrences. Given our number needed to treat of nine for 90-day recurrence prevention, fidaxomicin should be used first line in patients regularly to see this benefit. Reserving fidaxomicin for multiple recurrences or complicated patients who have seriously disrupted flora would likely decrease its effectiveness [21].
There are a number of study limitations. Results were limited by the completeness of information recorded in the EHR. However, there is anticipated low likelihood of misclassification error for exposure, outcomes, and covariates given that the diagnosis of CDI is based on laboratory values and clinical findings rather than subjective information. Generalizability may be limited given that this study was conducted at a single center. Quasi-experimental designs can be limited by maturation bias; therefore, deep vein thrombosis prophylaxis was utilized as a non-dependent control variable. Approximately 20% of patients in each of the pre- and post-implementation cohorts received mixed courses of fidaxomicin and vancomycin. This is likely reflective of real-world practice as patients were likely switched from fidaxomicin to vancomycin because of disposition and medication coverage. While utilizing CMRH’s cost data, we were able to supply numbers close to real-world application. However, cost may vary across institutions. Additionally, many methodologies exist for controlling outliers with economic data and it is possible that other modalities may have had a different impact on results. Finally, cost savings, while a useful metric, does not carry the weight of revenue that some hospital administrators prefer.
Conclusions
Findings suggest a CDI Treatment Optimization and Access Pathway directing more first-line use of fidaxomicin relative to oral vancomycin or metronidazole results in better clinical outcomes for patients having primary or secondary CDI episodes and is financially cost saving to the hospital. These findings validate real-world value of fidaxomicin for CDI disease management. Additionally, our use of institution-specific data to conduct budget impact analyses provides a practical approach that can be applied by other institutions to their own data.
Acknowledgements
Funding
This study and journal rapid service fee were funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Lauren McDaniel, Nathan Everson, Engels Obi, Melissa White; Methodology: Lauren McDaniel, Nathan Everson, Engels Obi, Rose Kohinke, Melissa White, Ellen Rachel Lockhart, Damian Chipriano & Yiyun Chen; Formal Analysis and investigation: Ellen Rachel Lockhart; Writing—review and editing: Lauren McDaniel, Nathan Everson, Engels Obi, Rose Kohinke, Melissa White, Ellen Rachel Lockhart, Damian Chipriano & Yiyun Chen.
Prior Presentation
The clinical data in this study was presented virtually via poster presentation at IDWeek 2021.
Disclosures
Engels Obi is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Rahway, NJ (MSD), and may own stock and/or stock options in Merck & Co., Inc., Rahway, NJ, USA. Yiyun Chen was an employee of MSD at the time this research was conducted and her current affiliation is Agios Pharmaceuticals, Cambridge, Massachusetts, USA. Lauren McDaniel, Nathan Everson, Melissa White, Rose Kohinke, Damian Chipriano, and Ellen Rachel Lockhart have nothing to disclose. Melissa White and Rose Kohinke were employees of Carilion Clinic, Roanoke, VA, USA at the time this research was conducted. Melissa White has changed affiliations to Penn Medicine Lancaster General Health, Lancaster, PA, USA. Rose Kohinke has changed affiliation to University of Pittsburgh Medical Center Pinnacle, Harrisburg, PA, USA.
Compliance with Ethics Guidelines
The study was reviewed by the institution’s internal review board prior to commencement and was in compliance with ethics guidelines. This study was determined to be exempt by the institutional review board at Carilion Clinic. Subjects were not consented as this was waived by IRB due to the nature of the retrospective chart review design.
Data Avalability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
The original online version of this article was revised. In table 3, number of patients with a 30-day CDI related readmission, the correct p-value for Pharmacy cost is 1 and number of patients with a 90-day CDI related readmission, the correct n value is 14.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12/2/2022
A Correction to this paper has been published: 10.1007/s40121-022-00721-w
References
- 1.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile Infection in the United States. N Engl J Med. 2015;372(9):825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prevention, C. for D. C. and. Antibiotic Resistance Threats in the United States. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed June 2021.
- 3.Prohaska L, Mahmoudjafari Z, Shune L, et al. Retrospective evaluation of fidaxomicin versus oral vancomycin for treatment of Clostridium difficile infections in allogeneic stem cell transplant. Hematol Oncol Stem Cell Ther. 2018;11(4):233–240. doi: 10.1016/j.hemonc.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Babakhani F, Gomez A, Robert N, Sears P. Postantibiotic effect of fidaxomicin and its major metabolite, op-1118, against Clostridium difficile. Antimicrob Agents Chemother. 2011;55(9):4427–4429. doi: 10.1128/AAC.00104-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of america (IDSA) and society for healthcare epidemiology of america(SHEA) Clin Infect Dis. 2018;66(7):e1–e48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson S, Lavergne V, Skinner AM, et al. Clinical practice guideline by the infectious diseases society of america (Idsa) and society for healthcare epidemiology of america (SHEA): 2021 focused update guidelines on management of clostridioides difficile infection in adults. Clin Infect Dis. 2021;73(5):e1029–e1044. doi: 10.1093/cid/ciab549. [DOI] [PubMed] [Google Scholar]
- 7.Nerandzic MM, Mullane K, Miller MA, Babakhani F, Donskey CJ. Reduced acquisition and overgrowth of vancomycin-resistant enterococci and candida species in patients treated with fidaxomicin versus vancomycin for Clostridium difficile infection. Clin Infect Dis. 2012;55(suppl 2):S121–S126. doi: 10.1093/cid/cis440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 9.Patel D, Senecal J, Spellberg B, et al. Fidaxomicin as first line: what will it cost in the USA and Canada? Infect Dis (except HIV/AIDS) 2021 doi: 10.1101/2021.11.03.21265881. [DOI] [Google Scholar]
- 10.AnalySource Online (selected from FDB MedKnowledge) data included with permission and copyrighted by First DataBank, Inc. Accessed April 1, 2021.
- 11.Bureau of Labor Statistics. Consumer Price Index. https://www.bls.gov/. Accessed 22 Mar 2021
- 12.Turner NA, Grambow SC, Woods CW, et al. Epidemiologic trends in clostridioides difficile infections in a regional community hospital network. JAMA Netw Open. 2019;2(10):e1914149. doi: 10.1001/jamanetworkopen.2019.14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clancy CJ, Buehrle D, Vu M, Wagener MM, Nguyen MH. Impact of revised infectious diseases society of America and society for healthcare epidemiology of America clinical practice guidelines on the treatment of clostridium difficile infections in the United States. Clin Infect Dis. 2021;72(11):1944–1949. doi: 10.1093/cid/ciaa484. [DOI] [PubMed] [Google Scholar]
- 14.Dubberke ER, Puckett JT, Obi EN, et al. 15. Real-world changes in Clostridioides difficile infection (CDI) treatment utilization and clinical outcomes associated with updated 2017 IDSA guidelines among medicare beneficiaries in the U.S. Open Forum Infect Dis. 2021;8(Supplement_1):S11–S11. doi: 10.1093/ofid/ofab466.015. [DOI] [Google Scholar]
- 15.Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345–354. doi: 10.1093/cid/ciu313. [DOI] [PubMed] [Google Scholar]
- 16.Nelson RL, Suda KJ, Evans C. Antibiotic treatment for Clostridium difficile-associated diarrhoea in adults. Cochrane Database Syst Rev. 2017 doi: 10.1002/14651858.CD004610.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Summers BB, Yates M, Cleveland KO, Gelfand MS, Usery J. Fidaxomicin compared with oral vancomycin for the treatment of severe Clostridium difficile–associated diarrhea: a retrospective review. Hosp Pharm. 2020;55(4):268–272. doi: 10.1177/0018578719844165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel N, Lowry C, Morgenson D, Shah V, Stornelli N, Lodise TP. Comparative effectiveness of early-targeted use of fidaxomicin versus oral vancomycin among hospitalized veterans’ affairs patients with infections due to Clostridioides difficile. Pharmacotherapy. 2021;41(2):212–219. doi: 10.1002/phar.2503. [DOI] [PubMed] [Google Scholar]
- 19.Gupta A, Anathakrishnan AN. Economic burden and cost-effectiveness of therapies for Clostridioides difficile infection: a narrative review. Ther Adv Gastroenterol. 2021;14:175628482110186. doi: 10.1177/17562848211018654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallagher JC, Reilly JP, Navalkele B, et al. Clinical and economic benefits of fidaxomicin compared to vancomycin for Clostridium difficile infection. Antimicro Agents and Chemot. 2015;59(11):7007–7010. doi: 10.1128/AAC.00939-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rinaldi A, Reed EE, Stevenson KB, Coe K, Smith JM. Effectiveness of fidaxomicin versus oral vancomycin in the treatment of recurrent clostridioides difficile. J Clin Pharm Ther. 2021;46(4):993–998. doi: 10.1111/jcpt.13387. [DOI] [PubMed] [Google Scholar]