Abstract
Background
Neuropathic pain (NP) is expected to increase due to the high risk of global population aging. Acupuncture has a definite clinical effect on NP. Therefore, a systematic review and meta-analysis were conducted to evaluate the effect on pain intensity and safety of acupuncture in patients with NP.
Methods
An encompassing search of specific authoritative databases in English, from their inception to 2022, was performed. The databases were as follows: Scopus, Ovid EMBASE, Ovid Cochrane Database of Systematic Reviews, Ovid Cochrane Central Register of Controlled Trials, Ovid MEDLINE(R) and Epub Ahead of Print, In-Process and Other Non-Indexed Citations, and Daily. All the randomized controlled trials regarding the acupuncture treatment of NP will be included. Methodological quality assessment of the included trials was assessed based on the risk of bias from the Cochrane handbook. A meta-analysis was performed for the main outcomes. In addition, sensitivity analysis, subgroup analysis, and funnel plot were also carried out.
Results
A total of 16 studies with 1,021 patients with NP were evaluated in a systematic review. According to the results of the overall meta-analysis in eight RCTs with 338 participants, the acupuncture group was better than the control group in improving changes in pain intensity (SMD −0.59, 95% CI: −0.95 to −0.23, P = 0.001). In subgroup analysis, five trials indicated that acupuncture was more effective in improving changes in pain intensity than sham acupuncture (SMD −0.54, 95% CI: −0.95 to −0.13, P = 0.01), two trials evaluated the effect on changes in pain intensity in the comparison of acupuncture and conventional treatments, no significant difference existed (SMD −0.61, 95% CI: −1.83 to 0.61, P = 0.33), and one trial compared acupuncture with blank control evaluating the effect of changes in pain intensity with a significant difference. Eleven studies mentioned the safety conditions and acupuncture-induced AEs were mild and reversible. Both the sensitivity analysis and funnel plot analysis showed that the meta-analysis was stable and irreversible without publication bias. The GRADE was rated as “very low.”
Conclusion
The acupuncture group had higher effectiveness than sham intervention or blank control for changes in pain intensity, but there is no significant difference between acupuncture and conventional treatments in treating NP. The acupuncture-induced adverse events were mild and reversible. However, the interpretation of our results should be performed cautiously due to the low methodological quality of selected publications.
Systematic review registration
https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022306461.
Keywords: acupuncture, neuropathic pain, alternative and complementary medicine, systematic review, meta-analysis
Introduction
The International Association for the Study of Pain (IASP) recently updated the definition of neuropathic pain (NP) as “pain caused by a lesion or disease of the somatosensory nervous system” (1). NP has both the “positive” symptoms (hyperalgesia, allodynia, shooting pain, burning pain, and especially at rest) that require therapy and the “negative” symptoms (sensory deficits such as hypalgesia and hypesthesia) that cannot be treated with medication (2). A survey of the general population sampled by multimodal recruitment in 2017 reported that about 10% of people in the United States suffered from NP (3). With the aging of the global population, NP is extremely likely to increase (4). Moreover, NP seriously affects patients' quality of life (5). Specifically, patients with NP have anxiety, depression, poor sleep, psychological disorder, physical disability, and social dysfunction (6–10). An observational study on the economic burden of patients with NP at all pain intensity levels in the United States showed that the annualized direct medical expenses to payers were $6,016, the annualized direct expenses to subjects were $2,219, and the annualized indirect expenses of each subject were $19,000 (11).
To date, the medications of NP focus on five categories including serotonin/norepinephrine-modulating antidepressants, Na-blocker anticonvulsants, Ca-modulator anticonvulsants, tramadol, and opioids, and two types of topical medicine including local anesthetics and capsaicin (12). However, pharmacological treatment is not very effective for NP for the reason that the patients keep reporting inadequate pain relief, and a progressive decrease in the estimated effect of NP drugs has been reported (13). In addition, a randomized controlled trial (RCT) on the safety of antiepileptics and antidepressants for NP showed that the incidence of any treatment-emergent adverse event (AE) ranged from 7 to 91.7% compared with the placebo groups, and the dizziness, drowsiness, nausea, and constipation were the most commonly reported AEs (14). Despite the lack of evidence to show beneficial effects, clinical trials on novel analgesic medicine to treat NP are lacking in recent years (15, 16).
Given the situation that NP is mostly chronic, which means that long-term management is required, therefore, it is critical to developing a treatment protocol concentrating on improving efficacy and safety monitoring is critical (17). As an alternative and complementary medicine, acupuncture refers to inserting needles into acupoints or specific parts of the human body at different depths by various manipulations (18). Widespread and effective applications of acupuncture were encouraged in the clinical treatment of NP in recent years (19–27). In addition, a meta-analysis reported that acupuncture could be considered the safer therapy in medications, with the reason that serious AEs were rare, and the most common AEs were mild (28).
However, some researchers consider the difference between acupuncture and sham acupuncture as not being clinically significant (29). A systematic review and meta-analysis (30) on acupuncture in the treatment of NP in adults published in 2017 suggested that it is challenging to either support or refute the effect of acupuncture for NP due to limited data available. Considering the widespread and effective applications of acupuncture in the clinical treatment of NP, the previous meta-analysis conclusions need to be further verified. Therefore, this study was conducted to explore the effect on pain intensity and safety of acupuncture in patients with NP.
Materials and methods
The protocol has been registered in the PROSPERO database with registration number: CRD42022306461. This study was carried out based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines updated in 2020 (31). A detailed description of the PRISMA 2020 checklist of this study is provided in the Supplementary material.
Search strategy
An encompassing search of specific authoritative databases was conducted. The search strategy was designed and performed by a professional librarian. The procedure was described in the Supplementary material of this article. The databases were as follows:
EBM Reviews—Cochrane Central Register of Controlled Trials April 2022;
EBM Reviews—Cochrane Database of Systematic Reviews 2005 to 5 May 2022;
Embase 1974 to 4 May 2022;
Ovid MEDLINE(R) and Epub Ahead of Print, In-Process, In-Data-Review and Other Non-Indexed Citations, Daily and Versions 1946 to 4 May 2022.
Selection criteria
Studies were eligible if met the following criteria: (1) RCTs evaluating acupuncture for NP; (2) participants with the diagnosis of NP; (3) acupuncture treatments as the main observational therapies [including traditional acupuncture, electroacupuncture (EA), auricular acupuncture, and abdominal acupuncture]; (4) the control group could be conventional treatment, sham acupuncture (close to the acupoints but not penetrating the skin), or blank control (no intervention during the treatment period); and (5) pain change variables including but not limited to visual analog scale (VAS) score, numeric rating scale (NRS), and Brief Pain Inventory-Short Form (BPI-SF) worst pain score. Limited to reports in English, AEs were used to assess the safety of acupuncture therapies. The following studies were excluded: (1) conference abstracts, case reports, protocols, reviews, and animal or cellular level experiments; (2) duplicated literature; (3) studies with insufficient data; (4) trials with acupuncture therapy in the control group; (5) studies using methods not based on Traditional Chinese Medicine (TCM) theory like a dry needle; (6) articles on moxibustion, cupping, herbal medicine, laser acupuncture, and any combination of the above; and (7) literature not published in English.
Full-text articles were retrieved after screening based on titles and abstracts of all articles, according to the criteria by two independent researchers. In addition, the discussion was carried out in case of disagreements, and a third party helped to reach a consensus if necessary.
Data extraction
Data were collected independently by two researchers using Excel tables from every included study and reviewed by a third party. The collected information contained the first author's name, year of publication, subjects, age, condition, sample size, interventions, sessions, outcome measures, follow-up, and the selected acupoints of treatment. The pain intensity outcomes were recorded as continuous variables. For each study, the mean difference (MD) before and after treatment was used to pool differences between experimental and control groups.
Risk of bias assessment
Studies were evaluated with the Cochrane risk of bias assessment tool (32) by the two researchers independently. Related evaluations were as follows: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources. Moreover, each part of the evaluation was defined as low, high, or unclear risk of bias. A third party helps reach a consensus if there was a conflict.
Statistical analysis
All analyses were carried out by RevMan 5.3. Qualitative analysis was carried out if extraction was insufficient to conduct a meta-analysis. Given the strong relevance among the scales of pain assessment (33), the results of the NRS, VAS, and BPI-SF worst pain score were used in the meta-analysis. Outcome data were performed with the standardized mean difference (SMD) and 95% confidence interval (CI) to standardize the study results into a unified scale. SMD with 95% CI was calculated with heterogeneity tested by the I2 test. Data was combined by a fixed effect model when I2 < 50%. Otherwise, a random effect model was carried out. There was a significant difference if the p-value was < 0.05 between the two groups. Subgroup analysis or sensitivity analysis could help find out the sources of heterogeneity. Furthermore, a descriptive analysis was conducted when the reasons for heterogeneity could not be determined. A funnel plot was applied to assess publication bias.
Quality of evidence
We rated the general quality of outcome with the classification of GRADEpro GDT (https://www.gradepro.org/) in the following areas: study design, risk of bias, inconsistency, indirectness, imprecision, and other considerations.
Results
Study selection
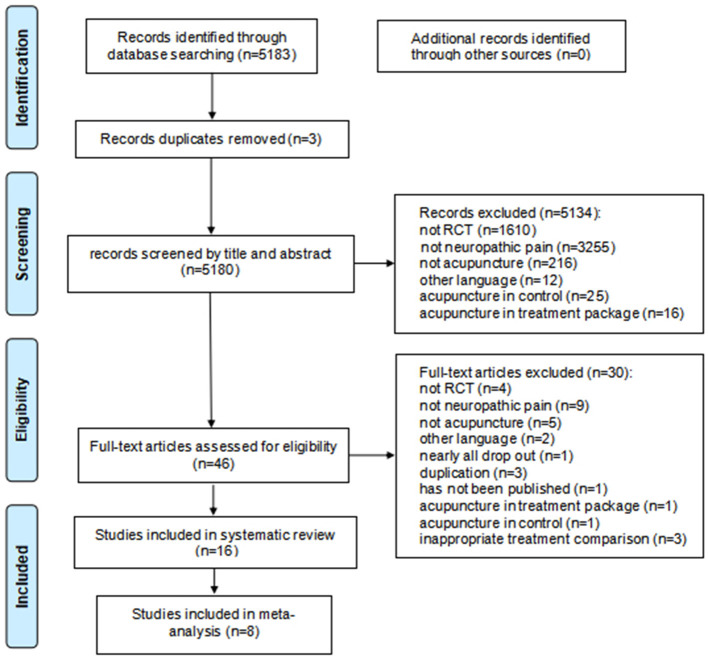
We obtained 5,813 studies after searching the databases. In addition to three duplicates, 5,134 records were removed for irrelevant results after screening. A total of 46 full-text articles were further screened. After excluding 30 reports that did not meet the inclusion criteria, 16 studies with 1,021 patients with NP in English were included in the systematic review. Finally, eight trials with 338 participants were conducted with a meta-analysis. The selection flow of trials is shown in Figure 1.
Figure 1.
Study flow diagram.
Description of the included studies
All the included studies were RCTs, with two (24, 26) of which were multicenter RCTs, including one study (24) located in four centers in South Korea and the other study (26) located in Iran and China. In addition, there were two studies (20, 21) from Taiwan, China, five studies (23, 27, 34–36) from the United States, three studies (19, 25, 37) from Iran, two studies (22, 38) from the United Kingdom, and one study from Croatia (39) and Italy (40), individually. The included studies contained various types of NP including one article on each of the following: postherpetic neuralgia (38), chronic sciatic pain (19), idiopathic neuropathy (40), burning mouth syndrome (39), spinal cord injury (23), and migraine (37), respectively; in addition, there were three studies on carpal tunnel syndrome (20, 21, 25) or diabetic painful neuropathy (22, 24, 40) and five studies (26, 27, 34–36) on chemotherapy-induced peripheral neuropathy. Conventional manual acupuncture was used in eight trials (20–22, 25–27, 38, 39), EA was used in four trials (19, 24, 34, 40), and auricular acupuncture was used in five trials (23, 35–38). The sessions of the interventions varied from 4 to 12 weeks, and the duration of treatments was from 20 to 30 min except for semi-permanent auricular acupuncture. A total of 10 studies (21, 23–27, 34–36, 40) reported follow-up investigations, nine (23–27, 34, 35, 40) of which ranged from 4 to 12 weeks, and one (21) was conducted for 1 year. Seven studies (20, 22, 25, 26, 34, 36, 37) mentioned the background of acupuncture practitioners, of which six (20, 22, 25, 34, 36, 37) were carried out by acupuncturists and one (26) was carried out by physicians with acupuncture experience. Moreover, 10 studies (19–27) reported positive effects, five studies (34, 36, 38–40) reported negative effects, and one study (35) did not report any clear effect on pain intensity. Detailed information is shown in Table 1.
Table 1.
General information of included trials.
| Author | Age (years old) | Type of NP | Sample size (TG:CG) | TG/CG | Outcome measures | Follow-up | Registration |
|---|---|---|---|---|---|---|---|
| Lewith et al. (38) | 49–87 | Post-herpetic neuralgia | 62 (30:32) | AA or BA/mock transcutaneous nerve stimulation | 7-point verbal pain scale | NR | NR |
| Hollisaz (19) | 20–50 | Chronic sciatic pain | 119 (41:38:40) | EA/physiotherapy/placebo (SEA) | Pain reduction percent of MMPC visual scale | NR | NR |
| Yang et al. (20) | TG:49.30 ± 8.90; CG:49.90 ± 10.30 | Carpal tunnel syndrome | 77 (38:39) | A/prednisolone | GSS | NR | NR |
| Penza et al. (40) | 43–75 | Axonal polyneuropathy (4 diabetes neuropathy and 12 idiopathic neuropathy) | 16 (11:5) | EA/SEA | VAS | 15, 30 d | NR |
| Yang et al. (21) | TG:49.30 ± 8.90; CG:49.90 ± 10.30 | Carpal tunnel syndrome | 77 (38:39) | A/prednisolone | GSS | 1 y | NCT01014221 |
| Garrow et al. (22) | TG:68 ± 11.10; CG:63 ± 10.80 | Diabetic painful neuropathy | 45 (24:21) | A/SA | LANNS, VAS | NR | ISRCTN39740785 |
| Jurisic Kvesic et al. (39) | TG:66.70 ± 12.00; CG:63.20 ± 14.00 | Burning mouth syndrome | 42 (20:22) | A/clonazepam | LANNS, VAS | NR | NR |
| Greenlee et al. (34) | TG:51.80 ± 10.70; CG:48.30 ± 12.00 | Stage I-III breast cancer with CIPN | 63 (31:32) | EA/SEA | BPI-SF subscale (intensity worst pain), NPS-4 | 4 w | NCT01163682 |
| Estores et al. (23) | 18–65 | Spinal cord injury | 24 (13:11) | AA/W (NI) | NRS | 4 w | NR |
| Shin et al. (24) | >19 | Painful diabetic peripheral neuropathy | 126 (63:63) | EA/UC (NI) | PI-NRS, McGill pain questionnaire | 4, 8 w | KCT0001135 |
| Lu et al. (27) | >18 | Stage I–III breast cancer with grade 1 or higher CIPN | 40 (20:20) | A/W (UC) | BPI-SF subscales (pain severity, pain interference, average pain) | 8 w | NCT02129686 |
| Bahrami-Taghanaki et al. (25) | 36.36 ± 7.74 | Mild-to-moderate carpal tunnel syndrome | 60 (30:30) | A/celebrex | GSS, GSS subscales (pain, numbness, tingling, muscle weakness, night awakening) | 12 w | IRCT2012122811912N1 |
| Bao et al. (35) | >18 | Solid tumors with moderate to severe CIPN | 75 (27:24:24) | AA + EA/SA/UC (NI) | NRS pain, NRS tingling, NRS numbness | 4 w | NCT03183037 |
| Iravani et al. (26) | TG:57.95 ± 10.39; CG:58.79 ± 8.36 | Cancers with CIPN | 40 (20:20) | A/vit B1 and gabapentin | NRS | 4 w | IRCT20190615043900N1 |
| Bao et al. (36) | >18 | Solid tumors with moderate to severe CIPN | 75 (27:24:24) | AA + EA/SA/UC (NI) | NRS | 4 w | NCT03183037 |
| Habibabadi et al. (37) | TG:37.10 ± 9.33; CG:36.65 ± 8.86 | Migraine | 80 (40:40) | AA/SAA | VAS | NR | IRCT20200213046477N1 |
TG, treatment group; CG, control group; NP, neuropathic pain; CIPN, chemotherapy-induced peripheral neuropathy; A, acupuncture; EA, electroacupuncture; AA, auricular acupuncture; SA, sham acupuncture; SEA, sham EA; SAA, sham auricular acupuncture; UC, usual care; NI, no intervention; R, rehabilitation; W, waiting, NR, not reported; GSS, global symptom score; LANSS, leeds assessment of neuropathic symptoms and signs; BPI-SF, brief pain inventory-short form; BPI, brief pain inventor; NRS, numeric rating scale; ADPS, average daily pain score; VAS, visual analog scale; PI-NRS, pain intensity numerical rating scale; y, year(s); w, week(s); d, day(s).
Risk of bias within trials
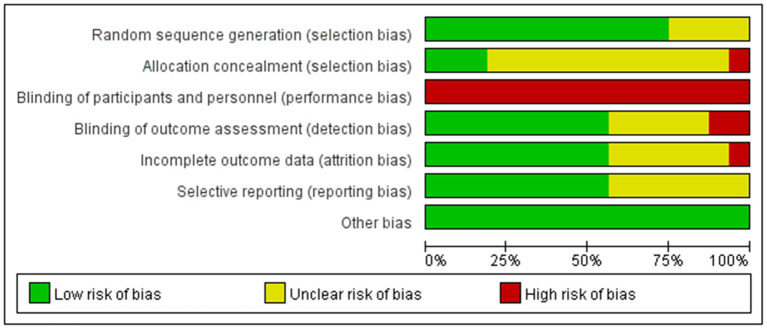
A total of 12 (19–22, 24–26, 35, 38, 39) of the included RCTs were evaluated with a low risk of bias of randomization sequence generation with a detailed description of randomization methods. Seven RCTs (20–22, 24, 35, 36, 38) used a computer-generated randomization list, one trial (39) used a simple randomization method of flipping a coin, one trial (25) used the random numbers table, and three trials (25, 27, 37) used a random allocation software. Four trials (19, 23, 34, 40) lack detailed information and resulted in an unclear risk of bias of randomization. Three trials (22, 24, 26), placing their information sequentially with sealed opaque envelopes, were evaluated with the low risk of bias of allocation concealment. One trial (25) used open randomization of random numbers table, which resulted in a high risk of bias of concealment. The remaining 12 trials (19–21, 23, 27, 34–40) without sufficient description in detail were regarded as unclear risk of bias of allocation concealment. None of the trials were double-blinded because the acupuncturists were not blinded. Nine trials (19, 21, 24, 26, 35–38, 40) were single-blinded in outcome assessment, five trials (20, 22, 25, 27, 39) did not describe methods of blinding, and two trials (23, 34) indicated that assessors were not blinded. Three trials (25, 26, 37) reported no attrition in follow-up studies, while seven trials (21–24, 27, 34, 36) have intention-to-treat (ITT) analysis. However, one trial (35) did not address the loss of follow-up treatments, while five trials (19, 20, 38–40) had no description of the loss of follow-up. Nine trials (20, 22, 25–27, 34–36) registered online previously with certain outcomes and led to a low risk of bias in selective reporting. One trial (24) did not report the complete outcome data. Therefore, we were unable to extract the data for meta-analysis, resulting in the unclear risk of bias. The left trials (19, 21, 23, 38–40) had an unclear risk of bias without detailed reports. In other sources of bias, all trials were evaluated with low risk. The brief information is shown in Figures 2, 3.
Figure 2.
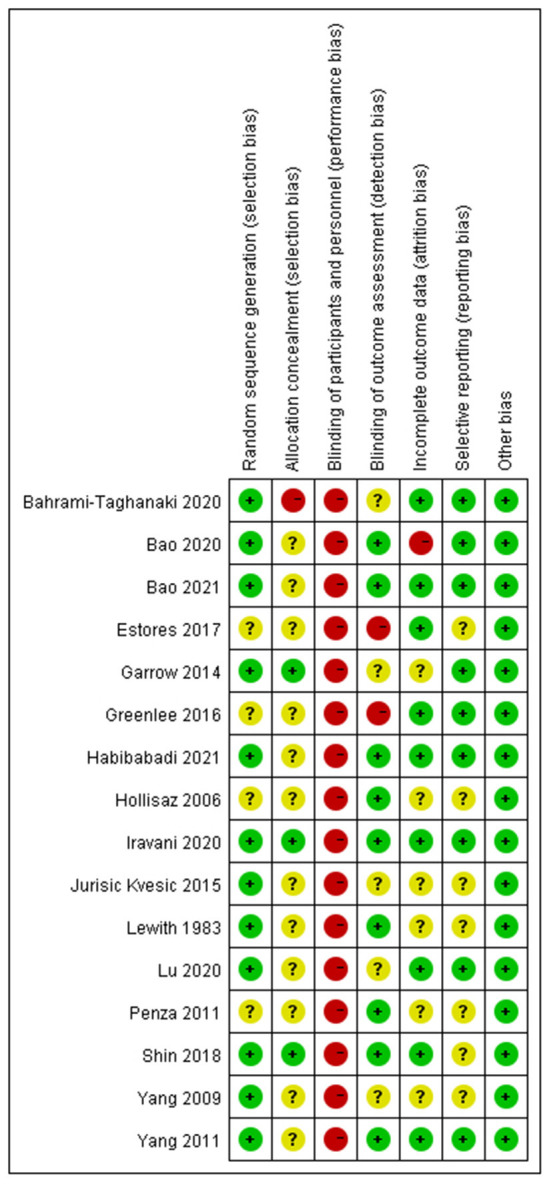
Risk of bias summary.
Figure 3.
Risk of bias graph.
Effects of interventions
Primary outcome (changes in pain intensity)
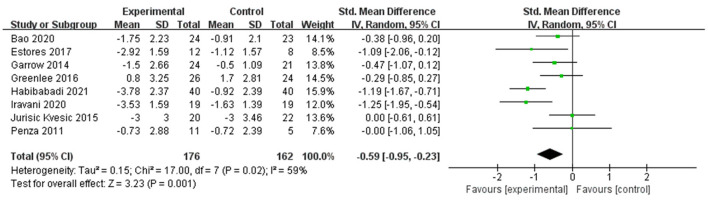
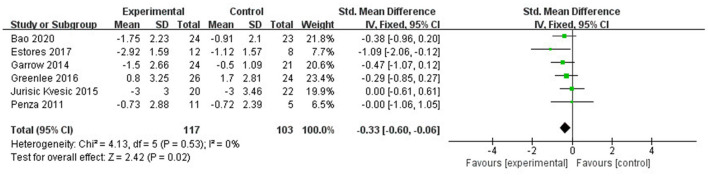
Changes in pain intensity (including changes in VAS, NRS, and BPI-SF worst pain score) occurred in eight RCTs (22, 23, 26, 34, 35, 37, 39, 40) with 338 participants. They investigated the effect of acupuncture on changes in pain intensity, including four trials (22, 26, 39, 40) on conventional manual acupuncture, three trials (23, 35, 37) on auricular acupuncture, and one trial (34) on EA. Using a random effect model among the results (P = 0.02, I2 = 59%), a significant effect was shown in changes in pain intensity in the acupuncture group (SMD −0.59, 95% CI: −0.95 to −0.23, P = 0.001) (Figure 4). Eight trials (19–21, 24, 27, 35, 36, 38) were not pooled in the meta-analysis because one trial (24) did not report complete data and seven trials (19–21, 27, 35, 36, 38) did not report the relative outcome.
Figure 4.
Forest plot and meta-analysis of changes in pain intensity.
Subgroup analysis
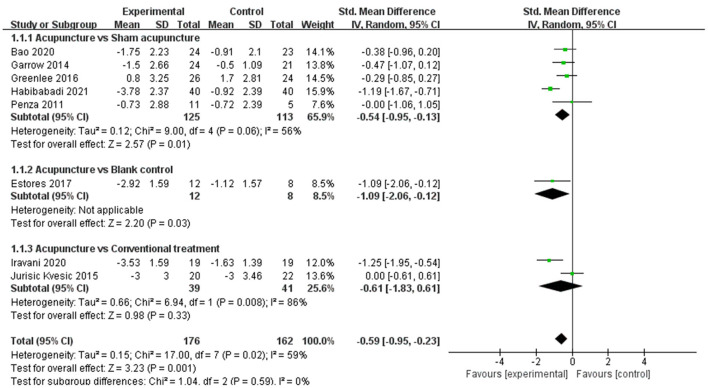
Subgroup analysis was used to verify if different interventions of the control group would affect the changes in pain intensity. Five trials (22, 34, 35, 37, 40) including 238 patients using a random effect model indicated that acupuncture was more effective in improving changes in pain intensity than sham acupuncture (SMD −0.54, 95% CI: −0.95 to −0.13, P = 0.01). Two trials (26, 39) evaluated the effect on changes in pain intensity with a random effect model among 80 patients in the comparison of acupuncture and conventional treatments including clonazepam in the study of Jurisic Kvesic et al. (39) and vitamin B1 and gabapentin in the study of Iravani et al. (26), and there was no significant difference (SMD −0.61, 95% CI: −1.83 to 0.61, P = 0.33). One trial (23) compared acupuncture with blank control evaluating the effect on changes in pain intensity with a significant difference (Figure 5).
Figure 5.
Subgroup analysis of changes in pain intensity.
According to the subgroup analysis, the acupuncture group had higher effectiveness than sham intervention or blank control for changes in pain intensity, but there is no significant difference between acupuncture and conventional treatments.
Sensitivity analysis and publication bias
The sensitivity analysis showed that studies of Iravani et al. (26) and Habibabadi et al. (37) may be the main cause of heterogeneity as I2 dropped to 0% after they were removed (Figure 6). The funnel plot of changes in pain intensity was symmetric, which means no publication bias was detected (Figure 7).
Figure 6.
Sensitivity analysis: Forest plot and meta-analysis of changes in pain intensity after removing the studies of Iravani et al. (26) and Habibabadi et al. (37).
Figure 7.

Funnel plots of changes in pain intensity.
Quality assessment
We evaluated the available evidence with the GRADE tool; the quality of evidence on acupuncture for NP was graded as “very low.” Details are shown in Table 2.
Table 2.
Effect of acupuncture on changes in pain intensity in patients with neuropathic pain.
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Acupuncture | Sham acupuncture or blank control or conventional treatments |
Relative (95% CI) |
Absolute (95% CI) |
||
| New outcome | ||||||||||||
| 8 | Randomized trials | Seriousa | Seriousb | Not serious | Seriousc | None | 176 | 162 | – | SMD 0.59 SD lower (0.95 lower to 0.23 lower) | ⊕○○○ Very low |
CRITICAL |
CI, confidence interval; SMD, standardized mean difference.
aHigh risk of bias due to the lack of allocation concealment, blinding, and incomplete outcome data.
bModerate heterogeneity.
cWide CI.
Adverse events
A total of 11 RCTs (20–22, 24, 26, 27, 34–36, 39, 40) reported safety conditions, while five RCTs (19, 23, 25, 37, 38) did not mention AE. Two RCTs (21, 40) reported that there was no AE, especially one RCT (21) reported that there was no AE during a 1-year follow-up. Three RCTs (24, 26, 39) reported that there was no AE associated with acupuncture treatments. AEs induced by acupuncture mentioned above involved pain, discomfort, paresthesia, minor swelling, bruising and ecchymosis of the acupoint sites, which were mild and reversible (20, 22, 27, 34, 36). Moreover, AEs in the control group were also reported, including nausea and epigastralgia caused by prednisolone (20); drowsiness, dizziness, and nausea induced by clonazepam (39); and somnolence and dizziness caused by vitamin B1 and gabapentin (26), which were mainly induced by side effects of conventional treatment for NP. Details are shown in Table 3.
Table 3.
Characteristic of included trials cont.
| Author | Acupoints | Session | Effect | Adverse events |
|---|---|---|---|---|
| Lewith et al. (38) | AA was used first with most tender point on the pinna, but if no improvement occurred after 2 or 3 treatments it was changed to BA with acupoints on the site and distribution of the pain. | 10 min, 8 times, 8 w | Negative | NR |
| Hollisaz (19) | NR | EA or placebo 20 min; physiotherapy 30 min, 15 times, 4w | Positive | NR |
| Yang et al. (20) | PC-7, PC-6 | A 30 min, 8 times, 4 w; prednisolone 20 mg qd 2 w | Positive | No serious adverse effects were noted. 5% patients in TG: local pain, ecchymosis and paresthesia; 18% patients in CG: nausea and epigastralgia |
| Penza et al. (40) | ST36, SP6, LR3, and BL60 | 30 min, 6 times, 6 w | Negative | No side effect was recorded |
| Yang et al. (21) | PC-7, PC-6 | A 30 min, 8 times, 4 w; prednisolone 20 mg qd, 2 w | Positive | No long-term AEs |
| Garrow et al. (22) | LR3, KI3, SP6, SP10, and ST36 | 30 min, 10 times, 10 w | Positive | One chest pain, one leg pain in TG; one localized swelling of leg in CG |
| Jurisic Kvesic et al. (39) | ST8, GB2, TE21, SI19, SI18, LI4, and GV20 | A 30 min, 12 times, 4 w; clonazepam 0.5 mg qd first 2 w and 0.5 mg bid further 2 w | Negative | Five drowsiness, dizziness, and nausea in CG |
| Greenlee et al. (34) | GB34, ST 36, LI4, LI10, L3, L5, C5, and C7 | 30 min, 12 times, 12 w | Negative | One needle site with grade one discomfort, minor swelling, and bruising in TG |
| Estores et al. (23) | Auricular acupuncture: anterior cingulate, thalamus, omega-2, Shen Men, and point zero | Semi-permanent, 8 times, 8 w | Positive | NR |
| Shin et al. (24) | ST36, GB39, SP9, SP6, LR3, GB41, and Bafeng (EX-LE10) | 30 min, 16 times, 8 w | Positive | 24 AEs and six serious AEs. None of them related to TG |
| Lu et al. (27) | LI-11, TW-5, SP-9, ST-36, SP-6, K-3, LR-3, Yin Tang, Baxie, and Qiduan (1st−5th) | 30 min, 18 times, 8 w | Positive | One grade one pruritis in feet, one grade two joint pain possibly related to the acupuncture |
| Bahrami-Taghanaki et al. (25) | LI-11, TB-5, PC-8, LI-4, PC-7, SI-3, TB-4, and ST-36 | 30 min, 12 times, 4 w; celebrex 100 mg, tid, 4 w | Positive | NR |
| Bao et al. (35) | Auricular acupuncture: Shen Men, point zero, and a third electrodermal active point; body acupuncture: LI-4, PC-6, SI-3, LR-3, GB-42, ST-40, Bafeng 2, and Bafeng 3 | 20 min, 10times, 8 w | Unclear | AEs were few and mild |
| Iravani et al. (26) | CV 6, GV 20, ST 36, SP 6, LI 4, LI 11, LR 3, Baxie (EX-UE 9), and Bafeng (EX-LE 10) | 20 min, 12 sessions, 4 w; vit B1 300 mg and gabapentin 900 mg, qd, 4 w | Positive | One somnolence and dizziness in CG |
| Bao et al. (36) | Auricular acupuncture: Shen Men, point zero, and a third electrodermal active point, and bilateral; body acupuncture: LI-4, PC-6, SI-3, LR-3, GB-43, ST-40, Bafeng 2, and Bafeng 3 | 30 min, 10 times, 8 w | Negative | Three grade one pain at the needling site, two bruising, one feel claustrophobic with the eye mask on in TG |
| Habibabadi et al. (37) | Auricular acupuncture: Sympathetic, Gallbladder, GB3, GB40, Lesser Occipital Nerve, Thalamus, Ear Apex, Forehead, Zero, Shen Men, PGE1, PGE2, Liver, Hypothalamus, Frustration, Temple, Occiput, local cervical point (back), local cervical point (front), and worry point | Semi-permanent, 2 times, 4 w | Positive | NR |
TG, treatment group; CG, control group; NR, not reported; AEs, adverse events; EA, electroacupuncture.
Discussion
This study included a systematic review of 16 RCTs with 1,021 patients and a meta-analysis of eight studies with 338 subjects assessing the effect on pain intensity and safety of acupuncture in patients with NP. Our findings from the qualitative analysis of the systematic review showed an unclear effect of acupuncture on improving pain intensity in patients with NP because 10 studies (19–27) reported positive effects, five studies (34, 36, 38–40) reported negative effects, and one study (35) did not report any clear effect on pain intensity. However, the results of the meta-analysis indicated that acupuncture was an effective intervention for patients with NP. In addition, 11 trials (20–22, 24, 26, 27, 34–36, 39, 40) reported safety conditions, and acupuncture-induced AEs were mild and reversible, indicating that acupuncture is a relatively safe intervention for patients with NP.
Sham acupuncture and blank control are conventionally designed to help reduce bias in assessing the specific effect of acupuncture. According to the results of the subgroup analysis, acupuncture had higher effectiveness than sham acupuncture or blank control for changes in pain intensity. This means that acupuncture is also an effective treatment for NP. However, there was no significant difference between acupuncture and conventional treatments for NP. Nevertheless, it is notable that compared with the side effects of conventional treatments, acupuncture-induced AEs were mild and reversible. Therefore, patients who respond to the limited effects of conventional treatments or feel difficult to withstand the side effects of conventional treatments may consider acupuncture as an alternative. In brief, acupuncture may be beneficial to improve the pain intensity of patients with NP in a relatively safe means, and as a complementary part to provide more specific evidence to improve clinical practice. The results of the sensitivity analysis and funnel plot showed that the effect of acupuncture on the changes in pain intensity in patients with NP was robust. Furthermore, the “very low” GRADE results of changes in pain intensity may suggest this treatment to clinical practice with a recommendation level of “very low.” Ultimately, the interpretation of our results should be performed cautiously due to the low methodological quality of selected publications.
Developing after nerve injuries, NP occurs in deleterious changes in damaged neurons and goes along with the nociceptive and descending modulatory pathways of the central nervous system (41). Sensitization of nociceptive pathways are mainly based on maladaptive structural alterations, cell interactions, and molecular signaling, including changes in the activation of immune cells, glial-derived mediators, ion channels, and epigenetic regulation (42). Ali et al. (43) found that EA can improve NP by stimulating the spinal microglial expression of IL-10 and subsequent β-endorphin. Liu et al. (44) indicated that EA can modulate miR-214 to suppress neuronal apoptosis by targeting Bax and inhibiting the expression of the Nav1.3 channel. Jang et al. (45) suggested that acupuncture can ameliorate chronic NP-induced comorbid conditions by changing the DNA methylation of Nr4a1, Rasgrp1, Rassf1, and Chkb in the PFC. Chen et al. (46) suggested that EA can ameliorate tactile allodynia after peripheral nerve injury by suppressing the excessive expression of IFN-γ in the spinal cord and subsequent P2X4R. In addition, several studies indicated that EA can relieve NP by suppressing PKC-dependent membrane P2X3 upregulation in DRG (47–49). Lee et al. (50) found that acupuncture can relieve pain by inhibiting JNK activation in astrocytes after SCI. Li et al. (51) suggested that EA can improve paclitaxel-induced peripheral NP by suppressing TLR4 signaling and TRPV1 upregulation in DRG neurons, which can further result in reduced spinal glial activation. Moreover, several studies found that opioid receptors or spinal muscarinic receptors can significantly suppress mechanical allodynia with NP (52–54). In addition, Napadow et al. (55) indicated that patients with carpal tunnel syndrome evaluated by fMRI respond to acupuncture through a coordinated limbic network including the hypothalamus and amygdala. Currently, the mechanism of acupuncture for NP has not met an agreement, and thus more concentration is needed to focus on how acupuncture relieves NP.
Study strengths and limitations
Our study has multiple strengths. First, our review focused on the effect of using acupuncture alone, so we excluded the studies of mixed therapies and conducted a subgroup study of sham acupuncture or blank control in the control group to verify whether acupuncture is effective for NP. Second, a previously published meta-analysis (30) showed that it is challenging to either support or refute the effect of acupuncture on NP. Nevertheless, the study only included two manual acupuncture RCTs (22, 56) on pain intensity in its meta-analysis. In contrast, we include more RCTs with a larger sample size and more acupuncture manipulations. Moreover, compared with the previous study (30), patients with central NP and peripheral NP were included in our study, which may more strongly support the hypothesis that acupuncture on NP is effective. Third, many studies were performed at multiple locations and in different countries, covering a more ethnically and culturally diverse sample, which may reduce selection bias and improve external validity. Fourth, sensitivity analysis and funnel plot were conducted, demonstrating that the meta-analysis was stable and irreversible without publication bias. Fifth, most of the studies were longitudinal and one of them was followed for 1 year. To some extent, our study provided supporting evidence for the clinical practice of acupuncture in the treatment of patients with NP.
However, there were also some limitations to this study. First, in the qualitative analysis of the systematic review, six (34–36, 38–40) of the 16 trials indicated a negative or ambiguous effect on pain intensity of acupuncture for NP. Second, the outcomes of life quality evaluation were inadequate to pool to perform the meta-analysis. Third, GRADE was rated as “very low.” The quality of total studies was low, especially in the area of allocation concealment and participant and personnel blindness.
Implication for further research
More high-quality studies on acupuncture for patients with NP are needed to enlarge the sample size and reduce bias. Longer follow-up trials are required to observe the long-term effect of acupuncture in the treatment of NP. Consolidated Standards of Reporting Trial (CONSORT) statement and STRICTA checklists (57, 58) should be followed in future studies. To achieve double-blinding, standardized trial design, a timely data storage system and a well-coordinated team are needed to help performed sham intervention successfully, which can refer to pragmatic-explanatory continuum indicator summary (PRECIS) or PRECIS-2 (59–61).
Conclusion
The acupuncture group had higher effectiveness than sham intervention or blank control for changes in pain intensity, but there is no significant difference between acupuncture and conventional treatments in treating NP. The acupuncture-induced adverse events were mild and reversible. However, the interpretation of our results should be performed cautiously due to the low methodological quality of selected publications.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MX conceptualized and designed the study. ZF and SC contributed to drafting the text and the analysis of data. JW searched and screened the data. HY, YW, LL, ZY, BH, and HZ identified relevant articles and extracted data. XZ analyzed data. All authors approved the final manuscript.
Acknowledgments
The authors would like to thank Juan Yang, MD. PhD., Kyung-Min Shin, MD. PhD., and Anli Weng, MD. PhD. for their support of this work.
Funding Statement
This study was supported by the Chinese Medicine Key Medical Specialties Construction Project of Shenzhen Municipal Health Commission (Grant No. ZYTS019).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.1076993/full#supplementary-material
References
- 1.Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, et al. Neuropathic pain. Nat Rev Dis Primers. (2017) 3:17002. 10.1038/nrdp.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlereth T. Guideline “diagnosis and non-interventional therapy of neuropathic pain” of the German society of neurology (Deutsche Gesellschaft Fur Neurologie). Neurol Res Pract. (2020) 2:16. 10.1186/s42466-020-00063-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiBonaventura MD, Sadosky A, Concialdi K, Hopps M, Kudel I, Parsons B, et al. The prevalence of probable neuropathic pain in the US: results from a multimodal general-population health survey. J Pain Res. (2017) 10:2525–38. 10.2147/JPR.S127014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmader KE, Baron R, Haanpaa ML, Mayer J, O'Connor AB, Rice AS, et al. Treatment considerations for elderly and frail patients with neuropathic pain. Mayo Clin Proc. (2010) 85:S26–32. 10.4065/mcp.2009.0646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue S, Taguchi T, Yamashita T, Nakamura M, Ushida T. The prevalence and impact of chronic neuropathic pain on daily and social life: a nationwide study in a Japanese population. Eur J Pain. (2017) 21:727–37. 10.1002/ejp.977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li KL, Chen YM, Wang XQ, Hu HY. Bibliometric analysis of studies on neuropathic pain associated with depression or anxiety published from 2000 to 2020. Front Hum Neurosci. (2021) 15:729587. 10.3389/fnhum.2021.729587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guntel M, Huzmeli ED, Melek I. Patients with neuropathic pain have poor sleep quality. J Nerv Ment Dis. (2021) 209:505–9. 10.1097/NMD.0000000000001325 [DOI] [PubMed] [Google Scholar]
- 8.Daniel HC, Narewska J, Serpell M, Hoggart B, Johnson R, Rice AS. Comparison of psychological and physical function in neuropathic pain and nociceptive pain: implications for cognitive behavioral pain management programs. Eur J Pain. (2008) 12:731–41. 10.1016/j.ejpain.2007.11.006 [DOI] [PubMed] [Google Scholar]
- 9.Melikoglu MA, Celik A. Does neuropathic pain affect the quality of sleep? Eurasian J Med. (2017) 49:40–3. 10.5152/eurasianjmed.2017.16261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarberg BH, Billington R. Consequences of neuropathic pain quality-of-life issues and associated costs. Am J Manag Care. (2006) 12(9 Suppl):S263–8. [PubMed] [Google Scholar]
- 11.Schaefer C, Sadosky A, Mann R, Daniel S, Parsons B, Tuchman M, et al. Pain severity and the economic burden of neuropathic pain in the United States: beat neuropathic pain observational study. Clinicoecon Outcomes Res. (2014) 6:483–96. 10.2147/CEOR.S63323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bannister K, Sachau J, Baron R, Dickenson AH. Neuropathic pain: mechanism-based therapeutics. Annu Rev Pharmacol Toxicol. (2020) 60:257–74. 10.1146/annurev-pharmtox-010818-021524 [DOI] [PubMed] [Google Scholar]
- 13.Serrano Afonso A, Carnaval T, Videla Cés S. Combination therapy for neuropathic pain: a review of recent evidence. J Clin Med. (2021) 10:533. 10.20944/preprints202106.0200.v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selvy M, Cuménal M, Kerckhove N, Courteix C, Busserolles J, Balayssac D. The safety of medications used to treat peripheral neuropathic pain, part 1 (antidepressants and antiepileptics): review of double-blind, placebo-controlled, randomized clinical trials. Exp Opin Drug Saf. (2020) 19:707–33. 10.1080/14740338.2020.1764934 [DOI] [PubMed] [Google Scholar]
- 15.Sachau J, Baron R. Neuropathic pain therapy: a puzzle of different approaches to stratify patients. Pain. (2021) 162:993–4. 10.1097/j.pain.0000000000002120 [DOI] [PubMed] [Google Scholar]
- 16.Knezevic NN, Cicmil N, Knezevic I, Candido KD. Discontinued neuropathic pain therapy between 2009 and 2015. Exp Opin Investig Drugs. (2015) 24:1631–46. 10.1517/13543784.2015.1099627 [DOI] [PubMed] [Google Scholar]
- 17.Rowin J. Integrative neuromuscular medicine: neuropathy and neuropathic pain: consider the alternatives. Muscle Nerve. (2019) 60:124–36. 10.1002/mus.26510 [DOI] [PubMed] [Google Scholar]
- 18.Musial F. Acupuncture for the treatment of pain: a mega-placebo? Front Neurosci. (2019) 13:1110. 10.3389/fnins.2019.01110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollisaz M. Use of electroacupuncture for treatment of chronic sciatic pain. Internet J Pain Symptom Control Palliat Care. (2007) 5:1–4. 10.5580/993 [DOI] [Google Scholar]
- 20.Yang CP, Hsieh CL, Wang NH Li TC, Hwang KL Yu SC, et al. Acupuncture in patients with carpal tunnel syndrome: a randomized controlled trial. Clin J Pain. (2009) 25:327–33. 10.1097/AJP.0b013e318190511c [DOI] [PubMed] [Google Scholar]
- 21.Yang C, Wang N, Li T, Hsieh C, Chang H, Hwang K, et al. A randomized clinical trial of acupuncture vs. oral steroids for carpal tunnel syndrome: a long-term follow-up. J Pain. (2011) 12:272–9. 10.1016/j.jpain.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 22.Garrow AP, Xing M, Vere J, Verrall B, Wang L, Jude EB. Role of acupuncture in the management of diabetic painful neuropathy (Dpn): a pilot rct. Acupunct Med. (2014) 32:242–9. 10.1136/acupmed-2013-010495 [DOI] [PubMed] [Google Scholar]
- 23.Estores I, Chen K, Jackson B, Lao L, Gorman PH. Auricular acupuncture for spinal cord injury related neuropathic pain: a pilot controlled clinical trial. J Spinal Cord Med. (2017) 40:432–8. 10.1080/10790268.2016.1141489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin KM, Lee S, Lee EY, Kim CH, Kang JW, Lee CK, et al. Electroacupuncture for painful diabetic peripheral neuropathy: a multicenter, randomized, assessor-blinded, controlled trial. Diabetes Care. (2018) 41:e141–e2. 10.2337/dc18-1254 [DOI] [PubMed] [Google Scholar]
- 25.Bahrami-Taghanaki H, Azizi H, Hasanabadi H, Jokar MH, Iranmanesh A, Khorsand-Vakilzadeh A, et al. Acupuncture for carpal tunnel syndrome: a randomized controlled trial studying changes in clinical symptoms and electrodiagnostic tests. Altern Ther Health Med. (2020) 26:10–6. [PubMed] [Google Scholar]
- 26.Iravani S, Kazemi Motlagh AH, Emami Razavi SZ, Shahi F, Wang J, Hou L, et al. Effectiveness of acupuncture treatment on chemotherapy-induced peripheral neuropathy: a pilot, randomized, assessor-blinded, controlled trial. Pain Res Manag. (2020) 2020:2504674. 10.1155/2020/2504674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu W, Giobbie-Hurder A, Freedman RA, Shin IH, Lin NU, Partridge AH, et al. Acupuncture for chemotherapy-induced peripheral neuropathy in breast cancer survivors: a randomized controlled pilot trial. Oncologist. (2020) 25:310–8. 10.1634/theoncologist.2019-0489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bäumler P, Zhang W, Stübinger T, Irnich D. Acupuncture-related adverse events: systematic review and meta-analyses of prospective clinical studies. BMJ Open. (2021) 11:e045961. 10.1136/bmjopen-2020-045961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGeeney BE. Acupuncture is all placebo and here is why. Headache. (2015) 55:465–9. 10.1111/head.12524 [DOI] [PubMed] [Google Scholar]
- 30.Ju ZY, Wang K, Cui HS, Yao Y, Liu SM, Zhou J, et al. Acupuncture for neuropathic pain in adults. Cochrane Database Syst Rev. (2017) 12:Cd012057. 10.1002/14651858.CD012057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. 10.1016/j.ijsu.2021.105906 [DOI] [PubMed] [Google Scholar]
- 32.JPT . H, S. G. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Cochrane Collabor. (2011) 91:25–32. Available online at: https://training.cochrane.org/handbook/archive/v5.1/ [Google Scholar]
- 33.Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. (2011) 41:1073–93. 10.1016/j.jpainsymman.2010.08.016 [DOI] [PubMed] [Google Scholar]
- 34.Greenlee H, Crew KD, Capodice J, Awad D, Buono D, Shi Z, et al. Randomized sham-controlled pilot trial of weekly electro-acupuncture for the prevention of taxane-induced peripheral neuropathy in women with early stage breast cancer. Breast Cancer Res Treat. (2016) 156:453–64. 10.1007/s10549-016-3759-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao T, Patil S, Chen C, Zhi IW Li QS, Piulson L, et al. Effect of acupuncture vs. sham procedure on chemotherapy-induced peripheral neuropathy symptoms: a randomized clinical trial. JAMA Netw Open. (2020) 3:e200681. 10.1001/jamanetworkopen.2020.0681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bao T, Baser R, Chen C, Weitzman M, Zhang YL, Seluzicki C, et al. Health-related quality of life in cancer survivors with chemotherapy-induced peripheral neuropathy: a randomized clinical trial. Oncologist. (2021) 26:e2070–e8. 10.1002/onco.13933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Habibabadi MR, Ashtari F, Raeisi I. Effect of auricular acupuncture with semi-permanent ear needles on controlling migraine symptoms: a single-blind randomized clinical trial. J Acupunct Meridian Stud. (2021) 14:58–66. 10.51507/j.jams.2021.14.2.58 [DOI] [PubMed] [Google Scholar]
- 38.Lewith GT, Field J, Machin D. Acupuncture compared with placebo in post-herpetic pain. Pain. (1983) 17:361–8. 10.1016/0304-3959(83)90167-7 [DOI] [PubMed] [Google Scholar]
- 39.Jurisic Kvesic A, Zavoreo I, Basic Kes V, Vucicevic Boras V, Ciliga D, Gabric D, et al. The effectiveness of acupuncture vs. clonazepam in patients with burning mouth syndrome. Acupunct Med. (2015) 33:289–92. 10.1136/acupmed-2015-010759 [DOI] [PubMed] [Google Scholar]
- 40.Penza P, Bricchi M, Scola A, Campanella A, Lauria G. Electroacupuncture is not effective in chronic painful neuropathies. Pain Med. (2011) 12:1819–23. 10.1111/j.1526-4637.2011.01230.x [DOI] [PubMed] [Google Scholar]
- 41.Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. (2014) 348:f7656. 10.1136/bmj.f7656 [DOI] [PubMed] [Google Scholar]
- 42.Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. (2021) 101:259–301. 10.1152/physrev.00045.2019 [DOI] [PubMed] [Google Scholar]
- 43.Ali U, Apryani E, Wu HY, Mao XF, Liu H, Wang YX. Low frequency electroacupuncture alleviates neuropathic pain by activation of spinal microglial Il-10/?-endorphin pathway. Biomed Pharmacother. (2020) 125:109898. 10.1016/j.biopha.2020.109898 [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Wu Y. Electro-acupuncture-modulated Mir-214 prevents neuronal apoptosis by targeting bax and inhibits sodium channel Nav13 expression in rats after spinal cord injury. Biomed Pharmacother. (2017) 89:1125–35. 10.1016/j.biopha.2017.02.077 [DOI] [PubMed] [Google Scholar]
- 45.Jang JH, Song EM, Do YH, Ahn S, Oh JY, Hwang TY, et al. Acupuncture alleviates chronic pain and comorbid conditions in a mouse model of neuropathic pain: the involvement of DNA methylation in the prefrontal cortex. Pain. (2021) 162:514–30. 10.1097/j.pain.0000000000002031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen XM, Xu J, Song JG, Zheng BJ, Wang XR. Electroacupuncture inhibits excessive interferon-Γ evoked up-regulation of P2x4 receptor in spinal microglia in a Cci rat model for neuropathic pain. Br J Anaesth. (2015) 114:150–7. 10.1093/bja/aeu199 [DOI] [PubMed] [Google Scholar]
- 47.Tu WZ, Cheng RD, Cheng B, Lu J, Cao F, Lin HY, et al. Analgesic effect of electroacupuncture on chronic neuropathic pain mediated by P2x3 receptors in rat dorsal root ganglion neurons. Neurochem Int. (2012) 60:379–86. 10.1016/j.neuint.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 48.Wang WS, Tu WZ, Cheng RD, He R, Ruan LH, Zhang L, et al. Electroacupuncture and a-317491 depress the transmission of pain on primary afferent mediated by the P2x3 receptor in rats with chronic neuropathic pain states. J Neurosci Res. (2014) 92:1703–13. 10.1002/jnr.23451 [DOI] [PubMed] [Google Scholar]
- 49.Zhou YF, Ying XM, He XF, Shou SY, Wei JJ, Tai ZX, et al. Suppressing Pkc-dependent membrane P2x3 receptor upregulation in dorsal root ganglia mediated electroacupuncture analgesia in rat painful diabetic neuropathy. Purinerg Signal. (2018) 14:359–69. 10.1007/s11302-018-9617-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JY, Choi DC, Oh TH, Yune TY. Analgesic effect of acupuncture is mediated via inhibition of Jnk activation in astrocytes after spinal cord injury. PLoS ONE. (2013) 8:e73948. 10.1371/journal.pone.0073948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, Yin C, Li X, Liu B, Wang J, Zheng X, et al. Electroacupuncture alleviates paclitaxel-induced peripheral neuropathic pain in rats via suppressing Tlr4 signaling and Trpv1 upregulation in sensory neurons. Int J Mol Sci. (2019) 20:5917. 10.3390/ijms20235917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meng X, Zhang Y, Li A, Xin J, Lao L, Ren K, et al. The effects of opioid receptor antagonists on electroacupuncture-produced anti-allodynia/hyperalgesia in rats with paclitaxel-evoked peripheral neuropathy. Brain Res. (2011) 1414:58–65. 10.1016/j.brainres.2011.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim JH, Min BI, Na HS, Park DS. Relieving effects of electroacupuncture on mechanical allodynia in neuropathic pain model of inferior caudal trunk injury in rat: mediation by spinal opioid receptors. Brain Res. (2004) 998:230–6. 10.1016/j.brainres.2003.11.045 [DOI] [PubMed] [Google Scholar]
- 54.Park JH, Kim SK, Kim HN, Sun B, Koo S, Choi SM, et al. Spinal cholinergic mechanism of the relieving effects of electroacupuncture on cold and warm allodynia in a rat model of neuropathic pain. J Physiol Sci. (2009) 59:291–8. 10.1007/s12576-009-0035-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Napadow V, Kettner N, Liu J, Li M, Kwong KK, Vangel M, et al. Hypothalamus and amygdala response to acupuncture stimuli in carpal tunnel syndrome. Pain. (2007) 130:254–66. 10.1016/j.pain.2006.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han X, Wang L, Shi H, Zheng G, He J, Wu W, et al. Acupuncture combined with methylcobalamin for the treatment of chemotherapy-induced peripheral neuropathy in patients with multiple myeloma. BMC Cancer. (2017) 17:40. 10.1186/s12885-016-3037-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MacPherson H, Altman DG, Hammerschlag R, Youping L, Taixiang W, White A, et al. Revised standards for reporting interventions in clinical trials of acupuncture (stricta): extending the consort statement. PLoS Med. (2010) 7:e1000261. 10.1371/journal.pmed.1000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. Consort 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. (2010) 340:c869. 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riddle DL, Johnson RE, Jensen MP, Keefe FJ, Kroenke K, Bair MJ, et al. The pragmatic-explanatory continuum indicator summary (precis) instrument was useful for refining a randomized trial design: experiences from an investigative team. J Clin Epidemiol. (2010) 63:1271–5. 10.1016/j.jclinepi.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The Precis-2 tool: designing trials that are fit for purpose. BMJ. (2015) 350:h2147. 10.1136/bmj.h2147 [DOI] [PubMed] [Google Scholar]
- 61.Zhang YQ, Jiao RM, Witt CM, Lao L, Liu J-P, Thabane L, et al. How to design high quality acupuncture trials—a consensus informed by evidence. BMJ. (2022) 376:e067476. 10.1136/bmj-2021-067476 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.