Abstract
Background
Whether Triglyceride-glucose (TyG) index is associated with 10-year risk of a first hard atherosclerotic cardiovascular disease (ASCVD) event in the United States remains unclear.
Methods
In this cross-sectional study, the participants, ranged from 40 to 79 years old, were from the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2018. TyG index was the independent variable and 10-year risk of a first hard ASCVD was the dependent variable. The other variables, such as age, gender, race, body mass index (BMI), hypertension treatment states, smoking states and low-density lipoprotein cholesterol (LDL-C) et al. were considered as the potential confounding factors. Multivariate linear regression models and smooth curve fittings were used to evaluate the association between TyG index and 10-year risk of a first hard ASCVD event.
Results
A total of 2,142 participants were included in the analysis. The results showed that TyG index was associated with an increased 10-year risk of a first hard ASCVD event [β = 2.208, 95% (1.716, 2.700), P < 0.00001]. The association had statistical significance in both men [β = 3.862 95% CI (3.274, 4.450), P < 0.00001] and women [β = 1.067, 95% CI (0.286, 1.849), P = 0.00756)] according to subgroup analysis. Smooth curve fittings revealed that TyG index was linearly associated with 10-year risk of ASCVD in both male and female.
Conclusion
Triglyceride-glucose index was associated with an increased 10-year risk of a first hard ASCVD event in the United States, suggesting it is necessary to monitor and control an appropriate range of TyG index.
Keywords: TyG index, 10-year risk, atherosclerotic cardiovascular disease, NHANES, cross-sectional study
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality all over the world (1). Despite the clinical application of anti-platelet agents and statins, the patients with CVD remain high risk of recurrent adverse cardiovascular events. Therefore, identifying the early indicator of CVD is significant, and will be beneficial for instructing further healthcare (2, 3).
The homeostasis model assessment estimated insulin resistance (HOMA-IR) index is used to evaluate β-cell function and insulin resistance (IR), but the effectiveness of HOMA-IR is influenced by insulin treatment (4). The triglyceride-glucose (TyG) index, calculated by Ln[fasting blood TG (mg/dL) × fasting glucose (mg/dL)/2)], has been confirmed more superior than HOMA-IR in assessing IR in individuals with or without diabetes (5). Recently, TyG index was also considered as an indicator of CVD or cardiovascular risk factors. Park et al.’s study showed that increased TyG index was associated with progression of carotid arterial stenosis, and Li et al. revealed that TyG index was positive related with the incidence of carotid artery plaques in patients with coronary heart disease (6, 7). Moreover, Sang et al. demonstrated that TyG index was independently associated with increased arterial stiffness (8). A multicenter retrospective study, including 731 patients with coronary heart disease, showed that TyG index influenced the severity of CAD, and increased TyG index could predict a high risk of multi-vessel lesions. Many clinical studies also showed that TyG index might be used as a hallmark of cardiovascular events in CVD patients (7, 9–13).
In addition, Ding et al. performed a meta-analysis of cohort studies which included more than 5 million participants, and the results demonstrated that compared with the lowest TyG index, the highest TyG index were independently associated with a higher incidence of atherosclerotic cardiovascular disease (ASCVD) in the participants without CVD at baseline (10). However, the included studies were performed in Asian or European, whether TyG index is associated with cardiovascular risk in the United States remains unclear.
Here, we conducted a cross-sectional study to estimate the association between TyG index and 10-year risk of a first hard ASCVD event (defined as non-fatal myocardial infarction or coronary heart disease death or stroke, over a 10-year period among people free from ASCVD at the beginning of the period) according to the 2013 ACC/AHA guideline on the Assessment of Cardiovascular Risk (14) using a large-scale database from National Health and Nutrition Examination Survey (NHANES).
Materials and methods
Study population
National Health and Nutrition Examination Survey is a population-based national survey that collected information regarding health and nutrition status in the United States with biennial cycles.1 Our analysis collected the data of 10 cycles between 1999 and 2018. The risk of a first hard ASCVD event in adults aged from 40 to 79 years without diagnosed ASCVD was estimated according to the 2013 ACC/AHA guideline (14). The participants were included if they met following criteria: (1) 40 to 79 years old, (2) without diagnosed ASCVD, (3) the level of high-density lipoprotein cholesterol (HDL-C) ranged from 20 mg/dl to 100 mg/dl, (4) the level of total cholesterol (TC) ranged from 130 to 320 mg/dl, and (5) the systolic blood pressure (SBP) ranged from 90 to 200 mmHg.
Finally, 2142 participants were included in the analyses after excluding the participants missing TyG data (n = 3136). The ethics review board of the National Center for Health Statistics approved all NHANES protocols and informed consents were obtained from all participants (15).
Variables
Continuous variables included age, body mass index (BMI), SBP, diastolic blood pressure (DBP), TC, TG, HDL-C, low density lipoprotein cholesterol (LDL-C), TyG index. Categorical variables included gender, race, smoking status, hypertension treatment status and diabetes status. The 10-year risk of a first hard ASCVD event was calculated according to the 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk, which can be accessed by https://tools.acc.org/ASCVD-Risk-Estimator-Plus/#!/calculate/estimate/ (14). The TyG index was calculated by Ln [fasting TG (mg/dL) × fasting glucose (mg/dL)/2)].
Statistical analysis
Continuous variables were represented as mean and standard deviation (SD) values if the data was normally distributed, or as median values and interquartile ranges otherwise; comparisons between groups were analyzed with Student’s t test or one-way analysis of variance. Categorical variables were described as percentages and compared by χ2 testing. We performed multicollinearity between variables, and the covariates were included as potential confounders in the final models if they changed the estimates of TyG index on 10-year risk of a first hard cardiovascular event by more than 10% or were significantly associated with 10-year risk of a first hard cardiovascular event (P < 0.01). Multivariable linear regression was used to assess the association between TyG index and 10-year risk of ASCVD event, and shown as model 1 (unadjusted), model 2 (adjusted for age, gender and race), model 2 (adjusted for m age, gender, race, BMI, SBP, DBP, hypertension treatment, smoking, diabetes, LDL-C, and fasting glucose). All statistical analyses were performed with R (The R Foundation; version 3.4.3)2 and EmpowerStats software (X&Y Solutions, Inc., Boston, MA, USA).3
Results
Characteristics of included participants
A total of 2,142 participants were included in our analyses, the characteristics of participants are presented in Table 1. The participants were classified based on TyG index quartiles (Q1, 7.069–8.448; Q2, 8.448–8.866; Q3, 8.866–9.318; Q4, 9.318–12.309). There were obvious differences in age, gender, race, BMI, lipid profiles, fasting glucose, SBP, DBP, and diabetes status among four groups (P < 0.05). And smoking status and hypertension treatment status are comparable among four groups (P > 0.05).
TABLE 1.
Baseline characteristic of participants.
| TyG index | Q1 (7.069–8.448) | Q2 (8.448–8.866) | Q3 (8.866–9.318) | Q4 (9.318–12.309) | P-value |
| N | 536 | 535 | 535 | 536 | |
| Age | 60.63 ± 10.12 | 60.16 ± 10.51 | 61.35 ± 9.69 | 58.82 ± 10.32 | <0.001 |
| Gender | 0.013 | ||||
| Men | 281 (52.43%) | 284 (53.08%) | 297 (55.51%) | 329 (61.38%) | |
| Women | 255 (47.57%) | 251 (46.92%) | 238 (44.49%) | 207 (38.62%) | |
| Race | <0.001 | ||||
| Non-Hispanic black | 214 (39.93%) | 144 (26.92%) | 93 (17.38%) | 73 (13.62%) | |
| Other | 322 (60.07%) | 391 (73.08%) | 442 (82.62%) | 463 (86.38%) | |
| BMI | 28.84 ± 6.93 | 30.39 ± 6.70 | 31.48 ± 6.54 | 31.92 ± 5.66 | <0.001 |
| Lipid profile | |||||
| TC (mg/dl) | 189.55 ± 32.75 | 197.09 ± 35.18 | 200.11 ± 36.46 | 208.28 ± 39.62 | <0.001 |
| HDL-C (mg/dl) | 62.01 ± 14.83 | 55.04 ± 13.66 | 48.97 ± 11.62 | 42.58 ± 11.38 | <0.001 |
| TG (mg/dl) | 71.92 ± 17.31 | 110.44 ± 19.63 | 153.19 ± 33.20 | 279.04 ± 155.12 | <0.001 |
| LDL-C (mg/dl) | 113.21 ± 30.25 | 120.14 ± 32.21 | 120.46 ± 33.62 | 114.55 ± 34.89 | <0.001 |
| Fasting glucose (mg/dl) | 99.39 ± 14.82 | 106.54 ± 17.23 | 118.99 ± 29.12 | 148.83 ± 65.06 | <0.001 |
| SBP | 132.60 ± 18.41 | 131.43 ± 18.76 | 134.32 ± 19.80 | 134.33 ± 19.46 | 0.032 |
| DBP | 71.37 ± 14.02 | 71.88 ± 13.80 | 72.63 ± 13.37 | 73.99 ± 14.32 | 0.013 |
| Hypertension treatment | 0.122 | ||||
| Yes | 455 (84.89%) | 452 (84.49%) | 472 (88.22%) | 473 (88.25%) | |
| No | 81 (15.11%) | 83 (15.51%) | 63 (11.78%) | 63 (11.75%) | |
| Diabetes | <0.001 | ||||
| Yes | 63 (11.75%) | 70 (13.08%) | 152 (28.41%) | 222 (41.42%) | |
| No | 473 (88.25%) | 465 (86.92%) | 383 (71.59%) | 314 (58.58%) | |
| Smoker | 0.414 | ||||
| Yes | 178 (33.21%) | 197 (36.82%) | 184 (34.39%) | 201 (37.50%) | |
| No | 358 (66.79%) | 338 (63.18%) | 351 (65.61%) | 335 (62.50%) | |
| 10-year risk | 14.43 ± 11.66 | 14.64 ± 12.14 | 18.26 ± 13.51 | 19.75 ± 14.43 | <0.001 |
Mean ± SD for continuous variables. (%) for categorical variables. TyG, triglyceride-glucose; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; LDL-C, low density lipoprotein cholesterol.
TyG index and 10-year risk of a first hard ASCVD event
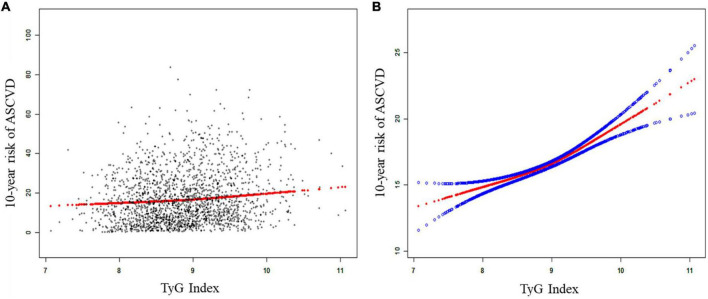
The results from regression analyses were described in Table 2. We adjusted no confounding factor in model 1; adjusted age, gender and race in model 2; and adjusted age, gender, race, BMI, SBP, DBP, hypertension treatment status, smoking status, diabetes and LDL-C in model 3. The results demonstrated that TyG index was associated with an increased 10-year risk of a first hard ASCVD event in model 1 [β = 3.680, 95% CI (2.847, 4.513)], model 2 [β = 4.950, 95% CI (4.393, 5.508)] or model 3[β = 2.208, 95% (1.716, 2.700)]. Compared with the TyG index in Q1 (the lowest quartile), the 10-year risk of a first hard ASCVD event increased in Q3 quartile [(β = 1.177, 95% CI (0.493, 1.860)], and in Q4 quartile [(β = 3.151, 95% CI (2.382, 3.920)]. We performed generalized additive models and smooth curve fittings to evaluate the associations between TyG index and the 10-year risk (Figure 1). There was a linear relationship between TyG index and the 10-year risk, which indicated that the 10-year risk of a first hard ASCVD event increased with TyG index.
TABLE 2.
Linear regression analyses of TyG index and 10-year risk of a first hard ASCVD event.
| Model 1 | Model 2 | Model 3 | |
| β (95% CI), P-value | β (95% CI), P-value | β (95% CI), P-value | |
| TyG index | 3.680 (2.847, 4.513), <0.00001 | 4.950 (4.393, 5.508), <0.00001 | 2.208 (1.716, 2.700) <0.00001 |
| TyG index (quartiles) | |||
| Q2 vs. Q1 | 0.214 (−1.341, 1.769), 0.78734 | 1.244 (0.213, 2.275), 0.01812 | 0.673 (0.021, 1.325) 0.04304 |
| Q3 vs. Q1 | 3.830 (2.275, 5.385), <0.00001 | 4.138 (3.094, 5.181), <0.00001 | 1.177 (0.493, 1.860) 0.00075 |
| Q4 vs. Q1 | 5.328 (3.774, 6.882), <0.00001 | 7.643 (6.590, 8.696), <0.00001 | 3.151 (2.382, 3.920) <0.00001 |
Model 1 adjust for none; Model 2 adjust for age, gender and race; model 3 adjust for age, gender, race, body mass index, systolic blood pressure, diastolic blood pressure, hypertension treatment, smoking, diabetes, low density lipoprotein cholesterol and fasting glucose. TyG, triglyceride-glucose; CI, confidence interval.
FIGURE 1.
The association between TyG index and the 10-year risk of a first hard ASCVD event. (A) The linear association between TyG index and the 10-year risk of a first hard ASCVD event. Black points represent samples, the red line represent the fitting line. (B) The smooth curve between TyG index and the 10-year risk of a first hard ASCVD event. Solid red line represents the smooth curve. Blue lines represent the 95% of confidence interval. Age, gender, race, body mass index, systolic blood pressure, diastolic blood pressure, hypertension treatment status, smoking status, diabetes status, and low-density lipoprotein cholesterol were adjusted. ASCVD, atherosclerotic cardiovascular disease; TyG, triglyceride-glucose.
Stratified analysis based on gender between TyG index and the 10-year risk of a first hard ASCVD event
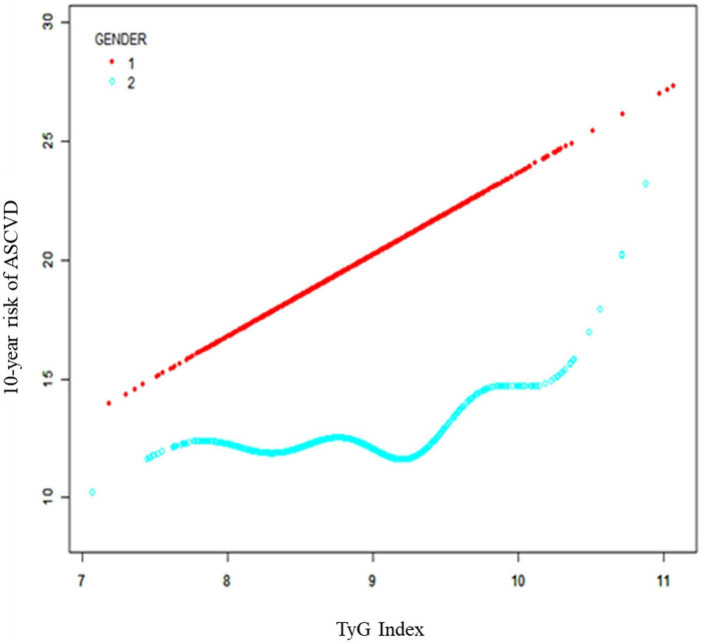
Subgroup analysis stratified by gender was performed, and significant associations between TyG index and 10-year risk of a first hard ASCVD event were observed in both men [β = 3.862, 95% CI (3.274, 4.450), P < 0.00001] and women [β = 1.067, 95% CI (0.286, 1.849), P = 0.00756)] (Table 3). Smooth curve fittings and generalized additive models were used to characterize the association between TyG index and the 10-year risk of a first hard ASCVD event (Figure 2).
TABLE 3.
Subgroup analysis stratified by gender between TyG index and 10-year risk of a first hard ASCVD event.
| Model 1 | Model 2 | Model 3 | |
| β (95% CI), P-value | β (95% CI), P-value | β (95% CI), P-value | |
| Male | 2.917 (1.791, 4.042), <0.00001 | 6.117 (5.360, 6.873), <0.00001 | 3.862 (3.274, 4.450) <0.00001 |
| Female | 3.894 (2.766, 5.021), <0.00001 | 3.825 (3.010, 4.641), <0.00001 | 1.067 (0.286, 1.849) 0.00756 |
Model 1 adjust for none; Model 2 adjust for age, gender and race; model 3 adjust for age, gender, race, body mass index, systolic blood pressure, diastolic blood pressure, hypertension treatment, smoking, diabetes, low density lipoprotein cholesterol. CI, confidence interval.
FIGURE 2.
Subgroup analysis stratified by gender between TyG index and 10-year risk of a first hard ASCVD event. Age, race, body mass index, systolic blood pressure, diastolic blood pressure, hypertension treatment, smoking, diabetes, and low-density lipoprotein cholesterol were adjusted. ASCVD, atherosclerotic cardiovascular disease; TyG, triglyceride-glucose.
Discussion
In the present study, 2,142 participants aged from 40 to 79 were included in the analyses to evaluate the associations between TyG index and the 10-year risk of a first hard ASCVD event. As we known, this is the first study to reveal the positive association between TyG index and 10-year risk of a first hard ASCVD event in the United States. In addition, the subgroup analysis based on gender showed that TyG index was associated with the 10-year risk of a first hard ASCVD event in both male and female. And we also identified the threshold effect of TyG index on ASCVD events in male was 9.812.
Despite significant advances in the prevention and treatment of ASCVDs, ASCVDs remain one of the leading causes of death worldwide (16, 17). Established risk factors of ASCVDs include age, the male sex, family history of ASCVDs, obesity, hypertension, hypercholesteremia, and diabetes (18, 19). However, recent studies also demonstrated that some patients without these risk factors may still develop ASCVDs, thus highlighting the importance of identifying novel risk factors for ASCVDs in the general population (20–22). TyG index has been identified as an indicator of cardiovascular events in Asia or Europe (10, 23), however, whether TyG index could be a hallmark of CVD incidence in the United States remains unclear because of the differences in the genes of CVD susceptibility and risk factors (24, 25). In the present study, we used the data from NHANES, which included nationally representative samples in the United States. And the results demonstrated that there is a positive association between TyG index and the 10-year risk of a first hard ASCVD event in the United States regardless of confounders, such as age, gender, race, BMI, SBP, DBP, hypertension treatment status, smoking status, diabetes status, and LDL-C. Additionally, even though significant associations between TyG index and 10-year risk of a first hard ASCVD event were observed in both men and women, the risk of ASCVD events increased more in male than those in female as each unit of TyG index increased (3.862 vs. 1.067%), indicating that TyG index might be more relevant to incidence of CVD in male than female. These difference between male and female may be due to the combinations of other risk factors (26) and these exposures could substantially increase 10-year risk of a first hard ASCVD event and enlarged the potentially effect of TyG index (27).
A strength of this study is that we firstly explored the association between TyG index and 10-year risk of a first hard ASCVD event in the United States. There were several limitations in our study. Firstly, we could not obtain the total covariates, such as atherosclerotic history, because some data were not collected in the survey. Secondly, races were divided into non-Hispanic Black people and others to calculate the 10-year risk of a first hard cardiovascular event, so other categories cannot be calculated. Finally, the present analysis was based on cross-sectional study, the specific causality relationship between TyG index and 10-year risk of a first hard cardiovascular event in the United States should be validated in prospective studies.
Conclusion
Triglyceride-glucose index was associated with an increased 10-year risk of a first hard ASCVD event in the United States using a cross-sectional database, suggesting it is necessary to monitor and control an appropriate range of TyG index.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the National Center for Health Statistics. The patients/participants provided their written informed consent to participate in this study.
Author contributions
HQ and L-ZL: writing the manuscript and data analysis. LC: revising the manuscript. H-TW: statistics and analyses. C-GF and S-SZ: revising and editing. All authors contributed to the article and approved the submitted version.
Acknowledgments
We appreciate the time and effort given by participants during the data collection phase of the NHANES project.
Funding Statement
This work was supported by Young scholar of China association for science and technology (No. 2020-QNRCI-02), Fundamental research funds for the central public Welfare Research Institutes (No. ZZ15-YQ-006), and the project of National Natural Science Foundation of China (Grant No. 82104678).
Footnotes
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.O’Hearn M, Lauren B, Wong J, Kim D, Mozaffarian D. Trends and disparities in cardiometabolic health among U.S. Adults, 1999-2018. J Am Coll Cardiol. (2022) 80:138–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrono C, Morais J, Baigent C, Collet J, Fitzgerald D, Halvorsen S, et al. Antiplatelet agents for the treatment and prevention of coronary atherothrombosis. J Am Coll Cardiol. (2017) 70:1760–76. [DOI] [PubMed] [Google Scholar]
- 3.Mortensen M, Tybjærg-Hansen A, Nordestgaard B. Statin eligibility for primary prevention of cardiovascular disease according to 2021 European prevention guidelines compared with other international guidelines. JAMA Cardiol. (2022) 7:836–43. 10.1001/jamacardio.2022.1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minh H, Tien H, Sinh C, Thang D, Chen C, Tay J, et al. Assessment of preferred methods to measure insulin resistance in Asian patients with hypertension. J Clin Hypertens. (2021) 23:529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simental-Mendía L, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. (2008) 6:299–304. [DOI] [PubMed] [Google Scholar]
- 6.Park K, Ahn C, Lee S, Kang S, Nam J, Lee B, et al. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. (2019) 42:1569–73. [DOI] [PubMed] [Google Scholar]
- 7.Cui H, Liu Q, Wu Y, Cao L. Cumulative triglyceride-glucose index is a risk for CVD: a prospective cohort study. Cardiovasc Diabetol. (2022) 21:22. 10.1186/s12933-022-01456-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S, Ahn C, Lee B, Kang S, Nam J, You J, et al. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. (2018) 17:41. 10.1186/s12933-018-0692-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian X, Zuo Y, Chen S, Liu Q, Tao B, Wu S, et al. Triglyceride-glucose index is associated with the risk of myocardial infarction: an 11-year prospective study in the Kailuan cohort. Cardiovasc Diabetol. (2021) 20:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. (2021) 20:76. 10.1186/s12933-021-01268-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao J, Hao Q, Gao M, Zhang K, Li X, Wang J, et al. Triglyceride-glucose index in the development of peripheral artery disease: findings from the Atherosclerosis Risk in Communities (ARIC) Study. Cardiovasc Diabetol. (2021) 20:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barzegar N, Tohidi M, Hasheminia M, Azizi F, Hadaegh F. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: tehran lipid and glucose study. Cardiovasc Diabetol. (2020) 19:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park B, Lee Y, Lee H, Jung D. The triglyceride-glucose index predicts ischemic heart disease risk in Koreans: a prospective study using National Health Insurance Service data. Cardiovasc Diabetol. (2020) 19:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goff D, Jr., Lloyd-Jones D, Bennett G, Coady S, D’Agostino R, Sr., Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College Of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. (2014) 63(25 Pt. B):2935–59. 10.1016/j.jacc.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zipf G, Chiappa M, Porter K, Ostchega Y, Lewis B, Dostal J. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat 1. (2013) 56:1–37. [PubMed] [Google Scholar]
- 16.Virani S, Alonso A, Benjamin E, Bittencourt M, Callaway C, Carson A, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. (2020) 141:e139–596. 10.1161/cir.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 17.Barquera S, Pedroza-Tobías A, Medina C, Hernández-Barrera L, Bibbins-Domingo K, Lozano R, et al. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res. (2015) 46:328–38. 10.1016/j.arcmed.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 18.Rosenblit P. Extreme atherosclerotic cardiovascular disease (ASCVD) risk recognition. Curr Diabetes Rep. (2019) 19:61. 10.1007/s11892-019-1178-6 [DOI] [PubMed] [Google Scholar]
- 19.Choi S. The potential role of biomarkers associated with ASCVD risk: risk-enhancing biomarkers. J Lipid Atheroscler. (2019) 8:173–82. 10.12997/jla.2019.8.2.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahtta D, Khalid U, Misra A, Samad Z, Nasir K, Virani S. Premature atherosclerotic cardiovascular disease: what have we learned recently? Curr Atheroscler Rep. (2020) 22:44. 10.1007/s11883-020-00862-8 [DOI] [PubMed] [Google Scholar]
- 21.Vikulova D, Grubisic M, Zhao Y, Lynch K, Humphries K, Pimstone S, et al. Premature atherosclerotic cardiovascular disease: trends in incidence, risk factors, and sex-related differences, 2000 to 2016. J Am Heart Assoc. (2019) 8:e012178. 10.1161/jaha.119.012178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayman L. Prevention of atherosclerotic cardiovascular disease in childhood. Curr Cardiol Rep. (2020) 22:86. 10.1007/s11886-020-01332-y [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, et al. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. (2022) 21:124. 10.1186/s12933-022-01546-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnett D, Blumenthal R, Albert M, Buroker A, Goldberger Z, Hahn E, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. (2019) 74:1376–414. 10.1016/j.jacc.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du X, Patel A, Anderson C, Dong J, Ma C. Epidemiology of cardiovascular disease in china and opportunities for improvement: JACC international. J Am Coll Cardiol. (2019) 73:3135–47. 10.1016/j.jacc.2019.04.036 [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Schoufour J, Wang D, Dhana K, Pan A, Liu X, et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ. (2020) 368:l6669. 10.1136/bmj.l6669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramdas Nayak V, Satheesh P, Shenoy M, Kalra S. Triglyceride glucose (TyG) index: a surrogate biomarker of insulin resistance. J Pak Med Assoc. (2022) 72:986–8. 10.47391/jpma.22-63 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.