Abstract
The outermost layer of Mycobacterium tuberculosis contains two major polysaccharides, arabinomannan (AM) and glucan (GC). We studied the in vitro and in vivo expression of an M. tuberculosis AM antigen using monoclonal antibody (MAb) 9d8 (2a), an isotype-switched variant of the immunoglobulin G3 (IgG3) MAb 9d8. MAb 9d8 had been previously shown to bind M. tuberculosis AM and the M. tuberculosis surface. Our in vitro experiments showed that MAb 9d8(2a) bound strongly to whole-cell M. tuberculosis Erdman but not to the CDC 1551 strain grown in medium for an extended period. However, AM antigen was detected in the culture supernatant of both strains, and its concentration increased in a time-dependent manner. The detection of AM antigen from both strains was decreased in the presence of Tween 80. In mice infected with M. tuberculosis Erdman, AM antigen accumulated in organ homogenates concomitant to an increase in bacterial organ burden and an increase in IgG and IgM titer to AM. These results (i) indicate that the surface expression of AM during in vitro growth changes with culture age, is strain dependent, and is affected by the presence of Tween 80 in the culture media; (ii) show that AM is produced by bacteria growth in vivo; and (iii) demonstrate that the amount of in vivo-detected AM can be dependent on the number of bacteria in the infected organ.
It is estimated that one-third of the human population is infected with Mycobacterium tuberculosis and that tuberculosis (TB) is a leading cause of death worldwide (9). TB in the setting of human immunodeficiency virus infection is a progressive disease that often responds poorly to therapy (18). There is an acute need for additional, effective, preventative and therapeutic modalities. A greater understanding of the M. tuberculosis surface could aid in the development of such modalities. Recent evidence from our group demonstrated that a monoclonal antibody (MAb) recognizing M. tuberculosis arabinomannan (AM) prolonged the life of mice infected with a lethal dose of M. tuberculosis after the bacilli were precoated with this antibody (34). This observation and a previous study that investigated the nature of the 9d8 antigen (14) have led to further studies regarding the in vivo and in vitro expression of the antigen recognized by MAb 9d8.
The outer layer of the mycobacteria contains mainly polysaccharides and some protein, with lipid components further inside (reviewed in reference 6). Dye studies of the mycobacterial envelope indicate that the outermost layer of the mycobacterial cell is composed of carbohydrate (28). This layer may correspond to the electron transparent zone (ETZ) described immediately outside the mycobacterial cell wall (6). Paired fibrils indicate the presence of an ETZ in surface structures of mycobacteria processed for electron microscopy (19) that can be found when the bacteria are intracellular (8). Recently, Lemassu and Daffe demonstrated that the two major components of the M. tuberculosis outermost layer are the polysaccharides AM and glucan (GC) (20). A third mannan component was also identified (20). AM is a neutral polysaccharide, of which two types have been described, either acetylated or nonacetylated (2). AMs have been reported to be structurally variable in different strains of M. tuberculosis (23). AM can also be found attached to a lipid, in the form of lipoarabinomannan (LAM) (2). The arabinose portion of the AM component of LAM from M. tuberculosis Erdman, H37Rv, H37Ra, Mycobacterium leprae, and Mycobacterium bovis BCG is capped extensively with mannose (2, 13). LAM has not been found in the outermost portion of the envelope (20) even though MAbs against LAM bind whole M. tuberculosis cells (12, 14). The reactivity of these MAbs with the outermost surface of M. tuberculosis is believed to be consequence of binding to AM in the ETZ (25). The polysaccharides that accumulate in the supernatant of M. tuberculosis cultures are predominantly AM and GC, providing additional evidence that supports the existence of a carbohydrate-rich outermost layer (7).
There is relatively little information regarding the antigenic nature of M. tuberculosis AM during growth of the bacilli. The expression of AM antigens during different in vitro growth stages and under different medium conditions has not been studied. Production of AM during the course of experimental infection has never been demonstrated, although inferred, as serological responses to M. tuberculosis LAM have been documented (24, 30; S. C. Arya, Letter, J. Clin. Microbiol. 31:2836–2838, 1993). In this study we generated a MAb to AM and used it to ascertain the presence of an AM antigen on whole-cell M. tuberculosis during in vitro growth. In addition, we designed a sensitive capture enzyme-linked immunosorbent assay (ELISA) for AM and used it to measure the amount of AM antigen in culture supernatant and in infected tissue. Our results indicate that AM is expressed in a dynamic and M. tuberculosis strain-dependent manner during in vitro culture and that this expression is affected by the presence of Tween 80 in the culture media. We also provide evidence that the amount of AM antigen produced in vivo can be dependent on the number of bacteria in the infected organ.
(The data in this paper are from a thesis to be submitted by J. Reid Schwebach in partial fulfillment of the requirements for the degree of doctor of philosophy from the Sue Golding Graduate Division of Medical Sciences, Albert Einstein College of Medicine, Yeshiva University, Bronx, N.Y. Portions of this work have been presented in poster format at the 2000 American Society for Microbiology General Meeting, Los Angeles, Calif.)
MATERIALS AND METHODS
M. tuberculosis for ELISA.
M. tuberculosis strains Erdman and CDC 1551 were obtained from the laboratory of Barry Bloom (Harvard School of Public Health, Boston, Mass.) and were grown in 7H9 medium (Difco, Detroit, Mich.) containing 1% glycerol. When indicated, the medium contained 0.05% Tween 80 (Sigma) (7H9-T). All M. tuberculosis cultures were grown in 490-cm2 roller bottles (Corning, Inc., Corning, N.Y.) rotated at 1.25 rpm in a 5% CO2 incubator at 37°C in the biohazard safety level 3 (BSL-3) facility. A vial of M. tuberculosis stored at −80°C was thawed and was then added to 25 ml of 7H9-T media and grown for 5 days to early log phase. A 5-ml volume of this starter culture was placed in a 15-ml conical tube and was sonicated for 15 s at 60% output, constant duty cycle, using a Branson Sonifier 250 sonicator (Danbury, Conn.) with a cuphorn sonicator (Branson 102 Convertor). The resulting sonicate was then added to 25 ml of 7H9-T media and grown for two more days. The absorbance value of this suspension was measured at 600 nm after fixation in an equal volume of 10% buffered formalin. Using the absorbance value of each bacterial culture, an equivalent amount of cells from either M. tuberculosis Erdman or CDC 1551 cultures was then added to roller bottles containing 50 ml of 7H9-T or 7H9. Previous studies had shown that 2 × 108 to 5 × 108 viable bacteria in 1 ml of 7H9-T give an absorbance of 1.0 at 600 nm after fixation. By this estimate, approximately 0.4 × 108 to 1 × 108 bacteria were used to begin each culture. The cultures were grown for 11, 20, or 25 days. Bacteria were collected at the various time intervals by centrifugation at 2,000 × g for 8 min, washed in 10 ml of phosphate-buffered saline and frozen at −80°C in approximately 2.0 ml of PBS (now concentrated >20-fold) until all samples were collected for analysis.
Whole-cell M. tuberculosis ELISA was performed as previously described (14), except that the number of bacteria used was standardized according to the amount of protein in a 100-μl volume of sedimented bacteria. For this ELISA, the concentrated frozen stocks of harvested M. tuberculosis (described above) were thawed and killed by heating to 80°C for 2 h. The concentrated bacterial samples were then suspended by persistent pipetting (using a shortened 200-μl pipette tip), creating a uniform and very dense suspension of bacteria. The suspension was then allowed to settle in a transparent 1.5-ml microcentrifuge tube, and the supernatant was removed, leaving 100 μl of settled bacteria. The day-11 M. tuberculosis samples grown in the presence of Tween 80 did not sediment as rapidly as other samples, so a more concentrated (approximately 100-fold) suspension of bacteria was used during sedimentation. Samples were then washed twice in 1 ml of PBS. The amount of protein in 20% of a 100-μl bacterial aliquot, collected by sedimentation as described above, was determined by boiling in 1 M NaOH, and the absorbance was measured at 230 and 260 nm (22). All bacterial samples, regardless of growth period or medium type, had similar amounts of protein (mean, 465 ± 39 μg, median, 466 μg; range, 390 to 665 μg) in 100 μl of settled bacteria. For each M. tuberculosis sample, an independent bacterial suspension was sedimented in a microcentrifuge tube as described above, washed twice in PBS, and added to 30 ml of Tris-buffered saline (TBS) (0.1 M Tris base, pH 7.2, 0.15 M NaCl, 0.001 M NaN3). This suspension was then sonicated with three 5-s bursts using a probe tip at 70% output, high setting, and a value of 280 (Braun Sonic 2000 U Sonifier). Prior to use in ELISA, samples were diluted approximately 1.7-fold in TBS (M. tuberculosis TBS); the volume was adjusted to account for the minor differences in protein concentration (using the values determined from the protein analysis of the other samples).
M. tuberculosis for capture ELISA.
M. tuberculosis cultures grown for 11, 20, or 25 days were started at different times and were harvested simultaneously. Culture media were then filtered twice using 0.2-μm-pore-size syringe filters (Fisher, Springfield, N.J.) before removal from the BSL-3 environment. Sodium azide was then added at a concentration of 1 mM, and the media were immediately analyzed by AM capture ELISA.
M. tuberculosis AM.
M. tuberculosis was isolated from a patient with confirmed TB and pleural effusion at Montefiore Medical Center (Bronx, N.Y.). AM from liquid culture supernatant (Sauton's media) of this strain was isolated by precipitation with 70% ethyl alcohol and was further purified by anion exchange and gel filtration chromatography, using methods similar to those described by Lemassu and Daffe (20). The AM contained only arabinose and mannose, as confirmed by gas liquid chromatography and [13C] magnetic resonance spectroscopy.
MAbs.
MAbs 9d8 (an immunoglobulin G3 [IgG3] recognizing AM) and 5c11 (an IgM recognizing LAM, AM, and mycolyarabinogalactan peptidoglycan complex), were previously described (14). Purified CS-40 MAb, an IgG1 recognizing Erdman mannose-capped LAM (manLAM) (3), was kindly supplied by J. T. Belisle, Colorado State University, through National Institutes of Health (NIH) contract NO1-A1-75320. The isolation of MAb 9d8(2a) is described below. Hybridomas expressing MAb 9d8, 9d8(2a), or 5c11 were grown in Dulbecco minimal essential medium (Cellgro, Herndon, Va.) containing 10% fetal calf serum (Harlan, Indianapolis, Ind.), and supernatants were concentrated under compressed N2 using an Amicon 8400 concentrator and YM100 membranes (Amicon/Millipore, Bedford, Mass.). Antibody concentration was determined by ELISA in comparison to isotype-matched control standards (ICN Biomedicals, Aurora, Ohio).
Isolation of 9d8(2a) from 9d8 hybridoma.
Spontaneous switch variants of the 9d8 hybridoma expressing the IgG2a isotype were recovered by the technique of sib selection using the ELISA spot assay (31). Briefly, polystyrene microtiter plates (Corning) coated with 2 μg of unlabeled goat anti-mouse (GAM) IgG2a antibody (Southern Biotechnology Associates Birmingham, Ala.)/ml were blocked with 3% bovine serum albumin (BSA) (fraction V; ICN Biomedicals, Aurora, Ohio) in TBS. Hybridoma cells producing MAb 9d8 were mixed with media and 1 μg of biotinylated GAM IgG2a antibody/ml, before addition to the microtiter plates for 4 h at 37°C. One-half of these cells had been kept separate for another cycle of enrichment or for cloning on soft agar. Extravidin (Sigma) diluted 1:5,000 in 1% BSA TBS was then added to the plates for incubation at room temperature for 1 h. Plates were washed as done for M. tuberculosis ELISA between steps. The assay was developed by addition of 5-bromo-4-chloro-3-indolylphosphate p-toluidine salt (BCIP) (Amresco, Solon, Ohio) in 2-amino-2-methyl-1-propanol (AMP) buffer (9.58% AMP, pH 9.8, 0.01% Triton X-405, and 1 M MgCl2 · 6H2O) (Sigma) for 4 h at room temperature. The microtiter plates were hand rinsed in deionized water, and the wells were inspected by using a dissecting microscope. Blue spots were considered indicative of cells producing IgG2a. After six enrichments by limiting dilution, the 9d8(2a) (IgG2a) hybridoma was cloned three times on soft agar to stabilize and purify a clonal population, and the isotype of the antibody was confirmed by ELISA.
ELISA.
Whole-cell M. tuberculosis ELISA was performed as follows: after addition of 50 μl of M. tuberculosis TBS suspension to microtiter wells (Corning) and incubation for 2 h at room temperature, the plates were blocked by adding 200 μl of 1% BSA in TBS and incubating overnight at 4°C. Primary antibody [MAb 9d8, 9d8(2a) or CS-40] in TBS was added, and the plate contents were incubated for 1 h at 37°C. For all ELISAs, primary antibody was serially diluted threefold between microplate wells. GAM alkaline phosphatase-conjugated (GAM-AP) IgG(2a) or IgG1 antibody (Southern Biotechnology) [recognizing MAb 9d8(2a) or CS-40, respectively] in TBS (1 μg/ml) was added, and plate contents were incubated for 1 h at 37°C. The ELISA was developed by adding 50 μl of 1 μg of p-nitrophenylphosphate (PNPP) (Sigma)/ml in substrate buffer (0.001 M MgCl2 and 0.05 M Na2CO3, pH 9.8) to each well. Plates were washed three times with a plate washer (Skatron, Sterling, Va.), using TBS containing 0.05% Tween 20 (Sigma), between all ELISA steps. The absorbance at 405 nm was then measured using a Multiskan MS microtiter plate reader (Labsys-tems, Finland). For comparison of different M. tuberculosis samples, M. tuberculosis ELISA plates were processed simultaneously. ELISA measurements were done in triplicate for each sample and averaged. Experiments were performed twice (including culture of bacteria) with similar results. The effects of any residual Tween 80 on AM detection were further investigated by washing 100-μl 7H9-T M. tuberculosis samples (sedimented as described above) 1, 3, or 9 times with 1 ml of TBS before M. tuberculosis ELISA. Day-20 M. tuberculosis Erdman, day-25 M. tuberculosis Erdman, and day-25 CDC-1551 M. tuberculosis grown in 7H9-T were used for this analysis.
For the AM ELISA, a 50-μl solution of 10 μg of purified AM/ml in carbonate buffer (0.015 M Na2CO3, 0.035 M NaHCO3, and 0.003 M NaN3, pH 9.8) was added to each well of a microtiter plate and incubated overnight at 4°C. The wells were then blocked by adding 200 μl of 3% BSA in TBS and incubating overnight at 4°C. MAb 9d8 [IgG3 or 9d8(2a)] or sera from M. tuberculosis-infected mice were added to the wells and incubated for 1 h at 37°C. For determining murine IgG titer to AM, sera were diluted (1:20) in TBS with 0.075 M β-mercaptoethanol and heated for 1 h at 37°C to dissociate IgM pentamers. The treated sera were then immediately assayed by ELISA. Plates were then washed, and 50 μl of 1 μg of GAM-AP antibody [GAM-AP IgG2a antibody recognizing MAb 9d8(2a) and GAM-AP IgG or IgM antibody for murine sera]/ml was diluted in TBS and added to each well for 1 h at 37°C. The ELISA plates were washed and developed as described above. For comparison of MAb 9d8(2a) and 9d8 (IgG3) binding to M. tuberculosis and AM, GAM-AP IgG (heavy plus light chains) was used as the secondary antibody. A LAM ELISA was performed as previously described (14), using Erdman manLAM and H37Rv manLAM supplied by J. T. Belisle, Colorado State University, through NIH contract NO1-A1-75320. Either 100 μg of MAb 9d8(2a)/ml or 10 μg of MAb CS-40/ml was serially diluted (threefold dilutions) in the LAM ELISA. Three separate measurements were made for each MAb in each ELISA. The most dilute concentration of MAb with absorbance 1.5 times the background absorbance at 405 nm was reported as the minimum concentration of MAb required to detect the antigen.
AM capture ELISA.
Capture ELISA was used to detect and measure the amount of AM antigen in culture supernatant or murine organ homogenate. These assays were modeled on capture assays developed for the detection of Cryptococcus neoformans glucuronoxylomannan (1) and M. tuberculosis LAM (15). ELISA plates were incubated with 50 μl of TBS containing 2 μg of unlabeled GAM IgG2a/ml antibody for 1 h at 37°C. Wells were blocked by adding 200 μl of 1% BSA in TBS and incubating their contents overnight at 4°C. Plates were then washed between steps with TBS-containing 0.05% Tween 20 as described above. After washing 1 μg of MAb 9d8(2a)/ml in TBS was added to each well of the 96-well plates and incubated for 1 h at 37°C. Aliquots of M. tuberculosis culture supernatant or diluted organ homogenates were added to the plates after washing. Purified AM, which added to 7H9 medium, 7H9-T medium, or PBS and was serially diluted in TBS, was used as a standard solution to determine the amount of detectable AM antigen in culture supernatants or organ samples. Following incubation at 37°C for 1 h, 10 μg of MAb 5c11 (IgM)/ml in TBS was added and the plate contents were incubated for 1 h at 37°C. After washing, 1 μg of GAM-AP IgM antibody/ml in TBS was added and the assay was developed as described above. The sensitivity of the capture ELISA was determined using threefold dilutions of antigen, and the dilution with an absorbance 1.5 times the background absorbance at 405 nm was reported as the minimum concentration of antigen required for detection.
Murine infection, organ/serum sample preparation, and CFU determination.
Six- to 8-week-old female BALB/cAnNCr1BR (Charles River Laboratories, Wilmington, Mass.) mice were infected with 106 Erdman bacilli via the intravenous route. Just prior to injection, mycobacteria were thawed from a frozen vial with a known quantity of CFU per milliliter and were added to 4 ml of PBS containing 0.05% Tween 80 (PBS-T) in a 15-ml conical tube. The suspension was sonicated for 5 s at 60% output, constant duty cycle, in a cuphorn sonicator (Branson, Danbury, Conn.) before inversion of the conical tube and sonication again for 10 s. Three mice were harvested at 0 (prior to infection), 7, 20, and 42 days after infection. Just prior to harvest of murine organs, mice were bled from the retroorbital plexus for serum collection. Serum was diluted in TBS containing 1 mM azide, filtered twice using a 0.2-μm-pore-size syringe filter, and removed from the BSL-3 facility for storage at 4°C until analysis with the AM ELISA. A small portion (approximately 10%) of the liver, spleen, and lung (the right upper lobe) was removed and immediately fixed in 10% formalin. The remaining organ portions were processed using a Seward Stomacher 80 Lab System (Seward, London, United Kingdom) in PBS-T for several minutes until homogenized. The homogenate was then serially diluted in PBS-T using 10-fold dilutions and was plated for CFU on Middlebrook 7H10 agar (Difco) containing 50 mg of cycloheximide (Sigma)/liter, and colonies were counted after 3 weeks of growth at 37°C. Portions of the organ homogenates (3 of 5 ml, starting volume for spleen and lung; 3 of 10 ml, starting volume for the liver) were treated with proteinase K (PK) to liberate carbohydrate antigens.
PK treatment was as follows: 60 μl of 100-mg/ml PK (Boehringer Mannheim, Indianapolis, Ind.) in PBS was added to the 3-ml organ homogenate (a final PK concentration of 2 mg/ml) before incubation at 37°C for 6 h. These samples were boiled for 15 min at 100°C (to inactivate the PK) before cooling to 80°C for 2 h (to ensure killing of M. tuberculosis) and were frozen at −20°C for storage. After collection, samples were thawed and centrifuged for 5 min at 13,000 × g. The supernatant was removed and vacuum concentrated 10-fold using a microcentrifuge vacuum concentrator (Savant, Farmingdale, N.Y.). Concentrated supernatants were then immediately tested in the AM capture ELISA. To assess the effect of PK digestion of whole-cell mycobacteria on AM detection in the capture ELISA, 100 μl of day-25 M. tuberculosis Erdman grown in the absence of Tween (sedimented as described above) was diluted 1:100 or 1:10 in 1 ml of TBS. These diluted samples were vigorously suspended by persistent pipetting, and either 2, 0.2, or 0.02% of the original 100-μl bacterial sample was added to 1-ml organ homogenates (lung, liver or spleen) prior to PK treatment and vacuum concentration. For comparison to untreated bacteria, equivalent amounts of diluted M. tuberculosis were added to PK-treated and vacuum-concentrated organ homogenates just before addition to the capture ELISA.
Immunohistochemistry.
Immunohistochemistry was performed as described elsewhere (16), except that the slides were steamed with citrate buffer (.082 M Na citrate and .0018 M citric acid) for 40 min and were cooled to room temperature for 20 min prior to blocking with TBS containing 1% BSA and 5% goat serum. MAb 9d8 (IgG3) supernatant was used as the primary antibody at 10 μg/ml. GAM IgG3 antibody conjugated to horseradish peroxidase (Southern Biotechnology) was used as the secondary antibody. As a control the reaction was done using both infected- and uninfected-tissue slides without primary antibody.
RESULTS
Generation and characterization of an IgG2a MAb to AM.
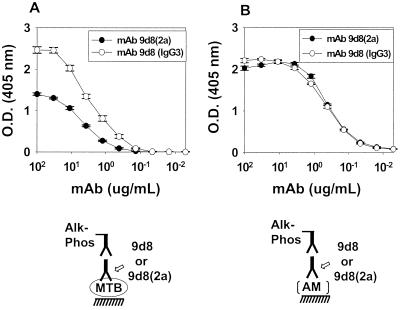
Although MAb 9d8 (IgG3) to AM was available (14), it was necessary to generate a better reagent because of difficulties in its production and purification. These difficulties are probably due to the tendency of this IgG3 MAb to self aggregate (34). An IgG2a switch variant of MAb 9d8 [9d8(2a)] was recovered after six rounds of enrichment by the sib selection technique. MAb 9d8(2a) bound to both M. tuberculosis and AM in a manner similar to that of the parent IgG3 MAb (Fig. 1). MAb 9d8 (IgG3) produced slightly higher absorbance than MAb 9d8(2a) in the M. tuberculosis ELISA, which we attribute to the higher apparent avidity of IgG3 MAbs due to their ability to polymerize after binding antigen (5). MAb 9d8(2a) (100 μg/ml) did not bind to purified Erdman manLAM or H37Rv manLAM (data not shown). This finding confirms that MAb 9d8(2a), like the parent MAb 9d8, is not able to bind purified LAM in ELISA.
FIG. 1.
ELISA of MAbs 9d8(2a) and 9d8 binding to M. tuberculosis and AM. MAb 9d8(2a) or 9d8 was serially diluted in an ELISA using M. tuberculosis Erdman (A) or purified AM (B). Each value represents the average of three measurements. Error bars show the standard deviations of the means. These growth experiments and the ELISA were repeated twice with similar results. O.D., optical density; MTB, M. tuberculosis; Alk-Phos, alkaline phosphatase.
Detection of AM antigen on bacteria during in vitro growth of M. tuberculosis Erdman.
M. tuberculosis cultures were grown in media with (7H9-T) or without (7H9) Tween 80 to compare bacteria grown as either a diffuse suspension or as a pellicle, respectively. By normalizing to equal volumes of settled bacteria, we were able to compare the amount of protein in cells of the Erdman and CDC 1551 strains at 11, 20, and 25 days of culture growth. The amount of protein in the samples was used to standardize the amount of M. tuberculosis (22) for comparative ELISA.
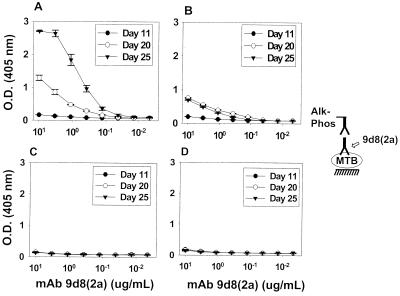
MAbs 9d8(2a) to AM and CS-40 to Erdman manLAM were used to study the presence of their respective antigens on whole-cell M. tuberculosis (Fig. 2 and 3). MAbs 9d8(2a) and CS-40 each bound to bacteria from both strains, but the reactivity of MAb 9d8(2a) with M. tuberculosis varied significantly with the age of the culture and the inclusion of Tween 80 in media. Furthermore, there was a significant difference in the reactivity of the MAb 9d8(2a) with the two strains of M. tuberculosis.
FIG. 2.
Binding of MAb 9d8(2a) to whole-cell M. tuberculosis Erdman and CDC 1551 at various culture times. M. tuberculosis Erdman (A and B) and CDC 1551 (C and D) were grown in the absence (A and C) or the presence (B and D) of Tween for periods of 11, 20, or 25 days before harvest. Each value represents the average of three measurements. Error bars show the standard deviations of the means. These growth experiments and the ELISA were repeated twice with similar results. O.D., optical density; MTB, M. tuberculosis; Alk-Phos; alkaline phosphatase.
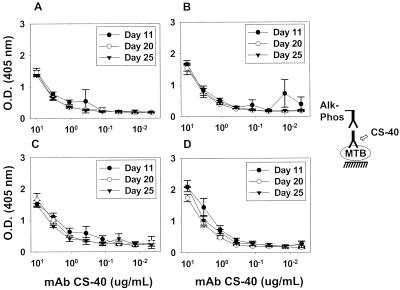
FIG. 3.
Binding of MAb CS-40 to whole-cell M. tuberculosis Erdman and CDC 1551 at various culture times. M. tuberculosis Erdman (A and B) and CDC 1551 (C and D) were grown in the absence (A and C) or the presence (B and D) of Tween for periods of 11, 20, or 25 days before harvest. Each value represents the average of three measurements. Error bars show the standard deviations of the means. These growth experiments and the ELISA were repeated twice with similar results. O.D., optical density; MTB, M. tuberculosis; Alk-Phos; alkaline phosphatase.
MAb 9d8(2a) demonstrated stronger reactivity with M. tuberculosis Erdman grown for longer times (days 20 and 25) in media with or without Tween 80 (Fig. 2A and B). The strongest binding was observed at day 25 for Erdman grown in the absence of Tween 80 (Fig. 2A). CDC 1551 M. tuberculosis bound MAb 9d8(2a) with relatively the same weak intensity regardless of the time of growth or the presence of Tween 80 (Fig. 2C and D). The CS-40 antibody, directed to manLAM (29), bound to either strain of M. tuberculosis in relatively the same manner, regardless of whether the strain had been grown in 7H9 or 7H9-T or of the age of the culture (Fig. 3). Extensive washing of M. tuberculosis Erdman or CDC 1551 grown in Tween 80 did not increase binding by MAb 9d8(2a) (data not shown). This suggests that weak binding to M. tuberculosis grown in Tween 80 is not the result of masking of AM by detergent.
MAb CS-40 bound to purified M. tuberculosis AM, M. tuberculosis Erdman manLAM, and M. tuberculosis H37Rv manLAM (10 μg/ml each) with similar apparent affinity (minimum dilution of MAb CS-40 required for detection was 3.3, 1.1, and 3.3 μg/ml, respectively). These results indicate that MAb CS-40 binds both M. tuberculosis AM and LAM (data not shown).
Development of capture ELISA for AM using MAbs 9d8(2a) and 5c11.
The availability of two MAbs of different isotypes that bound AM was exploited to design a sensitive capture ELISA for detection of AM. The ELISA uses the IgG2a MAb 9d8(2a) for capture of AM and the IgM MAb 5c11 for detection of immobilized AM. A diagram of the ELISA configuration is shown in Fig. 4. The lowest concentration of purified AM detected by this ELISA was 0.1 μg/ml. Since this assay was to be used for measurement of AM antigen in organ homogenates, we compared AM detection in organ homogenates treated with PK before and after addition of M. tuberculosis PK treatment allowed for approximately twice (2.6 ± 1.4-fold) (P = 0.03) as much antigen to be captured in this assay (data not shown). This result indicates that PK treatment could liberate slightly more detectable antigen in the capture ELISA assay than would otherwise be detectable.
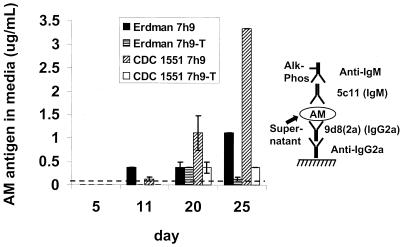
FIG. 4.
Capture ELISA of AM in M. tuberculosis culture medium. M. tuberculosis Erdman and CDC 1551 were grown in the absence or presence of Tween 80 for periods of 5, 11, 15, 20, or 25 days before harvest of bacterial supernatant. Each value represents the average of two separate measurements. Error bars represent the standard deviation of the means. These growth experiments and the ELISA were repeated twice with similar results. The detection limit of the assay is indicated by the dotted line. Alk-Phos, alkaline phosphatase.
M. tuberculosis culture supernatants contain AM antigen.
M. tuberculosis Erdman and CDC 1551 culture supernatants were tested for the presence of AM antigen using the AM capture ELISA (Fig. 4). Filtered supernatants from M. tuberculosis cultures grown in 7H9 or 7H9-T media were used. Overall, more antigen was detected in media from cultures of either Erdman or CDC 1551 M. tuberculosis grown in the absence of Tween 80 than in media from cultures grown in the presence of Tween 80 (Fig. 4). The CDC 1551 strain accumulated the largest amount of detectable antigen in the medium at day 25, while M. tuberculosis Erdman accumulated a lesser amount. Although some AM antigen accumulated in the media of cultures grown in the presence of Tween 80 from day 5 to day 20, no increase in AM antigen was detected in these cultures from day 20 to day 25.
MAb 9d8(2a) AM antigen is detected in the organs of M. tuberculosis-infected mice.
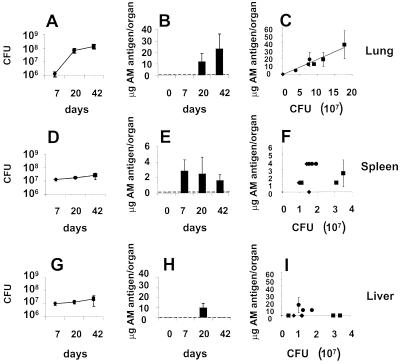
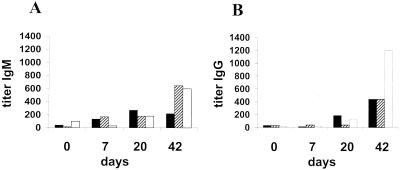
BALB/c mice were infected intravenously with 106 Erdman M. tuberculosis organisms, a dose that caused 50% mortality by day 57 (data not shown). Mice were sacrificed at 0, 7, 20, and 42 days for organ CFU determination (Fig. 5A, D, and G) and AM antigen capture ELISA (Fig. 5B, E, and H). As indicated in Fig. 5B, E, and H, the AM antigen was not detected in organs of uninfected mice (day 0) and became detectable at different times after infection in all organs. In the lung, the AM antigen was detected at days 20 and 42 but not at day 7 (Fig. 5B). The increase in AM antigen concentration in the lung paralleled the progressive increase in the number of CFU, and regression analysis demonstrates a linear relationship between the number of CFU and AM antigen concentration (Fig. 5C). In the spleen, the AM antigen was detected at day 7, with comparable amounts of antigen detected throughout days 20 and 42 (Fig. 5E). However, the amount of AM antigen per CFU in the spleen decreased when days 7 and 42 were compared (P = 0.03). Overall, the amount of AM antigen per CFU in the spleen (1.41 × 10−7 ± 9.4 × 10−8 μg/CFU) was similar to that in the lung (1.71 × 10−7 ± 4.8 × 10−8 μg/CFU) whenever the AM antigen was detected in these organs. In the liver, the number of CFU was not significantly different at 7, 20, or 42 days (Fig. 5G), although the AM antigen was detected on day 20 (8.36 × 10−7 ± 5.8 × 10−7 μg/CFU) but not at 7 or 42 days (Fig. 5H). These results suggest that the concentrations of AM antigen in the spleen (Fig. 5F) and the liver (Fig. 5I) do not rise in direct relationship to the CFU count. Both IgM and IgG to AM were detected in the sera of the mice. Higher titers were found at later times, thus indicating that the infection induced an immunological response to AM (Fig. 6).
FIG. 5.
Capture ELISA of AM in organ homogenate compared to organ CFU. The number of CFU in the lungs (A), spleens (D), or livers (G) of mice infected with M. tuberculosis Erdman was determined 7, 20, and 42 days after infection. Each value represents the average of three animals. Error bars show the standard deviation of the mean. Organ homogenates were assayed for micrograms of MAb 9d8(2a) AM antigen/organ in the lung (B), spleen (E), or liver (H) 0, 7, 20, and 42 days after infection. The detection limit of the assay is indicated by the dotted line. Each value represents the average of the three animals, using two measurements for each animal. Error bars represent the standard deviation of all six measurements. The CFU data for each animal organ were also related to the number of micrograms of MAb 9d8(2a) AM antigen in that animal organ (C, F, and I) for all day-7 (⧫), day-20 (●), and day-42 (■) mice. Each value represents the average of the two measurements of MAb 9d8(2a) AM antigen concentration per animal organ, and error bars represent the standard deviation of those two measurements. The point (⧫) at the bottom left of Panel C represents three mice that have the same apparent graphic value.
FIG. 6.
Antibody titer to AM during M. tuberculosis infection. Murine sera were assayed for IgM (A) and IgG (B) antibody titer to AM 0, 7, 20, and 42 days after infection of mice with M. tuberculosis Erdman. The three animals assayed for antibody titer at each period were the same animals used to generate the data for Fig. 5.
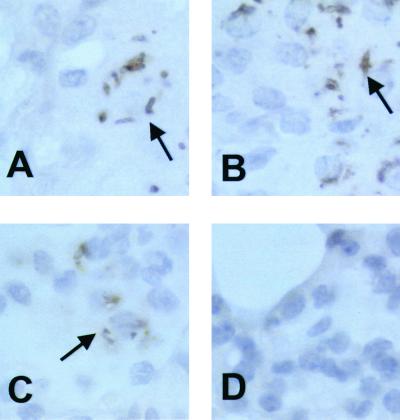
Immunohistochemistry reveals mycobacterial shapes and indicates antigen diffusion.
Immunohistochemistry of infected murine lung demonstrated that MAb 9d8 localized to mycobacterial structures, which were associated with cells. Some cells showed staining that was associated exclusively (Fig. 7A and B) with mycobacterial structures, while others demonstrated a more diffuse (Fig. 7C) cellular staining. Controls did not show specific staining (Fig. 7D).
FIG. 7.
MAb 9d8 immunohistochemistry. Infected (day 42) (A to C) and uninfected (day 0) (D) murine lung samples were stained for the AM antigen using MAb 9d8 and horseradish peroxidase-conjugated secondary antibody. Arrows indicate bacterial shapes (A and B) or more diffuse staining (C).
DISCUSSION
Earlier studies from our group demonstrated that a MAb to AM prolonged the survival of mice infected with a lethal dose of M. tuberculosis (34). These findings suggested that the AM antigen recognized by MAb 9d8 has biological importance. In this paper we demonstrate that M. tuberculosis Erdman expresses the 9d8 AM antigen in a dynamic manner in vitro and that the 9d8 AM antigen can be detected in vivo as a result of infection. AM and GC are the predominant polysaccharides isolated from the surface of M. tuberculosis H37Rv (ATCC 27294) bacteria grown as a pellicle for 7 days or more in Sauton's medium (26). These carbohydrates also appear to be on the surface of M. tuberculosis grown in 7H9 medium (J. R. Schwebach, A. Glatman-Freedman, J. B. Robbins, R. Schneerson, Z. Dai, W. R. Jacobs, and A. Casadevall, 100th Gen. Meet. Am. Soc. Microbiol., poster U-20, 2000). The amount of total M. tuberculosis carbohydrate reaches a maximum during early growth (35) and is greater for H37Rv than for H37Ra (35). After extended growth (>9 weeks), the amount of carbohydrate in the culture filtrate but not on or in the bacterium continues to increase without a concomitant increase in the dry weight of the bacterial pellet (35). In contrast, for BCG the amount of carbohydrate in the medium plateaus early in the stationary phase and remains constant during extended culture growth in Sauton's medium (35). These results imply that M. tuberculosis sheds polysaccharides when grown in liquid culture but do not indicate if the production of AM undergoes any specific changes during growth. Our goal was to study the expression of the AM antigen recognized by MAb 9d8 in a variety of in vitro as well as in vivo settings. To accomplish this, we generated an isotype switch variant of MAb 9d8 that specifically recognizes AM (34) and is more readily available than the parent antibody. Neither the parent 9d8 MAb (14) nor the 9d8(2a) MAb binds purified M. tuberculosis Erdman manLAM in ELISA. Using MAb 9d8(2a), we assessed the presence of AM antigen on M. tuberculosis Erdman and CDC 1551 cells and measured its concentration in culture supernatants. We also measured the concentration of this antigen in organs of mice infected with M. tuberculosis Erdman.
The reactivity of MAb 9d8(2a) with whole-cell M. tuberculosis increased as M. tuberculosis Erdman cells were grown in vitro for extended periods of time, even when the culture media contained Tween 80 (Fig. 2A and B). These results can be compared to the results obtained using MAb CS-40 (Fig. 3). The reactivity of MAb CS-40 with all M. tuberculosis samples was nearly the same, regardless of the period of growth or the addition of Tween 80 (Fig. 3). It has been previously reported that MAb CS-40 binds to manLAM and not to LAM devoid of mannose caps, or arabinose LAM (araLAM) (29). Our own observations indicate that MAb CS-40 is able to bind araLAM, although with less apparent affinity than to manLAM (unpublished observations). Although not central to this paper's work, this difference between the two studies may be due to differences in performing methods of ELISA (multiple dilutions of MAb were used in our study versus 1 dilution in the previous study). MAb CS-40 is also reactive with the carbohydrate portion of LAM alone (17) and bound M. tuberculosisAM. As AM structures are found alone or attached to LAM (2), it is probable that MAb CS-40 binds to carbohydrate epitopes within both LAM and AM in whole-cell M. tuberculosis ELISA. Regardless of the nature of the antigen recognized by MAb CS-40, the reactivity of MAb CS-40 with whole-cell M. tuberculosis did not change during culture of M. tuberculosis in contrast to that of MAb 9d8(2a). Because of the increased reactivity of MAb 9d8 with older M. tuberculosis Erdman cells, one of two possibilities is implied: either more AM is associated with the M. tuberculosis cell surface as the cultures age, or the structure of AM changes during growth. Since the AM antigen becomes more detectable on Erdman cells after extended growth, it is also conceivable that these epitopes are initially undetectable but later become detectable as a result of degradation or release of other surface components that prevent antibody recognition. Our methods cannot distinguish between these possibilities. However, given the increasing amount of AM antigen in the culture supernatant (Fig. 4) and an increasing amount of bacterial carbohydrate in M. tuberculosis culture supernatant (35), a likelier scenario is that the expression of AM antigens on the Erdman cell surface increases after extended culture.
Tween 80 diminished the reactivity of MAb 9d8(2a) with M. tuberculosis whole cells (Fig. 2). As this detergent has been used to partition the surface of M. tuberculosis for biochemical study (27), some Tween 80 effects on MAb 9d8(2a) binding were expected. M. tuberculosis strain differences were also noted, as the reactivity of MAb 9d8(2a) with M. tuberculosis Erdman whole cells was much greater than with CDC 1551 (Fig. 2). The ratio of arabinose to mannose in the AM extracted from the mycobacterial surface is known to vary among different strains of M. tuberculosis (25), and our results are consistent with AM variation between strains.
Our results indicate that the amount of the MAb 9d8(2a) AM antigen increases in the culture supernatant of cultures grown for longer periods (Fig. 4). Previous research has demonstrated that AM and GC are found in the culture medium (reviewed in reference 7), but the amount of AM-containing antigens during different growth stages had not been analyzed. Even though the amount of detectable MAb 9d8(2a) AM antigen on M. tuberculosis Erdman was significantly different from that on CDC 1551 (Fig. 2), the amount of detectable AM antigen in the media increased for both strains with the age of culture. Notably, the amount of AM antigen detected in the CDC 1551 culture media was greater than in the Erdman media. This raises the possibility that the CDC 1551 strain is shedding this antigen from the surface of the bacteria, while for the Erdman strain, more of the antigen remains associated with the bacillus during culture. M. tuberculosis cultures containing Tween 80 had a lower concentration of AM antigen than did those cultures grown in the absence of Tween 80. There are several possible explanations for this observation: Tween 80 may remove AM from the bacterial surface (27) during culture. It is also possible that Tween 80 affects the amount of AM produced by M. tuberculosis. (Tween 80 has been shown to increase the amount of fatty acids approximately twofold in Mycobacterium avium cells, indicating that culture in Tween 80 can affect the composition of the bacilli (32). In addition, M. tuberculosis grown in the presence of Tween 80 could have AM epitopes masked by the detergent. This latter possibility is unlikely, as extensive washing of the bacilli did not restore binding of MAb 9d8(2a) to bacteria in ELISA. A final possible explanation for the lower concentration of AM antigen, although we believe this possibility to be unlikely, is that AM cultured in the presence of Tween 80 for an extended amount of time undergoes structural change.
We were able to detect the MAb 9d8(2a) AM antigen in organ homogenates after bacterial burden had increased (Fig. 5). This result indicates that the AM antigen is produced in vivo by M. tuberculosis and is not solely a product of in vitro culture conditions. This observation is concomitant with an increased IgM and IgG antibody titer to M. tuberculosis AM (Fig. 6). Presumably, and as indicated by an increased amount of AM antigen in the lung and spleen, the bacteria continue to produce AM during their growth in vivo, a process that stimulates a humoral response. Production of antibody reactive with purified AM as a consequence of experimental infection had not been previously demonstrated. Nevertheless, it has been shown that sera from tuberculosis patients are reactive with LAM (24, 30; Arya, letter, 1993) and with Seibert polysaccharide fractions from M. tuberculosis (4). Such sera could therefore be reactive with AM, and our work supports this assumption, as sera from M. tuberculosis-infected mice bind purified AM.
The differences in the amount of detectable AM antigen in the various organs, as a function of time, warrant further discussion. The ratio of AM antigen to CFU in the lung remained similar at times when AM was detectable (days 20 and 42) (Fig. 5C), suggesting a direct correlation between the number of CFU and the ability to detect AM. However, in the spleen, such a correlation was not found. In the liver, the AM antigen became detectable by capture ELISA at day 20 and then disappeared by day 42 (Fig. 5H). No statistically significant difference between CFU counts in the liver was found for days 7, 20, and 42. Therefore, it is surprising that the AM antigen was not detected at days 7 and 42. Hence, these data indicate that the AM antigen was not always expressed as a function of the CFU count in the liver. These differences in the amount of AM antigen detected suggest in vivo changes in the expression of M. tuberculosis AM. One possible explanation for the lack of detection of the AM antigen in the liver at days 7 and 42 is that antibody is able to clear some of the AM antigen not associated with the bacteria, as was the case for administered LAM (15). In this regard, specific antibody effectively removes serum polysaccharide antigen and promotes deposition in the liver and spleen (21).
Immunohistochemistry of lung tissue reveals staining of both bacterium like structures as well as a more diffuse pattern of staining (Fig. 7). This suggests that the AM antigen is found on the surface of in vivo-grown M. tuberculosis and diffuses within infected cells. The diffuse pattern of staining is in accord with our in vitro findings that suggest shedding of the AM antigen from M. tuberculosis. It is possible that the diffused AM has a biological effect. In this respect, it has been shown that M. tuberculosis AM administered to human lymphocytes is immunosuppressive (11).
It has been demonstrated that MAb 9d8 binds strongly to AM (34) and that MAb 9d8 does not react with purified Erdman manLAM or other purified Erdman cell wall components in ELISA (14). It should be recognized that by immunoelectron microscopy, the MAb 9d8 antigen was found on the surface of M. tuberculosis Erdman cells, within an outer layer that is consistent with a capsule (14). This outer layer is composed almost entirely of carbohydrate (reviewed in references 6 and 26). Our observations are therefore consistent with the existence of a polysaccharide capsule containing AM, as proposed by Daffe and Draper (reviewed in reference 6).
In summary, we have demonstrated that AM recognized by MAb 9d8 is expressed in vitro and in vivo. Our data further suggest that AM expression is dynamic, M. tuberculosis strain specific, and regulated by the growth conditions. Changes in the surface carbohydrate of M. tuberculosis are potentially relevant with regard to studies involving M. tuberculosis interactions with cells as well as murine infection studies. Preparation of M. tuberculosis for these studies must take into consideration strain differences and culture differences that could affect the M. tuberculosis surface. M. tuberculosis surface carbohydrates have been shown to be important for the adherence of the bacteria to a complement receptor and macrophages (33). M. tuberculosis surface carbohydrate changes may influence adherence to macrophage receptors and phagocytosis of the bacteria, affecting the outcome of the infection (10). Biochemical studies on the structure of M. tuberculosis polysaccharide fractions have undoubtedly been influenced by the growth conditions used to prepare the bacteria. Our results strongly suggest that careful consideration must be given to culture conditions, since these can affect surface mycobacterial polysaccharides that may be critical for interaction with host cells, and also suggest that M. tuberculosis grown in vivo has the potential to undergo changes in the expression of AM.
ACKNOWLEDGMENTS
A.G-F. is supported by NIH grant 1K08A101691, a Department of Pediatrics Research Grant, and a grant from the Sequella Global Tuberculosis Foundation. A.C. is supported by NIH grants AI33142, AI33774, and HL59842. A.C. is also supported by a Burroughs-Wellcome Fund Scholar Award in Experimental Therapeutics. J.R.S. was supported by an NIH Training Grant in HIV, AIDS, and Opportunistic Infections (5T32A107501).
We thank John T. Belisle for supplying LAM and MAb CS-40 as part of NIH contract N01-AI-75320, “Tuberculosis Research Materials and Vaccine Testing.” We further thank the hybridoma facility at the Albert Einstein College of Medicine for technical advice, support, and material supplied by NIH grant CA13330. We are indebted to B. Chen and M. Chen for technical assistance in the Albert Einstein College of Medicine Howard Hughes BSL-3 facility and to W. R. Jacobs for the use of this facility. Thanks to R. G. Russell for advice regarding histological technique.
REFERENCES
- 1.Casadevall A, Mukherjee J, Scharff M D. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J Immunol Methods. 1992;154:27–35. doi: 10.1016/0022-1759(92)90209-c. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee D, Khoo K H. Mycobacterial lipoarabinomannan: an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology. 1998;8:113–120. doi: 10.1093/glycob/8.2.113. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee D, Lowell K, Rivoire B, McNeil M R, Brennan P J. Lipoarabinomannan of Mycobacterium tuberculosis. Capping with mannosyl residues in some strains. J Biol Chem. 1992;267:6234–6239. [PubMed] [Google Scholar]
- 4.Coates S R, Hansen D, Schecter G, Slutkin G, Hopewell P, Affronti L, Echenberg D F. Identification of Mycobacterium tuberculosis antigens in Seibert fractions by immunoblotting. J Clin Microbiol. 1986;24:126–130. doi: 10.1128/jcm.24.1.126-130.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper L J, Shikhman A R, Glass D D, Kangisser D, Cunningham M W, Greenspan N S. Role of heavy chain constant domains in antibody-antigen interaction. Apparent specificity differences among streptococcal IgG antibodies expressing identical variable domains. J Immunol. 1993;150:2231–2242. [PubMed] [Google Scholar]
- 6.Daffe M, Draper P. The envelope layers of mycobacteria with reference to their pathogenicity. Adv Microb Physiol. 1998;39:131–202. doi: 10.1016/s0065-2911(08)60016-8. [DOI] [PubMed] [Google Scholar]
- 7.Daniel T M. Soluble mycobacterial antigens. In: Kubica G P, Wayne L G, editors. The Mycobacteria, a sourcebook, part A. New York, N.Y: Marcel Dekker, Inc; 1984. pp. 417–465. [Google Scholar]
- 8.Draper P, Rees R J. Electron-transparent zone of mycobacteria may be a defense mechanism. Nature. 1970;228:860–861. doi: 10.1038/228860a0. [DOI] [PubMed] [Google Scholar]
- 9.Dye C, Scheele S, Dolin P, Pathania V, Raviglione R C. Consenus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 10.Ehlers M R, Daffe M. Interactions between Mycobacterium tuberculosis and host cells: are mycobacterial sugars the key? Trends Microbiol. 1998;6:328–335. doi: 10.1016/s0966-842x(98)01301-8. [DOI] [PubMed] [Google Scholar]
- 11.Ellner J J, Daniel T M. Immunosuppression by mycobacterial arabinomannan. Clin Exp Immunol. 1979;35:250–257. [PMC free article] [PubMed] [Google Scholar]
- 12.Gaylord H, Brennan P J, Young D B, Buchanan T M. Most Mycobacterium leprae carbohydrate-reactive monoclonal antibodies are directed to lipoarabinomannan. Infect Immun. 1987;55:2860–2863. doi: 10.1128/iai.55.11.2860-2863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilleron M, Bala L, Brando T, Vercellone A, Puzo G. Mycobacterium tuberculosis H37Rv parietal and cellular lipoarabinomannans. Characterization of the acyl- and glyco-forms. J Biol Chem. 2000;275:677–684. doi: 10.1074/jbc.275.1.677. [DOI] [PubMed] [Google Scholar]
- 14.Glatman-Freedman A, Martin J M, Riska P F, Bloom B R, Casadevall A. Monoclonal antibodies to surface antigens of Mycobacterium tuberculosis and their use in a modified enzyme-linked immunosorbent spot assay for detection of mycobacteria. J Clin Microbiol. 1996;34:2795–2802. doi: 10.1128/jcm.34.11.2795-2802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glatman-Freedman A, Mednick A J, Lendvai N, Casadevall A. Clearance and organ distribution of Mycobacterium tuberculosis lipoarabinomannan (LAM) in the presence and absence of LAM-binding immunoglobulin M. Infect Immun. 2000;68:335–341. doi: 10.1128/iai.68.1.335-341.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman D, Lee S C, Casadevall A. Pathogenesis of pulmonary Cryptococcus neoformans infection in the rat. Infect Immun. 1994;62:4755–4761. doi: 10.1128/iai.62.11.4755-4761.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamasur B, Kallenius G, Svenson S B. Synthesis and immunologic characterisation of Mycobacterium tuberculosis lipoarabinomannan specific oligosaccharide-protein conjugates. Vaccine. 1999;17:2853–2861. doi: 10.1016/s0264-410x(99)00124-3. [DOI] [PubMed] [Google Scholar]
- 18.Havlir D V, Barnes P F. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1999;340:367–373. doi: 10.1056/NEJM199902043400507. [DOI] [PubMed] [Google Scholar]
- 19.Imaeda T, Kanetsuna F, Galindo B. Ultrastructure of cell walls of genus mycobacterium. J Ultrastruct Res. 1968;25:46–63. doi: 10.1016/s0022-5320(68)80059-0. [DOI] [PubMed] [Google Scholar]
- 20.Lemassu A, Daffe M. Structural features of the exocellular polysaccharides of Mycobacterium tuberculosis. Biochem J. 1994;297:351–357. doi: 10.1042/bj2970351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lendvai N, Casadevall A, Liang Z, Goldman D L, Mukherjee J, Zuckier L. Effect of immune mechanisms on the pharmacokinetics and organ distribution of cryptococcal polysaccharide. J Infect Dis. 1998;177:1647–1659. doi: 10.1086/515329. [DOI] [PubMed] [Google Scholar]
- 22.Meyers P R, Bourn W R, Steyn L M, van Helden P, Beyers A D, Brown G D. Novel method for rapid measurement of growth of mycobacteria in detergent-free media. J Clin Microbiol. 1998;36:2752–2754. doi: 10.1128/jcm.36.9.2752-2754.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misaki A, Azuma I, Yamamura Y. Structural and immunochemical studies on d-arabino-d-mannans and d-mannans of Mycobacterium tuberculosis and other mycobacterium species. J Biochem (Tokyo) 1977;82:1759–1770. doi: 10.1093/oxfordjournals.jbchem.a131874. [DOI] [PubMed] [Google Scholar]
- 24.Near K A, Lefford M J. Use of serum antibody and lysozyme levels for diagnosis of leprosy and tuberculosis. J Clin Microbiol. 1992;30:1105–1110. doi: 10.1128/jcm.30.5.1105-1110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortalo-Magne A, Andersen A B, Daffe M. The outermost capsular arabinomannans and other mannoconjugates of virulent and avirulent tubercle bacilli. Microbiology. 1996;142:927–935. doi: 10.1099/00221287-142-4-927. [DOI] [PubMed] [Google Scholar]
- 26.Ortalo-Magne A, Dupont M-A, Lemassu A, Andersen A B, Gounon P, Daffe M. Molecular composition of the outermost capsular material of the tubercle bacillus. Microbiology. 1995;141:1609–1620. doi: 10.1099/13500872-141-7-1609. [DOI] [PubMed] [Google Scholar]
- 27.Ortalo-Magne A, Lemassu A, Laneelle M A, Bardou F, Silve G, Gounon P, Marchal G, Daffe M. Identification of the surface-exposed lipids on the cell envelopes of Mycobacterium tuberculosis and other mycobacterial species. J Bacteriol. 1996;178:456–461. doi: 10.1128/jb.178.2.456-461.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picard B, Frehel C, Rastogi N. Cytochemical characterization of mycobacterial outer surfaces. Acta Leprol. 1984;2:227–235. [PubMed] [Google Scholar]
- 29.Schlesinger L S, Hull S R, Kaufman T M. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–4079. [PubMed] [Google Scholar]
- 30.Sousa A O, Henry S, Maroja F M, Lee F K, Brum L, Singh M, Lagrange P H, Aucouturier P. IgG subclass distribution of antibody responses to protein and polysaccharide mycobacterial antigens in leprosy and tuberculosis patients. Clin Exp Immunol. 1998;111:48–55. doi: 10.1046/j.1365-2249.1998.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spira G, Scharff M D. Identification of rare immunoglobulin switch varients using the ELISA spot assay. J Immunol Methods. 1992;148:121–129. doi: 10.1016/0022-1759(92)90165-p. [DOI] [PubMed] [Google Scholar]
- 32.Stinson M W, Solotorovsky M. Interaction of Tween 80 detergent with mycobacteria in synthetic medium. I. Effect of Tween 80 on the growth and turbidimetric response of Mycobacterium avium cultures. Am Rev Respir Dis. 1971;104:717–727. doi: 10.1164/arrd.1971.104.5.717. [DOI] [PubMed] [Google Scholar]
- 33.Stokes R W, Doxsee D. The receptor-mediated uptake, survival, replication, and drug sensitivity of Mycobacterium tuberculosis within the macrophage-like cell line THP-1: a comparison with human monocyte-derived macrophages. Cell Immunol. 1999;197:1–9. doi: 10.1006/cimm.1999.1554. [DOI] [PubMed] [Google Scholar]
- 34.Teitelbaum R, Glatman-Freedman A, Chen B, Robbins J, Unanue E, Casadevall A, Bloom B R. A monoclonal antibody recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci USA. 1998;95:15688–15693. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turcotte R, Des Ormeaux Y. Influence of the age of mycobacterial cultures on the protein and carbohydrate composition of tuberculins. Can J Microbiol. 1972;18:637–645. doi: 10.1139/m72-101. [DOI] [PubMed] [Google Scholar]