Abstract
Colibacillosis caused by avian pathogenic Escherichia coli (APEC) is a very prevalent disease in poultry farms in China. The exploration of effective non-antibiotic substances is of great significance for the control of APEC infections. This experiment evaluated the efficacy of coated essential oil and organic acid (EOA) supplementation to prevent E.coli O78 infection in broiler chickens. A total of 288 one-day-old male broiler chicks were randomly distributed into 4 groups with 6 replicates per group. Chickens were fed a diet either supplemented with EOA (500 mg/kg feed) or not, and either uninfected or infected with E.coli O78 intratracheally. Results showed that E. coli O78 infection reduced body weight gain, increased mortality and the ratio of feed to gain along with cecal and liver E. coli load, damaged gut mucosa, induced local and systemic inflammation, and altered cecal microbial composition, diversity and function (P < 0.05). Supplemental EOA improved feed conversion efficiency, lowered gross lesion scores and cecal E. coli population, enhanced intestinal goblet cells and serum IgG concentration, and tended to decrease serum IL-12 production (P < 0.05). Essential oil and organic acid addition downregulated IFN-γ mRNA, tended to decrease mucin-2 mRNA levels while upregulating IL-10 mRNA, and tended to increase ZO-1 gene expression in the jejuna of infected birds at 7 d after E. coli O78 challenge (P < 0.05). The 16S rRNA gene sequencing indicated that both EOA addition and E. coli O78 challenge altered the diversity and composition of the cecal microbiota community. Furthermore, infected birds fed EOA showed decreased Bacteroidetes and genus Lactobacillus abundance compared with the infected control. LEfSe analysis showed that Firmicutes, Ruminococcaceae, Clostridiales, Clostridia, Lactobacillus, Lactobacilaceae, and cc-115 were enriched in the non-infected but EOA-treated group (P < 0.05). Collectively, dietary EOA supplementation could mildly alleviate E. coli-induced gut injury and inflammation.
Keywords: Encapsulated essential oil and organic acid mixture, Avian pathogenic Escherichia coli, Health, Broiler chicken
1. Introduction
Avian colibacillosis caused by avian pathogenic Escherichia coli (APEC) is the most prevalent and detrimental bacterial diseases in the poultry industry (Dho-moulin et al., 1999; Gibbs et al., 2004). In clinical cases of avian colibacillosis, the most frequently detected serotypes of APEC are O1:K1, O2:K1, O5, O8, O15, O35, O53, O55, O150 and O78:K80 (McPeake et al., 2005; Cordoni et al., 2016). APEC infection usually causes colibacillosis, a disease that can be localized or systemic, with various clinical symptoms ranging from respiratory tract infection stress, loose white or watery droppings to swollen head syndrome (Dho-moulin et al., 1999). Furthermore, necropsy has revealed that chickens with colibacillosis often exhibit typical lesions including perihepatitis, airsacculitis, pericarditis, egg peritonitis, salphingitis, coligranuloma, omphalitis, cellulitis, embryonic death, septicemia and osteomyelitis/arthritis in poultry (Dziva and Stevens, 2008). As a result, mild and moderate chronic APEC infection often causes reduced feed intake, decreases live weight, egg production and hatching rates, causes poor feed conversion rates, and increases contamination of carcasses at slaughter and thus risks human health (Dho-moulin et al., 1999; Collingwood et al., 2014; Kemmett et al., 2014). Severe and acute APEC infection is usually responsible for higher morbidity and mortality in young chickens and older birds as well as decreased production efficiency (Kemmett et al., 2014; Swelum et al., 2021). Furthermore, APEC can affect all species of poultry in all types of production systems. Colibacillosis in broilers is a critically important disease, which primarily affects broiler chickens between the ages of 3 and 6 wk and is considered the most important cause of growth depression, morbidity and mortality increase, leading to considerable economic losses every year in the global poultry industry (Dho-moulin et al., 1999; Abu-Basha et al., 2012; Zhang et al., 2012; Kemmett et al., 2014). Colibacillosis in broilers often occurs during respiratory stress caused by infection with Mycoplasma gallisepticum or viral agents such as infectious bronchitis, Newcastle disease and avian influenza. In addition, immunosuppressive disease (infectious bursal disease) and environmental stresses, such as overcrowding or high levels of dust and ammonia entering through oral and respiratory routes, also lead to APEC systemic infection in broiler chickens (Dho-moulin et al., 1999).
In order to reduce morbidity and mortality related to APEC systemic infection, antimicrobial drugs such as aminoglycosides, amoxicillin, blactams, cephalosporins, florfenicol, fluoroquinolones and tetracyclines, as well as the control of environmental contaminants are often used to manage colibacillosis infection and outbreak. The continuous or excessive use of these antimicrobial drugs in poultry, however, has contributed to the emergence of antimicrobial drug-resistant APEC populations in China and other countries (Zhang et al., 2013; Chen et al., 2014; Zhuang et al., 2014; Ceccarelli et al., 2020; Kim et al., 2020). This phenomenon not only poses new challenges to the rational and effective use of antibiotics, but also brings many difficulties to the prevention and control of APEC infection. Additionally, recent mandated reduction or bans in antimicrobial use in the poultry industry in some countries have impeded the treatment of avian colibacillosis. Consequently, APEC systemic infection incidence and mortality rates have increased gradually. Therefore, the exploration of effective and safe anti-infective substances and vaccines, which carry low risk of drug resistance, is of great significance and is valuable for the control of APEC infections (Swelum et al., 2021).
Essential oils (EO) from plants and Chinese herbal medicines are gathering increased attention and can be utilised as promising antibiotic alternatives due to their effective antimicrobial, anti-viral, anti-coccidial, anti-fungal, anti-inflammatory, anti-oxidative and immunomodulative capacities (reviewed by Bakkali et al., 2008; Yang et al., 2015; Zeng et al., 2015; El-Shall et al., 2020). In vitro studies have demonstrated that several plant-derived EO, such as thyme, carvacrol, cinnamaldehyde and citral, could inhibit or kill Gram-negative and Gram-positive bacteria including Salmonella, E. coli, Campylobacter and Clostridium perfringens, without harming beneficial bacteria like Lactobacillus spp. (Friedman et al., 2002, 2004; Burt, 2004; Mitsch et al., 2004; Jerzsele et al., 2012; Mathlouthi et al., 2012; Calo et al., 2015; Do et al., 2015; Lopez-Romero et al., 2015; Yang et al., 2015). Broiler chicken studies have indicated that several essential oil products with different kinds of EO components could improve growth performance and gut health (Cross et al., 2007; Khattak et al., 2014; Hashemipour et al., 2015; Pirgozliev et al., 2015; Peng et al., 2016; Adaszyńska-Skwirzyńska and Szczerbińska, 2018; Chowdhury et al., 2018; Liu et al., 2018; Wang et al., 2019), alleviate negative effects caused by pathogenic Salmonella (Alali et al., 2013), E. coli (Liu et al., 2018), C. perfringens (Mitsch et al., 2004; Jerzsele et al., 2012; Du et al., 2015, 2016; Yin et al., 2017) and coccidiosis (Mohiti-Asli and Ghanaatparast-Rashti, 2015; Gordillo Jaramillo et al., 2021).
Organic acids (OA) also could be used as antibiotic alternatives and feed preservatives due to their anti-microbial abilities. Some studies have demonstrated that OA supplementation could improve growth performance, feed conversion rate and feed protein utilization of broiler chickens (Nava et al., 2009; Adil et al., 2011), possibly via suppressing the growth of intestinal acid-intolerant potential pathogens such as E. coli, Salmonella and C. perfringens, increasing intestinal digestive enzyme activity and improving intestinal morphological structure (Gharib Naseri et al., 2012). Furthermore, adding organic acids in drinking water or feed could provide young chickens with protection against E. coli, Salmonella, C. perfringens and Campylobacter infection (Chaveerach et al., 2004; Van Immerseel et al., 2006; Dittoe et al., 2018; Lu et al., 2021).
Interestingly, previous studies have indicated that dietary essential oil and organic acid (EOA) supplementation not only showed synergistic beneficial effects on growth performance and gut health, but also exhibited higher efficacy in controlling harmful intestinal bacterial infection such as E. coli, Salmonella spp. and C. perfringens (Giannenas et al., 2014; Aristimunha et al., 2016; Basmacioğlu-Malayoğlu et al., 2016; Pham et al., 2020) compared with individual addition. The EOA mixture is a novel blend of coated EOA compounds containing thyme 4%, carvacrol 4%, hexanoic acid 0.5%, benzoic acid 3.5%, butyric acid 0.5% and carrier. However, the protective mechanism of dietary inclusion of the novel EOA mixture in broiler chickens challenged with APEC is unclear. Therefore, the purpose of this study was to evaluate whether adding EOA could protect broiler chickens against E. coli O78 infection through determining growth performance, immune response and gut health.
2. Materials and methods
2.1. Animal ethics statement
All study procedures were approved by the Animal Care and Use Committee of China Agricultural University (permit number: SYXK 2019-0031) and were in accordance with the Beijing Guidelines for Experimental Animals established by the Ministry of Science and Technology (Beijing, China). All efforts were made to minimize the suffering of the animals.
2.2. Experimental design, birds and diets.
A 2 × 2 completely randomized factorial design was used to evaluate the efficacy of EOA supplementation (0 or 500 mg/kg of diet) and 2 levels of E. coli challenge (challenged or unchallenged). A total of 228 one-day-old male (Arbor Acres) broiler chicks were obtained from a company hatchery. After arrival, all chicks were weighed and randomly allocated to 4 groups. There were 6 cages each group with 12 birds per cage per replicate. The treatment groups were as follows: (1) negative control group (A, neither EOA treatment nor E. coli infection), (2) the EOA-treated group (B, EOA treatment at 500 mg/kg of feed but without E. coli infection), (3) E. coli-infected control group (K, E. coli infection but without EOA treatment), and (4) the EOA-treated and E. coli-infected group (L, both EOA treatment and E. coli infection). The EOA product contained vegetable (palm) oil coated thyme 4% and carvacrol 4%, hexanoic acid 0.5%, benzoic acid 3.5%, butyric acid 0.5% and carrier which was provided by Menon Animal Nutrition Technology Co. Ltd, Shanghai, China. To avoid cross-contamination, the uninfected birds and E. coli-infected birds were reared in 2 separate areas equipped with three-tiered battery cages with a raised wire-netted floor. The room temperature was maintained at 32 to 34 °C during the first 3 d post–hatch followed by gradual reduction to reach a final room temperature of 22 to 24 °C. The birds received continuous light for the first 3 d (24 h) and then maintained under 23 h/day. In addition, the chickens were vaccinated against Newcastle disease virus (NDV) and infectious bronchitis virus (IBV) on d 7 and 22, respectively; and against infectious bursal disease virus (IBDV) according to the routine immunization program via drinking water on d 12 and 26. All birds were allowed ad libitum access to feed and water throughout the study. Based on the treatments assigned, chickens were fed either antibiotic-free and coccidiostat-free pellet basal diets which were formulated to meet or exceed the National Research Council (1994) requirements for starter (d 1 to 21) and grower (d 22 to 42) periods. The composition of the basal diet and nutrient levels are presented in Table 1.
Table 1.
Ingredients and nutrient levels of the basal diet (as-fed basis, %).
| Item | 1 to 21 d | 22 to 42 d |
|---|---|---|
| Ingredients | ||
| Corn (7.8% CP) | 39.70 | 57.0 |
| Wheat powder (15.3% CP) | 0 | 5 |
| Wheat (13.8% CP) | 19.0 | 0 |
| Soybean meal (46.0% CP) | 33.0 | 30.0 |
| Soybean oil | 4.00 | 4.40 |
| Limestone-calcium carbonate | 1.50 | 1.50 |
| Calcium hydrogen phosphate | 1.50 | 1.36 |
| Phytase | 0.02 | 0.03 |
| DL-Methionine (98%) | 0.27 | 0.19 |
| L-Lysine HCL (78%) | 0.20 | 0.11 |
| Sodium chloride | 0.30 | 0.30 |
| Vitamin Premix1 | 0.03 | 0.03 |
| Mineral Premix2 | 0.20 | 0.20 |
| Choline chloride (50%) | 0.25 | 0.15 |
| Ethoxyquin (33%) | 0.05 | 0.03 |
| Total | 100 | 100 |
| Calculated nutrient levels | ||
| Metabolizable energy, MJ/kg | 12.60 | 12.98 |
| Crude protein | 21.37 | 19.27 |
| Calcium | 0.99 | 0.93 |
| Available phosphorus | 0.45 | 0.43 |
| Lysine | 1.20 | 1.05 |
| Methionine | 0.57 | 0.46 |
| Methionine + Cysteine | 0.90 | 0.78 |
Vitamin premix provided per kilogram of diet: vitamin A (retinyl acetate), 12,500 IU; vitamin D3, 2,500 IU; vitamin E, 30 mg; vitamin K3, 3.0 mg; vitamin B1, 3.0 mg; vitamin B2, 6.0 mg; vitamin B6, 6 mg; vitamin B12, 0.025 mg; biotin, 0.30 mg; folic acid, 1.25 mg; nicotinic acid, 50 mg; D-pantothenic acid, 12 mg.
Mineral premix provided per kilogram of diet: iron, 80 mg; copper, 8 mg; manganese, 100 mg; zinc, 80 mg; iodine, 0.35 mg; selenium, 0.15 mg.
2.3. Escherichia coli O78 culture and challenge protocol
The E. coli O78 strain (CVCC1418) used in this study was purchased from the Chinese Veterinary Culture Collection Center, which was isolated from the heart of chicken with septicemia signs. The E. coli O78 was overnight cultured in Luria–Bertani broth (LB) at 37 °C, and then numerated by spread-plating the appropriate dilutions in duplicate on tryptose phosphate agar plates. The birds in different treatments were fed the corresponding diet for the first 21 d. On d 22, birds in the K and L groups were intratracheally challenged with 0.5 mL of E. coli O78 suspended in LB at a concentration of 106 CFU/mL using a polyethylene tube attached to a syringe, as previously described (Kariyawasam et al., 2004; Antão et al., 2008). The birds in the A and B groups were administered the same amount of sterile solution as a control.
2.4. Growth performance
Dead birds were recorded daily and mortality rates was calculated at every rearing stage. Each replicate cage had body weight and feed intake (FI) measured on d 1, 21 and 42. Average daily gain (ADG), average daily feed intake (ADFI) and feed conversion ratio (FCR) were calculated and corrected for mortality rate.
2.5. Sample collection
At 7 d post-E. coli infection, one bird per replicate was randomly selected, weighed, then blood samples were taken from the wing vein and centrifuged (3,000 × g, 10 min) at 4 °C, and then the serum was harvested and stored at −20 °C until analysis. The birds were euthanized by cervical dislocation. The middle intestinal sections of the jejunum were cut out (approximately 1 cm), carefully washed with sterile saline, then put into a sterile tube and immediately snap-frozen in liquid nitrogen solution and stored at −80 °C until subsequent analysis for mRNA expression. Another jejunal sample (approximately 2 cm) was rinsed in 0.9% physiological saline and stored in 4% paraformaldehyde buffer solution for later morphological analysis. Liver samples and cecal samples from each bird were aseptically collected into sterile tubes, then immediately snap-frozen in liquid nitrogen solution, stored at −40 °C and moved to the laboratory for microbial culture and microbial 16S rRNA analysis.
2.6. Gross lesion examination
At necropsy, tissues were scored for signs of inflammation and lesions in the air sac (0, normal; 1, slight edema; 2, slight diffuse thickening and neovascularization with slight fibrinous exudate; 3, moderate fibrinous exudate; and 4, severe extensive exudate), heart and pericardium (0, normal; 1, vascularization, opacity, cloudy fluid in the pericardial cavity; 2, acute pericarditis), and liver (0, normal; 1, mild fibrinous exudate; 2, severe perihepatitis), as described previously (Peighambari et al., 2000). Scores for heart plus pericardium and liver were combined for final analysis.
2.7. Histomorphological structure and goblet cells analysis of the jejunum
Villous height (VH), crypt depth (CD) and goblet cell analysis were performed as previously described (Shao et al., 2013).
2.8. Determination of bacterial concentration in the liver and cecal contents
Bacterial concentration in the liver and cecal contents was measured as previously described (Wu et al., 2018). Briefly, 6 birds per group were chosen at random for microbial analyses. The selected broilers were weighed and euthanized, and the liver and cecal contents were aseptically removed. The samples were then diluted 10-fold by weight in 0.90% sterile physiological saline and were mechanically massaged for 1 min. All samples were serially diluted to appropriate levels for plating. An aliquot from each sample (100 μL) was spiral plated on the following: EMB agar for the isolation and enumeration of total coliforms, Lactobacillus selection MRS agar for the enumeration of Lactobacillus spp.
2.9. ELISA for the measurement of immunoglobulins and cytokines
Immune globulin levels such as immunoglobulins (IgG, IgM and IgA), along with cytokines like interleukins (IL-2, IL-4, IL-6), TNF-α and IFN-γ were measured in the serum with the use of enzyme-linked immunosorbent assay (ELISA) kits particularly for chickens (R&D Systems, Minneapolis, MN, United States) according to the instructions of the manufacturer.
2.10. Quantitative real-time PCR analysis
Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA) was used to isolate total RNA of jejunal mucosa (about 60 mg) according to the manufacturer's instructions. An agarose gel was used to assess the RNA quality. The concentration and purity of the extracted RNA was assessed using a Nanodrop-2000 spectrophotometer (260 and 260/280 nm respectively) (Thermo Fisher Scientific, Waltham, MA, USA). Then, Primer Script RT reagent Kit (TaKaRa Bio Inc.) was used to reverse transcribe RNA to complementary DNA, which was stored at −20 °C until analysis. The target gene sequences and primers for the reference gene are shown in Table 2. Expressions of toll-like receptor (TLR) signal pathway-related genes, tight junction proteins, growth factors and mucin-2 and housekeeping gene β-actin were quantified with SYBR Premix Ex Taq kits (TaKaRa) on the Applied Biosystems 7500 Fast Real-Time PCR System. The specificity and efficiency of the primer was determined by melt curve analysis. The 2 −ΔΔCT method calculated the levels of mRNA expression of target genes using β-actin as a reference gene (Livak and Schmittgen, 2001).
Table 2.
Nucleotide sequences of primers for quantitative real-time PCR assay.
| Name | Primer sequence | GenBank accession |
|---|---|---|
| Receptors | ||
| TLR-4 | F: CCACTATTCGGTTGGTGGAC | NM_001030693.1 |
| R: ACAGCTTCTCAGCAGGCAAT | ||
| Adaptor proteins | ||
| MyD88 | F: GGATGGTGGTCGTCATTTCA | NM_001030962.1 |
| R: GAGATTTTGCCAGTCTTGTCCA | ||
| NF-κB | F: TGGAGAAGGCTATGCAGCTT | NM_205134.1 |
| R: CATCCTGGACAGCAGTGAGA | ||
| Pro-inflammatory cytokines | ||
| TNF-α | F: CCCCTACCCTGTCCCACAA | NM204267 |
| R: TGAGTACTGCGGAGGGTTCAT | ||
| IL-1β | F: CAGCAGCCTCAGCGAAGAG | NM_204524.1 |
| R: CTGTGGTGTGCTCAGAATCCA | ||
| IL-8 | F: GGCTTGCTAGGGGAAATGA | AJ009800 |
| R: AGCTGACTCTGACTAGGAAACTGT | ||
| IFN-γ | F: AAAGCCGCACATCAAACACA | NM_205149.1 |
| R: GCCATCAGGAAGGTTGTTTTTC | ||
| Ant-inflammatory cytokines | ||
| IL-10 | F: CGCTGTCACCGCTTCTTCA | NM_001004414.2 |
| R: CGTCTCCTTGATCTGCTTGATG | ||
| Tight junctions | ||
| Claudin-1 | F: AAGTGCATGGAGGATGACCA | NM_001013611.2 |
| R: GCCACTCTGTTGCCATACCA | ||
| Occlaudin | F: TCATCGCCTCCATCGTCTAC | NM_205128.1 |
| R: TCTTACTGCGCGTCTTCTGG | ||
| ZO-1 | F: TATGAAGATCGTGCGCCTCC | XM_015278981.1 |
| R: GAGGTCTGCCATCGTAGCTC | ||
| Mucin-2 | F: AGCGAGATGTTGGCGATGAT | NM_001318434.1 |
| R: AAGTTGCCACACAGACCACA | ||
| Growth factors | ||
| TGF-β3 | F: TGCGGCCAGATGAGCAT | NM_205454.1 |
| R: TGCACATTCCTGCCACTGA | ||
| IGF-2 | F: TGGCTCTGCTGGAAACCTAC | NM_001030342.2 |
| R: ACTTGGCATGAGATGGCTTC | ||
| EGFR | F: ACCAGCCTGCAGAGAATGTA | NM_205497 |
| R: CACCATGTTAAGCGCAATGA | ||
| GLP-2 | F: AAGCTTCCCAGTCTGAACCA | NM_205260.4 |
| R: ATCCTGAGCTCGTCTGCTGT | ||
| House-keeping genes | ||
| β-actin | F: GAGAAATTGTGCGTGACATCA | NM 205518 |
| R: CCTGAACCTCTCATTGCCA |
TLR = toll-like receptor; MyD88 = myeloid differential protein-88; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells; TNF-α = tumor necrosis factor α; IL = interleukin; IFN-γ = interferon γ; ZO-1 = zonula occludens-1; EGFR = epidermal growth factor receptor; GLP-2 = glucagon like peptide-2; IGF-2 = insulin-like growth factor-2; TGF-β3 = transforming growth factor beta 3.
2.11. Metagenomics analysis of gut microbiome
Frozen fecal samples were used for metagenomic studies by analyzing the 16S rDNA, as described previously (Pham et al., 2020). The 16S rRNA gene sequencing was performed using the Illlumina MiSeq PE 250 platform (Illumina, Santa Clara, CA) with a MiSeq Reagent Kit at Shanghai Personal Biotechnology Co., Ltd (Shanghai, China). The raw data and QIIME (Quantitative Insights into Microbial Ecology) were filtered and demultiplexed by using the Illumina MiSeq platform (version 1.8.0-dev). Raw tags were obtained by merging sequence data using FLAST (Caporaso et al., 2011). The sequences and reference operational taxonomic units (OTU; 97% similarity) were clustered and classified by the UCLUST algorithm and QIIME software. Afterwards, MOTHUR software was used to calculate alpha diversity and beta diversity analysis. The alpha diversity indices, including the observed OTU, ACE, Chao 1, Good's coverage, Shannon, and Simpson index were calculated by MOTHUR, and substantial variations between the microbial compositions in the control and EOA-treated chickens were determined via a nonparametric Mann–Whitney U test ranked using the percentage of representation of individual genera. A Venn diagram was created with R software to visualize the shared and unique OTU among samples based on the Silva taxonomic database (Zaura et al., 2009). Beta diversity analysis was performed to investigate the structural variation of microbial communities across samples using UniFrac distance metrics (Lozupone et al., 2007; Ramette, 2007), Partial least squares discriminant analysis (PLS-DA) (Chen et al., 2011) and nonmeric multidimensional scaling (NMDS) analyses. Linear discriminant analysis effect size (LEfSe) was performed to detect differentially abundant taxa across groups using the default parameters (LDA Score > 2.0, P < 0.05) (Segata et al., 2011). Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt 1.0.0) was used to predict metagenome functions associated with bacterial communities based on high-quality 16S rRNA sequencing data (Langille et al., 2013). The functions were deduced using Kyoto Encyclopedia of Genes and Genomes (KEGG) annotations for level 2 pathways.
2.12. Data analysis
Data regarding growth performance, gut lesion scores, intestinal bacterial concentration and liver E. coli, jejunal morphology and goblet cell numbers, blood parameters, the relative expression of mRNA, alpha diversity indices of Shannon and abundance-based coverage estimator (ACE) among the 4 groups were analyzed using one-way ANOVA in SPSS 20.0 (SPSS Inc., Chicago, IL, USA) in a 2 × 2 factorial design. Mean separations were carried out using Duncan's multiple comparison test when interactive effects differed significantly. The Kruskal–Wallis test as well as Benjamini-Hochberg P-value correction was used to compare the results of phylum and genus abundances. P ≤ 0.05 was regarded as significant, while 0.05 ≤ P ≤ 0.10 was regarded as a tendency.
3. Results
3.1. Growth performance
As illustrated in Table 3, dietary EOA addition (B and L) had no significant effects on ADG, ADFI and FCR of broiler chickens except for showing a numerical decrease in mortality rate compared with the un-treated birds (A and B) from 1 to 21 d. However, E. coli challenge retarded the growth of chickens with an obvious reduction in ADG (P < 0.05) from 22 to 42 d, and remarkably increased mortality rate (Fig. 1A) and tended to increase FCR from 22 to 42 d and 1 to 42 d (P < 0.05) relative the non-challenged groups. While birds fed with EOA showed decreased ADFI and numerically reduced mortality rate (Fig. 1A) from 22 to 42 d and 1 to 42 d, compared with the untreated groups (P < 0.05). There was a notable interaction effect for ADFI and FCR between EOA supplementation and E. coli challenge during the later and entire period (P > 005). Birds on L group exhibited improved FCR compared with infected control group.
Table 3.
Effects of dietary coated essential oil and organic mixture supplementation on growth performance of broiler chickens infected with Escherichia coli O78.
| Item | Experimental design |
Main effect |
P-value1 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | K | B | L | SEM | 0 | 500 mg/kg | Non-challenge | Challenged | EOA | Challenged | Interaction effects | |
| Day 1 to 21 | ||||||||||||
| ADG, g/d | 41.4 | 42.4 | 40.8 | 42.7 | 0.36 | 41.9 | 41.8 | 41.1 | 42.6 | 0.811 | 0.074 | 0.575 |
| ADFI, g/d | 59.5 | 60.0 | 59.3 | 60.7 | 0.43 | 59.8 | 60.0 | 59.4 | 60.4 | 0.792 | 0.337 | 0.672 |
| FCR, g/g | 1.44 | 1.41 | 1.45 | 1.42 | 0.01 | 1.43 | 1.44 | 1.44a | 1.42b | 0.294 | 0.046 | 0.677 |
| Day 22 to 42 | ||||||||||||
| ADG, g/d | 90.7 | 80.5 | 89.7 | 80.5 | 1.79 | 85.6 | 85.1 | 90.2 | 80.5 | 0.927 | 0.035 | 0.235 |
| ADFI, g/d | 147.4a | 144.8ab | 127.8b | 136.8ab | 3.10 | 146.1 | 132.3 | 137.6 | 140.8 | 0.027 | 0.582 | 0.036 |
| FCR, g/g | 1.66ab | 1.81a | 1.44c | 1.71ab | 0.06 | 1.73 | 1.58 | 1.55 | 1.76 | 0.193 | 0.098 | <0.001 |
| Day 1 to 42 | ||||||||||||
| ADG, g/d | 66.1 | 61.5 | 65.2 | 61.6 | 1.17 | 63.8 | 63.4 | 65.6 | 61.5 | 0.896 | 0.129 | 0.263 |
| ADFI, g/d | 103.4a | 102.4ab | 93.6b | 98.8ab | 1.56 | 102.9 | 96.2 | 98.5 | 100.6 | 0.031 | 0.476 | 0.034 |
| FCR, g/g | 1.58b | 1.67a | 1.44c | 1.61a | 0.04 | 1.63 | 1.52 | 1.51 | 1.64 | 0.216 | 0.129 | <0.001 |
A = neither infection nor coated essential oil and organic acid mixture addition negative control; K = E. coli infection positive control; B = coated essential oil and organic acid mixture addition at 500 mg/kg but without infection; L = both essential oil and organic acid mixture addition at 500 mg/kg and E. coli O78 infection; SEM = standard error of the mean; ADG = average daily weight gain; ADFI = average daily feed intake; FCR = feed-to-gain ratio.
a, b, c Means within the same row without a common superscript differ significantly (P < 0.05).
P-values represent the main effect of the diet, the main effect of E. coli O78 challenge, and the interaction between the dietary treatments and E. coli O78 challenge.
Fig. 1.
Effect of essential oil and organic acid mixture (EOA) on (A) mortality rate and (B) lesion scores of broiler chickens challenged with Escherichia coli O78. Treatment groups: A = neither infection nor EOA addition control, K = E. coli O78 infection control, B = EOA addition but without infection, L = both EOA addition and E. coli O78 infection.
3.2. Bacterial concentration of intestinal contents and liver
As shown in Table 4, a significant interaction effect between E. coli O78 infection and EOA addition on E. coli concentration in cecal chyme (P < 0.05). Both broilers on L and B groups displayed lower levels of E. coli in cecal chyme (P < 0.05) compared with the E. coli-challenged birds. In addition, E. coli challenge tended to increase E. coli concentration in liver (P = 0.057).
Table 4.
Effect of dietary coated essential oil and organic mixture supplementation on intestinal bacterial concentration and liver Escherichia coli numbers of broiler chickens challenged with E. coli O78.
| Item |
E. coli1 |
Cecal bacterial counts, CFU/g |
Liver E. coli, CFU/g |
|
|---|---|---|---|---|
| E. coli | Lactobacillus | |||
| EOA, mg/kg | ||||
| 0 | - | 6.59b | 9.55 | 0.00 |
| 0 | + | 8.24a | 9.97 | 0.79 |
| 500 | - | 6.60b | 10.41 | 0.30 |
| 500 | + | 5.84b | 9.30 | 0.52 |
| SEM | 0.24 | 0.24 | 0.13 | |
| Main effect | ||||
| 0 | 7.41 | 9.76 | 0.39 | |
| 500 | 6.22 | 9.63 | 0.41 | |
| Non-challenge | 6.59 | 9.73 | 0.15 | |
| Challenge | 7.04 | 9.66 | 0.65 | |
| P-value2 | ||||
| EOA | 0.005 | 0.825 | 0.954 | |
| Challenged | 0.257 | 0.907 | 0.057 | |
| EOA × Challenged | 0.004 | 0.305 | 0.265 | |
EOA = coated essential oil and organic mixture; SEM = standard error of the mean.
a, b Means within the same column without a common superscript differ significantly (P < 0.05).
“–”, without E. coli challenge; “+”, with E. coli challenge.
P-values represent the main effect of the diet, the main effect of E. coli O78 challenge, and the interaction between the dietary treatments and E. coli O78 challenge.
3.3. Gross lesion examination
Intratracheal inoculation of E. coli O78 not only caused higher mortality, but also lead to severe airsacculitis, pericarditis and perihepatitis. Fig. 1B illustrates that the highest gross lesion scores were observed in the E. coli O78-challenged birds (P < 0.05). However, EOA addition notably lowered gross lesion scores of birds on L group compared with the challenged control group (P < 0.05). No obvious pathological changes were observed in A and B groups.
3.4. Intestinal morphology
Based on the main effect of EOA supplementation, birds fed diets with EOA significantly increased villous height (VH), the villous height to crypt depth ratio (VH:CD) and goblet cell numbers compared with birds in the unsupplemented groups (A and K) (P < 0.05). On the other hand, compared with unchallenged groups (A and B), there was a great decrease in VH:CD ratio in birds challenged with E. coli (K and L) (P < 0.05). However, there was no interaction effect on VH and VH:CD ratio between EOA addition and E. coli challenge, but a significant interaction effect was found for goblet cell numbers between EOA supplementation and E. coli challenge in the jejunum (P = 0.010). Broilers on L group showed higher goblet cell concentrations than the K group (Table 5).
Table 5.
Effect of dietary coated essential oil and organic mixture supplementation on jejunal morphology and goblet cell numbers of broiler chickens challenged with Escherichia coli O78.
| Item | E. coli1 | Villous height, μm | Crypt depth, μm | VH:CD ratio | GC cells |
|---|---|---|---|---|---|
| EOA, mg/kg | |||||
| 0 | - | 439.66 | 61.93 | 7.07 | 23.57a |
| 0 | + | 377.68 | 79.94 | 4.92 | 19.30b |
| 500 | - | 507.07 | 59.00 | 8.66 | 23.03ab |
| 500 | + | 491.23 | 67.72 | 7.62 | 26.30a |
| SEM | 20.36 | 4.19 | 0.35 | 0.81 | |
| Main effect | |||||
| 0 | 408.67b | 70.94 | 5.99b | 21.43 | |
| 500 | 499.15a | 63.36 | 8.14a | 24.67 | |
| Non-challenge | 473.37 | 60.47 | 7.86a | 23.30 | |
| Challenge | 434.46 | 73.83 | 6.27b | 22.80 | |
| P-value2 | |||||
| EOA | 0.025 | 0.368 | <0.001 | 0.024 | |
| Challenged | 0.308 | 0.120 | 0.001 | 0.711 | |
| EOA × Challenged | 0.542 | 0.578 | 0.181 | 0.010 |
EOA = coated essential oil and organic mixture; VH:CD ratio = the villus height to crypt depth ratio; GC cells = goblet cell numbers per mm2; SEM = standard error of the mean.
a, b Means within the same column with different superscripts differ significantly (P < 0.05).
“–”, without E. coli O78 challenge; “+”, with E. coli O78 challenge.
P-values represent the main effect of the diet, the main effect of E. coli O78 challenge, and the interaction between the dietary treatments and E. coli O78 challenge.
3.5. Serum cytokines and immunoglobulin levels
Serum cytokines and IgG concentration are presented in Table 6. E. coli O78 infection significantly promoted the production of serum IgG, IL-4, IL-6, IL-8, IL-12, IFN-γ and TNF-α (P < 0.05) in comparison to the non-challenged groups. Supplemental EOA only increased serum IgG levels relative to the unsupplemented groups (P < 0.05). A considerable interaction effect was observed for serum IgG and IL-12 levels between EOA supplementation and E. coli O78 infection (P < 0.05). Feeding EOA to infected birds exhibited a significant increase in the production of serum IgG (P < 0.05), and tended to decrease serum IL-12 production compared with the birds on K group.
Table 6.
Effect of dietary coated essential oil and organic mixture supplementation on serum cytokine concentration and immunoglobulin levels of broiler chickens challenged with Escherichia coli O78.
| Item | E. coli1 | IL-1β, pg/mL | IL-4, pg/mL | IL-6, pg/mL | IL-8, pg/mL | IL-10, pg/mL | IL-12, pg/mL | IFN-γ, pg/mL | TNF-α, pg/mL | IgG, g/L |
|---|---|---|---|---|---|---|---|---|---|---|
| EOA, mg/kg | ||||||||||
| 0 | - | 12.13 | 25.74 | 153.57 | 26.68 | 46.12 | 21.42b | 29.55 | 4.06 | 3.81c |
| 0 | + | 19.03 | 53.23 | 794.79 | 80.48 | 47.77 | 166.52a | 454.74 | 36.41 | 3.75c |
| 500 | - | 18.25 | 17.30 | 172.17 | 31.61 | 59.85 | 46.86b | 73.00 | 9.66 | 4.29b |
| 500 | + | 10.15 | 36.28 | 369.97 | 41.20 | 56.90 | 82.81ab | 237.61 | 22.73 | 5.12a |
| SEM2 | 2.79 | 5.12 | 86.36 | 7.58 | 9.12 | 16.50 | 51.99 | 4.80 | 0.13 | |
| Main effect | ||||||||||
| 0 | 15.58 | 39.48 | 474.18 | 53.58 | 46.95 | 93.97 | 242.14 | 20.23 | 3.78 | |
| 500 | 14.20 | 26.79 | 271.07 | 36.41 | 58.38 | 64.83 | 155.30 | 16.20 | 4.71 | |
| Non-challenge | 15.19 | 21.52b | 162.87b | 29.15b | 52.99 | 34.14 | 51.27b | 6.86b | 4.05 | |
| Challenge | 14.59 | 44.75a | 582.38a | 60.84a | 52.33 | 124.66 | 346.17a | 29.57a | 4.44 | |
| P-value2 | ||||||||||
| EOA | 0.813 | 0.182 | 0.176 | 0.209 | 0.562 | 0.267 | 0.307 | 0.645 | <0.001 | |
| Challenged | 0.918 | 0.020 | 0.009 | 0.026 | 0.973 | 0.002 | 0.002 | 0.016 | 0.018 | |
| EOA × Challenged | 0.206 | 0.649 | 0.142 | 0.110 | 0.907 | 0.045 | 0.131 | 0.278 | 0.007 |
TNF-α = tumor necrosis factor α; IL = interleukin; IFN-γ = interferon γ; IgG = immunoglobulin G; EOA = coated essential oil and organic mixture; SEM = standard error of the mean.
a, b, c Means within the same column with different superscripts differ significantly (P < 0.05).
“–”, without E. coli O78 challenge; “+”, with E. coli O78 challenge.
P-values represent the main effect of the diet, the main effect of E. coli O78 challenge, and the interaction between the dietary treatments and E. coli O78 challenge.
3.6. Intestinal immune-related and barrier-related gene expression
As shown in Table 7, E. coli O78 infection notably upregulated TLR4, IFN-γ, IL-8, TGF-β and IGF-2 genes expression, while downregulating occludin and ZO-1 in the jejunum of broiler chickens compared with the unchallenged groups. Feeding EOA at 500 mg/kg of feed had no significant influence on intestinal immune-related and barrier-related gene mRNA levels relative to the unsupplemented groups. However, there were significant interactions with jejunal IFN-γ, IL-10, ZO-1 and mucin-2 mRNA levels between E. coli infection and EOA addition at 7 d after E. coli infection (P < 0.05, Table 8). E. coli-infected birds that received EOA displayed lower IFN-γ and higher IL-10 mRNA levels than the E. coli-infected control alone (P < 0.05) and tended to increase ZO-1 gene expression while decreasing mucin-2 mRNA levels relative to the infected control alone at 7 d post–challenge. Furthermore, the highest expression levels of mucin-2 were found in the uninfected and EOA treated group relative to that of the other three groups.
Table 7.
Effects of dietary coated essential oil and organic mixture supplementation on gene expressions of TLR signaling pathway-related genes in the jejunum of broiler chickens challenged with E. coli O78.
| Item | E. coli1 | TLR-4 | MyD88 | NF-κB | IFN-γ | TNF-α | IL-1β | IL-8 | IL-10 |
|---|---|---|---|---|---|---|---|---|---|
| EOA, mg/kg | |||||||||
| 0 | - | 1.07 | 1.02 | 1.06 | 1.01 | 1.00 | 1.05 | 1.04 | 1.07b |
| 0 | + | 1.92 | 1.18 | 1.04 | 2.74 | 1.04 | 1.12 | 1.38 | 0.72b |
| 500 | - | 1.18 | 1.11 | 1.16 | 1.13 | 1.32 | 1.02 | 0.75 | 0.81b |
| 500 | + | 1.31 | 1.22 | 1.03 | 1.06 | 1.13 | 1.09 | 1.96 | 1.84a |
| SEM2 | 0.10 | 0.08 | 0.09 | 0.09 | 0.06 | 0.14 | 0.32 | 0.11 | |
| Main effect | |||||||||
| 0 | 1.50 | 1.10 | 1.05 | 1.87 | 1.02 | 1.09 | 1.21 | 0.90 | |
| 500 | 1.20 | 1.17 | 1.10 | 1.09 | 1.23 | 1.05 | 1.36 | 1.33 | |
| Non-challenged | 1.13 | 1.07 | 1.11 | 1.07 | 1.16 | 1.04 | 0.90 | 0.94 | |
| Challenged | 1.66 | 1.20 | 1.03 | 1.90 | 1.09 | 1.10 | 1.67 | 1.28 | |
| P-value2 | |||||||||
| EOA | 0.377 | 0.663 | 0.805 | 0.408 | 0.095 | 0.905 | 0.821 | 0.055 | |
| Challenged | 0.032 | 0.407 | 0.676 | 0.016 | 0.524 | 0.803 | 0.013 | 0.126 | |
| Challenged × EOA | 0.567 | 0.877 | 0.747 | <0.01 | 0.353 | 0.998 | 0.237 | <0.01 |
TLR = toll-like receptor; MyD88 = myeloid differential protein-88; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells; TNF-α = tumor necrosis factor α; IL = interleukin; IFN-γ = interferon γ; SEM = standard error of the mean.
a, b Means within the same column with different superscripts differ significantly (P < 0.05).
“–”, without E. coli O78 challenge; “+”, with E. coli O78 challenge.
P-values represent the main effect of the diet, the main effect of E. coli O78 challenge, and the interaction between the dietary treatments and E. coli O78 challenge.
Table 8.
Effects of dietary coated essential oil and organic mixture supplementation on gene expressions of tight junction proteins, growth factors and mucin-2 in the jejunum of broiler chickens challenged with Escherichia coli O78.
| Item | E. coli1 | Claudin1 | Occludin | ZO-1 | TGF-β3 | IGF-2 | EGFR | GLP-2 | Mucin-2 |
|---|---|---|---|---|---|---|---|---|---|
| EOA, mg/kg | |||||||||
| 0 | - | 0.82 | 1.02 | 1.07a | 1.03 | 1.04 | 1.02 | 1.04 | 1.05b |
| 0 | + | 0.71 | 0.52 | 0.27c | 1.81 | 3.22 | 1.18 | 0.95 | 1.13ab |
| 500 | - | 0.50 | 1.10 | 0.64b | 1.18 | 1.21 | 1.45 | 1.26 | 1.44a |
| 500 | + | 0.80 | 0.72 | 0.48bc | 1.51 | 3.28 | 1.13 | 1.25 | 0.93b |
| SEM | 0.06 | 0.05 | 0.05 | 0.12 | 0.20 | 0.11 | 0.08 | 0.06 | |
| Main effect | |||||||||
| 0 | 0.76 | 0.77 | 0.67 | 1.42 | 2.13 | 1.10 | 1.00 | 1.09 | |
| 500 | 0.65 | 0.91 | 0.56 | 1.34 | 2.25 | 1.29 | 1.25 | 1.19 | |
| Non-challenged | 0.66 | 1.06a | 0.86 | 1.10b | 1.13b | 1.24 | 1.15 | 1.25 | |
| Challenged | 0.75 | 0.62b | 0.38 | 1.66a | 3.25a | 1.16 | 1.10 | 1.03 | |
| P-value2 | |||||||||
| EOA | 0.415 | 0.175 | 0.255 | 0.745 | 0.777 | 0.406 | 0.112 | 0.455 | |
| Challenged | 0.470 | <0.001 | <0.001 | 0.035 | <0.001 | 0.728 | 0.735 | 0.101 | |
| Challenged × EOA | 0.125 | 0.589 | 0.004 | 0.373 | 0.891 | 0.304 | 0.789 | 0.028 | |
ZO-1 = zonula occludens-1; EGFR = epidermal growth factor receptor; GLP-2 = glucagon like peptide-2; IGF-2 = insulin-like growth factor-2; TGF-β3 = transforming growth factor beta 3; EOA = coated essential oil and organic mixture; SEM = standard error of the mean.
a, b, c Means within the same column with different superscripts differ significantly (P < 0.05).
“–”, without E. coli O78 challenge; “+”, with E. coli O78 challenge.
P-values represent the main effect of the diet, the main effect of E. coli O78 challenge, and the interaction between the dietary treatments and E. coli O78 challenge.
3.7. Diversity and composition of cecal microbiota
In this study, we also determined cecal microbial communities of broiler chickens treated with EOA supplementation and challenged with E. coli O78. A total of 4,766 OTU were obtained from the cecal contents of all groups through high-throughput sequence analysis of the bacterial 16S rRNA V3–V4 region. Venn diagram analysis illustrated that 4,253 common OTU were shared by all 4 groups, whereas unique OTU varied from 40 to 75 in one group, respectively (Fig. 2).
Fig. 2.
The overall description of gut microorganism among the 4 groups. (A) Venn diagram, (B) Chao index, (C) Shannon index, (D) Simpson index, (E) abundance-based coverage estimator (ACE). Treatment groups: A = neither infection nor essential oil and organic acid mixture (EOA) addition control, K = Escherichia coli O78 infection control, B = EOA addition but without infection, L = both EOA addition and E. coli O78 infection.
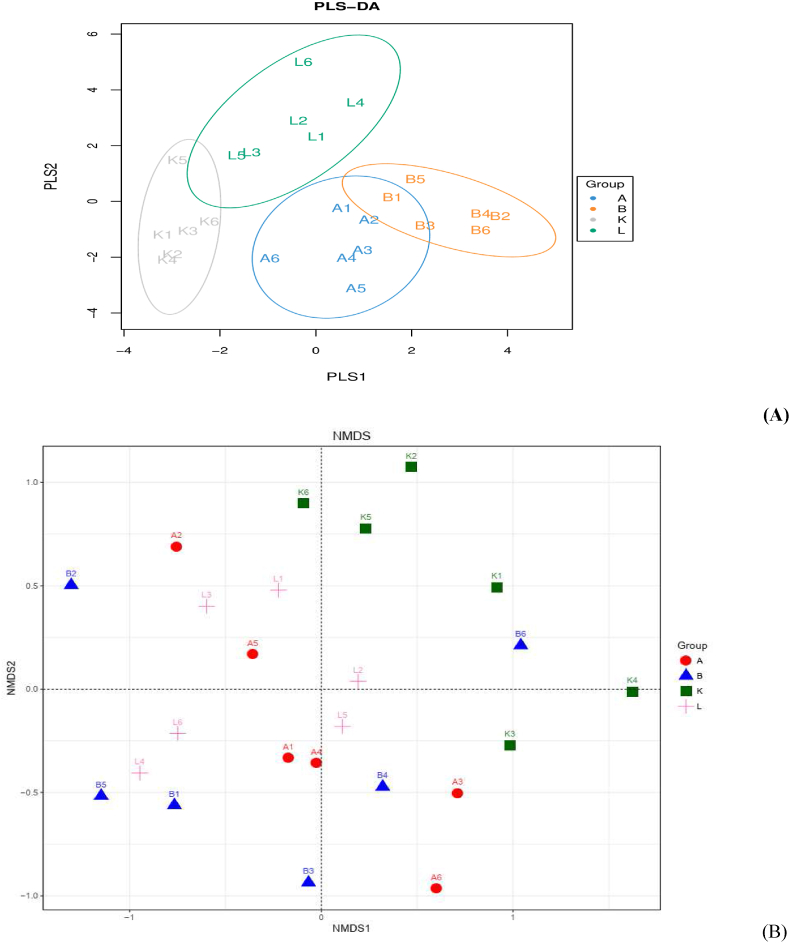
Results of alpha diversity (Fig. 3) showed that E. coli O78 infection remarkably increased Simpson index (P = 0.038), and tended to increase Shannon index (P = 0.053), while birds fed with EOA significantly increased Chao 1 and ACE indices compared with nonsupplemented groups (P < 0.05). There was a considerable interaction effect on Chao 1 and ACE indices between EOA supplementation and APEC challenge. Challenged birds given EOA had higher Chao 1 and ACE indices compared with the single challenged control. Results of alpha diversity indicated that EOA administration significantly increased richness of the intestinal microbial community, while the intestinal flora was more evenly distributed following E. coli O78 infection. Beta diversity of the cecal microbial community indicated that there was no obvious convergence among the 4 clusters of the microbial communities from the 4 groups. In addition, the clusters of the cecal microbial communities from the infected groups (K and L) were separated from those of the non-infected groups (A and B). Furthermore, the non-infected groups clustered closely together while the infected groups were highly dispersed.
Fig. 3.
Beta diversity analysis of cecal microbial community. (A) Partial least squares discriminant analysis (PLS-DA). (B) Nonmeric multidimensional scaling (NMDS). Treatment groups: A = neither infection nor essential oil and organic acid mixture (EOA) addition control, K = Escherichia coli O78 infection control, B = EOA addition but without infection, L = both EOA addition and E. coli O78 infection.
Cecal microbial composition analysis indicated that Firmicutes, Bacteroidetes and Proteobacteria dominated the cecal microbial community of chickens ( >90%) (Fig. 4). Birds fed with EOA significantly increased relative abundance of Firmicutes compared with the untreated groups, regardless of E. coli challenge. Infected birds that received EOA also notably suppressed the enrichment of Bacteroidetes compared with the single infected control (P = 0.015). However, E. coli infection alone decreased the relative population of Firmicutes (P = 0.007) relative to the negative control without EOA addition nor challenge (Fig. 4). Fig. 5 shows relative abundance of the cecal microbial composition at the genus level (Top 10). Results revealed that Faecalibacterium, Ruminococcus, Lactobacillus, Enterococcus, Oscillospira and Bilophila were the predominant genera, followed by Dorea and Coprococcus. Although there was no significant difference between the negative control group (A) and EOA group (B), the infected birds fed EOA showed a decrease in relative distribution of the genus Lactobacillus (P = 0.03) compared with the infected birds (K), whereas birds on K group remarkably enriched the relative fraction of the genus Lactobacillus and Oscillospira (Fig. 5B).
Fig. 4.
Composition of caecal microbiota of the broiler chickens among the 4 groups at phylum level. (A) Relative abundance of top 8 bacterial phyla. (B) Comparison of the relative abundances of the top 5 bacterial phyla among the 4 groups. Treatment groups: A = neither infection nor essential oil and organic acid mixture (EOA) addition control, K = Escherichia coli O78 infection control, B = EOA addition but without infection, L = both EOA addition and E. coli O78 infection.
Fig. 5.
Stacked bar chart of cecal microbial structure at the genus level. (A) Relative abundance of top 21 bacterial genera. (B) Comparison of the relative abundances of the bacterial genera which were greater than 1% among the 4 groups. Treatment groups: A = neither infection nor essential oil and organic acid mixture (EOA) addition control, K = Escherichia coli O78 infection control, B = EOA addition but without infection, L = both EOA addition and E. coli O78 infection.
LEfSe analysis was used to determine the statistically different biomarkers between groups. As presented in Fig. 6, significant differences were only observed between groups B and K.
Fig. 6.
Differential bacterial taxa in cecal microbiota of the B and K groups using LEfSe analysis (P ≤ 0.05 and LDA cutoff > 2.0) (n = 6/group). (A) Cladogram plot. (B)The histogram shows differentially abundant bacteria in the B and K groups ranked by linear discriminant analysis (LDA) scores. Treatment groups: A = neither infection nor essential oil and organic acid mixture (EOA) addition control, K = Escherichia coli O78 infection control, B = EOA addition but without infection, L = both EOA addition and E. coli O78 infection.
When compared with the B group, E. coli challenge alone (K) enriched the relative abundance of Rikenellaceae, Bacteroidales, Bacteroidia, Bacteroidetes, YS2, 4C0d_2, and Cyanobacteria, while Firmicutes, Ruminococcaceae, Clostridiales, Clostridia, Lactobacillus, Lactobacilaceae, and cc-115 were more abundant in the EOA-treated group (B) in contrast to the K group.
3.8. Predicted function of cecal microbiota
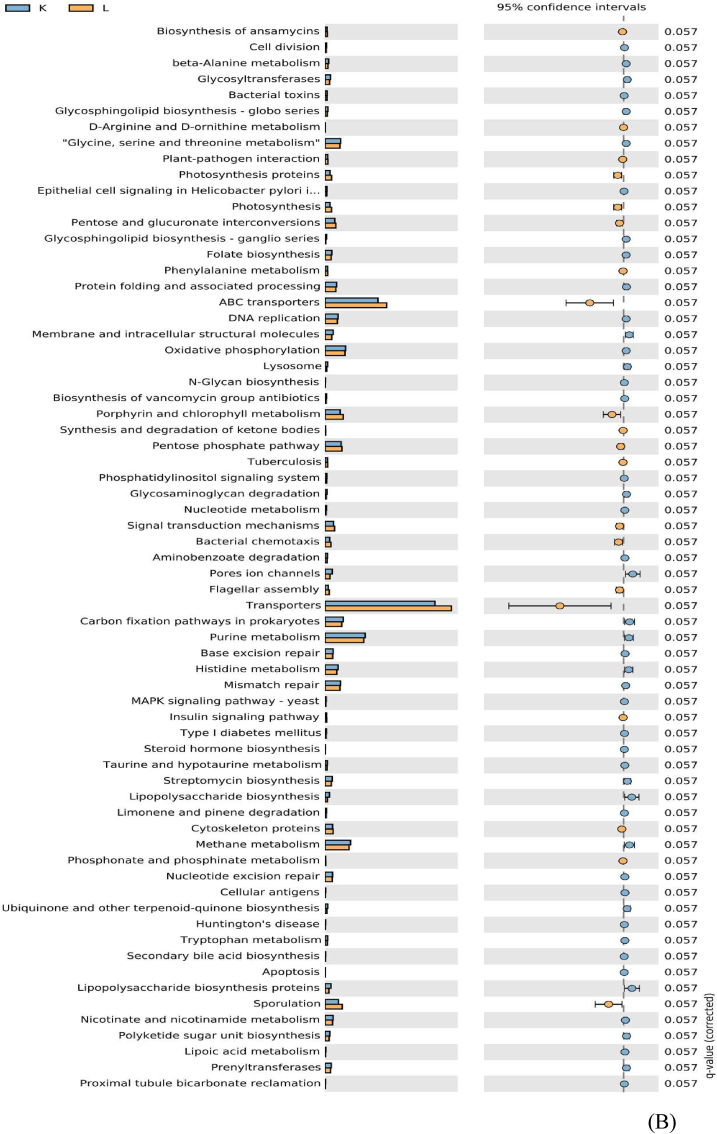
PICRUSt analysis showed significant distinctive differences between group A and K at KEGG level 2 (Fig. 7). We found that pentose and glucoronate interconversion (P = 0.022), and photosynthesis proteins (P = 0.057) were suppressed, while folate metabolism, methane metabolism, glycine, serine and threonine metabolism, MAPK signaling pathway, oxidative phosphorylation and beta-alanine metabolism were overrepresented (P = 0.057) in group K compared with the negative control A. However, there was no obvious difference between group K and L through PICRUSt analysis (P > 0.05).
Fig. 7.
Predictive functional profiles generated from 16S rRNA marker gene sequences using PICRUSt at KEGG levels 2. (A and B) Differentially regulated metabolic pathways in A vs. K group, K vs. L group, respectively. The corrected P-value is listed at the right. Treatment groups: A = neither infection nor essential oil and organic acid mixture (EOA) addition control, K = Escherichia coli O78 infection control, L = both EOA addition and E.coli O78 infection.
4. Discussion
This study evaluated the preventive efficacy of supplemental EOA on broiler chickens challenged intratracheally with E. coli O78. Results from the current study indicated that mortality, feed to gain, and gross lesion scores were remarkably increased by intra-tracheal infection of E. coli O78, which was consistent with results of previous studies (Kariyawasam et al., 2004; Horn et al., 2012), suggesting that intratracheal infection of pathogenic E. coli O78 can spread from the respiratory tract to various organs, resulting in severe systemic inflammation and negative effects on growth performance, livability and health. However, these negative impacts caused by E. coli O78 infection were attenuated by EOA supplementation. Additionally, although challenged birds given EOA showed a numerical reduction in mortality and had no improvement in body weight gain relative to the infected birds alone, EOA administration notably improved feed conversion efficiency, which was attributed to lower feed intake of challenged broiler chickens. In line with our findings, previous studies suggested that dietary essential oils and organic acid blend inclusion resulted in adverse or no effects on performance in chickens (Fascina et al., 2012, 2017), and early-weaned pigs (Manzanilla et al., 2004). Whereas other researches have reported that dietary EOA addition (containing thyme, carvacrol and butyric acid) could improve growth performance, as evidenced by an increase in average body weight gain, or improved FCR, when broiler chickens were either challenged or unchallenged with pathogens (Timbermont et al., 2010; Jerzsele et al., 2012; Abdelli et al., 2020; Stamilla et al., 2020; Stefanello et al., 2020; Pham et al., 2021). Variable results in growth performance of broiler chickens induced by EOA administration were possibly attributed to differences in chemical composition and dosage of EOA used; characteristics of infection pathogen and challenge route; birds’ health status; and the hygiene conditions of the experimental environments. However, our findings suggested that the novel EOA used in the study might elicit mild health-improving effects on broiler chickens intratracheally challenged with E. coli O78. Furthermore, this study indicated that dietary EOA supplementation improved feed efficiency of broilers and reduced the mortality after E. coli O78 challenge.
Gut morphology, goblet cell numbers, intestinal pathogen load and bacterial translocation are important indirect markers for assessing intestinal health, recovery and functionality (Kuttappan et al., 2015; Celi et al., 2017). The present study found that E. coli O78 intratracheal infection could damage gut health, as evidenced by decreased villus height and VH:CD ratio, lower goblet cell numbers, and higher E. coli carrier in the cecum and liver, which was similar to our previous observations (Huang et al., 2019). However, feeding EOA lowered E. coli counts in cecal chyme, prevented bacterial translocation and improved intestinal morphological structure regardless of E. coli O78 infection. Moreover, elevated goblet cell density was also observed in the infected birds treated with EOA relative to the single infected control. Similarly, previous studies from poultry and pig trials also indicated that supplemental EOA reduced the concentration of potential intestinal pathogens including E. coli, Salmonella and C. perfringens (Timbermont et al., 2010; Jerzsele et al., 2012; Cerisuelo et al., 2014; Basmacioğlu-Malayoğlu et al., 2016; Yang et al., 2019; Zhang et al., 2019; Abdelli et al., 2020; Stamilla et al., 2020; Stefanello et al., 2020), and attenuated gut impairment caused by pathogens (Timbermont et al., 2010; Jerzsele et al., 2012; Abdelli et al., 2020; Stamilla et al., 2020; Stefanello et al., 2020). Therefore, our results indicated that feeding EOA could mitigate gut impairment caused by E. coli O78 infection. Gut barrier-protecting effects of EOA in this study were possibly associated with the anti-bacterial capacity of EO or OA.
Serum immune parameters, such as cytokines, antibodies and complement proteins, were always used to evaluate systemic immune function or health status of the host (Lee et al., 2013). In this study, E. coli O78 infection induced a systemic inflammation response, indicated by higher levels of serum IgG and pro-inflammatory cytokines, such as IL-4, IL-6, IL-8, IL-12, IFN-γ and TNF-α. Overproduction of pro-inflammatory cytokines caused by pathogens may lead to damage in the body and gut (McKay and Baird, 1999). Higher gross lesion scores and damaged gut morphological structure caused by E. coli O78 infection were observed in this study, which supported our findings. Nevertheless, supplemental EOA significantly increased serum IgG levels and tended to decrease serum IL-12 production compared with the infected groups alone. IgG, secreted by B cells, directly contributes to an immune response by neutralizing of pathogens, toxins and viruses (Song et al., 2018). IL-12 is also involved in pro-inflammatory responses (Vignali and Kuchroo, 2014). Thus, lower pro-inflammatory cytokine IL-12 concentration and higher levels of serum IgG, accompanied by lower bacterial translocation found in the infected birds receiving EOA suggested that EOA supplementation could alleviate systemic inflammatory responses caused by E. coli O78 infection.
Tight junctions between intestinal epithelial cells form a barrier with selective permeability that not only allows nutrients, ions and solutes to be transferred but also helps to maintain the integrity of the intestinal mucosa barrier, immune hemostasis and intestinal health by preventing intestinal microorganisms, antigens and toxins from translocating into tissues (Schneeberger and Lynch, 2004; Turner, 2009; Gil-cardoso et al., 2016; Awad et al., 2017; Lee et al., 2018). Cytokines related to TLR-mediated signaling pathways and the energy status of enterocytes might all have an impact on tight junctions and intestinal epithelial barrier integrity (Capaldo and Nusrat, 2009; Lee, 2015). Pro-inflammatory cytokines have been shown to cause tight junction disruption, which results in increased intestinal permeability, whereas anti-inflammatory cytokines have been found to maintain intestinal integrity (Al-Sadi et al., 2009). In this study, we further examined the alterations in the gut mucosa TLR-mediated immune responses and tight junction proteins in E. coli-infected broiler chickens. Results indicated that E. coli O78 intratracheal infection induced intestinal inflammation and damaged intestinal barrier function, as evidenced by notable upregulation in gene expression of TLR4 and pro-inflammatory cytokines IFN-γ and chemokine IL-8, as well as downregulation in tight junction proteins Occludin and ZO-1 mRNA level in the jejunum. However, feeding EOA remarkably lowered jejunal pro-inflammatory cytokine IFN-γ abundance, but increased anti-inflammatory cytokine IL-10 mRNA levels and tended to increase ZO-1 gene expression of the E. coli-infected birds. In agreement with our findings, previous studies have demonstrated that a mixture of supplemental coated essential oils and organic acid (including thyme, carvacrol and butyric acid) could mitigate gut injury caused by oral C. perfringens infection (Timbermont et al., 2010; Jerzsele et al., 2012; Abdelli et al., 2020; Pham et al., 2020; Stamilla et al., 2020; Stefanello et al., 2020) through inhibiting intestinal inflammatory responses and improving the energy status of enterocytes. Thus, based on our results, we have suggested that dietary EOA supplementation protected against APEC-induced intestinal barrier junction injury in broilers, possibly through alleviating intestinal inflammatory responses caused by APEC.
The gut microbiota play an important role in host health, disease, feed digestion and nutrient absorption, and production of poultry (Waite and Taylor, 2015). This study revealed that E. coli challenge dramatically altered alpha diversity of the cecal microbial community, while the changes in cecal bacterial diversity induced by E. coli O78 challenge were reversed by EOA addition, suggesting that EOA supplementation could prevent intestinal microbial disturbances caused by E. coli O78 challenge. In contrast to our results, several previous studies have reported that alpha diversity was unaffected by essential oils (Zhu et al., 2019) or organic acids (Bortoluzzi et al., 2017) or an EOA mixture (Pham et al., 2020, 2021). The variability in results was possibly associated with different EOA products and challenge models. There could be a possibility that the EOA treatment effectively reduced the “bad” bacteria and promoted the “good” bacteria when broiler chickens were confronted with the pathogen challenge, but this hypothesis needs to be further evaluated. PLS-DA and NMDS analysis showed that EOA addition, E. coli O78 challenge, and both changed the beta diversity of the cecal microbial community, indicating that these treatments significantly affected the intestinal bacterial community profile. Furthermore, LEfSe analysis showed that EOA administration could improve cecal microbial composition in unchallenged birds, as evidenced by increased abundance of some beneficial bacteria, such as Ruminococcaceae, Lactobacilaceae and Lactobacillus, which was in accordance with several previous findings (Yin et al., 2017; Yang et al., 2019; Pham et al., 2020, 2021), indicating that EOA supplementation was good for gut health of broiler chickens reared under conditions without systemic infection.
Interestingly, in our study, E. coli intratracheal infection alone was observed to remarkably decrease the relative population of Firmicutes, but enrich the percentage of Lactobacillus and Oscillospira compared to the non-challenged control, while data from the infected birds fed EOA appeared to decrease cecal Bacteroidetes and Lactobacillus abundance compared with the single infected control, which was opposite to what was observed in the Lactobacillus population in the non-challenged broiler chickens supplemented with EOA. In the cecal microbiota, different trends in relative abundance of total Lactobacillus and different strains of Lactobacillus of cecal microbiota were been observed in non-challenged or challenged broiler chickens given EO, OA, or EOA from previous studies. Some authors have suggested that supplemental EO, OA or EOA could increase the proportion of Lactobacillus spp. in chickens without challenge (Abudabos and Al-Mufarrej, 2014; Yang et al., 2019; Dai et al., 2021). Other researchers found that with the appropriate dosage, EO addition could increase Lactobacillus populations, whereas a high dosage of EO inhibited the growth of Lactobacillus in birds challenged with C. perfringens, and this study also indicated that EO administration differentially modulated the abundance of different strains of Lactobacillus in challenged birds (Du et al., 2015; Yin et al., 2017). Conversely, several authors reported that supplementation with EO decreased cecal Lactobacillus abundance in the unchallenged broiler chickens (Zhu et al., 2019; Chen et al., 2020). Discrepancies in the reported results might be due to one or several factors, including the chemical composition of EO, OA, and EOA blend coating, challenged or not, challenge model, as well as time of sampling post challenge. Lactobacillus is the most abundant beneficial bacteria in the gut, which confers anti-inflammatory responses and affects gut integrity via modulating mucosal immune responses (Bermudez-Brito et al., 2012; Ashraf and Shah, 2014). Numerous studies have suggested that Oscillospira play an important role in gut health and metabolic diseases, and is both positively and negatively associated with many diseases (Konikoff and Gophna, 2016; Yang et al., 2021). Bacteroides species, the gut's most common anaerobes, have been documented to create lipopolysaccharides (LPS), impact host immunity, and inhibit pathogen colonization of the gastrointestinal tract (GIT) (Hiippala et al., 2018; Zafar and Saier, 2021). A higher proportion of Lactobacillus and Oscillospira was observed in the cecal contents of the single E. coli O78-challenged birds, suggesting that E. coli O78 infection in fact promotes the growth of intestinal benefical bacteria, resulting in an enhanced host anti-inflammatory response. However, a lower percentage of cecal Bacteroidetes and Lactobacillus which was discovered in APEC-infected broiler chickens after they were fed EOA, may be linked to the restoration of intestinal microbiota balance, reduced gut inflammation, and fewer gut lesions. As a result, the findings of this study found that EOA improved FCR and gut health in APEC-infected broiler chickens, as well as reducing gut inflammation, most likely due to favorable modification of intestinal microbiota composition. However, the correlation among gut microbiota, APEC challenge, EOA treatment and poultry gut health requires further investigation.
Most surprisingly, PICRUSt analysis revealed that only the pentose and glucoronate interconversion pathway of cecal microbiota was suppressed after E. coli O78 infection, while EOA addition had no significant influence on the function of cecal microbiota of broiler chickens regardless of E. coli O78 challenge. The pentose and glucoronate interconversion pathway have been reported to be involved in sugar utilization in microorganisms and redox status and therefore could provide nucleic acid synthesis substrates and keeps glutathione in its reducing state (Lakshmanan et al., 2021). Thus, the pentose and glucoronate interconversion pathway of cecal microbiota was suppressed after E. coli O78 infection, suggesting that E. coli O78 infection might cause oxidative stress in the gut through downregulating carbohydrate-related metabolic pathways. Whether the supplementation of EOA improves gut health through its antioxidant pathway requires further investigation. In addition, the impacts of EOA on fecal metabolite profiles should be investigated in order to understand the causal relationships between EOA, metabolites, and intestinal function.
5. Conclusion
The results of this study suggested that supplemental coated essential oil and organic acid mixture remarkably improved feed conversion efficiency, lowered gross lesion scores and cecal E. coli population, altered cecal microbiota composition, inhibited mucosal and systemic inflammation, enhanced intestinal goblet cells and serum IgG concentration along with ZO-1 gene expression of the infected birds compared with the infected groups alone. Together, the coated essential oil and organic acid mixture could be used as an antibiotic alternative to alleviate E. coli O78-induced gut injury and inflammation in the broiler industry.
Author contributions
Van Hieu Pham: Investigation, Conceptualization, Data curation, Writing - original draft, Validation. Jinyu Huang: Conceptualization, Methodology. Fangshen Guo: Conceptualization, Methodology, Writing - review & editing. Kaichen Zhang: Visualization, Investigation. Linhua Kong: Investigation. Wenrui Zhen: Methodology, Software. Waseem Abbas: Writing – review & editing. Zhong Wang: Conceptualization, Methodology, reviewing & editing, Supervision. Yuming Guo: Reviewing & editing, Supervision, Project administration.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This work was supported by a grant from Talent Plan of Zaozhuang City (2022), Shandong, China and Shanghai Menon Animal Nutrition Technology Co. Ltd., Shanghai, China. The company had no role in conducting the research, generating the data, interpreting the results, or writing the manuscript.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abdelli N., Pérez J.F., Vilarrasa E., Luna I.C., Melo-Duran D., D’angelo M., et al. Targeted-release organic acids and essential oils improve performance and digestive function in broilers under a necrotic enteritis challenge. Animals. 2020;10(2):1–31. doi: 10.3390/ani10020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Basha E.A., Gharaibeh S.M., Thabet A.M. In vitro susceptibility of resistant Escherichia coli field isolates to antimicrobial combinations. J Appl Poultry Res. 2012;21(3):595–602. [Google Scholar]
- Abudabos A.M., Al-Mufarrej S.I. Effects of organic acid supplementation on antioxidant capacity and immune responses of broilers challenged orally with Salmonella enterica subsp. enterica Typhimurium. South Afr J Anim Sci. 2014;44(4):342–349. [Google Scholar]
- Adaszyńska-Skwirzyńska M., Szczerbińska D. The antimicrobial activity of lavender essential oil (Lavandula angustifolia) and its influence on the production performance of broiler chickens. J Anim Physiol Anim Nutr. 2018;102(4):1020–1025. doi: 10.1111/jpn.12907. [DOI] [PubMed] [Google Scholar]
- Adil S., Banday T., Bhat G.A., Salahuddin M., Raquib M., Shanaz S. Response of broiler chicken to dietary supplementation of organic acids. J Cent Eur Agric. 2011;12(3):498–508. [Google Scholar]
- Al-Sadi R., Boivin M., Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci. 2009;14(7):2765–2778. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alali W.Q., Hofacre C.L., Mathis G.F., Faltys G. Effect of essential oil compound on shedding and colonization of Salmonella enterica serovar Heidelberg in broilers. Poultry Sci. 2013;92(3):836–841. doi: 10.3382/ps.2012-02783. [DOI] [PubMed] [Google Scholar]
- Antão E.M., Glodde S., Li G., Sharifi R., Homeier T., Laturnus C., et al. The chicken as a natural model for extraintestinal infections caused by avian pathogenic Escherichia coli (APEC) Microb Pathog. 2008;45(5–6):361–369. doi: 10.1016/j.micpath.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Aristimunha P.C., Rosa A.P., Boemo L.S., Garcez D.C., Rosa D.P., Londero A., et al. A blend of benzoic acid and essential oil compounds as an alternative to antibiotic growth promoters in broiler diets. J Appl Poultry Res. 2016;25(4):455–463. [Google Scholar]
- Ashraf R., Shah N.P. Immune system stimulation by probiotic microorganisms. Crit Rev Food Sci Nutr. 2014;54(7):938–956. doi: 10.1080/10408398.2011.619671. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Hess C., Hess M. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins. 2017;9(60):1–22. doi: 10.3390/toxins9020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils - a review. Food Chem Toxicol. 2008;46(2):446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Basmacioğlu-Malayoğlu H., Ozdemir P., Bağriyanik H.A. Influence of an organic acid blend and essential oil blend, individually or in combination, on growth performance, carcass parameters, apparent digestibility, intestinal microflora and intestinal morphology of broilers. Br Poultry Sci. 2016;57(2):227–234. doi: 10.1080/00071668.2016.1141171. [DOI] [PubMed] [Google Scholar]
- Bermudez-Brito M., Plaza-Díaz J., Muñoz-Quezada S., Gómez-Llorente C., Gil A. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61(2):160–174. doi: 10.1159/000342079. [DOI] [PubMed] [Google Scholar]
- Bortoluzzi C., Pedroso A.A., Mallo J.J., Puyalto M., Kim W.K., Applegate T.J. Sodium butyrate improved performance while modulating the cecal microbiota and regulating the expression of intestinal immune-related genes of broiler chickens. Poultry Sci. 2017;96(11):3981–3993. doi: 10.3382/ps/pex218. [DOI] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods - a review. Int J Food Microbiol. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Calo J.R., Crandall P.G., O'Bryan C.A., Ricke S.C. Essential oils as antimicrobials in food systems – a review. Food Control. 2015;54:111–119. [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2011;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli D., Hesp A., Van Der Goot J., Joosten P., Sarrazin S., Wagenaar J.A., et al. Antimicrobial resistance prevalence in commensal Escherichia coli from broilers, fattening turkeys, fattening pigs and veal calves in European countries and association with antimicrobial usage at country level. J Med Microbiol. 2020;69(4):537–547. doi: 10.1099/jmm.0.001176. [DOI] [PubMed] [Google Scholar]
- Celi P., Cowieson A.J., Fru-Nji F., Steinert R.E., Kluenter A.M., Verlhac V. Gastrointestinal functionality in animal nutrition and health: new opportunities for sustainable animal production. Anim Feed Sci Technol. 2017;234:88–100. [Google Scholar]
- Cerisuelo A., Marín C., Sánchez-Vizcaíno F., Gómez E.A., De La Fuente J.M., Durán R., et al. The impact of a specific blend of essential oil components and sodium butyrate in feed on growth performance and Salmonella counts in experimentally challenged broilers. Am Hist Rev. 2014;119(2):599–606. doi: 10.3382/ps.2013-03528. [DOI] [PubMed] [Google Scholar]
- Chaveerach P., Keuzenkamp D.A., Lipman L.J.A., Van Knapen F. Effect of organic acids in drinking water for young broilers on Campylobacter infection, volatile fatty acid production, gut microflora and histological cell changes. Poultry Sci. 2004;83(3):330–334. doi: 10.1093/ps/83.3.330. [DOI] [PubMed] [Google Scholar]
- Chen X., Zhang W., Yin J., Zhang N., Geng S., Zhou X., et al. Escherichia coli isolates from sick chickens in China: changes in antimicrobial resistance between 1993 and 2013. Vet J. 2014;202(1):112–115. doi: 10.1016/j.tvjl.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Chen Y., Wang J., Yu L., Xu T., Zhu N. Microbiota and metabolome responses in the cecum and serum of broiler chickens fed with plant essential oils or virginiamycin. Sci Rep. 2020;10:5382. doi: 10.1038/s41598-020-60135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Yang F., Lu H., Wang B., Chen Y., Lei D., et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54(2):562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- Chowdhury S., Mandal G.P., Patra A.K. Different essential oils in diets of chickens: 1. Growth performance, nutrient utilisation, nitrogen excretion, carcass traits and chemical composition of meat. Anim Feed Sci Technol. 2018;236:86–97. [Google Scholar]
- Collingwood C., Kemmett K., Williams N., Wigley P. Is the concept of avian pathogenic Escherichia coli as a single pathotype fundamentally flawed? Front Vet Sci. 2014;1(5):1–4. doi: 10.3389/fvets.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoni G., Woodward M.J., Wu H., Alanazi M., Wallis T., Ragione RM La. Comparative genomics of European avian pathogenic Escherichia coli (APEC) BMC Genom. 2016;17:1–21. doi: 10.1186/s12864-016-3289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross D.E., McDevitt R.M., Hillman K., Acamovic T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br Poultry Sci. 2007;48(4):496–506. doi: 10.1080/00071660701463221. [DOI] [PubMed] [Google Scholar]
- Dai D., Qiu K., Zhang H., Wu S., Han Y., Wu Y., et al. Organic acids as alternatives for antibiotic growth promoters alter the intestinal structure and microbiota and improve the growth performance in broilers. Front Microbiol. 2021;11:1–14. doi: 10.3389/fmicb.2020.618144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dho-moulin M., Fairbrother J.M., Dho-moulin M., Morris J., Avian F., Morris J. Avian pathogenic Escherichia coli (APEC) Vet Res. 1999;30:299–316. [PubMed] [Google Scholar]
- Dittoe D.K., Ricke S.C., Kiess A.S. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front Vet Sci. 2018;5:1–12. doi: 10.3389/fvets.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do T.K.T., Hadji-Minaglou F., Antoniotti S., Fernandez X. Authenticity of essential oils. Trends Anal Chem. 2015;66:146–157. [Google Scholar]
- Du E., Gan L., Li Z., Wang W., Liu D., Guo Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J Anim Sci Biotechnol. 2015;6(58):1–12. doi: 10.1186/s40104-015-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du E., Wang W., Gan L., Li Z., Guo S., Guo Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J Anim Sci Biotechnol. 2016;7:19. doi: 10.1186/s40104-016-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziva F., Stevens M.P. Colibacillosis in poultry: unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol. 2008;37(4):355–366. doi: 10.1080/03079450802216652. [DOI] [PubMed] [Google Scholar]
- El-Shall N.A., Shewita R.S., Abd El-Hack M.E., AlKahtane A., Alarifi S., Alkahtani S., et al. Effect of essential oils on the immune response to some viral vaccines in broiler chickens, with special reference to Newcastle disease virus. Poultry Sci. 2020;99:2944–2954. doi: 10.1016/j.psj.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fascina V.B., Pasquali G.A.M., Carvalho F.B., Muro E.M., Vercese F., Aoyagi M.M., et al. Effects of phytogenic additives and organic acids, alone or in combination, on the performance, intestinal quality and immune responses of broiler chickens. Brazilian J Poult Sci. 2017;19(3):497–508. [Google Scholar]
- Fascina V.B., Sartori J.R., Gonzales E., De F.B., Mailinch I., Pereira G., et al. Phytogenic additives and organic acids in broiler chicken diets. Rev Bras Zootec. 2012;41(10):2189–2197. [Google Scholar]
- Friedman M., Henika P.R., Levin C.E., Mandrell R.E. Antibacterial activities of plant essential oils and their components against Escherichia coli O157:H7 and Salmonella enterica in apple juice. J Agric Food Chem. 2004;52(19):6042–6048. doi: 10.1021/jf0495340. [DOI] [PubMed] [Google Scholar]
- Friedman M., Henika P.R., Mandrell R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Protect. 2002;65(10):1545–1560. doi: 10.4315/0362-028x-65.10.1545. [DOI] [PubMed] [Google Scholar]
- Gharib Naseri K., Rahimi S., Khaki P. Comparison of the effects of probiotic, organic acid and medicinal plant on Campylobacter jejuni challenged broiler chickens. J Agric Sci Technol. 2012;14(SUPPL):1485–1496. [Google Scholar]
- Giannenas I., Papaneophytou C.P., Tsalie E., Pappas I., Triantafillou E., Tontis D., et al. Dietary supplementation of benzoic acid and essential oil compounds affects buffering capacity of the feeds, performance of Turkey poults and their antioxidant status, pH in the digestive tract, intestinal microbiota and morphology. AJAS (Asian-Australas J Anim Sci) 2014;27(2):225–236. doi: 10.5713/ajas.2013.13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs P.S., Petermann S.R., Wooley R.E. Comparison of several challenge models for studies in avian colibacillosis. Avian Dis. 2004;48(4):751–758. doi: 10.1637/7176-030404R. [DOI] [PubMed] [Google Scholar]
- Gil-cardoso K., Ginés I., Ardévol A., Blay M., Terra X. Effects of flavonoids on intestinal in flammation , barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr Res Rev. 2016;29:234–248. doi: 10.1017/S0954422416000159. [DOI] [PubMed] [Google Scholar]
- Gordillo Jaramillo F.X., Kim D.H., Lee S.H., Kwon S.K., Jha R., Lee K.W. Role of oregano and Citrus species-based essential oil preparation for the control of coccidiosis in broiler chickens. J Anim Sci Biotechnol. 2021;12(1):1–9. doi: 10.1186/s40104-021-00569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemipour H., Khaksar V., Rubio L.A., Veldkamp T., van Krimpen M.M. Effect of feed supplementation with a thymol plus carvacrol mixture, in combination or not with an NSP-degrading enzyme, on productive and physiological parameters of broilers fed on wheat-based diets. Anim Feed Sci Technol. 2015;211:117–131. [Google Scholar]
- Hiippala K., Jouhten H., Ronkainen A., Hartikainen A., Kainulainen V., Jalanka J., et al. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients. 2018;10(988):1–23. doi: 10.3390/nu10080988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn F., Corrêa A.M.R., Barbieri N.L., Glodde S., Weyrauch K.D., Kaspers B., et al. Infections with avian pathogenic and fecal Escherichia coli strains display similar lung histopathology and macrophage apoptosis. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0041031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Luo L., Zhang Y., Wang Z., Xia Z. Effects of the dietary probiotic, enterococcus faecium NCIMB 11181, on the intestinal barrier and system immune status in Escherichia coli O78-challenged broiler chickens. Probiotics Antimicrob Proteins. 2019;11(3):946–956. doi: 10.1007/s12602-018-9434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Immerseel F., Russell J.B., Flythe M.D., Gantois I., Timbermont L., Pasmans F., et al. The use of organic acids to combat Salmonella in poultry: a mechanistic explanation of the efficacy. Avian Pathol. 2006;35(3):182–188. doi: 10.1080/03079450600711045. [DOI] [PubMed] [Google Scholar]
- Jerzsele A., Szeker K., Csizinszky R., Gere E., Jakab C., Mallo J.J., et al. Efficacy of protected sodium butyrate, a protected blend of essential oils, their combination, and Bacillus amyloliquefaciens spore suspension against artificially induced necrotic enteritis in broilers. Poultry Sci. 2012;91(4):837–843. doi: 10.3382/ps.2011-01853. [DOI] [PubMed] [Google Scholar]
- Kariyawasam S., Wilkie B.N., Gyles C.L. Resistance of broiler chickens to Escherichia coli respiratory tract infection induced by passively transferred egg-yolk antibodies. Vet Microbiol. 2004;98(3–4):273–284. doi: 10.1016/j.vetmic.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Kemmett K., Williams N.J., Chaloner G., Humphrey S., Wigley P., Humphrey T. The contribution of systemic Escherichia coli infection to the early mortalities of commercial broiler chickens. Avian Pathol. 2014;43(1):37–42. doi: 10.1080/03079457.2013.866213. [DOI] [PubMed] [Google Scholar]
- Khattak F., Ronchi A., Castelli P., Sparks N. Effects of natural blend of essential oil on growth performance, blood biochemistry, cecal morphology, and carcass quality of broiler chickens. Poultry Sci. 2014;93(1):132–137. doi: 10.3382/ps.2013-03387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y Bin, Yoon M.Y., Ha J.S., Seo K.W., Noh E.B., Son S.H., et al. Molecular characterization of avian pathogenic Escherichia coli from broiler chickens with colibacillosis. Poultry Sci. 2020;99(2):1088–1095. doi: 10.1016/j.psj.2019.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konikoff T., Gophna U. Oscillospira: a central, enigmatic component of the human gut microbiota. Trends Microbiol. 2016;24(7):523–524. doi: 10.1016/j.tim.2016.02.015. [DOI] [PubMed] [Google Scholar]
- Vignali D.A.A., Kuchroo V.K. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2014;13(8):722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttappan V.A., Berghman L.R., Vicuña E.A., Latorre J.D., Menconi A., Wolchok J.D., et al. Poultry enteric inflammation model with dextran sodium sulfate mediated chemical induction and feed restriction in broilers. Poultry Sci. 2015;94(6):1220–1226. doi: 10.3382/ps/pev114. [DOI] [PubMed] [Google Scholar]
- Lakshmanan A.P., Al Za’abi M., Ali B.H., Terranegra A. The influence of the prebiotic gum acacia on the intestinal microbiome composition in rats with experimental chronic kidney disease. Biomed Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110992. [DOI] [PubMed] [Google Scholar]
- Langille M.G.I., Zaneveld J., Caporaso J.G., McDonald D., Knights D., Reyes J.A., et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31(9):814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Moon K.M., Kim C.Y. Tight junction in the intestinal epithelium: its association with diseases and regulation by phytochemicals. J Immunol Res. 2018;2018:1–11. doi: 10.1155/2018/2645465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H. Intestinal permeability regulation by tight junction : implication on inflammatory bowel diseases. Int Res. 2015;13(1):11–18. doi: 10.5217/ir.2015.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Lillehoj H.S., Jang S.I., Lillehoj E.P., Min W., Bravo D.M. Dietary supplementation of young broiler chickens with capsicum and turmeric oleoresins increases resistance to necrotic enteritis. Br J Nutr. 2013;110(5):840–847. doi: 10.1017/S0007114512006083. [DOI] [PubMed] [Google Scholar]
- Liu S.D., Song M.H., Yun W., Lee J.H., Lee C.H., Kwak W.G., et al. Effects of oral administration of different dosages of carvacrol essential oils on intestinal barrier function in broilers. J Anim Physiol Anim Nutr. 2018;102(5):1257–1265. doi: 10.1111/jpn.12944. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopez-Romero J.C., González-Ríos H., Borges A., Simões M. Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus. Evid base Compl Alternative Med. 2015;(795435):1–9. doi: 10.1155/2015/795435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C.A., Hamady M., Kelley S.T., Knight R. Quantitative and qualitative β diversity measures lead to different insights into factors that structure microbial communities. Appl Environ Microbiol. 2007;73(5):1576–1585. doi: 10.1128/AEM.01996-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T., Marmion M., Ferone M., Wall P., Scannell A.G.M. On farm interventions to minimise Campylobacter spp. contamination in chicken. Br Poultry Sci. 2021;62(1):53–67. doi: 10.1080/00071668.2020.1813253. [DOI] [PubMed] [Google Scholar]
- Manzanilla E.G., Perez J.F., Martin M., Kamel C., Baucells F., Gasa J. Effect of plant extracts and formic acid on the intestinal equilibrium of early-weaned pigs. J Anim Sci. 2004;82(11):3210–3218. doi: 10.2527/2004.82113210x. [DOI] [PubMed] [Google Scholar]
- Mathlouthi N., Bouzaienne T., Oueslati I., Recoquillay F., Hamdi M., Urdaci M., et al. Use of rosemary, oregano, and a commercial blend of essential oils in broiler chickens: in vitro antimicrobial activities and effects on growth performance. J Anim Sci. 2012;90(3):813–823. doi: 10.2527/jas.2010-3646. [DOI] [PubMed] [Google Scholar]
- McKay D.M., Baird A.W. Cytokine regulation of epithelial permeability and ion transport. Gut. 1999;44(2):283–289. doi: 10.1136/gut.44.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPeake S.J.W., Smyth J.A., Ball H.J. Characterisation of avian pathogenic Escherichia coli (APEC) associated with colisepticaemia compared to faecal isolates from healthy birds. Vet Microbiol. 2005;110(3–4):245–253. doi: 10.1016/j.vetmic.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Mitsch P., Zitterl-Eglseer K., Köhler B., Gabler C., Losa R., Zimpernik I. The effect of two different blends of essential oil components on the proliferation of Clostridium perfringens in the intestines of broiler chickens. Poultry Sci. 2004;83(4):669–675. doi: 10.1093/ps/83.4.669. [DOI] [PubMed] [Google Scholar]
- Mohiti-Asli M., Ghanaatparast-Rashti M. Dietary oregano essential oil alleviates experimentally induced coccidiosis in broilers. Prev Vet Med. 2015;120(2):195–202. doi: 10.1016/j.prevetmed.2015.03.014. [DOI] [PubMed] [Google Scholar]
- National Research Council . Nutrient Requirements of Poultry. THE NATIONAL ACADEMIES PRESS; Washington, DC: 1994. pp. 1–176. [Google Scholar]
- Nava G.M., Attene-ramos M.S., Gaskins H.R., Richards J.D. Molecular analysis of microbial community structure in the chicken ileum following organic acid supplementation. Vet Microbiol. 2009;137:345–353. doi: 10.1016/j.vetmic.2009.01.037. [DOI] [PubMed] [Google Scholar]
- Capaldo C.T., Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009;1788(4):864–871. doi: 10.1016/j.bbamem.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peighambari S.M., Julian R.J., Gyles C.L. Experimental Escherichia coli respiratory infection in broilers. Avian Dis. 2000;44(4):759–769. [PubMed] [Google Scholar]
- Peng Q.Y., Li J.D., Li Z., Duan Z.Y., Wu Y.P. Effects of dietary supplementation with oregano essential oil on growth performance, carcass traits and jejunal morphology in broiler chickens. Anim Feed Sci Technol. 2016;214:148–153. [Google Scholar]
- Pham V.H., Abbas W., Huang J., He Q., Zhen W., Guo Y., et al. Effect of blending encapsulated essential oils and organic acids as an antibiotic growth promoter alternative on growth performance and intestinal health in broilers with necrotic enteritis. Poultry Sci. 2021;101(1) doi: 10.1016/j.psj.2021.101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham V.H., Kan L., Huang J., Geng Y., Zhen W., Guo Y., et al. Dietary encapsulated essential oils and organic acids mixture improves gut health in broiler chickens challenged with necrotic enteritis. J Anim Sci Biotechnol. 2020;11(1):1–18. doi: 10.1186/s40104-019-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirgozliev V., Bravo D., Mirza M.W., Rose S.P. Growth performance and endogenous losses of broilers fed wheat-based diets with and without essential oils and xylanase supplementation. Poultry Sci. 2015;94(6):1227–1232. doi: 10.3382/ps/peu017. [DOI] [PubMed] [Google Scholar]
- Ramette A. Multivariate analyses in microbial ecology. FEMS Microbiol Ecol. 2007;62(2):142–160. doi: 10.1111/j.1574-6941.2007.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger E.E., Lynch R.D. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Guo Y., Wang Z. β-1,3/1,6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella enterica serovar Typhimurium. Poultry Sci. 2013;92(7):1764–1773. doi: 10.3382/ps.2013-03029. [DOI] [PubMed] [Google Scholar]
- Song Z.H., Cheng K., Zheng X.C., Ahmad H., Zhang L.L., Wang T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poultry Sci. 2018;97(2):430–437. doi: 10.3382/ps/pex312. [DOI] [PubMed] [Google Scholar]
- Stamilla A., Messina A., Sallemi S., Condorelli L., Antoci F., Puleio R., et al. Effects of microencapsulated blends of organics acids (OA) and essential oils (EO) as a feed additive for broiler chicken. A focus on growth performance, gut morphology and microbiology. Animals. 2020;10:442–459. doi: 10.3390/ani10030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanello C., Rosa D.P., Dalmoro Y.K., Segatto A.L., Vieira M.S., Moraes M.L., et al. Protected blend of organic acids and essential oils improves growth performance, nutrient digestibility, and intestinal health of broiler chickens undergoing an intestinal challenge. Front Vet Sci. 2020;6:1–10. doi: 10.3389/fvets.2019.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swelum A.A., Elbestawy A.R., El-Saadony M.T., Hussein E.O.S., Alhotan R., Suliman G.M., et al. Ways to minimize bacterial infections, with special reference to Escherichia coli, to cope with the first-week mortality in chicks: an updated overview. Poultry Sci. 2021;100(5) doi: 10.1016/j.psj.2021.101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbermont L., Lanckriet A., Dewulf J., Nollet N., Schwarzer K., Haesebrouck F., et al. Control of Clostridium perfringens-induced necrotic enteritis in broilers by target-released butyric acid, fatty acids and essential oils. Avian Pathol. 2010;39(2):117–121. doi: 10.1080/03079451003610586. [DOI] [PubMed] [Google Scholar]
- Turner J.R. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Waite D.W., Taylor M.W. Exploring the avian gut microbiota: current trends and future directions. Front Microbiol. 2015;6:1–12. doi: 10.3389/fmicb.2015.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Liang S., Li X., Yang X., Long F., Yang X. Effects of encapsulated essential oils and organic acids on laying performance, egg quality, intestinal morphology, barrier function, and microflora count of hens during the early laying period. Poultry Sci. 2019;98(12):6751–6760. doi: 10.3382/ps/pez391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Shao Y., Song B., Zhen W., Wang Z., Guo Y., et al. Effects of Bacillus coagulans supplementation on the growth performance and gut health of broiler chickens with Clostridium perfringens-induced necrotic enteritis. J Anim Sci Biotechnol. 2018;9(1):1–14. doi: 10.1186/s40104-017-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Chowdhury M.A.K., Hou Y., Gong J. Phytogenic compounds as alternatives to in-feed antibiotics: potentials and challenges in application. Pathogens. 2015;4(1):137–156. doi: 10.3390/pathogens4010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Li Y., Wen Z., Liu W., Meng L., Huang H. Oscillospira - a candidate for the next-generation probiotics. Gut Microb. 2021;13(1) doi: 10.1080/19490976.2021.1987783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Liu Y., Yan F., Yang C., Yang X. Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens. Poultry Sci. 2019;98(7):2858–2865. doi: 10.3382/ps/pez031. [DOI] [PubMed] [Google Scholar]
- Yin D., Du E., Yuan J., Gao J., Wang Y.L., Aggrey S.E., et al. Supplemental thymol and carvacrol increases ileum Lactobacillus population and reduces effect of necrotic enteritis caused by Clostridium perfringes in chickens. Sci Rep. 2017;7:1–11. doi: 10.1038/s41598-017-07420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar H., Saier M.H. Gut Bacteroides species in health and disease. Gut Microb. 2021;13(1):1–20. doi: 10.1080/19490976.2020.1848158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaura E., Keijser B.J.F., Huse S.M., Crielaard W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009;9(259):1–12. doi: 10.1186/1471-2180-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z., Zhang S., Wang H., Piao X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: a review. J Anim Sci Biotechnol. 2015;6(7):1–10. doi: 10.1186/s40104-015-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Li W., Liu W., Zou L., Yan C., Lu K., et al. T4-like phage bp7, a potential antimicrobial agent for controlling drug-resistant escherichia coli in chickens. Appl Environ Microbiol. 2013;79(18):5559–5565. doi: 10.1128/AEM.01505-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Shen Y.R., Wu S., Xiao Y.Q., He Q., Shi S.R. The dietary combination of essential oils and organic acids reduces Salmonella enteritidis in challenged chicks. Poultry Sci. 2019;98(12):6349–6355. doi: 10.3382/ps/pez457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Wang C.G., Lv J.C., Wang R.S., Zhong X.H. Survey on tetracycline resistance and antibiotic-resistant genotype of avian Escherichia coli in North China. Poultry Sci. 2012;91(11):2774–2777. doi: 10.3382/ps.2012-02453. [DOI] [PubMed] [Google Scholar]
- Zhu N., Wang J., Yu L., Zhang Q., Chen K., Liu B. Modulation of growth performance and intestinal microbiota in chickens fed plant extracts or virginiamycin. Front Microbiol. 2019;10(1333):1–16. doi: 10.3389/fmicb.2019.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Q.Y., Wang S.C., Li J.P., Liu D., Liu S., Jiang W.M., et al. A clinical survey of common avian infectious diseases in China. Avian Dis. 2014;58(2):297–302. doi: 10.1637/10709-110113-ResNote.1. [DOI] [PubMed] [Google Scholar]