Abstract
Pulmonary arterial hypertension (PAH) is a progressive disease with no cure. Healthcare resource utilization (HCRU; hospitalization, outpatient visits, and drug utilization) before diagnosis and productivity loss (sick leave and disability pension) before and after PAH diagnosis are not well known. By linking several Swedish national databases, this study have estimated the societal costs in a national PAH cohort (n = 749, diagnosed with PAH in 2008−2019) 5 years before and 5 years after diagnosis and compared to an age, sex, and geographically matched control group (n = 3745, 1:5 match). HCRU and productivity loss were estimated per patient per year.
The PAH group had significantly higher HCRU and productivity loss compared to the control group starting already 3 and 5 years before diagnosis, respectively. HCRU peaked the year after diagnosis in the PAH group with hospitalizations (mean ± standard deviation; 2.0 ± 0.1 vs. 0.2 ± 0.0), outpatient visits (5.3 ± 0.3 vs. 0.9 ± 0.1), and days on sick leave (130 ± 10 vs. 13 ± 1) significantly higher compared to controls. Total costs during the entire 10‐year period were six times higher for the PAH group than the control group. In the 5 years before diagnosis the higher costs were driven by productivity loss (76%) and hospitalizations (15%), while the 5 years after diagnosis the main cost drivers were drugs (63%), hospitalizations (16%), and productivity loss (16%). In conclusion, PAH was associated with large societal costs due to high HCRU and productivity loss, starting several years before diagnosis. The economic and clinical burden of PAH suggests that strategies for earlier diagnosis and more effective treatments are warranted.
Keywords: healthcare resource utilization, national registry, productivity loss, pulmonary hypertension
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a rare and serious disease affecting all ages and both sexes. 1 The increased pulmonary pressure and resistance endured by the disease, lead to increased workload for the right ventricle and subsequent right ventricular failure and premature death. 1 Early symptoms include shortness of breath, exhaustion at rest or light exercise, fatigue, and, in later stages, chest pain and syncope. 1 The life expectancy is compromised with only 51%−55% surviving 5 years after diagnosis. 2 , 3 This, together with a high symptom burden, will affect the patient with PAH as well as their next of kin. 1 , 4 In Sweden, the incidence and prevalence of PAH are 5−7 and 40−50 patients per million inhabitants, respectively. 3 , 5
Due to the nonspecific symptoms and the progressive nature of the disease, patients with PAH have frequent contact with primary and specialist care both before, and after diagnosis. 1 , 6 , 7 , 8 This, together with disease targeted medical treatment, makes the economic burden of PAH substantial. 9 , 10 , 11 , 12 Estimations of the societal economic burden of PAH vary due to differences in data sources, selection of subjects, and cost components as well as changes in available treatments and treatment strategies over time. 12 Studies investigating indirect costs of PAH before and after diagnosis are scarce. However, previous studies have shown productivity loss regarding employment and sick leave after diagnosis of PAH 13 , 14 and that hospitalizations are a substantial driver of costs. 10 , 11 , 15 , 16 , 17
The aim of this study was to estimate the societal costs of PAH in Sweden, based on associated healthcare resource utilization (HCRU) and productivity loss 5 years before and 5 years after diagnosis compared to a matched control group. In Sweden, nationwide healthcare registries collect data from all citizens from birth to death, allowing for a comprehensive understanding of societal costs related to PAH.
MATERIALS AND METHODS
This was a retrospective registry‐based case‐control study including all adult (≥18 years) patients diagnosed with PAH (n = 759) registered in The Swedish PAH & CTEPH registry (SPAHR) between January 1, 2008 and June 30, 2019. SPAHR is a national quality registry that includes >90% of all patients diagnosed with PAH in Sweden. 3 All patients in SPAHR were informed locally about their participation in the registry and had the right to decline.
A control group (n = 3795, five controls per patient) was selected from the national population registry by Statistics Sweden. The control group were individuals without a PAH diagnosis and matched based on birth year (age), sex, and place of residence (municipality) at the date of the patients' PAH diagnosis (index date).
The study was approved by the Swedish Ethical Review Authority (Dnr 2020‐02573).
Societal setting and data sources
In Sweden, the healthcare system is mainly publicly funded, and the state, government, and municipalities share the responsibility for supplying equal healthcare to all citizens. 18 All employed individuals in Sweden are eligible for sick leave benefits from the Swedish Social Insurance Agency to cover any loss of income due to illness, disease, or disability. 19 The sick leave benefit can be combined with part‐time disability pension. The national pension system is mandatory for all citizens and constitutes the main source of an individual's age pension. 20
The study consists of individual data merged from several Swedish national registries. All data were anonymized by the national registries before delivery to the authors. Data were extracted 5 years before and 5 years after the index date for all study participants:
SPAHR 3 provided clinical data for the PAH group including date of PAH diagnosis.
Statistics Sweden's longitudinal integration database for health insurance and labor market studies (LISA) 21 provided socioeconomic data on employment status, education attainment, income level, and date of death. The Swedish Social Insurance Agency with the microdata on sickness‐ and activity compensation registry (MiDAS) 22 provided data on sick leave and disability pension.
The National Board of Health and Welfare provided data on HCRU from the National Patient Register 23 on outpatient visits and hospitalizations, and from the National Prescribed Drug Register 24 on prescription drug utilization. Outpatient visits included physician visits in hospital but not in primary care. Data on healthcare consumption and drug utilization were limited to predefined, related ICD‐10‐SE and ATC codes (Supporting Information: Tables S1 and S2). Drug utilization was measured as defined daily dose (DDD) of dispensed drugs. Comorbidities were based on ICD‐10‐SE codes in the National Patient Register 5 years before index date.
With the exception for drug utilization that was only available from 2005, all variables were available from 2003 (Supporting Information: Figure S1). Data on socioeconomics, in‐ and outpatient care, drug utilization, and cause of death were available through 2019 while data about date of death, sick leave, and disability pension were available through 2020.
Data management
Study participants were followed for 5 years after index date or until censoring at the date of last contact (time of death or loss to follow‐up), whichever occurred first. Annual mean HCRU (hospitalizations, outpatient visits, drug utilization) per patient included uncensored and living patients present at the beginning of each year. Total mean costs were calculated over the total 5 years pre‐ and 5 years post‐index date.
Direct costs related to in‐ and outpatient‐care were calculated using diagnosis related groups (DRG), a patient classification scheme for healthcare contacts providing a means of relating treatment of the groups to the costs incurred. 25 Each visit was attributed a DRG based cost, without adjusting for extreme outliers. Drug costs were based on pharmacy listing sales prices.
Indirect costs were restricted to productivity loss and estimated using the human capital approach. 26 Thus, the time spent absent from work, manifested as sick leave and disability pension, was valued as the mean gross salary plus payroll taxes per day in Sweden. The analysis of participants receiving disability pension included only individuals with employment, excluding individuals with age pension as the main source of income. The analysis of participants on sick leave further excluded participants with full time disability pension.
Total costs were adjusted for censoring using the Zhao and Tian censoring estimator. 27 The estimator handles censoring in the data set by weighting uncensored costs by the likelihood of being censored, that is, increased weight in parallel with risk of being censored. In addition, differences in costs between censored and uncensored individuals are adjusted. Costs were adjusted to 2020 prices using the consumer price index 28 and converted from SEK to EUR using the average exchange rate in 2020 (1 EUR = 10.4867 SEK). 29
Statistical methods
Participant characteristics at time of index date are shown as mean and standard deviation (SD) or median and interquartile range (IQR) for continuous variables, and frequency (n) and proportions (%) for categorical variables. Differences between the PAH and the control group were tested using Student's t‐tests, Mann−Whitney U tests, and χ 2 tests. p < 0.05 were considered significant. Microsoft Excel 365:2201, SPSS 28, and RStudio 2021.9.2.382 were used for all analyses. Overall survival was analyzed using Kaplan−Meier estimates with follow‐up from index date and censoring at the date of last contact or death.
RESULTS
Of 759 available patients with PAH (PAH group), 10 patients were excluded due to missing data leaving 749 patients with PAH and 3745 matched controls (control group) for analyses.
Baseline characteristics
The mean (SD) age of the study population was 62 (16) years and 64% were female (Table 1). The level of education was lower in the PAH group, mainly due to less individuals attending university than in the control group. In both groups, the main source of income was age pension (Table 1). The proportion with disability pension and sickness benefits was higher, and the proportion with income from employment was lower in the PAH group compared to the control group. The disposable income was lower in the PAH group.
Table 1.
Characteristics at index date for the PAH group and the control group
| PAH (n = 749) | Control (n = 3745) | P Value | |
|---|---|---|---|
| Age mean years (SD) | 62 (16) | 62 (16) | 0.984 |
| Sex female | 64% | 64% | 1.00 |
| Highest education level attained | <0.001 | ||
| Primary school | 30% | 27% | |
| Secondary school | 50% | 46% | |
| University (minimum 2 years) | 21% | 27% | |
| Missing values (n) | 77 | 69 | |
| Main source of incomea | <0.001 | ||
| Work | 15% | 34% | |
| Disability pension | 15% | 3% | |
| Age pension | 55% | 56% | |
| Sickness benefit | 7% | 1% | |
| Otherb | 8% | 6% | |
| Missing values (n) | 55 | 25 | |
| Disposable annual income, median (IQR), SEK | 164,404 (95,912) | 187,347 (137,021) | <0.001 |
| Disposable annual family income, median (IQR), SEK | 278,392 (255,181) | 318,886 (318,471) | <0.001 |
| Cohabitation | 50% | 55% | 0.024 |
| Proportion with children living at home | 17% | 23% | 0.001 |
| Comorbidities present at any time during the 5‐years before index date (ICD‐10c) | |||
| Essential (primary) hypertension (I10) | 31% | 13% | <0.001 |
| Diabetes (E10‐E14) | 15% | 5% | <0.001 |
| Atrial fibrillation and flutter (I48) | 13% | 4% | <0.001 |
| Stroke (I61, I63, I64) | 2% | 1% | 0.382 |
| Ischemic heart diseases (I20−I25) | 12% | 5% | <0.001 |
| Disorders of thyroid gland (E00−E07) | 9% | 3% | <0.001 |
| Acute kidney failure and chronic kidney disease (N17−N19) | 6% | 1% | <0.001 |
| Dialysis (Z49) | 0.4% | 0.1% | 0.114 |
Note: Data presented as mean (SD), proportion (%), or number (n).
Abbreviations: IQR, interquartile range; PAH, pulmonary arterial hypertension; SD, standard deviation.
During the (calendar) year of diagnosis.
Other: Student grants, reimbursement due to care of family member, unemployment benefits, unemployment program/training reimbursement, social security benefits, no known income.
ICD‐10‐SE, the National Board of Health and Welfare classifications of diseases. 30
Comorbidities were more common in the PAH group than in the control group; hypertension and ischemic heart disease were twice as common, diabetes three times as common, and kidney disease six times as common (Table 1).
Characteristics for the PAH group that specifically relate to the PAH diagnosis are shown in Table 2.
Table 2.
Characteristics of the PAH group (n = 749) at time of diagnosis
| PAH etiology | |
| Idiopathic PAH/hereditary PAH | 49% |
| Associated PAH‐connective tissue disease | 32% |
| Associated PAH‐congenital heart disease | 11% |
| Associated PAH‐other | 9% |
| Hemodynamic measurements, mean (SD) | |
| Mean pulmonary artery pressure (mmHg) | 46 (14) |
| Right atrial pressure (mmHg) | 8 (5) |
| Pulmonary artery wedge pressure (mmHg) | 9 (4) |
| Cardiac index (L/min/m)2 | 2.4 (0.8) |
| Pulmonary vascular resistance (Wood units) | 9.2 (4.7) |
| Echocardiography, mean (SD) | |
| Right atrial area (cm)2 | 24 (8) |
| Pericardial effusion (%) | 1.9 (0.3) |
| Clinical status | |
| Body mass index (kg/m)2, mean (SD) | 26 (5) |
| WHO functional class, I/II/III/IV (%) | 2/18/71/10 |
| Six‐min walk distance (m), mean (SD) | 299 (138) |
Note: Data presented as proportion (%) or mean (SD).
Abbreviations: PAH, pulmonary arterial hypertension; SD, standard deviation; WHO, world health organization.
HCRU
The mean number of hospitalizations per person and year were five times higher in the PAH group than in the control group over the whole study period (Figure 1a). Two years before the index date the mean number of hospitalizations were three times higher in the PAH group than in the control group (0.5 vs. 0.1 visits, p < 0.001), the year before the index date it was nine times higher (1.7 vs. 0.2 visits, p < 0.001), and the year after the index date it was 12 times higher (2.0 vs. 0.2 visits, p < 0.001; Supporting Information: Table S3a).
Figure 1.
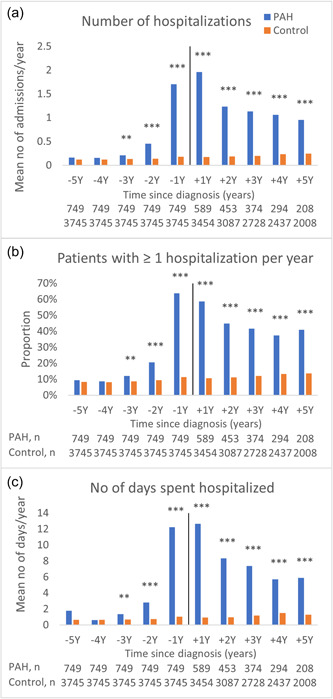
Healthcare resource utilization by hospitalizations. (a) Mean number of hospitalizations per person and year (analysis includes only surviving patients with complete follow‐up data per year). (b) Proportion with at least one hospitalization per year. (c) Mean number of days spent hospitalized per person and year. ***p < 0.001; **p < 0.01; *p < 0.05.
Similar trends were seen in the proportion of individuals with at least one hospitalization per year (Figure 1b) and the mean number of days spent hospitalized per person and year (Figure 1c). The mean number of days spent hospitalized per person and year over the whole study period was six times higher in the PAH group compared to the control group (5.9 vs. 1.0 days; Supporting Information: Table S3c).
The mean number of outpatient visits per person and year was higher in the PAH group than in the control group (0.9 vs. 0.7 visits, p = 0.004) already 5 years before the index date (Figure 2a and Supporting Information: Table S4). Two years before index date the PAH group used three times as much outpatient care as the control group (2.1 vs. 0.8 visits, p < 0.001), the year before the index date it was four times higher (3.5 vs. 0.9 visits, p < 0.001), and the first year after the index date, the mean number of outpatient visits was six times higher in the PAH group (5.3 vs. 0.9 visits, p < 0.001; Supporting Information: Table S4).
Figure 2.
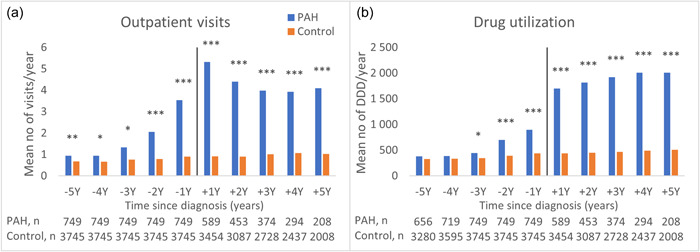
Healthcare resource utilization by outpatient visits and dispensed drugs (DDD). (a) Mean number of outpatient visits per person and year (analysis includes only surviving patients with complete follow‐up data per year). (b) Drug utilization, DDD of drugs per person and year. ***p < 0.001; **p < 0.01; *p < 0.05. DDD, defined daily dose.
Drug utilization measured as mean number of DDD per person and year did not differ between the PAH group and the control group 5 years before the index date (384 vs. 327 DDD, p = 0.191), but the mean cost of drugs was higher (514 vs. 200 EUR, p = 0.006; Supporting Information: Table S5). Three years before the index date the mean number of DDD was higher in the PAH group compared to the control group (446 vs. 347 DDD, p = 0.016). The year before the index date the mean number of DDD was twice as high in the PAH group and the year after the index date the DDD was four times higher than in the control group. Costs during the same periods increased from being four times higher in the PAH group than in the control group the year before the index date, to 113 times higher the year after the index date (Supporting Information: Table S5).
Sick leave and disability pension
For study participants eligible for sick leave, the number of days with sick leave per person and year was three times higher in the PAH group compared to the control group already 5 years before the index date (35 vs. 14, p < 0.001, Figure 3a). The year after the index date, the sick leave was 10 times higher in the PAH group (130 vs. 13, p < 0.001, Figure 3a).
Figure 3.
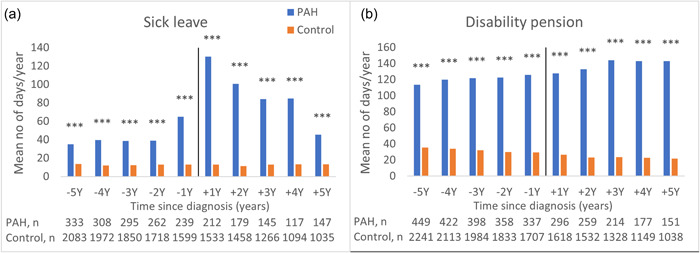
Productivity loss for individuals eligible for sick leave or disability pension, that is, not yet receiving age pension as main income. (a) Sick leave, mean days per person and year (full time equivalents). (b) Disability pension, mean days per person and year (full time equivalents). ***p < 0.001; **p < 0.01; *p < 0.05.
The study participants eligible for disability pension in the PAH group had three times more days with disability pension per person and year than the control group (114 vs. 36, p < 0.001; Figure 3b) already 5 years before the index date. This difference remained throughout the full 10‐year study period (Figure 3b). Sick leave or disability pension per person and year are presented in Supporting Information: Table S6.
Societal costs
The societal costs were considerably higher for the PAH group compared to the control group for the total 5‐year period before as well as the total 5‐year period after the index date (Table 3). The direct costs were higher while indirect costs were lower in both groups during the 5 years after index date compared to the 5 years before the index date (Table 3).
Table 3.
Societal costs, estimated over the total 5‐year period pre‐index date and 5‐year period post‐index date data using the Zhao and Tian (ZT) estimator to adjust for censoring
| PAH | Control | Difference | |
|---|---|---|---|
| The 5‐year period before index date | |||
| Hospitalizations | 11,300 (10,100−12,500) | 3200 (2800−3500) | 8100 (6900−9400) |
| Outpatient care | 3300 (2900−3800) | 1300 (1200−1500) | 2000 (1500−2500) |
| Drugs | 3500 (2400−4700) | 1100 (900−1200) | 2500 (1300−3600) |
| Productivity lossa | 58,300 (51,400−65,300) | 17,600 (15,900−19,300) | 40,700 (33,600−48,000) |
| Total societal costs | 76,500 (68,800−84,300) | 23,100 (21,300−25,000) | 53,400 (45,400−61,300) |
| The 5‐year period after index date | |||
| Hospitalizations | 28,800 (26,300−31,400) | 5000 (4600−5400) | 23,800 (21,200−26,300) |
| Outpatient care | 7200 (6700−7800) | 2000 (1800−2100) | 5300 (4800−5800) |
| Drugs | 112,100 (100,500−123,700) | 1600 (1400−1700) | 110,500 (98,900−122,200) |
| Productivity lossa | 28,500 (21,100−35,800) | 7700 (6100−9300) | 20,800 (13,200−28,300) |
| Total societal costs | 176,600 (160,200−193,000) | 16,300 (14,500−18,000) | 160,300 (143,900−176,800) |
Note: Data shown as mean (95% confidence interval), per patient, EUR.
Abbreviation: PAH, pulmonary arterial hypertension.
Sick leave and disability pension.
The main costs for the PAH group before the index date was productivity loss, constituting 76% of the total costs, while the cost for hospitalizations constituted 15%. After the index date, prescribed drugs accounted for two thirds of the total cost, whereof 95% were related to PAH specific treatment. Hospitalizations and productivity loss each constituted 16% of the total costs, while outpatient care accounted for 4% of total costs in the PAH group. The most common reasons for hospitalization were pulmonary hypertension, systemic sclerosis, and heart insufficiency.
Mean survival
The 1, 3, and 5‐year survival was 85%, 64%, and 49% in the PAH group and 98%, 95%, and 91% in the control group, respectively (Figure 4).
Figure 4.
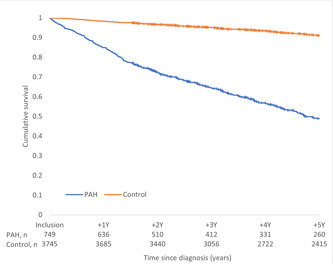
Overall survival for the PAH group and their matched control group by Kaplan−Meier estimates for 5 years with follow‐up from index date censored at the date of last contact or death. PAH, pulmonary arterial hypertension.
DISCUSSION
Patients with PAH had considerably higher HCRU and productivity loss compared to the matched control group, starting several years before the PAH diagnosis. In the PAH group, the main societal cost driver during the 5 years preceding the PAH diagnosis was productivity loss, while in the 5 years following the diagnosis, in order of magnitude, they were drugs, hospitalizations, and productivity loss.
That a debilitating disease like PAH have high HCRU after diagnosis is neither surprising nor unknown, 9 , 10 , 11 , 12 , 15 , 16 , 17 however, the costs for society before diagnosis is less studied 10 , 17 , 31 , 32 and the magnitude of productivity loss has not been shown before. Hospitalizations as a substantial cost driver after the PAH diagnosis 10 , 11 , 15 , 16 , 17 was confirmed by the present study and, importantly, productivity loss was found to be equally costly. However, the main cost driver in the present study was drug costs that were twice as high as the costs for hospitalizations and productivity loss combined.
Previous studies have shown diverging results of the main post‐diagnosis cost driver as being either related to drug costs 10 , 31 , 32 or to nondrug related healthcare costs. 14 , 15 , 16 This discrepancy might relate to that a majority of the previous studies are based on patients identified from insurance claim databases or databases using criteria such as treatment, procedures, hospitalization, and/or ICD‐codes and often include both prevalent and incident patients. 10 , 11 , 14 The present study includes only incident patients with a confirmed PAH diagnosis included in a national diagnose registry. 3 Furthermore, Swedish national registries allow the present study to estimate the societal direct and indirect costs using complete data. With the addition of five matched controls per patient and data available from 5 years before as well as 5 years after the PAH diagnosis (index date), this study adds valuable information.
The HCRU for the PAH patient group increased already 3 years before diagnosis, which is in line with previous research. 6 , 7 , 8 It is still unclear whether this is due to diagnostic delay, as early symptoms of PAH are unspecific 7 , 33 and may be interpreted and treated as heart or lung diseases, 7 or related to diseases that might be risk factors for PAH. A timely diagnosis of PAH and initiation of disease specific treatment will likely have prognostic benefit and lessen the burden and costs of living with PAH for the patient as well as the society. 7 The years before the PAH diagnosis warrants more in‐depth analyses to better understand the journey from symptom onset to diagnosis for patients with PAH and how this can be shortened. Contribution to societal costs by PAH subgroups associated to diseases such as connective tissue disease and congenital heart disease also need further exploration.
The cost for productivity loss was lower during the 5 years after diagnosis compared to the 5 years before diagnosis in both groups. This can be explained by an aging cohort, resulting in an increased proportion of deceased or retired persons. The higher mortality rate in the PAH group will affect the productivity loss in the PAH group to a higher degree than in the control group. The poor survival of patients with PAH in Sweden are in line with results from other pulmonary hypertension registries, 2 , 34 which may indicate international homogeneity in clinical practice as well as in treatment strategies. 1 The comparison with the matched control group in this study is unique and the results are alarming. The considerably higher mortality rate in the PAH group highlights the need for earlier diagnosis, a risk stratification tool easy to use in clinical practice as well as new and improved treatments, and likely, earlier referral for lung transplantation.
The present study adds new and important knowledge regarding the burden of PAH for the healthcare system, the society, and the patients with PAH. However, to understand how the burden can be reduced, further and more detailed studies of these direct and indirect costs are warranted. This information might also be useful for evaluation of present care including the effect of new treatments and treatment strategies and the effect of risk stratification and compliance to disease specific, international guidelines.
Strengths and limitations of the present study are the mainly tax funded national systems of healthcare, social insurance, and pension in Sweden that provide a high national coverage of all the included registries and the inclusion of an age, sex, and geographically matched control group, five controls per patient, are key strengths of the present study. All PAH‐specialist centers in Sweden participate in SPAHR allowing for a national coverage of >90% of all patients diagnosed with PAH in Sweden. Only incident patients with a confirmed PAH diagnosis were included. The presentation of indirect effects of PAH such as sick leave, disability pension, and the related productivity loss is another strength of the study.
Limitations include the censoring of data where patients diagnosed late in the study period had shorter follow‐up time and that the HCRU did not include primary care. To show the utilization progress in the living cohort, the HCRU analyses were limited to living and non‐censored individuals inducing a bias toward individuals diagnosed with PAH early in the data collection period.
In conclusion, PAH is associated with large societal costs, comprised of high HCRU and productivity loss, starting several years before diagnosis. Although PAH is a rare disease, the economic burden imposed on society and the high mortality rate, show that strategies for earlier diagnosis and more effective treatments are warranted.
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Hannes Runheim, Magnus Husberg, Barbro Kjellström, Lars Bernfort, and Lars‐Åke Levin. The first draft of the manuscript was written by Hannes Runheim and all authors commented on all versions of the manuscript. All authors have read and approved the final manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
CONFLICTS OF INTEREST
Amélie Beaudet and Nadia Pillai are employees of Actelion Pharmaceuticals, a Janssen Pharmaceutical Company of Johnson & Johnson, Allschwil, Switzerland. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Regional Ethics Committee in Lund, Sweden (LU 2016/766), and performed in accordance with the Declaration of Helsinki. The study used retrospective, anonymized data from Swedish National Registries and in accordance to Swedish law, no informed consent from patients was needed.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
We acknowledge the work of the SPAHR registrars at the PAH/CTEPH‐specialist clinics, the members of the SPAHR steering committee, and Uppsala Clinical Research Center for administrating SPAHR. This study was funded by Actelion Pharmaceuticals, a Janssen Pharmaceutical Company of Johnson & Johnson, Allschwil, Switzerland. Representatives of Actelion Pharmaceuticals, a Janssen Pharmaceutical Company of Johnson & Johnson, Allschwil, Switzerland, listed as authors of this paper, participated in study design, interpretation of data, and drafting the manuscript, but did not participate in data analyses. Additional funding was received in terms of a scholarship from SveFPH/SPAHR. Guarantor: Lund University, Lund, Sweden.
Runheim H, Kjellström B, Beaudet A, Ivarsson B, Husberg M, Pillai N, Levin L‐Å, Bernfort L. Societal costs associated with pulmonary arterial hypertension: a study utilizing linked national registries. Pulm Circ. 2023;13:e12190. 10.1002/pul2.12190
REFERENCES
- 1. Galiè N, Humbert M, Vachiery J‐L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document G. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: association for European paediatric and congenital cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119. [DOI] [PubMed] [Google Scholar]
- 2. National Health Service Digital . National pulmonary hypertension audit, 12th annual report. Accessed May 18, 2022. https://digital.nhs.uk/data-and-information/publications/statistical/national-pulmonary-hypertension-audit/12th-annual-report
- 3. SPAHR (Swedish Pulmonary Arterial Registry) . Årsrapport. 2020. Accessed June 1, 2022. https://www.ucr.uu.se/spahr/arsrapporter/arsrapporter/arsrapport-spahr-2020
- 4. Ivarsson B, Johansson A, Kjellström B. The odyssey from symptom to diagnosis of pulmonary hypertension from the patients and spouses perspective. J Primary Care Community Health. 2021;12:215013272110292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rådegran G, Kjellström B, Ekmehag B, Larsen F, Rundqvist B, Blomquist SB, Gustafsson C, Hesselstrand R, Karlsson M, Kornhall B, Nisell M, Persson L, Ryftenius H, Selin M, Ullman B, Wall K, Wikström G, Willehadson M, Jansson K. Characteristics and survival of adult Swedish PAH and CTEPH patients 2000−2014. Scand Cardiovasc J. 2016;50(4):243–50. [DOI] [PubMed] [Google Scholar]
- 6. Ginoux M, Turquier S, Chebib N, Glerant JC, Traclet J, Philit F, Sénéchal A, Mornex JF, Cottin V. Impact of comorbidities and delay in diagnosis in elderly patients with pulmonary hypertension. ERJ Open Res. 2018;4(4):00100‐2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khou V, Anderson JJ, Strange G, Corrigan C, Collins N, Celermajer DS, Dwyer N, Feenstra J, Horrigan M, Keating D, Kotlyar E, Lavender M, McWilliams TJ, Steele P, Weintraub R, Whitford H, Whyte K, Williams TJ, Wrobel JP, Keogh A, Lau EM. Diagnostic delay in pulmonary arterial hypertension: insights from the Australian and New Zealand pulmonary hypertension registry. Respirology. 2020;25(8):863–71. [DOI] [PubMed] [Google Scholar]
- 8. Brown LM, Chen H, Halpern S, Taichman D, McGoon MD, Farber HW, Frost AE, Liou TG, Turner M, Feldkircher K, Miller DP, Elliott CG. Delay in recognition of pulmonary arterial hypertension. Chest. 2011;140(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bergot E, De Leotoing L, Bendjenana H, Tournier C, Vainchtock A, Nachbaur G, Humbert M. Hospital burden of pulmonary arterial hypertension in France. PLoS One. 2019;14(9):e0221211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burger CD, Ozbay AB, Lazarus HM, Riehle E, Montejano LB, Lenhart G, White RJ. Treatment patterns and associated health care costs before and after treatment initiation among pulmonary arterial hypertension patients in the United States. J Managed Care Specialty Pharmacy. 2018;24(8):834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Exposto F, Hermans R, Nordgren Å, Taylor L, Sikander Rehman S, Ogley R, Davies E, Yesufu‐Udechuku A, Beaudet A. Burden of pulmonary arterial hypertension in England: retrospective HES database analysis. Therapeutic Adv Resp Dis. 2021;15:175346662199504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gu S, Hu H, Dong H. Systematic review of the economic burden of pulmonary arterial hypertension. Pharmacoeconomics. 2016;34(6):533–50. [DOI] [PubMed] [Google Scholar]
- 13. Fuge J, Park DH, von Lengerke T, Richter MJ, Gall H, Ghofrani HA, Kamp JC, Hoeper MM, Olsson KM. Impact of pulmonary arterial hypertension on employment, work productivity, and quality of life—results of a cross‐sectional multi‐center study. Front Psychiatry. 2022;12:781532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ogbomo A, Tsang Y, Mallampati R, Panjabi S. The direct and indirect health care costs associated with pulmonary arterial hypertension among commercially insured patients in the United States. J Managed Care Specialty Pharmacy. 2022;28(6):608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Copher R, Cerulli A, Watkins A, Laura Monsalvo M. Treatment patterns and healthcare system burden of managed care patients with suspected pulmonary arterial hypertension in the United States. J Med Economics. 2012;15(5):947–55. [DOI] [PubMed] [Google Scholar]
- 16. Kirson NY, Birnbaum HG, Ivanova JI, Waldman T, Joish V, Williamson T. Excess costs associated with patients with pulmonary arterial hypertension in a US privately insured population. Appl Health Econ Health Policy. 2011;9(5):293–303. [DOI] [PubMed] [Google Scholar]
- 17. Said Q, Martin BC, Joish VN, Kreilick C, Mathai SC. The cost to managed care of managing pulmonary hypertension. J Med Economics. 2012;15(3):500–8. [DOI] [PubMed] [Google Scholar]
- 18. Anell A, Glenngård AH, Merkur S. Sweden health system review. Health Syst Transition. 2012;14(5):1–159. [PubMed] [Google Scholar]
- 19. The Swedish Social Insurance Agency (FK) . Social insurance in figures 2021. 2021.
- 20. The Swedish Pensions Agency . Pension system in Sweden. Accessed June 17, 2022. https://www.pensionsmyndigheten.se/other-languages/english-engelska/english-engelska/pension-system-in-sweden
- 21. Statistics Sweden (SCB) . Longitudinal integrated database for health insurance and labour market studies (LISA). Accessed April 20, 2022. https://www.scb.se/en/services/ordering-data-and-statistics/ordering-microdata/vilka-mikrodata-finns/longitudinella-register/longitudinal-integrated-database-for-health-insurance-and-labour-market-studies-lisa/
- 22. The National Board of Health and Welfare (SoS) . Sjukpenning och Rehabiliteringspenning. Accessed April 20, 2022. https://www.forsakringskassan.se/wps/wcm/connect/f1e0dce5-e310-4d6d-8076-d4493534c10b/MiDAS_Sjukpenning_och_rehabiliteringspenning_Version_1_02.pdf?MOD=AJPERES
- 23. The National Board of Health and Welfare (SoS) . National patient register. Accessed April 20, 2022. https://www.socialstyrelsen.se/en/statistics-and-data/registers/national-patient-register/
- 24. The National Board of Health and Welfare (SoS) . National prescribed drug register. Accessed April 20, 2022. https://www.socialstyrelsen.se/en/statistics-and-data/registers/national-prescribed-drug-register/
- 25. The National Board of Health and Welfare (SoS) . Viktlistor för NordDRG. Accessed May 12, 2022. https://www.socialstyrelsen.se/statistik-och-data/klassifikationer-och-koder/drg/viktlistor/
- 26. Office of Health Economics . Productivity costs: principles and practice in economic evaluation. 2000.
- 27. Zhao H, Tian L. On estimating medical cost and incremental cost‐effectiveness ratios with censored data. Biometrics. 2001;57(4):1002–8. [DOI] [PubMed] [Google Scholar]
- 28. Statistics Sweden (SCB) . Consumer price index (CPI). Accessed Decemebr 16, 2021. http://www.scb.se/pr0101-en
- 29. The Riksbank . Annual average exchange rates (aggregate). Accessed June 30, 2022. https://www.riksbank.se/en-gb/statistics/search-interest--exchange-rates/annual-average-exchange-rates/?y=2020&m=12&s=Dot&f=y
- 30. The National Board of Health and Welfare (SoS) . Klassifikationen ICD‐10. Accessed May 12, 2022. https://www.socialstyrelsen.se/statistik-och-data/klassifikationer-och-koder/icd-10/
- 31. Pizzicato LN, Nadipelli VR, Governor S, Mao J, Lanes S, Butler J, Pepe RS, Phatak H, El‐Kersh K. Real‐world treatment patterns, healthcare resource utilization, and cost among adults with pulmonary arterial hypertension in the United States. Pulm Circ. 2022;12(2):e12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sikirica M, Iorga SR, Bancroft T, Potash J. The economic burden of pulmonary arterial hypertension (PAH) in the US on payers and patients. BMC Health Serv Res. 2014;14:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bergemann R, Allsopp J, Jenner H, Daniels FA, Drage E, Samyshkin Y, Schmitt C, Wood S, Kiely DG, Lawrie A. High levels of healthcare utilization prior to diagnosis in idiopathic pulmonary arterial hypertension support the feasibility of an early diagnosis algorithm: the SPHInX project. Pulm Circ. 2018;8(4):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoeper MM, Pausch C, Grünig E, Staehler G, Huscher D, Pittrow D, Olsson KM, Vizza CD, Gall H, Distler O, Opitz C, Gibbs JSR, Delcroix M, Ghofrani HA, Rosenkranz S, Park D‐E, Ewert R, Kaemmerer H, Lange TJ, Kabitz H‐J, Skowasch D, Skride A, Claussen M, Behr J, Milger K, Halank M, Wilkens H, Seyfarth H‐J, Held M, Dumitrescu D, Tsangaris I, Vonk‐Noordegraaf A, Ulrich S, Klose H. Temporal trends in pulmonary arterial hypertension: results from the COMPERA registry. Eur Respir J. 2022;59:2102024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.


