Abstract
Background
Coronavirus disease 2019 (COVID-19) vaccine effectiveness (VE) studies are increasingly reporting relative VE (rVE) comparing a primary series plus booster doses with a primary series only. Interpretation of rVE differs from traditional studies measuring absolute VE (aVE) of a vaccine regimen against an unvaccinated referent group. We estimated aVE and rVE against COVID-19 hospitalization in primary-series plus first-booster recipients of COVID-19 vaccines.
Methods
Booster-eligible immunocompetent adults hospitalized at 21 medical centers in the United States during December 25, 2021–April 4, 2022 were included. In a test-negative design, logistic regression with case status as the outcome and completion of primary vaccine series or primary series plus 1 booster dose as the predictors, adjusted for potential confounders, were used to estimate aVE and rVE.
Results
A total of 2060 patients were analyzed, including 1104 COVID-19 cases and 956 controls. Relative VE against COVID-19 hospitalization in boosted mRNA vaccine recipients versus primary series only was 66% (95% confidence interval [CI], 55%–74%); aVE was 81% (95% CI, 75%–86%) for boosted versus 46% (95% CI, 30%–58%) for primary. For boosted Janssen vaccine recipients versus primary series, rVE was 49% (95% CI, −9% to 76%); aVE was 62% (95% CI, 33%–79%) for boosted versus 36% (95% CI, −4% to 60%) for primary.
Conclusions
Vaccine booster doses increased protection against COVID-19 hospitalization compared with a primary series. Comparing rVE measures across studies can lead to flawed interpretations of the added value of a new vaccination regimen, whereas difference in aVE, when available, may be a more useful metric.
Keywords: absolute vaccine effectiveness, booster vaccine series, COVID-19, primary vaccine series, relative vaccine effectiveness
Although relative vaccine effectiveness can be a useful measure to understand incremental benefit of a vaccine booster regimen compared with a primary series alone, absolute vaccine effectiveness estimates are needed to fully understand their benefit.
Observational coronavirus disease 2019 (COVID-19) vaccine effectiveness (VE) studies have generally assessed absolute VE (aVE) of a vaccine regimen by comparing the frequency of the outcome (eg, infection, hospitalization, death) in vaccinated (primary series [1, 2] or first booster [3, 4]) versus unvaccinated groups to estimate risk reduction for disease based on vaccination [1–4]. Relative VE (rVE), in contrast, has often been used to compare the risk reduction benefits of different influenza vaccine products (eg, adjuvanted vs high-dose vaccines) based on their effectiveness versus an unvaccinated group [5, 6]. As booster doses were added to COVID-19 vaccination schedules, rVE was also increasingly used to assess the VE of booster regimens by comparing disease incidence between those receiving a booster dose and those receiving the primary series alone [7, 8].
Observations of waning effectiveness for first booster doses during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron-predominant period [9, 10] have prompted ongoing evaluation of the additional benefit of (1) a first booster compared with a primary series and, increasingly, (2) a second booster dose compared with a first. However, both widespread primary series and first booster vaccination in some settings and population groups (eg, older adults) have led to some COVID-19 VE reporting solely rVE to characterize their added value, without the context of an unvaccinated comparator group [11, 12]. Assessing rVE results in terms of absolute improvement in protection for a population can be challenging.
Relative VE for recipients of a vaccine primary series plus booster dose versus the primary series alone can be expressed as follows:
Relative VE can also be expressed in relation to aVE estimation:
Therefore, rVE is the proportion of residual disease remaining after the first vaccine regimen that is prevented by the second, new vaccine regimen. The increased reporting of rVE estimates poses several interpretive challenges. Estimates of rVE might not be comparable across outcomes or studies when the absolute VE varies for the comparator vaccine. That is, for the same rVE reported in 2 different studies, the absolute reduction in disease burden provided by the newer regimen (eg, the primary series plus first booster dose) compared with the older one (eg, the primary series) can be quite different depending on the aVE of the older regimen [6]. We sought to better contextualize the rVE of COVID-19 boosters against COVID-19 hospitalization and to develop the interpretation of rVE as an increasingly common metric in COVID-19 booster studies. To achieve these goals, we estimated rVE for boosted versus primary-series-only COVID-19 vaccine recipients as well as aVE for each of the 2 regimens, among booster-eligible patients during the Omicron-predominant period.
METHODS
Setting
During December 25, 2021–April 4, 2022, a period in which the B.1.1.529 (Omicron) variant of SARS-CoV-2 was close to 100% predominant (including an estimated >90% BA.1 or BA.2 lineages) [13], adults admitted to 21 hospitals in 18 US states within the Influenza or Other Viruses in the Acutely Ill (IVY) Network [2, 4, 14, 15] who received testing for SARS-CoV-2 were included in a VE analysis. The analysis start date of December 25, 2021 was approximately 1 month after the emergency use authorization of a first booster dose (following a primary series of 2 mRNA doses or 1 Janssen dose) was expanded to include all adults aged ≥18 years [16] and coincided with the start of the period when the SARS-CoV-2 Omicron variant dominated in the United States. This activity was determined to be public health surveillance by each participating site and the Centers for Disease Control and Prevention (CDC) and was conducted consistent with applicable federal law and CDC policy (see 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq).
Participants
Patients were eligible for analysis if they were immunocompetent adults (≥18 years old) hospitalized with COVID-19-like illness (CLI), defined as having 1 or more of the following: fever, cough, shortness of breath, loss of taste, loss of smell, use of respiratory support for the acute illness, or new pulmonary findings on chest imaging consistent with pneumonia. Test-positive case patients had CLI and tested positive for SARS-CoV-2 by a molecular or antigen test within 14 days of illness onset. Test-negative control patients had CLI and received negative SARS-CoV-2 test results by molecular test [16]. Control patients were time matched to cases within 2 weeks and could not have been previously enrolled during the prior 30 days; no cases appeared more than once during the study period.
Data Collection
Patients or their proxies were interviewed regarding demographic and clinical characteristics, and medical record searches were completed to collect information about chronic medical conditions. Information about receipt of prior COVID-19 vaccination doses, including dates and vaccine product received, was obtained through self-report and review of source documentation (including state vaccination registries, medical records, and vaccination cards). The COVID-19 vaccination was considered verified with information such as dates of vaccination, vaccine products, and lot numbers using a systematic search of hospital electronic medical records, state vaccination registries, or vaccination cards (when available).
Patient Consent Statement
The IVY Network surveillance program is approved as a public health surveillance activity. This study is conducted with a waiver of informed consent granted by the Institutional Review Boards at the US Centers for Disease Control and Prevention, the IVY Network coordinating center at Vanderbilt University, and at each participating institution.
Vaccination Groups
For immunocompetent adults, a mRNA primary series refers to a 2-dose series of an mRNA vaccine (BNT162b2 [Pfizer] or mRNA-1273 [Moderna]) with the second dose ≥14 days before illness onset, and a Janssen primary series refers to 1 dose of Ad26.COV2 (Janssen [Johnson & Johnson]) ≥14 days before illness onset. Three vaccination groups were considered: (1) unvaccinated patients: received no COVID-19 vaccine doses before illness onset; (2) primary series only recipients - mRNA primary series recipients or Janssen primary series recipients who were eligible for but had not received a booster vaccine dose (≥150 days since mRNA primary series or ≥60 days since Janssen primary series) or had received the booster dose <7 days before illness onset; and (3) primary series plus booster recipients - boosted mRNA vaccine recipients or boosted Janssen recipients who received any single booster dose ≥7 days before illness onset. All other vaccine recipients outside of these 3 groups were excluded from the analysis, including recipients who received the single-dose mRNA vaccine, recipients who received 2 booster doses, and recipients who were not ineligible for booster in the primary series.
Statistical Analysis
We reported differences in sociodemographic characteristics and baseline clinical conditions by vaccination status. We summarized continuous variables as medians and interquartile ranges, and categorical variables were reported as counts and percentages. Differences in distribution and association, respectively, were tested using the Wilcoxon rank-sum test for continuous variables and χ2 tests with continuity correction for categorical variables. A test-negative design was used to evaluate aVE and rVE. Estimates of rVE against COVID-19 hospitalization were calculated among 2 subgroups: (1) booster-eligible mRNA primary series recipients versus booster recipients and (2) booster-eligible Janssen primary series recipients versus booster recipients.
We estimated aVE and rVE using multivariable logistic regression, where the odds ratio (OR) is modeled with case-status as the outcome and vaccination group (vaccinated without booster, vaccinated with booster, or unvaccinated, depending on analysis) as the exposure of interest while adjusting for sex, race/ethnicity, US Census region of the admitting hospital, age, number of pre-existing conditions, and admission date (biweekly intervals). Covariates were selected a priori based on clinical knowledge and past IVY analyses [2, 4, 13, 14]. The VE was calculated as (1-OR) × 100% [17]. Profile likelihood confidence intervals (CIs) not containing 0 (zero) were considered statistically significant. Analyses were conducted using R software (Vienna, Austria) [18].
Scenario Analysis
We demonstrated, through hypothetical numerical examples, how a fixed rVE could have different practical meanings under differing estimates of aVE. We assumed (1) 2 different vaccines each with a different aVE estimate for the primary series, (2) a constant rVE estimate for a booster dose of either vaccine relative to the primary series alone, and (3) completion of both primary series and booster within a hypothetical population of 2000 people. Using these inputs, we calculated outputs as the number of events averted by (1) implementation of the primary series alone and (2) implementation of the primary series plus booster dose. To isolate the effects of rVE on events averted and for purposes of illustration, we assumed that all input parameters were true unbiased estimates not requiring adjustment for confounding variables. To contextualize the results of the current study and the scenario analysis, we used the International Vaccine Access Center database [19] to identify extant studies using rVE to compare COVID-19 vaccine regimens.
RESULTS
Current Study
During December 25, 2021–April 4, 2022, a period during which the Omicron variant of SARS-CoV-2 predominated, the IVY Network enrolled 2105 patients, 45 of whom were excluded due to missing one of the covariates of age, sex, race, ethnicity, region, number of conditions, or admission date, leaving 2060 hospitalized patients included in the analytic dataset for this study (1104 case-patients and 956 non-COVID-19 controls). Among all participants, median age was 63 years, 48% of patients were female, and 61% were non-Hispanic White. Among 1104 case patients with laboratory-confirmed COVID-19, 309 (28%) were vaccinated with 2-dose mRNA primary series only, 148 (13%) with 3 doses of mRNA vaccine (primary series plus 1 booster); 58 (5%) with 1-dose Janssen primary series only, 25 (2%) with 1-dose Janssen plus any booster (primary series plus 1 booster); and 564 (51%) were unvaccinated. Among 956 controls without COVID-19, 245 (26%) were vaccinated with mRNA primary series, 372 (39%) with mRNA primary series plus mRNA booster; 38 (4%) with Janssen primary series, 40 (4%) with Janssen primary series plus any booster; and 261 (27%) were unvaccinated.
Patients who received 2 doses of COVID-19 mRNA vaccine (compared with those unvaccinated) were older (P < .001), had more chronic medical conditions (P < .001), and were less likely to test positive for SARS-CoV-2 (P < .001); recipients of a booster dose (compared to those who received the primary series only) were older (P < .001), and booster status was not independent of race/ethnicity (P = .002) (Table 1). Adults hospitalized with COVID-19 after 1 dose of Janssen (compared with being unvaccinated) had more chronic medical conditions (P < .001); those who received a Janssen primary series plus any booster (compared to those who received the primary series only) had more chronic medical conditions (P < .001), were older (P = .049), and were less likely to test positive for SARS-CoV-2 (P = .007). For mRNA vaccine recipients, median days since the last full dose of vaccine (P < .001) were greater among primary series recipients (274 days [interquartile range {IQR}, 238–310 days]) than for boosted recipients (77 days [IQR, 52–107 days]; the trend was similar for Janssen recipients (255 days [IQR, 203–293 days] vs 56 days [IQR, 33–87 days], P < .001). Case patients (compared with control patients) also differed by race/ethnicity distribution (P = .018) (see Supplemental Table 1). Time since last full vaccine dose was longer for vaccinated case patients compared with vaccinated control patients (median 237 days [IQR, 102–289 days] vs 124 days [IQR, 64–259 days]).
Table 1.
Characteristics of Unvaccinated Patients, mRNA Vaccine Recipients, and Janssen Vaccine Recipients (n = 2060), IVY Network, December 2021–April 2022
| Group (n) | |||||
|---|---|---|---|---|---|
| Characteristic, n (%) | Unvaccinated (n = 825) | mRNA Primary Series (n = 554) | mRNA Primary Series Plus Booster (n = 520)a | Janssen Primary Series (n = 96) | Janssen Primary Series Plus Booster (n = 65)b |
| Clinical Group | |||||
| COVID-19 Case | 564 (68.4) | 309 (55.8) | 148 (28.5) | 58 (60.4) | 25 (38.5) |
| Age in Years | |||||
| Median (IQR) | 58 (44–70) | 65 (53–74) | 68 (58–78) | 61 (51–69) | 62 (52–70) |
| 18–49 years | 265 (32.1) | 106 (19.1) | 58 (11.2) | 22 (22.9) | 12 (18.5) |
| 50–64 years | 269 (32.6) | 158 (28.5) | 153 (29.4) | 38 (39.6) | 29 (44.6) |
| ≥65 years | 291 (35.3) | 290 (52.3) | 309 (59.4) | 36 (37.5) | 24 (36.9) |
| Sex | |||||
| Female | 370 (44.8) | 277 (50.0) | 263 (50.6) | 41 (42.7) | 30 (46.2) |
| Race/ethnicity | |||||
| Non-Hispanic White | 465 (57.9) | 316 (58.3) | 351 (68.3) | 52 (54.2) | 35 (56.5) |
| Non-Hispanic Black | 173 (21.5) | 112 (20.7) | 91 (17.7) | 21 (21.9) | 13 (21.0) |
| Hispanic, Any Race | 120 (14.9) | 85 (15.7) | 52 (10.1) | 19 (19.8) | 9 (14.5) |
| Non-Hispanic, all other races | 45 (5.6) | 29 (5.4) | 20 (3.9) | 4 (4.2) | 5 (8.1) |
| Unknown | 22 (2.6) | 12 (2.1) | 6 (1.1) | 0 (0.0) | 3 (4.4) |
| US Census Region | |||||
| Northeast | 182 (22.1) | 151 (27.3) | 120 (23.1) | 24 (25.0) | 16 (24.6) |
| South | 307 (37.2) | 206 (37.2) | 162 (31.2) | 40 (41.7) | 16 (24.6) |
| Midwest | 165 (20.0) | 81 (14.6) | 95 (18.3) | 7 (7.3) | 14 (21.5) |
| West | 171 (20.7) | 116 (20.9) | 143 (27.5) | 25 (26.0) | 19 (29.2) |
| Number of chronic medical conditionsc, median (IQR) | 1 (1–2) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| Days from vaccine dose 1 to illness onset, median (IQR) | – | 303 (266–338) | 346 (316–372) | 255 (203–293) | 292 (257–329) |
| Days from last full dose to illness onset, median (IQR) | – | 274 (238–310) | 77 (52–107) | 255 (203–293) | 56 (33–87) |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; mRNA, messenger ribonucleic acid.
Among the 520 who received an mRNA primary series plus mRNA booster, 191 received a Moderna primary series and booster, 295 received a Pfizer primary series and booster, 16 received a Moderna primary series with Pfizer booster, and 18 received a Pfizer primary series with Moderna Booster.
Among the 65 who received the Janssen primary series plus any booster, 21 received a Moderna booster, 27 received a Pfizer booster, and 17 received a Janssen Booster.
Chronic medical conditions are defined as chronic cardiovascular disease (heart failure, peripheral vascular disease that limits mobility, prior myocardial infarction, cardiac arrhythmias including atrial fibrillation and ventricular arrhythmias, vascular heart disease, or hypertension), chronic lung disease (asthma, chronic obstructive pulmonary disease cystic fibrosis, pulmonary fibrosis, pulmonary hypertension, home oxygen use [except at night for sleep disorder], tracheostomy, home noninvasive ventilation use [except at night for sleep disorder], home invasive mechanical ventilation), diabetes mellitus (diabetes mellitus without end organ damage, diabetes mellitus with end organ damage).
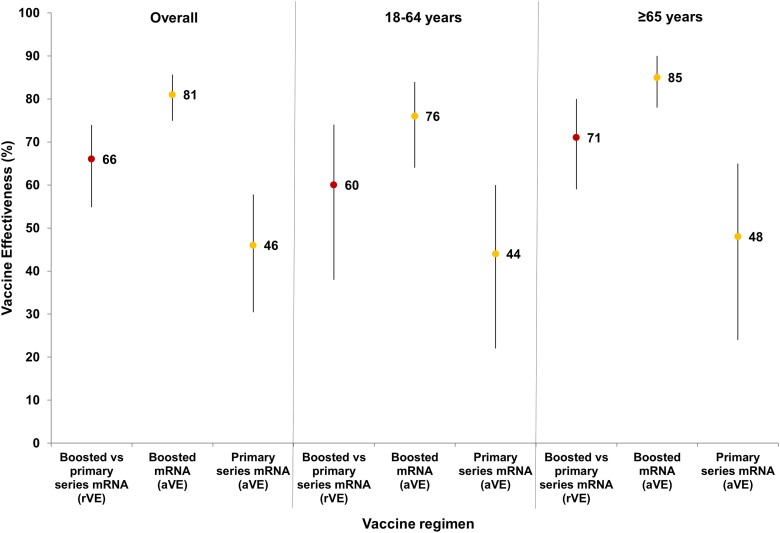
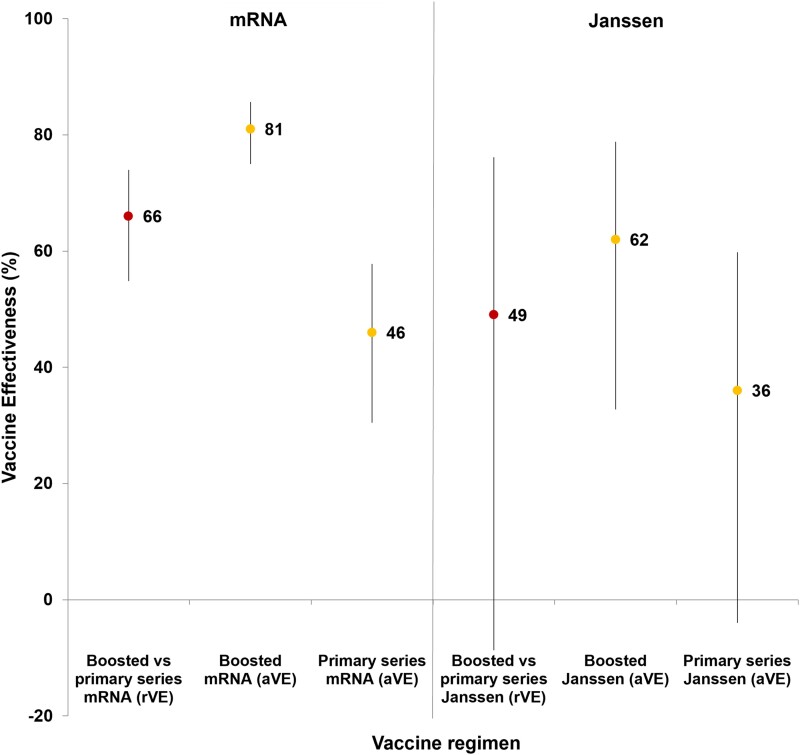
Relative VE against COVID-19 hospitalization in mRNA primary series plus booster recipients (vs primary series recipients) was 66% (95% CI, 55%–74%); aVE (vs unvaccinated patients) was 81% (95% CI, 75%–86%) for mRNA primary series plus booster recipients vs 46% (95% CI, 30%–58%) for primary series recipients (difference, 35); rVE for patients aged ≥65 years was 71% (rVE = 71% [95% CI, 59% vs 80%], aVE = 85% [95% CI, 78%–90%] vs 48% [95% CI, 24%–65%]; difference, 37), whereas those aged 18–64 years had an rVE of 60% (rVE = 60% [95% CI, 38%–74%]), aVE = 76% [64%–84%] vs 44% [95% CI, 22%–60%]; difference, 32) (Figure 1). In comparison, for Janssen primary series plus booster recipients (vs primary series recipients), rVE was 49% (95% CI, −9% to 76%); aVE (vs unvaccinated patients) was 62% (33%–79%) for primary series plus booster recipients versus 36% (95% CI, −4% to 60%) for primary series recipients (difference, 26) (Figure 2).
Figure 1.
Absolute and relative vaccine effectiveness (rVE) against hospitalization (point estimates [95% confidence intervals]) for primary series plus first mRNA booster dose and mRNA vaccine primary series alone (overall, 18–64 years and ≥65 years), December 2021–April 2022. The rVE point estimates at the left of each age category are denoted by red dots, absolute vaccine effectiveness (aVE) point estimates in the middle and at the right of each age category are denoted by yellow dots, and 95% confidence intervals are delineated by black vertical lines going through the corresponding dots for each point estimate.
Figure 2.
Absolute and relative vaccine effectiveness (rVE) against hospitalization (point estimates [95% confidence intervals]) for mRNA and Janssen vaccine primary series plus first booster dose and primary series alone, December 2021–April 2022. The rVE point estimates are denoted by red dots at the left of each vaccine type category, absolute vaccine effectiveness (aVE) point estimates are denoted by yellow dots in the middle and at the right of each vaccine type, and 95% confidence intervals are delineated by black vertical lines going through the corresponding dots for each point estimate.
Scenario Analysis
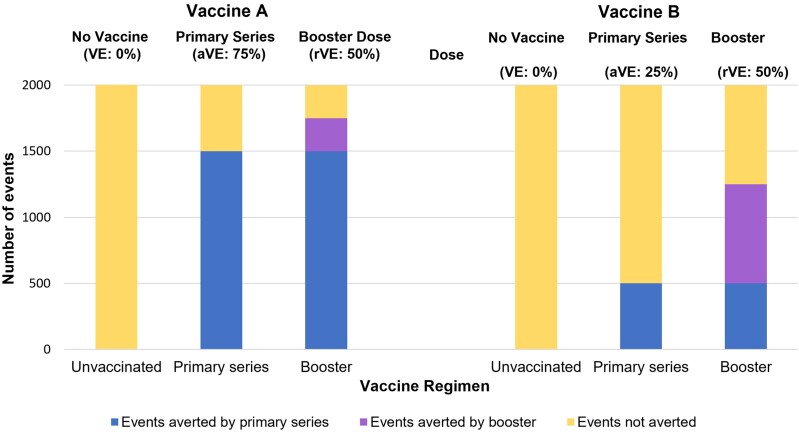
The actual reduction in disease associated with a given rVE depends on difference between the aVE of the primary series plus booster dose and the aVE of the primary series alone. As an illustration, we assumed that the primary series of vaccine A (aVE, 75%) and the primary series of vaccine B (aVE, 25%) are each delivered in a population of 2000 people (Figure 3), resulting in effectiveness against an event/outcome for 1500 in the population receiving vaccine A and for 500 in the population receiving vaccine B. When we assume an rVE of 50% for the corresponding booster dose for each vaccine primary series, an additional 250 events are averted by a booster dose of vaccine A while an additional 750 are averted by a booster dose of vaccine B.
Figure 3.
Scenario exercise comparing events averted by primary series alone and primary series plus first booster vaccine dose (n = 2000). Clustered bars show the additional number of events averted by adding a vaccine booster (purple/middle section of each bar) to a primary series alone (blue/bottom section of each bar). Unvaccinated persons are represented by the yellow/top section of each bar. Abbreviations: aVE, absolute vaccine effectiveness; rVE, relative vaccine effectiveness; VE, vaccine effectiveness.
Current studies using rVE to assess the effectiveness of primary series plus 2 booster doses versus a primary series plus 1 booster dose report rVE of 11%–85% against infection and 54%–87% against hospitalization (Table 2). However, many studies assessing both infection and hospitalization as outcomes report rVE estimates for infection and hospitalization within 10 points of one another, whereas aVE estimates for these outcomes diverge considerably [7–10]. These metrics underline that rVE cannot be translated into a precise reduction in risk from a booster dose or taken as evidence of greater or lesser vaccine effectiveness in one study versus another.
Table 2.
Studies Using Relative Effectiveness to Evaluate the Effectiveness of a COVID-19 Vaccine Booster Dose (n = 16)a, 2021–2022
| Study (Country), Study Type | Dates (Predominant Variants) | Study Design | Population (%Fully Vaccinated) | Primary Series or 1st Booster Evaluated (No. of Recipients) | Follow-up Time Point, Primary Series or 1st Booster | 1st or 2nd Booster Evaluated (No. of Recipients) | Follow-up Time Point, 1st or 2nd Booster | Outcomes Evaluated | Estimand (Formula), Estimator | rVE Reported (95% CI) | aVE Reported (95% CI), Primary or 1st Booster vs 1st or 2nd Booster |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies Reporting rVE and aVE (primary series vs 1st booster) | |||||||||||
| 1. Lewis et al [30] (USA), primary series vs 1st booster (current study) | December 25, 2021–April 4, 2022 (Omicron) | Test-negative | 1357 immunocompetent, booster-eligible adults aged ≥18 y (62% fully vaccinated) | BNT162b2 or mRNA-1273 primary series only (n = 669) | ≥150 d | BNT162b2 or mRNA-12 731st booster (n = 520) | ≥7 d | Hospitalization | OR (1-OR), multivariable logistic regression | 66% (55%–74%) | 81% (75%–86%) vs 46% (31%–58%) |
| Adenovirus vector vaccine primary series only (n = 103) | ≥150 d | BNT162b2 or mRNA-1273 1st booster (n = 65) | ≥7 d | Hospitalization | 49% (−9% to 76%) | 62% (33%–79%) vs 36% (−4%–60%) | |||||
| 2. Andrews et al [7] (UK), primary series vs 1st booster | September 13, 2021 (Delta) | Test-negative case control | 374 795 adults aged ≥50 y (96% fully vaccinated) | ChAdOx1-S primary series (n = 266 505) | ≥140 d | BNT162b2 1st booster (n = 25 672) | ≥14 d | Symptomatic disease | OR (1-OR), multivariable logistic regression | 89% (88%–90%) | 94% (93%–94%) vs 50% (48%–53%) |
| Hospitalization | 89% (75%–95%) | 99% (97%–100%) vs 90% (88%–91%) | |||||||||
| BNT162b2 primary series only (n = 108 290) | ≥140 d | BNT162b2 1st booster (n = 42 013) | ≥14 d | Symptomatic disease | OR (1-OR), multivariable logistic regression | 85% (84%–85%) | 94% (94%–95%) vs 63% (62%–65%) | ||||
| Hospitalization | 83% (73%–90%) | 99% (98%- 99%) vs 93% (92%- 94%) | |||||||||
| 3. Tartof et al [8] (USA), primary series vs 1st booster | December 14, 2020–December 5, 2021 (Alpha, Delta) | Retrospective cohort | 3 133 075 adults aged ≥18 y (35% fully vaccinated) | BNT162b2 primary series only (n = 829 100) | ≥6 mo | BNT162b2 1st booster (n = 276 037) | ≥14 d | Infection | RR (1-RR), Kaplan-Meier | 75% (71%−78%) | 88% (86%–89%) vs 49% (46%–51%) |
| … | Hospitalization | 70% (48%–83%) | 97% (95%–98%) vs 88% (85%–90%) | ||||||||
| 4. Plumb et al [20] (USA), primary series vs 1st booster | June 20, 2021–February 4, 2022 (Delta, Omicron) | Test-negative | 10 754 adults aged ≥18 y with previous SARS-CoV-2 infection (45% fully vaccinated) | BNT162b2 or mRNA-1273 primary series only (n = 3534) | ≥150 d | BNT162b2 or mRNA-1273 1st booster (n = 1301) | ≥14 d | Hospitalization | OR (1-OR), conditional logistic regression | 56% (44%–66%) | 67% (61%–72%) vs 39% (33%–45%) |
| Studies Reporting rVE and aVE (1st booster vs 2nd booster) | |||||||||||
| 5. Grewal et al [21] (Canada), 1st booster vs 2nd booster | December 30, 2021–March 2, 2022 (Omicron) | Test-negative | 56 806 LTCF residents aged ≥60 y old (93% fully vaccinated) | BNT162b2 or mRNA-1273 primary series plus 1st booster (BNT162b2 or mRNA-1273) (n = 30 222) | ≥84 d | BNT162b2 or mRNA-1273 2nd booster (n = 7548) | ≥7 d | Infection | OR (1-OR), multivariable logistic regression | 40% (34%–45%) | 65% (60%–70%) vs 42% (35%–48%) |
| Symptomatic infection | … | 63% (51%–71%) | 87% (81%–91%) vs 66% (54%–75%) | ||||||||
| Severe outcomes | … | 54% (31%–70%) | 92% (87%–95%) vs 82% (75%–88%) | ||||||||
| Studies Reporting rVE only (primary series vs 1st booster) | |||||||||||
| 6. Abu-Raddad et al [24] (Qatar), primary series vs 1st booster | January 5, 2021–January 9, 2022 (Alpha, Delta) | Retrospective cohort | 511 348 persons, all age groups (100% fully vaccinated) | BNT162b2 primary series only (n = 189 483) | 49 d | BNT162b2 1st booster (n = 66 191) | 49 d | Symptomatic disease | HR (1-HR) Cox proportional hazards | 50% (47%–53%) | … |
| mRNA-1273 primary series only (n = 189 483) | 35 d | mRNA-1273 1st booster (n = 66 191) | 35 d | Symptomatic disease | 51% (43%–57%) | … | |||||
| 7. Sharma et al [11] (USA), primary series vs 1st booster | January 1– November 25, 2021 (Alpha, Delta) | Observational cohort | 258 260 adult veterans aged ≥18 y (100% fully vaccinated) | BNT162b2 primary series only (n = 74 032) | ≥14 d | BNT162b2 1st booster (n = 74 032) | ≥14 d | Infection | RR (1-RR), Kaplan-Meier | 46% (38%–53%) | … |
| Hospitalization | 47% (36%–55%) | … | |||||||||
| mRNA-1273 primary series only (n = 55 098) | ≥8 d | mRNA-1273 1st booster (n = 55 098) | ≥8 d | Infection | 45% (27%–58%) | … | |||||
| Hospitalization | 50% (26%–66%) | … | |||||||||
| 8. Barda et al [12] (Israel), primary series vs 1st booster | July 30, 2020–September 23, 2021 (Delta) | Observational cohort | 1 456 642 immunocompetent persons aged ≥12 y (100% fully vaccinated) | BNT162b2 primary series only (n = 728 231) | ≥5 mo | BNT162b2 1st booster (n = 728 231) | ≥7 d | Hospitalization | RR (1-RR), Kaplan-Meier | 93% (88%–97%) | … |
| Severe disease | 92% (82%–97%) | … | |||||||||
| COVID-19-related death | 81% (59%–97%) | … | |||||||||
| 9. Butt et al [22] (USA), primary series vs 1st booster | September 22–December 25, 2021 (Omicron) | Retrospective cohort | 791 372 adult veterans aged 66–77 y (IQR) (100% fully vaccinated) | BNT162b2 primary series only (n = 236 693) | ≥4.5 mo | BNT162b2 1st booster (n = 236 693) | ≥14 d | Symptomatic infection | HR (1-HR), Cox proportional hazards | 84% (78%–88%) | … |
| Hospitalization | 77% (65%–85%) | … | |||||||||
| mRNA-1273 primary series only (n = 158 993) | ≥4.5 mo | mRNA-1273 1st booster (n = 158 993) | ≥14 d | Symptomatic infection | 87% (83%–90%) | … | |||||
| Hospitalization | 94% (93%–95%) | … | |||||||||
| 10. Patalon et al [25] (Israel), primary series vs 1st booster | August 1–October 4, 2021 (Delta) | Retrospective case control | 500 323 adults aged ≥40 y (100% fully vaccinated) | BNT162b2 primary series only (n = 227 380) | ≥150 d | BNT162b2 primary series only (n = 272 852) | 7–65 d | Infection | OR (1-OR), GEE logistic regression | 12% (8%–17%)–85% (83%–86%)b | … |
| 11. McConeghy et al [23] (USA), primary series vs 1st booster | September 22–December 18, 2021 (Delta, Omicron) | Observational cohort | 14 210 nursing home residents aged 69–87 y (IQR) (100% fully vaccinated) | BNT162b2 or mRNA-1273 primary series only (n = 8538) | ≥6 mo | BNT162b2 or mRNA-1273 booster (n = 5672) | 10–66 d | Infection | RR (1-RR), Kaplan-Meier with IPTW | 50% (29%–65%)–58% (32%–78%)b | … |
| Hospitalization | 97% (88%–100%)b | … | |||||||||
| 12. Patalon et al [9] (Israel), primary series vs 1st booster | January 1–21, 2021 (Omicron) | Observational cohort | 389 265 persons aged ≥16 y (100% fully vaccinated) | BNT162b2 primary series only (n = 54 221) | ≥5 mo | BNT162b2 1st booster (n = 335 044) | 0–5 mo | Infection | OR (1-OR), conditional logistic regression | 16% (12%–20%)–59% (55%–64%)b | … |
| 13. Korves et al [29] (USA), primary series vs 1st booster | September 23, 2021–March 3, 2022 (Delta, Omicron) | Self-controlled case series | 259 Veterans aged 60–71 y (med) in care for ≥2 y (100% fully vaccinated) | BNT162b2 or mRNA-1273 primary series only (n = 179) | 247 d (average) | BNT162b2 or mRNA-1273 1st booster (n = 80) | 4–6 d (control interval), 4–6 d (exposure interval) | Infection | OR (1-OR), conditional logistic regression | 50% (20%–68%)–70% (42%–84%)b | … |
| Studies Reporting rVE only (1st booster vs 2nd booster) | |||||||||||
| 14. Magen et al [27] (Israel), 1st booster vs 2nd booster | January 3– February 18, 2022 (Omicron) | Retrospective case control | 364 244 adults aged ≥60 y (100% fully vaccinated) | BNT162b2 primary series plus 1st booster (n = 182 122) | ≥4 mo | BNT162b2 2nd booster (n = 182 122) | 7–30 d | Symptomatic infection | RR (1-RR), Kaplan-Meier | 55% (53%–58%) | … |
| Hospitalization | 68% (59%–74%) | … | |||||||||
| Severe COVID-19 | 62% (50%–74%) | … | |||||||||
| 15. Gazit et al [26] (Israel), 1st booster vs 2nd booster | January 10– March 13, 2022 (Omicron) | Retrospective case control | 97 499 adults aged ≥60 y (100% fully vaccinated) | BNT162b2 primary series plus 1st booster (n = 69 623) | ≥4 mo | BNT162b2 2nd booster (n = 27 876) | 7–69 d | Infection | OR (1-OR), conditional logistic regression | 27% (4%–45%)–64% (62%–67%)b | … |
| Hospitalization | 73% (37%–86%) –87% (0%–98%)b | … | |||||||||
| 16. Regev-Yochay et al [28] (Israel), 1st booster vs 2nd booster | December 27, 2021–January 30, 2022 (Omicron) | Open-label nonrandom-ized trial | 725 healthcare workers aged ≥18 y (100% fully vaccinated) | BNT162b2 primary series plus 1st booster (n = 307) | ≥5 mo | BNT162b2 2nd booster (n = 153) | ≥8 d | Infection | RR (1-RR), Poisson regression | 30% (−9% to 55%) | … |
| Symptomatic infection | 43%b | … | |||||||||
| mRNA-1273 primary series plus 1st booster (n = 149) | ≥5 mo | mRNA 2nd booster (n = 116) | ≥8 d | Infection | 11% (−43% to 44%) | … | |||||
| Symptomatic infection | 31%c | … | |||||||||
Abbreviations: aVE, absolute vaccine effectiveness; CI, confidence interval; COVID-19, coronavirus disease 2019; d, days; HR, hazard ratio; IPTW, inverse probability treatment weighting; IQR, interquartile range; LTCF, long-term care facility; mRNA, messenger ribonucleic acid; mo, months; OR, odds ratio; RR, rate ratio or risk ratio; rVE, relative effectiveness; y, years.
Studies were selected during April–June 2022 through review of the International Vaccine Access Center database [18] in the category of COVID-19 Data—Effectiveness Studies. To be included, the studies had to (1) analyze effectiveness of a first booster dose compared with a primary series or a second booster dose compared with a first booster dose and (2) use rVE as a measure of vaccine effectiveness, either alone or in conjunction with aVE of each option.
Range of rVE reported; rVE varies by follow-up time point.
Confidence intervals not reported.
DISCUSSION
This study adds to the body of evidence that a recent first booster dose of mRNA vaccine for recipients of an mRNA primary series results in lower risk of COVID-associated hospitalization (aVE, 81%) compared with the primary series alone (aVE, 46%). An increase in aVE was observed both for patients aged ≥65 years (85% vs 48%) and those aged 18–64 years (76% vs 44%). We also found that the J&J primary series followed by any booster resulted in lower risk of hospitalization (aVE, 62%) compared with the 1-dose J&J primary series alone (36%). These aVE estimates translated into rVE of 66% for boosted mRNA primary series recipients (71% among patients aged ≥65 years and 60% among those aged 18–64 years, respectively) and 49% for boosted Janssen primary series recipients. A positive rVE was observed for the booster in every category, although it spanned zero for boosted Janssen vaccine recipients. However, a positive rVE does not always result in a substantial reduction in disease burden, and there are several factors involved in evaluating the public health utility of a booster dose. For example, the relatively lower rVE reported for boosted Janssen primary series recipients (compared with mRNA primary series recipients) does not indicate poor effectiveness, rather it reflects a large improvement in effectiveness over the low effectiveness observed for the primary series alone.
Comparing the findings of the current study to other similarly designed studies highlights some interpretive challenges. Among 16 studies additional studies, only 4 (25%) reported aVE; 3 were studies of primary series versus first booster [7,8,20] and 1 was first versus second booster [21]. The mRNA rVE reported in the current study (66%) against hospitalization (compared with primary series alone) was lower than in 2 of the 3 studies (83% [7] and 70% [8], respectively) comparing the primary series and the first booster. However, the absolute increase in aVE observed for the first mRNA booster in the current study (difference: 36) is highest among the 3 studies. In the other 2 studies, the aVE of the primary series against hospitalization was already high and the point estimate change in aVE (differences 6 and 9, respectively) was small, which is typical of studies conducted before the Omicron-predominant period. In the third study [20], where rVE was lower than the current study, the aVE of the primary series was low and the resulting difference with aVE from the first booster dose was large (difference, 28). This result was similar to the current study and more typical of studies covering the Omicron-predominant period. Higher rVE reported in several studies [7, 8, 10, 22] could also be associated with larger reductions in risk that might be more common in older populations [7, 10, 22, 23] or could be associated with smaller reductions in risk typical of studies in locales with near-universal vaccination or during earlier variant-predominant periods (eg, Alpha, Delta) with generally higher aVE for the primary series alone [8, 24]. Two studies that demonstrate large changes in rVE observed based on time elapsed after a 1st booster dose (12%–85% during 7–65 days after a first booster dose in one study [11], 27%–64% during 7–69 days after a second booster dose in another study [25]) show that follow-up period can also substantially influence rVE metrics. As studies of second boosters of mRNA vaccines proliferate [20, 6–8] and novel study designs to estimate rVE are introduced [29], it will be important to consider these details and interpret rVE with caution.
We have also shown through a simple scenario analysis that with an equal rVE and baseline disease burden, the actual reduction in disease burden with the introduction of a new vaccination regimen can differ substantially. Because rVE is a measure of incremental effectiveness of the second vaccine option relative to the first option, its interpretation depends on the aVE of the first vaccine option (eg, primary series or first booster). With higher aVE, the residual disease burden will be lower than a vaccination regimen with a lower aVE. Consequently, the actual benefits in terms of events averted by a new vaccination regimen, such as a booster, will be greater in populations with lower aVE before the introduction of the booster. These issues create challenges in the interpretation of rVE across factors such as different vaccines, populations, and time periods. Without an unvaccinated control group against which the effectiveness of each option can be evaluated, the additional reduction in risk provided by one option over the other is often unclear. In instances in which aVE of a booster dose cannot be estimated, studies should ideally report the aVE of the primary series, which, in combination with rVE, can be used to estimate the aVE of the booster. However, this estimate may become less accurate when the primary series and booster have been evaluated during different strain-predominant periods. Alternatively, published studies can provide data from similarly designed studies to provide the risk reduction context for the series against which the newer regimen is being compared. Despite these difficulties in interpretation, studies may be compelled to use rVE as the sole measure of effectiveness when there is no unvaccinated comparison group with which to create an aVE estimate. However, rVE can also be useful (when provided alongside aVE) as a snapshot of additional, incremental effectiveness offered by a new dosing regimen or product compared with the original option.
The results have some limitations given the complexities of estimating rVE and aVE. Although booster doses were associated with better protection against COVID-19 hospitalization than the primary series, understanding the durability of protection over time or against emerging SARS-CoV-2 variants will require ongoing surveillance. Second, although adjustments were made for calendar time, age, and race/ethnicity, among other potential confounders, unmeasured or residual confounding is possible. A complete case analysis was performed, and bias may be present based on strong missing data mechanism assumptions. Third, most hospitalized patients had multiple chronic medical conditions, and the overall VE observed in this analysis might underestimate protection in healthier populations. Although chronic conditions have been observed to reduce effectiveness [30], sample size was insufficient to differentiate VE by number of conditions. Fourth, the influence of prior infection on VE is not fully known due to a lack of definitive prior infection history among hospitalized patients (eg, inability or refusal to answer) and the likelihood of undercapture [31]; however, a previous sensitivity analysis showed that effectiveness did not change after removing patients with prior infection [13]. Fifth, it was not possible to estimate potential waning of VE due to the time-limited nature of the study, but previous analyses have shown waning for booster doses with increasing time since vaccination [6]. Finally, the broader parameters for ascertaining case status (ie, both molecular and antigen tests) and vaccination status (ie, including self-reported only, although these patients accounted for ≤5% of the total) could potentially overestimate totals of cases and vaccinated patients, respectively.
CONCLUSIONS
We found COVID vaccine primary series plus booster continues to provide substantial protection against COVID-19 hospitalization. However, we found that interpretation of the actual benefits of COVID-19 vaccine booster doses is difficult when considering rVE alone without the benefit of additional contextual data including aVE of the vaccine regimens being compared. As public health agencies evaluate rVE estimates across studies, understanding the rVE of booster doses for COVID-19 vaccines against COVID-19 hospitalization and its relationship to aVE will be important for assessing the potential for additional booster doses to further reduce risk of severe illness and hospitalization.
Supplementary Material
Acknowledgments
Financial support. This work was funded by the United States Centers Disease Control and Prevention (Grants 75D30120F00002 and 75D30122C12914; to W. H. S.).
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor Information
Nathaniel M Lewis, CDC COVID-19 Response Team, Atlanta, Georgia, USA.
Nancy Murray, CDC COVID-19 Response Team, Atlanta, Georgia, USA.
Katherine Adams, CDC COVID-19 Response Team, Atlanta, Georgia, USA.
Diya Surie, CDC COVID-19 Response Team, Atlanta, Georgia, USA.
Manjusha Gaglani, Baylor Scott and White Health, Texas A&M University College of Medicine, Temple, Texas, USA.
Adit A Ginde, Department of Emergency Medicine, University of Colorado School of Medicine, Aurora, Colorado, USA.
Tresa McNeal, Baylor Scott and White Health, Texas A&M University College of Medicine, Temple, Texas, USA.
Shekhar Ghamande, Baylor Scott and White Health, Texas A&M University College of Medicine, Temple, Texas, USA.
David J Douin, Department of Anesthesiology, University of Colorado School of Medicine, Aurora, Colorado, USA.
H Keipp Talbot, Departments of Medicine and Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Jonathan D Casey, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Nicholas M Mohr, Department of Emergency Medicine, University of Iowa, Iowa City, Iowa, USA.
Anne Zepeski, Department of Emergency Medicine, University of Iowa, Iowa City, Iowa, USA.
Nathan I Shapiro, Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Kevin W Gibbs, Department of Medicine, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA.
D Clark Files, Department of Medicine, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA.
David N Hager, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Harith Ali, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Matthew E Prekker, Department of Emergency Medicine and Medicine, Hennepin County Medical Center, Minneapolis, Minnesota, USA.
Anne E Frosch, Department of Medicine, Hennepin County Medical Center, Minneapolis, Minnesota, USA.
Matthew C Exline, Department of Medicine, The Ohio State University, Columbus, Ohio, USA.
Michelle N Gong, Department of Medicine, Montefiore Health System, Albert Einstein College of Medicine, Bronx, New York, USA.
Amira Mohamed, Department of Medicine, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, New York, USA.
Nicholas J Johnson, Department of Emergency Medicine and Division of Pulmonary, Critical Care and Sleep Medicine, University of Washington, Seattle, Washington, USA.
Vasisht Srinivasan, Department of Emergency Medicine, University of Washington, Seattle, Washington, USA.
Jay S Steingrub, Department of Medicine, Baystate Medical Center, Springfield, Massachusetts, USA.
Ithan D Peltan, Department of Medicine, Intermountain Medical Center, Murray, Utah and University of Utah, Salt Lake City, Utah, USA.
Samuel M Brown, Department of Medicine, Intermountain Medical Center, Murray, Utah and University of Utah, Salt Lake City, Utah, USA.
Emily T Martin, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Arnold S Monto, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Adam S Lauring, Departments of Internal Medicine and Microbiology and Immunology, University of Michigan, Ann Arbor, Michigan, USA.
Akram Khan, Department of Medicine, Oregon Health and Sciences University, Portland, Oregon, USA.
Catherine L Hough, Department of Medicine, Oregon Health and Sciences University, Portland, Oregon, USA.
Laurence W Busse, Department of Medicine, Emory University, Atlanta, Georgia, USA.
William Bender, Department of Medicine, Emory University, Atlanta, Georgia, USA.
Abhijit Duggal, Department of Medicine, Cleveland Clinic, Cleveland, Ohio, USA.
Jennifer G Wilson, Department of Emergency Medicine, Stanford University School of Medicine, Stanford, California, USA.
Alexandra June Gordon, Department of Emergency Medicine, Stanford University School of Medicine, Stanford, California, USA.
Nida Qadir, Department of Medicine, University of California-Los Angeles, Los Angeles, California, USA.
Steven Y Chang, Department of Medicine, University of California-Los Angeles, Los Angeles, California, USA.
Christopher Mallow, Department of Medicine, University of Miami, Miami, Florida, USA.
Carolina Rivas, Department of Medicine, University of Miami, Miami, Florida, USA.
Hilary M Babcock, Department of Medicine, Washington University, St. Louis, Missouri, USA.
Jennie H Kwon, Department of Medicine, Washington University, St. Louis, Missouri, USA.
James D Chappell, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Natasha Halasa, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Carlos G Grijalva, Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Todd W Rice, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
William B Stubblefield, Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Adrienne Baughman, Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Christopher J Lindsell, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Kimberly W Hart, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Jillian P Rhoads, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Meredith L McMorrow, CDC COVID-19 Response Team, Atlanta, Georgia, USA.
Mark W Tenforde, CDC COVID-19 Response Team, Atlanta, Georgia, USA.
Wesley H Self, Department of Emergency Medicine and Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Manish M Patel, CDC COVID-19 Response Team, Atlanta, Georgia, USA.
for the Influenza and Other Viruses in the Acutely Ill (IVY) Network:
Manjusha Gaglani, Tresa McNeal, Shekhar Ghamande, Nicole Calhoun, Kempapura Murthy, Judy Herrick, Amanda McKillop, Eric Hoffman, Martha Zayed, Michael Smith, Jay Steingrub, Lori-Ann Kozikowski, Lesley De Souza, Scott Ouellette, Nathan I Shapiro, Michael Bolstad, Brianna Coviello, Robert Ciottone, Arnaldo Devilla, Ana Grafals, Conor Higgins, Carlo Ottanelli, Kimberly Redman, Douglas Scaffidi, Alexander Weingart, Manish Patel, Mark Tenforde, Nathaniel Lewis, Samantha Olson, Meagan Stephenson, Katherine Adams, Diya Surie, Meredith McMorrow, Maraia Tremarelli, Caitlin Turbyfill, Abhijit Duggal, Omar Mehkri, Megan Mitchell, Zachary Griffith, Connery Brennan, Kiran Ashok, Bryan Poynter, Laurence Busse, William Bender, Caitlin ten Lohuis, Nicholas Stanley, Sophia Zhang, Matthew Prekker, Heidi Erickson, Anne Frosch, Audrey Hendrickson, Sean Caspers, Walker Tordsen, Olivia Kaus, Tyler Scharber, Ithan Peltan, Samuel Brown, Jenna Lumpkin, Cassie Smith, Hunter Marshall, David N, Hager, Arber Shehu, Harith Ali, Richard E Rothman, Michelle Gong, Amira Mohamed, Rahul Nair, Jen-Ting (Tina) Chen, Matthew Exline, Sarah Karow, Maryiam Khan, Preston So, Madison So, Elizabeth Schwartz, Mena Botros, Akram Khan, Catherine L Hough, Haeun Jung, Jesus Martinez, Andrea Luong, Bao Huynh, Habiba Ibrahim, Cynthia Villanueva-Vargas, Juliana Villanueva-Vargas, Suha Quadri, Jennifer G Wilson, Alexandra June Gordon, Cynthia Perez, Nida Qadir, Steven Chang, Trevor Frankel, Omai Garner, Sukantha Chandrasekaran, Adit Ginde, David Douin, David Huynh, Aimee Steinwand, Cori Withers, Conner Driver, Shelby Wright, Nicholas Mohr, Anne Zepeski, Paul Nassar, Shannon Landers, Karin Nielsen, Noble Briggs, Cathy Fairfield, Chris Mallow, Hayley Gershengorn, Carolina Rivas, Emily Martin, Arnold Monto, Adam Lauring, EJ McSpadden, Rachel Truscon, Anne Kaniclides, Lara Thomas, Ramsay Bielak, Weronika Damek Valvano, Rebecca Fong, William J Fitzsimmons, Christopher Blair, Julie Gilbert, Leigh Baker, Nicholas Johnson, Vasisht Srinivasan, Christine D Crider, Kyle A Steinbock, Thomas C Paulsen, Layla A Anderson, Wesley H Self, H Keipp Talbot, Chris Lindsell, Carlos Grijalva, Ian Jones, Natasha Halasa, James Chappell, Kelsey Womack, Jillian Rhoads, Adrienne Baughman, Christy Kampe, Jakea Johnson, Jake Sturgill, Kim Hart, Robert McClellan, Todd Rice, Jonathan Casey, William B Stubblefield, Yuwei Zhu, Laura L Short, Lauren J Ezzell, Margaret E Whitsett, Rendie E McHenry, Samarian J Hargrave, Marcia Blair, Jennifer L Luther, Claudia Guevara Pulido, Bryan P M Peterson, D Clark Files, Kevin Gibbs, Mary LaRose, Leigha Landreth, Madeline Hicks, Lisa Parks, Hilary Babcock, Jennie Kwon, Jahnavi Bongu, David McDonald, Candice Cass, Sondra Seiler, David Park, Tiffany Hink, Meghan Wallace, Carey-Ann Burnham, and Olivia G Arter
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tenforde MW, Patel MM, Ginde AA, et al. Effectiveness of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccines for preventing coronavirus disease 2019 hospitalizations in the United States. Clin Infect Dis 2022; 74:1515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA 2022; 327:639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tenforde MW, Patel MM, Gaglani M, et al. Effectiveness of a third dose of Pfizer-Biontech and Moderna vaccines in preventing COVID-19 hospitalization among immunocompetent and immunocompromised adults United States, August–December 2021. MMWR Morb Mortal Wkly Rep 2022; 71:118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Izurieta HS, Lu M, Kelman J, et al. Comparative effectiveness of influenza vaccines among US Medicare beneficiaries ages 65 years and older during the 2019–2020 season. Clin Infect Dis 2021; 73:e4251–9. [DOI] [PubMed] [Google Scholar]
- 6. Lewis NM, Chung JR, Uyeki TM, et al. Interpretation of relative efficacy and effectiveness for influenza vaccines. Clin Infect Dis 2021; 75:170–5. [DOI] [PubMed] [Google Scholar]
- 7. Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med 2022; 28:831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tartof SY, Slezak JM, Puzniak L, et al. Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: a retrospective cohort study. Lancet Reg Health Am 2022; 9:100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patalon R, Saciuk Y, Peretz A. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat Commun 2022; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022; 71:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma A, Oda G, Holodniy M. Effectiveness of mRNA-based vaccines during the emergence of SARS-CoV-2 omicron variant. Clin Infect Dis 2022; 75:2186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 2021; 398:2093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams K, Rhoads JP, Surie D, et al. Vaccine effectiveness of primary series and booster doses against COVID-19 associated hospital admissions in the United States: living test negative design study. BMJ 2022; 379:e072065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lauring AS, Tenforde MW, Chappell JD. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ 2022; 376:e069761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tenforde MW, Patel MM, Gaglani M, et al. Effectiveness of a third dose of Pfizer-BioNTech and Moderna vaccines in preventing COVID-19 hospitalization among immunocompetent and immunocompromised adults—United States, August–December 2021. MMWR Morb Mortal Wkly Rep 2022; 71:118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013; 2013:2165–8. [DOI] [PubMed] [Google Scholar]
- 17. US Food and Drug Administration . Coronavirus (COVID-19) update: FDA expands eligibility for COVID-19 vaccine boosters. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-expands-eligibility-covid-19-vaccine-boosters. Accessed 19 November 2021.
- 18. R Core Team . R: a language and environment for statistical computing. Vienna, Austria. Available at: https://www.R-project.org/. Accessed 18 August 2022.
- 19. International Vaccine Access Center (IVAC) . Johns Hopkins Bloomberg School of Public Health. VIEW-hub. Available at: www.view-hub.org. Accessed 18 August 2022.
- 20. Plumb ID, Feldstein LR, Barkley E, et al. Effectiveness of COVID-19 mRNA vaccination in preventing COVID-19-associated hospitalization among adults with previous SARS-CoV-2 infection—United States. MMWR Morb Mortal Wkly Rep 2022; 71:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grewal R, Kitchen SA, Nguyen L, et al. Effectiveness of a fourth dose of COVID-19 vaccine among long-term care residents in Ontario, Canada. BMJ 2022; 378:e071502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Butt AA, Talisa VB, Shaikh OS, et al. Relative vaccine effectiveness of a SARS-CoV-2 mRNA vaccine booster dose against the omicron variant. Clin Infect Dis 2022; 75:2161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McConeghy KW, Bardenheier B, Huang AW, et al. Infections, Hospitalizations, and Deaths Among US Nursing Home Residents With vs Without a SARS-CoV-2 Vaccine Booster. JAMA Netw Open 2022; 5:e2245417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N Engl J Med 2022; 386:1804–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patalon T, Gazit S, Pitzer VE, Prunas O, Warren JL, Weinberger DM. Odds of testing positive for SARS-CoV-2 following receipt of 3 vs 2 doses of the BNT162b2 mRNA vaccine. JAMA Intern Med 2022; 182:179–84.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gazit S, Saciuk Y, Perez G, et al. Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study. BMJ 2022; 377:e071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Magen O, Waxman JG, Makov-Assif M, et al. Fourth dose of BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med 2022; 386:1603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Regev-Yochay G, Mandelboim M, Amit S, et al. Efficacy of a fourth dose of COVID-19 mRNA vaccine against omicron. N Engl J Med 2022; 386:1377–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Korves C, Izurieta HS, Smith Jet al. Relative effectiveness of booster vs. 2-dose mRNA Covid-19 vaccination in the Veterans Health Administration: Self-controlled risk interval analysis. Vaccine 2022; 40:4742–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lewis NM, Naioti EA, Self WH, et al. Effectiveness of mRNA vaccines against COVID-19 hospitalization by age and chronic medical conditions burden among immunocompetent US adults, March–August 2021. J Infect Dis 2022; 225:1694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clarke KE, Jones JM, Deng Y, et al. Seroprevalence of infection-induced SARS-CoV-2 antibodies—United States, September 2021–February 2022. MMWR Morb Mortal Wkly Rep 2022; 71:606–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.