Abstract
Background
FK506 binding protein 51 (FKBP5) is a co-chaperone regulator of the glucocorticoid receptor (GR). Recent studies have reported increased FKBP5 mRNA in the circulation from patients with Cushing disease (CD) which returned to comparable levels seen in healthy controls following successful trans-nasal trans-sphenoidal (TNTS) surgical corticotroph tumor removal. However, the expression of circulating FKBP5 mRNA levels in other pituitary tumor subtypes and its specificity to corticotroph tumors is unknown.
Methods
Pre-operative blood was collected from consecutive patients undergoing TNTS for pituitary tumors (n = 57) at our center between 2015 and 2019. Total RNA was isolated from whole blood using RiboPure blood RNA isolation kit and real-time qPCR was used to quantitate circulating FKBP5 mRNA expression.
Results
Consistent with the prior report, higher circulating FKBP5 mRNA levels were observed in 20 patients with CD prior to surgical tumor removal, compared to 21 healthy controls (p < 0.0005) and compared to 8 patients harboring gonadotroph pituitary tumors (p < 0.05) and 6 patients with silent corticotroph pituitary tumors (p < 0.05). However, circulating FKBP5 mRNA levels were higher in 10 patients with prolactin (PRL)-secreting pituitary tumors compared to healthy controls (p < 0.05), and did not differ between patients with CD and patients with growth hormone secreting tumors (GH-omas).
Conclusions
Although we confirm that circulating FKBP5 mRNA is higher in patients with corticotroph tumors compared to healthy subjects, measurement of circulating FKBP5 does not appear to be helpful to distinguish corticotroph tumors from other pituitary tumor sub-types.
Keywords: Circulating biomarker, FKBP5, Pituitary tumors, Cushing disease (CD)
1. Introduction
The diagnosis of autonomous endogenous hypercortisolism is often challenging due to the dysregulation of hypothalamic-pituitary-adrenal axis function that can be observed in other more common disorders [1]. Additional improved specific biomarkers to diagnose hypercortisolism, monitor response to therapies and enable early detection of recurrence of hypercortisolism would be very helpful [2].
Recently, FK506 binding protein 51 (FKBP5) has been identified as a sensitive biomarker of glucocorticoid (GC) responsiveness [5]. FKBP5 belongs to a subfamily of immunophilins and along with heat shock protein 90 (hsp 90), hsp 70, and the co-chaperone identified as p23 (encoded by the prostaglandin E synthase 3, PTGES3 gene), FKBP5 forms a multiprotein chaperone complex. In the absence of ligand, this complex prevents degradation of the cytosolic GR and assists in GR maturation in the cytosol, where it facilitates high affinity ligand binding. When local cortisol levels increase and bind GR, the complex changes its conformation from FKBP5- to the FKBP4-associated state, releasing ligand-bound GR and exposing two nuclear localization signals that enable rapid GR transport into the nucleus [3]. In turn, GR activation increases FKBP5 expression, thereby dampening GR-ligand affinity and resulting in an ultra-short negative feedback loop to carefully regulate GR activity.
Several lines of evidence support a connection between GC action and FKBP5. Firstly, dose-dependent alterations in FKBP5 mRNA have been demonstrated in human peripheral blood mononuclear cells (PBMC) following oral administration of glucocorticoids to healthy subjects compared to their baseline levels and co-administration of the GR antagonist mifepristone blunted this GC-induced FKBP5 mRNA increase in PBMC [11]. Additionally, ∼3-fold higher circulating PBMC FKBP5 mRNA level was reported in 12 patients with Cushing disease (CD) compared to 26 healthy controls, and following surgical-induced CD remission the circulating FKBP5 mRNA levels fell to values similar to healthy controls [4].
A role for FKBP5 in GR signaling is further supported by the association of FKBP5 variants with several psychiatric disorders associated with GR dysregulation. For example, the FKBP5 TT genotype single-nucleotide polymorphism rs1360780 has been associated with increased GR-induced FKBP5 upregulation and may contribute to post-traumatic stress disorder, depression, and altered cognitive function through non-suppression of the hypothalamic-pituitary-adrenal (HPA) axis [[5], [6], [7], [8], [9], [10]]. In contrast, patients carrying rs1360780 genotypes which confer lower FKBP5 expression exhibit blunted responses to antidepressants [11,12].
Collectively, these studies support a link between FKBP5 expression and HPA axis function. We measured FKBP5 mRNA levels in circulating blood to investigate the potential broader utility of FKBP5 mRNA as a biomarker of GR action to diagnose and differentiate corticotroph pituitary tumors from other pituitary tumor sub-types.
2. Methods and materials
2.1. Blood sample collection and preparation
This research study was conducted under UCLA Institutional Review Board (IRB#20–002235) approval with informed consent obtained from all subjects. We recruited fifty seven consecutive patients [24 (42%) were male, mean age 46.0 ± 15.4 years (range, 15–72 years)], diagnosed with a pituitary adenoma according to standard criteria which included evaluation of clinical symptoms, biochemical parameters and imaging studies. Thirty-seven/57 (65%) had macroadenomas (Table 1). Fasting early morning blood samples were drawn from the patients with pituityary tumors undergoing trans-nasal-trans-sphenoidal (TNTS) into EDTA tubes and stored at −80C for measurement of circulating FKBP5 mRNA levels as described below. Pituitary tumor subtypes were confirmed histopathologically in all cases following TNTS pituitary tumor resection. Control blood samples (n = 21, 50% males) were collected from healthy blood donors at the UCLA transfusion medicine service laboratory.
Table 1.
Patient clinical characteristics.
| N | Age/Year (Mean ± SD) | Gender |
Tumor size |
|||
|---|---|---|---|---|---|---|
| M(%) | F(%) | Macro (%) | Micro (%) | |||
| PA |
57 |
46.0 ± 15.4 |
24(42%) |
33(58%) |
37 (65%) |
20 (35%) |
| CD | 20 | 46.0 ± 15.2 | 3 | 17 | 7 | 13 |
| SCA | 6 | 40.5 ± 16.3 | 2 | 4 | 6 | 0 |
| Gonadotroph | 8 | 49.5 ± 14.6 | 7 | 1 | 8 | 0 |
| PRL | 10 | 38.8 ± 13.3 | 5 | 5 | 5 | 5 |
| GH | 9 | 46.0 ± 17.0 | 4 | 5 | 8 | 1 |
| TSH | 1 | 52 | 1 | 0 | 0 | 1 |
| NCA | 3 | 55.0 ± 17.4 | 2 | 1 | 3 | 0 |
PA: pituitary adenoma, CD: Cushing disease, SCA: silent corticotroph adenoma, PRL: prolactin secreting tumor, GH: growth hormone secreting tumor, TSH: thyroid-stimulating hormone secreting tumor, NCA: null cell adenoma.
2.2. FKBP5 mRNA measurement
Total RNA was purified from whole blood using the RiboPure blood RNA isolation kit (Thermo Fisher, AM9128), according to manufacturer's instructions. RNA was eluted using 50 μl Elution Solution followed by DNase treatment to remove genomic DNA and RNA purity was confirmed and concentration was measured using NanoDrop. cDNA synthesis was then performed using a cDNA reverse transcription kit with random hexamers. FKBP5 mRNA expression was quantified using TaqMan gene expression assay and compared with the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA (Life Technologies, Hs01561006_m1 and Hs02758991_g1). Relative FKBP5 expression was determined using the 2−ΔΔCT method normalized to GAPDH expression [13].
2.3. Statistics analysis
All experiments were performed in triplicate and statistical analyses were conducted with SPSS for Mac Version 22.0 (SPSS Inc, Chicago, IL) and Prism 7 Version 7.0 (Graphpad Inc, San Diego, CA, USA). The Shapiro-Wilk test was used to assess normality distribution; and normalized data are expressed as mean ± standard deviations (SD), and non-normalized data are reported as median ± interquartile ranges. Two-way ANOVA was used for multiple comparisons taking a p-value <0.05 as significant.
3. Results
3.1. FKBP5 mRNA expression in patients with pituitary tumors
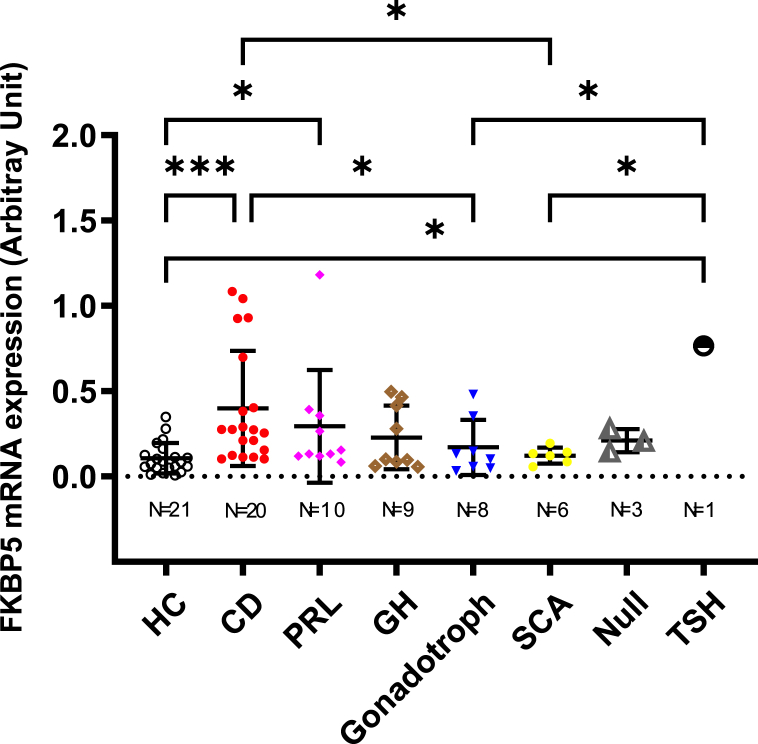
As depicted in Fig. 1, median FKBP5 mRNA levels were higher in 20 patients with CD (median [IQR], 0.27 [0.13–0.63]) compared to the 21 healthy controls (0.085 [0.05–0.14], p < 0.0005), 8 patients with gonadotroph tumors (0.12 [0.05–0.30], p < 0.05) and 6 patients with silent corticotroph pituitary tumors (SCA, 0.13 [0.08–0.16], p < 0.05, Fig. 1). However, circulating FKBP5 mRNA levels in patients with CD did not differ from 10 patients with PRL-secreting pituitary tumors (0.14 [0.12–0.37]) which were also higher compared to levels measured in healthy controls (p < 0.05), even after exclusion of one outlying patient that who had concomitant depression and exhibited a particularly elevated FKBP5 mRNA level of 1.18 arbitrary units (p = 0.008). Circulating FKBP5 mRNA expression did not differ between nine patients with GH-secreting (0.10 [0.07–0.44]) pituitary tumors and healthy controls. Interestingly, a single patient with a TSH-secreting pituitary tumor also exhibited higher FKBP5 mRNA expression compared to healthy controls (p < 0.05), and patients who harbored either a gonadotroph (p < 0.05), or a SCA (p < 0.05, Fig. 1).
Fig. 1.
Whole blood FKBP5 mRNA expression in patients with various pituitary tumor subtypes and healthy controls. Relative quantification of whole blood FKBP5 mRNA expression was assessed in multiple pituitary adenoma subtypes prior to pituitary tumor resection and 21 healthy controls. Each data point indicates an individual sample, expressed as arbitrary 2−ΔΔCT values. FKBP5 mRNA levels were higher in 20 patients with CD compared to 21 healthy controls (p < 0.0005), 8 patients harboring gonadotroph- (p < 0.05) and 6 patients with silent corticotroph-pituitary tumors (SCA, p < 0.05). Higher FKBP5 mRNA levels were also observed in 10 patients with lactotroph pituitary tumors (PRL) compared to healthy controls (p < 0.05). A single patient with a TSH-secreting pituitary tumor also exhibited higher FKBP5 mRNA expression compared to healthy controls (p < 0.05), or patients harboring gonadotroph (p < 0.05) or SCA (p < 0.05). All other comparisons did not attain statistical significance. HC, Healthy Controls.
4. Discussion
FKBP5 is a key regulator of glucocorticoid receptor (GR) sensitivity and action. Given it is expressed on circulating lymphocytes, it can be relatively easily quantitated in peripheral blood samples obtained in clinical practice. Prior studies have demonstrated elevated circulating FKBP5 mRNA expression in patients with several psychiatric disorders [14], where it correlated with elevated glucocorticoid levels and one prior study reported higher circulating FKBP5 mRNA levels in 12 patients with CD compared to healthy subjects. These authors also noted that circulating FKBP5 mRNA levels fell following corticotroph tumor resection to levels comparable to healthy controls [4].
We sought to investigate the potential broader utility of FKBP5 mRNA quantitation in peripheral blood to distinguish corticotroph pituitary tumors causing hypercortisolism from other pituitary tumor sub-types. Consistent with the prior study, we did observe elevated circulating FKBP5 mRNA expression in a series of 20 patients with CD although we noted high variability in the circulating FKBP5 mRNA levels with significant overlap between patients with CD and healthy controls (Fig. 1).
Interestingly, we also observed high circulating FKBP5 mRNA levels in 10 patients with prolactin-secreting pituitary tumors compared to healthy controls. One of these patients had a history of depression but the elevated FKBP5 mRNA level we observed in patients with PRL-omas persisted even when this patient was eliminated from the analysis. Given increased circulating FKBP5 expression has been reported in several other conditions including relatively common psychiatric disorders such as anxiety, major depression and cognitive impairment [15], this may in part explain our observations. It is important to note that none of these patients were receiving dopamine agonist therapy, the latter of which can cause depressive symptoms as a side effect [16,17]. We also observed comparatively increased circulating FKBP5 mRNA in a single patient with a TSH-secreting pituitary tumor. Expression of FKBP5 can be increased by several nuclear receptor ligands including progestins and androgens, whereby FKBP5 expression is significantly lower in the follicular phase of the menstrual cycle. This is a further confounding factor that may contribute to the variability in FKBP5 expression we observed in some of our female patients. The association between thyroid hormone receptor activation and increased FKBP5 mRNA, though uncharacterized, is plausible given the connection between FKBP5 and many other steroid receptors [18].
Although lymphocytes harbor the highest FKBP5 mRNA expression in peripheral blood, absolute lymphocyte number is often decreased in patients with Cushing Syndrome and variable lymphocyte GR number and affinity have been described, all of which could also influence relative circulating FKBP5 expression [18]. Furthermore, it is important to note that the cytosolic MR-containing complex is highly similar to the cytosolic GR complex and in addition to including hsp 90, and other common co-chaperones, FKBP5 is also a component of that complex. Therefore co-existing hypertension, and potentially drug therapy may affect FKBP5 expression.
Overall, our study indicates that measurement of circulating FKBP5 is quite non-specific and does not reliably distinguish a corticotroph pituitary tumor from other pituitary tumor sub-types. Limitations to our study include a relatively small sample size and single sample collection. That said, our goal was to examine the potential utility of circulating FKBP5 mRNA measurement in a “real-world” clinical setting to distinguish patients with pituitary corticotroph tumors causing hypercortisolism from other pituitary tumor sub-types. We conclude that due to the many factors and medical disorders that affect FKBP5 expression, measurement of circulating FKBP5 is not helpful to distinguish corticotroph tumors from other pituitary tumor sub-types.
References
- 1.Findling J.W., Raff H. Diagnosis of endocrine disease: differentiation of pathologic/neoplastic hypercortisolism (Cushing's syndrome) from physiologic/non-neoplastic hypercortisolism (formerly known as pseudo-Cushing’s syndrome) Eur. J. Endocrinol. 2017;176(5):R205–R216. doi: 10.1530/EJE-16-0946. [DOI] [PubMed] [Google Scholar]
- 2.Nieman L.K., Biller B.M., Findling J.W., Newell-Price J., Savage M.O., Stewart P.M., et al. The diagnosis of cushing's syndrome: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2008;93(5):1526–1540. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wochnik G.M., Ruegg J., Abel G.A., Schmidt U., Holsboer F., Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 2005;280(6):4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- 4.Bancos I., Hatipoglu B.A., Yuen K.C., Chandramohan L., Chaudhari S., Moraitis A.G. Evaluation of FKBP5 as a cortisol activity biomarker in patients with ACTH-dependent Cushing syndrome. J. Clin. Transl. Endocrinol. 2021;24 doi: 10.1016/j.jcte.2021.100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menke A., Klengel T., Rubel J., Bruckl T., Pfister H., Lucae S., et al. Genetic variation in FKBP5 associated with the extent of stress hormone dysregulation in major depression. Gene Brain Behav. 2013;12(3):289–296. doi: 10.1111/gbb.12026. [DOI] [PubMed] [Google Scholar]
- 6.Binder E.B., Salyakina D., Lichtner P., Wochnik G.M., Ising M., Putz B., et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 2004;36(12):1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 7.Binder E.B., Bradley R.G., Liu W., Epstein M.P., Deveau T.C., Mercer K.B., et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szczepankiewicz A., Leszczynska-Rodziewicz A., Pawlak J., Narozna B., Rajewska-Rager A., Wilkosc M., et al. FKBP5 polymorphism is associated with major depression but not with bipolar disorder. J. Affect. Disord. 2014;164:33–37. doi: 10.1016/j.jad.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Rao S., Yao Y., Ryan J., Li T., Wang D., Zheng C., et al. Common variants in FKBP5 gene and major depressive disorder (MDD) susceptibility: a comprehensive meta-analysis. Sci. Rep. 2016;6 doi: 10.1038/srep32687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii T., Ota M., Hori H., Hattori K., Teraishi T., Matsuo J., et al. The common functional FKBP5 variant rs1360780 is associated with altered cognitive function in aged individuals. Sci. Rep. 2014;4:6696. doi: 10.1038/srep06696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stamm T.J., Rampp C., Wiethoff K., Stingl J., Mossner R.G.O.M., et al. The FKBP5 polymorphism rs1360780 influences the effect of an algorithm-based antidepressant treatment and is associated with remission in patients with major depression. J. Psychopharmacol. 2016;30(1):40–47. doi: 10.1177/0269881115620459. [DOI] [PubMed] [Google Scholar]
- 12.Ising M., Maccarrone G., Bruckl T., Scheuer S., Hennings J., Holsboer F., et al. FKBP5 gene expression predicts antidepressant treatment outcome in depression. Int. J. Mol. Sci. 2019;20(3):485. doi: 10.3390/ijms20030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hameed N., Yedinak C.G., Brzana J., Gultekin S.H., Coppa N.D., Dogan A., et al. Remission rate after transsphenoidal surgery in patients with pathologically confirmed Cushing's disease, the role of cortisol, ACTH assessment and immediate reoperation: a large single center experience. Pituitary. 2013;16(4):452–458. doi: 10.1007/s11102-012-0455-z. [DOI] [PubMed] [Google Scholar]
- 14.Binder E.B. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Zannas A.S., Wiechmann T., Gassen N.C., Binder E.B. Gene-stress-epigenetic regulation of FKBP5: clinical and translational implications. Neuropsychopharmacology : Off. Publ. Am. Coll. Neuropsychopharmacol. 2016;41(1):261–274. doi: 10.1038/npp.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ioachimescu A.G., Fleseriu M., Hoffman A.R., Vaughan T.B., III, Katznelson L. Psychological effects of dopamine agonist treatment in patients with hyperprolactinemia and prolactin-secreting adenomas. Eur. J. Endocrinol. 2019;180(1):31–40. doi: 10.1530/EJE-18-0682. [DOI] [PubMed] [Google Scholar]
- 17.Hinojosa-Amaya J.M., Johnson N., González-Torres C., Varlamov E.V., Yedinak C.G., McCartney S., et al. Depression and impulsivity self-assessment tools to identify dopamine agonist side effects in patients with pituitary adenomas. Front. Endocrinol. 2020;11 doi: 10.3389/fendo.2020.579606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinouchi S., Iga J., Ueno S., Yamauchi K., Numata S., Song H., et al. FKBP5, SERT and COMT mRNA expressions in the peripheral leukocytes during menstruation cycle in healthy reproductive females. Neurosci. Lett. 2008;434(1):124–128. doi: 10.1016/j.neulet.2008.01.039. [DOI] [PubMed] [Google Scholar]