Abstract
Background
Up to 80% of children with autism experience behavioural sleep problems, predominantly relating to bedtime resistance, sleep anxiety, sleep dysregulation, and shorter duration, which are associated with increased autistic symptom expression and emotional and behavioural difficulties. Researchers predicted the COVID-19 pandemic would worsen sleep and behavioural difficulties for autistic children, due to their need for routine and certainty. This systematic review is the first to focus on delineating the role of sleep disturbance in exacerbating autistic symptoms and internalising and externalising behaviours during the pandemic.
Method
In this PROSPERO registered systematic review, we aggregated and synthesised findings from empirical studies from 2020 onwards that included children with autism and examined sleep outcomes, using narrative and framework synthesis due to the variety of methods and designs employed. We identified additional relevant themes through inductive thematic analysis.
Results
Seventy-one studies met the search criteria, and we selected seventeen for review following screening and quality assessment. These studies reported mixed findings; with strongest support for worsening of sleep problems typically experienced by autistic children, including difficulties with sleep regulation and shorter sleep duration. Further, sleep problems were associated with increased expression of autistic characteristics.
Conclusions
Preliminary findings of worsening sleep and increased autistic characteristics for autistic children throughout the COVID-19 pandemic highlight the need for ongoing, accessible and flexible service provision during exposure to environmental stressors. We propose that behavioural sleep interventions are well suited to telehealth adaptation and play an important role in supporting families when in-person treatment for sleep problems is not possible.
Keywords: Autism, Children, Sleep, COVID-19, Telehealth
1. Background
1.1. Autism spectrum disorder
Autism spectrum disorder, hereafter referred to as ‘autism’,1 is diagnosed in children who present with differences and challenges across two domains: (1) social/communication, e.g. relationships, non-verbal communication, and social-emotional reciprocity; and (2) restricted and repetitive behaviours, e.g. insistence on ‘sameness’, stereotyped/repetitive behaviours, special interests, and unusual sensory symptoms/behaviours (American Psychiatric Association, 2013). Emotional and behavioural difficulties are common for children with autism (Chandler et al., 2016), the expressions of which are categorised as internalising (i.e., anxiety/depression/withdrawal) or externalising behaviours (i.e., dysregulation/impulsivity/aggression; Bauminger, Solomon, & Rogers, 2010). Further, psychiatric and mental-health conditions, intellectual disability, and sleep-wake disorders are more prevalent in autistic populations (Baio et al., 2018, Lai et al., 2019).
1.2. Sleep problems in autism
Behavioural sleep problems are the most common type of sleep-wake disorder experienced by autistic children, affecting 50–80% of autistic children as compared to 25–51% of children overall (Chen et al., 2021, de Almeida and Nunes, 2019, Richdale and Schreck, 2009). Behavioural sleep problems include sleep regulation difficulties such as irregular patterns of sleeping and waking, trouble falling or staying asleep, and early morning waking; as well as anxiety related to sleep and bedtime (sleep anxiety), and bedtime resistance (Chen et al., 2021, Díaz-Román et al., 2018, Krakowiak et al., 2008, Richdale and Schreck, 2009). Given this prevalence, it is important to identify the impact of sleep problems and address treatment needs.
Sleep problems can result in poorer sleep quality, with increased activity and awakenings during sleep time, poorer sleep efficiency, longer sleep latency (time to fall asleep), and shorter sleep duration common for autistic children (Souders et al., 2009). Sleep duration for autistic children was 32.8 min less than for non-autistic children according to a meta-analysis of ten studies that used objective measures of sleep (Elrod & Hood, 2015). Sleep problems and poorer sleep quality have been associated with increased severity of core autistic symptoms, increased internalising and externalising behaviours, poorer executive and daytime functioning, and social functioning problems (Han et al., 2022, Whelan et al., 2022). For autistic individuals, sleep problems in childhood can impact later quality of life (Deserno et al., 2019). Behavioural sleep problems may arise from the reliance of autistic children on particular stimuli to fall or remain asleep, and be precipitated by environmental, psychological and physical stressors (Souders, Taylor, & Zavodny Jackon, 2020). The impact of the environment on sleep is often exacerbated by core autistic symptoms such as insistence on ‘sameness’, hyperarousal and sensory sensitivities, alongside internalising behaviours such as anxiety and challenging externalising behaviours (Hollway and Aman, 2011, Mazurek and Petroski, 2015, Richdale and Schreck, 2009, Richdale et al., 2014, Souders et al., 2020).
However, the relationship between sleep problems and core autistic symptoms is complex and multifaceted. A theoretical framework by Hollway and Aman (2011) describes a bidirectional relationship between vulnerability factors, environmental stressors, and coping strategies for insomnia in children with autism. In this framework, core autistic symptoms and environmental stressors increase internalising and externalising behaviours, leading to over-arousal and insomnia and adverse behavioural outcomes (Hollway & Aman, 2011). This framework would predict a significant impact on both sleep and emotional and behavioural functioning in autistic children from chronic environmental stressors such as the recent COVID-19 pandemic.
In assessing sleep problems, paediatric consultation is often required to rule out underlying medical causes such as sleep apnoea or other medical conditions associated with sleep disruption, such as epilepsy. With medical conditions ruled out, the importance of timely intervention to support children with behavioural sleep problems in clinical care is well established. Behavioural sleep intervention is recommended as the first line of treatment (Malow et al., 2012, National Institute for Health and Care Excellence, 2013), and can be effective in improving sleep as well as emotional and behavioural difficulties (Hunter et al., 2020, Papadopoulos et al., 2019, Papadopoulos et al., 2022). However, sleep problems often remain untreated (Cohen, Conduit, Lockley, Rajaratnam, & Cornish, 2014) and families can find it difficult to access services (Lord et al., 2022). The COVID-19 pandemic worsened access to services for many families (McNally Keehn, Tomlin, & Ciccarelli, 2021), potentially adding to the impact on sleep for autistic children. Behavioural sleep interventions, particularly those that are brief, show promise for scalability to more flexible delivery modes such as telehealth, to improve accessibility (McLay et al., 2020, Papadopoulos et al., 2022, Pattison et al., 2020, Tan-MacNeill et al., 2020).
1.3. The impact of the COVID-19 pandemic
The COVID-19 virus emerged in December 2019 and was declared a global pandemic by the World Health Organisation in March 2020 (Cucinotta & Vanelli, 2020). In response, governments world-wide implemented a range of restrictions on travel, movement, and gatherings; closed or moved education to an online format; or instituted broader lockdowns (Haug et al., 2020). Children and adolescents were impacted by restrictions on engaging with in-person schooling, therapy, social gatherings, and community activities such as sport, which are crucial for healthy development. Researchers immediately forecast a huge negative impact on children with neurodevelopmental conditions such as autism (Ameis et al., 2020, Becker and Gregory, 2020).
Autistic children are particularly vulnerable to change in their environments, preferring routine and certainty; with chronic and unpredictable changes contributing greatly to the daily struggles for families, including behavioural sleep disturbance (Ameis et al., 2020, Becker and Gregory, 2020, Hollway and Aman, 2011). Nonetheless, some of the pandemic impacts such as ‘school closures’ and more time at home may also offer a sanctuary to autistic children who struggled with school attendance and community social interaction pre-pandemic (Ameis et al., 2020). In support, whilst research reviews report study findings of negative impacts on wellbeing and behaviour for autistic children, the findings are not always consistent (e.g., Ahmed et al., 2022; Alonso-Esteban, López-Ramón, Moreno-Campos, Navarro-Pardo, & Alcantud-Marín, 2021; Shorey, Lau, Tan, Ng, & Ramkumar, 2021). Some studies have also shown that autistic children were not greatly impacted by the pandemic or even experienced improvements in functioning with the burden of engaging in daily life reduced during pandemic related restrictions (e.g., Asbury, Fox, Deniz, Code, & Toseeb, 2021; Asbury & Toseeb, 2022; Colizzi et al., 2020). However, the influence of the pandemic is likely to be more complex, as indicated by recent findings that whilst autistic individuals enjoyed the release from social challenges, they also experienced deep social loss and reduced mental health (Pellicano et al., 2021); which both relate to disrupted sleep (Freeman et al., 2020, Griffin et al., 2020, Schreck and Richdale, 2020).
The impact of this pandemic specifically on sleep for autistic children is even less clear. The literature has predominantly employed survey based cross-sectional designs to report a mix of worsened, improved or unchanged sleep outcomes (e.g., Bruni, Breda, Ferri, & Melegari, 2021; Bruni et al., 2022; Huang et al., 2021; Hosokawa et al., 2021; Lugo-Marin et al., 2021). However, the overall direction of findings has not yet been clearly established. To our knowledge, no aggregation or synthesis of these studies has been conducted to provide a cumulative account of the state of the evidence regarding the effect of the COVID-19 pandemic, as an environmental stressor, on sleep for children with autism.
This pandemic provides a unique context to further develop understanding about the relationship between symptoms of autism, environmental stressors, emotional and behavioural difficulties, and sleep in children predicted by the Hollway and Aman (2011) bidirectional theoretical framework. Understanding the bidirectional relationship proposed by Hollway and Aman (2011) may elucidate an approach to assessment, treatment, and broader scaffolding for children with autism and their families when increased environmental stressors are sustained. The use of telehealth to deliver health services grew exponentially during the COVID-19 pandemic (Taylor et al., 2021) and is predicted to improve longer term support for autistic individuals and their families (Ameis et al., 2020, Ellison et al., 2021). Emerging research suggests that sleep treatment delivered via telehealth is effective (e.g., Ali et al., 2018; Davenport, Berry, Mazurek, & McCrae, 2021; McCrae et al., 2020; Rigney et al., 2018; Tan-MacNeill, Smith, & Weiss, 2020). However, firm conclusions are precluded by methodological limitations. For example, these limitations include: (1) studies not being controlled and blinded, (2) the variety of intervention structure and delivery used in each study making it difficult to compare outcomes (e.g., long versus short interventions, live intervention sessions versus pre-recorded resources etc.), (3) broad inclusion of participants with a range of neurodevelopmental disorders that include but are not exclusive to autism (e.g., Stuttard, Clarke, Thomas, & Beresford, 2015; Tan-MacNeill, Smith, & Weiss, 2020), or (4) single case study design (e.g., Davenport et al., 2021). Accordingly, there is a need to better understand the impact of environmental stressors such as a pandemic on sleep for autistic children, as well as how to best support impacted families when access to treatment is a challenge.
1.4. Study aim
This study aimed to systematically review published empirical findings to ascertain characteristics of sleep that are reported to have worsened, not changed, or improved for autistic children during the COVID-19 pandemic. We also sought to identify whether changes in sleep were associated with changes in internalising and externalising behaviours, as well as severity of autistic symptoms, as predicted by the Hollway and Aman (2011) bidirectional theoretical framework.
2. Method
2.1. Design
This systematic review was registered with PROSPERO (CRD42021296012) and adhered to the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA 2020 Statement’ (Page et al., 2021). The primary outcome of interest was sleep, and secondary outcomes drawn from primary outcome studies as they relate to sleep, were internalising and externalising behaviours and autism symptom severity.
2.2. Search strategy
The literature search was conducted in December 2021 in the PsycInfo, Medline, and Embase electronic databases. Keywords associated with autism spectrum disorder, sleep, and the COVID-19 pandemic with appropriate truncation and Boolean operators, as well as their related subject headings, were incorporated in each search of titles and abstracts. Each search was additionally limited to English language where possible. Additional papers were discovered using similar search terms in Scopus, via automated alerts from databases, or backwards searching reference lists of other publications. Search results were uploaded to Endnote and duplicates removed. De-duplicated results were uploaded to Covidence for screening. See supplementary file for full search strategy.
2.3. Study selection and data extraction
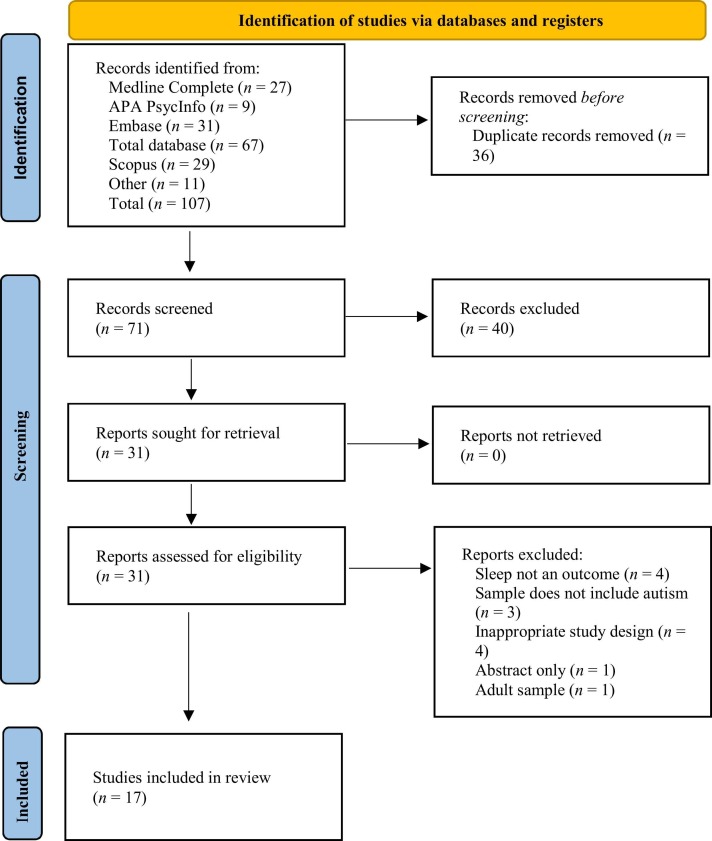
The inclusion criteria comprised: (1) data collected during the COVID-19 pandemic, (2) sample includes autistic children (0–18 years; parent/caregiver-report or official diagnosis), and (3) subjective (parent/caregiver or child) quantitative or qualitative report or objective measure of sleep. Studies that had not been peer-reviewed, non-empirical studies, and those that were abstract only, conference proceedings, or not written in English were to be excluded. Using Covidence, two independent researchers (SL and MW) screened titles and abstracts then full texts against the eligibility criteria. Any disagreements were discussed and consensus was reached. Articles excluded following full text screening were noted with the reason for exclusion. See Fig. 1 for study selection flow diagram (Page et al., 2021). The first author extracted data including design, sample, measures, context of study (setting, country, data collection time period, and COVID-19 restrictions), and primary and secondary outcome results.
Fig. 1.
PRISMA 2020 Flow Diagram.
2.4. Quality assessment
The ‘Checklist for Prevalence Studies’ or ‘Checklist for Qualitative Research’ was used to critically appraise each study for inclusion based upon study specific details including sample, setting, measurement, statistical analysis, and response rate (The Joanna Briggs Institute, 2017). Then the Weight of Evidence framework was used to weight the suitability of each study to address the aims of this systematic review based upon trust in the quality of the evidence (which was derived from the previously mentioned checklists), appropriateness of the design to address the aims, and relevance of the focus to the topic of our systematic review (Gough, 2007). Each study was given a combined weight of low, medium, or high (see Table 1). The combined weight was derived from averaging the weights across trust, appropriateness and relevance. For example, a study weighted low in trust, medium in appropriateness and high in relevance was given an overall weight of medium or a study that was weighted low in trust and medium in appropriateness and relevance was given a combined weight of medium. The quality assessments were conducted in Covidence independently by two authors (SL and MW), who met to resolve any inconsistencies and were able to reach consensus.
Table 1.
Weight of Evidence for Included Studies.
| Study | Trust | Appropriateness | Relevance | Combined Weight |
|---|---|---|---|---|
| Berard et al. (2021) | low | low | medium | low |
| Bruni et al. (2021) | medium | medium | high | medium |
| Bruni et al. (2022)a | medium | medium | high | medium |
| Di Renzo et al. (2020) | low | medium | medium | medium |
| Dondi et al. (2021) | low | low | low | low |
| Garcia, Lawrence, Brazendale, Leahy, and Fukuda (2021) | low | medium | medium | medium |
| Guller, Yaylaci, and Eyuboglu (2021) | low | low | medium | low |
| Hosokawa et al. (2021) | low | low | low | low |
| Huang et al. (2021) | low | medium | medium | medium |
| Lugo-Marin et al. (2021) | low | low | medium | low |
| Miniarkova et al. (2021) | medium | low | low | low |
| Panjwani, Bailey, and Kelleher (2021) | low | medium | low | low |
| Polónyiová et al. (2021) | medium | medium | medium | medium |
| Rabbani et al. (2021) | medium | medium | medium | medium |
| Stankovic et al. (2021) | low | low | low | low |
| Tokatly Latzer, Leitner, and Karnieli-Miller (2021) | medium | medium | low | medium |
| Turkoglu, Ucar, Cetin, Guler, and Tezcan (2020) | medium | high | high | high |
Whilst this study re-analysed data from Bruni et al. (2021), the weight of evidence for each study was calculated separately due to differing analyses and outcomes.
2.5. Data analysis
As a large variety of methods and designs, including both quantitative and qualitative, were used in selected studies, the narrative synthesis approach was adopted to summarise and explain findings with text (Booth, 2016). The narrative synthesis process adhered to the guidance provided by Popay, Roberts, Sowden, Petticrew, and Ara (2006), and included a preliminary synthesis of the data using tabulation and thematic analysis, exploring relationships in the data using a mind map, and assessing the robustness of the synthesis by incorporating quality assessment into the discussion and conclusions on direction of findings. Further, a framework synthesis approach was incorporated (Snilstveit, Oliver, & Vojtkova, 2012), using key concepts from the Hollway and Aman (2011) bidirectional theoretical framework a priori to organise and aggregate findings. Additionally, an inductive thematic synthesis of the content of each included study was conducted using NVIVO, to ensure other themes grounded in the data (beyond the identified primary and secondary outcomes) were not overlooked (Snilstveit et al., 2012).
3. Results
We identified seventeen studies for inclusion in this systematic review (see Fig. 1). However, two studies are inter-related and analyse data originally collected during 2020 (Bruni et al., 2021, Bruni et al., 2022), with the second study (Bruni et al., 2022) presenting a re-analysis of the original data with slightly different outcomes (e.g., percentages of modified bedtimes and risetimes, and statistical differences in duration and sleep latency before and during lockdown) from a sample with a slightly broader age-range. We predominantly cite findings from the Bruni et al. (2021) study, and where appropriate we cite findings from each study separately.
Of the included studies only one had a high combined weight of evidence (see Table 1). One neurodevelopmentally diverse study sample included 43.8% autistic participants and reported sleep outcomes for this sub-set of the sample separately (Guller et al., 2021). Another study sample (N = 6210) only included 49 autistic participants, reporting data for these participants separately (Dondi et al., 2021). Three studies employed a comparison group of either non-autistic children (Hosokawa et al., 2021, Polónyiová et al., 2021), or both an attention deficit hyperactivity disorder (ADHD) group and comparison group without autism or ADHD (Bruni et al., 2021).
Six studies did not report intelligence quotient (IQ), level of functioning, or schooling type of participating autistic children (Bruni et al., 2021, Dondi et al., 2021, Guller et al., 2021, Huang et al., 2021, Panjwani et al., 2021, Polónyiová et al., 2021). Of the remaining studies, eight studies reported a range of IQ, functioning, or type of schooling (Berard et al., 2021, Di Renzo et al., 2020, Hosokawa et al., 2021, Lugo-Marin et al., 2021, Miniarikova et al., 2021, Rabbani et al., 2021, Stankovic et al., 2021, Tokatly Latzer et al., 2021). One study excluded participants who had physical or psychological disorders (Garcia et al., 2021); and another excluded participants who had chronic physical disorders, additional neurodevelopmental disorder diagnosis, or medication use (Turkoglu et al., 2020). Whilst the studies arise from a range of countries worldwide, only one explicitly reported race (Panjwani et al., 2021). Of the studies that reported on socio-economic status or income related variables, there was a range of statuses or regions/settings (Berard et al., 2021, Bruni et al., 2021, Bruni et al., 2022, Dondi et al., 2021, Huang et al., 2021, Miniarikova et al., 2021, Panjwani et al., 2021, Rabbani et al., 2021, Tokatly Latzer et al., 2021), and a range of educational attainment of parents (Berard et al., 2021, Bruni et al., 2021, Bruni et al., 2022, Dondi et al., 2021, Guller et al., 2021, Huang et al., 2021, Miniarikova et al., 2021, Panjwani et al., 2021, Polónyiová et al., 2021, Rabbani et al., 2021, Tokatly Latzer et al., 2021).
Six studies compared data collected on sleep and other behaviours before the pandemic to data collected during pandemic related restrictions (Di Renzo et al., 2020, Garcia et al., 2021, Lugo-Marin et al., 2021, Polónyiová et al., 2021, Rabbani et al., 2021, Turkoglu et al., 2020), which we refer to hereon as ‘pre–post’ study design, but could also be described as prospective cohort or longitudinal design. Most included studies were cross-sectional (n = 10; Berard et al., 2021; Bruni et al., 2021; Bruni et al., 2022; Dondi et al., 2021; Guller et al., 2021; Hosokawa et al., 2021; Huang et al., 2021; Miniarikova et al., 2021; Panjwani et al., 2021; Stankovic et al., 2021), retrospectively reporting changes from before to during the pandemic. One study reported qualitative data on changes in primary and secondary outcomes, collected by semi-structured interview during the restriction period (Tokatly Latzer et al., 2021). No studies incorporated objective data (i.e., data collected from polysomnography or actigraphy) in their findings. Only three studies utilised validated sleep measures (Di Renzo et al., 2020, Lugo-Marin et al., 2021, Turkoglu et al., 2020), with the remaining studies using modified or study developed measures. The results of the pre–post studies or those using validated sleep measures are given precedence as part of the assessment of robustness of the synthesis, due to the increased strength of this evidence compared to retrospectively reported data (Setia, 2016, Thiese, 2014). See Table 2 for summary of study characteristics.
Table 2.
Characteristics of Included Studies.
| Study | Design | Sample | Study Setting | Country | Data Collection Period | Reported COVID-19 restrictions |
|---|---|---|---|---|---|---|
| Berard et al. (2021) | Cross-sectional |
N = 239 ASDa, 2–21 yrs. (Mage = 9.1, SD = 4.0) 21% female |
Research cohort | France | 27 April to 13 May 2020 | Restrictions on movement Loss of access to children’s usual education and therapy |
| Bruni et al. (2021) | Cross-sectional and comparison groups |
n = 100 ASD, 4–18 yrs 16% female n = 236 ADHDb, 4–18 years 19% female n = 340 control group, 17% female 4–18 yrs. |
Community-based | Italy | 7 May to 15 June 2020 | Lockdown for 3 months from March 2020. Loss of access to children’s usual education and therapy. Restrictions on movement |
| Bruni et al. (2022)c | Cross-sectional |
N = 111 ASD, 1–18 yrs. 16% female |
Community-based | Italy | 7 May to 15 June 2020 | Lockdown not otherwise described |
| Di Renzo et al. (2020) | Observational, single group pre–post |
N = 63 ASD, 2.7–9.4 yrs. (Mage = 5.9, SD = 1.7) 13% female |
Clinical | Italy | February and April 2020 | Stay at home orders Loss of access to children’s usual education and therapy |
| Dondi et al. (2021) | Cross-sectional |
n = 49 ASD, N = 6210, ≤ 18 yrs. Mean age unknown % female unknown |
Community-based | Italy | 1 September to 15 October 2020 | Not provided |
| Garcia et al. (2021) | Observational, single group pre–post |
N = 9 ASD, 14–19 yrs. (Mage = 16.87, SD = 1.36) 11% female |
Educational | United States | March to April 2020 | Stay at home orders Loss of access to children’s usual education and therapy |
| Guller et al. (2021) | Cross-sectional |
N = 299, 2–18 yrs. (Mage = 10.32, SD = 4.57), 29% female n = 131 ASD (43.8%), (Mage = 10.31, SD = 4.32) 16% female |
Clinical | Turkey | 18–30 April 2020 | Stay at home orders Loss of access to children’s usual education and therapy Curfew |
| Hosokawa et al. (2021) | Cross-sectional, non-autistic comparison group |
n = 84 ASD, 6–18 yrs. (Mage = 11.6, SD = 3.1), n = 361 control 25% female |
Clinical | Japan | 30 April to 8 May 2020 | School closures |
| Huang et al. (2021) | Cross-sectional |
N = 406 ASD, (Mage = 4.6 yrs., SD = 2.3) 19% female |
Clinical | China | 12–31 May 2020 Restrictions easing |
Social distancing Loss of access to children’s usual education and therapy |
| Lugo-Marin et al. (2021) | Observational, single group pre–post and cross-sectional |
N = 37 ASD, 3–17.11 yrs. (Mage = 10.7, SD = 3.4) 14% female |
Clinical | Spain | Not clear | Restrictions on movement Social distancing |
| Miniarkova et al. (2021) | Cross-sectional | N = 134 ASD (Mage = 8.6, SD = 4.0) 18% female |
Research cohort | France | 27 April to 13 May 2020 | School, university, and public venue closure |
| Panjwani et al. (2021) | Cross-sectional |
N = 197, 2–17 yrs. (Mage = 7.7, SD = 4.1) 24% female |
Community-based | United States | 6 May to 23 June 2020 | Stay at home or shelter in place orders for most states |
| Polónyiová et al. (2021) | Pre–post with comparison group | First wave: n = 84 ASD, 8% female (Male Mage = 7.7, SD = 3.5; female Mage = 7.7, SD = 2.5) n = 95 control 23% female (Male Mage = 8.4, SD = 4.3; female Mage = 9.3, SD = 4.1) Second wave: n = 71 ASD, 12% female (Male Mage = 8.6, SD = 3.9; female Mage = 10.8, SD = 5.1) n = 82 control 25% female (Male Mage = 9.5, SD = 4.8; female Mage = 8.6, SD = 4.9) |
Community-based | Slovakia | June to July 2020 | First wave (March to June 2020): Face-masks, social distancing, travel quarantine, all service closed excluding essential services but including education and therapy. Second-wave (October to December 2020) less severe restrictions |
| Rabbani et al. (2021) | Prospective longitudinal |
N = 150 ASD, n = 86 reported on sleep, 2–9 yrs. 20% female |
Research cohort | Bangladesh | November 2019 to February 2020 March to May 2020 June to November 2020 |
Lockdown, not otherwise described |
| Stankovic et al. (2021) | Cross-sectional | N = 85, 2–24 yrs. (Mage = 9.2, SD = 4.5) | Community-based | Serbia | Data collected across 18 days in 2020 (6 days after commencement of state of emergency) | State of emergency, with lockdown from 9 to 12 h per day |
| Tokatly et al. (2021) | Qualitative |
N = 25 ASD, (Mage = 5 yrs., 11 mths) 12% female |
Clinical | Israel | April 2020 | Restrictions on movement Loss of access to children’s usual education and therapy |
| Turkoglu et al. (2021) | Observational, single group pre–post |
N = 46 ASD, 4–17 yrs. (Mage = 7.89) 17% female |
Clinical | Turkey | 7–14 May 2020 | Stay at home orders Loss of access to children’s usual education and therapy |
ASD = autism spectrum disorders; bADHD = Attention Deficit Hyperactivity Disorder; c This sample overlaps with Bruni et al. (2021).
3.1. Primary outcomes: impact on sleep
The synthesis of sleep outcomes is detailed below under the following headings: (1) General Sleep Quality: outcomes broadly related to sleep or described as sleep quality, (2) Sleep Regulation: specific outcomes related to problems with sleep regulation, (3) Sleep Duration: specific outcomes related to the duration of sleep, and (4) Other Sleep Problems: a variety of specific behavioural and physiological/medical sleep outcomes that do not fit the other categories. Outcomes are further grouped according to worsened, no change, or improved, excluding Sleep Duration which is grouped according to decreased, no change, or increased. See Table 3 for detailed results reported by each study, including purely cross-sectional findings not summarised here (e.g., Guller et al., 2021).
Table 3.
Broad Summary of Primary Outcomes.
| Study | Measures | Sleep Outcomes |
|---|---|---|
| Berard et al. (2021) | Study developed surveys/ questionnaires |
Worsening 32.5%, no change 55.5%, improvement 12% (approximately) Improved versus worsened sleep, p = .003. Improved versus no change, p = .01. |
| Bruni et al. (2021) | Study developed surveys/ questionnaires including modified SDSCa | In depth analyses comparing sleep for ASDb, ADHDc and control group pre and during lockdown Summary provided below ASD group more likely to maintain similar bedtime* and risetime* compared to ADHD and control group For ASD sample:Sleep duration* weekday decreased 25.5%, no change 52%, increased 22.4%, ASD v control x2 = 7.214, p = .027 ASD children sleeping < 7 h before 11.2%, ASD v control, x2 = 5.588, p < .018; and during 20.2%, ASD v control, x2 = 18.888, p < .001 Sleep duration* weekend decreased 25%, no change 58%, increased 17%, ASD v control x2 = 22.627, p < .001 Sleep latency weekday increased 57.7%, no change 40.8%, decreased, 1.4%, ASD v control x2 = 12.058, p = .002 Sleep latency weekend increased 61.3%, no change 36.3%, decreased, 2.5%, ASD v control x2 = 14.101, p < .001 Increased difficulty falling asleep, 23–35%, p = .029; anxiety at bedtime 12–22%, p = .006; daytime sleepiness 4–15%, p = .003 Sleep disorder comparison* ASD v Control: Bedtime weekdays 10–11 pm 25% v 35.9%, x2 = 4.122, p = .042 Risetime weekdays < 7 am 19.6% v 4.5%, x2 = 23.901, p < .001; 8–9 am 22.7% v 42.7%, x2 = 12.891, p < .001; > 10 am 12.4% v 5.9%, x2 = 4.569, p = .033. Difficulty falling asleep pre-lockdown 23% v 21.2%, x2 = 0.152, no significant difference; during lockdown 35% v 19.1%, x2 = 11.099, p = .001 Anxiety at bedtime pre-lockdown 12% v 5.6%, x2 = 4.831, p = .028; during lockdown 22% v 11.2%, x2 = 7.687, p = .006 Night awakenings pre-lockdown 10% v 3.2%, x2 = 7.780, p = .005; during lockdown 18% v 6.2%, x2 = 13.373, p < .001 Restless sleep pre-lockdown 28% v 22.1%, x2 = 1.521, no significant difference; during lockdown 34% v 20.9%, x2 = 7.318, p = .007 Daytime sleepiness pre-lockdown 4% v 4.4%, no significant difference; during lockdown 15% v 7.9%, x2 = 4.459, p = .035 ASD group increase pre-during lockdown in hypnic jerks, rhythmic movement disorder, snoring/apnoeas, sleep walking, sleep terrors, bruxism and nightmares; all significantly greater compared to control during lockdown excluding bruxism and nightmares* |
| Bruni et al. (2022)d | Study developed surveys/ questionnaires including modified SDSCa | 57.8% modified bedtime weekdays (56.9% delayed or 0.9% advanced) 49.1% modified bedtime weekends (44.4% delayed or 4.6% advanced) 69.2% modified risetime weekdays (61.7% delayed or 7.5% advanced) 44% modified risetime weekends (31.2% delayed or 12.8% advanced) Additional results regarding changes to specific bed and risetimes* Change in sleep duration weekdays*: significant decrease in children sleeping 7–8 h, p < .05 Change in sleep duration weekends*: significant increase in children sleeping 6–7 h, p < .05 Sleep latency before v during lockdown: Weekdays: 5–15 mins 37.5–14.7%, p < .05; 15–30 mins 34.1–31.2%, p < .05; 30–60 mins 22.7–33.9%, p < .05; > 60 mins 5.7–20.2%, p < .05 Weekends: 5–15 mins 28.8–14.5%, p < .05; 15–30 mins 44.1–32.7%; 30–60 mins 21.6–30%; > 60 mins 5.4–22.7%, p < .05 |
| Di Renzo et al. (2020) | ABAS-IIe ASDBIf SSPg |
Time One to Time Two Increased sleep regulation problems: F = 15.645; p < .01; η2 = .20 Increased report of sleep regulation problems experienced ‘often’: difficulty falling asleep, x2 = 19.70, p = .001, waking unusually early, x2 = 62.26, p = .001; difficulty waking, x2 = 11.28, p = .01; and awakening during night, x2 = 50.77, p = .001 |
| Dondi et al. (2021) | Study developed surveys/ questionnaires |
ASD sample: Difficulty falling asleep: OR = 1.05 (SE = 0.38), not significant Difficulty staying asleep: OR = 1.42(SE = 0.45), not significant |
| Garcia et al. (2021) | Study developed surveys/questionnaires | Pre–post changes Hours of sleep weekdays: p = .16 Hours of sleep weekends: p = .20 44% reported a later bedtime (n = 4), 3 of these participants reported a later risetime |
| Guller et al. (2021) | Study developed surveys/ questionnaires |
ASD sample: Sleep problems 62.6%, delayed sleep phase 49.6%, dyssomnia 28.2%, insomnia 19.8%, not able to sleep alone 12.2%, advanced sleep phase 9.2%, frequent awakenings 9.2%, not wanting to sleep in own bed 3.8%, bed-wetting 2.3%, nightmares 0.8% |
| Hosokawa et al. (2021) | Study developed surveys/ questionnaires |
Change of sleep pattern: ASD group 40.5%, control group 55.1%, non-sig difference crude OR 0.69, 95% CI [0.39, 1.21] |
| Huang et al. (2021) | Study developed surveys/ questionnaires |
One or more child sleep problems 50.3%, no sleep problems 49.7% Sleep quality: 9.6% worsened, 78.6% no change, 11.8% improved Sleep duration: 10.8% decreased, 70.7% no change, 18.5% increased Most common sleep problems: difficulty falling asleep 29.3%, night waking 14.3%, and difficulty falling back to sleep after waking 16.5%. |
| Lugo-Marin et al. (2021) | CBCLh & study developed surveys/questionnaires | Sleep quality: 30% worsened, 38% no change, 32% improved |
| Miniarkova et al. (2021) | Study developed surveys/ questionnaires |
Children’s sleep: 32.3% worsened, 7.5% improved, 60.2% unchanged Improved versus unchanged sleep, OR = 0.85, 95% CI [0.15, 4.78], p = .85 Worsened versus unchanged sleep, OR = 2.41, 95% CI [0.97, 6.00], p = 0.06. |
| Panjwani et al. (2021) | Study developed surveys/ questionnaires |
Disrupted sleep: approximately 60% increase, 34% no change, 6% decrease Nightmares: approximately 18% increase, 74% no change, 8% decrease |
| Polonyiova et al. (2021) | Study developed surveys/questionnaires for sleep outcome | Later bedtime: first-wave greater for ASD v control, t(177) = 2.124, p = .035, d = 0.031; but not for second-wave Waking late: second-wave no difference ASD v control t(177) = 0.614, p = .540, d = 0.088 |
| Rabbani et al. (2021) | Study developed mCare application | Authors report increased sleep problems during lockdown Mean score pre-lockdown 5.7, 95% CI [5.4, 6.3], lockdown 5.9, 95% CI [5.1, 6.0], post-lockdown 4.2, 95% CI [3.7, 4.8]. Statistical significance not stated |
| Stankovic et al. (2020) | Modified version of Caregiver Needs Survey | 30.6% of caregivers reported sleep problems as one of the main challenges experienced in the pandemic |
| Tokatly et al. (2021) | Study developed surveys/ questionnaires |
Sleep was a common topic, with sleep problems (difficulty falling, staying, or going back to sleep and night terrors) and improvements (family sleeping better) reported |
| Turkoglu et al. (2021) | AuBCi CSHQj CCQk |
Differences from pre to post: Total CSHQ, t = − 3.612, p = .001; Bedtime Resistance, t = − 4.453, p < .001; Delay in falling asleep t = − 3.889 p < .001; Sleep duration, t = − 4.850, p < .001; Night waking, t = − 2.290, p = .027 No significant differences for Sleep anxiety, Parasomnias, Breathing Disturbance, Sleepiness during the day Total CCQ scores, t = − 3.098, p = .003 |
*Additional details reported in the journal article.
a = Sleep Disturbance Scale for Children. b = Autism Spectrum Disorder. c = Attention Deficit Hyperactivity Disorder. d = This study inter-relates with Bruni et al. (2021) and only additional outcomes reported are included. e = The Adaptive Behaviour Assessment System-II. f = ASD Behaviour Inventory. g = Short Sensory Profile. h = The Child Behavior Checklist (CBCL). i = The Autism Behavior Checklist. j = Children’s Sleep Habits Questionnaire. k = Children’s Chronotype Questionnaire.
3.1.1. General sleep quality
Four studies reported the percentage of participants who indicated that the COVID-19 pandemic either worsened, improved or made no change in sleep quality using study developed surveys, showing a spread of responses (Berard et al., 2021, Huang et al., 2021, Lugo-Marin et al., 2021, Miniarikova et al., 2021), with strongest evidence from the large cross-sectional study (n = 406, M age = 4.6 yrs., SD = 2.3) by Huang et al. (2021) whose weight of evidence is medium and reported 9.6% worsened, 78.6% no change, and 11.8% improvemed via their study developed questionnaire. In their pre–post study, Rabbani et al. (2021) reported overall worsening sleep problems pre–post pandemic from increased mean scores on their study developed questionnaire for their research cohort (n = 86, 2–9 years). However, although the overall weight of evidence for this study is medium, the questionnaire was not a validated measure of sleep problems and the statistical difference between the mean scores from each time point was not reported. Whilst one family in the qualitative study by Tokatly Latzer et al. (2021) reported an improvement in sleep, the high weight of evidence pre–post study by Turkoglu et al. (2020) reported a significant worsening of sleep for their clinical sample of autistic children (n = 46, 4–17 years, Mage = 7.89) from the total Children’s Sleep Habits Questionnaire (CSHQ) score (t = −3.612, p = .001), a validated measure of sleep. Findings are categorised below.
3.1.1.1. Worsened
Three pre–post studies reported worse sleep quality during the pandemic (Lugo-Marin et al., 2021, Rabbani et al., 2021, Turkoglu et al., 2020), as did three cross-sectional studies by retrospective report (Berard et al., 2021, Huang et al., 2021, Miniarikova et al., 2021). Another cross-sectional study reported that some caregivers found sleep to be one of the main challenges for their autistic children during the pandemic (Stankovic et al., 2021).
3.1.1.2. No change
One pre–post study reported no change in sleep quality during the pandemic for some participants (Lugo-Marin et al., 2021), which was supported by retrospective report in three cross-sectional studies (Berard et al., 2021, Huang et al., 2021, Miniarikova et al., 2021).
3.1.1.3. Improved
One small pre–post study (N = 37, 3–17.11 yrs, M age = 10.7) reported improvement in sleep quality during the pandemic for some participants (Lugo-Marin et al., 2021), which was supported by retrospective report from three cross-sectional studies (Berard et al., 2021, Huang et al., 2021, Miniarikova et al., 2021), and one qualitative study (Tokatly Latzer et al., 2021). The study by Berard et al. (2021) compared the severity score for improved, worsened or unchanged sleep, finding that the improved sleep score was significantly lower and confirming the magnitude of the difference in these outcomes for participants (Berard et al., 2021). Conversely, the study by Miniarikova et al. (2021) found the odds for improved versus unchanged sleep were not significantly different. However, as both studies were cross-sectional and the weight of evidence of each was low, and the samples were somewhat similar in size and age, the differences in findings may be attributed to differences in the way the outcomes were measured in their study developed surveys or some other unknown confounding factor.
3.1.2. Sleep regulation
Ten studies reported findings related to sleep regulation, with a majority of these findings indicating that sleep regulation did worsen during the COVID-19 pandemic. These findings are categorised and compared below; however, extensive findings on bedtimes and risetimes reported by a cross-sectional study (Bruni et al., 2021) are summarised further in Table 3.
3.1.2.1. Worsened
One pre–post study found significantly increased sleep regulation problems for participants in their clinical sample (N = 63, 2.7–9.4 yrs, M age = 5.9) during the pandemic, using the standardised ASD Behaviour Inventory (Di Renzo et al., 2020). Similarly, one cross-sectional study retrospectively reported modified sleep patterns for their clinical sample (n = 84, 6–18 yrs, M age = 11.6) using a study developed questionnaire (Hosokawa et al., 2021). In addition, a range of studies reported worsening of specific aspects of sleep regulation. Increased difficulty in falling asleep or delayed or advanced bedtimes for autistic children was reported by four pre–post studies with medium-high weight of evidence and small to large clinical, educational, and community-based samples (Di Renzo et al., 2020, Garcia et al., 2021, Polónyiová et al., 2021, Turkoglu et al., 2020), and supported by large cross-sectional (Bruni et al., 2021, Bruni et al., 2022) and qualitative (Tokatly Latzer et al., 2021) studies. Bruni et al. (2022) found that the percentage of children taking 5–15 min to fall asleep significantly decreased, whereas the percentage of children taking > 30 min to fall asleep significantly increased from before to during the lockdown. Increased frequent or early awakenings was reported by two pre–post studies with medium to high weight of evidence in their clinical samples (Di Renzo et al., 2020, Turkoglu et al., 2020), and supported by cross-sectional (Bruni et al., 2021, Panjwani et al., 2021) and qualitative (Tokatly Latzer et al., 2021) studies. Greater difficulty or later waking was reported by two medium weighted and sized pre–post studies with community-based and clinical samples (Di Renzo et al., 2020, Polónyiová et al., 2021) and one cross-sectional study (Bruni et al., 2021). Sleep regulation problems (sleep latency, difficulty falling asleep, restless sleep and frequent awakenings) were greater in the autistic (n = 100, 4–18 yrs) than comparison group (n = 340, 4–18 yrs) participants during pandemic related restrictions in a community-based sample of a cross-sectional study, according to scores on a modified version of the Sleep Disturbance Scale for Children (Bruni et al., 2021); whereas other studies reported that non-autistic participants were also waking later (n = 82; Polónyiová et al., 2021) or had modified sleep patterns (n = 361; Hosokawa et al., 2021), although these studies did not use validated or standardised measures of sleep patterns.
3.1.2.2. No change
None of the pre–post studies that compared data collected before to during the pandemic reported no change in sleep regulation. Conversely, one cross-sectional study found that there were no significant odds for difficulty falling or staying asleep for 49 autistic participants from a broader sample of participants with a neurodevelopmental disorder diagnosis, using a study developed questionnaire (Dondi et al., 2021). However, the weight of evidence for this study was low. Similarly, no change was also retrospectively reported for specific elements of sleep regulation, including sleep disruption, for some participants in a community-based sample (N = 197, 2–17 yrs, M age = 7.7) of a cross-sectional study with low weight of evidence (Panjwani et al., 2021). Bedtimes and risetimes (see Table 3 for a more detailed summary of the extensive findings from this cross-sectional study) remained more stable for autistic participants during the pandemic (n = 100, 4–18 years) compared to ADHD (n = 236, 4–18 yrs) and comparison group (n = 340, 4–18 yrs) participants, which the authors attribute to greater fixed and stereotyped behaviours in autistic children (Bruni et al., 2021). Further, no change in sleep latency for autistic participants was reported for 40.8% on weekdays and 36.3% on weekends in this cross-sectional study (Bruni et al., 2021).
3.1.2.3. Improved
No pre–post studies reported improvement in sleep regulation during the pandemic. However, sleep regulation was retrospectively reported to improve for only a very small proportion of participants in two community-based cross-sectional studies, including a decrease in disrupted sleep reported in a study developed survey for 6% of participants (N = 197, 2–17 yrs, M age = 7.7) in a low weight of evidence study (Panjwani et al., 2021) and sleep latency according to a modified version of the Sleep Disturbance Scale for Children for 1.4% of participants (n = 100, 4–18 years) in a medium weight of evidence study (Bruni et al., 2021).
3.1.3. Sleep duration
Less focus was given to the impact of the COVID-19 pandemic on sleep duration in the empirical research literature. Two cross-sectional studies provided percentages of participants in community-based or clinical samples whose parents/caregivers reported a range of decreased, no change, or increased sleep duration (Bruni et al., 2021, Huang et al., 2021). The largest percentage of responses was for no change in duration for 52% (Bruni et al., 2021) and 70.7% (Huang et al., 2021) of participants. The studies by Bruni et al., 2021, Bruni et al., 2022 provided detailed analysis of retrospectively reported changes in sleep duration (see Table 3 for additional detail). Bruni et al. (2021) reported that autistic children (n = 100, 4–18 yrs), had a significantly greater decrease in duration than children in their non-autistic comparison group (n = 340, 4–18 yrs). Two pre–post studies compared sleep duration pre–post pandemic, providing a more rigorous measure and analysis (Garcia et al., 2021, Turkoglu et al., 2020). Garcia et al. (2021) found no significant difference in hours of sleep. However, this data was collected from only nine adolescent students, who had previously enrolled in a health promotion class at one private school. The strongest evidence is from the high weight of evidence pre–post study by Turkoglu et al. (2020), which found a significant decrease in sleep duration according to that CSHQ subscale, for their clinical sample of autistic children (N = 46, 4–17 yrs, M age = 7.89). The findings are categorised below.
3.1.3.1. Decreased
One pre–post study (Turkoglu et al., 2020) and two cross-sectional studies (Bruni et al., 2021, Huang et al., 2021) reported decreased hours of sleep per night for children with autism. Bruni et al. (2021) reported that the decrease in duration was greater for autistic compared to comparison group participants and a greater number of autistic children slept < 7 h per night during lockdown. Bruni et al. (2022) reported that the percentage of autistic children sleeping only 6–7 h on weekends and 7–8 h on weekdays significantly increased from before to during the lockdown, suggesting a decrease in sleep duration.
3.1.3.2. No change
One small pre–post study found no significant differences in hours slept per night on weekdays and weekends during the pandemic for nine adolescent participants with autism (Garcia et al., 2021), which was supported by two cross-sectional studies’ retrospective report for a percentage of their samples (Bruni et al., 2021, Huang et al., 2021).
3.1.3.3. Increased
An increase in hours slept per night that was attributed to the pandemic was retrospectively reported by two cross-sectional studies for some participants (Bruni et al., 2021, Huang et al., 2021), but not by any pre–post studies.
3.1.4. Other sleep problems
A range of other behavioural and physiological/medical sleep problem outcomes were reported by included studies. These findings are categorised below, with greater attention given to the behavioural sleep problems reported.
3.1.4.1. Worsened
One clinical pre–post study with a high weight of evidence reported increased bedtime resistance according to a CSHQ subscale (Turkoglu et al., 2020). A community-based cross-sectional study with medium weight of evidence retrospectively reported a significant increase in anxiety at bedtime and daytime sleepiness (Bruni et al., 2021). However, it is not clear how these constructs were measured, and data may have been derived from single items of the modified Sleep Disturbance Scale for Children or from their study developed questionnaire. Bruni et al. (2021) found a greater increase in retrospective report of movement related sleep difficulties such as hypnic jerks, rhythmic movement disorder and sleep walking, as well as snoring/apnoeas and bruxism. Increase in nightmares or night terrors were also reported for a percentage of participants by cross-sectional (Bruni et al., 2021, Panjwani et al., 2021) and qualitative (Tokatly Latzer et al., 2021) studies. Additionally, the Bruni et al. (2021) study found more autistic than comparison group participants experienced many of these problems during lockdown, excluding nightmares.
3.1.4.2. No change
One high weight of evidence pre–post study reported no change in the CSHQ Sleep Anxiety, Parasomnias, Sleep Disordered Breathing, and Daytime Sleepiness subscale scores for their clinical sample of autistic participants (N = 46; Turkoglu et al., 2020). Two medium–large community-based cross-sectional studies reported no change in nightmares for most participants (Bruni et al., 2021, Panjwani et al., 2021).
3.1.4.3. Improved
Only a few participants retrospectively reported a decrease in nightmares in one community-based cross-sectional study that has a low weight of evidence (Panjwani et al., 2021).
3.2. Secondary outcomes: relationship between sleep and other factors
Relationships between sleep and autism symptom severity and internalising and externalising behaviours during the pandemic were reported in five of the included studies (see Table 4 for a summary of findings). A synthesis of these findings is provided below. These studies either reported on sleep and autism symptom severity or sleep and internalising and externalising behaviours, but not all three outcomes together.
Table 4.
Broad Summary of Secondary Outcomes.
| Study | Measures | Autistic symptoms and internalising and externalising behaviours | Relationship |
|---|---|---|---|
| Berard et al. (2021) | Study developed surveys/ questionnaires VABS-IIa ADOS-2b |
Challenging behaviours: no change 36%, worsened 45%, improved 19%. Communicative abilities: no change 57%, worsened 14%, improved 29%. Stereotyped behaviours: no change 55%, worsened 39%, improved 6% |
Association between higher ADOS-2 comparison score and decreased probability of sleep improvement, OR = 0.70, 95% CI [0.53, 0.91], p = .01 No association with communication, socialisation and daily living skills* |
| Bruni et al. (2021) | n/a | Nil | Nil |
| Bruni et al. (2022)c | n/a | Nil | Nil |
| Di Renzo et al. (2020) | ABAS-IId ASDBIe SSPf |
Increasing autistic symptoms* 33.6%, restricted and repetitive behaviours 14%, motor stereotypies 14% Pre–post worsening ASDBI subscales Hyperactivity: F = 49.00; p < .01, η2 = .44 Fear of New Situation, F = 11.698, p < .0, η2 = .16 Moodiness, F = 11.737, p < .01, η2 = .16 Irritability: F = 26.481; p < .01; η2 = .30 No significant differences Self-Other-Directed Aggression ASDBI subscales; Taste/Smell Sensitivity SSP subscale; and Self-Care ABAS-II subscale |
Severity of autistic symptomology as a covariate for Sleep Regulation Problems ASDBI subscale not significant |
| Dondi et al. (2021) | Study developed surveys/ questionnaires |
ASDg sample: Increase in unusual repetitive movements: OR = 7.24, p < .001 |
Whole sample (0.80% ASD): Relationship between mood problems and difficulty falling asleep, OR = 3.16, p < .01, and staying asleep, OR = 4.85, p < .01. |
| Garcia et al. (2021) | n/a | Nil | Nil |
| Guller et al. (2021) | Study developed surveys/questionnaires | ASDg sample: Cross-sectional report of emotional* 64.1% and behavioural* 77.1% problems |
Whole sample (43.8% ASD): Disruption of child's sleep routine associated with increased odds for children's emotional problems, OR = 2.73, 95% CI [1.5, 4.7], p < .001 and behavioural problems, OR = 4.07, 95% CI [2.2, 7.2], p < .001 |
| Hosokawa et al. (2021) | Study developed surveys/ questionnaires | ASD v Control group Increased restricted and repetitive behaviours: ASD group 26.2%, Control group 4.4%, OR = 7.20, 95% CI [3.56, 14.6], p < .01. Frustration due to change of schedule: ASD group 45.2%, Control Group 31%, p = .013 No statistically significant differences in following outcomes: Stress from COVID 19 changes: ASD group 76.2%, Control group 77.8% More unstable: ASD group 16.7%, Control group 4.7% More irritated: ASD group 42.9%, Control group 31.9% Decreased motivation: ASD group 52.4%, control group 41.6% Increased sensory issues: ASD group 12%, Control group 2.8% Decreased calmness: ASD group 25%, Control group 9.4% |
Nil |
| Huang et al. (2021) | Study developed surveys/ questionnaires | Emotional and social performance: 36.2% worsened, 25.1% improved Aggressive behaviour: 5.9% worsened, 1.5% improved Self-stimulating behaviour: 17.2% worsened, 4.0% improved Language comprehension: 15% worsened, 40.2% improved Language expression: 19.2% worsened, 38.7% improved |
Nil |
| Lugo-Marin et al. (2021) | CBCLh | Survey results: Anxiety 38% worse, 32% no change, 30% better. Externalising 30% worse, 35% no change, 35% better. Internalising 27% worse, 30% no change, 45% better. Mood/Irritability 57% worse, 19% no change, 24% better. No statistically significant difference between time 1 and time 2 on CBCL total and subscale scores* (Anxious/Depressed, Withdrawn/Depressed, Somatic Complaints, Social Problems, Thought Problems, Attention Problems, Rule-Breaking Behaviour, Aggressive Behaviour, Internalising Problems, Externalising Problems.) |
Nil |
| Miniarkova et al. (2021) | Study developed surveys/ questionnaires | Challenging behaviours: 50.5% worsened, 17.2% improved, 32.3% unchanged | Nil |
| Panjwani et al. (2021) | Study developed surveys/ questionnaires |
70% increased distractibility, arguing, stubbornness 60% increased hyperactivity and tantrums < 30% increased self-injurious behaviours 10% (approximately) decreased hyperactivity, aggression, tantrums, and withdrawing |
Nil |
| Polonyiova et al. (2021) | Study developed surveys/ questionnaires VABS-IIIi |
Internalising behaviour increased for ASD group t(153) = –2.324, p = .021, d = 1.45; but not control group t(175) = –0.225, p = .822, d = 0.03 | ASD group Significant positive correlation between late bedtime and both internalising, r = .162, p < .05, and externalising, r = .255, p < .01, behaviour, but not late waking up |
| Rabbani et al. (2021) | Study developed mCare application | Negative impact for 43.3% of the 30 included behavioural parameters across communication, social interaction, problematic behaviour, and sensory sensitivities Authors report unchanged mood swings Mean score before lockdown 8.9, 95% CI [8.8, 9.04], during lockdown 7.9, 95% CI [7.7, 8.2], after lockdown 6.5, 95% CI [6.3, 6.8]. Authors report unchanged aggressive behaviour frequency Mean score before lockdown 6.8, 95% CI [6.2, 7.2], during lockdown 5.7, 95% CI [5.4, 6.03], after lockdown 5.1, 95% CI [4.9, 5.4]. No impact by lockdown |
Nil |
| Stankovic et al. (2020) | Modified version of Caregiver Needs Survey | The strongest concern reported by caregivers was worsening of autistic symptoms (54.1%). Top challenges reported also include insistence on sameness/special interests/repetitive behaviour (50.6%), problematic behaviour (35.3%), communication (29.4%), and social interaction (31.8%) |
Nil |
| Tokatly et al. (2021) | Study developed surveys/ questionnaires |
Increased externalising behaviours reported | Discussion about relationship between anxiety and sleep, where sleep impeded by fear for one child and anger for another child. |
| Turkoglu et al. (2021) | AuBCj CSHQk CCQl |
Differences from pre to post Frequency and severity of autistic symptoms AuBC: t = − 6.231, p < .001 |
During home confinement: Correlation between total CCQ and AuBC.354, p = .016 (mild positive). Correlation between total CSHQ scores and AuBC.591, p < .001 (not significant before COVID-19). Significant effect of sleep problems on severity of autistic symptoms, β = 1.91, t = 3.85, p < .001. Significant effect of chronotype score on severity of autistic symptoms, β = 1.39, t = 2.51, p = .015. Sleep problems mediate the relationship between chronotype score and severity of autism symptoms, β = 1.42, 95% CI [0.57, 2.38]. Sleep problems as a mediator: the total effect of chronotype score on the severity of autism symptoms becomes non-significant, β = −0.03, t = 0.06, p = .951, when CSHQ score entered into path analysis. |
*Additional details reported in the journal article.
a = Vineland Adaptive Behavior Scales, Second edition. b = Autism Diagnostic Observation Schedule-2. c = This study inter-relates with Bruni et al. (2021). d = The Adaptive Behaviour Assessment System-II. e = ASD Behaviour Inventory. f = Short Sensory Profile. g = Autism Spectrum Disorder. h Child Behavior Checklist. i = Vineland Adaptive Behaviour Scales – Third Edition. j = The Autism Behavior Checklist. k = Children’s Sleep Habits Questionnaire. l = Children’s Chronotype Questionnaire.
3.2.1. Sleep and autism symptom severity
One pre–post study with a high weight of evidence found stronger relationships between increased autism symptoms and both increased sleep problems and later chronotype (sleep-wake cycle) for their clinical sample (N = 46, 4–17 yrs, M age = 7.89) during the COVID-19 pandemic (Turkoglu et al., 2020). During home confinement only, later chronotype as measured by the Children’s Chronotype Questionnaire, had a significant effect on the severity of autism symptoms as measured by the Autism Behavior Checklist, which was mediated by sleep problems as measured by the CSHQ (Turkoglu et al., 2020). Further, the direct relationship between later chronotype and autism symptom severity was no longer significant when sleep problems were included in the path analysis, suggesting that this association relies on the presence of sleep problems (Turkoglu et al., 2020). In contrast, another pre–post study found that whilst parent-report of autism symptom severity intensified during restrictions for their clinical sample (N = 63, 2.7–9.4 yrs, M age = 5.9); the severity was not a significant covariate for scores on the Sleep Regulation subscale of the ASD Behaviour Inventory (Di Renzo et al., 2020). Whilst both study designs were similar, the mean age for participants in the study by Di Renzo et al. (2020) was younger and the study by Turkoglu et al. (2020) excluded participants with co-occurring conditions or medication use. In support of the findings by Turkoglu et al. (2020), one cross-sectional study with a low weight of evidence found that increased autism symptom severity for their large research cohort (N = 239, 2–21 yrs, M age = 9.1) was associated with a decreased probability of sleep improvement (Berard et al., 2021).
3.2.2. Sleep and internalising and externalising problems
A community-based pre–post study with medium weight of evidence reported a significant relationship between later bedtime and increased internalising and externalising behaviours in the autistic group, and both later bed and waking time and increased externalising behaviours in the comparison group (Polónyiová et al., 2021). Internalising and externalising behaviours were measured by the Vineland Adaptive Behaviour Scales – Third Edition. However, it is not clear whether these correlation analyses were calculated using data reported about the first wave (n = 84 ASD, male M age = 7.7, female M age = 7.7) or second wave (n = 71 ASD, male M age = 8.6, female M age = 10.8) of the coronavirus outbreak in Slovakia, or a combined dataset. Further, data was not compared pre–post pandemic. This finding was supported by a cross-sectional study that found disruption to sleep routine was associated with increased odds for emotional problems for their whole sample of children with neurodevelopmental disorders (N = 299, Mage 10.3 years, 43.8% autistic; Dondi et al., 2021). However, it is not clear whether these findings would be specific to autism or to other diagnoses. In Tokatly et al. (2021), parents/caregivers reported an association between fear and difficulty falling asleep, and anger and the ability to go back to sleep via semi-structured telephone interviews. Moreover, a cross-sectional study attributed sleep problems to psychological distress caused by the pandemic, although they did not present data to support this attribution (Bruni et al., 2021).
3.3. Other common themes
In addition to the above secondary outcomes, several common themes associated with child outcomes more generally during COVID-19 related pandemic restrictions were additionally identified. The most common protective factors against sleep problems reported by included studies were transition to online or ongoing learning and intervention (Berard et al., 2021, Di Renzo et al., 2020, Garcia et al., 2021, Guller et al., 2021, Huang et al., 2021, Rabbani et al., 2021). The most common vulnerability factors to sleep problems reported across studies included parent psychological distress (Dondi et al., 2021, Guller et al., 2021, Miniarikova et al., 2021, Mutluer et al., 2020, Polónyiová et al., 2021, Rabbani et al., 2021) and decreased learning, intervention, or extra-curricular activities (Bruni et al., 2021, Dondi et al., 2021, Guller et al., 2021, Rabbani et al., 2021). Additional support for parents to manage the pandemic and/or negative impact on sleep was the most common recommendation of the included studies (Guller et al., 2021, Hosokawa et al., 2021, Huang et al., 2021, Lugo-Marin et al., 2021, Miniarikova et al., 2021, Stankovic et al., 2021, Tokatly Latzer et al., 2021), as was the need for the structure of usual learning, intervention, or extra-curricular activities for children (Di Renzo et al., 2020, Dondi et al., 2021, Garcia et al., 2021, Guller et al., 2021, Hosokawa et al., 2021, Stankovic et al., 2021, Tokatly Latzer et al., 2021). See Table 5 for complete summary.
Table 5.
Other Common Themes Identified.
Note. This table is a compilation of additional themes identified in the included papers via inductive thematic synthesis that relate to the primary and secondary outcomes
4. Discussion
Conducted during the COVID-19 pandemic, this systematic review synthesised empirical findings from data collected during 2020 on sleep for children with autism, providing an insight into the role of protracted environmental stress that systematically affected this already susceptible population. Review findings suggest that children with autism may be particularly vulnerable to disrupted sleep. Further, as anticipated, sleep problems were associated with increased expression of autistic characteristics, but the evidence was less clear on the association with internalising and externalising behaviours. These findings align with the challenges this population are known to experience in adapting to change and coping with stress (see Lai, Anagnostou, Wiznitzer, Allison, & Baron-Cohen, 2020; Lord et al., 2022).
4.1. Impact on sleep
Whilst findings were mixed overall, those that suggest a worsening of sleep for autistic children during the COVID-19 pandemic were the most consistent and identified from the strongest weight of evidence; providing an insight into the challenges that many families experienced. Some findings relate to reduced overall sleep quality, including a clinical pre–post study that had a high weight of evidence (e.g., Turkoglu et al., 2020). However, most findings relate to the types of behavioural sleep problems commonly experienced by autistic children. For example, sleep regulation problems including difficulty or delay in falling asleep, frequent or early awakening, and difficulty waking in the morning, were widely reported to have worsened or increased during the pandemic by most studies, including the more rigorous pre–post studies across community-based, clinical and educational samples and a range of age groups (e.g., Di Renzo et al., 2020; Garcia et al., 2021; Polónyiová et al., 2021; Turkoglu et al., 2020). Additionally, the number of hours slept per night was reported to have decreased for many autistic children across a wide age-range and from community-based and clinical samples (Bruni et al., 2021, Huang et al., 2021, Turkoglu et al., 2020). However, it is not clear that this reduction in sleep duration equates to insufficient sleep or whether developmental stage affected outcomes with regards to sleep duration, although sleep for autistic children may not follow usual developmental patterns (e.g., Hodge, Carollo, Lewin, Hoffman, & Sweeney, 2014; Humphreys et al., 2013; Sivertsen, Posserud, Gillberg, Lundervold, & Hysing, 2012). The strength of many of these findings relates to their medium to high weight of evidence quality assessment, which distinguishes them from the overall mixed findings that also incorporate studies weighted as low. Nonetheless, it is important to acknowledge that not all types of sleep problems experienced by autistic children worsened, or worsened for all participating children.
Some reported findings from reviewed studies differed, describing no change or even improvement in aspects of sleep. For example, contrary to findings that sleep regulation worsened during the pandemic for autistic children, a community-based cross-sectional study with medium weight of evidence reported that autistic children were more likely to maintain usual bed and risetimes during the pandemic as compared to children with ADHD or without either diagnosis (Bruni et al., 2021). This finding may relate to autistic children’s preference for routine (Ameis et al., 2020) and indicate this preference as a protective factor. Another cross-sectional study reported worsening of sleep regulation that was not statistically significant (Dondi et al., 2021), but may still be clinically significant, as this sample of children also showed greater odds for increased unusual repetitive movements that the authors propose may be related to increased psychological distress and we suggest could also relate to disrupted sleep. Accordingly, managing sleep may have a collateral effect on emotional and behavioural difficulties (see Hunter et al., 2020). However, evidence from this study was weighted ‘low’, and the stronger evidence supports a worsening of sleep regulation.
There were also conflicting findings reported for sleep anxiety. Autistic children often experience sleep problems specifically related to anxiety and hyperarousal at bedtime (Mazurek & Petroski, 2015). However, the study with the strongest weight of evidence reported that sleep anxiety did not increase during the pandemic (Turkoglu et al., 2020), whereas a cross-sectional study found that anxiety at bedtime did increase (Bruni et al., 2021). Anxiety at bedtime in the cross-sectional study was retrospectively reported and it is not clear how it was measured. Whilst the use of a validated subscale designed to specifically measure sleep anxiety alongside the pre–post design strengthens confidence in the findings by Turkoglu et al. (2020), which had a high weight of evidence; although we acknowledge that the clinical sample size was relatively small. Accordingly, the impact of the COVID-19 pandemic on sleep anxiety, that is anxiety specifically related to bedtime and sleep, has not been clearly established in this systematic review. Irrespective of these mixed or inconclusive findings, we highlight the support needed for families who do experience worsening sleep.
4.2. Relationship between sleep and secondary outcomes
Hollway and Aman’s bidirectional theoretical framework (2011) informed additional analyses of included studies to illuminate an approach to identifying support needs. There was insufficient evidence from included studies to support a relationship between sleep and internalising and externalising behaviours. Although one pre–post study reported a small but positive relationship between later bedtime and internalising and externalising behaviours (Polónyiová et al., 2021), the evidence provided does not demonstrate whether this relationship changed due to pandemic related restrictions. In addition, the cross-sectional study that supported these findings analysed the relationship for their whole sample of participants with neurodevelopmental disorders (Guller et al., 2021) and their evidence was weighted as low. Further, the supporting qualitative study reported a related finding from one family only (Tokatly Latzer et al., 2021). These inconclusive findings may reflect the lower weight of evidence of included studies due to challenges associated with conducting empirical research that relies on a specific and fleeting context of environmental stress, or indicate that the pandemic as an environmental stressor was not sufficient to influence this relationship with these participants.
The additional analyses of included studies against the bidirectional theoretical framework did, however, find some evidence to support a relationship between increased severity of autistic symptoms and sleep problems during the COVID-19 pandemic. The strongest evidence was from two studies with conflicting findings, with Di Renzo et al. (2020) reporting that autism symptom severity was not a covariate for sleep problems and; conversely, Turkoglu et al. (2020) reporting increased autism symptom severity and increased sleep problems and later chronotype during the pandemic. Whilst both studies employed a pre–post design and were conducted with similar sized clinical samples, the findings by Turkoglu et al. (2020) are emphasised here due to the study’s higher weight of evidence and use of validated scales that specifically measure the sleep related outcomes reported. However, these findings do not establish a directional relationship, although they do suggest implications that will be discussed further.
4.3. Limitations and future studies
The inclusion of heterogeneous study designs and contexts, and the assessment of the robustness of this synthesis when drawing conclusions is a strength of this systematic review. Nevertheless, in assessing the robustness of this synthesis, a range of limitations must be acknowledged. Firstly, this review relies on predominantly cross-sectional studies or studies that were not initially designed to measure the pre–post impact of the COVID-19 pandemic. In addition, no studies used an objective measure of sleep and few used validated scales. Accordingly, the weight of evidence for many studies was low (n = 8), with only one weighted high, and any inferences we make from the data when formulating implications and recommendations must be qualified to err on the side of caution (see Hawkins, Elsworth, Nolte, & Osborne, 2021; Hawkins, Elsworth, & Osborne, 2018).
Secondly, there are limitations to the generalisability of these findings. The broad-age range of participants, the differences in ability and types of challenges experienced by participants across each sample, and inclusion of participants with co-occurring conditions in some but not all study samples may limit generalisability of findings to either broad or specific clinical populations due to potential developmental differences in sleep and behaviour (Hiscock and Davey, 2018, Lord et al., 2022, Schreck and Richdale, 2020), notwithstanding that autistic children may not experience the developmental changes in sleep that non-autistic children do (Mazurek, Dovgan, Neumeyer, & Malow, 2019). Further, many studies either provided no or limited family demographic data, although included studies were conducted worldwide and those that did provide such data appeared to include families from a range of socio-economic statuses and geographical and developmental contexts. Furthermore, this systematic review involved only English language articles, although included studies were conducted worldwide and no articles were identified and excluded on this basis (see Table 2). Finally, these studies were conducted across a range of countries with different pandemic related restrictions and levels of ongoing intervention and education, and different contexts and settings (e.g., ongoing studies, interventional studies, and clinical versus community-based samples). Therefore, quality and variability may have affected results of individual studies as well as our synthesis and comparison.
As additional studies emerge from this pandemic period, it may be possible to update this review with a focus on longitudinal sleep outcomes. However, due to the finite time period engendered by a pandemic context, it is unlikely that studies can now be designed and conducted to address the above limitations. Accordingly, it may not be possible to obtain sufficient rigorous evidence to support causal pathways corroborating the Hollway and Aman (2011) bidirectional theoretical framework, during the COVID-19 pandemic.
4.4. Implications and recommendations
A key message from the recent Lancet Commission on care and research in autism (Lord et al., 2022) is the need to ensure that effective treatment and services for autistic individuals are provided efficiently and with equity of access, to optimise outcomes. The findings of this systematic review illustrate this point; with implications arising from the additionally generated themes (see Table 5) across two key areas of focus: (1) supporting caregivers and (2) managing children’s sleep problems. Firstly, the most frequent recommendation identified in the reviewed studies was for additional support for parents to manage changes to their children’s routines and behaviours. Parents of children with autism may require specific and targeted support to help them care for their children when environmental stressors increase or are prolonged, including support to maintain their children’s usual sleep patterns or manage sleep problems. Notably, sleep interventions that improve child sleep may also improve parent sleep, psychosocial functioning, and quality of life (e.g., Martin, Papadopoulos, Chellew, Rinehart, & Sciberras, 2019; Martin et al., 2020; McLay et al., 2021; Papadopoulos et al., 2019; Papadopoulos et al., 2022; Varma, Conduit, Junge, Lee, & Jackson, 2021). Therefore, sleep could be a target for assessment and intervention when unpredictable environmental stressors are prolonged.
Secondly, worsening sleep for children with autism in response to increased environmental stress, alongside the reduction in usual in-person intervention and schooling reported by studies during the COVID-19 pandemic (see Table 2), highlights the importance of continued participation in ongoing, accessible and flexible intervention and schooling during times of change or upheaval. Indeed, a range of included studies described restrictions and changes to routine as vulnerability factors for families (see Table 5). Behavioural intervention for sleep problems, individualised to a child’s presenting needs, can improve overall behaviour and functioning for children (Hunter et al., 2020, Pattison et al., 2020), enhancing resilience to stress and change. Further, brief behavioural sleep interventions are practical (e.g., Sleeping Sound; see Papadopoulos et al., 2019; Papadopoulos et al., 2022), and could be scaled to more novel modes of treatment delivery such as telehealth to improve access (see recommendations by Kazdin, 2019). In the Australian context, the government’s commitment to continuing Medicare funding for telehealth consultations (Australian Government, 2022) increases adaptability of services to unpredictable environmental stressors. Further, given the importance of optimising the ‘person-environment fit’ for autistic individuals emphasised by Lai et al. (2020), by providing a choice in access to services, telehealth delivery of behavioural sleep treatment holds great promise in supporting families with autistic children in the future.
5. Conclusion
The findings of this systematic review suggest that, as predicted, sleep problems typically experienced by autistic children can worsen for many during times of great stress and change; and that worsening sleep may negatively impact other child and parent outcomes. Fortunately, behavioural sleep problems experienced by autistic children can be treated (Cuomo et al., 2017) and sleep can improve alongside behavioural difficulties (Hunter et al., 2020, Pattison et al., 2020). This study highlights the need for sleep treatment to be individualised, ongoing, accessible, and flexible to adapt to periods of significant and unpredictable environmental stress. Indeed, the COVID-19 pandemic, by offering a preview of how treatment can be feasibly scaled to other modes of delivery such as telehealth, provides impetus for future research into modifying treatment to be autism-friendly and adaptable in the long term.
Acknowledgements
We gratefully acknowledge funding received from Jonathan and Simone Wenig (Grant ID 379652105, Australia) . The present study forms part of the first author’s (Samantha Lewis) doctoral thesis, which is supported by an Australian Government Research Training Program (RTP) Scholarship (Australia). Neither funding source had a role in study design, collection, analysis or interpretation of data, writing of the report, or decision to submit the article for publication.
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
This study uses both person first (i.e., child with autism) and identity first (i.e., autistic child) language to describe autism/autism spectrum disorder as considered appropriate in Australia and the United Kingdom (Bury et al., 2020, Kenny et al., 2016).
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.rasd.2023.102110.
Appendix A. Supplementary material
Supplementary material
.
Data availability
Data will be made available on request.
References
- Ahmed S., Hanif A., Khaliq I., Ayub S., Saboor S., Shoib S.…Mahmood Khan A. Psychological impact of the COVID-19 pandemic in children with autism spectrum disorder - A literature review. International Journal of Developmental Disabilities. 2022:1–11. doi: 10.1080/20473869.2022.2066248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N., Rigney G., Weiss S.K., Brown C.A., Constantin E., Godbout R.…Corkum P.V. Optimizing an eHealth insomnia intervention for children with neurodevelopmental disorders: a Delphi study. Sleep Health. 2018;4(2):224–234. doi: 10.1016/j.sleh.2017.12.008. [DOI] [PubMed] [Google Scholar]
- Alonso-Esteban Y., López-Ramón M.F., Moreno-Campos V., Navarro-Pardo E., Alcantud-Marín F. A systematic review on the impact of the social confinement on people with autism spectrum disorder and their caregivers during the COVID-19 pandemic. Brain Sciences. 2021;11(11) doi: 10.3390/brainsci11111389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameis S.H., Lai M.C., Mulsant B.H., Szatmari P. Coping, fostering resilience, and driving care innovation for autistic people and their families during the COVID-19 pandemic and beyond. Molecular Autism. 2020;11(1):61. doi: 10.1186/s13229-020-00365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association,; 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Asbury K., Fox L., Deniz E., Code A., Toseeb U. How is COVID-19 affecting the mental health of children with special educational needs and disabilities and their families. Journal of Autism and Developmental Disorders. 2021;51(5):1772–1780. doi: 10.1007/s10803-020-04577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbury K., Toseeb U. A longitudinal study of the mental health of autistic children and adolescents and their parents during COVID-19: Part 2, qualitative findings. Autism. 2022 doi: 10.1177/13623613221086997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Government. (2022). MBS Telehealth Services from 1 July 2022 [online]. 〈http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Factsheet-telehealth-1July22〉.
- Baio J., Wiggins L., Christensen D.L., Warren Z., Kurzius-Spencer M., Zahorodny W.…Dowling N.F. Prevalence of autism spectrum disorder among children aged 8 Years — Autism and developmental disabilities monitoring network, 11 sites, United States, 2014. Morbidity and Mortality Weekly Report Surveillance Summaries. 2018;67(6):1. doi: 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauminger N., Solomon M., Rogers S.J. Externalizing and internalizing behaviors in ASD. Autism Research. 2010;3(3):101–112. doi: 10.1002/aur.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S.P., Gregory A.M. Editorial Perspective: Perils and promise for child and adolescent sleep and associated psychopathology during the COVID-19 pandemic. Journal of Child Psychology and Psychiatry. 2020;61(7):757–759. doi: 10.1111/jc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berard M., Rattaz C., Peries M., Loubersac J., Munir K., Baghdadli A. Impact of containment and mitigation measures on children and youth with ASD during the COVID-19 pandemic: Report from the ELENA cohort. Journal of Psychiatric Research. 2021;137:73–80. doi: 10.1016/j.jpsychires.2021.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. EVIDENT Guidance for Reviewing the Evidence: a compendium of Methodological Literature and websites. 2016 doi: 10.13140/RG.2.1.1562.9842. [DOI] [Google Scholar]
- Bruni O., Breda M., Ferri R., Melegari M.G. Changes in sleep patterns and disorders in children and adolescents with attention deficit hyperactivity disorders and autism spectrum disorders during the COVID-19 lockdown. Brain Sciences. 2021;11(9) doi: 10.3390/brainsci11091139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni O., Melegari M.G., Breda M., Cedrone A., Finotti E., Malorgio E.…Ferri R. Impact of COVID-19 lockdown on sleep in children with autism spectrum disorders. Journal of Clinical Sleep Medicine. 2022 doi: 10.5664/jcsm.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury S.M., Jellett R., Spoor J.R., Hedley D. "It defines who I am" or "it's something I have": What language do [autistic] Australian adults [on the autism spectrum] prefer? Journal of Autism and Developmental Disorders. 2020 doi: 10.1007/s10803-020-04425-3. [DOI] [PubMed] [Google Scholar]
- Chandler S., Howlin P., Simonoff E., O'sullivan T., Tseng E., Kennedy J.…Baird G. Emotional and behavioural problems in young children with autism spectrum disorder. Developmental Medicine & Child Neurology. 2016;58(2):202–208. doi: 10.1111/dmcn.12830. [DOI] [PubMed] [Google Scholar]
- Chen H., Yang T., Chen J., Chen L., Dai Y., Zhang J.…Li T. Sleep problems in children with autism spectrum disorder: a multicenter survey. BMC Psychiatry. 2021;21(1):406. doi: 10.1186/s12888-021-03405-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Conduit R., Lockley S.W., Rajaratnam S.M., Cornish K. The relationship between sleep and behaviour in autism spectrum disorder (ASD): A review. Journal of Neurodevelopmental Disorders. 2014;6(44):1–10. doi: 10.1186/1866-1955-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colizzi M., Sironi E., Antonini F., Ciceri M.L., Bovo C., Zoccante L. Psychosocial and behavioral impact of COVID-19 in autism spectrum disorder: An online parent survey. Brain Science. 2020;10(6) doi: 10.3390/brainsci10060341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta bio-medica: Atenei Parmensis. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuomo B.M., Vaz S., Lee E.A.L., Thompson C., Rogerson J.M., Falkmer T. Effectiveness of sleep-based interventions for children with autism spectrum disorder: A meta-synthesis. Pharmacotherapy. 2017;37(5):555–578. doi: 10.1002/phar.1920. [DOI] [PubMed] [Google Scholar]
- Davenport M.A., Berry J.R., Mazurek M.O., McCrae C.S. Using telehealth to deliver family-based cognitive behavioral treatment of insomnia in a school-aged child with autism spectrum disorder. Journal of Cognitive Psychotherapy. 2021;35(4):235–254. doi: 10.1891/jcpsy-d-20-00062. [DOI] [PubMed] [Google Scholar]
- de Almeida G.M.F., Nunes M.L. Sleep characteristics in Brazilian children and adolescents: A population-based study. Sleep Medicine. 2019;1 doi: 10.1016/j.sleepx.2019.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deserno M.K., Borsboom D., Begeer S., Agelink van Rentergem J.A., Mataw K., Geurts H.M. Sleep determines quality of life in autistic adults: A longitudinal study. Autism Research. 2019;12(5):794–801. doi: 10.1002/aur.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Renzo M., Di Castelbianco F.B., Vanadia E., Petrillo M., D’Errico S., Racinaro L., Rea M. Parent-reported behavioural changes in children with autism spectrum disorder during the COVID-19 lockdown in Italy. Continuity in Education. 2020;1(1):117–125. doi: 10.5334/cie.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Román A., Zhang J., Cortese S., Delorme R., Beggiato A. Sleep in youth with autism spectrum disorders: Systematic review and meta-analysis of subjective and objective studies [Article] Evidence-Based Mental Health. 2018;21(4):146–154. doi: 10.1136/ebmental-2018-300037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondi A., Fetta A., Lenzi J., Morigi F., Candela E., Rocca A.…Lanari M. Sleep disorders reveal distress among children and adolescents during the Covid-19 first wave: Results of a large web-based Italian survey. Italian Journal of Pediatrics. 2021;47(1):130. doi: 10.1186/s13052-021-01083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison K.S., Guidry J., Picou P., Adenuga P., Davis T.E., 3rd Telehealth and autism prior to and in the age of COVID-19: A systematic and critical review of the last decade. Clinical Child and Family Psychology Review. 2021 doi: 10.1007/s10567-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod M.G., Hood B.S. Sleep differences among children with autism spectrum disorders and typically developing peers: a meta-analysis. Journal of Developmental and Behavioral Pediatrics. 2015;36(3):166–177. doi: 10.1097/dbp.0000000000000140. [DOI] [PubMed] [Google Scholar]
- Freeman D., Sheaves B., Waite F., Harvey A.G., Harrison P.J. Sleep disturbance and psychiatric disorders. The Lancet Psychiatry. 2020;7(7):628–637. doi: 10.1016/S2215-0366(20)30136-X. [DOI] [PubMed] [Google Scholar]
- Garcia J.M., Lawrence S., Brazendale K., Leahy N., Fukuda D. Brief report: The impact of the COVID-19 pandemic on health behaviors in adolescents with autism spectrum disorder. Disability and Health Journal. 2021;14(2) doi: 10.1016/j.dhjo.2020.101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough D. Weight of Evidence: a framework for the appraisal of the quality and relevance of evidence. Research Papers in Education. 2007;22(2):213–228. doi: 10.1080/02671520701296189. [DOI] [Google Scholar]
- Griffin S.C., Williams A.B., Ravyts S.G., Mladen S.N., Rybarczyk B.D. Loneliness and sleep: A systematic review and meta-analysis. Health Psychology Open. 2020;7(1) doi: 10.1177/2055102920913235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guller B., Yaylaci F., Eyuboglu D. Those in the shadow of the pandemic: impacts of the COVID-19 outbreak on the mental health of children with neurodevelopmental disorders and their parents. International Journal of Developmental Disabilities. 2021:1–13. doi: 10.1080/20473869.2021.1930827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G.T., Trevisan D.A., Abel E.A., Cummings E.M., Carlos C., Bagdasarov A.…McPartland J.C. Associations between sleep problems and domains relevant to daytime functioning and clinical symptomatology in autism: A meta-analysis. Autism Research. 2022 doi: 10.1002/aur.2758. [DOI] [PubMed] [Google Scholar]
- Haug N., Geyrhofer L., Londei A., Dervic E., Desvars-Larrive A., Loreto V.…Klimek P. Ranking the effectiveness of worldwide COVID-19 government interventions. Nature Human Behaviour. 2020;4(12):1303–1312. doi: 10.1038/s41562-020-01009-0. [DOI] [PubMed] [Google Scholar]
- Hawkins M., Elsworth G.R., Nolte S., Osborne R.H. Validity arguments for patient-reported outcomes: justifying the intended interpretation and use of data. Journal of Patient-Reported Outcomes. 2021;5(1):64. doi: 10.1186/s41687-021-00332-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins M., Elsworth G.R., Osborne R.H. Application of validity theory and methodology to patient-reported outcome measures (PROMs): building an argument for validity. Quality of Life Research. 2018;27(7):1695–1710. doi: 10.1007/s11136-018-1815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock H., Davey M.J. Sleep disorders in infants and children. Journal of Paediatrics and Child Health. 2018;54(9):941–944. doi: 10.1111/jpc.12033. [DOI] [PubMed] [Google Scholar]
- Hodge D., Carollo T.M., Lewin M., Hoffman C.D., Sweeney D.P. Sleep patterns in children with and without autism spectrum disorders: Developmental comparisons. Research in Developmental Disabilities. 2014;35(7):1631–1638. doi: 10.1016/j.ridd.2014.03.037. [DOI] [PubMed] [Google Scholar]
- Hollway J.A., Aman M.G. Sleep correlates of pervasive developmental disorders: a review of the literature. Research in Developmental Disabilities. 2011;32(5):1399–1421. doi: 10.1016/j.ridd.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Hosokawa R., Kawabe K., Nakachi K., Yoshino A., Horiuchi F., Ueno S.I. Behavioral affect in children with autism spectrum disorder during school closures due to the COVID-19 pandemic in Japan: A case-controlled study. Developmental Neuropsychology. 2021:1–10. doi: 10.1080/87565641.2021.1939350. [DOI] [PubMed] [Google Scholar]
- Huang S., Sun T., Zhu Y., Song S., Zhang J., Huang L.…Jing J. Impact of the COVID-19 pandemic on children with ASD and their families: An online survey in China. Psychology Research and Behavior Management. 2021;14:289–297. doi: 10.2147/PRBM.S293426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys J.S., Gringras P., Blair P.S., Scott N., Henderson J., Fleming P.J., Emond A.M. Sleep patterns in children with autistic spectrum disorders: A prospective cohort study. Archives of Disease in Childhood. 2013;99:114–118. doi: 10.1136/archdischild-2013-304083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J.E., McLay L.K., France K.G., Blampied N.M. Systematic review of the collateral effects of behavioral sleep interventions in children and adolescents with autism spectrum disorder. Research in Autism Spectrum Disorders. 2020:79. doi: 10.1016/j.rasd.2020.101677. [DOI] [Google Scholar]
- Kazdin A.E. Annual research review: Expanding mental health services through novel models of intervention delivery. Journal of Child Psychology and Psychiatry. 2019;60(4):455–472. doi: 10.1111/jc. [DOI] [PubMed] [Google Scholar]
- Kenny L., Hattersley C., Molins B., Buckley C., Povey C., Pellicano E. Which terms should be used to describe autism? Perspectives from the UK autism community. Autism. 2016;20(4):442–462. doi: 10.1177/1362361315588200. [DOI] [PubMed] [Google Scholar]
- Krakowiak P., Goodlin-Jones B., Hertz-Picciotto I., Croen L.A., Hansen R.L. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: a population-based study. Journal of Sleep Research. 2008;17(2):197–206. doi: 10.1111/j.1365-2869.2008.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.-C., Anagnostou E., Wiznitzer M., Allison C., Baron-Cohen S. Evidence-based support for autistic people across the lifespan: maximising potential, minimising barriers, and optimising the person–environment fit. Lancet Neurology. 2020;19(5):434–451. doi: 10.1016/S1474-4422(20)30034-X. [DOI] [PubMed] [Google Scholar]
- Lai M.-C., Kassee C., Besney R., Bonato S., Hull L., Mandy W.…Ameis S.H. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. The Lancet Psychiatry. 2019;6(10):819–829. doi: 10.1016/s2215-0366(19)30289-5. [DOI] [PubMed] [Google Scholar]
- Lord C., Charman T., Havdahl A., Carbone P., Anagnostou E., Boyd B.…McCauley J.B. The Lancet Commission on the future of care and clinical research in autism. The Lancet. 2022;399(10321):271–334. doi: 10.1016/s0140-6736(21)01541-5. [DOI] [PubMed] [Google Scholar]
- Lugo-Marin J., Gisbert-Gustemps L., Setien-Ramos I., Espanol-Martin G., Ibanez-Jimenez P., Forner-Puntonet M.…Ramos-Quiroga J.A. COVID-19 pandemic effects in people with autism spectrum disorder and their caregivers: Evaluation of social distancing and lockdown impact on mental health and general status. Research in Autism Spectrum Disorders. 2021;83 doi: 10.1016/j.rasd.2021.101757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malow B.A., Byars K., Johnson K., Weiss S., Bernal P., Goldman S.E.…Sleep Committee of the Autism Treatment N. A practice pathway for the identification, evaluation, and management of insomnia in children and adolescents with autism spectrum disorders. Pediatrics. 2012;130:S106–S124. doi: 10.1542/peds.2012-0900I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C.A., Papadopoulos N., Chellew T., Rinehart N.J., Sciberras E. Associations between parenting stress, parent mental health and child sleep problems for children with ADHD and ASD: Systematic review. Research in Developmental Disabilities. 2019;93 doi: 10.1016/j.ridd.2019.103463. [DOI] [PubMed] [Google Scholar]
- Martin C.A., Sciberras E., Papadopoulos N., Engel L., Hiscock H., Williams K.…Rinehart N.J. Associations between child sleep problem severity and maternal well-being in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2020 doi: 10.1007/s10803-020-04726-7. [DOI] [PubMed] [Google Scholar]
- Mazurek M.O., Dovgan K., Neumeyer A.M., Malow B.A. Course and predictors of sleep and co-occurring problems in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2019;49(5):2101–2115. doi: 10.1007/s10803-019-03894-5. [DOI] [PubMed] [Google Scholar]
- Mazurek M.O., Petroski G.F. Sleep problems in children with autism spectrum disorder: examining the contributions of sensory over-responsivity and anxiety. Sleeping Medicine. 2015;16(2):270–279. doi: 10.1016/j.sleep.2014.11.006. [DOI] [PubMed] [Google Scholar]
- McCrae C.S., Chan W.S., Curtis A.F., Nair N., Deroche C.B., Munoz M.…Mazurek M.O. Telehealth cognitive behavioral therapy for insomnia in children with autism spectrum disorder: A pilot examining feasibility, satisfaction, and preliminary findings. Autism. 2020 doi: 10.1177/1362361320949078. [DOI] [PubMed] [Google Scholar]
- McLay L., Sutherland D., Machalicek W., Sigafoos J. Systematic review of telehealth interventions for the treatment of sleep problems in children and adolescents. Journal of Behavioral Education. 2020;29(2):222–245. doi: 10.1007/s10864-020-09364-8. [DOI] [Google Scholar]
- McLay L.L., France K.G., Blampied N.M., Hunter J.E., van Deurs J.R., Woodford E.C.…Lang R. Collateral child and parent effects of function based behavioral interventions for sleep problems in children and adolescents with autism. Journal of Autism and Developmental Disorders. 2021 doi: 10.1007/s10803-021-05116-3. [DOI] [PubMed] [Google Scholar]
- McNally Keehn R., Tomlin A., Ciccarelli M.R. COVID-19 pandemic highlights access barriers for children wspectrum disorder. Journal of Developmental & Behavioral Pediatrics. 2021;42(7) doi: 10.1097/DBP.0000000000000988. 〈https://journals.lww.com/jrnldbp/Fulltext/2021/09000/COVID_19_Pandemic_Highlights_Access_Barriers_for.11.aspx〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniarikova E., Vernhet C., Peries M., Loubersac J., Picot M.C., Munir K., Baghdadli A. Anxiety and depression in parents of children with autism spectrum disorder during the first COVID-19 lockdown: Report from the ELENA cohort [Article in Press] Journal of Psychiatric Research. 2021 doi: 10.1016/j.jpsychires.2021.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutluer T., Doenyas C., Aslan Genc H. Behavioral implications of the Covid-19 process for autism spectrum disorder, and individuals' comprehension of and reactions to the pandemic conditions. Frontiers in Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.561882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. (2013). Autism spectrum disorder in under 19s: Support and management. 〈https://www.nice.org.uk/guidance/cg170/chapter/Key-priorities-for-implementation〉. [PubMed]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D.…Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. British Medical Journal. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjwani A.A., Bailey R.L., Kelleher B.L. COVID-19 and behaviors in children with autism spectrum disorder: Disparities by income and food security status [Article] Research in Developmental Disabilities. 2021;115 doi: 10.1016/j.ridd.2021.104002. Article 104002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos N., Sciberras E., Hiscock H., Mulraney M., McGillivray J., Rinehart N. The efficacy of a brief behavioral sleep intervention in school-aged children with ADHD and comorbid autism spectrum disorder. Journal of Attention Disorders. 2019;23(4):341–350. doi: 10.1177/1087054714568565. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N., Sciberras E., Hiscock H., Williams K., McGillivray J., Mihalopoulos C.…Rinehart N. Sleeping Sound Autism Spectrum Disorder (ASD): a randomised controlled trial of a brief behavioural sleep intervention in primary school-aged autistic children. Journal of Child Psychology and Psychiatry. 2022 doi: 10.1111/jc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison E., Papadopoulos N., Marks D., McGillivray J., Rinehart N. Behavioural treatments for sleep problems in children with autism spectrum disorder: A review of the recent literature. Current Psychiatry Reports. 2020;22(9):46. doi: 10.1007/s11920-020-01172-1. [DOI] [PubMed] [Google Scholar]
- Pellicano E., Brett S., den Houting J., Heyworth M., Magiati I., Steward R.…Stears M. COVID-19, social isolation and the mental health of autistic people and their families: A qualitative study. Autism. 2021 doi: 10.1177/13623613211035936. [DOI] [PubMed] [Google Scholar]
- Polónyiová K., Belica I., Celušáková H., Janšáková K., Kopčíková M., Szapuová Ž., Ostatníková D. Comparing the impact of the first and second wave of COVID-19 lockdown on Slovak families with typically developing children and children with autism spectrum disorder. Autism. 2021 doi: 10.1177/13623613211051480. [DOI] [PubMed] [Google Scholar]
- Popay J., Roberts H., Sowden A., Petticrew M., Ara I.L. Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC Methods Programme. 2006;1(1):b92. doi: 10.13140/2.1.1018.4643. [DOI] [Google Scholar]
- Rabbani M., Haque M.M., Das Dipal D., Zarif M.I.I., Iqbal A., Schwichtenberg A.…Ahamed S.I. An mCARE study on patterns of risk and resilience for children with ASD in Bangladesh. Scientific Reports. 2021;11(1):21342. doi: 10.1038/s41598-021-00793-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richdale A.L., Baker E., Short M., Gradisar M. The role of insomnia, pre-sleep arousal and psychopathology symptoms in daytime impairment in adolescents with high-functioning autism spectrum disorder. Sleep Medicine. 2014;15(9):1082–1088. doi: 10.1016/j.sleep.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Richdale A.L., Schreck K.A. Sleep problems in autism spectrum disorders: prevalence, nature, & possible biopsychosocial aetiologies. Sleep Medicine Reviews. 2009;13(6):403–411. doi: 10.1016/j.smrv.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Rigney G., Ali N.S., Corkum P.V., Brown C.A., Constantin E., Godbout R.…Weiss S.K. A systematic review to explore the feasibility of a behavioural sleep intervention for insomnia in children with neurodevelopmental disorders: A transdiagnostic approach. Sleep Medicine Reviews. 2018;41:244–254. doi: 10.1016/j.smrv.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Schreck K.A., Richdale A.L. Sleep problems, behavior, and psychopathology in autism: inter-relationships across the lifespan. Current Opinion in Psychology. 2020;34:105–111. doi: 10.1016/j.copsyc.2019.12.003. [DOI] [PubMed] [Google Scholar]
- Setia M.S. Methodology series module 3: Cross-sectional studies. Indian Journal of Dermatology. 2016;61(3):261–264. doi: 10.4103/0019-5154.182410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorey S., Lau L.S.T., Tan J.X., Ng E.D., Ramkumar A. Families with children with neurodevelopmental disorders during COVID-19: A scoping review. Journal of Pediatric Psychology. 2021 doi: 10.1093/jpepsy/jsab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivertsen B., Posserud M.B., Gillberg C., Lundervold A.J., Hysing M. Sleep problems in children with autism spectrum problems: a longitudinal population-based study. Autism. 2012;16(2):139–150. doi: 10.1177/1362361311404255. [DOI] [PubMed] [Google Scholar]
- Snilstveit B., Oliver S., Vojtkova M. Narrative approaches to systematic review and synthesis of evidence for international development policy and practice. Journal of Development Effectiveness. 2012;4(3):409–429. doi: 10.1080/19439342.2012.710641. [DOI] [Google Scholar]
- Souders M.C., Mason T.B.A., Valladares O., Bucan M., Levy S.E., Mandell D.…Pinto-Martin J. Sleep behaviors and sleep quality in children with autism spectrum disorders. Sleep. 2009;32(12):1566–1578. doi: 10.1093/sleep/32.12.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souders, M.C., Taylor, B.J., & Zavodny Jackon, S. (2020). Sleep Problems in Autism Spectrum Disorder. In S.W. White, B.B. Maddox, & C.A. Mazefsky. (Eds.), The Oxford Handbook of Autism and Co-Occurring Psychiatric Conditions (pp. 257–283). 10.1093/oxfordhb/9780190910761.013.14. [DOI]
- Stankovic M., M., Stojanovic A., Stojanov J., Stankovic M., Shih A., Stankovic S. The Serbian experience of challenges of parenting children with autism spectrum disorder during the COVID-19. pandemic and the state of emergency with the police lockdown. European Child & Adolescent Psychiatry. 2021;31(4) doi: 10.1007/s00787-021-01917-0. 693-669. https://doi.org/https://doi.org/10.1007/s00787-021-01917-0(0123456789.,-volV)(0123456789,-.volV) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuttard L., Clarke S., Thomas M., Beresford B. Replacing home visits with telephone calls to support parents implementing a sleep management intervention: findings from a pilot study and implications for future research. Child: Care, Health and Development. 2015;41(6):1074–1081. doi: 10.1111/cch.12250. [DOI] [PubMed] [Google Scholar]
- Tan-MacNeill K.M., Smith I.M., Jemcov A., Keeler L., Chorney J., Johnson S.…Corkum P.V. Barriers and facilitators to treating insomnia in children with autism spectrum disorder and other neurodevelopmental disorders: Parent and health care professional perspectives. Research in Developmental Disabilities. 2020;107 doi: 10.1016/j.ridd.2020.103792. [DOI] [PubMed] [Google Scholar]
- Tan-MacNeill K.M., Smith I.M., Weiss S.K., Johnson S.A., Chorney J., Constantin E.…Corkum P.V. An eHealth insomnia intervention for children with neurodevelopmental disorders: Results of a usability study. Research in Developmental Disabilities. 2020;98 doi: 10.1016/j.ridd.2020.103573. [DOI] [PubMed] [Google Scholar]
- Taylor A., Caffery L.J., Gesesew H.A., King A., Bassal A.-r, Ford K.…Ward P.R. How Australian health care services adapted to telehealth during the COVID-19 pandemic: A survey of telehealth professionals [Original Research] Frontiers in Public Health. 2021;9(121) doi: 10.3389/fpubh.2021.648009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Joanna Briggs Institute. (2017). Critical Appraisal Checklists. 〈https://jbi.global/critical-appraisal-tools〉.
- Thiese M.S. Observational and interventional study design types; an overview. Biochemia Medica. 2014;24(2):199–210. doi: 10.11613/BM.2014.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokatly Latzer I., Leitner Y., Karnieli-Miller O. Core experiences of parents of children with autism during the COVID-19 pandemic lockdown. Autism. 2021;25(4):1047–1059. doi: 10.1177/1362361320984317. [DOI] [PubMed] [Google Scholar]
- Turkoglu S., Ucar H.N., Cetin F.H., Guler H.A., Tezcan M.E. The relationship between chronotype, sleep, and autism symptom severity in children with ASD in COVID-19 home confinement period. Chronobiology International. 2020;37(8):1207–1213. doi: 10.1080/07420528.2020.1792485. [DOI] [PubMed] [Google Scholar]
- Varma P., Conduit R., Junge M., Lee V.V., Jackson M.L. A systematic review of sleep associations in parents and children. Journal of Child and Family Studies. 2021;30(9):2276–2288. doi: 10.1007/s10826-021-02002-5. [DOI] [Google Scholar]
- Whelan S., Mannion A., Madden A., Berger F., Costello R., Ghadiri S., Leader G. Examining the relationship between sleep quality, social functioning, and behavior problems in children with autism spectrum disorder: A systematic review. Nature and Science of Sleep. 2022;14:675–695. doi: 10.2147/NSS.S239622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.