Abstract
Enteropathogenic Escherichia coli (EPEC) is an important human intestinal pathogen, especially in infants. EPEC adherence to intestinal epithelial cells induces the accumulation of a number of cytoskeletal proteins beneath the bacteria, including the membrane-cytoskeleton linker ezrin. Evidence suggests that ezrin can participate in signal transduction. The aim of this study was to determine whether ezrin is activated following EPEC infection and if it is involved in the cross talk with host intestinal epithelial cells. We show here that following EPEC attachment to intestinal epithelial cells there was significant phosphorylation of ezrin, first on threonine and later on tyrosine residues. A significant increase in cytoskeleton-associated ezrin occurred following phosphorylation, suggesting activation of this molecule. Nonpathogenic E. coli and EPEC strains harboring mutations in type III secretion failed to elicit this response. Expression of dominant-negative ezrin significantly decreased the EPEC-elicited association of ezrin with the cytoskeleton and attenuated the disruption of intestinal epithelial tight junctions. These results suggest that ezrin is involved in transducing EPEC-initiated signals that ultimately affect host physiological functions.
Infection by enteropathogenic Escherichia coli (EPEC) is associated with significant morbidity and mortality, especially in infants (3, 40). Although EPEC was one of the first pathogenic strains of E. coli to be linked to diarrheal disease, its mechanisms of pathogenesis have yet to be completely elucidated. Following EPEC adherence to enterocytes, a characteristic histological lesion is formed (26, 39). This lesion, termed attaching and effacing (A/E), is characterized by the accumulation of a number of cytoskeletal proteins beneath the adherent bacteria, including actin, talin, α-actinin, and ezrin (17). The involvement of these proteins in EPEC pathogenesis has not been explored. Several host signal transduction pathways are activated following EPEC attachment (4, 15), and host intestinal epithelial functions, including ion transport (12, 23), immune response, (49) and tight junction (TJ) barrier function (10, 44, 53, 61), are perturbed. The host cell proteins involved in mediating the cross talk between adherent bacteria and/or bacterial proteins are not known. One potential candidate is the membrane-cytoskeleton linker and signal transducer, ezrin.
Ezrin, a member of the ezrin-radixin-moesin (ERM) family of proteins, is concentrated in the microvilli of epithelial cells (6) and redistributes to A/E lesions induced by EPEC adherence (17). Ezrin functions as a membrane-cytoskeleton linker (47) through the binding of its N terminus directly to integral membrane proteins such as CD44, CD43, and ICAM-2 (54, 60) or indirectly through ezrin binding protein 50 (45). The C terminus houses an F-actin binding site (57). Ezrin sites responsible for actin and membrane binding also interact intramolecularly, thus rendering the molecule inactive. Hence, specific activation signals are required to unmask the N- and C-terminal ERM-associated domains so that respective membrane and cytoskeletal interactions ensue. One event involved in activating ezrin is the phosphorylation of a C-terminal threonine residue, Thr 567. Phosphorylation of this critical threonine residue maintains ezrin in an active state by suppressing the intramolecular interaction (36).
Although threonine phosphorylation is crucial for unmasking F-actin and ezrin binding protein 50 binding sites (51), specific conserved tyrosine residues, 145 and 353, are phosphorylated by various stimuli as well (8, 29). In addition to its role as a structural component of the cytoskeleton, mounting evidence suggests that ezrin is also involved in signal transduction (9, 56). Both threonine and tyrosine phosphorylation appear to be key requirements for this function. For example, tyrosine phosphorylation has been shown to be essential for hepatocyte growth factor (HGF)-induced cell spreading (14) and HGF- and epidermal growth factor-stimulated changes in cell shape (8, 29). Recent studies have also shown that ERM proteins act in the upstream activation of the Rho pathway and as downstream targets of activated Rho and Rac (31). Together, these results provide evidence that ezrin possesses activities that control both cell shape and signaling.
In view of the observations that EPEC redistributes ezrin, alters host cell membrane morphology, and induces a number of different signaling cascades, the aims of this study were to determine whether EPEC activates ezrin, as assessed by enhanced threonine and tyrosine phosphorylation and cytoskeletal association, and to investigate whether ezrin is involved in EPEC-induced changes in a functional endpoint, TJ barrier function. For these studies, we utilized the human intestinal epithelial cell line T84, as well as the porcine kidney epithelial cell line LLC-PK1, transfected to express full-length or dominant-negative ezrin. Together, our findings suggest that ezrin is exploited by EPEC to effect functional changes in its host target, the intestinal epithelial cell.
MATERIALS AND METHODS
Chemicals and antibodies.
Antibiotics, protease inhibitors, monoclonal anti-vesicular stomatitis virus glycoprotein (anti-VSVG) antibody, polyclonal anti-rabbit immunoglobulin G alkaline phosphate-conjugated antibodies and protein A immobilized on Sepharose were purchased from Sigma Chemical Company (St. Louis, Mo.). The Bradford protein assay reagent was purchased from Bio-Rad (Hercules, Calif.). Rabbit polyclonal antiezrin antibody was raised against the entire ezrin molecule produced in bacteria as previously described (1). Commercially available antibody to ZO-1 (Zymed Laboratories, San Francisco, Calif.) was used. Rhodamine-labeled phalloidin and Antifade used for immunofluorescence studies were purchased from Molecular Probes (Eugene, Oreg.). Blocking solution, polyclonal rabbit antiphosphotyrosine and antiphosphothreonine antibodies, and the nitroblue tetrazolium–5-bromo-4-chloro-3-indoylphosphate premixed solution were purchased from Zymed Laboratories, Inc.
Cell culture.
T84 polarized human intestinal epithelial cells and LLC-PK1, a polarized epithelial cell line derived from the proximal tubules of pig kidney (43), were used for these studies. LLC-PK1 stable transfectants expressing full-length ezrin (E17) or the N-terminal domain of ezrin (amino acids [aa] 1 to 309), serving as a dominant-negative (N12), (1) were also used. T84 cells were grown in a 1:1 (vol/vol) mixture of Dulbecco-Vogt modified Eagle medium and Ham's F-12 with 6% newborn calf serum at 37°C in 5% CO2 as previously described (33). Passages 40 to 50 were used for these studies. LLC-PK1 cells were grown in Dulbecco-Vogt modified Eagle medium with 10% fetal calf serum and high glucose. Stable transfectants were grown in the presence of G418 (400 μg/ml).
Growth of bacteria and infection of host cells.
The EPEC strain E2348/69 pMAR 7, a derivative of the wild-type strain E2348/69 into which an ampicillin-resistant transposon had been inserted, a generous gift of James Kaper (Center for Vaccine Development, University of Maryland, Baltimore), was used for these studies. This modified wild-type strain was used to prevent the loss of the 60-MDa plasmid, which encodes the bundle-forming pilus responsible for the initial, nonintimate phase of attachment. This strain induces the characteristic A/E lesions in cultured T84 cells (53). Escherichia coli HB101, a nonpathogenic strain, was also obtained from James Kaper. Mutant strains UMD864 and UMD876 were provided by Michael Donenberg (Infectious Diseases, University of Maryland, Baltimore). UMD864 harbors a deletion of the espB gene, whose product is essential for the activation of signal transduction (18). UMD876 fails to express EspF, which is required for full disruption of the host intestinal epithelial barrier function (38). Both proteins are translocated via type III secretion.
Wild-type EPEC was grown overnight in Luria-Bertani broth in the presence of ampicillin (100 μg/ml). HB101 was similarly grown but in the absence of ampicillin. On the day of experimentation, overnight cultures were inoculated 1:50 into serum- and antibiotic-free T84 medium containing 0.5% mannose and grown to mid-log growth phase (optical density at 610 nm, 0.35 to 0.4; ca. 5 × 108 cells/ml) at 37°C with aeration. The bacterial suspension was pelleted and resuspended in culture medium. EPEC was added to the apical surface of T84 monolayers grown on glass coverslips, 60-mm-diameter dishes or collagen-coated permeable supports (Transwells; Costar, Cambridge, Mass.) at an MOI of ∼50 to 100. Monolayers and bacteria were coincubated in antibiotic- and serum-free medium at 37°C in a 5% CO2 water jacket incubator for 1 h. Medium was aspirated, and nonadherent bacteria were removed by gentle washing with phosphate-buffered saline (PBS). Monolayers were incubated for additional time periods as indicated. This well-characterized model has been employed to study the impact of EPEC infection on various intestinal epithelial functions (23, 48, 61).
Quantitation of adherent bacteria.
To determine the efficiency of EPEC attachment to the transfected LLC-PK1 cell lines, cells were infected as described above. After extensive washing to remove nonadherent bacteria, serial dilutions of scraped cells with attached bacteria were plated on Luria-Bertani agar plates containing ampicillin (100 μg/ml). Plates were incubated at 37°C overnight, and the number of CFU was determined.
Protein extraction and fractionation.
To obtain total cell lysates, monolayers were washed on ice with cold PBS three times, and scraped with a rubber policeman in the presence of PBS and 1 mM phenylmethylsulfonyl fluoride. Clarified cell extracts were obtained by centrifuging for 10 min at 300 × g at 4°C. Pellets were resuspended in Laemmli buffer, and samples were heated to 100°C for 5 min. Equal volumes were separated by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS–10% PAGE). To obtain detergent-soluble and -insoluble fractions, monolayers were lysed in the Triton X-100 extraction buffer as previously described (29) [50 mM 2-(N-morpholino)ethanesulfonic acid, 3 mM EGTA, 5 mM MgCl2, 0.5% Triton X-100 (pH 6.4), 1 mM phenylmethylsulfonyl fluoride, pepstatin (20 μg/ml), leupeptin (20 μg/ml), aprotinin (20 μg/ml), 1 mM NaVO3, 50 mM NaF] for 10 min at room temperature. The extract was fractionated by centrifugation at 14,000 × g for 10 min at 4°C. Pellets, representing the insoluble fraction, were washed twice with PBS and resuspended in Laemmli buffer. Equal volumes of protein from the insoluble fraction were separated by SDS-PAGE and probed by immunoblotting.
Immunoprecipitation.
Clarified whole-protein extracts (1.0 mg) were rotated for 2 h at 4°C with 5.0 μg of polyclonal rabbit antiphosphotyrosine or antiphosphothreonine antibodies or with 5 μg of ezrin antibody. Protein extracts and antibody were then rotated for 2 h at 4°C with protein A-Sepharose beads. Beads were washed three times with radioimmunoprecipitation assay extraction buffer, and the samples were eluted by boiling for 10 min in SDS sample buffer containing 100 mM dithiothreitol. Eluted proteins were resolved by SDS-PAGE and analyzed by immunoblotting as described below.
Immunoblot analysis.
Proteins from total cell lysates, cellular fractions, or immunoprecipitation were subjected to SDS–10% PAGE (10% acrylamide) as previously described (30). The proteins were transferred electrophoretically onto 0.45–μm-pore-size nitrocellulose membranes using a Trans-Blot Cell apparatus (Bio-Rad Laboratories, Richmond, Calif.). The membranes were blocked with Zymed blocking solution for 1 h at room temperature. Following three 5-min washes in Tris-buffered saline-Tween, membranes were subjected to sequential incubation with appropriate primary antibodies for 1 h followed by corresponding alkaline phosphatase-conjugated secondary antibody at appropriate dilutions for 1 h. Color development was achieved with a nitroblue tetrazolium–5-bromo-4-chloro-3-indolyl phosphate premixed solution purchased from Zymed Laboratories.
Immunofluorescence microscopy.
Uninfected control and EPEC-infected monolayers of T84 cells were processed for immunofluorescence to visualize total ezrin or the VSVG tag of transfected ezrin. A previously published fixation protocol for immunofluorescence detection of ezrin was used (22). Cells were fixed with 10% trichloroacetic acid, rinsed with G-PBS (30 mM glycine in PBS), and permeabilized with 0.2% Triton X-100 in G-PBS for 15 min. Blocking was achieved by incubation with G-PBS containing 10% fetal calf serum and 1% bovine serum albumin for 1 h. Monolayers were incubated with ezrin antibody for 1 h followed by rhodamine-conjugated anti-rabbit immunoglobulin G antibody for 1 h. Adherent EPEC cells were visualized by incubating with E. coli antibody (raised against E. coli lysates-AXL480; Accurate Chemical and Scientific Corp., Westbury, N.Y.) for 1 h, followed by fluorescein-conjugated goat anti-rabbit secondary antibody for 1 h. The protocol used for ZO-1 staining was as previously published (49). Monolayers were washed and mounted with Antifade reagent (Molecular Probes). Stained monolayers were visualized and photographed using a Nikon Opti-Phot inverted microscope equipped with the Spot-RT digital imaging system (Diagnostic Instruments, Sterling Heights, Mich.).
Electrophysiological studies.
Cells were grown to confluence on 0.33-cm2 collagen-coated permeable supports (Transwells; Costar). Using a simplified apparatus described by Madara et al. (32) transepithelial electrical resistance (TER) was determined by passing 25 μA of current, measuring the resulting voltage deflection, and applying Ohm's law (V = IR) to calculate resistance.
Computer imaging and densitometric analysis of immunoblots.
Immunoblots were scanned using DeskScan II (Hewlett-Packard, Palo Alto, Calif.) and the images were compiled in Power Point. Densitometry was performed on immunoblots using NIH Immage 1.61 software. Densitometric values are represented as the fold increase in densitometry compared to the values from uninfected control cells.
Statistical analysis.
Data were analyzed using Student's t test for unpaired data. Data were considered significant if P was ≤0.05.
RESULTS
Concentration of ezrin does not change following infection with EPEC.
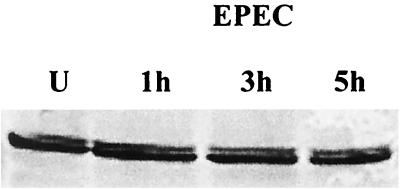
Since ezrin is one of the host cytoskeletal proteins that redistributes following infection with EPEC, we initially evaluated the effect of EPEC infection on the total T84 cell ezrin. Immunoblot analysis was performed on proteins extracted from both uninfected control monolayers and monolayers infected with EPEC for 1, 3, or 5 h. Following infection, total cell lysates were collected and resolved by SDS-PAGE. Immunoblot analysis of T84 cells with polyclonal ezrin antibody revealed a band migrating at 82 kDa (Fig. 1). No change was observed in the density of this band following EPEC infection, demonstrating that ezrin concentration does not change.
FIG. 1.
Concentration of ezrin in intestinal epithelial cells is not altered following EPEC infection. Immunoblot analysis of ezrin from uninfected controls (U) and cells infected with EPEC for 1, 3, and 5 h revealed no change in migration or density of this band. The immunoblot is representative of three separate experiments.
EPEC infection increases threonine phosphorylation of ezrin.
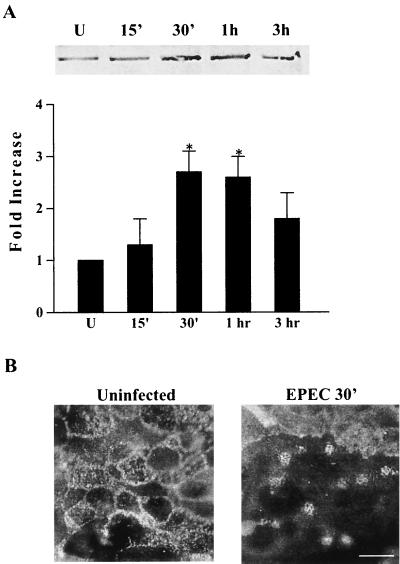
Evidence suggests that phosphorylation of a critical threonine residue, Thr 567, in the C terminus of ezrin converts this protein from a dormant state to an active state (36). Phosphorylation of this specific residue is also believed to maintain ERM proteins in the active state by suppressing the intramolecular interactions (58). To examine the impact of EPEC infection on the threonine phosphorylation of ezrin, proteins from uninfected T84 cells or cells infected with EPEC for 15 min, 30 min, 1 h, and 3 h were subjected to immunoprecipitation using antiphosphothreonine antibodies. Immunoprecipitates were separated by SDS–10% PAGE and then immunoblotted with polyclonal ezrin antibody. Threonine-phosphorylated ezrin peaked at 2.6 ± 0.4-fold above uninfected controls at 30 min postinfection. Threonine phosphorylation then began to diminish but remained elevated for at least 3 h postinfection (Fig. 2A). Protein extracts were also immunoprecipitated using ezrin antibody and immunoblotted with antiphosphothreonine antibody. A similar increase in threonine phosphorylation (threefold) was seen at 30 min using this approach. These findings demonstrate that threonine phosphorylation of ezrin is an early event associated with EPEC infection. To correlate this early change in ezrin phosphorylation with bacterial adherence and EPEC-induced A/E lesion formation, monolayers were infected for 30 min and immunofluorescence staining for ezrin was performed. Ezrin localization within A/E lesions was apparent in cells infected with EPEC for this short period of time (Fig. 2B).
FIG. 2.
Threonine phosphorylation of ezrin is rapidly enhanced following EPEC infection and corresponds with A/E lesion formation. (A) Immunoprecipitation using antiphosphothreonine antibody was performed on extracts from uninfected controls (U) and cells infected with EPEC for the designated times. Separation by SDS-PAGE and immunoblot analysis using polyclonal ezrin antibody revealed an increase in ezrin threonine which peaked at 30 min postinfection. The bar graph represents means + standard errors of the means (error bars) of three separate experiments. *, P ≤ 0.05. (B) Uninfected monolayers and monolayers infected with EPEC for 30 min were subjected to immunofluorescence staining for ezrin. In uninfected controls, ezrin is distributed throughout the cells. After 30 min of EPEC infection, characteristic A/E lesions have formed and ezrin redistribution to these sites is evident. Bar,10 μm.
EPEC infection induces tyrosine phosphorylation of ezrin.
Phosphorylation of ezrin on tyrosine residues is essential for its role as a membrane-cytoskeleton linker as well as for its role in signal transduction (9, 55, 56). To examine the level of tyrosine-phosphorylated ezrin in response to EPEC infection, clarified extracts from T84 control uninfected cells and cells infected with EPEC for 1, 3, and 5 h were subjected to immunoprecipitation. Extracts were immunoprecipitated using polyclonal antiphosphotyrosine or ezrin antibody followed by immunoblotting with polyclonal ezrin or antiphosphotyrosine antibody, respectively. An increase in tyrosine-phosphorylated ezrin was detected by 1 h following EPEC infection and peaked at 2.9-fold 3 h postinfection (Fig. 3A). In contrast to EPEC, nonpathogenic E. coli (HB101) failed to enhance the tyrosine phosphorylation of ezrin (Fig. 3B).
FIG. 3.
Tyrosine phosphorylation of ezrin is induced by EPEC but not nonpathogenic E. coli. (A) Whole-cell extracts from monolayers were immunoprecipitated with antiphosphotyrosine antibodies and immunoblotted for ezrin. An increase in ezrin tyrosine phosphorylation was seen at 1 h and peaked after 3 h of infection. This blot is representative of three separate experiments, and the corresponding graph shows the means + standard errors of the means (error bars) of densitometry measurements from these experiments. *, P ≤ 0.05. (B) Using the same experimental approach, no significant change in tyrosine-phosphorylated ezrin was detected in cells infected with nonpathogenic E. coli strain HB101.
EPEC infection enhances the association of ezrin with the cytoskeleton.
Phosphorylation of ezrin on threonine and tyrosine residues increases its association with the actin cytoskeleton. We therefore determined whether infection of T84 cells with EPEC increased cytoskeleton-associated ezrin, a commonly employed assay used to infer ezrin activation (27). Cytoskeletal and cytoskeleton-associated proteins are more resistant to solubilization by nonionic detergents (52). Detergent-insoluble fractions from uninfected cells and those infected for 1, 3, and 5 h were obtained using the Triton X-100 extraction procedure as previously published (28). Proteins from the insoluble pool were separated by SDS-PAGE, and then immunoblotted using ezrin antibody. As shown in Fig. 4A, there was a progressive and significant increase in the Triton X-100-insoluble pool of ezrin following EPEC infection—an increase of fourfold at 5 h. In contrast, nonpathogenic E. coli strain HB101 induced no change in cytoskeleton-associated ezrin (Fig. 4B). These findings show that there is a significant increase in the pool of cytoskeletal ezrin following infection with EPEC but not following infection with nonpathogenic E. coli strain HB101. The increase in cytoskeleton-associated ezrin correlated temporally with the EPEC-induced drop in transepithelial electrical resistance, a measure of barrier function (Fig. 4C).
FIG. 4.
EPEC infection enhances the association of ezrin with the cytoskeleton. (A) Protein extracts from uninfected cells (U) and cells infected with EPEC for the designated times were separated into detergent-soluble and -insoluble fractions. The insoluble fraction was separated by SDS-PAGE and immunoblotted for ezrin. A gradual increase in detergent-insoluble ezrin, representing the cytoskeleton-associated pool, was evident by 1 h postinfection and progressively increased over time. The immunoblot is representative of three separate experiments. Densitometry data represent the means ± standard errors of the means (error bars) of the same three experiments. *, P ≤ 0.05. (B) Nonpathogenic E. coli strain HB101 had no effect on ezrin association with the cytoskeleton. (C) Time course of TER changes in T84 cells following infection with EPEC or HB101.
Expression of dominant-negative ezrin attenuates ezrin association with the cytoskeleton following EPEC infection.
In an attempt to understand the role of EPEC-induced ezrin phosphorylation and cytoskeletal association in functional alterations in host cells, studies were performed with stable LLC-PK1 transfectants expressing full-length (E17) or dominant-negative (N12) ezrin. To determine whether EPEC attachment to different LLC-PK1 transfectants varied, the number of attached organisms was assessed as described in Materials and Methods. The number of CFU attached to E17 and N12 did not differ significantly (10% of the initial inoculum for both after 1 h).
EPEC infection did not alter the total ezrin content in LLC-PK1 E17 cells (Fig. 5A), but did enhance threonine phosphorylation of ezrin by 2.2-fold at 30 min (Fig. 5B) as was seen in T84 monolayers. Expression of the N terminus of ezrin (aa 1 to 309) has been previously characterized as producing a dominant-negative effect (1). Using the same approach of cell fractionation described for T84 cells, the effect of EPEC infection on the cytoskeletal association of ezrin in LLC-PK1 cells overexpressing full-length (E17) and dominant-negative (N12) ezrin was examined. LLC-PK1 transfected cells, E17 and N12, were infected with EPEC for 15 min, 30 min, 1 h, and 5 h. The extracted proteins were fractionated, and the Triton X-100-insoluble pool was immunoblotted for ezrin. The expected shift of ezrin to the insoluble pool after EPEC infection was seen in cells expressing full-length ezrin as early as 15 min and by 5 h had increased 18-fold (Fig. 5C). In contrast, analysis of the insoluble protein fraction from cells expressing dominant-negative ezrin showed no significant change in cytoskeleton-associated ezrin (Fig. 5D).
FIG. 5.
Effect of EPEC infection on cytoskeleton-associated ezrin in stable transfectants of LLC-PK1 cells expressing full-length (E17) and dominant-negative (N12) ezrin. (A) Immunoblot analysis of LLC-PK1 E17 cells showed no change in total ezrin following EPEC infection. (B) Immunoprecipitation with antiphosphothreonine antibody followed by immunoblotting for ezrin revealed that threonine phosphorylation of ezrin in E17 cells increased 2.3-fold following 30 min of EPEC infection. (C) Analysis of the detergent-insoluble pool of ezrin from uninfected control and EPEC-infected LLC-PK1 E17 monolayers revealed a gradual increase in the insoluble fraction of ezrin. Increased ezrin-cytoskeleton association was evident by 15 min postinfection and progressively increased for up to 5 h. Using the same approach, EPEC infection of N12 revealed no change in cytoskeleton-associated ezrin, consistent with its dominant-negative effect. (D) The bar graph represents means + standard errors of the means of three separate experiments. *, P ≤ 0.02 for the ezrin insoluble fraction in E17 versus N12.
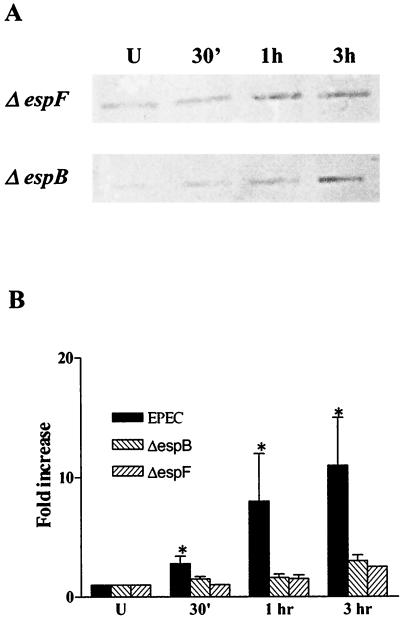
Virulence factors associated with EPEC type III secretion are required for cytoskeletal association of ezrin.
The type III secretion apparatus is critical to EPEC pathogenesis (24). Several EPEC-secreted proteins are translocated into host cells using this machinery. We examined the effect of deleting two genes that express EPEC-secreted proteins, EspB and EspF, on ezrin activation. UMD864 harbors a deletion of the espB gene whose product is believed to form pores in the host cell membrane (59) and is essential for the activation of signal transduction (18). Bacterial strain UMD876 does not express EspF, a protein recently characterized as an effector molecule required for disrupting intestinal epithelial barrier function (38). LLC-PK1 cells expressing full-length ezrin were infected with these mutant strains for 30 min, 1 h, and 3 h, and the insoluble protein fraction was immunoblotted for ezrin. In contrast to wild-type EPEC, the impact of the espB and espF mutant strains on cytoskeleton-associated ezrin was significantly reduced (Fig. 6B). Also, EspB and EspF mutant strains were significantly (P < 0.05) less efficient in reducing TER in T84 monolayers compared to the wild-type EPEC (40% ± 8%, 5% ± 10%, and 15% ± 8% decrease in TER at 5 h for wild-type EPEC, espB, and espF mutant strains, respectively).
FIG. 6.
Virulence genes associated with the type III secretion are crucial for ezrin association with the cytoskeleton. (A) The detergent insoluble fractions of protein extracts from E17 uninfected cells and cells infected with two EPEC type III secretion mutants, espB and espF, were analyzed for ezrin-cytoskeleton association. (B) The increase of ezrin in the cytoskeletal pool in response to infection by either the espB or espF mutant strain was markedly less compared to wild type EPEC, as shown in the bar graph.
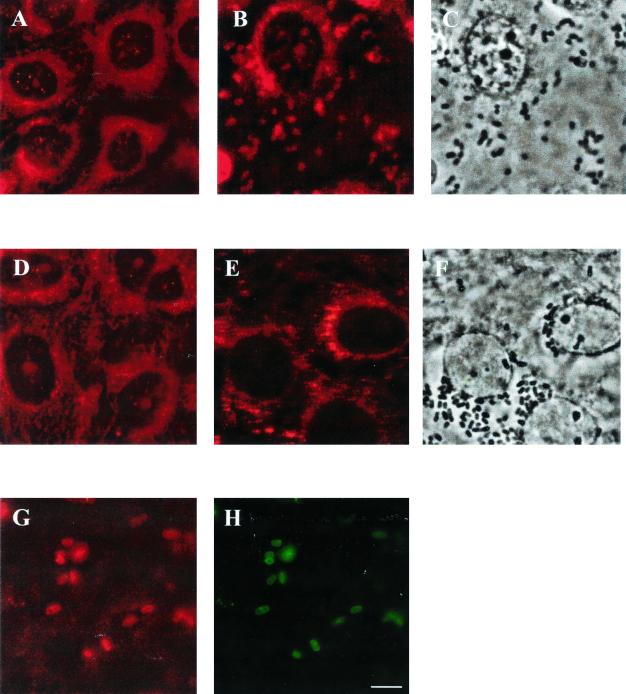
EPEC infection redistributes both full-length and dominant-negative ezrin into A/E lesions.
As shown in T84 cells (Fig. 2B), ezrin also redistributed to A/E lesions in LLC-PK1 cells infected with EPEC (Fig. 7). Using the same approach of immunofluorescence staining, we followed changes in ezrin localization in E17 and N12 cells after infection. In control, uninfected cells immunofluorescently stained for ezrin (Fig. 7A and F) there was uniform distribution throughout the cells, as has been previously shown (14). After EPEC infection, accumulation of ezrin in A/E lesions was evident in both E17 and N12 monolayers (Fig. 7B and E). The association of ezrin with attached bacteria is evident by comparing the distribution of attached organisms, seen by phase-contrast microscopy (Fig. 7C and D), to the distribution of ezrin (Fig. 7B and E). To determine whether dominant-negative ezrin localized to A/E lesions, N12 monolayers were dually labeled using VSVG antibody, which labeled only the transfected pool of ezrin (Fig. 7G), and antibody against E. coli (Fig. 7H). The staining pattern for these two antibodies is identical, indicating that dominant-negative ezrin accumulates in A/E lesions.
FIG. 7.
EPEC infection causes the redistribution of ezrin in both E17 and N12 monolayers into A/E lesions. E17 and N12 monolayers were immunostained using ezrin antibody. (A and D) Uninfected E17 and N12 monolayers, respectively. The distribution of ezrin is similar in both cell lines. (B and E) Following 30 min of EPEC infection, ezrin localized to A/E lesions in both E17 and N12 monolayers. The relocalization of ezrin beneath attached organisms (C and F) (shown by phase microscopy) is demonstrated by comparing panel B to C and panel E to F. N12 monolayers infected with EPEC for 30 min were also dual-stained for the VSVG tag of dominant-negative ezrin (G) and EPEC (H). The identical distribution of transfected dominant-negative ezrin and attached EPEC is evident.
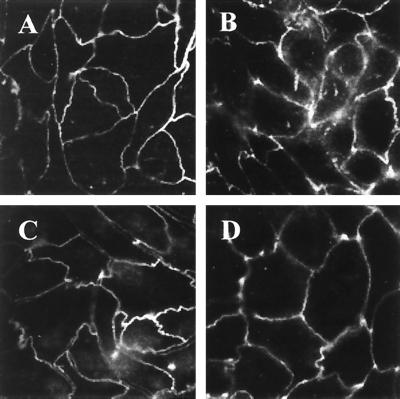
Dominant-negative ezrin diminishes the effect of EPEC on TJs.
Some proteins of the ERM family have been shown to play an important role in the organization and function of TJs by establishing a link between TJ proteins and the actin cytoskeleton (37). Also, EPEC infection has been shown to alter the TJ proteins (44, 50). To examine if ezrin is involved in these changes, we used immunofluorescence microscopy to study ZO-1 distribution in E17 and N12 cells in response to EPEC infection. In uninfected E17 monolayers, ZO-1 localized primarily to the cell membrane at the level of TJ in a uniform manner (Fig. 8A). Following EPEC infection, breaks in ZO-1 staining were seen (Fig. 8B) as was previously reported (44). The same pattern was observed in T84 cells following EPEC infection (reference 44 and our data [not shown]). The localization of ZO-1 in cells overexpressing dominant-negative ezrin was the same as in E17 monolayers (Fig. 8C). However, the EPEC-induced disruption of ZO-1 was significantly reduced in N12 monolayers compared to E17 (Fig. 8D). To determine whether ezrin plays a role in mediating an EPEC-induced functional response, monolayers of E17 and N12 ezrin transfectants were infected with EPEC, and TER, a measure of TJ barrier function, was assessed. Consistent with the ZO-1 findings described above, the expression of dominant-negative ezrin significantly attenuated the effect of EPEC on TER compared to the expression of full-length ezrin (Fig. 9).
FIG. 8.
Dominant-negative ezrin diminishes the effect of EPEC on ZO-1. Confluent monolayers of E17 and N12 cells were subjected to immunofluorescence staining for ZO-1. Uniform distribution of ZO-1 at the level of TJ was observed in both E17 (A) and N12 (C) monolayers. (B) After 3 h of EPEC infection, breaks in the staining of ZO-1 were seen in E17 cells. (D) The alterations in ZO-1 staining in response to EPEC infection were markedly attenuated in N12 monolayers expressing dominant-negative ezrin.
FIG. 9.
Expression of dominant-negative ezrin attenuates the drop in TER resulting from EPEC infection. Monolayers of E17 or N12 cells grown on permeable supports were infected with EPEC as described in Materials and Methods. TER was serially measured, and following 5 h of infection, the TER of E17 monolayers decreased approximately 50%, similar to the response in T84 monolayers. The impact of EPEC on the TER of N12 monolayers, however, was significantly attenuated (P = 0.009 for E17 versus N12; data represent means − standard errors of the means (error bars) for three experiments performed in triplicate or quadruplicate) despite no difference in the number of attached organisms. The absolute TER value of uninfected E17 monolayers was 134 ± 20 Ωcm2, and that of N12 monolayers was 148 ± 13 Ωcm2 (n = 9 for each group).
DISCUSSION
In an attempt to clarify the complex nature of interactions between the cortical actin cytoskeleton and integral membrane proteins, recent studies brought into focus the ERM proteins, which serve as cross-linkers between specific plasma membrane proteins and cortical actin filaments. For ERM protein activation, specific signals, such as phosphorylation or binding of phosphatidylinositol 4,5-bisphosphate (lipid signaling molecule) to the N-terminal domain is required (8, 25, 36). Activation of ERM proteins may be triggered by physiological (14, 29) and pathophysiological (52, 58) processes. Ezrin is one of the host cytoskeletal proteins reorganized following EPEC infection (17). Here we examine the impact of this important enteric bacterial pathogen on ezrin activation and explore its involvement in EPEC pathogenesis.
In contrast to prototypic enteric bacterial pathogens, EPEC produces no recognized toxin and is essentially noninvasive. Instead, through a series of complex steps, EPEC intimately attaches to its host cell and, by the expression of a type III secretory apparatus, is able to transport and deliver proteins into that cell (24). EPEC attachment and/or injection of prokaryotic proteins into the host cell activates several signal transduction pathways (13, 35, 46, 61). Considering the role of ezrin in signal transduction (7, 11, 19, 21) and its involvement in organizing cell shape, we were interested in examining the effect of EPEC on ezrin. Our findings show that EPEC infection immediately induces a threefold increase in the threonine phosphorylation of ezrin, corresponding with its redistribution to A/E lesions. Of the other stimuli that induce ERM threonine phosphorylation and cytoskeletal association, thrombin treatment of human erythro-leukemia cells causes a twofold increase in phosphorylation of moesin (51), while HGF-treated LLC-PK1 cells show a 10 to 20% increase in cytoskeleton-associated ezrin. In contrast to the simultaneous stimulation of all cells by growth factors, EPEC infection causes an asynchronous localized stimulation, highlighting the level of ezrin activation in this system. Phosphorylation of a critical C-terminal threonine residue appears to be required for ERM protein activation. Recent insight into crystal structure of dormant moesin showed that Thr558 phosphorylation weakens the interactions between C- and N-terminal ERM-associated domains through both electrostatic and steric effects (42). Since EPEC enhances the cytoskeletal association of ezrin, and dominant-negative ezrin attenuates this event as well as its functional consequences, we speculate that EPEC induces the phosphorylation of critical threonine residues. In addition to threonine phosphorylation, phosphorylation of specific tyrosine sites is also deemed important for ezrin activation (14, 29). For instance, epidermal growth factor stimulation of A431 cells concomitantly caused the tyrosine phosphorylation of ezrin and its translocation from the cytoplasm to the cortical actin layer. EPEC also induces ezrin tyrosine phosphorylation, but subsequent to the phosphorylation of threonine residues. The sequence of EPEC-induced phosphorylation of ezrin with respect to barrier disruption is intriguing. Threonine phosphorylation, a crucial step in opening the ezrin molecule, is an early step associated with EPEC infection. Tyrosine phosphorylation, required for maintaining ERMs in their active state as well as inducing signal transduction events leading to cytoskeletal rearrangements, is apparent at later stages of infection and just precedes the increased association of ezrin with the cytoskeleton. Changes in TER are not detected until approximately 2 h postinfection and progressively increase over time, temporally correlating with increases in cytoskeleton-associated ezrin.
Nonpathogenic E. coli failed to induce these events, suggesting that specific virulence genes are important for this process. Since type III secretion is central to EPEC pathogenicity (24), it is possible that one of the EPEC virulence factors delivered into the host cell activates ezrin. Indeed, mutants lacking either EspB or EspF, two type III secreted proteins, were significantly attenuated for ezrin activation compared to wild-type EPEC. Consistent with this, these mutants also failed to reduce TER in T84 cells as efficiently as the wild-type strain (38).
Finally, we show that ezrin is important in mediating EPEC-induced signals that ultimately result in perturbation of the TJ barrier. One possible link between ezrin and TJs was suggested by studies regarding the downstream signaling pathways activated by the small GTPase protein Rho (2). Rho-kinase and the myosin-binding subunit of myosin phosphatase, two downstream targets of Rho, interact with ERM proteins and myosin light chain (MLC), resulting in their phosphorylation. MLC is also phosphorylated following EPEC infection (34), triggering cytoskeletal contraction and the opening of TJs (61). In part, EPEC-induced phosphorylation of MLC is a consequence of MLC kinase activation (61). Whether inhibition of MLC phosphatase or direct phosphorylation of MLC by Rho kinase is also triggered by EPEC has not been determined. Although A/E lesion formation by EPEC was shown to be independent of Rho (5, 16) other EPEC-induced events in the host cell, such as protein phosphorylation, may be regulated by Rho. In fact, we recently demonstrated that EPEC-associated alterations in TJ permeability occur independent of A/E lesion formation (38).
Indirect evidence from other model systems suggests that ezrin may be directly involved in regulating TJ permeability. In MDCK cells, HGF has been shown to increase cytoskeleton-associated ezrin (14) and cause the disassembly of ZO-1 (20), a peripheral TJ-associated protein speculated to organize TJ proteins so that signaling events can be generated. An independent study showed that HGF stimulation of intestinal epithelial monolayers perturbed the TJ barrier (41). EPEC infection alters the distribution of TJ proteins (44, 50). Thus, both HGF- and EPEC-induced changes in TJ permeability may result from ezrin-mediated signaling. The role of EPEC-activated ezrin in altering TJ function was evaluated by studying ZO-1 distribution in stable transfectants expressing either full-length (E17) or dominant-negative (N12) ezrin. Dominant-negative ezrin reduced the effect of EPEC on the cytoskeletal association of ezrin, ZO-1 distribution, and barrier function, suggesting an active role for ezrin in EPEC-induced TJ alterations. A similar approach revealed that ezrin was important in mediating Shigella invasion (52).
Truncated N-terminal ezrin localized to A/E lesions, suggesting that the dominant-negative effect may be due to the absence of the C-terminally mediated transduction of signals. Since ERM proteins share 85% identity in their N-terminal halves, we cannot exclude the possibility that other ERMs may also participate in this process. In fact, the presence of these proteins may partly account for the residual effect of EPEC on TER in N12 monolayers. Nevertheless, this work highlights the importance of ezrin in mediating the events that lead to physiological alterations in the host, specifically intestinal epithelial barrier function. It also gives further insight into the complex nature of cytoskeletal rearrangements and signaling events in host cells triggered by EPEC infection.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (grant DK50694 to G.H.) and from the Department of Veterans Affairs (Merit Review and Research Enhancement Awards Program to G.H.).
REFERENCES
- 1.Algrain M, Turunen O, Vaheri A, Louvard D, Arpin M. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J Cell Biol. 1993;120:129–139. doi: 10.1083/jcb.120.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amano M, Fukata Y, Kaibuchi K. Regulation of cytoskeleton and cell adhesions by small GTPase Rho and its targets. Trends Cardiol Med. 1998;8:162–168. doi: 10.1016/S1050-1738(97)00145-X. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin T J. Pathogenicity of enteropathogenic Escherichia coli. J Med Microbiol. 1998;47:283–293. doi: 10.1099/00222615-47-4-283. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin T J, Knutton S, Haigh R, Williams P H, Palmer H M, Aitken A, Borriello S P. Hijacking host cell signal transduction mechanisms during infection with enteropathogenic Escherichia coli. Biochem Soc Trans. 1996;24:552–558. doi: 10.1042/bst0240552. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Ami G, Ozeri V, Hanski E, Hofmann F, Aktories K, Hahn K M, Bokoch G M, Rosenshine I. Agents that inhibit Rho, Rac, and Cdc42 do not block formation of actin pedestals in HeLa cells infected with enteropathogenic Escherichia coli. Infect Immun. 1998;66:1755–1758. doi: 10.1128/iai.66.4.1755-1758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berryman M, Franck Z, Bretscher A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci. 1993;105:1025–1043. doi: 10.1242/jcs.105.4.1025. [DOI] [PubMed] [Google Scholar]
- 7.Bonilha V L, Finnemann S C, Rodriguez-Boulan E. Ezrin promotes morphogenesis of apical microvilli and basal infoldings in retinal pigment epithelium. J Cell Biol. 1999;147:1533–1547. doi: 10.1083/jcb.147.7.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bretscher A. Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. J Cell Biol. 1989;108:921–930. doi: 10.1083/jcb.108.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bretscher A, Reczek D, Berryman M. Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J Cell Sci. 1997;110:3011–3018. doi: 10.1242/jcs.110.24.3011. [DOI] [PubMed] [Google Scholar]
- 10.Canil C, Rosenshine S, Ruschkowski S, Donnenberg M S, Kaper J B, Finlay B B. Enteropathogenic Escherichia coli decreases the transepithelial electrical resistance of polarized epithelial monolayers. Infect Immun. 1993;61:2755–2762. doi: 10.1128/iai.61.7.2755-2762.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Cohn J A, Mandel L J. Dephosphorylation of ezrin as an early event in renal microvillar breakdown and anoxic injury. Proc Natl Acad Sci USA. 1995;92:7495–7499. doi: 10.1073/pnas.92.16.7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collington G K, Booth I W, Knutton S. Rapid modulation of electrolyte transport in Caco-2 cell monolayers by enteropathogenic Escherichia coli (EPEC) infection. Gut. 1998;42:200–207. doi: 10.1136/gut.42.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crane J K, Oh J S. Activation of host cell protein kinase C by enteropathogenic Escherichia coli. Infect Immun. 1997;65:3277–3285. doi: 10.1128/iai.65.8.3277-3285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crepaldi T, Gautreau A, Comoglio P M, Louvard D, Arpin M. Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J Cell Biol. 1997;138:423–434. doi: 10.1083/jcb.138.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dytoc M, Fedorko L, Sherman P M. Signal transduction in human epithelial cells infected with attaching and effacing E. coli in vitro. Gastroenterology. 1994;106:1150–1161. doi: 10.1016/0016-5085(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 16.Ebel F, von Eichel-Streiber C, Rohde M, Chakraborty T. Small GTP-binding proteins of the Rho- and Ras-subfamilies are not involved in the actin rearrangements induced by attaching and effacing Escherichia coli. FEMS Microbiol Lett. 1998;163:107–112. doi: 10.1111/j.1574-6968.1998.tb13033.x. [DOI] [PubMed] [Google Scholar]
- 17.Finlay B B, Rosenshine I, Donnenberg M S, Kaper J B. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1992;60:2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foubister V, Rosenshine I, Donnenberg M, Finlay B B. The eaeB gene of enteropathogenic Escherichia coli is necessary for signal transduction in epithelial cells. Infect Immun. 1994;62:3038–3040. doi: 10.1128/iai.62.7.3038-3040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gautreau A, Louvard D, Arpin M. Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane. J Cell Biol. 2000;150:193–203. doi: 10.1083/jcb.150.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grisendi S, Arpin M, Crepaldi T. Effect of hepatocyte growth factor on assembly of zonula occludens-1 protein at the plasma membrane. J Cell Physiol. 1998;176:465–471. doi: 10.1002/(SICI)1097-4652(199809)176:3<465::AID-JCP3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 21.Hanzel D, Reggio H, Bretscher A, Forte J G, Mangeat P. The secretion-stimulated 80K phosphoprotein of parietal cells is ezrin, and has properties of a membrane cytoskeletal linker in the induced apical microvilli. EMBO J. 1991;10:2363–2373. doi: 10.1002/j.1460-2075.1991.tb07775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi K, Yonemura S, Matsui T, Tsukita S, Tsukita S. Immunofluorescence detection of exrin/radixin/moesin (ERM) proteins with their carboxyl-terminal threonine phosphorylated in cultured cells and tissues. Application of a novel fixation protocol using trichloroacetic acid (TCA) as a fixative. J Cell Sci. 1999;112:1149–1158. doi: 10.1242/jcs.112.8.1149. [DOI] [PubMed] [Google Scholar]
- 23.Hecht G, Koutsouris A. Enteropathogenic E. coli attenuates secretagogue-induced net intestinal ion transport but not Cl45 ∴φ ∴ς 12 secretion. Am J Physiol. 1999;276:G781–G788. doi: 10.1152/ajpgi.1999.276.3.G781. [DOI] [PubMed] [Google Scholar]
- 24.Jarvis K G, Giron J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang W G, Hiscox S K, Singhrao M C, Puntis M D, Nakamura T, Mansel R E, Hallett M B. Induction of tyrosine phosphorylation and translocation of ezrin by hepatocyte growth factor/scatter factor. Biochem Biophys Res Commun. 1995;217:1062–1069. doi: 10.1006/bbrc.1995.2877. [DOI] [PubMed] [Google Scholar]
- 26.Knutton S, Lloyd D R, McNeish A S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987;55:69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondo T, Takeuchi K, Doi Y, Yonemura S, Nagata S, Tsukita S, Tsukita S. ERM (ezrin/radixin/moesin)-based molecular mechanism of microvillar breakdown at an early stage of apoptosis. J Cell Biol. 1997;139:749–758. doi: 10.1083/jcb.139.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreis T E. Microinjected antibodies against the cytoplasmic domain of vesicular stomatitis virus glycoprotein block its transport to the cell surface. EMBO J. 1986;5:931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krieg J, Hunter T. Identification of the two major epidermal growth factor-induced tyrosine phosphorylation sites in the microvillar core protein ezrin. J Biol Chem. 1992;267:19258–19265. [PubMed] [Google Scholar]
- 30.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Mackay D J G, Esch F, Furthmayr H, Hall A. Rho- and Rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for ezrin/radixin/moesin proteins. J Cell Biol. 1997;138:927–938. doi: 10.1083/jcb.138.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madara J L, Colgan S P, Nusrat A, Delp C, Parkos C A. A simple approach to measurement of electrical parameters of cultured epithelial monolayers: use in assessing neutrophil epithelial interactions. J Tissue Cult Res. 1992;14:209–216. [Google Scholar]
- 33.Madara J L, Stafford J, Dharmsathaphorn K, Carlson S. Structural analysis of a human intestinal epithelial cell line. Gastroenterology. 1987;92:1133–1145. doi: 10.1016/s0016-5085(87)91069-9. [DOI] [PubMed] [Google Scholar]
- 34.Manjarrez-Hernandez H A, Amess B, Sellers L, Baldwin T J, Knutton S, Williams P H, Aitken A. Purification of a 20 kDA phosphoprotein from epithelial cells and identification as myosin light chain. FEBS Lett. 1991;292:121–127. doi: 10.1016/0014-5793(91)80848-w. [DOI] [PubMed] [Google Scholar]
- 35.Manjarrez-Hernandez H A, Baldwin T J, Williams P H, Haigh R, Knutton S, Aitken A. Phosphorylation of the myosin light chain at distinct sites and its association with the cytoskeleton during enteropathogenic Escherichia coli infection. Infect Immun. 1996;64:2368–2370. doi: 10.1128/iai.64.6.2368-2370.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S. Rho-Kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattagajasingh S N, Huang S C, Hartenstein J S, Benz E J., Jr Characterization of the interaction between protein 4.1R and ZO-2. J Biol Chem. 2000;275:30573–30585. doi: 10.1074/jbc.M004578200. [DOI] [PubMed] [Google Scholar]
- 38.McNamara B P, Koutsouris A, O'Connell C B, Nougayrede J-P, Donnenberg M S, Hecht G. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J Clin Investig. 2001;107:621–629. doi: 10.1172/JCI11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moon H W, Whipp S C, Argenzio R A, Levine M M, Giannella R A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nusrat A, Parkos C, Bacarra A E, Godowski P J, Delp-Archer C, Rosen E M, Madara J L. Hepatocyte growth factor/scatter factor effects on epithelia. J Clin Investig. 1994;93:2056–2065. doi: 10.1172/JCI117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearson M A, Reczek D, Bretscher A, Karplus P A. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell. 2000;101:259–270. doi: 10.1016/s0092-8674(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 43.Pfaller W, Gstraunthaler G, Loidl P. Morphology of the differentiation and maturation of LLC-PK1 epithelia. J Cell Physiol. 1990;142:247–254. doi: 10.1002/jcp.1041420205. [DOI] [PubMed] [Google Scholar]
- 44.Philpott D J, McKay D M, Sherman P M, Perdue M H. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport. Am J Physiol. 1996;270:G634–G645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- 45.Reczek D, Berryman M, Bretscher A. Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol. 1997;139:169–179. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato N, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. A gene family consisting of ezrin, radixin, and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci. 1992;103:131–143. doi: 10.1242/jcs.103.1.131. [DOI] [PubMed] [Google Scholar]
- 48.Savkovic S, Koutsouris A, Hecht G. Activation of NF-107 ∴φ ∴ς 12B in intestinal epithelial cells by enteropathogenic Escherichia coli. Am J Physiol. 1997;273:C1160–C1167. doi: 10.1152/ajpcell.1997.273.4.C1160. [DOI] [PubMed] [Google Scholar]
- 49.Savkovic S D, Koutsouris A, Hecht G. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect Immun. 1996;64:4480–4487. doi: 10.1128/iai.64.11.4480-4487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simonovic I, Rosenberg J, Koutsouris A, Hecht G. Enteropathogenic E. coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol. 2000;2:305–315. doi: 10.1046/j.1462-5822.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- 51.Simons P, Pietromonaco S, Reczek D, Bretscher A, Elias L. C-terminal threonine phosphorylation activates ERM proteins to link the cell's cortical lipid bilayer to the cytoskeleton. Biochem Biophys Res Commun. 1998;253:561–565. doi: 10.1006/bbrc.1998.9823. [DOI] [PubMed] [Google Scholar]
- 52.Skoudy A, Van Nhieu G T, Mantis N, Arpin M, Mounier J, Gounon P, Sansonetti P. A functional role for ezrin during Shigella flexneri entry into epithelial cells. J Cell Sci. 1999;112:2059–2068. doi: 10.1242/jcs.112.13.2059. [DOI] [PubMed] [Google Scholar]
- 53.Spitz J, Yuhan R, Koutsouris A, Blatt C, Alverdy J, Hecht G. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol. 1995;268:G374–G379. doi: 10.1152/ajpgi.1995.268.2.G374. [DOI] [PubMed] [Google Scholar]
- 54.Tsukita S, Oishi K, Sato N, Sagara J, Kawai A, Tsukita S. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsukita S, Yonemura S, Tsukita S. ERM proteins: head-to-tail regulation of actin-plasma membrane interactions. Trends Biol Sci. 1997;22:53–58. doi: 10.1016/s0968-0004(96)10071-2. [DOI] [PubMed] [Google Scholar]
- 56.Tsukita S, Yonemura Y, Tsukita S. ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction. Curr Opin Cell Biol. 1997;9:70–75. doi: 10.1016/s0955-0674(97)80154-8. [DOI] [PubMed] [Google Scholar]
- 57.Turunen O, Wahlstrom T, Vaheri A. Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J Cell Biol. 1994;126:1445–1453. doi: 10.1083/jcb.126.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vaheri A, Carpen O, Heiska L, Helander T S, Jaaskelainen J, Majander-Nordenswan P, Sainio M, Timonen T, Turunen O. The ezrin protein family: membrane-cytoskeleton interactions and disease associations. Curr Opin Cell Biol. 1997;9:659–666. doi: 10.1016/s0955-0674(97)80119-6. [DOI] [PubMed] [Google Scholar]
- 59.Wachter C, Beinke C, Mattes M, Schmidt M A. Insertion of EspD into epithelial target cell membranes by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1999;31:1695–1707. doi: 10.1046/j.1365-2958.1999.01303.x. [DOI] [PubMed] [Google Scholar]
- 60.Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S, Tsukita S. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J Cell Biol. 1998;140:885–895. doi: 10.1083/jcb.140.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuhan R, Koutsouris A, Savkovic S D, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]