Abstract
Objective
To analyze the impact of the COVID-19 pandemic on medical care and vaccination acceptance of vasculitis patients in Germany.
Methods
A web-based national survey was developed by rheumatology centers and vasculitis patient advocacy groups. The survey was distributed nationwide by mail and flyers and could be accessed via a QR-code or weblink from December 2021 to April 2022. Descriptive statistics [mean, median, standard derivation (SD), 25%, 75% quantile] were calculated. 95% confidence intervals were presented for responses that were directly related to the impact of COVID-19 on parameters associated with vasculitis patient care.
Results
The online survey was completed by 117 patients with small and large vessel vasculitis [granulomatosis with polyangiitis (n = 69), eosinophilic granulomatosis with polyangiitis (n = 16), microscopic polyangiitis (n = 12), giant cell arteritis (n = 17) and Takayasu's arteritis (n = 3)]. Prescheduled rheumatological appointments had been canceled due to the COVID-19 pandemic in 12.6% of the respondents [95% confidence interval (CI), 7.3–20.0%); in 9% (95% CI, 4.5–15.6%)] appointments had been replaced by digital services. Therapeutic regimens were changed (shifted, reduced, or discontinued) due to the pandemic in 15.5% (95% CI 9.5–22.2%). Vaccination coverages were generally high compared to patients with other rheumatic diseases and the general population. Highest vaccination coverage was observed against COVID-19 (98.1% 95% CI 93.9–99.6%).
Conclusion
Vasculitis patients experienced changes in medical care during COVID-19 pandemic such as cancelation of prescheduled rheumatology appointments and modifications in therapeutic regimens. The overall acceptance rate for vaccination was comparatively high, particularly for vaccination against COVID-19.
Keywords: systemic vasculitis, COVID-19, vaccination coverage, health care, patient survey
Introduction
The care of vasculitis patients during the ongoing COVID-19 pandemic represents an extraordinary challenge: Once diagnosed, patients require regular follow-up visits to assess treatment responses, track symptoms, perform blood tests, monitor potential adverse events and comorbidities, and re-evaluate treatment indications (1).
At the beginning of the pandemic, risk factors associated with mortality and morbidity associated with COVID-19 infections were unknown and optimal management of immunosuppressive therapy was uncertain. This resulted in widespread anxiety and a high rate of self-isolation among patients diagnosed with rheumatic and musculoskeletal diseases (RMDs) (2, 3).
German (Deutsche Gesellschaft für Rheumatologie, DGRh) and European (European Alliance of Associations for Rheumatology, EULAR) organizations ultimately developed a set of recommendations and provided guidelines to address these concerns (4–7).
Specifically, these guidelines recommended to balance the benefits of regular visits (monitoring of disease activity, complications and comorbidities) and the risks associated with direct in-person consultations. Consequently, extended follow-up intervals could be considered for patients in a stable disease status (4).
Ultimately, vaccinations against COVID-19 were approved and recommended for patients diagnosed with RMDs (7–9). Reported vaccination rates in these patients ranged from 30.2 to 80% between June and October 2021 (10–12).
Against this background, our study aims to examine the impact of COVID-19 on the medical care of vasculitis patients across specialized centers in Germany and to evaluate their overall acceptance of COVID-19 vaccination.
Methods
A web-based national survey was developed by rheumatology centers in collaboration with vasculitis patient advocacy groups. An expert panel identified two areas of interest to investigate: (1) medical care of vasculitis patients during the COVID-19 pandemic and (2) vaccine acceptance.
After performing a literature search, members of the panel designed a questionnaire based on the standard operating procedures outlined by the EULAR recommendation task force (13). The questionnaire was shared with members of vasculitis patient advocacy groups who reviewed the draft version and provided critical insight and patient perspectives.
The web-based survey (LimeSurvey, https://www.limesurvey.org/) was distributed nationwide by members of patient advocacy groups and clinical rheumatologists via flyers and mail. The survey was accessible via a QR code or weblink from December 9, 2021, to April 19, 2022. Informed consent was obtained from all participants prior to study participation. Patients with self-reported diagnoses of small and large vessel vasculitis, including granulomatosis with polyangiitis (GPA), eosinophilic granulomatosis with polyangiitis (EGPA), microscopic polyangiitis (MPA), giant cell arteritis (GCA) and Takayasu arteritis (TAK) were included in the analysis. Participation was voluntary and there were no incentives provided. Internet protocol (IP) addresses were used to identify potential duplicate entries. Responses from incomplete questionnaires were included in the analysis as appropriate. This resulted in differences of the overall number of patients for different questions within the questionnaire. The respective total numbers are indicated in the text.
The study methodology and results are reported according to the Checklist for Reporting Results of Internet E-Surveys (14).
Statistical analysis
The data was collected anonymously. For data description, either absolute or relative numbers given by the percentage of all observations for binary covariates were used. For continuous covariates, the distribution for symmetry and potential outliers were visually checked. If both conditions were sufficiently met, covariate was calculated as mean (±SD), otherwise as median [25%; 75%quantile]. 95% confidence intervals were calculated using Jeffreys equal-tailed intervals as it showed improved performance in comparison with confidence intervals based on the normal approximation in settings with low prevalence (15). All missing data were assumed as missing at random.
Daily rates of COVID-19 prevalence in Germany were provided by the Robert-Koch-Institute (available at https://corona.rki.de) and used to evaluate findings on days corresponding to the time points the patients answered the questionnaire1. Average prevalence rates were compared with the prevalence rates determined for study participants.
To interpret the COVID-19 vaccination rates in our cohort, patients were asked about other recommended vaccinations. Recommended standard vaccinations (16) against tetanus and diphteria were used to compare vaccination status among participants. Influenza and pneumococcal vaccination are only recommended for selected patients in Germany. The frequencies presented were calculated based on the number of persons eligible for the respective vaccination according to the recommendations of the German Society for Rheumatology and the German Standing Committee on Vaccination. Our findings were compared to data obtained from the Association of Statutory Health Insurances from 2020 to 2021 presenting the vaccination coverage of persons eligible for the respective vaccination in the total population (Kassenärztliche Vereinigungen, RKI Vaccination Surveillance) (17).
All calculations were performed using the “R” software environment Version 4.1.2.
The study was approved by the Ethics Committee of the medical faculty of the University of Duesseldorf, Germany (2021–1405).
Patient and public involvement
The research question and questionnaire was developed by rheumatology centers in collaboration with vasculitis patient advocacy groups who provided critical insight and patient perspectives. The web-based survey was distributed nationwide by members of patient advocacy groups and clinical rheumatologists via flyers and mail. Results will be disseminated via the patient advocacy groups using graphical presentation of the results (Supplementary material 1).
Results
Participant details
The online survey was answered by 116 patients, who were diagnosed with small and large vessel vasculitis (GPA, EGPA, MPA, GCA or TAK). GPA was the most prevalent disease (n = 69/117, 59.0%), followed by GCA (n = 17/117, 14.5%). The mean age of the study respondents was 56.3 (±15.6) years. The majority of study participants were female (n = 73/117, 62.4%). Additional demographics and patient characteristics are presented in Table 1.
Table 1.
Characteristics of the patient cohort.
| Overall | Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis | Large vessel vasculitis | |
|---|---|---|---|
| n (%) | 117 (100) | 97 (82.9) | 20 (17.1) |
| Granulomatosis with polyangiitis (GPA) | 69 (71.1) | ||
| Eosinophilic granulomatosis with polyangiitis (EGPA) | 16 (16.5) | ||
| Microscopic polyangiitis (MPA) | 12 (12.4) | ||
| Giant cell arteritis (GCA) | 17 (85.0) | ||
| Takayasu's arteritis | 3 (15.0) | ||
| Age; mean (SD) | 56.3 (15.6) | 55.5 (15.4) | 60.0 (16.3) |
| Sex; n (%) | |||
| Female | 73 (62.4) | 56 (57.7) | 17 (85.0) |
| Male | 43 (36.8) | 40 (41.2) | 3 (15.0) |
| Non-binary | 1 (0.9) | 1 (1.0) | 0 (0.0) |
| Body mass index; kg/m2 mean (SD) | 26.3 (5.4) | 26.6 (5.6) | 24.9 (4.2) |
| Immunosuppressive therapy; n (%) | |||
| Rituximab Infusion (past six months) | 38 (32.5) | 38 (39.2) | 0 (0.0) |
| Cyclophosphamide | 7 (6.0) | 6 (6.2) | 1 (5.0) |
| Tocilizumab | 6 (5.1) | 0 (0.0) | 6 (30.0) |
| Mepolizumab | 4 (3.4) | 4 (4.1) | 0 (0.0) |
| Azathioprine | 16 (13.7) | 16 (16.5) | 0 (0.0) |
| Methotrexate | 25 (21.4) | 21 (21.6) | 4 (20.0) |
| Aprelimast | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Prednisolone; n (%) | |||
| >7.5 mg/day | 11 (9.5) | 8 (8.3) | 3.0 (15.0) |
| ≥5 and ≤ 7.5 mg/day | 35 (30.2) | 31 (32.3) | 4.0 (20.0) |
| < 5 mg/day | 33 (28.4) | 25 (26.0) | 8.0 (40.0) |
| Inhaled corticosteroids (alone or in combination) | 4 (3.4) | 4 (4.2) | 0.0 (0.0) |
| Other | 10 (8.6) | 6 (8.3) | 1 (10.0) |
| Main provider of vasculitis care; n (%) | |||
| General practitioner | 7 (6.5) | 2 (2.2) | 5 (26.3) |
| Nephrologist | 13 (12.0) | 13 (14.6) | 0 (0.0) |
| Rheumatologist | 87 (80.6) | 73 (82.0) | 14 (73.7) |
| Main provider of other medical issues; n (%) | |||
| Dermatologist | 1 (0.9) | 1 (1.1) | 0 (0.0) |
| General practitioner | 77 (71.3) | 63 (70.8) | 14 (73.7) |
| Nephrologist | 5 (4.6) | 5 (5.6) | 0 (0.0) |
| Neurologist | 1 (0.9) | 0 (0.0) | 1 (5.3) |
| Rheumatologist | 10 (9.3) | 7 (7.0) | 3 (15.8) |
| Other | 14 (13.0) | 13 (14.6) | 1 (5.3) |
| Current status; n (%) | |||
| Disease in remission | 76 (76.0) | 66 (79.5) | 10 (58.8) |
| Active disease | 24 (24.0) | 17 (20.5) | 7 (41.2) |
| Disease progression; n (%) | |||
| Relapsing | 43 (44.3) | 35 (43.2) | 8 (50.0) |
| Persistently active | 10 (10.3) | 7 (8.6) | 3 (18.8) |
| In remission after initial therapy | 44 (45.4) | 39 (48.1) | 5 (31.2) |
| Overall health status; n (%) | |||
| Excellent | 1 (1.0) | 1 (1.1) | 0 (0.0) |
| Very good | 12 (11.4) | 10 (11.5) | 2 (11.1) |
| Good | 43 (41.0) | 36 (41.4) | 7 (38.9) |
| Less good | 39 (37.1) | 30 (34.5) | 9 (50.0) |
| Poor | 10 (9.5) | 10 (11.5) | 0 (0.0) |
Values shown are means ± SD or medians followed by the IQR, as indicated. The bold values indicate the numbers of the overall study population.
Medical care during the COVID-19 pandemic
Most of the patients who participated in our study reported that their drug regimen included prednisolone at doses of < 5 mg/day (n = 33; 28.4%), 5–7.5 mg/day (n = 35/117; 30.2%), or >7.5 mg/day (n = 11/116; 9.5%). Rituximab was the most commonly used immunosuppressive drug (n = 38/117; 32.5%), followed by methotrexate (n = 25/117; 21.4%). Management of vasculitis was mainly provided by university hospitals (n = 53/106; 50.0%) and physicians in private practices (n = 37/106, 34.9%). Patient care was provided primarily by rheumatologists (n = 87/107; 81.3%) and to a lesser extent by nephrologists (n = 13/107; 12.1%). Most respondents identified general practitioners (GPs) as their main contact for all other medical concerns (n = 77/108; 71.3%). The patients reported that they needed to travel a median distance of 17 km (25%;75%quantile 10.0–40.0 km) to obtain care from the physician providing vasculitis care and 2.5 km (1.0–5.0 km) to receive care from their GPs (n = 108).
At the time of survey completion, 76% (n = 76/100) of the patients stated a stable disease status (remission). By contrast, 24% (n = 24/100) reported their disease status as active; 13 of these patients required recent adjustments to their therapeutic regimens to control the disease (n = 13/100, 13%). A relapsing disease was claimed by 43 of the patients (n = 43/97, 44.3%), while 44 patients (44/97; 45.4%) reported that they experienced only a single flare at the time of disease onset. The disease was classified as persistently active by 10 patients (n = 10/97, 10.3%).
Changes in medical care due to the COVID-19 pandemic
Appointments were canceled due to COVID-19 in 13 patients (n = 13/103, 12.6%, 95% CI 7.3–20.0%); nine participants reported that their in-person appointments were replaced by digital services (e.g., remote consultations; n = 9/101, 8.9%, 95% CI 4.5–15.6%). Therapy was changed due to the pandemic in 18 respondents (n = 18/102, 17.6%; 95% CI 9.5–22.2%), including regimens that were shifted (n = 8/102; 7.8%), reduced (n = 6/102; 5.9%) or discontinued (n = 4/102; 3.9%). Therapy was changed in 16 of 84 patients (19.0%) with AAV and 2 of 18 patients with GCA (11.1%). In patient with a persistently active or relapsing disease (n = 53) therapy was changed due to COVID-19 in 9.4% compared to 27.3% (12/44) in patients with a stable disease. Table 2 displays the reported therapeutic modifications based on specific drug regimens received. Compared to other immunosuppressive therapies, therapy with Rituximab was more often postponed (17.2%) due to COVID-19.
Table 2.
Modifications to therapeutic regimens due to COVID-19.
| Drug regimen | Yes, the start of therapy was postponed | Yes, the dosage or frequency was reduced | Yes, medication(s) was stopped | No modification | Total |
|---|---|---|---|---|---|
| Azathioprine | 0 | 1 | 0 | 14 | 15 |
| Cyclophosphamide | 1 | 0 | 0 | 0 | 1 |
| Mepolizumab | 1 | 0 | 0 | 2 | 3 |
| Methotrexate | 1 | 3 | 1 | 18 | 23 |
| Rituximab | 5 | 1 | 1 | 22 | 29 |
| Tocilizumab | 0 | 0 | 1 | 4 | 5 |
| Others | 0 | 0 | 0 | 10 | 10 |
| Total | 8 | 5 | 3 | 60 | 76 |
By the time the questionnaire was completed, 7.9% of the participants (n = 8/101, 95% CI 4.2–15.7%) had already had a COVID-19 infection. Interestingly, prevalence rates of COVID-19 among the general population in Germany were slightly higher (13%) compared to the participants included in this study1. Concerns regarding potential increased susceptibility to COVID-19 was stated by 70 participants (n = 70/104, 67.3%; 95% CI 57.9–75.8%); these concerns were most prevalent among patients undergoing treatment with azathioprine (13 of 15; 86.7%) or rituximab (24 of 29; 82.8%) (Figure 1). Concerns were not associated with patients age or gender. Patients with small-vessel vasculitis were more likely to report concerns (n = 61/85, 71.8%, 95% CI 61.6%; 80.5%) than patients with large-vessel vasculitis (n = 9/19, 47.4%, 95% CI 26.6%; 68.8%).
Figure 1.
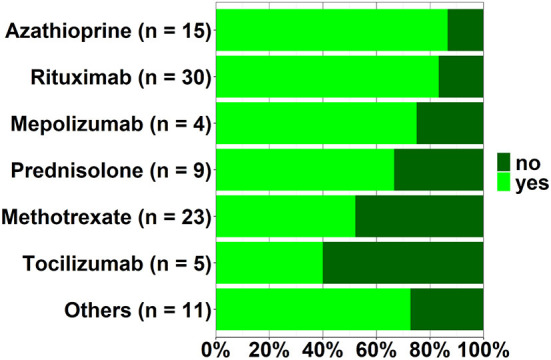
Respondents managed with specific drug regimens who reported concerns regarding increased susceptibility to COVID-19.
Vaccination
Owning a vaccine record card was reported by 103 of the respondents (n = 103/104, 99.0%; 95% CI 95.6–99.9%). Vaccination status was checked most frequently by their GPs (n = 80/104; 76.9%; 95% CI 68.2–84.2%) or rheumatologists (n = 27/104; 26.0%; 95% CI 18.3–35.0%]). Vaccination status had not been checked by any physician in 15 patients (n = 15/104, 14.4%; 95% CI 8.7–22.1%). While eight participants (n = 8/100, 8.0%; 95% CI 3.7–14.0%) reported that they refused to undergo vaccination because of concerns regarding disease flares and/or side effects, the vast majority of patients (n = 92/104; 88.5%; 95% CI 81.3–93.5%) stated they had never refused vaccinations that were offered to them.
Patients were also asked about coverage for selected vaccinations (e.g., tetanus, pneumococcus, influenza, COVID-19; Table 3).
Table 3.
Vaccination status.
| Vaccination | Eligible participants (n) |
Eligible participants who were vaccinated n (%; 95% CI) |
Vaccination coverage in the general population (%) |
|---|---|---|---|
| COVID-19 | 103 | 100 (98.1; 93.9–99.6) | 76.5* |
| Influenza (past year)a | 78 | 65 (83.33; 73.9–90.3) | 39.3** |
| Pneumococcus (past 5 years)b | 78 | 49 (62.8; 51.8–72.9) | 17.6** |
| Tetanus (past 10 years) | 103 | 47c (45.6; 36.2–55.3) | 53.9** |
| Diphtheria (past 10 years) | 103 | 23c (22.3; 15.1–31.1) | 52.7** |
The reported frequencies are based on the number of persons eligible for the respective vaccination according to the recommendations of the German Society for Rheumatology and the German Standing Committee on Vaccination. The findings are compared to data obtained from the Association of Statutory Health Insurances from 2020/2021 (Kassenärztliche Vereinigungen, RKI Vaccination Surveillance);
patients ≥60 years of age, currently managed with immunosuppressive therapy, or diagnosed with specific comorbidities (chronic cardiovascular, liver, renal or lung disease and/or diabetes);
patients managed with immunosuppressive therapy;
patients were asked if they have been vaccinated in the past 5 years;
data obtained from the Robert-Koch institute,
data obtained from Rieck et al. (17).
We used these data to compare their rates of vaccination with individuals in the general population. Compared with data from the Association of Statutory Health Insurances for the general population, the vaccination coverages were high in our cohort [e.g., 83% for influenza (n = 64/77, 95% CI 73.6–09.2) and 62.3% for pneumococcus (n = 58/77, 95% CI 51.2–72.5)] The highest vaccination coverage was reported for COVID-19 (n = 100/102, 98.0% 95% CI 93.9–99.6%). The coverage for COVID-19 vaccination was significantly higher compared to the general population in Germany (76.5%) at the time of survey completion on April 19, 2022, (Robert-Koch-Institute).
Seventeen patients (n = 17/94, 18%; 95% CI 10.9–25.7%) reported that they had not been informed by any of their physicians about COVID-19 vaccination; by contrast, 80 participants reported that they received information from their rheumatologists (n = 39/96; 40%; 95% CI 31.5–50.7%), GPs (n = 23/96; 24%; 95% CI 15.9–32.5%), or other physicians (n = 18/96; 19%; 95% CI 11.7–26.9%).
Discussion
We report findings from a patient survey focused on the medical care provided to vasculitis patients during the COVID-19 pandemic. To the best of our knowledge, this is the first patient survey designed to assess (1) the changes in medical care provided to vasculitis patients in the COVID-19 pandemic and (2) vaccination acceptance in this patient cohort. While several published reports document the results of surveys that included patients with RMDs, the proportion of vasculitis patients was small and their responses were not analyzed separately; in other studies, only data from the first month of the pandemic were presented (18–22).
Participating patients in this study reported that prescheduled appointments for the disease vasculitis had been canceled due to COVID-19 (10.3%) or had been replaced by digital services (7.1%). The reported proportions are lower than those reported in a survey of rheumatologists throughout Europe that included patients with various RMD in which 82% stated that they had canceled patient appointments due to COVID-19 (23). Therefore, vasculitides might be considered as diseases that require closer monitoring compared to other RMDs.
At the beginning of the pandemic, patients and physicians faced substantial uncertainty due to the lack of data regarding the clinical course of COVID-19 in patients diagnosed with RMDs, most notably those managed with immunosuppressive therapy. The first findings to emerge suggested that rituximab therapy was associated with an increased risk of severe COVID-19 (8, 24, 25). Consequently, recommendations for a more stringent risk-benefit analysis were brought forward. However, recent studies have highlighted the extraordinary efficacy of rituximab for induction of remission and maintenance therapy in small vessel vasculitides (26–28). Rituximab was the most commonly used immunosuppressive drug in our patient cohort for patients diagnosed with AAV and remained the treatment of choice for 75.9% of the participants despite the pandemic.
For other immunosuppressive therapies, it was recommended to continue necessary treatments because ongoing disease activity was considered to pose a greater risk than COVID-19 infections (8). Only 17.6% (n = 18) of the respondents of our study reported changes in their therapeutic regimens (i.e., shifts, reductions, or discontinuations) due to COVID-19. Our results are consistent with previous reports that documented no major changes in the management of vasculitis patients during the COVID-19 pandemic (20, 29). This might be explained by the profound importance of current drug regimens used to prevent flare-ups in order to prevent irreversible damage or death in patients with uncontrolled vasculitis (30, 31).
Interestingly, the proportion of respondents who had already contracted and recovered from COVID-19 at the time the survey data was collected was slightly lower than that reported in the general population. These differences might be explained by the high proportion of participants who reported concerns regarding their potentially higher susceptibility to COVID-19. These individuals may have more stringently reduced their in-person social interactions and taken other safety precautions (2). Although the level of concern regarding their potentially higher susceptibility to COVID-19 was high in our cohort, a meta-analysis of data collected from studies that documented the prevalence of immunosuppression among patients with COVID-19 (n = 10,049) revealed that these patients were at no increased risk of contracting this infection (32). However, the meta-analysis did not explore the impact of different immunosuppressive therapies and diseases.
Overall, the study participants exhibited a positive attitude toward vaccinations. The majority (n = 92/104; 88.5%) stated they had never refused any vaccinations. The rate of COVID-19 vaccination was exceptionally high compared to previously reported studies focused on RMD patients (10–12) as well as in the general population in Germany1.
To interpret the COVID-19 vaccination rates in our cohort, patients were asked about other recommended vaccinations. We used these data to compare their rates of vaccination with individuals in the general population. Apart from the high COVID-19 vaccination rate in our study population, our findings also revealed a generally high rate of vaccination among members of our study, e.g. against influenza and pneumococcus (two- and three-fold higher, respectively). Compared to 2018 (i.e., before the COVID-19 pandemic), Harrison et al. (33) reported lower vaccination rates against influenza in RMDs patients. The increased acceptance of vaccination, in general, might be due to the increased information, public discussion and awareness of the need for vaccination during the COVID-19 pandemic as already observed by Starrostzik (34).
Among the strengths of our study, we note the comparatively large cohort size, patient recruitment based at multiple centers and collaboration with vasculitis patient advocacy groups to develop the survey. We do recognize the potential bias inherent in an online survey. In addition, there seems to be a bias toward patients with small-vessel vasculitis, as this disease is less common than large-vessel vasculitis in the general population (35, 36), but more prevalent in our cohort. This also explains the large proportion of patients reporting a therapy with rituximab, which is used as one standard therapy in ANCA-associated vasculitis. Since our survey was voluntary, more patients with concerns about COVID-19 infections may have participated in the survey.
Conclusion
Participants of our self-reported survey experienced changes in medical care during the COVID-19 pandemic. Compared to the general population, acceptance of vaccinations, especially against COVID-19, was considerably high on our cohort.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Medical Faculty of the University of Duesseldorf, Germany (2021-1405). The patients/participants provided their written informed consent to participate in this study.
Author contributions
AK and TF performed the data analyses. AK and GC drafted the first version of the manuscript. All co-authors were involved in the critical interpretation of the results, discussed the findings together, critically reviewed the manuscript and approved its final version. All authors designed the study and contributed to data collection.
Acknowledgments
The authors would like to thank the following persons and societies for their great effort and distribution of the online survey: J. Mucke, J. Richter, I. Frohne, I. Haase, O. Sander, R. Fischer-Betz, I. Kötter, W. Berchem Selbsthilfegruppe Morbus Wegener, S. Engel Rheuma-Liga Schleswig Holstein e.V., and U. Garske Rheuma-Liga Hamburg e.V.
Footnotes
1https://experience.arcgis.com/unsupported-browser/index.html (accessed November 11, 2022).
Conflict of interest
KG was employed by Sana Klinikum Offenbach GmbH. NR was employed by Klinikum Bad Bramstedt GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1103694/full#supplementary-material
References
- 1.Yates M, Watts RA, Bajema IM, Cid MC, Crestani B, Hauser T, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. (2016) 75:1583–94. 10.1136/annrheumdis-2016-209133 [DOI] [PubMed] [Google Scholar]
- 2.Glintborg B, Jensen DV, Engel S, Terslev L, Jensen MP, Hendricks O, et al. Self-protection strategies and health behaviour in patients with inflammatory rheumatic diseases during the COVID-19 pandemic: results and predictors in more than 12 000 patients with inflammatory rheumatic diseases followed in the Danish DANBIO registry. RMD Open. (2021) 7:e001505. 10.1136/rmdopen-2020-001505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassan MS, Mostafa DI, Abdelhady EI, Sarhan SA, Abdelghani M, Seleem DA. Psychosocial and clinical impact of COVID-19 pandemic and its relationship to the quality of life in patients with rheumatoid arthritis: a cross-sectional study, Egypt. Middle East Curr Psychiatry. (2022) 29:16. 10.1186/s43045-022-00184-2 [DOI] [Google Scholar]
- 4.Schulze-Koops H, Holle J, Moosig F, Specker C, Aries P, Burmester G, et al. Current guidance of the German Society of Rheumatology for the care of patients with rheumatic diseases during the SARS-CoV-2/Covid-19 pandemic. Z Rheumatol. (2020) 79:385–8. 10.1007/s00393-020-00799-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulze-Koops H, Specker C, Iking-Konert C, Holle J, Moosig F, Krueger K, et al. Preliminary recommendations of the German Society of Rheumatology (DGRh eV) for the management of patients with inflammatory rheumatic diseases during the SARS-CoV-2/COVID-19 pandemic. Ann Rheum Dis. (2020) 79:840–2. 10.1136/annrheumdis-2020-217628 [DOI] [PubMed] [Google Scholar]
- 6.Schulze-Koops H, Krueger K, Specker C. Response to: ‘Treatment adherence of patients with sytemic rheumatic diseases in COVID-19 pandemic' by Fragoulis et al. Ann Rheum Dis. (2021) 80:e61. 10.1136/annrheumdis-2020-217987 [DOI] [PubMed] [Google Scholar]
- 7.Landewé RB, Machado PM, Kroon F, Bijlsma HW, Burmester GR, Carmona L, et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann Rheum Dis. (2020) 79:851–8. 10.1136/annrheumdis-2020-217877 [DOI] [PubMed] [Google Scholar]
- 8.Specker C, Aries P, Braun J, Burmester G, Fischer-Betz R, Hasseli R, et al. Updated recommendations of the German Society for Rheumatology for the care of patients with inflammatory rheumatic diseases in the context of the SARS-CoV-2/COVID-19 pandemic, including recommendations for COVID-19 vaccination. Z Rheumatol. (2021) 80:33–48. 10.1007/s00393-021-01055-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vygen-Bonnet S, Koch J, Bogdan C. Beschluss der STIKO zur 8. Aktualisierung der COVID-19-Impfempfehlung und die dazugehörige wissenschaftliche Begründung. Epid Bull. (2021) 4:14–31. 10.25646/8776 [DOI] [Google Scholar]
- 10.Krasselt M, Baerwald C, Seifert O. COVID-19 vaccination coverage in patients with rheumatic diseases in a german outpatient clinic: an observational study. Vaccines. (2022) 10:253. 10.3390/vaccines10020253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goulenok T, Francois C, Mendes C, Farhi F, Alexandra JF, Rouzaud D, et al. Improving COVID-19 vaccine coverage in patients with autoimmune and inflammatory diseases. J Rheumatol. (2022) 49:118–9. 10.3899/jrheum.210534 [DOI] [PubMed] [Google Scholar]
- 12.Li YK, Lui MP, Yam LL, Cheng CS, Tsang TH, Kwok WS, et al. COVID-19 vaccination in patients with rheumatic diseases: Vaccination rates, patient perspectives, and side effects. Immun Inflamm Dis. (2022) 10:e589. 10.1002/iid3.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van der Heijde D, Aletaha D, Carmona L, Edwards CJ, Kvien TK, Kouloumas M, et al. 2014 Update of the EULAR standardised operating procedures for EULAR-endorsed recommendations. Ann Rheum Dis. (2015) 74:8–13. 10.1136/annrheumdis-2014-206350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet e-surveys (CHERRIES). J Med Internet Res. (2004) 6:e34. 10.2196/jmir.6.3.e34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci. (2001) 16:101–33. 10.1214/ss/1009213286 [DOI] [Google Scholar]
- 16.Ständige Impfkommission. Empfehlungen der Ständigen Impfkommission (STIKO) beim Robert Koch-Institut 2022. Epid Bull. (2022) 4:3–67. 10.25646/9285.3 [DOI] [Google Scholar]
- 17.Rieck T, Steffen A, Schmid-Küpke N, Feig M, Wichmann O, Siedler A. Impfquoten bei Erwachsenen in Deutschland - Aktuelles aus der KV-Impfsurveillance. Epid Bull. (2021) 50:3–22. 10.25646/9436 [DOI] [Google Scholar]
- 18.Hausmann JS, Kennedy K, Simard JF, Liew JW, Sparks JA, Moni TT, et al. Immediate effect of the COVID-19 pandemic on patient health, health-care use, and behaviours: results from an international survey of people with rheumatic diseases. Lancet Rheumatol. (2021) 3:e707–14. 10.1016/S2665-9913(21)00175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernández-Ávila DG, Barahona-Correa J, Romero-Alvernia D, Kowalski S, Sapag A, Cachafeiro-Vilar A, et al. Impact of COVID-19 pandemic on rheumatology practice in latin America. J Rheumatol. (2022) 48:1616–22. 10.3899/jrheum.201623 [DOI] [PubMed] [Google Scholar]
- 20.Salas A, Kant S, Floyd L, Kratky V, Brix SR, Prendecki M, et al. ANCA Vasculitis induction management during the COVID-19 pandemic. Kidney Int Rep. (2021) 6:2903–7. 10.1016/j.ekir.2021.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopp CR, Naidu GS, Misra DP, Deo P, Jakulla RS, Makan K, et al. Managing ANCA-associated vasculitis during the COVID-19 pandemic: results from an online survey. Rheumatol Int. (2021) 41:1941–7. 10.1007/s00296-021-04975-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ince B, Bektaş M, Koca N, Agargün BF, Zarali S, Güzey DY, et al. A single center survey study of systemic vasculitis and COVID-19 during the first months of pandemic. Turk J Med Sci. (2021) 51:2243–7. 10.3906/sag-2010-267 [DOI] [PubMed] [Google Scholar]
- 23.Dejaco C, Alunno A, Bijlsma JW, Boonen A, Combe B, Finckh A, et al. Influence of COVID-19 pandemic on decisions for the management of people with inflammatory rheumatic and musculoskeletal diseases: a survey among EULAR countries. Ann Rheum Dis. (2021) 80:518–26. 10.1136/annrheumdis-2020-218697 [DOI] [PubMed] [Google Scholar]
- 24.Strangfeld A, Schäfer M, Gianfrancesco MA, Lawson-Tovey S, Liew JW, Ljung L, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. (2021) 80:930–42. 10.1136/annrheumdis-2020-219498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avouac J, Drumez E, Hachulla E, Seror R, Georgin-Lavialle S, El Mahou S, et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol. (2021) 3:e419–26. 10.1016/S2665-9913(21)00059-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. (2010) 363:221–32. 10.1056/NEJMoa0909905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaître O, Cohen P, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. (2014) 371:1771–80. 10.1056/NEJMoa1404231 [DOI] [PubMed] [Google Scholar]
- 28.Jones RB, Furuta S, Tervaert JW, Hauser T, Luqmani R, Morgan MD, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann Rheum Dis. (2015) 74:1178–82. 10.1136/annrheumdis-2014-206404 [DOI] [PubMed] [Google Scholar]
- 29.Kronbichler A, Geetha D, Smith RM, Egan AC, Bajema IM, Schönermarck U, et al. The COVID-19 pandemic and ANCA-associated vasculitis – reports from the EUVAS meeting and EUVAS education forum. Autoimmun Rev. (2021) 28:102986. 10.1016/j.autrev.2021.102986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallace ZS, Fu X, Harkness T, Stone JH, Zhang Y, Choi H. All-cause and cause-specific mortality in ANCA-associated vasculitis: overall and according to ANCA type. Rheumatol Oxf Engl. (2020) 59:2308–15. 10.1093/rheumatology/kez589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dagostin MA, Nunes SLO, Shinjo SK. Mortality predictors in ANCA-associated vasculitis. Medicine. (2021) 100:e28305. 10.1097/MD.0000000000028305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tassone D, Thompson A, Connell W, Lee T, Ungaro R, An P, et al. Immunosuppression as a risk factor for COVID-19: a meta-analysis. Intern Med J. (2021) 51:199–205. 10.1111/imj.15142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison N, Poeppl W, Miksch M, Machold K, Kiener H, Aletaha D, et al. Predictors for influenza vaccine acceptance among patients with inflammatory rheumatic diseases. Vaccine. (2018) 36:4875–9. 10.1016/j.vaccine.2018.06.065 [DOI] [PubMed] [Google Scholar]
- 34.Starostzik C. Impfquoten bei Erwachsenen: Höher, aber nicht hoch genug. MMW Fortschr Med. (2021) 163:18–9. 10.1007/s15006-020-9517-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geetha D, Jefferson JA. ANCA-Associated Vasculitis: Core Curriculum 2020. Am J Kidney Dis Off J Natl Kidney Found. (2020) 75:124–37. 10.1053/j.ajkd.2019.04.031 [DOI] [PubMed] [Google Scholar]
- 36.Muratore F, Boiardi L, Mancuso P, Restuccia G, Galli E, Marvisi C, et al. Incidence and prevalence of large vessel vasculitis (giant cell arteritis and Takayasu arteritis) in northern Italy: a population-based study. Semin Arthritis Rheum. (2021) 51:786–92. 10.1016/j.semarthrit.2021.06.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


