Abstract
Porphyromonas gingivalis is a periodontal pathogen that also localizes to atherosclerotic plaques. Our previous studies demonstrated that P. gingivalis is capable of invading endothelial cells and that intracellular bacteria are contained in vacuoles that resemble autophagosomes. In this study, we have examined the trafficking of P. gingivalis 381 to the autophagic pathway. P. gingivalis 381 internalized by human coronary artery endothelial (HCAE) cells is located within vacuoles morphologically identical to autophagosomes. The progression of P. gingivalis 381 through intracellular vacuoles was analyzed by immunofluorescence microscopy. Vacuoles containing P. gingivalis colocalize with Rab5 and HsGsa7p early after internalization. At later times, P. gingivalis colocalizes with BiP and then progresses to a vacuole that contains BiP and lysosomal glycoprotein 120. Late endosomal markers and the lysosomal cathepsin L do not colocalize with P. gingivalis 381. The intracellular survival of P. gingivalis 381 decreases over 8 h in HCAE cells pretreated with the autophagy inhibitors 3-methyladenine and wortmannin. In addition, the vacuole containing P. gingivalis 381 lacks BiP but contains cathepsin L in the presence of wortmannin. These results suggest that P. gingivalis 381 evades the endocytic pathway to lysosomes and instead traffics to the autophagosome.
Porphyromonas gingivalis is a gram-negative, anaerobic rod that is considered to be among the major pathogens associated with adult periodontitis (64). A possible mechanism of pathogenesis may be cellular invasion. P. gingivalis has been demonstrated to be internalized within gingival epithelial cells in vitro (19, 34, 53) and buccal epithelial cells in vivo (51). Recent epidemiological studies have demonstrated a strong relationship between periodontal disease and coronary heart disease (2, 3, 17, 38, 39). Oral bacteria have a direct route to the circulatory system in periodontitis patients due to transient bacteremias produced by flossing, mastication, and toothbrushing (11, 57, 62). P. gingivalis localizes to atherosclerotic plaques (12, 30) and is capable of invasion of coronary artery cells in vitro (16, 18). Therefore, invasion and intracellular parasitism of endothelial cells by P. gingivalis in vivo may exacerbate the inflammatory response of atherosclerosis.
Invasion of nonphagocytic cells is a common strategy of evading the immune system for many pathogens (23). Once within the cell, these pathogens have developed various mechanisms for survival (28, 40). Legionella pneumophila and virulent Brucella abortus gain access to and replicate in vacuoles that resemble autophagosomes and are associated with endoplasmic reticulum proteins (46, 47). Autophagosomes, multimembranous vacuoles formed from invaginations of ribosome-free regions of the rough endoplasmic reticulum (RER) (20), are the organelles of the autophagic process. Autophagy is a process whereby cytosol and organelles are sequestered for lysosome degradation in response to nutrient deprivation (20). Under normal conditions, the autophagosome matures into an autolysosome, where the contents are degraded. The autophagosome-like vacuoles containing these bacterial species do not acquire lysosomal hydrolases (46, 66). Bacterial trafficking to the autophagic pathway has been proposed to be a mechanism of increasing the concentration of free amino acids to be utilized by the bacteria for their biochemical pathways and/or to inhibit host cell protein synthesis in order to reduce the cellular response to the pathogen (63).
In a previous study, we demonstrated that the vacuoles containing P. gingivalis in human coronary artery endothelial (HCAE) cells morphologically resembled autophagosomes, similar to the vacuoles containing L. pneumophila in macrophages and virulent B. abortus in HeLa cells (18). The first goal of this study was to characterize and delineate the trafficking of P. gingivalis within endothelial cells using strain 381. The second goal was to determine whether the autophagosome-like vacuole was the intracellular niche for P. gingivalis in the HCAE cell or whether the HCAE cell utilized the autophagic pathway to rid itself of this intracellular intruder.
MATERIALS AND METHODS
Bacterial and cell culture conditions.
P. gingivalis strain 381 was subcultured on tryptic soy agar (Difco Laboratories, Detroit, Mich.) supplemented with 5.0% sheep blood (Lampire Biological Laboratories, Pipersville, Pa.), 0.5% yeast extract (Difco), hemin (5 μg/ml), and vitamin K (5 μg/ml). Liquid cultures were grown in brain heart infusion broth (Difco) supplemented with 0.5% yeast extract, 0.1% cysteine (Sigma), hemin (5 μg/ml), and vitamin K (5 μg/ml) under anaerobic conditions. These strains were grown at 37°C in a Coy (Ann Arbor, Mich.) anaerobic chamber with an atmosphere of 5% CO2, 10% H2, and 85% N2. Escherichia coli MC1061was subcultured at 37°C aerobically on Luria-Bertani (LB) plates consisting of Bacto Agar (15 g/liter; Difco), Bacto Tryptone (10 g/liter; Difco), yeast extract (5 g/liter), and sodium chloride (10 g/liter; Fisher Scientific, Springfield, N.J.) and was also grown in LB broth media. The HCAE cells are a primary cell culture line purchased from Clonetics Inc. (San Diego, Calif.), cryopreserved on third passage, and were passaged an additional two or three times before use. The HCAE cells were maintained in endothelial growth medium-2 (EGM-2), which consisted of endothelial basal medium-2 supplemented with fetal bovine serum, hydrocortisone, human recombinant fibroblast growth factor, vascular endothelial growth factor, recombinant insulin growth factor-1, ascorbic acid, human recombinant epidermal growth factor, gentamicin, and amphotericin (Clonetics).
Antibodies.
Rabbit polyclonal anti-BiP (provided by S. Frost); rabbit polyclonal anti-cathepsin L (C. Gabel); rabbit polyclonal anti-HsGSA7p (human-specific Gsa7p); 61BG1.3, a mouse monoclonal anti-P. gingivalis hemagglutinin A (R. Gmür) (7); rabbit polyclonal antilysosomal glycoprotein 120 (LGP120) (22); rabbit polyclonal anti-mannose 6-phosphate receptor (MPR) (P. Nissley); rabbit polyclonal anti-Rab5 (Stressgen Biotechnologies Corp., Vancouver, British Columbia, Canada); rabbit polyclonal anti-Rap1 (Stressgen); and rabbit polyclonal anti-2,6 Gal β1,4-GlcNAc sialyltransferase (G. Hart) antibodies were used in this study. Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse antibody (Sigma Chemical Co., St. Louis, Mo.) and tetramethyl rhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit antibody (Sigma) were used as secondary antibodies.
Persistence assay.
The numbers of intracellular bacteria at various times and in the absence or presence of autophagy inhibitors were quantitated. Approximately 105 HCAE cells per well in a 24-well tissue culture plate were washed three times with phosphate-buffered saline (PBS). For inhibition of autophagy, both 10 mM 3-methyladenine (Sigma) and 10 nM wortmannin (Sigma) in EGM-2 were preincubated with HCAE cells for 60 min prior to the start of the invasion assay. For wells not receiving the autophagy inhibitors, media were replaced with fresh antibiotic-free EGM-2. Following preincubation, broth-grown bacteria were centrifuged at low speed and resuspended in antibiotic-free medium to a concentration of 107 cells/ml as determined spectrophotometrically (Shimadzu UV-1201; VWR, Marietta, Ga.). Then 1.0 ml of the bacterial suspension was added to each well and the HCAE cells plus bacteria were incubated at 37°C in 5% CO2 for 90 min. The autophagy inhibitors were present during the time of infection in the appropriate wells. The media were removed from infected cells after 90 min, and the cells were washed three times with PBS. Medium containing gentamicin (300 μg/ml) and metronidazole (200 μg/ml) was then added to each well to kill any extracellular bacteria, and the plates were incubated for an additional 60 min in 5% CO2 at 37°C. The autophagy inhibitors were also present during the antibiotic incubation in the appropriate wells. The media were removed, and the cells were washed three times with PBS. The HCAE cells from one set of wells were then lysed by a 20-min incubation with 1.0 ml of sterile distilled water at 37°C. This was the 2.5-h time point. For the wells to be assessed at 4, 6, and 8 h postinfection, the media were replaced with antibiotic-free EGM-2 without autophagy inhibitors and further incubated at 37°C in 5% CO2. At the appropriate time points, the HCAE cells were lysed by the aforementioned protocol. Dilutions of the lysates of cells infected with P. gingivalis were plated in triplicate on tryptic soy agar (Difco) plates supplemented with 5.0% sheep blood, 0.5% yeast extract, hemin (5 μg/ml), and vitamin K (5 μg/ml) and were cultured anaerobically. The dilutions of the lysates of E. coli MC1061, the negative control, were cultured on LB plates at 37°C aerobically. The CFU of invasive bacteria were then enumerated. Each condition was performed in duplicate, and each assay was performed three times independently. All of the inhibitors were also tested at the appropriate concentration for adverse effects on the viability of the bacteria by plate count. The viability of the HCAE cells were tested by trypan blue exclusion and by examining the confluency of the monolayer.
Transmission electron microscopy.
The bacteria were incubated with the HCAE cells for 30, 60, and 90 min and were subsequently fixed in 2% glutaraldehyde in PBS at room temperature for 1 h. For inhibition studies, the HCAE cells were preincubated with 10 nM wortmannin and 10 mM 3-methyladenine in EGM-2 for 1 h, and the wortmannin was also present during the infection. After the cells were centrifuged and the pellet was washed with PBS (pH 7.3), 3 drops of 3% low-gelling-point agarose was added, and the pellet was solidified at 4°C for 10 min. The agarose-embedded pellet was then washed twice for 10 min in PBS, postfixed in 1% osmium tetroxide for 1 h, and washed three times in distilled H2O for 10 min. The specimens were then dehydrated in a graded series of ethanol and stained overnight en bloc in 2% uranyl acetate. Following the last ethanol treatment, the specimens were infiltrated and embedded in EM Bed-812 (Electron Microscopy Sciences, Fort Washington, Pa.). Thin sections were cut, poststained with uranyl acetate and lead citrate, and examined on a Hitachi 7000 transmission electron microscope.
Immunofluorescence microscopy.
HCAE cells were grown on glass coverslips in a six-well tissue culture plate. The HCAE cells were then washed three times with PBS prior to infection with P. gingivalis 381 at different time points at a multiplicity of infection of approximately 100. The media were removed, and the HCAE cells were washed vigorously three times with PBS. Wortmannin (10 nM) was added 1 h before infection and was present during infection. The infected HCAE cells were then fixed in 4% paraformaldehyde in PBS for 30 min at room temperature. This was followed by washing twice in PBS and quenching in 50 mM NH4Cl–0.3% Tween 20–PBS for 10 min at room temperature. After quenching, the HCAE cells were washed two times in PBS. The primary antibodies, 1/50 dilution in PBS–5% normal goat serum–0.3% Tween 20, were applied for 2 h at room temperature. The HCAE cells were then washed four times in PBS for 5 min each wash. In all cases, the bacteria were detected and organelle and host protein markers were detected with FITC-conjugated goat anti-mouse antibody and TRITC-conjugated goat anti-rabbit antibody, respectively, as the secondary antibodies. The secondary antibodies (1/200 dilution in PBS–5% normal goat serum–0.3% Tween 20) were applied for 1 h at room temperature. The HCAE cells were then washed twice with PBS before mounting with Fluoromount-G (Southern Biotechnology Associates, Inc., Birmingham, Ala.) onto glass microscope slides.
Images were viewed using a Zeiss Axiophot fluorescence photomicroscope with a spot camera and Adobe Photoshop imaging software. Each time point was analyzed by examining at least 100 internalized bacteria (entire fields of view under the microscope were counted, so totals exceeded 100) except for 15 min postinfection, at which time too few bacteria had invaded to view 100 internalized P. gingivalis. All immunofluorescence tests were performed at least twice. These same colocalization experiments were also examined by deconvolution microscopy using an Olympus IX70 microscope and Deltavision software (Applied Precision, Inc., Wepahah, Wash.). Raw images were captured by a charge-coupled device camera in both color channels in a series of 10- to 0.2-μm increments. The Deltavision software converted the stack of images into a computational three-dimensional view using a constrained iterative deconvolution algorithm.
Statistical analysis.
Student's t test (Microsoft Excel; Microsoft, Redmond, Wash.) was used to compare differences in colocalization data between untreated and wortmannin-treated time points. Due to similar comparisons over multiple time points, Bonferroni's correction was utilized, and statistical significance was achieved when P was <0.01 (0.05/5). The percentages of colocalization from each individual field of view were compared for this analysis.
RESULTS
Morphological analysis of vacuoles containing P. gingivalis.
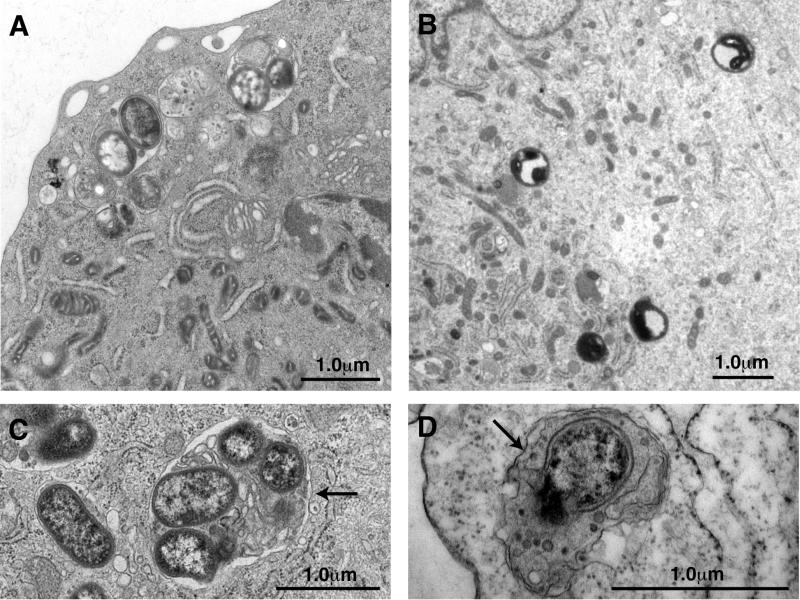
Internalized P. gingivalis was found within structures resembling autophagosomes at 90 min postinfection (Fig. 1A). These vacuoles were bound by one or two membranes and contained undegraded cytoplasm and vesicles of cytoplasmic origin (Fig. 1C and D). The presence of cytoplasmic components in these vacuoles suggests that the vacuoles arose via autophagic events. Profiles of RER were routinely observed in close association with these vacuoles. The morphological data suggest that the internalized P. gingivalis 381 cells reside in autophagosomes, which have been derived from the RER. We next examined the intracellular fate of P. gingivalis when 3-methyladenine or wortmannin suppresses autophagy. Internalized P. gingivalis was observed within vacuoles in wortmannin-treated HCAE cells at 30 min postinfection (Fig. 1B). However, these vacuoles were morphologically distinct from autophagosomes. They were bounded by only a single membrane and lacked undegraded cytoplasmic ground substance. These inhibitors appeared to have no effect on bacterial internalization. At later time points only what appeared to be bacterial fragments were observed in HCAE cells preincubated with 3-methyladenine and wortmannin.
FIG. 1.
Internalized P. gingivalis in HCAE cells. HCAE cells were infected with P. gingivalis in the presence (A, C, and D) and absence (B) of wortmannin. After 90 min of infection, P. gingivalis could be observed in vacuoles within the cytoplasm of HCAE cells (A). These vacuoles were bound by one or two membranes (arrows) and contained undegraded vesicles and cytoplasmic ground substance (C and D). After 30 min of infection of wortmannin-treated HCAE cells, P. gingivalis appears to be in the process of degradation and is within vacuoles that resemble lysosomes (B).
Trafficking through the autophagic pathway.
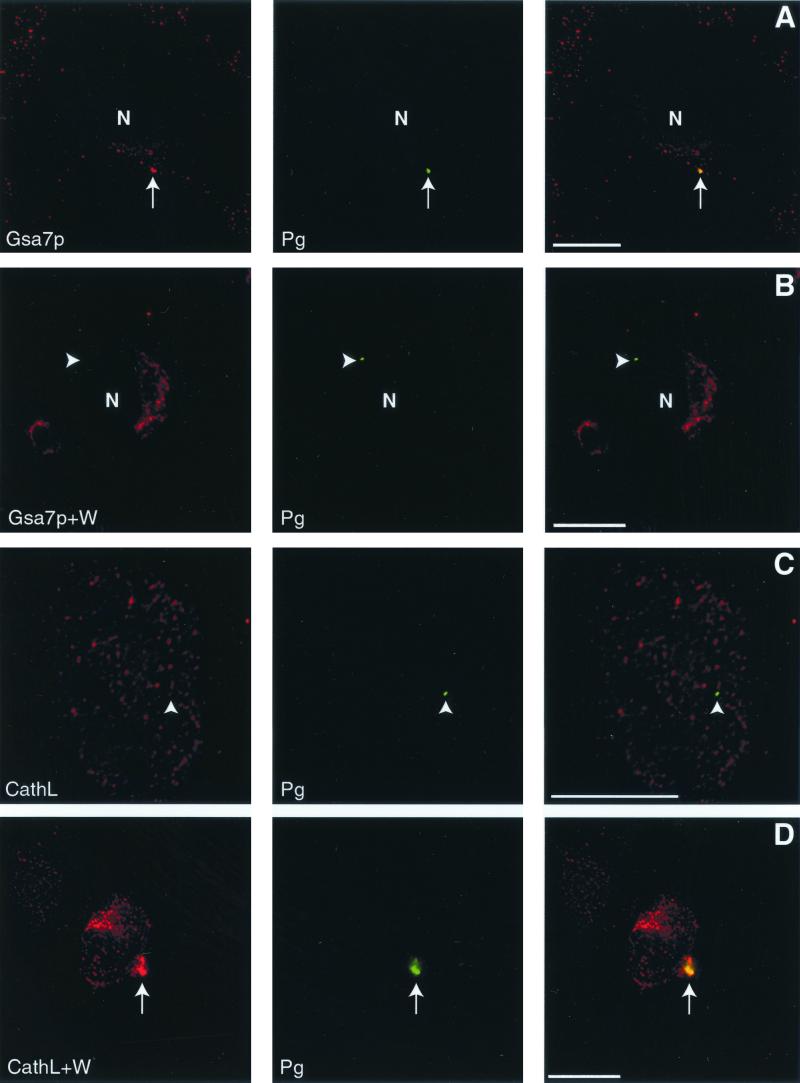
Autophagy is responsible for the sequestration and delivery of cytoplasmic proteins and organelles to the autolysosome, where they are degraded. Our data suggest that internalized P. gingivalis enters the autophagic pathway. Therefore, we utilized protein markers of autophagy to investigate the intracellular trafficking of P. gingivalis in HCAE cells. We found that P. gingivalis resided in a vacuole that contained HsGsa7p but lacked cathepsin L (Fig. 2A and C). We next examined the trafficking of P. gingivalis in HCAE cells treated with 10 nM wortmannin, an inhibitor of autophagy. Under these conditions, the bacterial vacuole contained cathepsin L (Fig. 2D) but not HsGsa7p (Fig. 2B). We also observed that the cellular distribution of HsGsa7p differed in the absence and presence of wortmannin (Fig. 2). HsGsa7p resided in a Golgi-like vacuole when autophagy was suppressed by wortmannin (Fig 2B). During bacterial invasion in the absence of wortmannin, the HsGsa7p was found in small unknown organelles distributed throughout the cell. We believe that this alteration in the cellular distribution of HsGsa7p reflects the onset of autophagy. Indeed, we found HsGsa7p colocalized with Golgi markers in fed HuH7 cells when autophagy is suppressed but distributed to round organelles in starved HuH7 cells when autophagy is enhanced (data not shown).
FIG. 2.
Localization of HsGsa7p and cathepsin L to vacuoles containing P. gingivalis in HCAE cells using deconvolution microscopy. (A) P. gingivalis colocalized with HsGsa7p (arrow). (B) In the presence of wortmannin, P. gingivalis did not colocalize with HsGsa7p (arrowhead). HsGsa7p resided in a Golgi-like vacuole in the wortmannin-treated HCAE cells (arrowhead). (C) P. gingivalis did not colocalize with cathepsin L (arrowhead). (D) P. gingivalis colocalized with cathepsin L (arrow) in the presence of wortmannin. Abbreviations: Gsa7p, HsGsa7p; W, wortmannin; CathL, cathepsin L; Pg, P. gingivalis; N, nucleus. Bar, 15 μm.
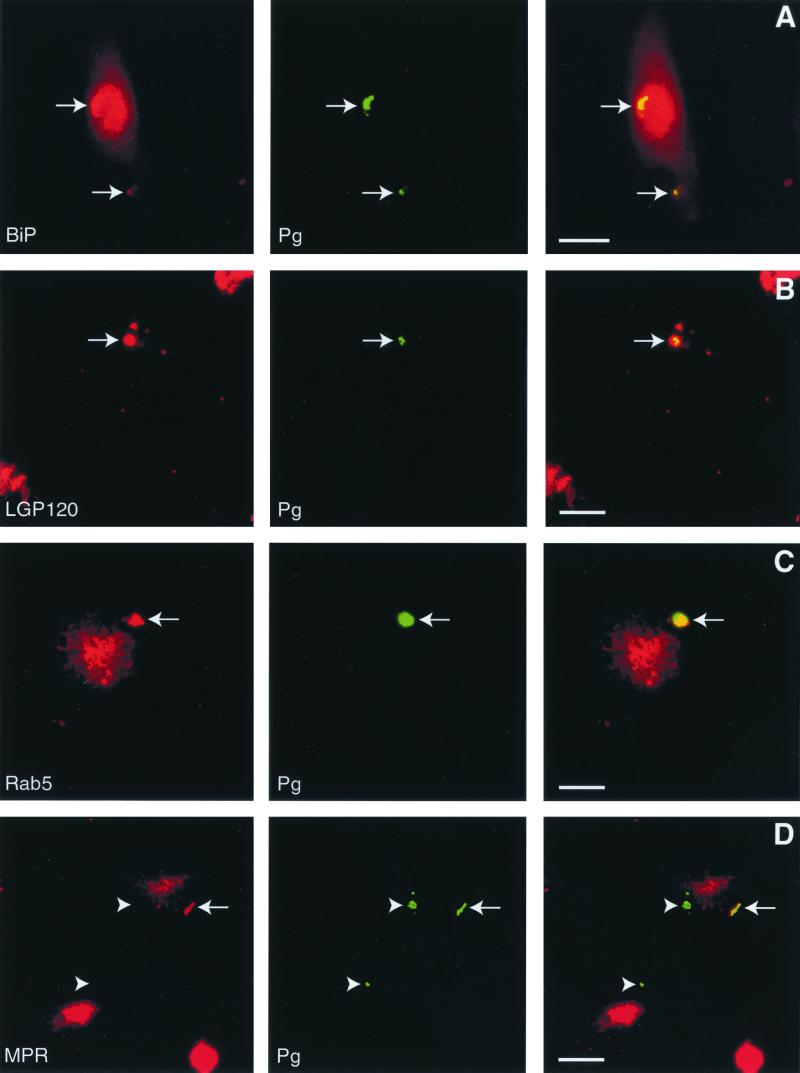
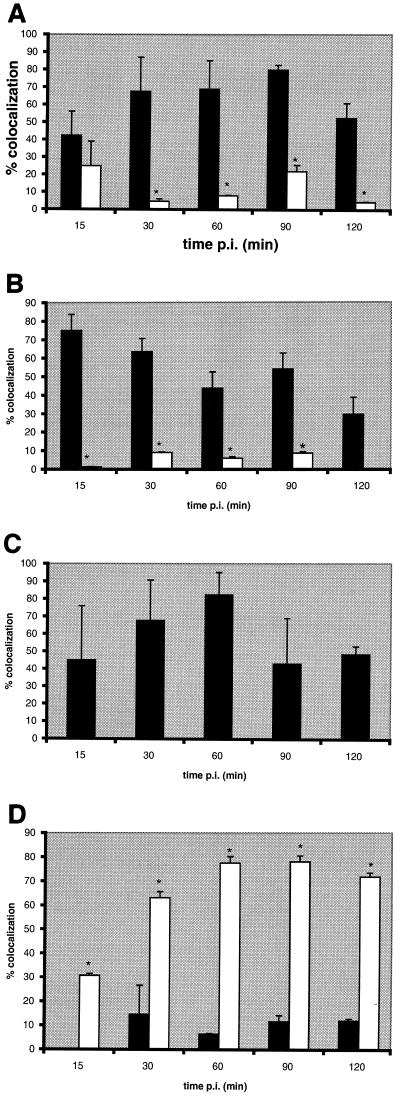
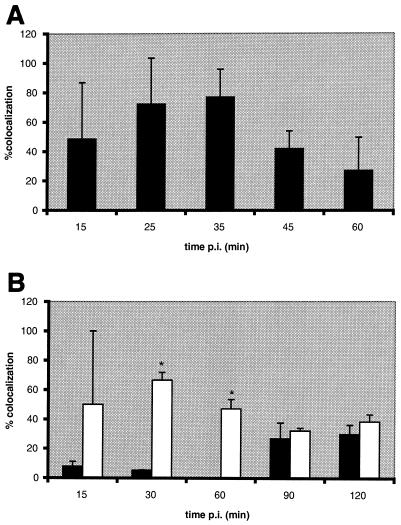
At 90 min postinfection, we observed that HsGsa7p, BiP (Fig. 3A), and LGP120 (Fig. 3B) colocalized with the P. gingivalis vacuoles. We next quantified the colocalization between the bacteria and cellular markers over a time course to delineate the intracellular trafficking of P. gingivalis. Within 15 min of infection, 42% of the internalized bacteria colocalized with BiP, an RER luminal protein (Fig. 4A) (71). At 30 to 90 min, 68 to 80% of the P. gingivalis was found in BiP-positive compartments. Even at 120 min, 52% of the bacteria were localized to the BiP compartment. In contrast, P. gingivalis was not found in this BiP-positive compartment when the cells were treated with wortmannin. We also examined the distribution of HsGsa7p in the infected HCAE cells in the absence and presence of wortmannin (Fig. 4B). Within 15 min, 75% of the internalized P. gingivalis cells were localized to vacuoles containing HsGsa7p, and this value decreased over time to 30 at 120 min. However, in the presence of wortmannin, less than 9% of the internalized P. gingivalis localized to the HsGsa7p vacuole. The data suggest that soon after invasion P. gingivalis becomes associated with an early autophagosome containing BiP and HsGsa7p but over time loses HsGsa7p.
FIG. 3.
Localization of BiP, LGP120, Rab5, and MPR to vacuoles containing P. gingivalis in HCAE cells. At 90 min postinfection, the P. gingivalis (Pg) vacuoles contained BiP (A) and LGP120 (B) (arrows). (C) At 35 min after infection, P. gingivalis colocalized with Rab5 (arrow). (D) MPR was absent from a majority of the vacuoles observed up to 2 h postinfection (arrowheads indicate colocalization). Bar, 15 μm.
FIG. 4.
Colocalization of P. gingivalis with protein markers of the autophagic pathway. HCAE cells were incubated with P. gingivalis 381 in the absence (solid bars) and presence (open bars) of 10 nM wortmannin for 15 to 120 min. The data represent the percentage of P. gingivalis vacuoles that contained BiP (A), HsGsa7p (B), LGP120 (C), and cathepsin L (D), expressed as the mean + the standard deviation (error bars). The data suggest that the P. gingivalis vacuole first acquires HsGsa7p and then BiP and LGP120; however, the vacuole fails to acquire cathepsin L. In the presence of wortmannin, the vacuole does not acquire HsGsa7p or BiP but rather acquires cathepsin L rapidly. The effects of wortmannin at each time point were evaluated by Student's t test utilizing Bonferroni's correction. Statistical significance (∗) was achieved when P was <0.01.
The above data suggest that P. gingivalis is sequestered into the early autophagosome after invasion. We next examined whether P. gingivalis trafficked to the late autophagosome and eventually to the autolysosome for degradation. LGP120 is a membrane protein present in late autophagosomes, autolysosomes, and lysosomes (22, 36, 58). Cathepsin L is a thiol-proteinase present in autolysosomes and lysosomes. P. gingivalis was observed in vacuoles that contained LGP120 but not cathepsin L. Within 60 min of invasion, 82% of the P. gingivalis cells were in an LGP120-positive vacuole (Fig. 4C). At this same time point, only 6% of the bacteria were in an autolysosome or lysosome as determined by the presence of cathepsin L (Fig. 4D). In contrast, 78% of the internalized P. gingivalis cells were localized to a cathepsin L-positive vacuole when the cells were treated with wortmannin. These data suggest that P. gingivalis traffics to a late autophagosome that fails to mature to an autolysosome, but the bacterium is transported to phagolysosomes when autophagy is inhibited by wortmannin.
Trafficking along the endocytic pathway.
We have demonstrated that P. gingivalis is internalized and sequestered into a late autophagosome containing BiP and LGP120 but lacking cathepsin L. P. gingivalis must either promote its entry into the autophagic pathway or block its entry into the endocytic pathway, thereby channeling itself into the autophagic pathway. When autophagy was inhibited by wortmannin, the bacteria were found in a cathepsin L-positive, lysosome-like vacuole. Hence, we propose that P. gingivalis does not block the endocytic pathway but rather promotes its entry into the autophagosome. Therefore, we suggest that bacteria are trafficked through the endocytic pathway to lysosomes during autophagy inhibition. To test this hypothesis, we traced the trafficking of P. gingivalis through the endocytic pathway in the presence and absence of wortmannin using antibody markers to endosomal vacuoles.
A majority of the P. gingivalis vacuoles contained Rab5, a GTP-binding protein that is a marker of early endosomes (26) (Fig. 3C), but few contained the late endosomal marker MPR (Fig. 3D). Within 15 min of invasion, 49% of the P. gingivalis vacuoles contained Rab5 (Fig. 5A). This value increased to >70% at 25 and 35 min postinfection and then decreased to 27% at 60 min. A majority of these vacuoles did not acquire the endosome marker MPR (Fig. 5B). This receptor is normally found in the Golgi apparatus and late endosomes or prelysosomes but not in autophagosomes (21, 25, 27, 35, 65). However, 46% of the bacteria colocalized with this receptor at the final 120-min time point in wortmannin-treated HCAE cells. This labeling is probably not due to its distribution to the Golgi apparatus, since α2,6 Gal β1,4-GlcNAc sialyltransferase did not colocalize to these vacuoles (data not shown). Similar results were obtained with Rap1, a Ras-related protein localized to late endosomes (48). At 90 min postinfection, 27% of the vacuoles contained Rap1, while as much as 67% (30 min postinfection) of the vacuoles contained Rap1 when the cells were treated with wortmannin (data not shown). These data suggest that the majority of P. gingivalis initially traffic to a Rab5 vacuole and then to the autophagosome. However, a few of the P. gingivalis cells reach a Rap1 vacuole. This is consistent with our observations that a minority of the internalized bacteria ultimately traffic to a cathepsin L-positive lysosome (Fig. 4D). When the autophagic pathway is blocked, the majority of the bacteria traffic from a Rab5 vacuole to a MPR vacuole and then to a cathepsin L vacuole.
FIG. 5.
Colocalization of P. gingivalis with protein markers of the endocytic pathway. HCAE cells were incubated with P. gingivalis 381 in the absence (solid bars) and presence (open bars) of 10 nM wortmannin for 15 to 120 min. The data represent the percentage of P. gingivalis vacuoles that contained Rab5 (A) and MPR (B), expressed as the mean + the standard deviation (error bars). The data suggest that the P. gingivalis vacuole acquires Rab5 early but does not acquire the late endocytic marker MPR. In the presence of wortmannin, a higher percentage of P. gingivalis vacuoles acquire MPR. The effects of wortmannin at each time point were evaluated by Student's t test utilizing Bonferroni's correction. Statistical significance (*) was achieved when P was <0.01.
Bacterial persistence
Since autophagy is a degradative pathway, endothelial cells could use this pathway as a defensive mechanism against invading bacterial aggregates. However, if the autophagosome is the intracellular niche of P. gingivalis (at least initially), then inhibition of autophagy should result in a decrease in the number of bacteria able to survive. A modification of the antibiotic protection assay was used to test the effects of the autophagy inhibitors 3-methyladenine and wortmannin on the persistence of P. gingivalis within HCAE cells.
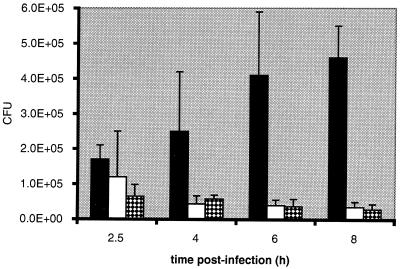
The number of CFU of P. gingivalis recovered from untreated HCAE cells at 2.5 h postinfection was (1.7 ± 0.4) × 105 (Fig. 6). The number of CFU continued to increase from 4 to 8 h postinfection. At 8 h postinfection, the number of CFU was (4.6 ± 0.9) × 105, 2.7-fold higher than that seen at 2.5 h postinfection. When the HCAE cells were treated with 3-methyladenine or wortmannin, bacterial persistence decreased dramatically. At 8 h postinfection, the number of CFU from wortmannin-treated cells was 6.3% of that from untreated infected HCAE cells. The decreased persistence in the presence of these drugs is consistent with our immunofluorescence data that reveal the bacteria colocalizing with cathepsin L (Fig. 4D). This is also consistent with the ultrastructural analyses that showed degraded bacteria at later time points in 3-methyladenine- or wortmannin-treated HCAE cells. The data suggest that these autophagy inhibitors prevented the establishment of the intracellular niche for the replication of P. gingivalis.
FIG. 6.
Persistence of P. gingivalis within HCAE cells over 8 h. HCAE cells were preincubated for 1 h at 37°C with either fresh antibiotic-free EGM-2 (solid bars), 10 mM 3-methyladenine (open bars), or 10 nM wortmannin (checkered bars) (n = 3). P. gingivalis 381 cells were incubated with EGM-2 with or without the appropriate inhibitor. The number of CFU of HCAE cells infected with P. gingivalis in the absence of autophagy inhibitors grew over the 8 h, whereas the number of CFU of HCAE cells infected with P. gingivalis in the presence of either autophagy inhibitor declined over the 8 h. The number of CFU for E. coli MC1061, the negative control, at 2.5 h postinfection was <200 (n = 3).
It is possible that 3-methyladenine or wortmannin may affect bacterial viability, adhesion, and/or invasion. Neither 3-methyladenine nor wortmannin inhibited the growth of P. gingivalis on blood agar plates (data not shown). The data presented here and reported by Sandros et al. show that internalization of P. gingivalis proceeds via endocytosis (52). Araki et al. and Reaves et al. have also demonstrated that the phosphoinositide 3-kinase (PI3-kinase) targeted by these drugs is not required for endocytosis (1, 49). Nevertheless, to allay concerns that these drugs may affect adherence and/or invasion, the HCAE cells were preincubated for 60 min with the inhibitors, at which time these drugs were withdrawn during the 90-min infection. The drugs were then reintroduced during the antibiotic incubation and were also present during the remainder of the incubation time (up to 6.5 h). These changes in the protocol did not appreciably alter the results in Fig. 6 (data not shown).
DISCUSSION
P. gingivalis is invasive of a variety of cells in vitro. The intracellular location of P. gingivalis has not been singularly defined within the variety of cells tested for invasion. Deshpande et al. have demonstrated that P. gingivalis resides within uncharacterized vacuoles, which may or may not be autophagosomes, in fetal bovine heart endothelial cells, bovine aortic endothelial cells, and human umbilical vein endothelial cells (16). P. gingivalis was reported free in the cytoplasm in gingival epithelial cells (33). Sandros et al. also found evidence of P. gingivalis free in the cytoplasm and within endosomes in KB cells, human pocket epithelium, and human junctional epithelium (44, 53, 54). The data suggest that the intracellular niche of P. gingivalis may be host cell specific. We have examined the trafficking of P. gingivalis in primary HCAE cells to better understand the proposed relationship between periodontal disease and coronary heart disease.
P. gingivalis traffics to the late autophagosome.
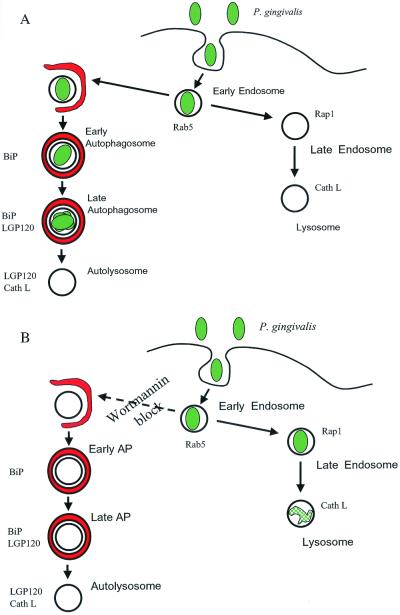
The data presented here are consistent with P. gingivalis being targeted to autophagosomes shortly after invasion of HCAE cells. We have shown that P. gingivalis cells are internalized into Rab5-positive vacuoles that rapidly acquire HsGsa7p. This likely represents the first step in the autophagic sequestration of P. gingivalis. The bacteria eventually traffic to a vacuole that contains both the RER protein BiP and the lysosomal protein LGP120 but is devoid of cathepsin L. This vacuole has been previously defined as a late autophagosome that contains RER and lysosomal membrane proteins but lacks hydrolytic enzymes (22). Then the late autophagosome containing P. gingivalis does not appear to acquire cathepsin L and thereby become an autolysosome, at least during the 2-h time course. The data suggest that P. gingivalis is sequestered into an early autophagosome which then matures into a late autophagosome, where the bacteria presumably replicate (Fig. 7A). These observations are substantiated by our ultrastructural observations. We observed the bacteria in a double-membrane-bound vacuole containing undegraded ribosomes and cytoplasmic ground substance. Profiles of dividing P. gingivalis can be observed in these vacuoles.
FIG. 7.
Model of P. gingivalis trafficking in HCAE cells. P. gingivalis is initially found within an early endosome after internalization. (A) The bacteria promote their own entry into the autophagic pathway. The P. gingivalis 381-containing vacuole acquires BiP and later LGP120. However, this vacuole does not acquire cathepsin L. (B) Upon inhibition of autophagy with wortmannin, P. gingivalis enters the endocytic pathway. The vacuole matures into a late endosome and then a lysosome, as characterized by the presence of Rap1 and cathepsin L.
In contrast, when 3-methyladenine or wortmannin suppresses autophagy, internalized P. gingivalis cells transit a population of single-membrane-bound vacuoles that lack HsGsa7p and BiP and contain Rap1, MPR, and cathepsin L (Fig. 7B). The localization of P. gingivalis in cathepsin L-positive vacuoles is consistent with the profiles of degraded bacteria observed in these cells as well as the decrease in bacterial persistence within these cells. The ability of the HCAE cells to enter the endocytic pathway when autophagy is inhibited further suggests that this bacterium does not suppress the endocytic pathway but rather promotes its sequestration into a late autophagosome that provides a beneficial environment for its survival and growth.
Comparison to L. pneumophila and virulent B. abortus.
Studies of B. abortus and L. pneumophila trafficking demonstrate several similarities to P. gingivalis intracellular trafficking. All three bacterial species reside in vacuoles bound by multiple membranes surrounded by ribosomes (47, 66). The data are consistent with all three of these microorganisms evading the endocytic pathway and entering the autophagic pathway. Additionally, both virulent B. abortus- and L. pneumophila-containing vacuoles do not acquire Rab7, which is characteristic of late endosomes (46, 50). Both P. gingivalis and B. abortus are present in early endosome-like vacuoles as assessed by Rab5 and EEA1, respectively (46). There does exist a rapid convergence between autophagosomes and early endosomes (37, 69). However, L. pneumophila completely avoids the endocytic system and does not colocalize with Rab5 (13). Additionally, virulent B. abortus does not colocalize with MPR (46). Each of the three wild-type, bacterial species also fails to traffic to vesicles that fuse with lysosomal hydrolases, but vesicles containing attenuated B. abortus or growth mutants of L. pneumophila fuse with lysosomes (47, 72).
Although very similar, there are some differences among these three species in colocalization with components of the autophagic pathway. All three bacteria are found in vacuoles surrounded by RER, but B. abortus does not colocalize with BiP or ribophorin (46). However, B. abortus colocalizes with two other RER proteins, s61β and the protein disulfide isomerase. Both L. pneumophila and P. gingivalis are present in vacuoles that colocalize with BiP (66). Vacuoles containing P. gingivalis acquire LGP120, and virulent B. abortus-containing vacuoles acquire both LAMP1 and LAMP2 (46). However, the majority of L. pneumophila phagosomes are negative for LGP120 during its interaction with the autophagic pathway (50, 67). From the data presented elsewhere and here, we propose that vacuoles containing P. gingivalis and virulent B. abortus mature into late autophagosomes whereas the vacuoles containing L. pneumophila remain early autophagosomes. Although these three bacteria have evolved similar strategies to survive in the host, there appear to be some differences in the exact mechanism of trafficking through the autophagic pathway.
Bacterial survival.
The specific genes of P. gingivalis required for its intracellular survival have not yet been identified. However, studies have indicated specific mechanisms by which the other two species survive intracellularly. For example, the functional secretion systems Icm-Dot and VirB in L. pneumophila and B. abortus, respectively, are necessary for their intracellular replication (8, 61). When the replication vacuole and host cell are depleted of amino acids, L. pneumophila converts to a virulent phenotype (29). The virulence traits, e.g., motility and cytotoxicity, allow the bacteria to escape the host cell and infect adjacent cells to start this cycle anew. Similar to P. gingivalis, L. pneumophila uses short peptides and amino acids as its carbon and energy source (24). We expect that P. gingivalis has evolved similar mechanisms to survive, which we are just beginning to elucidate.
Regulation of autophagy.
Our data suggest that P. gingivalis promotes its entry into the autophagosome. At this time, we are uncertain whether this is directly mediated by the bacterium or a consequence of cellular signals transduced during invasion. A functional secretion system in both L. pneumophila and B. abortus is necessary for the establishment of an intracellular niche divergent from the endocytic pathway (14, 15). Once established within this replication vacuole, the Icm-Dot secretion system is no longer expressed (9). This suggests that a secreted protein interacts with the host cell to induce autophagy and is essential for the biogenesis of the replication vacuole. However, the precise mechanism for the sequestration of the bacterium into the autophagosome-like vacuole is unknown.
A bacterial protein may regulate autophagy at several different points. For example, it could activate the class III PI 3-kinase or cause the redistribution of HsGsa7p. In mammalian cells, the class I PI 3-kinase has been shown to negatively regulate autophagy, whereas the class III PI-3 kinase is a positive regulator (45). Wortmannin and 3-methyladenine inhibit autophagy by suppressing the class III PI 3-kinases (5, 59, 70). In addition to the kinase regulation, an amide linkage between the C terminus of Apg12p and an ɛ-amino group of Apg5p has been shown to be required for autophagosome formation in Saccharomyces cerevisiae (60). An ubiquitin E1-like activating enzyme that has been identified in S. cerevisiae (Apg7p), Pichia pastoris (Gsa7p), and humans (HsGsa7p [also known as hAPG7]) activates Apg12p (32, 68, 73). The conjugation of the activated Apg12p to Apg5p is performed by Apg10p, a ubiquitin E2-like conjugation enzyme (60). The human homologues for Apg10p and Apg5p have been identified (55). Thus, this conjugation process appears to be conserved throughout eukaryotes, including humans (41, 42).
Another possible mechanism could be the inactivation of TOR (target of rapamycin) or the class I PI-3 kinase. TOR, a phosphatidylinositol kinase, is a negative regulator of autophagy in S. cerevisiae (43). It is believed that rapamycin stimulates autophagy by inhibiting TOR in yeast and RAFT (rapamycin and FKBP12 target) or FRAP (FKBP12 and rapamycin-associated protein) in mammalian cells, thereby suppressing phosphorylation of the ribosomal protein S6 (6, 56). TOR stimulates the expression of some genes and represses the expression of others under conditions of nutrient deprivation by controlling the repressor Ure2 and the transcription factor Gln3 (10). TOR phosphorylation of Gln3 enhances its binding to Ure2, thereby preventing transcription (4). Gln3 phosphorylation is also dependent on Tap42, which is phosphorylated by TOR (31). A bacterial protein could also interact at one or more of these steps in the autophagic pathway.
In summary, this study demonstrates that P. gingivalis traffics to late autophagosomes in primary HCAE cells. The ability to persist within HCAE cells and thus establish a chronic infection could exacerbate the immune response at sites along the vascular tree. Whether the P. gingivalis at the sites of atherosclerosis contributes to disease or constitutes a “bystander effect” has yet to be determined. This work is the beginning of a series of studies to investigate the cellular interactions between P. gingivalis and HCAE cells that could provide molecular explanations for the association between periodontal disease and cardiovascular disease.
ACKNOWLEDGMENTS
We are very grateful to and thank all of the people mentioned in Materials and Methods for their generous gifts of antibodies. We also thank Paul Gulig, Martin Handfield, Jeffrey Hillman, and William McArthur for useful discussion; Jacob Burks and Todd Barnash for assistance; Amy Perwien for consultations on statistics; the Optical Microscopy Facility of the Center for Structural Biology at the University of Florida Brain Institute for the use of the deconvolution microscope; and R. Davis, S. Whittaker, and the University of Florida Electron Microscopy Core Laboratory of the Interdisciplinary Center for Biotechnology Research for the transmission electron microscopy.
This study was supported by NIDCR grant DE 07496 (A.P.-F.) and NSF grant MCB 9817002 (W.A.D.).
REFERENCES
- 1.Araki N, Johnson M T, Swanson J A. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck J, Garcia R, Heiss G, Vokonas P S, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 3.Beck J D, Pankow J, Tyroler H A, Offenbacher S. Dental infections and atherosclerosis. Am Heart J. 1999;138:528–533. doi: 10.1016/s0002-8703(99)70293-0. [DOI] [PubMed] [Google Scholar]
- 4.Beck T, Hall M N. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature. 1999;402:689–692. doi: 10.1038/45287. [DOI] [PubMed] [Google Scholar]
- 5.Blommaart E F, Krause U, Schellens J P, Vreeling-Sindelarova H, Meijer A J. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem. 1997;243:240–246. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]
- 6.Blommaart E F, Luiken J J, Blommaart P J, van Woerkom G M, Meijer A J. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem. 1995;270:2320–2326. doi: 10.1074/jbc.270.5.2320. [DOI] [PubMed] [Google Scholar]
- 7.Booth V, Lehner T. Characterization of the Porphyromonas gingivalis antigen recognized by a monoclonal antibody which prevents colonization by the organism. J Periodontal Res. 1997;32:54–60. doi: 10.1111/j.1600-0765.1997.tb01382.x. [DOI] [PubMed] [Google Scholar]
- 8.Brand B C, Sadosky A B, Shuman H A. The Legionella pneumophila icm locus: a set of genes required for intracellular multiplication in human macrophages. Mol Microbiol. 1994;14:797–808. doi: 10.1111/j.1365-2958.1994.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 9.Byrne B, Swanson M S. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardenas M E, Cutler N S, Lorenz M C, Di Como C J, Heitman J. The TOR signaling cascade regulates gene expression in response to nutrients. Genes Dev. 1999;13:3271–3279. doi: 10.1101/gad.13.24.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll G C, Sebor R J. Dental flossing and its relationship to transient bacteremia. J Periodontol. 1980;51:691–692. doi: 10.1902/jop.1980.51.12.691. [DOI] [PubMed] [Google Scholar]
- 12.Chiu B. Multiple infections in carotid atherosclerotic plaques. Am Heart J. 1999;138:S534–S536. doi: 10.1016/s0002-8703(99)70294-2. [DOI] [PubMed] [Google Scholar]
- 13.Clemens D L, Lee B-Y, Horwitz M A. Deviant expression of Rab5 on phagosomes containing the intracellular pathogens Mycobacterium tuberculosis and Legionella pneumophila is associated with altered phagosomal fate. Infect Immun. 2000;68:2671–2684. doi: 10.1128/iai.68.5.2671-2684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coers J, Kagan J C, Matthews M, Nagai H, Zuckman D M, Roy C R. Identification of Icm protein complexes that play distinct roles in the biogenesis of an organelle permissive for Legionella pneumophila intracellular growth. Mol Microbiol. 2000;38:719–736. doi: 10.1046/j.1365-2958.2000.02176.x. [DOI] [PubMed] [Google Scholar]
- 15.Comerci D J, Martinez-Lorenzo M J, Sieira R, Gorvel J P, Ugalde R A. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell Microbiol. 2001;3:159–168. doi: 10.1046/j.1462-5822.2001.00102.x. [DOI] [PubMed] [Google Scholar]
- 16.Deshpande R G, Khan M B, Genco C A. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun. 1998;66:5337–5343. doi: 10.1128/iai.66.11.5337-5343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeStefano F, Anda R F, Kahn H S, Williamson D F, Russell C M. Dental disease and risk of coronary heart disease and mortality. Br Med J. 1993;306:688–691. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorn B R, Dunn W A, Jr, Progulske-Fox A. Invasion of human coronary artery cells by periodontal pathogens. Infect Immun. 1999;67:5792–5798. doi: 10.1128/iai.67.11.5792-5798.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan M J, Nakao S, Skobe Z, Xie H. Interactions of Porphyromonas gingivalis with epithelial cells. Infect Immun. 1993;61:2260–2265. doi: 10.1128/iai.61.5.2260-2265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn W A., Jr Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol. 1994;4:139–143. doi: 10.1016/0962-8924(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 21.Dunn W A., Jr Studies on the mechanisms of autophagy: formation of the autophagic vacuole. J Cell Biol. 1990;110:1923–1933. doi: 10.1083/jcb.110.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn W A., Jr Studies on the mechanisms of autophagy: maturation of the autophagic vacuole. J Cell Biol. 1990;110:1935–1945. doi: 10.1083/jcb.110.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George J R, Pine L, Reeves M W, Harrell W K. Amino acid requirements of Legionella pneumophila. J Clin Microbiol. 1980;11:286–291. doi: 10.1128/jcm.11.3.286-291.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geuze H J, Stoorvogel W, Strous G J, Slot J W, Bleekemolen J E, Mellman I. Sorting of mannose 6-phosphate receptors and lysosomal membrane proteins in endocytic vesicles. J Cell Biol. 1988;107:2491–2501. doi: 10.1083/jcb.107.6.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorvel J P, Chavrier P, Zerial M, Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- 27.Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- 28.Hackstadt T. Redirection of host vesicle trafficking pathways by intracellular parasites. Traffic. 2000;1:93–99. doi: 10.1034/j.1600-0854.2000.010201.x. [DOI] [PubMed] [Google Scholar]
- 29.Hammer B K, Swanson M S. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol Microbiol. 1999;33:721–731. doi: 10.1046/j.1365-2958.1999.01519.x. [DOI] [PubMed] [Google Scholar]
- 30.Haraszthy V I, Zambon J J, Trevisan M, Zeid M, Genco R J. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554–1560. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Y, Broach J R. Tor proteins and protein phosphatase 2A reciprocally regulate Tap42 in controlling cell growth in yeast. EMBO J. 1999;18:2782–2792. doi: 10.1093/emboj/18.10.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J, Dalton V M, Eggerton K P, Scott S V, Klionsky D J. Apg7p/Cvt2p is required for the cytoplasm-to-vacuole targeting, macroautophagy, and peroxisome degradation pathways. Mol Biol Cell. 1999;10:1337–1351. doi: 10.1091/mbc.10.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamont R J, Chan A, Belton C M, Izutsu K T, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamont R J, Oda D, Persson R E, Persson G R. Interaction of Porphyromonas gingivalis with gingival epithelial cells maintained in culture. Oral Microbiol Immunol. 1992;7:364–367. doi: 10.1111/j.1399-302x.1992.tb00637.x. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence B P, Brown W J. Inhibition of protein synthesis separates autophagic sequestration from the delivery of lysosomal enzymes. J Cell Sci. 1993;105:473–480. doi: 10.1242/jcs.105.2.473. [DOI] [PubMed] [Google Scholar]
- 36.Lewis V, Green S A, Marsh M, Vihko P, Helenius A, Mellman I. Glycoproteins of the lysosomal membrane. J Cell Biol. 1985;100:1839–1847. doi: 10.1083/jcb.100.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liou W, Geuze H J, Geelen M J, Slot J W. The autophagic and endocytic pathways converge at the nascent autophagic vacuoles. J Cell Biol. 1997;136:61–70. doi: 10.1083/jcb.136.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattila K J, Nieminen M S, Valtonen V V, Rasi V P, Kesaniemi Y A, Syrjala S L, Jungell P S, Isoluoma M, Hietaniemi K, Jokinen M J. Association between dental health and acute myocardial infarction. Br Med J. 1989;298:779–781. doi: 10.1136/bmj.298.6676.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattila K J, Valtonen V V, Nieminen M, Huttunen J K. Dental infection and the risk of new coronary events: prospective study of patients with documented coronary artery disease. Clin Infect Dis. 1995;20:588–592. doi: 10.1093/clinids/20.3.588. [DOI] [PubMed] [Google Scholar]
- 40.Meresse S, Steele-Mortimer O, Moreno E, Desjardins M, Finlay B, Gorvel J P. Controlling the maturation of pathogen-containing vacuoles: a matter of life and death. Nat Cell Biol. 1999;1:E183–E188. doi: 10.1038/15620. [DOI] [PubMed] [Google Scholar]
- 41.Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George M D, Klionsky D J, Ohsumi M, Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395:395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- 42.Mizushima N, Sugita H, Yoshimori T, Ohsumi Y. A new protein conjugation system in human. J Biol Chem. 1998;273:33889–33892. doi: 10.1074/jbc.273.51.33889. [DOI] [PubMed] [Google Scholar]
- 43.Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273:3963–3966. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- 44.Papapanou P N, Sandros J, Lindberg K, Duncan M J, Niederman R, Nannmark U. Porphyromonas gingivalis may multiply and advance within stratified human junctional epithelium in vitro. J Periodontal Res. 1994;29:374–375. doi: 10.1111/j.1600-0765.1994.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 45.Petiot A, Ogier-Denis E, Blommaart E F, Meijer A J, Codogno P. Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem. 2000;275:992–998. doi: 10.1074/jbc.275.2.992. [DOI] [PubMed] [Google Scholar]
- 46.Pizarro-Cerda J, Meresse S, Parton R G, van der Goot G, Sola-Landa A, Lopez-Goni I, Moreno E, Gorvel J P. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect Immun. 1998;66:5711–5724. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pizarro-Cerda J, Moreno E, Sanguedolce V, Mege J L, Gorvel J P. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect Immun. 1998;66:2387–2392. doi: 10.1128/iai.66.5.2387-2392.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pizon V, Desjardins M, Bucci C, Parton R G, Zerial M. Association of Rap1a and Rap1b proteins with late endocytic/phagocytic compartments and Rap2a with the Golgi complex. J Cell Sci. 1994;107:1661–1670. doi: 10.1242/jcs.107.6.1661. [DOI] [PubMed] [Google Scholar]
- 49.Reaves B J, Bright N A, Mullock B M, Luzio J P. The effect of wortmannin on the localisation of lysosomal type I integral membrane glycoproteins suggests a role for phosphoinositide 3-kinase activity in regulating membrane traffic late in the endocytic pathway. J Cell Sci. 1996;109:749–762. doi: 10.1242/jcs.109.4.749. [DOI] [PubMed] [Google Scholar]
- 50.Roy C R, Berger K H, Isberg R R. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol. 1998;28:663–674. doi: 10.1046/j.1365-2958.1998.00841.x. [DOI] [PubMed] [Google Scholar]
- 51.Rudney J D, Chen R, Sedgewick G J. Intracellular Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in buccal epithelial cells collected from human subjects. Infect Immun. 2001;69:2700–2707. doi: 10.1128/IAI.69.4.2700-2707.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandros J, Madianos P N, Papapanou P N. Cellular events concurrent with Porphyromonas gingivalis invasion of oral epithelium in vitro. Eur J Oral Sci. 1996;104:363–371. doi: 10.1111/j.1600-0722.1996.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 53.Sandros J, Papapanou P, Dahlen G. Porphyromonas gingivalis invades oral epithelial cells in vitro. J Periodontal Res. 1993;28:219–226. doi: 10.1111/j.1600-0765.1993.tb01072.x. [DOI] [PubMed] [Google Scholar]
- 54.Sandros J, Papapanou P N, Nannmark U, Dahlen G. Porphyromonas gingivalis invades human pocket epithelium in vitro. J Periodontal Res. 1994;29:62–69. doi: 10.1111/j.1600-0765.1994.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 55.Schmeiser K, Armstrong S, Hammond E M, Grand R J. Assignment of the yeast APG5 human homologue APG5L to chromosome band 6q21 by fluorescence in situ hybridisation. Cytogenet Cell Genet. 1999;87:213–214. doi: 10.1159/000015471. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt A, Beck T, Koller A, Kunz J, Hall M N. The TOR nutrient signalling pathway phosphorylates NPR1 and inhibits turnover of the tryptophan permease. EMBO J. 1998;17:6924–6931. doi: 10.1093/emboj/17.23.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sconyers J R, Crawford J J, Moriarty J D. Relationship of bacteremia to toothbrushing in patients with periodontitis. J Am Dent Assoc. 1973;87:616–622. doi: 10.14219/jada.archive.1973.0453. [DOI] [PubMed] [Google Scholar]
- 58.Seglen P O, Berg T O, Blankson H, Fengsrud M, Holen I, Stromhaug P E. Structural aspects of autophagy. Adv Exp Med Biol. 1996;389:103–111. doi: 10.1007/978-1-4613-0335-0_12. [DOI] [PubMed] [Google Scholar]
- 59.Seglen P O, Gordon P B. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shintani T, Mizushima N, Ogawa Y, Matsuura A, Noda T, Ohsumi Y. Apg10p, a novel protein-conjugating enzyme essential for autophagy in yeast. EMBO J. 1999;18:5234–5241. doi: 10.1093/emboj/18.19.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sieira R, Comerci D J, Sanchez D O, Ugalde R A. A homologue of an operon required for DNA transfer in Agrobacterium is required in Brucella abortus for virulence and intracellular multiplication. J Bacteriol. 2000;182:4849–4855. doi: 10.1128/jb.182.17.4849-4855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Silver J G, Martin A W, McBride B C. Experimental transient bacteraemias in human subjects with varying degrees of plaque accumulation and gingival inflammation. J Clin Periodontol. 1977;4:92–99. doi: 10.1111/j.1600-051x.1977.tb01888.x. [DOI] [PubMed] [Google Scholar]
- 63.Sinai A P, Joiner K A. Safe haven: the cell biology of nonfusogenic pathogen vacuoles. Annu Rev Microbiol. 1997;51:415–462. doi: 10.1146/annurev.micro.51.1.415. [DOI] [PubMed] [Google Scholar]
- 64.Socransky S S, Haffajee A D. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63:322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 65.Stromhaug P E, Berg T O, Fengsrud M, Seglen P O. Purification and characterization of autophagosomes from rat hepatocytes. Biochem J. 1998;335:217–224. doi: 10.1042/bj3350217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Swanson M S, Isberg R R. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swanson M S, Isberg R R. Identification of Legionella pneumophila mutants that have aberrant intracellular fates. Infect Immun. 1996;64:2585–2594. doi: 10.1128/iai.64.7.2585-2594.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanida I, Mizushima N, Kiyooka M, Ohsumi M, Ueno T, Ohsumi Y, Kominami E. Apg7p/Cvt2p: a novel protein-activating enzyme essential for autophagy. Mol Biol Cell. 1999;10:1367–1379. doi: 10.1091/mbc.10.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tooze J, Hollinshead M, Ludwig T, Howell K, Hoflack B, Kern H. In exocrine pancreas, the basolateral endocytic pathway converges with the autophagic pathway immediately after the early endosome. J Cell Biol. 1990;111:329–345. doi: 10.1083/jcb.111.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ueno T, Ishidoh K, Mineki R, Tanida I, Murayama K, Kadowaki M, Kominami E. Autolysosomal membrane-associated betaine homocysteine methyltransferase. Limited degradation fragment of a sequestered cytosolic enzyme monitoring autophagy. J Biol Chem. 1999;274:15222–15229. doi: 10.1074/jbc.274.21.15222. [DOI] [PubMed] [Google Scholar]
- 71.Vogel J P, Misra L M, Rose M D. Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J Cell Biol. 1990;110:1885–1895. doi: 10.1083/jcb.110.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiater L A, Dunn K, Maxfield F R, Shuman H A. Early events in phagosome establishment are required for intracellular survival of Legionella pneumophila. Infect Immun. 1998;66:4450–4460. doi: 10.1128/iai.66.9.4450-4460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuan W, Stromhaug P E, Dunn W A., Jr Glucose-induced autophagy of peroxisomes in Pichia pastoris requires a unique E1-like protein. Mol Biol Cell. 1999;10:1353–1366. doi: 10.1091/mbc.10.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]