Abstract
Background
Aflatoxin B1 and ochratoxin A are mycotoxins produced by filamentous fungi that attack crops on field and storage. Both mycotoxins present a risk on public health since aflatoxin B1 is a hepatotoxic and hepatocarcinogenic agent while ochratoxin A can be nephrotoxic. Those mycotoxins can be found in several food items including spices, herbs, and nuts.
Objectives
In Lebanon, few studies address aflatoxin B1 and ochratoxin A contamination in spices, herbs, and nuts. So, the aim of this study is to investigate the concentrations of those two mycotoxins particularly in spices and herbs and the concentration of aflatoxin B1 in nuts, and to determine the dietary exposure of the Lebanese population and their possible attribution to liver cancer and renal damage.
Methods
In this work, a total of 198 samples of spices, herbs, and nuts were collected from different sites. Aflatoxin B1 and ochratoxin A were quantified using immune-affinity columns. A food frequency questionnaire was used to quantify the consumption of spices, herbs, and nuts in Lebanon. Exposure to aflatoxin B1 and ochratoxin A was calculated accordingly and liver and kidney cancer risks were evaluated.
Results
Aflatoxin B1 was respectively found in 100, 20.4, and 98.6% of the spices, herbs, and nuts samples, while ochratoxin A was found in 100 and 44.4% of spices and herbs, respectively. Aflatoxin B1 was found at mean concentration of 0.97, 0.27, and 0.40 μg/kg in spices, herbs, and nuts, respectively while ochratoxin A was found at mean concentrations of 38.8 and 1.81 μg/kg in spices and herbs, respectively. Aflatoxin B1 occurrence was shown to be associated in this study with 0.017 additional cancer cases per 100,000 persons per year, and ochratoxin A weekly exposure was shown to be 5.04 ng/kg bw less than the Provisional Tolerable Weekly Intake of 100 ng/kg bw which indicates low risk of renal damage from spices and herbs consumption.
Conclusion
The consumption of spices, herbs, and nuts in Lebanon could lead to an increase in health risks associated with aflatoxin B1 and ochratoxin A, specifically spices. The reported occurrence may be directly related to poor storage conditions.
Keywords: contamination, daily exposure, cancer risk, AFB1, OTA
1. Introduction
Mycotoxins are secondary derivatives of filamentous fungi produced on-field or during storage due to climatic conditions such as humidity and temperature. Aflatoxins and ochratoxin A (OTA) are types of mycotoxins that can contaminate several foods such as cereals, legumes, nuts, spices, herbs, coffee, cocoa beans, beer, etc. (1–3). Aflatoxins are produced by Aspergillus species mainly Aspergillus flavus and Aspergillus parasiticus (4, 5) while ochratoxin A is produced by Aspergillus ochraceus, Aspergillus carbonarius, Aspergillus niger, and Penicillium verrucosum (6).
Aflatoxin B1 (AFB1) is the most potent of all mycotoxins. It has been classified by the International Agency for Research on Cancer (IARC) as carcinogenic to human (group 1) (7) as it affects the liver and can be the main cause for cases of “acute toxicity, chronic toxicity, carcinogenicity, teratogenicity, genotoxicity, and immunotoxicity.” As for OTA it was classified by IARC as a possible carcinogen to humans (group 2B) (7). Though it is less toxic than AFB1, however, exposure to OTA can cause several health consequences like nephrotoxic, hepatotoxic, neurotoxic, teratogenic, and immunotoxic effects (8, 9). OTA was also suspected to be implicated in the Balkan Endemic Nephropathy (BEN) that was characterized by a high prevalence of a unique chronic renal disease in the Balkan region (10–13).
Aflatoxins and OTA are the main mycotoxin contaminants of spices and herbs, while nuts are usually more prone to contamination with aflatoxins. The “Rapid Alert System for Food and Feed” (RASFF) 2018 annual report showed that aflatoxin notifications were mostly reported in nuts while in spices and herbs the main contaminant was OTA (14).
In particular, spices, herbs, and nuts are produced in tropical regions where the climate is characterized by high temperatures, humidity, and rainfalls (15). This climate increases the susceptibility of fungal invasion and mycotoxin production in crops, therefore, increasing the risk of AFB1 and OTA contamination. Furthermore, improper harvest, drying, transport, and storage practices can result in further contamination (16).
In Lebanon, spices, herbs, and nuts are very popular and are used extensively in culinary activities so they are frequently imported in large quantities to accomplish the market need (17). Mainly those are imported in batches to get processed and packaged in factories before market distribution or to be handled in bulk for local shops and roasteries. Before admission to the country, the imported products get inspected and tested by the inspectors of the Ministry of Agriculture (MOA) for aflatoxins and OTA contamination (18, 19). Unlike spices, herbs can be produced locally and they are particularly popular due to their wide usage in Lebanese cuisine and due to their medicinal properties (20). So, based on traditional practices, herbs are used in Lebanon in many regions especially rural ones, as therapeutic agents for several health problems (21).
Nonetheless, the weather in Lebanon is characterized by high temperature and humidity (22) so it can induce mycotoxin contamination in spices and herbs in the prevalence of improper agricultural, harvest, drying, and storage practices.
Worldwide, regulations were set to minimize the introduction of contaminated spices to any market. The European Commission (EC) has set a maximum tolerable limit (MTL) of AFB1 and OTA in spices at 5 and 15 μg/kg, respectively (23). As for herbs, no MTL is defined by the EC. On the other hand, AFB1 MTL in nuts intended for direct human consumption is set at 2 μg/kg for peanuts and other tree nuts, and at 8 μg/kg for almonds and pistachios (23). In Lebanon, “The Lebanese Standards Institution—LIBNOR” follows the European regulations and sets the same MTL for AFB1 and OTA in some types of spices and nuts accordingly (19, 24).
On the national level, limited studies have shown the contamination of spices, herbs, and nuts and recommended continuous monitoring of aflatoxins and OTA in the Lebanese market (25–27). A recent study done on spices in Lebanese markets also showed the prevalence of fungal species mostly A. flavus even in packaged samples which increases the risk of contamination with AFB1 (20). In addition, the RASFF portal reported that several rejection notifications have been issued in multiple European countries to imported batches of spices and nuts from Lebanon due to contamination with AFB1 (28).
Therefore, assessing the risk of AFB1 and OTA in Lebanon is particularly important especially, since hepatic cancer and kidney disease cases have been increasing (29–31). Accordingly, the aim of this study is to investigate the concentrations of AFB1 and OTA particularly in spices and herbs and the concentration of AFB1 in nuts, and to determine the dietary exposure of the Lebanese population to those toxins and their possible attribution to liver cancer and renal damage.
2. Materials and methods
2.1. Sampling
2.1.1. Spices
A total of 73 spices samples were collected from supermarkets and shops in all Lebanese regions including sweet pepper (thirteen), white pepper (ten), black pepper (nine), cinnamon (eight), nutmeg (six), turmeric (three), cumin (three), paprika (four), and spices mix (seventeen). Among the samples collected 51 were vacuum packaged while 22 were purchased unpackaged from traditional local shops.
2.1.2. Herbs
A total of 54 herbs samples were collected from supermarkets and shops in all Lebanese regions including sumac (ten), thyme (ten), coriander (eight), dried mint (six), oregano (six), basil (three), anise (eight), and infused herbs (three). Among the samples collected 34 were vacuum packaged while 20 were purchased unpackaged from traditional local shops.
2.1.3. Nuts
A total of 71 nuts samples were collected from supermarkets and shops in all Lebanese regions including mixed nuts (nine), peanuts (twenty), pistachios (fifteen), cashews (six), almonds (eleven), chickpeas (eight), and others (two). Among the samples collected 28 were vacuum packaged while 43 were purchased unpackaged from traditional local shops.
2.2. Sampling area, period, size, and technique
The samples used in the study were collected during the month of January 2020. Samples withdrawn from bulk were collected from all Lebanese governorates, while packaged samples were purchased from different brands representative of the Lebanese market.
Samples were collected according to two different methods. First, if the sample was collected from bulk, the items were mixed thoroughly and 100 g were randomly obtained, however in case the sample was packaged, one package was purchased from every sample. The samples were transferred to the lab afterwards and stored at −18°C until the time of analysis.
2.3. Chemicals
Standards of AFB1 (2 μg/ml in acetonitrile) were purchased from Sigma-Aldrich (Steinheim, Germany), standards of OTA (50 μg/ml in benzene: acetic Acid 99:1) were purchased from Supelco (Pennsylvania, USA), acetonitrile, methanol, and water (HPLC grade) were purchased from Sigma-Aldrich (Steinheim, Germany), and Aflaochra prep immunoaffinity columns (IAC) specific to aflatoxins and OTA were purchased from R-Biopharm Rhone Ltd. (Glasgow, Scotland, UK). All other chemicals and reagents were of analytical grade.
2.4. Sample preparation
2.4.1. Primary preparation
Samples were prepared according to the following method. In case the sample was ground it was mixed thoroughly and then 25 g of the sample were randomly withdrawn to be analyzed. In case the sample was solid, first the sample was grinded and mixed thoroughly, then 25 g were randomly withdrawn to be used as the test sample for analysis.
2.4.2. AFB1 and OTA extraction and purification in spices and herbs samples
The extraction of AFB1 and OTA was carried out according to the method specified in the Aflaochraprep immunoaffinity columns manual supplied by R-Biopharm Rhone Ltd. (32). First, 25 g of the ground sample were blended using a high speed blender with 5 g of sodium chloride and 100 ml of 80% methanol at a high speed for 2 min. Then the samples were centrifuged at 4,000 rpm for 10 min. Next, 2 ml of the filtrate were diluted with 18 ml of 10% Tween 20 in PBS and filtered through glass microfiber filter paper. After that, 10 ml of the filtrate were passed through the IAC at a slow steady flow rate (2 ml per minute) to purify the toxins. The IAC were then washed with 20 ml PBS to get rid of any sample residues. Finally the toxins were eluted by passing 1 ml of methanol into the IAC followed by 1 ml of distilled water to attain a final volume of 2 ml. The final eluted volumes were collected and stored in sealed vials at proper temperatures until the time of HPLC analysis.
2.4.3. AFB1 extraction and purification in nuts samples
The extraction of AFB1 and OTA was carried out according to the method specified in the Aflaprep immunoaffinity columns manual supplied by R-Biopharm Rhone Ltd. (33). First, 50 g of the ground sample were blended using a high speed blender with 5 g of sodium chloride and 100 ml of water at a high speed for 2 min. Then 150 ml of methanol were added and the mixture was blended for 2 min. Then the samples were centrifuged at 4,000 rpm for 10 min. Next, 5 ml of the filtrate were diluted with 5 ml of PBS and passed through the IAC at a slow steady flow rate (2 ml per minute) to purify the toxins. The IAC were then washed with 20 ml PBS to get rid of any sample residues. Finally, the toxins were eluted by passing 1 ml of methanol into the IAC followed by 1 ml of distilled water to attain a final volume of 2 ml. The final eluted volumes were collected and stored in sealed vials at proper temperatures until the time of HPLC analysis.
2.5. HPLC conditions
2.5.1. AFB1 and OTA quantification by HPLC in spices and herbs samples
HPLC conditions were adopted from the instructions in the Aflaochraprep immunoaffinity columns manual supplied by R-Biopharm Rhone Ltd. (32). Reverse phase HPLC (Waters 2690®, Waters Corp., MA, USA) coupled with a fluorescence detector (Waters 2475®) and a HS C18 column (250 mm × 4.6 mm I.D., 5 μm particle diameter, Supelco Discovery®) fitted with a C18 guard column (Supelco Supelguard®, Sigma-Aldrich Co., MO, USA) at 25°C was used for analysis. The method for HPLC analysis was performed as described by the Aflaochra Prep IAC supplier R-Biopharm Rhone Ltd. Two mobile phases were used for analysis: solution A was composed of water: methanol (55:45 v:v), while solution B was composed of water: methanol (20:80 v:v). For both solutions, 119 mg potassium bromide and 350 μl of 4 M nitric acid were added for each 1 L. The mobile phases were prepared and filtrated on the same day of HPLC analysis, and they were passed at a flow rate of 0.8 ml/min. The sample injection volume was 100 μl and the wavelengths for excitation and emission at the beginning of the analysis were 365 and 442 nm, respectively, but after 17 min the excitation and emission were changed to 333 and 463 nm, respectively.
2.5.2. AFB1 quantification by HPLC in nuts samples
HPLC conditions were adopted from the instructions in the Aflaprep immunoaffinity columns manual supplied by R-Biopharm Rhone Ltd. (33). Reverse phase HPLC (Waters 2690®, Waters Corp., MA, USA) coupled with a fluorescence detector (Waters 2475®) and a HS C18 column (250 mm × 4.6 mm I.D., 5 μm particle diameter, Supelco Discovery®) fitted with a C18 guard column (Supelco Supelguard®, Sigma-Aldrich Co., MO, USA) at 40°C temperature was used for analysis. The method for HPLC analysis was performed as described by the Aflaprep IAC supplier R-Biopharm Rhone Ltd. A mobile phase made of water: methanol (60:40 v:v) with 119 mg potassium bromide and 350 μl of 4 M nitric acid per liter was used for analysis. The mobile phase was prepared and filtrated on the same day of HPLC analysis, and it was passed at a flow rate of 1.0 ml/min. The sample injection volume was 100 μl and the wavelengths for excitation and emission were 362 and 425 nm, respectively.
2.6. Validation and quality assurance methods
A calibration curve was established using AFB1 and OTA standards at eight different concentrations ranging from 0 to 10 ppb. The limits of detection (LOD) and the limits of quantification (LOQ) were determined according to signal-to-noise ratios (S/N) of 3:1 and 6:1, respectively (34). The LOD and LOQ of AFB1 were 0.0054 and 0.018 μg/kg and for OTA 0.0030 and 0.014 μg/kg, respectively. Recovery analysis was performed were samples of spices, herbs, and nuts were spiked at three different levels of AFB1 and OTA. The average recovery for AFB1 and OTA were 82 and 90%, respectively. All tests, samplings, and operations in this study were conducted using the “Data Quality Assessment” checklist (35).
2.7. Population exposure to AFB1 and OTA
In order to assess the exposure of the Lebanese population to AFB1 and OTA, the food consumption data with regards to spices, herbs, and nuts was used to estimate the dietary exposure to AFB1 and OTA (35). In that context, a food frequency questionnaire (FFQ) was developed and verified to estimate the consumption rates of spices, herbs, and nuts in Lebanon. A random representative sample of 770 people was selected from all Lebanese governorates to participate in this study. Before starting the questionnaire, participants were clearly informed about the nature of the study, its objectives, methods, requirements, and guidelines. Additionally, an informed consent was obtained from every participant before filling the questionnaire. After obtaining the consumption data of every item, the exposure to AFB1 and OTA was evaluated accordingly:
The average daily exposure per person was then converted into average daily exposure to AFB1 and OTA per kg body weight per day based on the average weight of the Lebanese adult population found in this study which is 75.49 kg. This weight was used to calculate the average daily exposure to AFB1 and OTA per kg of body weight for adults of both genders.
The average daily exposure to AFB1 and OTA from every item was summed up to get the total daily exposure (ng/kg body weight/day) from spices, herbs, and nuts in Lebanon.
The risk associated with exposure to AFB1 and OTA were analyzed according to the Joint FAO/WHO Expert Committee of Food Additives (JECFA) method. It is estimated that, for non-European countries, the ingestion of 1 ng per kg of body weight per day of aflatoxins would induce 0.083 liver cancer cases per year per 100,000 persons (36). So the liver cancer risk based on the total daily exposure to AFB1 (ng/kg body weight/day) from spices, herbs, and nuts was calculated as follows:
As for OTA, JECFA established a provisional tolerable weekly intake (PTWI) of 100 ng/kg bw, so the risk resulting from OTA exposure was compared to this value (37).
2.8. Statistical analysis
The IBM® SPSS® software was used to compute means, range, standard deviations, and perform independent samples t-test to test significant difference on a p-value < 0.05.
3. Results
3.1. Spices
3.1.1. AFB1
A summary of AFB1 results in spices is presented in Table 1. All of the 73 samples (100%) were found to contain AFB1 in the range of 0.13–18.35 μg/kg and at a mean of 0.97 μg/kg. Out of 73 samples only two nutmeg samples were contaminated above the MTL set by the EC.
Table 1.
Incidence and occurrence of AFB1 in different kinds of spices in Lebanon.
| Spices |
Samples analyzed (n) |
Contamination level (μg/kg) | ||
|---|---|---|---|---|
| Number (%) of samples exceeding MTL | Range of contamination | Mean ±SD | ||
| Sweet pepper | 13 | 0 | 0.14–0.96 | 0.23 ± 0.22 |
| White pepper | 10 | 0 | 0.15–3.11 | 0.51 ± 0.92 |
| Black pepper | 9 | 0 | 0.15–0.32 | 0.19 ± 0.06 |
| Cinnamon | 8 | 0 | 0.15–0.84 | 0.27 ± 0.23 |
| Nutmeg | 6 | 2 (33.3%) | 1.65–18.35 | 7.28 ± 6.76 |
| Turmeric | 3 | 0 | 0.14–0.16 | 0.15 ± 0.01 |
| Cumin | 3 | 0 | 0.14–1.90 | 0.74 ± 1.00 |
| Paprika | 4 | 0 | 0.15–0.94 | 0.42 ± 0.37 |
| Spices Mix | 17 | 0 | 0.13–3.87 | 0.63 ± 0.93 |
| Total | 73 | 2 (2.7%) | 0.13–18.35 | 0.97 ± 2.68 |
MTL for AFB1 in spices indicated in this table is 5 μg/kg.
Comparing different types of spices, all AFB1 mean contamination levels were found to be less than the MTL of 5 μg/kg, while only AFB1 mean contamination in nutmeg was found to be 7.28 μg/kg higher than the MTL.
3.1.2. OTA
A summary of OTA contamination in spices is presented in Table 2. All of the 73 samples (100%) were found to contain OTA in the range of 0.12–452.46 μg/kg. The mean contamination of OTA in all spice samples was high at 38.8 μg/kg that exceeds the MTL set by the EC at 15 μg/kg. Out of 73 samples 22 samples (30.1%) were contaminated at levels above the MTL.
Table 2.
Incidence and occurrence of OTA in different kinds of spices in Lebanon.
| Spices |
Samples analyzed (n) |
Contamination level (μg/kg) | ||
|---|---|---|---|---|
| Number (%) of samples exceeding MTL | Range of contamination | Mean ±SD | ||
| Sweet pepper | 13 | 1 (7.7%) | 0.47–44.22 | 15.08 ± 44.22 |
| White pepper | 10 | 8 (80%) | 0.12–452.46 | 132.03 ± 141.79 |
| Black pepper | 9 | 6 (66.7%) | 0.85–238.95 | 77.53 ± 89.08 |
| Cinnamon | 8 | 0 | 0.02–2.99 | 0.72 ± 0.95 |
| Nutmeg | 6 | 4 (66.7%) | 1.39–236.26 | 68.05 ± 90.16 |
| Turmeric | 3 | 0 | 0.47–3.09 | 1.38 ± 1.50 |
| Cumin | 3 | 1 (33.3%) | 0.12–31.99 | 11.11 ± 18.10 |
| Paprika | 4 | 0 | 0.13–3.62 | 1.36 ± 1.55 |
| Spices mix | 17 | 2 (11.7%) | 0.49–79.45 | 9.33 ± 19.52 |
| Total | 73 | 22 (30.1%) | 0.12–452.46 | 38.8 ± 80.5 |
MTL for OTA in spices indicated in this table is 15 μg/kg.
Comparing different types of spices, the highest mean contamination that exceeded the MTL of 15 μg/kg was recorded in white pepper (132.03 μg/kg), followed by black pepper (77.53 μg/kg), nutmeg (68.05 μg/kg), and sweet pepper (15.08 μg/kg). As for other types, the mean OTA level was below the MTL in cumin (11.11 μg/kg), spices mix (9.33 μg/kg), turmeric (1.38 μg/kg), paprika (1.36 μg/kg), and cinnamon (0.72 μg/kg).
3.2. Herbs
3.2.1. AFB1
A summary of AFB1 results in herbs is presented in Table 3. Out of 54 samples, only 11 samples (20.4%) were found to contain AFB1 in the range of 0.72–4.87 μg/kg. The total mean of the 54 samples was low at 0.27 μg/kg.
Table 3.
Incidence and occurrence of AFB1 in different kinds of herbs in Lebanon.
| Herbs |
Samples analyzed (n) |
Positive samples n (%) |
Contamination level (μg/kg) | |
|---|---|---|---|---|
| Range of contamination | Mean ±SD | |||
| Sumac | 10 | 1 (10%) | BDL−0.84 | 0.08 ± 0.26 |
| Thyme | 10 | 3 (30%) | 0.72–0.96 | 0.25 ± 0.41 |
| Coriander | 8 | 2 (25%) | 0.85–2.16 | 0.38 ± 0.78 |
| Dried mint | 6 | 1 (16.7%) | BDL−0.89 | 0.15 ± 0.36 |
| Oregano | 6 | 1 (16.7%) | BDL−0.82 | 0.14 ± 0.33 |
| Basil | 3 | 0 | – | – |
| Anise | 8 | 2 (25%) | 0.84–4.87 | 0.71 ± 1.71 |
| Infused herbs | 3 | 1 (33.3%) | BDL−0.82 | 0.27 ± 0.47 |
| Total | 54 | 11 (20.4%) | 0.72–4.87 | 0.27 ± 0.76 |
3.2.2. OTA
A summary of OTA results in herbs is presented in Table 4. Out of 54 samples, only 23 samples (42.6%) were found to contain OTA in the range of 0.02–23.82 μg/kg. The total mean of the 54 samples was low at 1.81 μg/kg.
Table 4.
Incidence and occurrence of OTA in different kinds of herbs in Lebanon.
| Herbs |
Samples analyzed (n) |
Positive samples n (%) |
Contamination level (μg/kg) | |
|---|---|---|---|---|
| Range of contamination | Mean ±SD | |||
| Sumac | 10 | 3 (30%) | 0.02–1.24 | 0.24 ± 0.50 |
| Thyme | 10 | 3 (30%) | 0.017–1.94 | 0.20 ± 0.61 |
| Coriander | 8 | 4 (50%) | 0.017–10.98 | 1.50 ± 3.83 |
| Dried mint | 6 | 0 | – | – |
| Oregano | 6 | 2 (33.3%) | 0.67–4.59 | 0.88 ± 1.84 |
| Basil | 3 | 2 (66.7%) | 0.45–0.71 | 0.39 ± 0.36 |
| Anise | 8 | 8 (100%) | 0.02–23.82 | 5.62 ± 8.30 |
| Infused herbs | 3 | 1 (33.3%) | BDL−20.18 | 10.04 ± 10.09 |
| Total | 54 | 23 (42.5%) | 0.02–23.82 | 1.81 ± 4.78 |
3.3. Nuts
3.3.1. AFB1
A summary of AFB1 results in nuts is presented in Table 5. Out of 71 samples, 70 were found to contain AFB1 at low levels in the range of 0.06–1.78 μg/kg and at a mean of 0.40 μg/kg. None of the samples were found to exceed the MTL set by the EC at 2 μg/kg for processed tree nuts and at 8 μg/kg for almonds and pistachios.
Table 5.
Incidence and occurrence of AFB1 in different kinds of nuts in Lebanon.
| Product |
Samples analyzed (n) |
Positive samples n (%) |
Contamination level (μg/kg) | ||
|---|---|---|---|---|---|
| Number (%) of samples exceeding MTL | Range of contamination | Mean ±SD | |||
| Mixed | 9 | 9 (100%) | 0 | 0.10–0.55 | 0.32 ± 0.16 |
| Peanuts | 20 | 19 (95%) | 0 | 0.08–1.78 | 0.50 ± 0.46 |
| Pistachiosa | 15 | 15 (100%) | 0 | 0.18–0.84 | 0.41 ± 0.19 |
| Cashews | 6 | 6 (100%) | 0 | 0.14–0.62 | 0.37 ± 0.18 |
| Almondsa | 11 | 11 (100%) | 0 | 0.06–0.90 | 0.34 ± 0.28 |
| Chickpeas | 8 | 8 (100%) | 0 | 0.06–0.50 | 0.34 ± 0.16 |
| Others | 2 | 2 (100%) | 0 | 0.25–0.36 | 0.30 ± 0.08 |
| Total | 71 | 70 (98.6%) | 0 | 0.06–1.78 | 0.40 ± 0.30 |
MTL for AFB1 in nuts indicated in this table is 2 μg/kg.
The item specified has a MTL of 8 μg/kg.
3.4. Comparison between vacuum packed and un-packed samples
Table 6 shows the mean and range of contamination in vacuum packaged vs. unpackaged samples that were purchased from bulk, which is a common practice in Lebanon.
Table 6.
Mean and range of contamination of AFB1 and OTA in vacuum packaged vs. unpackaged samples of spices, herbs, and nuts.
| Spices | Herbs | Nuts | |||||
|---|---|---|---|---|---|---|---|
|
Packed (51) |
Unpacked (22) |
Packed (34) |
Unpacked (20) |
Packed (28) |
Unpacked (43) |
||
| AFB1 | Mean ± SD (μg/kg) | 0.86 ± 1.97 | 1.22 ± 3.91 | 0.26 ± 0.92 | 0.29 ± 0.41 | 0.34 ± 0.20 | 0.44 ± 0.34 |
| Range (μg/kg) | 0.13–12.90 | 0.14–18.40 | 0.07–4.87 | 0.84–0.96 | 0.08–0.90 | 0.06–1.78 | |
| p-value (packed vs. unpacked) | 0.59* | 0.11* | 0.143* | ||||
| OTA | Mean ± SD (μg/kg) | 40.8 ± 90.0 | 34.0 ± 80.50 | 2.23 ± 5.52 | 1.10 ± 3.18 | ||
| Range (μg/kg) | 0.12–452 | 0.02–162 | 0.02–23.8 | 0.02–10.8 | |||
| p-value (packed vs. unpacked) | 0.741* | 0.881* | |||||
All p-values are >0.05 indicating no significant difference between packed and unpacked sample means.
For AFB1, it was evident that in spices, herbs, and nuts the mean contamination levels found in unpackaged samples were higher than packaged ones, however, those differences were not statistically significant (p > 0.05).
As for OTA, the mean contamination levels found in packaged samples of spices and herbs were higher than unpackaged ones, however, those differences were also not statistically significant (p > 0.05).
3.5. Exposure level
The consumption levels of spices, herbs, and nuts, the average contamination of each item, and the exposure to AFB1 and OTA are presented in Table 7.
Table 7.
Average consumption of spices, herbs, and nuts, average contamination of AFB1 and OTA, and exposure level to AFB1 and OTA.
| Type | Consumption (g/person/day) | AFB1 contamination (ng/kg) | Exposure to AFB1 (ng/person/day) | OTA contamination (ng/kg) | Exposure to OTA (ng/person/day) |
|---|---|---|---|---|---|
| Spices | 1.33 | 967 | 1.29 | 38,765 | 51.6 |
| Herbs | 2.56 | 164 | 0.42 | 1,205 | 3.08 |
| Nuts | 33.6 | 398 | 13.4 | 0 | 0 |
| Total | 15.1 | 54.6 |
The total exposure to AFB1 was shown to be 15.1 ng/person/day so accordingly, the daily exposure was calculated in our study to be 0.2 ng/kg bw/day. Therefore, this exposure would be associated with 0.017 additional liver cancer cases per 100,000 persons per year.
On the other hand, the total exposure to OTA was shown to be 54.6 ng/person/day so accordingly, the daily exposure was calculated in our study to be 0.72 ng/kg bw/day, and therefore the weekly exposure is 5.04 ng/kg bw much less than the PTWI of 100 ng/kg bw set by JECFA.
4. Discussion
In this study, AFB1 was found in 100% and 20.4% of spices and herbs, respectively, with two nutmeg samples (2.7% of total spices samples) exceeding MTL of 5 μg/kg in spices. The mean concentration of AFB1 in spices was reported to be 0.97 μg/kg higher than that in herbs of 0.27 μg/kg. All types of spices had mean concentrations at levels below the limit of 5 μg/kg except nutmeg samples in which the AFB1 mean of 7.28 μg/kg exceeded the limit.
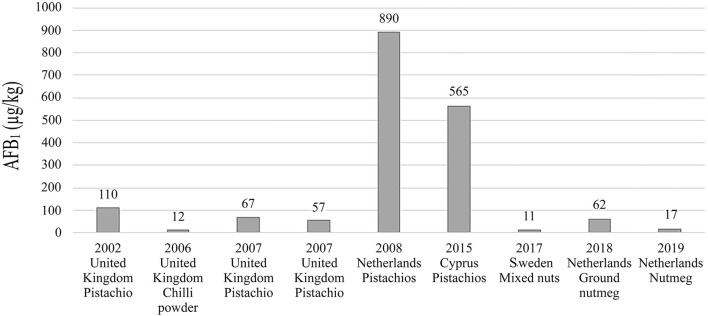
While, In Lebanon, a previous study done, reported the contamination of 15.9 and 8% of spices and herbs samples with AFB1, respectively, with 14 and 8% of samples exceeding MTL set by the EC (25). As mentioned previously, AFB1 contamination in Lebanese products of spices and nuts was also reported on the RASFF portal (28). Figure 1 shows examples of rejected imports and the concentration of AFB1 in each.
Figure 1.
Examples of reported border rejections on RASFF portal of Lebanese imports to the European Union due to high levels of AFB1 in spices, herbs, and nuts.
Several studies around the world reported contamination with AFB1 in spices, for example, studies done in Iran and Hungary showed that 13.3 and 16.5% of samples, respectively, exceeded MTL, which was much higher than that reported in this study (2.7%) (38, 39). In Morocco all tested samples were found contaminated, which is similar to our result (40). However, data from another countries in the world reported less contamination such as in Turkey, Italy, Spain, Portugal, and Brazil where AFB1 was found in 96, 46, 90, 43, and 61% of spices samples, respectively (41–45). As for herbs, few studies were performed worldwide; for example, AFB1 was found in 29% and 93.3% of herb samples in Egypt and India, respectively (46, 47).
In nuts, 98.6% of samples contained AFB1 at levels below MTL of 2 μg/kg and a low mean concentration of 0.40 μg/kg. Previously, Raad et al. reported contamination of nuts with AFB1 in Lebanon in which a composite group made up of nuts, seeds, olives, and dried dates had AFB1 at a mean concentration of 0.1 μg/kg (27). While Soubra et al. found that contamination with AFB1 in nuts ranged from 0.5 to 8.0 μg/kg and the mean was 1.0 μg/kg higher than the level reported in this study (26). The variation of results between this study and other ones might be due to differences in sampling periods, methods, preparation, and analysis. Moreover, high levels of AFB1 have been also reported in Lebanese nut imports to the European Union (Figure 1) (28). Around other parts of the world contamination studies reported, as well, the occurrence of AFB1 in nuts. For example, similar levels as this study were found in Iran and Brazil, where 96.5% of samples were found contaminated in both countries (48, 49). While in Libya and Pakistan lower contamination levels were found with 31.1% and 45% of samples contaminated with AFB1 (50, 51). However, in Pakistan and Brazil AFB1 contamination exceeded MTL in 39.5% and 2.8% of samples (48, 50), respectively, while in this study no sample was reported to contain AFB1 at levels above this limit.
Comparing the packaged and unpackaged samples, AFB1 mean concentration was higher in unpackaged samples for spices, herbs, and nuts, but this difference was not statistically significant.
On the other hand, OTA was prevalent in 100% of spices with 30.1% exceeding MTL (one sweet pepper, eight white pepper, six black pepper, four nutmeg, one cumin, and two spices mix samples). The mean concentration of OTA in spices was high at 38.8 μg/kg. The highest mean OTA concentration was reported in white pepper (132.03 μg/kg), followed by black pepper (77.53 μg/kg), nutmeg (68.05 μg/kg), sweet pepper (15.08 μg/kg), cumin (11.11 μg/kg), spices mix (9.33 μg/kg), turmeric (1.38 μg/kg), paprika (1.36 μg/kg), and cinnamon (0.72 μg/kg).
OTA was found in 44.4% of herb samples at a low mean concentration of 1.81 μg/kg, however, two kinds of herbs, namely, infused herbs and anise, had a higher mean concentration of OTA than other kinds at 10.04 and 5.62 μg/kg, respectively.
Previously in Lebanon, Darra et al., reported that 29.8% of spices samples contained OTA with 3% of samples exceeding MTL, while 10.5% of herbs were contaminated with no sample exceeding MTL. Furthermore, spices were contaminated with OTA at a range of 0.7–33.9 μg/kg (25), lower than the contamination range found in this study that was between 0.02 and 452 μg/kg. As for herbs, the range of contamination reported by Darra et al. (25) of 0.7–9.8 μg/kg was lower as well than that reported in this study between 0.02 and 23.8 μg/kg. The variation between this study and the previous one is related to differences in sampling period, method, and analysis.
Other studies done around the world reported contamination with OTA in spices, for example, in Hungary, 71.4% of samples were contaminated with 17.6% exceeding MTL (38). In other countries studies reported less contamination as well, such as in Tunisia, Spain, Korea, Malaysia, and Brazil where OTA was found in 70, 98, 22, 82, and 86 of spices samples, respectively (45, 52–55). As for herbs, 42.6% of samples were found contaminated with OTA in India, similar to this study with 44.4% positive samples, while lower levels were reported in Croatia where 14.3% of samples were contaminated (56, 57).
Since Lebanon imports spices, the high occurrence levels of OTA might indicate local contamination due to improper storage practices such as inappropriate temperature and humidity conditions in factories and/or in the market. This may allow for further contamination with OTA while in storage especially in traditional local shops where batches of spices and herbs are kept mostly in unsealed containers at room temperatures.
Exposure to AFB1 and OTA, from the tested samples, was also reported in this study. The exposure to AFB1 from spices, herbs, and nuts was found to be 0.2 ng/kg bw/d which would be associated with 0.017 additional liver cancer cases per 100,000 persons per year. On the other hand, the total exposure to OTA was shown to be 54.6 ng/person/day so accordingly, the daily exposure was calculated in our study to be 0.72 ng/kg bw/day, and therefore the weekly exposure is 5.04 ng/kg bw much less than the PTWI of 100 ng/kg bw set by JECFA indicating low risk of renal damage from spices and herbs consumption.
Compared to findings from other studies, the exposure to AFB1 from spices was found to be 0.017 ng/kg bw/d which is much lower than the value of 1.55 ng/kg bw/d reported in Lebanon by Al Ayoubi et al. (58). On the other hand, OTA exposure form spices was reported in this study to be 0.68 ng/kg bw/d at six times fold greater than the one determined by Al Ayoubi et al. (58) and reported to be 0.11 ng/kg bw/d. This variance in findings between the two studies could be due to the difference in tested sample types, methods of testing, and analysis.
5. Implications and control strategies
In this study AFB1 was reported at low levels in spices, herbs, and nuts in which the mean concentration was 1.0, 0.3, and 0.4 μg/kg, respectively. However, OTA was reported at high levels in spices at a mean concentration of 38.8 μg/kg, unlike herbs that had OTA at a mean concentration of 1.8 μg/kg. Therefore, the consumption of these commodities in Lebanon, especially spices, could lead to an increase in health risks associated with AFB1 and OTA knowing that these food products are extensively consumed by the Lebanese population. The exposure and effect of AFB1 and OTA are studied separately in this study, but nonetheless, their co-occurrence could lead to synergistic effects on health, therefore, causing additional health risks on Lebanese consumers. Added to that, other food products than spices, herbs, and nuts could contain AFB1 and OTA such as wheat, wheat-derivatives, dried fruits, coffee, wine, beer… and could add to the exposure to these toxins, therefore, increasing the health risks correlated with them.
The reported occurrence of AFB1 and OTA may be directly related to poor storage conditions that promote fungal invasion and mycotoxin production. Therefore, regular inspections are required especially on warehouses, factories, supermarkets, roasteries, and local shops to enforce the application of good storage practices.
Finally, continuous monitoring of AFB1 and OTA in spices, herbs, and nuts in Lebanon should be implemented and studies should be done routinely to ensure their safety.
6. Conclusion
AFB1 and OTA contamination in food can present a risk to public health and this study showed contamination of spices, herbs, and nuts in Lebanon at different levels. Therefore, assuring the safety of those food items consumed at regular basis by Lebanese population is essential to prevent health problems related specifically to chronic exposure to AFB1 and OTA. Hence, continuous monitoring and inspections are recommended.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RD contributed to application, statistical analysis, and writing of the study. AK contributed to the planning, supervision, project administration, reviewing, writing, and approving of final version. RM and AI contributed to supervision, reviewing, and approving of final version. MH, KB, KJ, and LK contributed to reviewing and approving of final version. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Daou R, Joubrane K, Khabbaz LR, Maroun RG, Ismail A, El Khoury A. Aflatoxin B1 and ochratoxin A in imported and Lebanese wheat and -products. Food Addit Contam Part B. (2021) 14:1–9. 10.1080/19393210.2021.1933203 [DOI] [PubMed] [Google Scholar]
- 2.Assaf JC, Nahle S, Louka N, Chokr A, Atoui A, El Khoury A. Assorted methods for decontamination of aflatoxin. Toxins. (2020) 11:1–23. 10.3390/toxins11060304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchese S, Polo A, Ariano A, Velotto S, Costantini S, Severino L. Aflatoxin B1 and M1: biological properties and their involvement in cancer development. Toxins. (2018) 10:214. 10.3390/toxins10060214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bullerman L, Bianchini A. Stability of mycotoxins during food processing. Int J Food Microbiol. (2007) 119:140–6. 10.1016/j.ijfoodmicro.2007.07.035 [DOI] [PubMed] [Google Scholar]
- 5.Flores-Flores ME, Lizarraga E, López de. Cerain A, González-Peñas E. Presence of mycotoxins in animal milk: a review. Food Control. (2015) 53:163–76. 10.1016/j.foodcont.2015.01.020 [DOI] [Google Scholar]
- 6.Bui-Klimke T, Wu F. Ochratoxin A and human health risk: a review of the evidence. Crit Rev Food Sci Nutr. (2014) 55:1860–9. 10.1080/10408398.2012.724480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer . International agency for research on cancer iarc monographs on the evaluation of carcinogenic risks to humans. Iarc Monogr Eval Carcinog Risks Hum. (2002) 80:273–338. [Google Scholar]
- 8.el Khoury A, Atoui A. Ochratoxin A: general overview and actual molecular status. Toxins. (2010) 2:461–93. 10.3390/toxins2040461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liew W-P-P, Mohd-Redzwan S. Mycotoxin: its impact on gut health and microbiota. Front Cell Infect Microbiol. (2018) 8:60. 10.3389/fcimb.2018.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barta F, Levova K, Hodek P, Schmeiser HH, Arlt VM, Stiborova M. The effects of heavy metal ions, phthalates and ochratoxin A on oxidation of carcinogenic aristolochic acid I causing Balkan endemic nephropathy. Neuro Endocrinol Lett. (2015) 36(Suppl 1):13–21. [PubMed] [Google Scholar]
- 11.Malir F, Ostry V, Pfohl-Leszkowicz A, Malir J, Toman J. Ochratoxin A: 50 years of research. Toxins. (2016) 8:191. 10.3390/toxins8070191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlovic N. Balkan endemic nephropathy - current status and future perspectives. Clin Kidney J. (2013) 6:257–65. 10.1093/ckj/sft049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klingelhöfer D, Braun M, Schöffel N, Oremek GM, Brüggmann D, Groneberg DA. Ochratoxin – characteristics, influences and challenges of global research. Food Control. (2020) 114:107230. 10.1016/j.foodcont.2020.107230 [DOI] [Google Scholar]
- 14.European Commission . Directorate-General for Health Food Safety. RASFF Annual Report 2018, Publications Office (2019). Available online at: https://data.europa.eu/doi/10.2875/914558
- 15.Thanushree MP, Sailendri D, Yoha KS, Moses JA, Chinnaswamy A. Mycotoxin contamination in food: an exposition on spices. Trends Food Sci Technol. (2019) 93:69–80. 10.1016/j.tifs.2019.08.010 [DOI] [Google Scholar]
- 16.Daou R, Joubrane K, Maroun RG, Khabbaz LR, Ismail A, El Khoury A. Mycotoxins: factors influencing production and control strategies. AIMS Agric Food. (2021) 6:416–47. 10.3934/agrfood.2021025 [DOI] [Google Scholar]
- 17.Sami Halabi A, Ghanem N. Strategic Review of Food and Nutrition Security in Lebanon. United Nations-ESCWA; (2016), 1−108. [Google Scholar]
- 18.El-Jardali F, Hammoud R, Kamleh R, Jurdi M. Briefing Note Protecting Consumers in Lebanon: The Need for Effective Food Safety System. (2014) 1–27. Available online at: https://www.aub.edu.lb/k2p/Documents/K2PBNFoodSafetyEnglish.pdf (accessed August 10, 2022).
- 19.Ministry of Agriculture . Decision No. 322/1 Permissible Levels of Aflatoxin in Some Foodstuffs. Beirut: (2004). [Google Scholar]
- 20.Makhlouf J, Carvajal-Campos A, Querin A, Tadrist S, Puel O, Lorber S, et al. Morphologic, molecular and metabolic characterization of Aspergillus section Flavi in spices marketed in Lebanon. Sci Rep. (2019) 9:5263. 10.1038/s41598-019-41704-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baydoun S, Chalak L, Dalleh H, Arnold N. Ethnopharmacological survey of medicinal plants used in traditional medicine by the communities of Mount Hermon, Lebanon. J Ethnopharmacol. (2015) 173:139–56. 10.1016/j.jep.2015.06.052 [DOI] [PubMed] [Google Scholar]
- 22.Joubrane K, Mnayer D, el Khoury A, El Khoury A, Awad E. Co-occurrence of aflatoxin B1 (AFB1) and ochratoxin A (OTA) in Lebanese stored wheat. J Food Prot. (2020) 83:1547–52. 10.4315/JFP-20-110 [DOI] [PubMed] [Google Scholar]
- 23.European Commission. Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union. (2006) 364:5. [Google Scholar]
- 24.LIBNOR . NL 546 : 2005, Mixed spices and condiments. Dekwneh, Lebanon: (2005). [Google Scholar]
- 25.Darra N, Gambacorta L, Solfrizzo M. Multimycotoxins occurrence in spices and herbs commercialized in Lebanon. Food Control. (2018) 95:63–70. 10.1016/j.foodcont.2018.07.033 [DOI] [Google Scholar]
- 26.Soubra L, Sarkis D, Hilan C, Verger P. Occurrence of total aflatoxins, ochratoxin A and deoxynivalenol in foodstuffs available on the Lebanese market and their impact on dietary exposure of children and teenagers in Beirut. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. (2009) 26:189–200. 10.1080/02652030802366108 [DOI] [PubMed] [Google Scholar]
- 27.Raad F, Nasreddine L, Hilan C, Bartosik M, Parent-Massin D. Dietary exposure to aflatoxins, ochratoxin A and deoxynivalenol from a total diet study in an adult urban Lebanese population. Food Chem Toxicol. (2014) 73:35–43. 10.1016/j.fct.2014.07.034 [DOI] [PubMed] [Google Scholar]
- 28.RASFF Portal . (2021). Available online at: https://webgate.ec.europa.eu/rasff-window/portal/?event=searchResultList (accessed September 3, 2022).
- 29.Aoun M, Helou E, Sleilaty G, Zeenny RM, Chelala D. Cost of illness of chronic kidney disease in Lebanon: from the societal and third-party payer perspectives. BMC Health Serv Res. (2022) 22:586. 10.1186/s12913-022-07936-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shamseddine A, Saleh A, Charafeddine M, Seoud M, Mukherji D, Temraz S, et al. Cancer trends in Lebanon: a review of incidence rates for the period of 2003-2008 and projections until 2018. Popul Health Metr. (2014) 12:4. 10.1186/1478-7954-12-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebanese Ministry of Public Health NCR . Frequency (nb) of Incident Cases by Primary Site & Age Group. Available online at: https://www.moph.gov.lb/userfiles/files/Esu_data/Esu_ncr/BA2016.HTM (accessed December 5, 2022).
- 32.R-Biopharm Rhone LTD. AFLAOCHRA PREP® Product Code: P89/P89B. Glasgow, Scotland. [Google Scholar]
- 33.R-Biopharm Rhone LTD . AFLAPREP® Product Code: DP07/P07. Glasgow, Scotland. [Google Scholar]
- 34.Asghar MA, Iqbal J, Ahmed A, Khan MA, Shamsuddin ZA, Jamil K. Development and validation of a high-performance liquid chromatography method with post-column derivatization for the detection of aflatoxins in cereals and grains. Toxicol Ind Health. (2016) 32:1122–34. 10.1177/0748233714547732 [DOI] [PubMed] [Google Scholar]
- 35.USAID . Data Quality Assessment Checklist. (2014). [Google Scholar]
- 36.Joint FAO/WHO Expert Comittee on Food Additives. 49th JECFA Meeting. Evaluation of Certain Food Additives and Contaminants. (1999). p. 1–5. [Google Scholar]
- 37.Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain food Additives and Contaminants. WHO Technical Report Series (2007). [Google Scholar]
- 38.Fazekas B, Tar A, Kovacs M. Aflatoxin and ochratoxin A content of spices in Hungary. Food Addit Contam. (2005) 22:856–63. 10.1080/02652030500198027 [DOI] [PubMed] [Google Scholar]
- 39.Khazaeli P, Mitra M, HEIDARI M, Asadikaram G, Lari Najafi M. Prevalence of aflatoxin contamination in herbs and spices in different regions of Iran. Iran J Public Health. (2017) 46:1540–5. [PMC free article] [PubMed] [Google Scholar]
- 40.Zinedine A, Brera C, Elakhdari S, Catano C, Debegnach F, Angelini S, et al. Natural occurrence of mycotoxins in cereals and spices commercialized in Morocco. Food Control. (2006) 17:868–74. 10.1016/j.foodcont.2005.06.001 [DOI] [Google Scholar]
- 41.Ardic M, Karakaya Y, Atasever M, Durmaz H. Determination of aflatoxin B-1 levels in deep-red ground pepper (isot) using immunoaffinity column combined with ELISA. Food Chem Toxicol. (2008) 46:1596–9. 10.1016/j.fct.2007.12.025 [DOI] [PubMed] [Google Scholar]
- 42.Hernandez-Hierro JM, Garcia-Villanova RJ, Torrero P, Fonseca I. Aflatoxins and ochratoxin A in red paprika for retail sale in spain: occurrence and evaluation of a simultaneous analytical method. J Agric Food Chem. (2008) 56:751–6. 10.1021/jf073002c [DOI] [PubMed] [Google Scholar]
- 43.Martins M, Martins H, Fernando B. Aflatoxins in spices marked in Portugal. Food Addit Contam. (2001) 18:315–9. 10.1080/02652030120041 [DOI] [PubMed] [Google Scholar]
- 44.Romagnoli B, Menna V, Gruppioni N, Bergamini C. Aflatoxins in spices, aromatic herbs, herb-teas and medicinal plants marketed in Italy. Food Control. (2007) 18:697–701. 10.1016/j.foodcont.2006.02.020 [DOI] [Google Scholar]
- 45.Shundo L, Palma de Almeida A, Alaburda J, Lamardo L, Navas S, Ruvieri V, et al. Aflatoxins and ochratoxin A in Brazilian paprika. Food Control. (2009) 20:1099–102. 10.1016/j.foodcont.2009.02.008 [DOI] [Google Scholar]
- 46.Selim MI, Popendorf W, Ibrahim MS, el Sharkawy S, el Kashory ES. Aflatoxin B1 in common Egyptian foods. J AOAC Int. (1996) 79:1124–9. 10.1093/jaoac/79.5.1124 [DOI] [PubMed] [Google Scholar]
- 47.Roy AK, Sinha KK, Chourasia HK. Aflatoxin contamination of some common drug plants. Appl Environ Microbiol. (1988) 54:842–3. 10.1128/aem.54.3.842-843.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milhome M, Lima CG, Lima LK, Lima F, Sousa D, do Nascimento R. Occurrence of aflatoxins in cashew nuts produced in northeastern Brazil. Food Control. (2014) 42:34–7. 10.1016/j.foodcont.2014.01.033 [DOI] [Google Scholar]
- 49.Rezaei M, Karimi F, Parviz M, Behzadi AA, Javadzadeh M, Mohammadpourfard I, et al. An empirical study on aflatoxin occurrence in nuts consumed in Tehran, Iran 2013. Health. (2014) 6:649–53. 10.4236/health.2014.68084 [DOI] [Google Scholar]
- 50.Iqbal S, Rafique Asi M, Mahmood Z, Misbah S, Shahid M, Malik N. Co-occurrence of aflatoxins and ochratoxin A in nuts, dry fruits and nuty products. J Food Saf . (2018) 38:1–7. 10.1111/jfs.12462 [DOI] [Google Scholar]
- 51.Abushahma H, Najii S, Amra HA. Natural incidence of aflatoxins and ochratoxin A nuts collected from local market in Tripoli. Int J Curr Microbiol Appl Sci. (2017) 6:1479–86. 10.20546/ijcmas.2017.603.170 [DOI] [Google Scholar]
- 52.Zaied C, Abid S, Bouaziz C, Chouchane S, Jomaa M, Bacha H. Ochratoxin A levels in spices and dried nuts consumed in Tunisia. Food Addit Contam Part B, Surveill. (2010) 3:52–7. 10.1080/19440041003587302 [DOI] [PubMed] [Google Scholar]
- 53.Santos L, Marín S, Ramos A. Co-occurrence of aflatoxins, ochratoxin A and zearalenone in Capsicum powder samples available on the Spanish market. Food Chem. (2010) 122:826–30. 10.1016/j.foodchem.2010.03.070 [DOI] [Google Scholar]
- 54.Ahn J, Kim D, Jang H-S, Kim Y, Shim W-B, Chung D-H. Occurrence of ochratoxin A in Korean red paprika and factors to be considered in prevention strategy. Mycotoxin Res. (2010) 26:279–86. 10.1007/s12550-010-0067-2 [DOI] [PubMed] [Google Scholar]
- 55.Jalili M, Jinap S. Natural occurrence of aflatoxins and ochratoxin A in commercial dried chili. Food Control. (2012) 24:160–4. 10.1016/j.foodcont.2011.09.020 [DOI] [Google Scholar]
- 56.Halt M. Moulds and mycotoxins in herb tea and medicinal plants. Eur J Epidemiol. (1998) 14:269–74. 10.1023/A:1007498613538 [DOI] [PubMed] [Google Scholar]
- 57.Kumar S, Roy AK. Occurrence of aflatoxin in some liver curative herbal medicines. Lett Appl Microbiol. (2008) 17:112–4. 10.1111/j.1472-765X.1993.tb01437.x34574455 [DOI] [Google Scholar]
- 58.Al Ayoubi M, Solfrizzo M, Gambacorta L, Watson I, Darra N. Risk of exposure to aflatoxin B1, ochratoxin A, and fumonisin B1 from spices used routinely in Lebanese cooking. Food Chem Toxicol. (2021) 147:111895. 10.1016/j.fct.2020.111895 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.